SUMMARY
This study aimed to evaluate the risk factors for tuberculosis (TB) treatment default in a priority city for disease control in Brazil. A cohort of TB cases diagnosed from 2008 to 2009 was followed up from patients’ entry into three outpatient sites, in Juiz de Fora, Minas Gerais (Brazil), until the recording of the outcomes. Drug addiction, alcoholism and treatment site appeared to be independently associated with default. Current users of crack as the hardest drug (odds ratio (OR) 12·25, 95% confidence interval (CI) 3·04–49·26) were more likely to default than other hard drug users (OR 5·67, 95% CI 1·34–24·03), former users (OR 4·12, 95% CI 1·11–15·20) and those not known to use drugs (reference group). Consumers at high risk of alcoholism (OR 2·94, 95% CI 1·08–7·99) and those treated in an outpatient hospital unit (OR 8·22, 95% CI 2·79–24·21%) also were more likely to default. Our results establish that substance abuse was independently associated with default. National TB programmes might be more likely to achieve their control targets if they include interventions aimed at improving adherence and cure rates, by diagnosing and treating substance abuse concurrently with standard TB therapy.
Key words: Alcoholism, risk factors, substance abuse, treatment default, tuberculosis
INTRODUCTION
In the 21st century, tuberculosis (TB) is still one of the greatest challenges in the public health field. In 2015, there were an estimated 10·4 million new TB cases worldwide and 1·4 (13·5%) million died from the disease, 0·4 million of whom were HIV-positive. People living with HIV accounted for 1·2 million (11·5%) of all new TB cases [1].
In Brazil, there were an estimated 84 000 new cases in 2015, corresponding to an incidence of 41/100 000 inhabitants. The mortality rate in 2014 was 2·2 deaths/100 000 inhabitants. These indicators put Brazil among the 30 high TB burden countries (HBCs) that accounted together for more than 80% of the world's TB cases in 2015. Of the 30 HBCs, 13 did not reach a treatment success rate of 85% among all new and relapse TB cases in 2014, including Brazil (71% success) [1].
Default from TB treatment is common in high-incidence urban areas in Brazil, pointing to the need for strategies to address adherence. de Queiroz and Bertolozzi stated that health professionals should recognise patients as people who have specific needs that are not limited to the TB treatment [2]. Similarly, a study carried out in Colombia concluded that family support during treatment is a protective factor for the successful cure [3]. In this context, the WHO recommends the Directly Observed Treatment Short (DOTS) course as a highly efficient and cost-effective strategy and the central element in a comprehensive patient-centred approach to prevent non-adherence [1]. Services linked to the Brazilian Unified Health System (SUS), however, due to scarcities of human and financial resources, have encountered operational difficulties in expanding this strategy to a greater number of patients.
One study found that substance abuse and the side effects of treatment were some of the factors associated with TB treatment default in Brazil, although drug users exhibited the highest risk [4]. This is a worrying issue since the last household survey on psychotropic drug use in this country confirmed that 22·8% of the population had used some psychotropic drug in their lifetime [5].
However, most of the Brazilian studies assessed TB treatment outcomes through a secondary database, instead of validated instruments, and only retrospective analyses are currently available. Since the available data have limitations, further studies with more detailed information are needed. Therefore, the aim of the present study was to evaluate the risk factors for TB treatment default through a prospective cohort study in Juiz de Fora, state of Minas Gerais, Brazil, an area where default is currently one of the biggest challenges for TB control [6, 7]. Knowledge of these risk factors is of utmost importance for guiding future actions intended to increase the effectiveness of TB control programmes worldwide.
STUDY POPULATION AND METHODS
Setting
The study was carried out in Juiz de Fora, an urban area in the state of Minas Gerais, Brazil. This municipality has a population of approximately 500 000 inhabitants, and has the second highest prevalence of TB cases in Minas Gerais. Along with 24 other municipalities, they are responsible for 54·38% of the total notified cases statewide. Therefore, this municipality was considered by the Brazilian Health Ministry a priority location for TB control in Brazil [6]. Most TB cases (75%) reported to the Brazilian Notifiable Diseases Information System (SINAN) by this municipality are managed at three public health facilities. They are considered the main referral service for patient care and drug supply in this area, and were included in this study to facilitate logistical issues, which required regular follow-up of patients. The centres included were classified as follows: centres 1 and 2 are of secondary complexity and attend general TB patients and HIV/AIDS patients, respectively; centre 3 is a hospital unit that attends patients after discharge from the same establishment. Cases of TB were treated through health centre-based, with treatment provided cost-free to the patient.
