SUMMARY
We sought to comprehensively assess the prevalence and outcomes of complications associated with Staphylococcus aureus bacteremia (SAB) in children. Secondarily, prevalence of methicillin resistance and outcomes of complications from methicillin-resistant S. aureus (MRSA) vs. methicillin-susceptible S. aureus SAB were assessed. This is a single-center cross-sectional study of 376 patients ⩽18 years old with SAB in 1990–2014. Overall, 197 (52%) patients experienced complications, the most common being osteomyelitis (33%), skin and soft tissue infection (31%), and pneumonia (25%). Patients with complications were older (median 3 vs. 0·7 years, P = 0·05) and more had community-associated SAB (66% vs. 34%, P = 0·001). Fewer patients with complications had a SAB-related emergency department or hospital readmission (10% vs. 19%, P = 0·014). Prevalence of methicillin resistance increased from 1990–1999 to 2000–2009, but decreased in 2010–2014. Complicated MRSA bacteremia resulted in more intensive care unit admissions (66% vs. 47%, P = 0·03) and led to increased likelihood of having ⩾2 foci (58% vs. 26%, P < 0·001). From multivariate analysis, community-associated SAB increased risk and concurrent infections decreased risk of complications (odds ratio (OR) 1·82 (1·1–3·02), P = 0·021) and (OR 0·58 (0·34–0·97), P = 0·038), respectively. In conclusion, children with SAB should be carefully evaluated for complications. Methicillin resistance remains associated with poor outcomes but have decreased in overall prevalence.
Key words: Antibiotic resistance, bloodstream infections, epidemiology, pediatrics, Staphylococcus aureus
INTRODUCTION
Staphylococcus aureus is a leading cause of bacteremia in pediatrics, causing 9–12% of all bloodstream infections in children in the USA at the turn of the 21st century [1–5]. In addition, resistance to methicillin have approached as high as 60% among staphylococcal isolates obtained from hospitalized adult patients with nosocomial bloodstream infections in hospitals across the USA in 2001 [6,7]. Once confined to the hospital setting, community-associated methicillin-resistant S. aureus (CA-MRSA) has now emerged as an important pathogen in the community as well [8–11].
The morbidities associated with S. aureus bacteremia (SAB) consist of focal and systemic complications that may occur at any bodily site with MRSA twice as likely as methicillin-susceptible S. aureus (MSSA) to cause complications in SAB [12]. Different studies have evaluated complications in children with SAB, including osteomyelitis, septic arthritis, pneumonia, meningitis, infective endocarditis, and toxic-shock syndrome [13–23]. Other complications, including septic shock, respiratory distress syndrome, and splenic abscess have not been extensively described in the pediatric population. More importantly, patient outcomes including visit to the emergency department (ED) and readmission to the hospital after infection have not been evaluated.
This study was uniquely designed to comprehensively evaluate the many focal and systemic complications associated with SAB in the pediatric population. Our primary objective was to determine the incidence and outcomes (including ED visit or hospital readmission) of focal and systemic complications of SAB. Our secondary objectives were to evaluate the prevalence of methicillin resistance as well as compare clinical characteristics and outcomes of SAB complications caused by MRSA and MSSA.
METHODS
This was a 25-year cross-sectional study of pediatric patients who were diagnosed with SAB from 1 January 1990 to 31 December 2014 at Miller Children's and Women's Hospital of Long Beach, a 308-bed, not-for-profit, community-based teaching hospital featuring pediatric and neonatal intensive care units (ICUs), cancer, general surgery and cardiology centers. Patients ⩽18 years of age, with at least one positive S. aureus blood culture anytime during hospitalization were included in the study. Patients were initially screened using microbiology records maintained by the microbiology department. Only the first episode was considered in subjects with multiple positive S. aureus blood cultures within the same hospital stay. The institutional review boards at Miller Children's Hospital and University of California, San Diego approved the study to ensure protection of health information.
Definitions
A standardized form was used to collect patient demographics, comorbidities, including concurrent infections with organisms other than S. aureus, microbiology data, diagnostic studies, antibiotic therapy, and clinical outcomes, including duration of hospitalization, ICU stay, and S. aureus-related admission to the ED or hospital within 2 years subsequent to the SAB episode. The study period was defined as the time from the first positive culture to hospital discharge or death, with an additional review for ED or hospital admission within 2 years after hospital discharge. Community-associated bacteremia was defined as onset of infection initiating within the community as evident by first positive blood culture occurring ⩽48 h after hospitalization, with no recent admission to a healthcare institution or antibiotic use within 90 days. A suspected focus of infection was one that was identified concomitantly with the bacteremia. An infectious focus was deemed unsuspected when diagnostic workup occurred ⩾2 days after the first positive culture. Days of bacteremia was defined as the time from first positive to last positive cultures.
