PURPOSE
Timely lung cancer surgery is a metric of high-quality cancer care and improves survival for early-stage non–small-cell lung cancer. Historically, Black patients experience longer delays to surgery than White patients and have lower survival rates. Antiracism interventions have shown benefits in reducing racial disparities in lung cancer treatment.
METHODS
We conducted a secondary analysis of Accountability for Cancer Care through Undoing Racism and Equity, an antiracism prospective pragmatic trial, at five cancer centers to assess the impact on overall timeliness of lung cancer surgery and racial disparities in timely surgery. The intervention consisted of (1) a real-time warning system to identify unmet care milestones, (2) race-specific feedback on lung cancer treatment rates, and (3) patient navigation. The primary outcome was surgery within 8 weeks of diagnosis. Risk ratios (RRs) and 95% CIs were estimated using log-binomial regression and adjusted for clinical and demographic factors.
RESULTS
A total of 2,363 patients with stage I and II non–small-cell lung cancer were included in the analyses: intervention (n = 263), retrospective control (n = 1,798), and concurrent control (n = 302). 87.1% of Black patients and 85.4% of White patients in the intervention group (P = .13) received surgery within 8 weeks of diagnosis compared with 58.7% of Black patients and 75.0% of White patients in the retrospective group (P < .01) and 64.9% of Black patients and 73.2% of White patients (P = .29) in the concurrent group. Black patients in the intervention group were more likely to receive timely surgery than Black patients in the retrospective group (RR 1.43; 95% CI, 1.26 to 1.64). White patients in the intervention group also had timelier surgery than White patients in the retrospective group (RR 1.10; 95% CI, 1.02 to 1.18).
CONCLUSION
Accountability for Cancer Care through Undoing Racism and Equity is associated with timelier lung cancer surgery and reduction of the racial gap in timely surgery.
INTRODUCTION
Lung cancer is the leading cause of cancer deaths in the United States with an estimated 131,880 deaths expected in 2021.1 Surgical resection is the standard treatment for early-stage non–small-cell lung cancer (NSCLC) and results in an overall 5-year survival rate of 80%.2 Timely surgery also improves survival for early-stage NSCLC and is a key indicator of high-quality cancer care.3-8 Treatment delays > 8 weeks may lead to pathologic upstaging, progression of disease, and increased mortality.3,9,10 Although optimal timing of lung cancer surgery has not been precisely defined, the RAND corporation recommends surgery within 6 weeks of diagnosis11 and several studies suggest that surgery within 6-8 weeks improves outcomes.3-5,7,12,13
CONTEXT
Key Objective
Racial inequities in lung cancer surgery and timely lung cancer care have been well documented and may contribute to poor survival among Black patients. The Accountability for Cancer Care through Undoing Racism and Equity (ACCURE), an antiracism system–based intervention, which included race-specific reporting on treatment completion rates to clinical teams, racial equity training, and patient navigation, previously demonstrated near elimination of the treatment gap between Black and White patients with early-stage non–small-cell lung cancer. A secondary analysis was conducted to assess the impact of ACCURE on racial disparities in timely lung cancer surgery.
Knowledge Generated
Black patients experienced longer delays to surgery than White patients in the preintervention period. During ACCURE, both Black and White patients experienced timelier surgery and the racial gap in timely lung cancer surgery was no longer observed.
Relevance
Antiracism system–based interventions may reduce racial disparities in timely lung cancer care.
Black patients with early-stage lung cancer are more likely to experience treatment delays4,14,15 and have higher mortality rates than White patients.4,6,7,14,16-21 Black race is associated with lung cancer treatment delays averaging 6.7 days.13 Factors contributing to delays in timely care include inadequate access to specialty care, poor care coordination, and poor patient-provider communication.3,5,12,17,22-24 Differential access to quality cancer care on the basis of race is a result of systemic racism, a fundamental cause of racial health inequities.25-28 Although racial disparities in lung cancer treatment, timely care, and outcomes have been well documented, few system-based interventions have addressed them.
The Accountability for Cancer Care through Undoing Racism and Equity (ACCURE) study, a pragmatic prospective quality improvement trial, was one of the first studies of its kind to address systemic racism and essentially eliminated the treatment gap between Black and White patients with stage I and II NSCLC.18,29 The ACCURE study was developed by the Greensboro Health Disparities Collaborative, a community-academic-medical partnership with expertise in antiracism community–based participatory research.18,30 The intervention was designed to enhance cancer care systems' transparency and accountability for race-specific inequities by reporting race-specific treatment outcomes to the care teams and racial equity training for patient navigators and physician champions. Given the success in eliminating the treatment gap between Black and White patients, we evaluated whether the ACCURE intervention was associated with timely lung cancer surgery among Black patients and reduced any racial gap.