Patients were provided with adherence counselling and TB health education by physicians at the start of TB treatment and on recurrent visits. During counselling, patients were informed about TB, signs and symptoms, how the disease spreads, consequences of not following treatment guidelines, why treatment is long and why completion of treatment is critical, likely adverse events during therapy, and that free treatment and public services are available in Brazil.
Design and study population
A prospective cohort study was carried out with all adults with a first episode of TB. Patients were enrolled between 2008 and 2009 and followed up until treatment completion (6 months) or some episode of default (at any time up to 6 months).
The inclusion and exclusion criteria were as follows. Patients with TB who were attended at the three centres were eligible for the study if they were at least13 years old and had the start of TB treatment recorded in SINAN with at least one diagnostic test confirming TB, such as sputum smear microscopy, microbiological culture or histopathology examination with suggestive aspects (granulomatous lesions with caseous necrosis). Participants had to be able to respond to an interview and give their consent.
Data collection and main study variables
Field workers interviewed each patient to collect socio-demographic, medical (e.g. treatment site), individual and behavioural information using a structured questionnaire. Complementary information was abstracted from the SINAN database (treatment supervision variable) or from treatment centre medical records (HIV diagnosis and information on hospitalisation variables).
The treatment site variable refers to one of the three centres included in the study where the treatment was undertaken and the participants were recruited for this study. The Cut down, Annoyed, Guilty, Eye-opener (CAGE) questionnaire was used to screen persons at high risk of alcoholism, as described by Ewing [8]. The risk of alcoholism was defined as low when a score was lower than 3 and high when the score was 3 or 4 [9]. Tobacco addiction was considered to be the consumption of 10 or more cigarettes per day. The instruments to assess illicit drug consumption were already tested for validity and reliability in previous Brazilian studies, PESSOAS and ATAR [10, 11], which included several questions related to type (e.g. cocaine, crack, etc.), routes of administration (e.g. smoke, freebase/smoke, inject) and quantities of current and lifetime illicit drug use. The answers to these questions were grouped into a summary variable in the present analyses, called illicit drug use. The treatment supervision variable used a general classification of ‘yes or no’. When this variable has a positive record in SINAN, this indicates that some degree of supervision of the TB treatment has occurred, regardless of the time used during the supervision of drug intake, either semi-supervised, supervised or strictly supervised. Serodiagnosis of HIV infection was obtained using data from the most recent tests at the time of interview.
The TB treatment outcome was the dependent variable and the explanatory variables were grouped into three hierarchical levels:
distal (socio-demographic): skin colour, marital status, income, education level and type of housing;
middle (health care): treatment site and treatment supervision;
proximal (individual and behavioural features): gender, clinical features of TB, cigarette smoking, alcoholism, illegal drugs, comorbid HIV/AIDS and if the patient was hospitalised at some stage during TB treatment.
Definition of the outcomes
The outcomes, defined according to the Brazilian TB Guidelines [12], were recorded during the monthly patient follow-up appointments at each service and were detailed in the first publication of this cohort study [7]. Briefly, default and cure were defined as follows:
Default was defined as a patient having missed treatment by the health unit for at least 30 consecutive days, prior to physician-approved treatment completion. In the case of supervised treatment, the period of 30 days is from the last medication intake;
Cure for sputum smear-positive cases was defined as either of two criteria being met at the completion of the treatment period: a patient presented two negative smears – in the last month of treatment and on at least one previous occasion (bacteriological cure proven); and a patient who did not do the sputum examination due to the absence of expectoration and who was classified as cured based on clinical and complementary examinations such as radiography (clinical cure unproven);
Cure for sputum smear negative or extrapulmonary cases at the completion of the treatment was defined based on clinical, radiological and other complementary examinations such as radiography and culture.
The definition of outcomes was in line with the WHO, but Brazil made some modifications for operational issues [12]. The main modification was that this country considers at least 30 days without taking the necessary medications as default, while the WHO has defined it as being at least 60 days. The Brazilian Health Ministry believes that with this modification they would avoid permanently losing a patient under treatment.
Statistical analyses
Descriptive statistics were used to summarise general data about the study patients. The cumulative incidences of TB treatment default and cure were calculated.