Using physician-assessed clinical features and appropriate diagnostic procedures (e.g. abdominal ultrasonography or radiography, echocardiogram, lumbar puncture, bone radiography, gallium study, computed tomography, or magnetic resonance imaging), a complication of bacteremia was defined as having one of the following conditions: endocarditis, osteomyelitis, pneumonia, meningitis, septic shock, toxic shock syndrome, septic arthritis, skin–soft tissue infection (including necrotizing fasciitis), splenic abscess, bacteriuria, respiratory distress syndrome, and septic thrombophlebitis.
Statistical analyses
Data were entered into an Excel database and formatted for analysis. The cumulative prevalence of complications and period prevalence as well as percentage yearly incidences of methicillin resistance were determined. Logistic regression was used to determine statistical significance between time periods: 1990–1999 vs. 2000–2009 as well as 1990–1999 vs. 2010–2014.
Study subjects were divided according to the presence or absence of SAB-related complications as well as presence or absence of methicillin resistance (i.e. MRSA vs. MSSA) in those with complications. Subjects divided into presence or absence of SAB-related complications were also analyzed with premature infants excluded. Demographic, clinical, and outcome variables were compared between two groups using appropriate statistical methods (i.e. χ2 tests or Fisher's exact test for cross-tabulations with nominal variables and independent samples and Student t test for continuous variables). For instances where non-parametric analyses were required, Cochran's Mantel–Haenszel tests were used for categorical data, and the Mann–Whitney test with two independent samples for continuous data. To determine odds ratio (OR), a correction of 0·5 was used in cells with values of zero. All statistical tests were performed with two-tailed analyses. Statistical differences between groups were considered significant when P-values were ⩽0·05.
Univariable and bivariable analyses were conducted to identify clinical explanatory variables associated with development of SAB-related complications, our binary outcome variable. Statistically significant factors (P ⩽ 0·05) that were not considered an aftermath of SAB-related complications were identified. Multivariate analysis to identify clinical parameters that represent potential predictors of SAB-related complications was performed using logistic regression. The Kaplan–Meier survival method with log-rank test was used to display the cumulative probability of patients who survived during hospitalization after first positive blood cultures [21]. All analyses were performed using SPSS PASW 20 (SPSS Inc., Chicago, Illinois, USA).
RESULTS
The medical records of 424 neonatal and pediatric patients with positive blood cultures for S. aureus were reviewed. A total of 48 patients were excluded from analysis for the following reasons: 45 had incomplete or unavailable medical records, 2 were >21 years old at the time of first positive culture and 1 had contaminated blood cultures as reported by the microbiology laboratory. The remaining 376 patients (i.e. 98 in 1990–1999, 216 in 2000–2010, and 62 in 2010–2014) were included in our study.
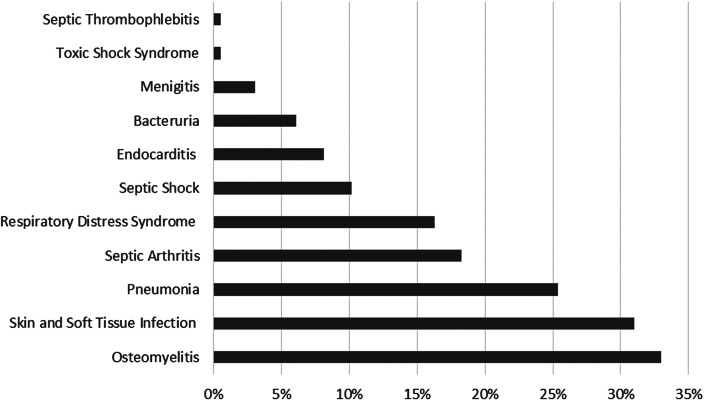
The overall prevalence of complications among hospitalized pediatrics with SAB during the 25-year period was 52% (197 of 376). The most common complications observed were skin and soft tissue infections, osteomyelitis, pneumonia, and septic arthritis (Fig. 1). Septic shock, infective endocarditis, bacteriuria, meningitis, toxic-shock syndrome, as well as septic thrombophlebitis were more infrequent. Among subjects who experienced complications, 63 (32% of 197) had ⩾2 foci and 48 (34% of 140 evaluable patients) had an unsuspected focus with osteomyelitis (19%), septic arthritis (9%), pneumonia (9%), and endocarditis (5%) being the most common infectious foci. Forty-two percent of patients with an unsuspected focus had ⩾2 complications, and median time to identification of an unsuspected focus was 5 days (Table 1).
