SUMMARY
Cats are known to be the main reservoir for Bartonella henselae and Bartonella clarridgeiae, which are the agents of ‘cat-scratch disease’ in humans. In the present study, we investigated the prevalence of the two Bartonella species on 1754 cat bloods collected from all prefectures in Japan during 2007–2008 by a nested-polymerase chain reaction (PCR) targeting the 16S–23S rRNA internal transcribed spacer region. Overall, Bartonella DNA was detected in 4·6% (80/1754) of the cats examined. The nested-PCR showed that 48·8% (39/80) of the positive cats were infected with B. henselae mono-infection, 33·8% (27/80) with B. clarridgeiae mono-infection and 17·5% (14/80) were infected with both species. The prevalence (5·9%; 65/1103) of Bartonella infection in the western part of Japan was significantly higher than that (2·3%; 15/651) of eastern Japan (P < 0·001). Statistical analysis of the cats examined suggested a significant association between Bartonella infection and FeLV infection (OR = 1·9; 95% CI = 1·1–3·4), but not with FIV infection (OR = 1·6; 95% CI = 1·0–2·6).
Key words: Bartonella clarridgeiae, Bartonella henselae, Japan, species-specific nested-PCR
INTRODUCTION
Bartonella bacteria are haemotropic Gram-negative organisms that persistently infect a wide variety of mammalian erythrocytes [1] and several Bartonella species have been recognized to be pathogenic for humans [2]. Bartonella henselae is known to be the agent of ‘cat-scratch disease’ (CSD) and B. clarridgeiae has also been suspected as causing a few cases of CSD in humans [3]. In addition, Bartonella koehlerae has been associated with culture-negative endocarditis in human [4].
Cats are recognized to be the reservoir for B. henselae, Bartonella clarridgeiae and B. Koehlerae, which are transmitted by cat fleas between cats [5–7]. In contrast to humans, cats infected with the Bartonella species do not usually develop any symptoms but present relapsing bacteraemia for months or years [8–10]. Epidemiological studies have been conducted in cats in many countries and the prevalence of Bartonella varies from 24·0% (65/271) to 39·5% (81/205) in USA [11, 12], 8·1% (8/99) to 16·5% (72/436) in France [13, 14], 13·0% (13/100) in Germany [15], 61·3% (19/31) in the Philippines [16], 27·6% (76/275) in Thailand [17] and 19·1% (25/131) in Taiwan [18]. In these studies, B. henselae tends to be the more dominant species than B. clarridgeiae in many countries. In Japan, the prevalence of Bartonella infection in cats from 10 prefectures was reported as 7·2% (50/690) and B. henselae was the predominant species among the isolates identified in 2000 [19]. On the other hand, B. koehlerae has never been isolated from any cats not only in Japan, but also in other Asian countries to date. Therefore, surveillance of B. henselae and B. clarridgeiae infection in cats in Japan seems to be more crucial for preventing cat-associated bartonellosis rather than that of B. koehlerae.
Isolation of Bartonella bacteria is the most important method for detection of current status of bacteraemic cats. However, primary culture of Bartonella requires long incubation periods since the bacteria are fastidious and the growth is considerably slow [20]. Several conventional and real-time polymerase chain reactions (PCRs) have been applied as alternative diagnostic tools for the isolation of Bartonella bacteria from clinical specimens [21]. On the other hand, identification of Bartonella species by DNA sequencing with PCR amplicons takes a long time and at considerable cost [22,23]. Consequently, it is necessary to develop an easy and rapid identification method for Bartonella instead of DNA sequencing at the clinical site.
The objectives of the present study are to investigate the prevalence of CSD-associated Bartonella species by a nested-PCR capable of discriminating between B. henselae and B. clarridgeiae and to evaluate correlation between FIV or FeLV and Bartonella infection in pet cats from all prefectures in Japan.
MATERIALS AND METHODS
Cat blood and DNA extraction
Between 2007 and 2008, blood samples were collected from 1754 cats in animal clinics from all (47) prefectures of Japan. In total 14–41 samples were collected from each prefecture. Western and eastern parts of Japan were divided at a longitude of approximately 137° east. The samples were transferred into commercial blood collection tubes and stored at 4 °C or −20 °C, until the DNA extraction.