Univariate and multivariate logistic regression analyses were conducted to evaluate the risk factors for TB treatment default, comparing those defaulting from treatment with those who completed it. We excluded from the risk factors analyses patients who failed, died during the study period or had not completed TB treatment in time for the outcome, because if any variables were associated with both treatment default and death or another negative outcome, this dichotomisation could bias resulting odds ratios (ORs) towards the null, as previously stated [13].
In the univariate analyses, the χ2 test was used to assess which explanatory variables were associated with the outcome, with a significance level of 5%. The dose–response trend between the score of addiction to illicit drugs and TB treatment default was evaluated using χ2 for linear trend.
In the multivariate analyses, theoretical models with three hierarchical levels (distal, middle and proximal) were constructed according to Victora et al. [14]. The variables of each hierarchical level were detailed in the topic ‘Data collection and main study variables’.
In the multivariate analyses, the initial criteria for a variable of any hierarchical level being tested in the multivariate model was having P-values <0·20 in the univariate analyses. All the variables with P-values <0·20 in the univariate analyses were hierarchically included in the multiple logistic regressions and adjusted from a further towards a closer hierarchical level. A backward stepwise method was used to reach a satisfactory level of fit. Crude and adjusted ORs and 95% confidence intervals (CIs) were calculated using a backward stepwise method and goodness-of-fit was assessed by the Hosmer and Lemeshow's test. The OR obtained from logistic regression was used in this study as an estimate of relative risk.
Data management was performed using Epi Info version 6 [15] and statistical analyses carried out with SPSS version 20.0 [16].
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Furthermore, this study was approved by the Brazilian National Research Ethics Committee (protocol 166/2006).
RESULTS
Descriptive analyses
Patients of potential interest (n = 287) were followed up during their TB treatment. From this group, 172 (60·0%) were successfully treated, 48 (16·7%) defaulted and 67 (23·3%) were not eligible for current analyses, because they had outcomes other than cure or default. This study therefore consisted of 220 patients (172 cures and 48 defaults). Among them, the present instruments identified that: (i) 129 (58·6%) and 59 (26·8%) presented low (CAGE <3) and high (CAGE 3–4) risks of alcoholism at the start of treatment, respectively, while 32 (14·6%) did not have such information recorded; (ii) 136 (61·8%) had never used illicit drugs, 21 (9·5%) were former users, 11 (5·0%) were current users of cocaine as the hardest drug and 19 (8·7%) were current users of crack as the hardest drug, while 33 (15·0%) did not have such information recorded (Table 1).
Table 1.
Univariate analysis for tuberculosis treatment default, Juiz de Fora, state of Minas Gerais, Brazil, 2008–2009
| Treatment default | ||||
|---|---|---|---|---|
| Variables | Total | TD* (%) | OR (95% CI) | P-value |
| Distal level | ||||
| Marital status | 0·034 | |||
| Never lived with sexual partner | 46 | 5 (10·9) | 1·00 | |
| Lives with sexual partner | 83 | 14 (16·9) | 1·66 (0·55–4·95) | |
| Has lived with sexual partner | 64 | 19 (29·7) | 3·46 (1·18–10·11) | |
| Education | 0·011 | |||