Fig. 1.
Overall prevalence (%) by type of complication. Of 376 pediatric subjects, 197 (52%) had complications associated with Staphylococcus aureus bacteremia and 63 patients experienced ⩾2 types of complications.
Table 1.
Complications of unsuspected focus
| Unsuspected focus, no. (%) | n = 140a |
|---|---|
| Osteomyelitis | 27 (19) |
| Septic arthritis | 13 (9) |
| Pneumonia | 13 (9) |
| Endocarditis | 7 (5) |
| ⩾2 foci, no. (%) | 20/48 (42%) |
| Time to complication from hospital admission, median, days | 5 |
no., number.
Of 140 evaluable patients, 48 (34%) had complications of unsuspected focus.
All subjects by presence vs. absence of complications
Table 2 shows the baseline characteristics and demographic information of study patients. Patients with complications were older than patients without (P = 0·05). There were no significant differences between subjects in these two groups in terms of gender, race, comorbid conditions (except prematurity (P = 0·002)), presence of central venous catheters (CVC), and prosthetic devices. Compared with those without, more patients with complications had community-associated SAB (66% vs. 34%, P = 0·001). On the contrary, significantly less patients with complications had concurrent infections by organisms other than S. aureus (31% vs. 47%, P = 0·01).
Table 2.
Baseline characteristics by complications and methicillin resistance
| Variable | With complications (n = 197)a | Without complications (n = 179) | P-valueb | MRSA complications (N = 38) | MSSA complications (N = 159) | P-valuea |
|---|---|---|---|---|---|---|
| Age, mean ± s.d. (median, range), years | 5 ± 5·5 (3, 0·003–20) | 3·8 ± 5·7 (0·7, 0·003–22) | 0·05 | 5·8 ± 6·3 (2·3, 0·003–20) | 4·8 ± 5·2 (3, 0·003–20) | 0·32 |
| Male gender, no. (%) | 132 (67) | 107 (60) | 0·15 | 23 (61) | 109 (69) | 0·35 |
| Race, no. (%) | ||||||
| Hispanic | 92 (47) | 78 (44) | 0·45 | 18 (47) | 74 (47) | 0·54 |
| White | 41 (21) | 42 (24) | 9 (24) | 32 (20) | ||
| Black | 35 (18) | 35 (20) | 7 (18) | 28 (18) | ||
| Asian | 11 (6) | 15 (8) | 0 (0) | 11 (7) | ||
| Other | 16 (8) | 8 (5) | 4 (11) | 12 (8) | ||
| Comorbid conditionsc, no. (%) | ||||||
| Prematurity | 46 (23) | 68 (38) | 0·002 | 9 (24) | 37 (23) | 0·99 |
| Neurologic | 17 (9) | 18 (10) | 0·65 | 5 (14) | 12 (8) | 0·33 |
| Cancer | 12 (6) | 17 (10) | 0·22 | 0 (0) | 12 (8) | 0·13 |
| Hemophilia A | 2 (1) | 6 (3) | 0·16 | 0 (0) | 2 (1) | 0·99 |
| SLE with CNS involvement | 2 (1) | 5 (3) | 0·27 | 0 (0) | 2 (1) | 0·99 |
| Trauma/burn | 5 (3) | 2 (1) | 0·45 | 0 (0) | 5 (3) | 0·59 |
| Connective tissue | 5 (3) | 2 (1) | 0·45 | 0 (0) | 5 (3) | 0·59 |
| Diabetes | 3 (2) | 0 (0) | 0·25 | 1 (3) | 2 (1) | 0·47 |
| Cirrhosis | 1 (1) | 0 (0) | 0·99 | 0 (0) | 1 (1) | 0·99 |
| Aplastic anemia | 2 (1) | 2 (1) | 0·99 | 1 (3) | 1 (1) | 0·34 |
| Community-associated infection, no. (%) | 114 (66) | 58 (34) | 0·001 | 17 (44) | 97 (63) | 0·036 |
| Concurrent infection, no (%) | 45 (31) | 55 (47) | 0·01 | 9 (27) | 36 (32) | 0·67 |
| Central venous catheters, no. (%) | 94 (76) | 106 (80) | 0·53 | 21 (84) | 73 (75) | 0·32 |
| Prosthetic device, no. (%) | 3 (2) | 6 (5) | 0·5 | 1 (4) | 2 (2) | 0·5 |
CI, confidence interval; CNS, central nervous system; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive S. aureus; s.d., standard deviation; SLE, systemic lupus erythematosus.