Before collecting the samples, the physical condition of cats was checked and the demographic information, including age and sex were obtained by clinical veterinarians. In addition, FeLV and FIV infections in the cats were examined using the Snap Combo FeLV Ag/FIV Ab test kit (Idexx Laboratories, ME, USA). The genomic DNA of Bartonella was extracted from 200 µl of each blood sample by using QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany) and kept at −20 °C until further examination.
Detection of B. henselae and B. clarridgeiae DNAs by nested-PCR
A nested-PCR was applied to detect Bartonella DNAs by modifying the PCR targeting a part of the 16S-23S rRNA internal transcribed spacer (ITS) region of Bartonella species. The genus-specific primers (URBarto1 and URBarto2) reported in the previous study [24] were used for the first PCR. The primer sets used for the second PCR were newly designed in silico to be specific to B. henselae (URBhen-f and URBhen-r) or B. clarridgeiae (URBcla-f and URBcla-r), respectively (Table 1). B. henselae strains of Houston-IT (Accession No. L35101), CAL-1 (Accession No. AF369527) and San Ant 2 (Accession No. AF369529) and B. clarridgeiae strains of Houston-IIT (Accession No. AF312497) and Kyoto19-2 (Accession No. AB674239) were used for design of the species-specific primers. The DNA sequences obtained from GenBank database were analyzed by using GENETYX-win software, version 9 (Genetyx Corp., Tokyo, Japan).
Table 1.
Primer information for the first and second PCRs used in this study
| PCR | Target species | Primer | Sequence (5′→3′) | Reference |
|---|---|---|---|---|
| First | Bartonella species | URBarto1 | CTTCGTTTCTCTTTCTTCA | [24] |
| URBarto2 | CTTCTCTTCACAATTTCAAT | |||
| Second | B. henselae | URBhen-f | TTGCTTCTAAAAAGCTTATCAA | This study |
| URBhen-r | CAAAAGAGGGATTACAAAATC | |||
| Second | B. clarridgeiae | URBcla-f | ATGCTAAAAGTTGCTATATTGG | This study |
| URBcla-r | CCTCACACTAAAATATAAAAAAC |
First and second steps of the nested-PCRs were carried out in a 20 µl volume of mixture as follows: 1 µl of a 10 µM solution of each primer, 10 µl of 2 × Go Taq DNA polymerase master mix (Promega, Madison, WI, USA), 7 µl of nuclease-free distilled water and 1 µl of a sample DNA (1–10 ng/μl) for the first PCR or an amplicon of the first PCR for the second PCR. The first PCR was performed in the same way as the previous report [24]. Conditions for the second PCR were as follows: 3 min at 95 °C followed by 40 cycles of 30 s at 95 °C, 30 s at 50 °C, 1 min at 72 °C and a final extension of 3 min at 68 °C. Amplicons of the PCRs were separated by electrophoresis on 3% agarose gels and visualized by staining with ethidium bromide. Any sample showing the product size of 700–722 bp by the first PCR was considered to be positive for genus Bartonella and used for the following second PCR. B. henselae or B. clarridgeiae-specific primers for the second PCR were predicted to amplify products of 254 bp or 283 bp to 285 bp, respectively. Positive controls were the genomic DNAs from B. henselae Houston-IT and B. clarridgeiae Houston-IIT and negative control was nuclease-free distilled water in the first PCR.
Statistical analysis
Chi-square test was applied to analyze the association between Bartonella infection and demographic factors. A P < 0·05 was considered as statistically significant in this study. In relation to the association between Bartonella and FeLV or FIV infections, the odds ratio was calculated with a 95% confidence interval.
RESULTS
Prevalence of Bartonella DNA in pet cats
The first PCR detected Bartonella DNA in 4·6% (80/1754) of the cats examined. The following second PCR demonstrated that the DNAs of B. henselae, B. clarridgeiae and both species accounted for 48·8% (39/80), 33·8% (27/80) and 17·5% (14/80) of the DNA-positive cats, respectively.