METHODS
Data Source and Study Population
We conducted a secondary analysis of two multi-institutional prospective pragmatic clinical trials conducted at five cancer centers: Lung Cancer Surgery: Decisions against Life Saving Care—The Intervention and ACCURE to assess the impact on timely surgery. The cancer centers were academic affiliate or community practices located in urban and rural regions of Pennsylvania, South Carolina, and North Carolina. Both trials had comparable study designs and were thus treated as a single trial (herein ACCURE) for all analyses. The intervention consisted of (1) a real‐time warning system derived from electronic health records (EHRs) for missed appointments and time-sensitive clinical milestones to enhance race-specific transparency in adhering to standards of care; (2) a physician champion who reviewed quarterly reports with surgeons and their clinical teams on race-specific treatment completion rates to enhance transparency; and (3) a nurse navigator trained in racial equity analysis who accessed the warning system on a daily basis, informed the clinical team of care delays, and addressed patients' barriers to care, such as lack of transportation or misinformation about surgery, to enhance accountability. Missed clinical milestones that triggered an alert for the navigator included the following: patients' missed appointments, care delays such as no follow‐up appointment or diagnostic testing scheduled within 30 days of the initial patient visit, no surgery or radiation scheduled within 90 days, and no surgery or radiation received within 120 days of the initial visit.
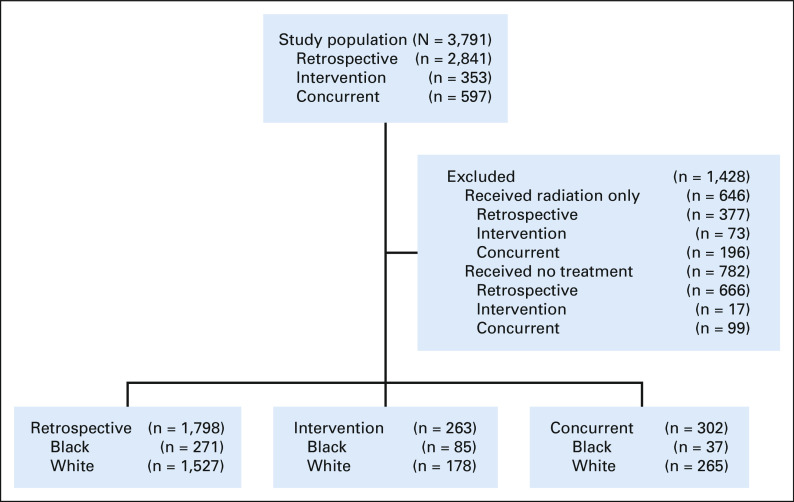
In the primary study, research staff identified study participants using the EHR to screen multidisciplinary clinic schedules. Inclusion criteria for this secondary analysis were patients with stage I and II NSCLC treated with surgery (n = 2,363). Patients were excluded from the analyses if they received no treatment (n = 782) or were treated with radiation alone (n = 646). There were three separate cohorts: (1) consented patients enrolled on the intervention arm from April 2013 to December 2016; (2) a retrospective (historical) control group composed of patients diagnosed and treated before the intervention period from January 1, 2007, to December 31, 2012; and (3) a concurrent control arm that included patients not enrolled in the trial but were diagnosed in 2014 and 2015 and received surgery at two participating study sites that had informatics teams that could provide automated data for nonstudy participants.18 The research associates who enrolled and consented patients during the intervention could not provide concurrent coverage for all potential enrollment sites and thus covered sites on a rolling basis. Concurrent controls were those that the research associates did not enroll or approach for the study. Figure 1 shows a schematic representation of participants included in this secondary analysis. The study was approved by the governing institutional review board of each site. All patients in the intervention arm provided informed consent.
FIG 1.
Flow diagram.
Study Outcome Variables and Comparison Groups
The primary outcome variable was completion of surgery within 8 weeks of diagnosis. The time from diagnosis (radiographic or histologic) to surgery was dichotomized into < 56 days or ≥ 56 days. A secondary outcome, median time from diagnosis to surgery measured in days, was also assessed. Baseline characteristics and outcomes in the intervention group were compared with the two control groups. The retrospective cohort served as the baseline comparison of the primary and secondary study outcomes. Comparison with the concurrent control group was to evaluate for secular trends. Estimates of racial differences in the study outcomes between the intervention and retrospective groups and between intervention and concurrent groups were also assessed. For between-group and within-group comparisons, data from each study group were included in the model where race-study arm combinations were used to estimate outcome differences.