Percentages presented for nominal variables are based on number of evaluable patients, which may represent a different denominator than the sample size in each category.
Only P-values were provided for continuous variables.
Percentages do not add up to 100% as some patients had no or more than one concomitant disease.
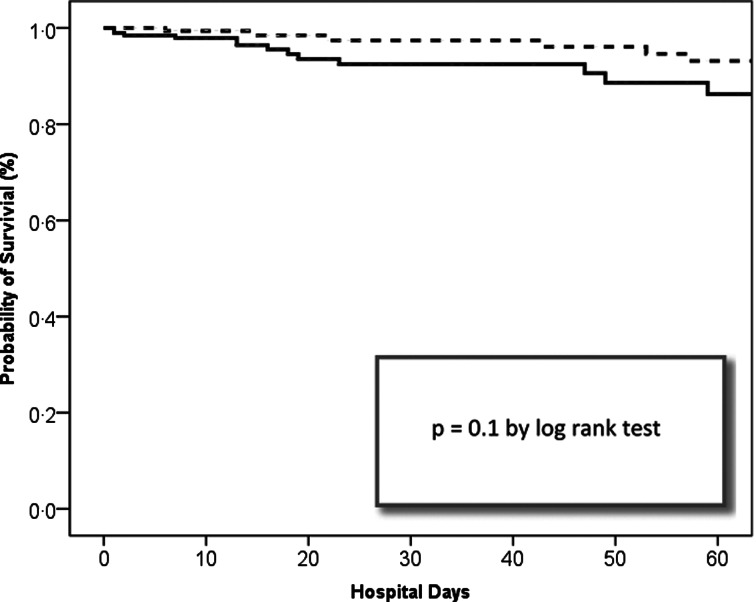
With respect to treatment and clinical outcomes in patients with complications vs. those without, as shown in Table 3, ICU stay, mean duration of SAB, and death during hospitalization (both SAB-related and all-cause) were not significantly different. No differences were seen in antibiotic initiations within 24 h and vancomycin therapy (empiric use and duration of therapy) between these two groups. Fewer patients with complications had a SAB-related ED or hospital readmission within 2 years (10% vs. 19%, P = 0·014). Hospital stay, both total and after first positive culture, were shorter for patients with complications (median 18 vs. 24 days, P = 0·006; median 16 vs. 18 days, P = 0·02). When analysis of demographic and outcomes for patients with and without complications was performed with the exclusion of all premature neonates, there were no longer any significant differences in age (median 5 vs. 3 years, P = 0·59), total hospital stay (median 15 vs. 14 days, P = 0·32), and stay after positive culture (median 15 vs. 13 days, P = 0·84) (not shown). Kaplan–Meier analysis did not demonstrate a significant relationship between patient survival during hospital stay within 60 days after first positive cultures and complications (Fig. 2).
Table 3.