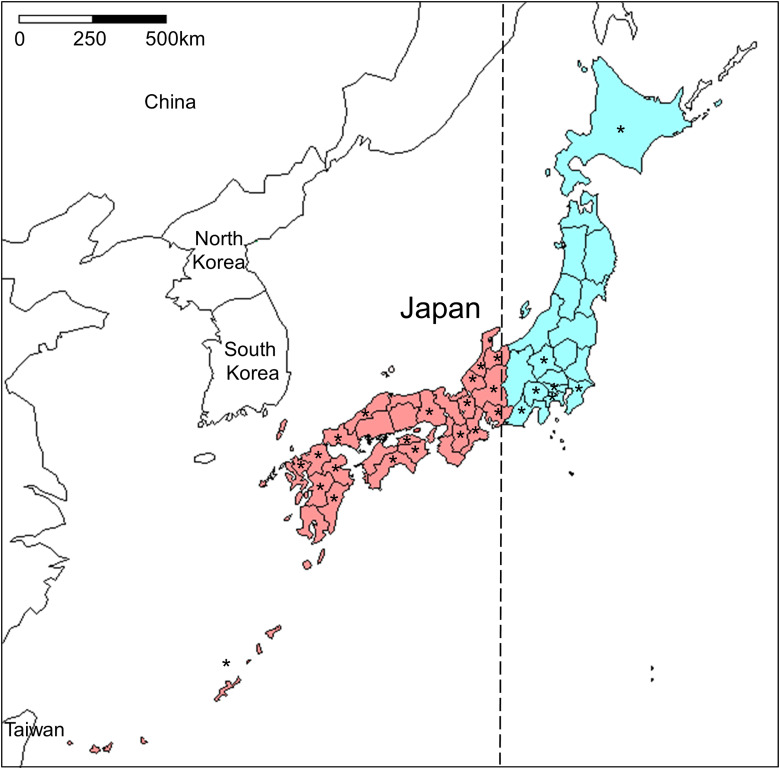
The Bartonella DNA by regions was detected in 2·3% (15/651) of cats from 7 of 18 prefectures in the eastern part of Japan (Fig. 1). In contrast, the positivity rate was found to be 5·9% (65/1103) in cats from 20 of 29 prefectures in the western part of Japan. The prevalence of Bartonella DNA in the western part was significantly higher than that observed in the eastern part of Japan (P < 0·001; Table 2).
Fig. 1.
Geographical distribution of the Bartonella-infected cats. Blue and red colors indicate the areas of eastern and western parts of Japan, respectively. The areas are segregated by a vertical dashed line located at a longitude of approximately 137° east. Asterisks represent the prefectures where Bartonella-infected cats were found.
Table 2.
Bartonella DNA prevalence in cats in Japan by respective demographic factors
| Demographic factor | No. examined | No. positive for Bartonella DNA (%) | P value | |
|---|---|---|---|---|
| Area of Japan | East | 651 | 15 (2·3) | <0·001 |
| West | 1103 | 65 (5·9) | ||
| Sex | Male | 931 | 43 (4·6) | 0·840 |
| Female | 815 | 36 (4·4) | ||
| Unknown | 8 | 1 (12·5) | – | |
| Age | 1 year > | 218 | 14 (6·4) | 0·153 |
| 1 year ⩽ | 1523 | 65 (4·3) | ||
| No record | 13 | 1 (7·7) | – | |
The prevalence of Bartonella DNA by gender was 4·6% (43/931) in male cats and 4·4% (36/815) in female cats. Only one of eight cats was positive for Bartonella, where gender was unknown. Prevalence by age was 6·4% (14/218) in cats under 1 year of age and 4·3% (65/1523) in cats over 1 year of age. Only one of 13 cats was positive for Bartonella where age was unknown. No significant difference was observed in the prevalence when sex and age of the cats were examined and compared (Table 2).
Table 3 summarizes the prevalence of Bartonella DNA in relation to FeLV or FIV infections of cats. Prevalence of Bartonella DNA in cats that were positive or negative for FeLV infection were 7·7% (16/209) and 4·1% (64/1545), respectively; in cats with FIV infection positive or negative the prevalence was 6·4% (26/407) and 4·0% (54/1347), respectively. Statistical analysis demonstrated a significant association between Bartonella infection and FeLV infection (OR = 1·9; 95% CI = 1·1–3·4) but not with FIV infection (OR = 1·6; 95% CI = 1·0–2·6).
Table 3.