Covariates
Patient demographics including age, sex, race, mean household income by zip code, and clinical stage at diagnosis were extracted from EHRs. Charlson comorbidity scores were derived from the cancer registry for retrospective and concurrent controls but were individually assessed for intervention participants using EHRs.
Statistical Analyses
Patient-level demographic characteristics were summarized with descriptive statistics. Median time to surgery was compared by race and across the three study groups using Mann-Whitney tests. Pearson's chi-squared tests were used to compare crude proportions of patients who received surgery within 6 and 8 weeks, respectively. Separate univariate and multivariable log-binomial regression models within each study group assessed the association between race-study arm combinations with the likelihood of surgery within 56 days of diagnosis, adjusting for age, sex, Charlson score, mean household income, site of care, and clinical stage. Sensitivity analysis was performed to assess surgery within 42 days using a narrower treatment window on the basis of RAND corporation recommendations for timely surgery.11 A single multivariable log-binomial regression analysis comparing the intervention arm with the retrospective and concurrent controls was adjusted for age, race, sex, Charlson score, mean household income, site of care, clinical stage of diagnosis, and race by study group interaction. All statistical analyses were performed in SAS (version 9.4).
RESULTS
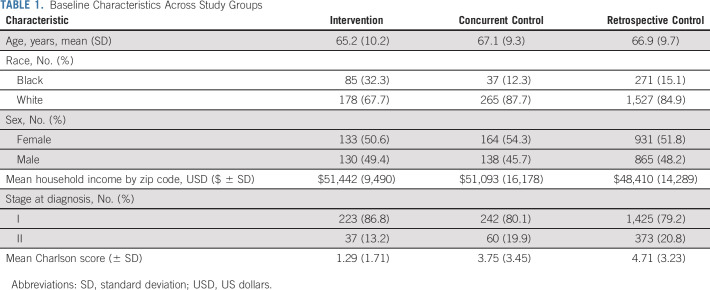
A total of 2,363 patients underwent surgery. Across all study arms, 393 (19.9%) were Black, with higher representation of Black patients within the intervention group (n = 85, 33.5%) relative to both retrospective (n = 271, 15.1%) and concurrent (n = 37, 12.3%) control groups. Baseline demographics by study arm are presented in Table 1. Age and sex were similar across all groups, whereas the intervention group had a lower Charlson score and a higher proportion of patients with stage I NSCLC.
TABLE 1.
Baseline Characteristics Across Study Groups
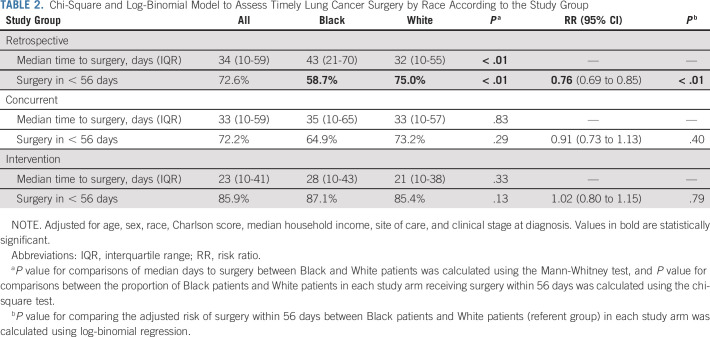
The median time to surgery, as summarized in Table 2, was 34 days in the retrospective group, 33 days in the concurrent group, and 23 days in the intervention group. When evaluated by race, the median time to surgery in the retrospective group was 43 days for Black patients compared with 32 days for White patients. During the intervention, the median time to surgery decreased to 28 days for Black patients and 21 days for White patients. For the concurrent arm, the median time to surgery was 35 days for Black patients and 33 days for White patients.
TABLE 2.
Chi-Square and Log-Binomial Model to Assess Timely Lung Cancer Surgery by Race According to the Study Group
In assessments of timely surgery, 85.9% of all patients in the intervention group received surgery within 8 weeks of diagnosis compared with 72.6% of patients in the retrospective arm and 72.2% in the concurrent arm. In stratified analyses by race and study group, 87.1% of Black patients in the intervention group received surgery within 56 days compared with 58.7% of Black patients in the retrospective group and 64.9% in the concurrent control group. Among White patients, 85.4% received surgery within 56 days in the intervention arm compared with 75.0% in the retrospective arm and 73.2% in the concurrent control arm. Multivariable analyses showed a racial gap in timely surgery in the retrospective (risk ratio [RR] 0.76; 95% CI, 0.69 to 0.85) and concurrent (RR 0.91; 95% CI, 0.73 to 1.13) control groups with Black patients less likely to receive timely surgery than White patients. However, in the intervention group, Black patients were equally likely to have timely surgery (RR 1.03; 95% CI, 0.93 to 1.14) as White patients (Table 2).