Treatment and clinical outcomes by complications and methicillin resistance
| Variable | With complication (n = 197)a | Without complication (n = 179) | Odds ratio (95% CI, P-value)b | MRSA complication (n = 38) | MSSA complication (n = 159) | Odds ratio (95% CI, P-value)a |
|---|---|---|---|---|---|---|
| No. of complications, mean ± s.d. (median, range) | 1·5 ± 0·9 (1, 1–6) | – | – | 2·2 ± 1·4 (2, 1–6) | 1·3 ± 0·6 (1, 1–4) | 0·01 |
| > 1 Foci, no. (%) | 63 (32) | 22 (58) | 41 (26) | <0·001 | ||
| Second focus in patients with osteomyelitis/septic arthritis, no. (%) | 29/57 (51) | – | – | 12 (92) | 17 (39) | 0·001 |
| Antibiotics initiated ⩽24 h of first positive culture, no. (%) | 175 (92) | 160 (93) | 0·74 | 35 (92) | 140 (92) | 0·99 |
| Empiric vancomycin, no. (%) | 134 (69) | 135 (76) | 0·14 | 33 (87) | 101 (65) | 0·009 |
| Days of vancomycin use, mean ± s.d. (median, range) | 8·9 ± 9·6 (4, 1–47) | 8·2 ± 10·2 (4, 1–75) | 0·56 | 17·9 ± 12·3 (14, 1–47) | 5·6 ± 5·5 (3, 1–26) | <0·001 |
| Days of bacteremiac, mean ± s.d. (median, range) | 2·5 ± 2·8 (1, 1–20) | 2·4 ± 6·9 (1, 1–88) | 0·98 | 2·8 ± 3·5 (1, 1–20) | 2·4 ± 2·7 (1, 1–20) | 0·42 |
| Days to clear bacteremiad, mean ± s.d. (median, range) | 4·4 ± 3·3 (4, 1–20) | 4·2 ± 8·2 (3, 1–96) | 0·8 | 5·4 ± 4 (4·5, 1–20) | 4·1 ± 3 (3, 1–19) | 0·07 |
| Total hospital days, mean ± s.d. (median, range) | 38·2 ± 50·8 (18, 1–382) | 53·8 ± 55·5 (24, 2–309) | 0·006 | 41·1 ± 46·5 (24·5, 1–248) | 37·4 ± 51·9 (16, 1–382) | 0·69 |
| Hospital days after first positive culture, mean ± s.d. (median, range) | 29·1 ± 40·5 (16, 0–381) | 39·7 ± 45·6 (18, 0–309) | 0·02 | 28·5 ± 19·5 (22·5, 1–62) | 29·2 ± 44·2 (15, 0–381) | 0·92 |
| ICU stay, no. (%) | 99 (50) | 97 (54) | 0·45 | 25 (66) | 74 (47) | 0·03 |
| Subsequent ED or hospital readmission for Staphylococcus aureus infection, no. (%) | 19 (10) | 33 (19) | 0·014 | 5 (14) | 14 (9) | 0·37 |
| All-cause case fatality, no. (%) | 18 (9) | 14 (8) | 0·648 | 5 (13) | 13 (8) | 0·35 |
| SAB-related case fatality, no. (%)e | 10 (56) | 4 (29) | 0·165 | 3 (60) | 7 (54) | 0·99 |
CI, confidence interval; ED, emergency department; ICU, intensive care unit; MRSA, methicillin-resistant S. aureus; MSSA, methicillin-sensitive S. aureus; s.d., standard deviation; SAB, S. aureus bacteremia.
Percentages presented for nominal variables are based on number of evaluable patients, which may represent a different denominator than the sample size in each category.
Only P-values were provided for continuous variables.
Days of bacteremia was defined as time from first positive to last positive cultures.
Days to clear bacteremia was defined as time from first positive to first negative cultures.
The total number of patients was based on those who died.
Fig. 2.
Stratified Kaplan–Meier curve of hospital days after first positive Staphylococcus aureus blood cultures.
Prevalence of methicillin resistance
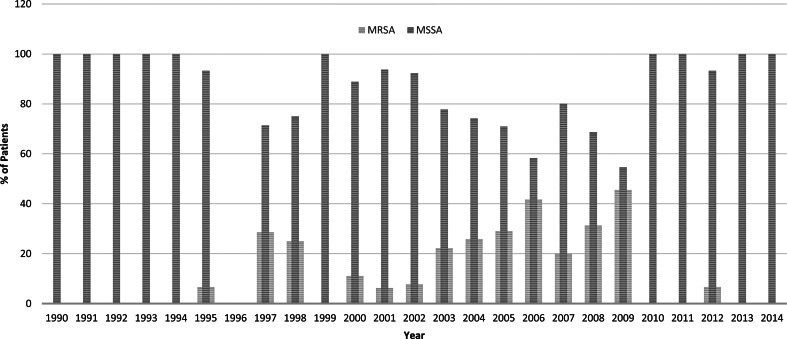
Compared with the first 10-year study period from 1990 to 1999, the second 10-year period from 2000 to 2009 had significant increases in the prevalence of methicillin resistance (26% of 216 vs. 5% of 98, P < 0·001) and complications (53% vs. 36%, P = 0·004) (Fig. 3). However, there was a decrease of methicillin resistance in 2010–2014 and the prevalence did not differ significantly from 1990 to 1999 (2% of 62 vs. 5% of 98, P = 0·31).