Correlation between FeLV or FIV infections and Bartonella infection in cats
| Feline immunosuppressive viral diseases | No. examined | No. positive for Bartonella (%) | OR (95% CI) | |
|---|---|---|---|---|
| FeLV infection | Positive | 209 | 16 (7·7) | 1·9 (1·1–3·4) |
| Negative | 1545 | 64 (4·1) | ||
| FIV infection | Positive | 407 | 26 (6·4) | 1·6 (1·0–2·6) |
| Negative | 1347 | 54 (4·0) | ||
OR, odds ratio; CI, confidence interval.
DISCUSSION
The nested-PCR used in this study could differentiate B. henselae and B. clarridgeiae infections in cat blood. Bacterial culture with blood samples and PCR and DNA sequencing with the DNA of the isolates are considered as gold-standard diagnosis methods for Bartonella infection in cats and the identification of Bartonella species. In addition, the indirect immunofluorescence assay with serum samples is also used for diagnosis of Bartonella infection. However, primary culture of Bartonella bacteria requires a long period of time, which can extend to 4 weeks because of the fastidious property of the organism [20]. Furthermore, serological tests are not adequate in understanding the present status (vs. historic legacy) of infection in cats [25]. The nested-PCR applied in this study can be a useful tool for estimating active infection in cats without culture of Bartonella bacteria. Furthermore, the nested-PCR can differentiate B. henselae and B. clarridgeiae infection in cats. Consequently, the nested-PCR may contribute to the rapid detection and identification of Bartonella in cats at the clinical site.
In the present study, Bartonella DNA was detected from 4·6% (80/1754) of the cats collected from all the prefectures in Japan using PCR. Maruyama et al. [19] showed that the prevalence of Bartonella by culture was 7·2% (50/690) in pet cats derived from 10 prefectures in Japan. It has been reported that the detection of Bartonella DNA by PCR is more sensitive than culture when performed on clinical specimens [26]. However, the positivity rate of the previous study by culture was higher than that of the present study by PCR, suggesting that the difference may be due to epidemiological biases, such as the number of prefectures examined and the study date reflecting a change in hygienic status of rearing environment. We investigated the Bartonella prevalence from cat samples collected from all prefectures in Japan, whereas only 10 out of 47 prefectures had been examined in the previous study [19]. Thus, the data of the present study may reflect the accurate status of Bartonella infection in domestic cats throughout Japan.
Our data showed that Bartonella prevalence in cats in the western part of Japan was significantly higher than that in the eastern part. According to previous studies in Asian countries excepting Japan, the prevalence of Bartonella infection in cats ranged from 19·1% in Taiwan [18] to 64·3% in Indonesia [27]. Bartonella prevalence in Asian cats varies by country, suggesting that the prevalence in tropical and sub-tropical countries tend to be higher than those of the countries with temperate climates, such as Japan. It has also been reported that cats raised in countries with high temperatures and humid climates tend to exhibit a higher seroprevalence of B. henselae than those cats, which dwell in cold and dry climates [28]. High temperature and humid climate is thought to be suitable for growth of ectoparasites such as cat fleas, which is the vector for B. henselae and B. clarridgeiae [5, 26]. Maruyama et al. [29] reported that a significantly higher seroprevalence of B. henselae was observed in cats with flea infestations than that in flea-free cats. Bartonella prevalence in cats in Kagoshima and Okinawa Prefectures located in the most south-western part of Japan was significantly higher than the other areas in Japan and flea infestation rates in the cats have also been reported to be 46·0% in Kagoshima Prefecture and 58·0% in Okinawa Prefecture. Of which, Okinawa Prefecture belong geographically to the sub-tropical region [19]. In the present study, although we could not investigate flea infestation in cats examined, the higher prevalence of this infection in the western part of Japan may possibly be attributed to the status of flea infestation in the cat population.
The percentages of PCR positive cats for B. henselae, B. clarridgeiae and both species were found to be 48·8% (39/80), 33·8% (27/80) and 17·5% (14/80), respectively. In contrast, it has been reported that B. henselae is much more prevalent (88·0%: 44/50) in cats followed by B. clarridgeiae (10·0%: 5/50), while co-infection with both species was detected only on one cat (2·0%: 1/50) [19]. The previous report suggested that [3, 30] isolation of B. clarridgeiae from cat blood appears to be more difficult than that of B. henselae. Then, the data of the present study based on PCR detection may reflect the accurate infection status of the two Bartonella species in pet cats in Japan.