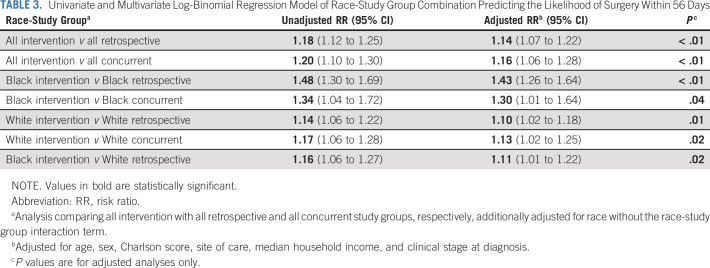
Multivariable analyses comparing all patients in the intervention group with those in the retrospective and concurrent control groups demonstrated a 14% and 16% higher likelihood of timely surgery, respectively (P < .01 for both). Black patients in the intervention group had a higher likelihood of receiving surgery within 8 weeks than Black patients in the retrospective (RR 1.43; 95% CI, 1.26 to 1.64) and concurrent control groups (RR 1.30; 95% CI, 1.01 to 1.64) and White patients in the retrospective group (RR 1.11; 95% CI, 1.01 to 1.22). White patients in the intervention group were also more likely to have surgery within 8 weeks than White patients in the retrospective group (RR 1.10; 95% CI, 1.02 to 1.18) and concurrent group (RR 1.13; 95% CI, 1.02 to 1.25; Table 3).
TABLE 3.
Univariate and Multivariate Log-Binomial Regression Model of Race-Study Group Combination Predicting the Likelihood of Surgery Within 56 Days
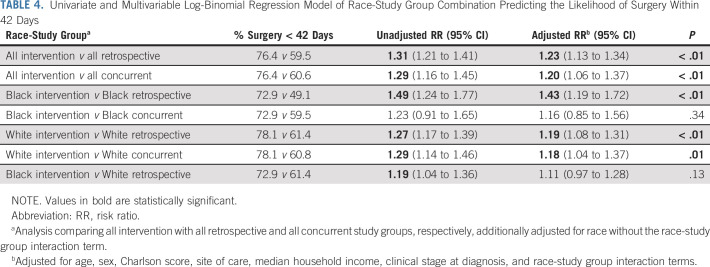
As a sensitivity analysis, surgery within 6 weeks of diagnosis was assessed. The proportion of patients who received surgery within 6 weeks of diagnosis was 59.5% in the retrospective arm, 60.6% in the concurrent control, and 76.4% in the intervention arm. Patients in the intervention group had a > 20% higher likelihood of surgery within 6 weeks than those in each of the control groups (P < .01 for both). Black patients in the intervention group had a higher likelihood of surgery within 6 weeks of diagnosis compared with Black patients in the retrospective arm (RR 1.43; 95% CI, 1.19 to 1.72) and concurrent control group (RR 1.16; 95% CI, 0.85- to 1.56). White patients in the intervention group also had a higher likelihood of completing surgery within 6 weeks compared with White patients in the retrospective group (RR 1.19; 95% CI, 1.08 to 1.25) and concurrent group (RR 1.18; 95% CI, 1.04 to 1.37; Table 4).
TABLE 4.
Univariate and Multivariable Log-Binomial Regression Model of Race-Study Group Combination Predicting the Likelihood of Surgery Within 42 Days
DISCUSSION
Our study demonstrated two key findings: The ACCURE intervention was associated with improved timely lung cancer surgery for Black and White patients and a reduction in the racial gap in timely care. Patients who were part of a system-based, multifaceted intervention on average received surgery nearly 2 weeks sooner than those in the retrospective control group. When considering the recommended 8-week timeframe to achieve better survival outcomes, more than 85% of patients from both racial groups received surgery on time although that threshold was not achieved in either racial group across retrospective and concurrent controls.
Beyond the racial disparity lens, there has been increased scrutiny on the time from diagnosis to treatment in lung cancer in recent years. With the advent of increasingly complex workup and staging procedures including molecular testing and PET imaging, the median time to treatment in early-stage lung cancer has increased from 26 days in 2004 to 34 days in 2013. Thus, the secular trend has been moving in the wrong direction for all patients. This is especially troubling since each week of treatment delay results in a 3.2% drop in survival for stage I NSCLC.13 Our findings suggest that the ACCURE intervention may be a powerful tool to not only reduce racial disparities in timely care but also may likely improve cancer survival for all.