| Incomplete high school to complete undergraduate | 64 | 6 (9·4) | 1·00 | |
| None to complete elementary school | 138 | 34 (24·6) | 3·16 (1·25–7·97) | |
| Dwelling type | 0·020 | |||
| House, apartment, shack, room | 177 | 33 (18·6) | 1·00 | |
| Penitentiary, shelter, home for elderly or homeless | 26 | 10 (38·5) | 2·72 (1·13–6·54) | |
| Middle level | ||||
| Treatment site | <0·0000001 | |||
| Centre 1† | 124 | 8 (6·5) | 1·00 | |
| Centre 2‡ | 19 | 4 (21·0) | 5·8 (1·48–22·66) | |
| Centre 3§ | 77 | 32 (41·6) | 10·31 (4·41– 24·07) | |
| Treatment supervision | <0·0000001 | |||
| No | 108 | 6 (5·6) | 1·00 | |
| Yes | 106 | 41 (38·7) | 10·72 (4·31– 26·67) | |
| Internment at some stage of the treatment | 0·0000006 | |||
| No | 104 | 7 (6·7) | 1·00 | |
| Yes | 116 | 41 (35·3) | 7·57 (3·21–17·83) | |
| Proximal level | ||||
| Gender | 0·0002 | |||
| Female | 72 | 5 (6·9) | 1·00 | |
| Male | 148 | 43 (29·1) | 5·48 (2·06–14·55) | |
| Cigarette consumption | 0·001 | |||
| Never in life | 48 | 6 (12·5) | 1·00 | |
| Former to current moderate (<10/day) | 80 | 10 (12·5) | 1·00 (0·33–2·95) | |
| Current abuse (⩾10/day) | 69 | 24 (34·8) | 3·73 (1·38–10·03) | |
| Current alcohol use | 0·000002 | |||
| Low risk of alcoholism (CAGE <3)|| | 129 | 13 (10·1) | 1·00 | |
| High risk of alcoholism (CAGE 3–4)|| | 59 | 24 (40·7) | 6·11 (2·82–13·26) | |
| Serological diagnosis for HIV | 0·005 | |||
| Negative | 136 | 20 (14·7) | 1·00 | |
| Testing not conducted | 65 | 22 (33·8) | 2·96 (1·47–5·97) | |
| Positive | 19 | 6 (31·6) | 2·67 (9·91– 7·86) | |
| Illicit drug use | <0·0000001¶ | |||
| Never in life | 136 | 10 (7·4) | 1·00 | |
| Former user | 21 | 7 (33·3) | 6·30 (2·07–19·16) | |
| Current user of cocaine as the hardest drug | 11 | 5 (45·5) | 10·50 (2·72– 40·51) | |
| Current user of crack as the hardest drug | 19 | 11 (57·9) | 17·32 (5·68– 52·84) | |
Number of tuberculosis treatment default cases.
General outpatient unit.
Outpatient unit specialised in the HIV/AIDS patient care.
Outpatient unit of a public tuberculosis referral hospital.
Cut-Annoyed-Guilty-Eye questionnaire.
Pearson's χ2 = 40·50 (P < 0·0000001), χ2 for linear trend = 39·45 (P < 0·0000001), χ2 for non-linearity = 39·45 (P = 0·59).
Univariate regression analyses
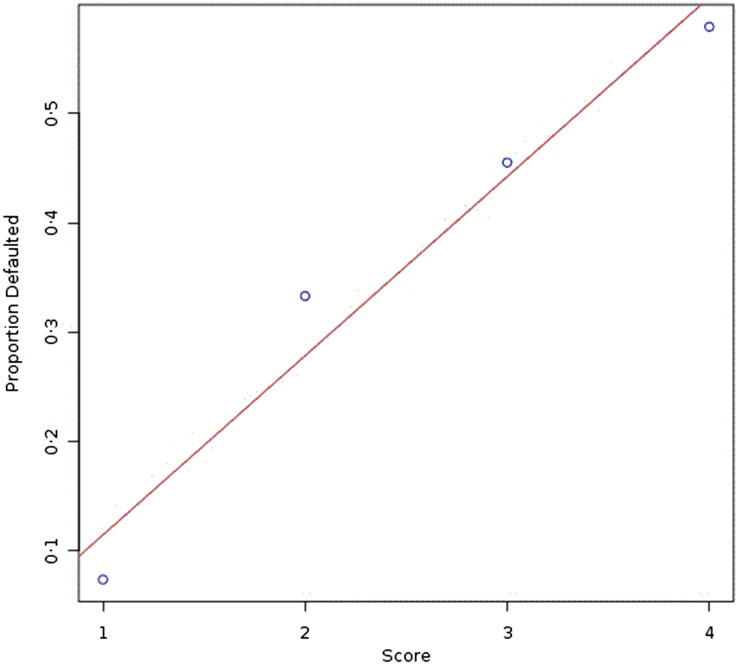
Table 1 presents all the variables associated (P ⩽ 0·05) with default, and shows a linear trend between illicit drug use scores and TB treatment default, which is displayed in Figure 1.
Fig. 1.
Dose–response trend between the score of addiction to illicit drugs and TB treatment default. Score 1: never addicted to illicit drugs, score 2: former user, score 3: current user of cocaine as the hardest drug, score 4: current user of crack as the hardest drug.
Multivariate regression analyses
In the final model, the illicit drug use, current alcohol use and treatment site variables remained as independent variables associated with default (P ⩽ 0·05). Current users of crack as the hardest drug (OR 12·25, 95% CI 3·04–49·26) were more likely to default from treatment compared with other current users of cocaine as the hardest drug (OR 5·67, 95% CI 1·34–24·03), those who were former users (OR 4·12, 95% CI 1·11–15·20) and those not known to use drugs (reference group). Additionally, consumers at high risk of alcoholism at the start of treatment were more likely to default from treatment (OR 2·94, 95% CI 1·08–7·99) and those treated in an outpatient hospital unit were more likely to default (OR 8·22, 95% CI 2·79–24·21%) than those treated in a general outpatient unit (Table 2).