Though no significant association was observed between prevalence and sex or age of the cats examined, cats <1 year-old tended to show higher prevalence than those aged over 1 year-old. Previous reports in the USA and the Netherlands also indicated that cats under 1 year of age showed a higher prevalence of B. henselae by culture and serology than the older cats [11, 31]. These facts suggest that juvenile cats rather than the adults may be more important reservoirs of CSD for humans. In fact, an association between owning a kitten was demonstrated in CSD patients in Connecticut, USA when compared with healthy persons [32].
In the present study, Bartonella infection was significantly associated with FeLV infection but not with FIV infection in the cats examined. The present data may support the hypothesis that susceptibility of Bartonella infection is possibly increased in cats with FeLV infection, regardless of whether infection is latent or progressive [33]. In contrast, it has also been suggested that Bartonella infection in cats was not affected by FIV [29, 34] nor by FeLV infections [35]. Therefore, the association between feline Bartonella bacteria and the immunosuppressive viruses remains controversial. Additional epidemiological studies should be conducted to assess the cause-and-effect relationship in other countries.
ACKNOWLEDGEMENTS
This work was supported by the Strategic Research Base Development Program, International Research on Epidemiology of Zoonoses and Training for Young Researchers by the Ministry of Education, Culture, Sports, Science and Technology, Japan. We thank the Japanese Advisory Board on Feline Infectious Diseases for their help with sample collection.
REFERENCES
- 1.Dehio C. Bartonella-host-cell interactions and vascular tumour formation. Nature Reviews Microbiology 2005; 3: 621–631. [DOI] [PubMed] [Google Scholar]
- 2.Chomel BB, Kasten RW. Bartonellosis, an increasingly recognized zoonosis. Journal of Applied Microbiology 2010; 109: 743–750. [DOI] [PubMed] [Google Scholar]
- 3.Chomel BB, et al. Bartonella spp. in pets and effect on human health. Emerging Infectious Diseases 2006; 12: 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avidor B, et al. Bartonella koehlerae, a new cat-associated agent of culture-negative human endocarditis. Journal of Clinical Microbiology 2004; 42: 3462–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chomel BB, et al. Experimantal transmission of Bartonella henselae by the cat flea. Journal of Clinical Microbiology 1996; 34: 1952–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai YL, et al. Bartonella species and their ectoparasites: selective host adaptation or strain selection between the vector and the mammalian host? Comparative Immunology Microbiology and Infectious Diseases 2011; 34: 299–314. [DOI] [PubMed] [Google Scholar]
- 7.Droz S, et al. Bartonella koehlerae sp. nov., isolated from cats. Journal of Clinical Microbiology 1999; 37: 1117–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guptill L, et al. Experimental infection of young specific pathogen-free cats with Bartonella henselae. Journal of Infectious Diseases 1997; 176: 206–216. [DOI] [PubMed] [Google Scholar]
- 9.Kordick DL, Breitschwerdt EB. Relapsing bacteremia after blood transmission of Bartonella henselae to cats. American Journal of Veterinary Research 1997; 58: 492–497. [PubMed] [Google Scholar]
- 10.Kabeya H, et al. Genomic variations among Bartonella henselae isolates derived from naturally infected cats. Veterinary Microbiology 2002; 89: 211–221. [DOI] [PubMed] [Google Scholar]
- 11.Chomel BB, et al. Bartonella henselae prevalence in domestic cats in California: risk factors and association between bacteremia and antibody titers. Journal of Clinical Microbiology 1995; 33: 2445–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guptill L, et al. Prevalence, risk factors, and genetic diversity of Bartonella henselae infections in pet cats in four regions of the United States. Journal of Clinical Microbiology 2004; 42: 652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurfield AN, et al. Epidemiology of Bartonella infection in domestic cats in France. Veterinary Microbiology 2001; 80: 185–198. [DOI] [PubMed] [Google Scholar]