To our knowledge, a significant improvement in timely lung cancer surgery for both Black and White patients with early-stage NSCLC and the reduced racial gap have not been previously demonstrated. Other studies have demonstrated improvements in timely lung cancer treatment more broadly, but all stages of lung cancer and multiple treatment modalities were included, thus limiting comparison with our findings.31,32 Moreover, these studies did not assess timeliness of care specifically for Black patients. However, the treatment delays observed in our retrospective and concurrent controls, with a median of 34 and 33 days to surgery, respectively, are very consistent with findings from the literature.33-35
Another distinct feature of this analysis compared with others is the multilevel study design. Multilevel interventions can address the interplay of provider-, interpersonal-, and system-level factors that contribute to racial inequities in health outcomes.36,37 The multifaceted ACCURE system–based intervention combined patient navigation with a warning system that recognized the timing of care milestones in real time (interpersonal- and system-level factors) and enhanced transparency of race-specific reporting of surgery completion rates to clinical teams (provider-level).18 Quarterly reporting of race-specific feedback to the clinical team likely contributed to timely care for Black patients by increasing transparency and accountability for racial differences in treatment completion rates. Race-specific data transparency in ACCURE increases the likelihood of reversing the unintentional consequence of colorblind practices in medical settings.38 This audit and feedback mechanism likely led to more timely discussions about surgery with Black patients, resulting in less delay. Audit and feedback have been previously shown to improve quality of care when feedback is provided regularly and is delivered by a trusted colleague, such as the physician champion employed in ACCURE.39
Patient navigation was also likely an important driver of timely surgery. Patient navigation is a well-known intervention with demonstrated evidence for improving patient adherence to care across the cancer continuum for multiple cancer types.40,41 However, few studies have specifically evaluated the benefit of patient navigation on improving timely lung cancer treatment.31,32 Nurse navigators in ACCURE received an alert when time-sensitive care milestones were missed and were specially trained to address patient barriers to care, such as distrust and misconceptions about lung cancer surgery.18 The automated warning system derived from the EHRs likely improved timely care for all patients by providing an efficient mechanism for the nurse navigators to address time-sensitive barriers to care. A study on an EHR trigger–based intervention targeting primary care providers demonstrated reduced delays in time to diagnostic evaluation of cancer.42 The nurse navigators' specialized communication training, which included an intensive study of historical and contemporary barriers that Black Americans face from institutional racism, in addition to enhanced communication methods such as teach-back,43 might have also improved timeliness of surgery specifically for Black patients. Patient navigation services have been shown to foster trust, which is particularly critical for Black patients given their experiences of mistreatment and disrespect by health providers.44
Although this study demonstrated improvement in overall timeliness of lung cancer surgery and a reduction in the racial gap in timely care, there were some limitations. First, this was a secondary analysis, and several demographic and clinical characteristics were not well balanced across the three groups such as Black race, mean Charlson score, and clinical lung cancer stage as the analyses were limited to patients receiving surgery. The percentage of Black patients in the intervention group was three times as high as the control groups because of intentional oversampling since the primary aim of the ACCURE was powered to address Black-White treatment disparities.18 Mean Charlson score was also notably different across the three study groups with lower mean scores in the intervention arm. Different data sources were used to tabulate the score, with more stringent comorbidity assessment in the intervention arm, compared with unfiltered comorbidity data extracted from the cancer registry in the retrospective and concurrent control groups.18 To address these differences, we adjusted for mean Charlson score and clinical stage in multivariable analyses. Another potential limitation is the lack of adjustment for insurance status. Most study participants (96%) were insured, largely by Medicare; data on secondary insurance such as Medicaid or Medicare supplement were not available. However, given near universal health coverage for our study population, secondary insurance type would likely have a small effect on the timeliness of surgery.45 We also did not assess the distance from patient home or census tract to the site of care, a potentially important indicator for timely care,46,47 as geo-coded data were not collected in the primary study. There were also limitations related to the sample size and study design. The Black-White comparisons for median time to surgery and receipt of surgery within 8 weeks in the concurrent control group were likely underpowered to detect a statistically significant difference because of the small sample size of Black patients (n = 37) in that study arm. However, the a priori purpose of the concurrent control arm was to ensure that improvements in timely care or reduction in the racial disparities, if any, between the intervention and retrospective group were not due to secular trends. Point estimates in all multivariable analyses suggest a higher likelihood of timely surgery for all study participants associated with ACCURE.
The cost of developing the ACCURE system relative to the achievement of cancer treatment equity at five participating institutions was low. Costs included time for one full-time analyst to build the real-time warning system (9 months for initial build), 10% of local analysts time at each of the five sites to map and automate uploads of EHR data to an umbrella system and system maintenance, and racial equity training. Two participating institutions are committed to embedding the real-time warning system program into their own EHR system, saving additional costs.