Fig. 3.
Annual incidences (%) of methicillin resistance by year. Compared with 1990–1999, in 2000–2009, there were significant increases in methicillin resistance (5% of 98 vs. 26% of 216, P < 0·001) and 10-year period prevalence of complications (36% vs. 53%, P = 0·004). In 2010–2014, methicillin resistance decreased to 2% and did not significantly differ compared with 1990–1999 (5% of 98 vs. 2% of 62, P = 0·31).
Subjects with complications by methicillin resistance
Among subjects with complications, all baseline characteristics were similar between those with MRSA vs. MSSA SAB-related complications (Table 2). More complicated SAB originating from the community were caused by MSSA (63% vs. 44%, P = 0·036). The number of complications per episode (2·2 vs. 1·3, P = 0·01) and proportion of patients with multiple foci (58% vs. 26%, P < 0·001) were significantly higher in those with MRSA SAB than MSSA SAB (Table 3). Among those with SAB and septic arthritis or osteomyelitis, 12 of 13 of subjects with MRSA and 17 of 44 with MSSA had a second focus (92% vs. 39%, P = 0·001). Compared with those with MSSA, more patients with complications associated with MRSA SAB had ICU stay (66% vs. 47%, P = 0·03). More of these patients also were initiated on vancomycin empirically (87% vs. 65%, P = 0·009) and had longer vancomycin therapy (17·9 vs. 5·6 days, P < 0·001) than patients with complications from MSSA SAB.
Multivariate analysis
By multivariate regression analysis (Table 4), patients with community-associated infections were twice as likely to develop SAB-related complications (OR 1·82; 95% confidence interval (CI) 1·1–3·02, P = 0·02). Conversely, patients with concurrent infections were almost twice as less likely to develop complications (OR 0·58; 95% CI 0·97–0·34, P = 0·038). Admission to the ICU, age, and methicillin resistance were not associated with development of complications.
Table 4.
Multivariate analysis of risk factors associated with complicationsa
| Factor | Odds ratio (95% confidence interval) | P-value |
|---|---|---|
| Concurrent infections | 0·58 (0·34–0·97) | 0·038 |
| Community-associated infection | 1·82 (1·1–3·02) | 0·021 |
Age, methicillin-resistant Staphylococcus aureus, and admission to the intensive care unit were analyzed but found to be not significant.
DISCUSSION
The morbidities associated with SAB consist of complications that may occur at any bodily site. Our study was unique as it comprehensively evaluated these distinct complications and readmission to the ED or hospital associated with SAB. Overall, more than half of the children with SAB in our cohort experienced complications. Similar to other published pediatric studies, the most common complications identified in our study were osteomyelitis, skin and soft tissue infection, pneumonia, and septic arthritis [14,20,22–24]. Respiratory distress syndrome was also a common complication in our study, observed in 16% of children. Infrequent complications in our study were infective endocarditis and toxic-shock syndrome, which is also similar to existing evidence [16,24]. Of note, almost 80% of our patients had CVC. The primary origin of SAB in one study was from CVC and prolonged bacteremia was also reported in the presence of CVC [13,22].
Unsuspected foci may contribute significantly to the mortality and morbidity of SAB as failure to identify complications timely, particularly endocarditis and vertebral osteomyelitis, may result in calamitous outcomes, including neurologic events, multi-organ failure, paralysis, and death [25,26]. Among children with SAB-related complications in our study, one-third had unsuspected foci, which included osteomyelitis, septic arthritis, and pneumonia. Similarly, unsuspected foci of infection were identified in 20% of pediatric patients (excluding neonates) with SAB [14]. Another study that evaluated only deep-seated foci of infections (primarily consisted of osteomyelitis and endocarditis) observed unsuspected complications in only 3·7% of children [18]. This difference was most likely attributed to our distinct definition for unsuspected focus, defined as ⩾2 days after first positive blood cultures in our study as opposed to before hospital discharge in the other study. Furthermore, in our study, one-third of children with SAB-related complications also had multiple foci of infection.