- 14.Rolain JM, et al. Prevalence of Bartonella clarridgeiae and Bartonella henselae in domestic cats from France and detection of the organisms in erythrocytes by immunofluorescence. Clinical and Diagnostic Laboratory Immunology 2004; 11: 423–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sander A, et al. Detection and identification of two Bartonella henselae variants in domestic cats in Germany. Journal of Clinical Microbiology 1997; 35: 584–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chomel BB, et al. Bartonella henselae and Bartonella clarridgeiae infection in domestic cats from The Philippines. American Journal of Tropical Medicine and Hygiene 1999; 60: 593–597. [DOI] [PubMed] [Google Scholar]
- 17.Maruyama S, et al. Prevalence of Bartonella species and 16s rRNA gene types of Bartonella henselae from domestic cats in Thailand. American Journal of Tropical Medicine and Hygiene 2001; 65: 783–787. [DOI] [PubMed] [Google Scholar]
- 18.Chang CC, et al. Cat-scratch disease in veterinary-associated populations and in its cat reservoir in Taiwan. Veterinary Research 2006; 37: 565–577. [DOI] [PubMed] [Google Scholar]
- 19.Maruyama S, et al. Prevalence of Bartonella henselae, Bartonella clarridgeiae and the 16S rRNA gene types of Bartonella henselae among pet cats in Japan. Journal of Veterinary Medical Science 2000; 62: 273–279. [DOI] [PubMed] [Google Scholar]
- 20.Brenner SA, et al. Isolation of Bartonella (Rochalimaea) henselae: effects of methods of blood collection and handling. Journal of Clinical Microbiology 1997; 35: 544–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fenollar F, Raoult D. Molecular genetic methods for the diagnosis of fastidious microorganisms. Acta Pathologica, Microbiologica, et Immunologica Scandinavica 2004; 112: 785–807. [DOI] [PubMed] [Google Scholar]
- 22.Angelakis E, et al. Real-time PCR strategy and detection of bacterial agents of lymphadenitis. European Journal of Clinical Microbiology and Infectious Diseases 2009; 28: 1363–1368. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez R, et al. Bartonellae in domestic and stray cats from Israel: comparison of bacterial cultures and high-resolution melt real-time PCR as diagnostic methods. Vector-Borne and Zoonotic Diseases 2013; 13: 857–864. [DOI] [PubMed] [Google Scholar]
- 24.Rolain JM, et al. Molecular detection of Bartonella quintana, B. koehlerae, B. henselae, B. clarridgeiae, Rickettsia felis, and Wolbachia pipientis in cat fleas, France. Emerging Infectious Diseases 2003; 9: 338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergmans AM, et al. Pitfalls and fallacies of cat scratch disease serology: evaluation of Bartonella henselae-based indirect fluorescence assay and enzyme-linked immunoassay. Journal of Clinical Microbiology 1997; 35: 1931–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciervo A, Ciceroni L. Rapid detection and differentiation of Bartonella spp. by a single-run real-time PCR. Molecular and Cellular Probes 2004; 18: 307–312. [DOI] [PubMed] [Google Scholar]
- 27.Marston EL, et al. Prevalence of Bartonella henselae and Bartonella clarridgeiae in an urban Indonesian cat population. Clinical and Diagnostic Laboratory Immunology 1999; 6: 41–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jameson P, et al. Prevalence of Bartonella henselae antibodies in pet cats throughout regions of North America. Journal of Infectious Diseases 1995; 172: 1145–1149. [DOI] [PubMed] [Google Scholar]
- 29.Maruyama S, et al. Seroprevalence of Bartonella henselae, Toxoplasma gondii, FIV and FeLV infections in domestic cats in Japan. Microbiology and Immunology 2003; 47: 147–153. [DOI] [PubMed] [Google Scholar]
- 30.Breitschwerdt EB, et al. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clinical Microbiology Reviews 2000; 13: 428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergmans AM, et al. Prevalence of Bartonella species in domestic cats in the Netherlands. Journal of Clinical Microbiology 1997; 35: 2256–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zangwill KM, et al. Cat scratch disease in Connecticut. Epidemiology, risk factors, and evaluation of a new diagnostic test. New England Journal of Medicine 1993; 329: 8–13. [DOI] [PubMed] [Google Scholar]
- 33.Buchmann AU, et al. Does a feline leukemia virus infection pave the way for Bartonella henselae infection in cats? Journal of Clinical Microbiology 2010; 48: 3295–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ueno H, et al. Does coinfection of Bartonella henselae and FIV induce clinical disorders in cats? Microbiology and Immunology 1996; 40: 617–620. [DOI] [PubMed] [Google Scholar]
- 35.Glaus T, et al. Seroprevalence of Bartonella henselae infection and correlation with disease status in cats in Switzerland. Journal of Clinical Microbiology 1997; 35: 2883–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]