In conclusion, our results suggest that a multifaceted system change intervention grounded in an antiracism framework likely reduced racial disparities in timely lung cancer surgery. Both Black and White patients with stage I and II NSCLC benefited from this intervention. Given the ubiquitous availability of EHRs and the demonstrated value of patient navigation in cancer care across cancer centers in the United States, implementation of the ACCURE system change could be transferrable in reducing delays and closing the racial gap in timely care for other cancers.
ACKNOWLEDGMENT
We thank the members of the Greensboro Health Disparities Collaborative and Hayley Morris for their contribution to this study.
Emily Damone
Research Funding: Genentech/Roche
Cleo Samuel-Ryals
Employment: Flatiron Health
Uncompensated Relationships: Voluntis
Matthew Manning
Stock and Other Ownership Interests: Fuse Oncology
Samuel Cykert
Consulting or Advisory Role: Pfizer
No other potential conflicts of interest were reported.
See accompanying editorial on page 1718
DISCLAIMER
The statements contained herein are solely those of the authors and do not represent or imply concurrence or endorsement by the University of North Carolina or NCI. The funding source had no role in the review of the literature, preparation of the manuscript, or decision to submit the manuscript for publication.
PRIOR PRESENTATION
Presented in part at the ASCO Virtual Annual Meeting, June 4-8, 2021.
SUPPORT
Supported by the University of North Carolina Simmons Scholar program (M.C.), the National Cancer Institute (NCI)–funded Cancer Health Disparities Training grant No. (T32CA128582) (J.N.S.), and the NCI-funded Mentored Research Scientist Award (K01CA218473-04) (C.A.S.-R.).
AUTHOR CONTRIBUTIONS
Conception and design: Marjory Charlot, Stephanie Baker, Eugenia Eng, Matthew Manning, Samuel Cykert
Administrative support: Marjory Charlot, Samuel Cykert
Provision of study materials or patients: Christina Yongue, Samuel Cykert
Collection and assembly of data: Eugenia Eng, Samuel Cykert
Data analysis and interpretation: Marjory Charlot, Jacob Newton Stein, Emily Damone, Isabella Wood, Moriah Forster, Stephanie Baker, Marc Emerson, Cleo Samuel-Ryals, Christina Yongue, Allison Deal, Samuel Cykert
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Effect of an Antiracism Intervention on Racial Disparities in Time to Lung Cancer Surgery
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Emily Damone
Research Funding: Genentech/Roche
Cleo Samuel-Ryals
Employment: Flatiron Health
Uncompensated Relationships: Voluntis
Matthew Manning
Stock and Other Ownership Interests: Fuse Oncology
Samuel Cykert
Consulting or Advisory Role: Pfizer
No other potential conflicts of interest were reported.
REFERENCES
- 1. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2. Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: A systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg. 2008;86:2008–2016. doi: 10.1016/j.athoracsur.2008.07.009. discussion 2016. [DOI] [PubMed] [Google Scholar]
- 3. Samson P, Patel A, Garrett T, et al. Effects of delayed surgical resection on short-term and long-term outcomes in clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2015;99:1906–1912. doi: 10.1016/j.athoracsur.2015.02.022. discussion 1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holmes JA, Chen RC. Racial disparities in time from diagnosis to treatment for stage I non-small cell lung cancer. JNCI Cancer Spectr. 2018;2:pky007. doi: 10.1093/jncics/pky007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bukhari A, Kumar G, Rajsheker R, et al. Timeliness of lung cancer diagnosis and treatment. Fed Pract. 2017;34(suppl 1):24S–29S. [PMC free article] [PubMed] [Google Scholar]
- 6. Yang C-FJ, Wang H, Kumar A, et al. Impact of timing of lobectomy on survival for clinical stage IA lung squamous cell carcinoma. Chest. 2017;152:1239–1250. doi: 10.1016/j.chest.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 7. Samson P, Crabtree T, Broderick S, et al. Quality measures in clinical stage I non-small cell lung cancer: Improved performance is associated with improved survival. Ann Thorac Surg. 2017;103:303–311. doi: 10.1016/j.athoracsur.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: Systematic review and meta-analysis. BMJ. 2020;371:m4087. doi: 10.1136/bmj.m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gomez DR, Liao K-P, Swisher SG, et al. Time to treatment as a quality metric in lung cancer: Staging studies, time to treatment, and patient survival. Radiother Oncol. 2015;115:257–263. doi: 10.1016/j.radonc.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 10. Bott MJ, Patel AP, Crabtree TD, et al. Pathologic upstaging in patients undergoing resection for stage I non-small cell lung cancer: Are there modifiable predictors? Ann Thorac Surg. 2015;100:2048–2053. doi: 10.1016/j.athoracsur.2015.05.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vidaver RM, Shershneva MB, Hetzel SJ, et al. Typical time to treatment of patients with lung cancer in a multisite, US-based study. J Oncol Pract. 2016;12:e643–e653. doi: 10.1200/JOP.2015.009605. [DOI] [PubMed] [Google Scholar]