Table 2.
Final multivariate regression model* in hierarchical levels for tuberculosis treatment default, Juiz de Fora, state of Minas Gerais, Brazil, 2008–2009
| Variable | OR (95% CI) | P-value |
|---|---|---|
| Treatment site | 0·001 | |
| Centre 1 | 1·00 | |
| Centre 2 | 1·79 (0·24–13·37) | 0·56 |
| Centre 3 | 8·22 (2·79–24·21) | <0·001 |
| Illicit drug use | 0·001 | |
| Never in life | 1·00 | |
| Former user | 4·12 (1·11–15·20) | 0·033 |
| Current user of cocaine as the hardest drug | 5·67 (1·34–24·03) | 0·018 |
| Current user of crack as the hardest drug | 12·25 (3·04–49·26) | <0·001 |
| Current alcohol use | 0·034 | |
| Low risk of alcoholism (CAGE <3)† | 1·00 | |
| High risk of alcoholism (CAGE 3–4) | 2·94 (1·08–7·99) | 0·034 |
Model summary: Cox & Snell's R2 = 0·267; Nagelkerke's R2 = 0·441; Hosmer and Lemeshow's test χ2 = 1·597 (P = 0·902); score test for significance of the model = 57·689 (P < 0·001); likelihood ratio test for significance of the model = 56·819 (P < 0·001).
Cut-Annoyed-Guilty-Eye questionnaire.
DISCUSSION
We found that substance abuse was significantly associated with defaulting from TB treatment in Brazil, with adjusted ORs for consumers at high risk of alcoholism at the start of treatment 2·94 (95% CI 1·08–7·99); past use of illicit drugs 4·12 (95% CI 1·11–15·20); current use of cocaine as the hardest drug 5·67 (95% CI 1·34–24·03) and current use of crack as the hardest drug 12·25 (95% CI 3·04–49·26).
Addictive behaviours also have been reported by other studies as associated with non-adherence to, and defaulting from, TB treatment. A prospective cohort study showed that patients who defaulted were more likely to have used illegal drugs and abused alcohol [13]. A retrospective cohort study found that baseline alcohol dependence and intravenous drug use were strongly associated with non-adherence-or-default and non-adherence, respectively [17]. A case–control study found an association between drug addiction and non-adherence to treatment regimen or default from treatment altogether [18]. A matched case–control study also found alcoholism as a risk factor for default [19].
The authors do not know of any other prospective cohort study with risk factor analyses for default among TB patients in Brazil. Furthermore, most studies in this country exclusively used a secondary database (SINAN) [4, 20, 21] instead of validated instruments for more appropriately assessing explanatory variables and outcome, as the current study did.
Non-adherence to, and default from, anti-TB treatments are serious challenges that are jeopardizing TB control worldwide, because these patients required longer treatment regimens, were more likely to acquire drug resistance and were less likely to complete treatment [22].
Hypothetical explanations for the association between substance abuse and TB treatment default have been proposed. Chemically dependent individuals do not invest in their self-care, use less health services, eat poorly, have precarious family and institutional relationships [23], and are prone to escapism and forgetfulness when confronting real-life situations [24, 25].
Our multivariate analyses showed that current users of crack as the hardest drug were more likely to default from treatment than other current or former hard drug users, and those not known to use drugs. Another study also found a similar association, even using only univariate analyses [18]. This higher incidence of both default and non-adherence among crack users could be attributed to a more addictive power of crack [26]. Those who inject or freebase/smoke cocaine tend to use cocaine more frequently and experience higher rates of dependence than traditional intranasal users [27, 28].
Compared with patients treated in a general outpatient unit, those treated in an outpatient hospital unit were more likely to default, in this study. This could be attributed to a worse quality of patient care found in this unit, where physicians often were not complying with the schedule times and patients waited for a long time (approximately 4 h) to be examined. A close doctor–patient relationship (humanisation) and a better organisation of healthcare services, leading to a reduction in waiting time for consultations, were reinforced as important factors related to satisfactory adherence [29–31].
The DOTS is directed mainly at patients with drug and alcohol abuse [32], and although high rates of illicit drugs consumption and persons at high risk of alcoholism were revealed in this study, strictly supervised treatment strategy, surprisingly, was not applied for any study patient. Additionally, the term supervised treatment was used indiscriminately by the health system for any TB patient who had hospitalisation at some stage, regardless of the monitoring time. Perhaps for this reason, there was an unexpected direct association between treatment supervision and TB treatment default in the univariate analyses. This could be due to the effect of possible confounding variables such as: (i) hospitalisation at some stage during treatment and (ii) place of treatment, both of which were associated with both the treatment supervision and outcome variables.
The high incidence of default (16·7%) among the study population during the study period (2008–2009) was above that recommended by the WHO, at 5% [1]. In 2015, Juiz de Fora still presented a 19% rate of default from treatment [33], and remained in second place in number of TB cases statewide [34], and one of the 181 priority municipalities of Brazil for TB control [35] as of the study period. Treatment default has been an overlooked issue in this municipality, where a TB control programme based on the effective implementation of the DOTS strategy is not a local reality yet, despite the availability of free anti-TB medicines in this country. Brazilian TB surveillance has passively worked even in priority municipalities, including Juiz de Fora. National surveillance has been presenting fragilities, such as inadequate active search for patients with respiratory symptoms and incipient decentralisation of both the DOTS strategy and TB treatments [1, 4, 7, 36–38]. The prospective follow-up of patients to identify exposure and detect outcomes with appropriate instruments, rather than mere reuse of secondary data, strengthened the results of this study. These instruments identified significantly higher incidences of both substance abuse and TB treatment default, and lower frequencies of missing data than recorded by SINAN, which results were previously reported by our team [7]. However, this study also had some limitations, which we will address. The TB patients followed up in the three centres (n = 287) represented 57·05% of the total reported (n = 503) in the municipality during the study period. Nevertheless our sample was representative, since it did not differ (P > 0·05) from the total in terms of gender, age, race, educational level, HIV diagnosis or municipality of residence [7]. The lack of a better and more detailed definition of the treatment supervision variable assigned according to the degree of supervision as self-administered, semi-supervised, supervised and strictly supervised could be another limitation of this study. There was also a loss of follow-ups resulting in some outcomes missed; however, we confirmed that the losses were few (2·1%). Some associations, especially those related to socio-economic conditions present in the univariate analyses, may not have been identified in multivariate analyses because of the lack of power of this study. Munro et al. [39], using the reconceptualised model of factors influencing adherence to TB treatment, concluded that adherence to the long course of TB treatment is a complex, dynamic phenomenon with a wide range of factors having impact on treatment-taking behaviour. They emphasised four major factors that interact to affect adherence to TB treatment: structural factors, including poverty and gender discrimination; the social context; health service factors and personal factors. Some of these factors, such as characteristics of healthcare service providers and individual perception for the reasons of defaulting were not contemplated by the present study and could hamper a more detailed exploration of predictors.
In conclusion, our results establish that substance abuse was independently associated with default and suggest that programmes might be more likely to achieve their control targets if they include interventions aimed at improving adherence and cure rates by diagnosing and treating substance abuse concurrently with standard TB therapy. The training of health care professionals to identify the symptoms of substance abuse in order to support and treat patients earlier, and the adoption of DOTS among users, should be incorporated into the strategies to improve the effectiveness of TB control programmes.
ACKNOWLEDGEMENTS
M. R. Silva and J. P. Cruz received fellowships from the Brazilian Agricultural Research Corporation (Embrapa) and Federal University of Juiz de Fora, respectively. This work was supported by the National Research Council of Brazil – CNPq (grant number 410595/2006-3).
DECLARATION OF INTEREST
None.