- 12. Kanarek NF, Hooker CM, Mathieu L, et al. Survival after community diagnosis of early-stage non-small cell lung cancer. Am J Med. 2014;127:443–449. doi: 10.1016/j.amjmed.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khorana AA, Tullio K, Elson P, et al. Time to initial cancer treatment in the United States and association with survival over time: An observational study. PLoS One. 2019;14:e0213209. doi: 10.1371/journal.pone.0213209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heiden BT, Eaton DB, Engelhardt KE, et al. Analysis of delayed surgical treatment and oncologic outcomes in clinical stage I non-small cell lung cancer. JAMA Netw Open. 2021;4:e2111613. doi: 10.1001/jamanetworkopen.2021.11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shugarman LR, Mack K, Sorbero MES, et al. Race and sex differences in the receipt of timely and appropriate lung cancer treatment. Med Care. 2009;47:774–781. doi: 10.1097/MLR.0b013e3181a393fe. [DOI] [PubMed] [Google Scholar]
- 16. Bach PB, Cramer LD, Warren JL, et al. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198–1205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 17. Cykert S, Dilworth-Anderson P, Monroe MH, et al. Factors associated with decisions to undergo surgery among patients with newly diagnosed early-stage lung cancer. JAMA. 2010;303:2368–2376. doi: 10.1001/jama.2010.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cykert S, Eng E, Walker P, et al. A system-based intervention to reduce Black-White disparities in the treatment of early stage lung cancer: A pragmatic trial at five cancer centers. Cancer Med. 2019;8:1095–1102. doi: 10.1002/cam4.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lathan CS, Neville BA, Earle CC. The effect of race on invasive staging and surgery in non-small-cell lung cancer. J Clin Oncol. 2006;24:413–418. doi: 10.1200/JCO.2005.02.1758. [DOI] [PubMed] [Google Scholar]
- 20. Esnaola NF, Gebregziabher M, Knott K, et al. Underuse of surgical resection for localized, non-small cell lung cancer among whites and African Americans in South Carolina. Ann Thorac Surg. 2008;86:220–226. doi: 10.1016/j.athoracsur.2008.02.072. discussion 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taioli E, Flores R. Appropriateness of surgical approach in Black patients with lung cancer—15 years later, little has changed. J Thorac Oncol. 2017;12:573–577. doi: 10.1016/j.jtho.2016.08.119. [DOI] [PubMed] [Google Scholar]
- 22. McCann J, Artinian V, Duhaime L, et al. Evaluation of the causes for racial disparity in surgical treatment of early stage lung cancer. Chest. 2005;128:3440–3446. doi: 10.1378/chest.128.5.3440. [DOI] [PubMed] [Google Scholar]
- 23. Yorio JT, Xie Y, Yan J, et al. Lung cancer diagnostic and treatment intervals in the United States: A health care disparity? J Thorac Oncol. 2009;4:1322–1330. doi: 10.1097/JTO.0b013e3181bbb130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Samuel CA, Landrum MB, McNeil BJ, et al. Racial disparities in cancer care in the Veterans Affairs health care system and the role of site of care. Am J Public Health. 2014;104(suppl 4):S562–S571. doi: 10.2105/AJPH.2014.302079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lavizzo-Mourey RJ, Besser RE, Williams DR. Understanding and mitigating health inequities—Past, current, and future directions. N Engl J Med. 2021;384:1681–1684. doi: 10.1056/NEJMp2008628. [DOI] [PubMed] [Google Scholar]
- 26. Bailey ZD, Krieger N, Agénor M, et al. Structural racism and health inequities in the USA: Evidence and interventions. Lancet. 2017;389:1453–1463. doi: 10.1016/S0140-6736(17)30569-X. [DOI] [PubMed] [Google Scholar]
- 27. Bailey ZD, Feldman JM, Bassett MT. How structural racism works—Racist policies as a root cause of U.S. racial health inequities. N Engl J Med. 2021;384:768–773. doi: 10.1056/NEJMms2025396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boyd RW, Lindo EG, Weeks LD, et al. On racism: A new standard for publishing on racial health inequities. Health Affairs Blog. 2020 https://www-healthaffairs-org.libproxy.lib.unc.edu/do/10.1377/hblog20200630.939347/full/ [Google Scholar]
- 29. Cykert S, Eng E, Manning MA, et al. A multi-faceted intervention aimed at Black-White disparities in the treatment of early stage cancers: The ACCURE pragmatic quality improvement trial. J Natl Med Assoc. 2020;112:468–477. doi: 10.1016/j.jnma.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Eng E, Schaal J, Baker S, et al. Partnership, transparency, and accountability: Changing systems to enhance racial equity in cancer care and outcomes in Wallerstein N, Duran B, Oetzel JG, et al. (eds): Community-Based Participitory Research for Health: Advancing Social and Health Equity San Francisco, CA: Jossey-Bass; 2018 [Google Scholar]
- 31. Hunnibell LS, Rose MG, Connery DM, et al. Using nurse navigation to improve timeliness of lung cancer care at a veterans hospital. Clin J Oncol Nurs. 2012;16:29–36. doi: 10.1188/12.CJON.29-36. [DOI] [PubMed] [Google Scholar]
- 32. Alsamarai S, Yao X, Cain HC, et al. The effect of a lung cancer care coordination program on timeliness of care. Clin Lung Cancer. 2013;14:527–534. doi: 10.1016/j.cllc.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 33. Stone CJL, Robinson A, Brown E, et al. Improving timeliness of oncology assessment and cancer treatment through implementation of a multidisciplinary lung cancer clinic. J Oncol Pract. 2019;15:e169–e177. doi: 10.1200/JOP.18.00214. [DOI] [PubMed] [Google Scholar]
- 34. Olsson JK, Schultz EM, Gould MK. Timeliness of care in patients with lung cancer: A systematic review. Thorax. 2009;64:749–756. doi: 10.1136/thx.2008.109330. [DOI] [PubMed] [Google Scholar]
- 35. Gould MK, Ghaus SJ, Olsson JK, et al. Timeliness of care in veterans with non-small cell lung cancer. Chest. 2008;133:1167–1173. doi: 10.1378/chest.07-2654. [DOI] [PubMed] [Google Scholar]
- 36. Gorin SS, Badr H, Krebs P, et al. Multilevel interventions and racial/ethnic health disparities. J Natl Cancer Inst Monogr. 2012;2012:100–111. doi: 10.1093/jncimonographs/lgs015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Agurs-Collins T, Persky S, Paskett ED, et al. Designing and assessing multilevel interventions to improve minority health and reduce health disparities. Am J Public Health. 2019;109:S86–S93. doi: 10.2105/AJPH.2018.304730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Penner LA, Dovidio JF.Racial color blindness and Black-White health care disparities In Neville HA, Gallardo ME, Sue DW. (eds): The Myth of Racial Color Blindness: Manifestations, Dynamics, and Impact Washington, DC: American Psychological Association; 2016. pp 275–293. [Google Scholar]
- 39. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: Effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012:CD000259. doi: 10.1002/14651858.CD000259.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Freund KM, Battaglia TA, Calhoun E, et al. Impact of patient navigation on timely cancer care: The Patient Navigation Research Program. J Natl Cancer Inst. 2014;106:dju115. doi: 10.1093/jnci/dju115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Freeman HP, Rodriguez RL. History and principles of patient navigation. Cancer. 2011;117(15 suppl):3539–3542. doi: 10.1002/cncr.26262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Murphy DR, Wu L, Thomas EJ, et al. Electronic trigger-based intervention to reduce delays in diagnostic evaluation for cancer: A cluster randomized controlled trial. J Clin Oncol. 2015;33:3560–3567. doi: 10.1200/JCO.2015.61.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yen PH, Leasure AR. Use and effectiveness of the teach-back method in patient education and health outcomes. Fed Pract. 2019;36:284–289. [PMC free article] [PubMed] [Google Scholar]
- 44. Natale-Pereira A, Enard KR, Nevarez L, et al. The role of patient navigators in eliminating health disparities. Cancer. 2011;117(15 suppl):3543–3552. doi: 10.1002/cncr.26264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Neroda P, Hsieh M-C, Wu X-C, et al. Racial disparity and social determinants in receiving timely surgery among stage I-IIIA non-small cell lung cancer patients in a U.S. Southern state. Front Public Health. 2021;9:662876. doi: 10.3389/fpubh.2021.662876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sanchez R, Zhou Y, Sarrazin MSV, et al. Lung cancer staging at diagnosis in the Veterans Health Administration: Is rurality an influencing factor? A cross-sectional study. J Rural Health. 2020;36:484–495. doi: 10.1111/jrh.12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Muslim Z, Baig MZ, Weber JF, et al. Travelling to a high-volume center confers improved survival in stage I non-small cell lung cancer. Ann Thorac Surg. 2022;113:466–472. doi: 10.1016/j.athoracsur.2021.02.028. [DOI] [PubMed] [Google Scholar]