REFERENCES
- 1.World Health Organization. Global Tuberculosis Report 2016. Geneva, Switzerland: World Health Organization, 2016, 214pp. [Google Scholar]
- 2.de Queiroz EM, Bertolozzi MR. Tuberculosis: supervised treatment in North, West and East Health Departments of São Paulo. Revista da Escola de Enfermagem da USP 2010; 44: 453–461. [DOI] [PubMed] [Google Scholar]
- 3.Cáceres FM, Orozco LC. Incidencia y factores asociados al abandono del tratamiento antituberculoso. Biomédica 2007; 27: 498–504. [PubMed] [Google Scholar]
- 4.Paixão LMM, Gontijo ED. Profile of notified tuberculosis cases and factors associated with treatment dropout. Revista de Saúde Pública 2007; 41: 205–213. [DOI] [PubMed] [Google Scholar]
- 5.Carlini EA, et al. II levantamento domiciliar sobre o uso de drogas psicotrópicas no Brasil: estudo envolvendo as 108 maiores cidades do Brasil: 2005. São Paulo, Brazil: Centro Brasileiro de Informações sobre Drogas Psicotrópicas: Universidade Federal de São Paulo, 2006, 473pp. [Google Scholar]
- 6.Ministério da Saúde, Secretaria de Vigilância em Saúde (MS). Sistema Nacional de Vigilância em Saúde, Relatório de Situação, Minas Gerais, série c, projetos, programas e relatórios. Brasília, DF, Brazil: Ministério da Saúde, Secretaria de Vigilância em Saúde, 2006, 24pp (http://bvsms.saude.gov.br/bvs/publicacoes/relatorio_snvs_mg_2ed.pdf). Accessed 08 May 2017. [Google Scholar]
- 7.Pereira JC, et al. Profile and follow-up of patients with tuberculosis in a priority city in Brazil. Revista de Saúde Pública 2015; 49: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ewing JA. Detecting alcoholism. The CAGE questionnaire. Journal of the American Medical Association 1984; 252: 1905–1907. [DOI] [PubMed] [Google Scholar]
- 9.Magruder-Habib K, Stevens HA, Alling WC. Relative performance of the MAST, VAST, and CAGE versus DSM-III-R criteria for alcohol dependence. Journal of Clinical Epidemiology 1993; 46: 435–441. [DOI] [PubMed] [Google Scholar]
- 10.Guimarães MDC, et al. Reliability and validity of a questionnaire on vulnerability to sexually transmitted infections among adults with chronic mental illness: PESSOAS project. Revista Brasileira de Psiquiatria 2008; 30: 55–59. [DOI] [PubMed] [Google Scholar]
- 11.Ministério da Saúde (MS). Secretaria de Vigilância em Saúde. Departamento de DST, Aids e Hepatites Virais. Adesão ao tratamento antirretroviral no Brasil: coletânea de estudos do Projeto Atar, série B, textos básicos de saúde. Brasília, DF, Brazil: Ministério da Saúde, 2010, 408pp (http://www.aids.gov.br/sites/default/files/atar-web.pdf). Accessed 08 May 2017. [Google Scholar]
- 12.Silva JB. Jr. Tuberculose: Guia de Vigilância Epidemiológica. Jornal Brasileiro de Pneumologia 2004; 30(Suppl.): S57–S86. [Google Scholar]
- 13.Lackey B, et al. Patient characteristics associated with tuberculosis treatment default: a cohort study in a high-incidence area of Lima, Peru. PLoS ONE 2015; 10: e0128541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Victora CG, et al. The role of conceptual frameworks in epidemiological analysis: a hierarchical approach. International Journal of Epidemiology 1997; 26: 224–227. [DOI] [PubMed] [Google Scholar]
- 15.Dean AG, et al. Epi Info Version 6: A Word Processing Database and Statistics Program for Epidemiology on Microcomputers. Atlanta, GA, USA: Centers for Diseases Control and Prevention, 1994. [Google Scholar]
- 16.International Business Machines (IBM). IBM SPSS Statistics 20 [Software], version 20.0. New York, NY, USA: IBM, 2012. [Google Scholar]
- 17.Gelmanova IY, et al. Barriers to successful tuberculosis treatment in Tomsk, Russian Federation: non-adherence, default and the acquisition of multidrug resistance. Bulletin of the World Health Organization 2007; 85: 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Story A, Bothamley G, Hayward A. Crack cocaine and infectious tuberculosis. Emerging Infectious Diseases 2008; 14: 1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slama K, et al. Factors associated with treatment default by tuberculosis patients in Fez, Morocco. Eastern Mediterranean Health Journal 2013; 19: 687–693. [PubMed] [Google Scholar]
- 20.Bergel FS, Gouveia N. Frequent return as a novel strategy for tuberculosis treatment adherence. Revista de Saúde Pública 2005; 39: 898–905. [DOI] [PubMed] [Google Scholar]
- 21.Ribeiro Macedo L, et al. Treatment outcomes of tuberculosis patients in Brazilian prisons: a polytomous regression analysis. The International Journal of Tuberculosis and Lung Disease 2013; 17: 1427–1434. [DOI] [PubMed] [Google Scholar]
- 22.Pablos-Méndez A, et al. Nonadherence in tuberculosis treatment: predictors and consequences in New York City. The American Journal of Medicine 1997; 102: 164–170. [DOI] [PubMed] [Google Scholar]
- 23.Souza SS, Silva DMGV. Experiencing treatment for tuberculosis (in Portuguese). Texto & Contexto Enfermagem 2010; 19: 636–643. [Google Scholar]
- 24.Sadava SW, Thistle R, Forsyth R. Stress, escapism and patterns of alcohol and drug use. Journal of Studies on Alcohol 1978; 39: 725–736. [DOI] [PubMed] [Google Scholar]
- 25.Sumartojo E. When tuberculosis treatment fails. a social behavioral account of patient adherence. The American Review of Respiratory Disease 1993; 147: 1311–1320. [DOI] [PubMed] [Google Scholar]
- 26.Jekel JF, et al. Epidemic freebase cocaine abuse. Case study from the Bahamas. The Lancet 1986; 1: 459–462. [DOI] [PubMed] [Google Scholar]
- 27.Gossop M, et al. Cocaine: patterns of use, route of administration, and severity of dependence. The British Journal of Psychiatry 1994; 164: 660–664. [DOI] [PubMed] [Google Scholar]
- 28.Chen K, Kandel D. Relationship between extent of cocaine use and dependence among adolescents and adults in the United States. Drug and Alcohol Dependence 2002; 68: 65–85. [DOI] [PubMed] [Google Scholar]
- 29.Natal LS, et al. Prediction model for the pulmonary tuberculosis treatment default (in Portuguese). Boletim de Pneumologia Sanitária 1999; 7: 65–78. [Google Scholar]
- 30.Lima MB, et al. Non-adherence to tuberculosis treatment: a study on perceptions and knowledge of the disease and evaluation of health services from the patient perspective (Fortaleza, Ceará, Brazil) (in Portuguese). Cadernos de Saúde Pública 2001; 7: 877–885. [DOI] [PubMed] [Google Scholar]
- 31.Deheinzelin D, et al. Predictive factors of abandoning treatment in tuberculosis patients (in Portuguese). Revista do Hospital das Clínicas 1996; 51: 131–135. [PubMed] [Google Scholar]
- 32.Santos J. Brazilian response to tuberculosis control. Revista de Saúde Pública 2007; 41(Suppl.): 89–94. [DOI] [PubMed] [Google Scholar]
- 33.Anon. Saúde realiza semana de conscientização contra tuberculose. Portal de Notícias 2017; 21 March (http://www.diarioregionaljf.com.br/cidade/14651-secretaria-saude-realiza-semana-de-conscientizacao-contra-tuberculose). Accessed 07 September 2017. [Google Scholar]
- 34.Anon. JF é a 2ª em casos de tuberculose em Minas. Tribuna de Minas 2016; 24 March (http://tribunademinas.com.br/noticias/cidade/24-03-2016/jf-e-a-2a-em-casos-de-tuberculose-em-minas.html). Accessed 07 September 2017. [Google Scholar]
- 35.Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância das Doenças Transmissíveis (MS). Panorama da tuberculose no Brasil: indicadores epidemiológicos e operacionais. Brasília, DF, Brazil: Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância das Doenças Transmissíveis, 2014, 92pp (http://bvsms.saude.gov.br/bvs/publicacoes/panorama%20tuberculose%20brasil_2014.pdf). Accessed 07 September 2017. [Google Scholar]
- 36.Froes GC, et al. Profile and follow-up of patients with Mycobacterium sp. at the Hospital das Clínicas da Universidade Federal de Minas Gerais. Jornal Brasileiro de Pneumologia 2003; 29: 365–370. [Google Scholar]
- 37.Muniz JN, et al. Active search for individuals with respiratory symptoms as part of community health workers’ role in tuberculosis control (in Portuguese). Ciência e Saúde Coletiva 2005; 10: 315–321. [Google Scholar]
- 38.Silva HO, Gonçalves MLC. Tuberculosis/HIV co-infection in Brazilian state capitals: comments from the data of the Information System of Notifiable Diseases. Revista Brasileira em Promoção da Saúde 2009; 22: 172–178. [Google Scholar]
- 39.Munro SA, et al. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. PLoS Medicine 2007; 4: e238. [DOI] [PMC free article] [PubMed] [Google Scholar]