We observed a rise in MRSA causing bacteremia in children between 1990–1999 and 2000–2009 and conversely a decrease in methicillin resistance in 2010–2014. Similar drops in incidences of methicillin resistance have also been documented in recent years. Both hospital-acquired and CA-MRSA SAB decreased in incidence from 2005 to 2011 in the USA and MRSA SAB was shown to have decreased from 11·5% to 7·8% of SAB from 2002 to 2007 in the UK [27,28]. Furthermore, significantly more patients with MRSA bacteremia in our study had multiple foci of infection and ICU admission. An association between multiple infectious foci and ICU stay has been demonstrated previously in a 10-year single-center study, in which 67% of pediatrics with SAB requiring ICU admission had multifocal complications [29]. In another study, ICU admission was more common in children with MRSA vs. MSSA SAB [30].
Our findings from multivariate regression modeling indicated that those with complications, as compared with those without, were two times more likely to have community-associated SAB. This corroborates with one previously published study that demonstrated significantly more children with suspected complications, compared with those without, had community-onset SAB (83% vs. 57%, P < 0·01) [18]. While this trend was observed in another study of children with bone and joint infections, it was not apparent in children with SAB complicated by endocarditis [20,21]. Interestingly, one study observed 95% of 58 cases of S. aureus infections requiring pediatric ICU admission were community-associated [31]. Although studies have found age, MRSA, and delayed antibiotic therapy to be associated with SAB complications in multivariate models [16,28,30], these associations were not found to be significant in our analysis. However, we did show that the presence of concurrent infections was associated with a lower risk of SAB complications.
Case-fatality rates associated with SAB have ranged from 1·4% to 8% in children (8% observed in our study) [13,14,17,20]. Furthermore, in children, increased mortality has been observed in those with SAB-related endocarditis (20%) and potentially, toxic shock syndrome (~5%) [16,32].
In our study, the exclusion of all premature patients yielded differing results for age and hospital stay. However, this is not unexpected as a large proportion of premature patients had uncomplicated SAB and all tended to have longer hospital stay due to their underlying conditions. Approximately 14% of our pediatric population were subsequently admitted to the ED or hospital for SAB within 2 years. Significantly fewer children with complications, as compared with those without, had subsequent SAB-related ED or hospital visit, an interesting finding in our study that has not been previously assessed in other pediatric studies. This observation, along with our observation from multivariate analysis that patients with concurrent infections were less likely to develop SAB complications may be attributed to more aggressive treatment and monitoring or differences in innate immunity response to infection.
Limitations of this study were irretrievable data, particularly those in the early study period to determine whether or not the foci of infection were unsuspected (i.e. ⩾2 days after diagnosis of SAB). We also did not record whether patients have had a recent episode of SAB before their first positive culture. In addition, although recent evidence has suggested the need to classify bloodstream infections into community- and healthcare-associated categories, we did not know with certainty about all healthcare interactions of study patients outside of our institution before the study period [33]. Furthermore, molecular analysis was not performed to truly determine the presence of community-associated strains as isolates were not consistently saved for analysis throughout the study period. Lastly, readmission to the hospital or ED was also only captured at our hospital; therefore, this may have been underestimated since admission to other healthcare facilities was not assessed.
In conclusion, with the high prevalence of complications, both concurrent and unexpected, hospitalized children with SAB should be carefully evaluated and monitored, including those with community-associated and nosocomial SAB. Our interesting discoveries showing less patients with SAB complications subsequently requiring ED or hospital admission as well as children with concurrent infections by other organisms being less likely to develop SAB complications deserve further exploration to determine potential contributory roles of treatment differences or innate immunity. In addition, future studies should evaluate the impact on mortality and infection-related hospital readmission of treatment strategies and other clinical outcomes, such as antibiotic selection, duration and outpatient adherence, source control, as well as infectious disease consult utilization. Lastly, the prevalence of MRSA sharply decreased during the last 5 years of our study, and this appears to be a part of a larger trend of decreased prevalence of methicillin resistance in recent years worldwide. Although MRSA may be less prevalent, clinicians should still be vigilant as MRSA bacteremia was associated with a greater number of complications and ICU stay.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Dr Sarah Hwang, Dr Helen Le, Dr Wendy Dinh, Dr Gene Nguyen, and Dr Yvonne Tongoc for assisting in data collection. In addition, the authors would like to thank Jane Hodding, Pharm.D. for providing the census data.
This work was supported, in part, by Cubist Pharmaceuticals, Inc., Lexington, Massachusetts, USA; and the Memorial Medical Center Foundation, Long Beach, CA (J.L.).
DECLARATION OF INTEREST
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. J.L. receives support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Institute of Allergy and Infectious Diseases (1U54HD090259 and 1K23AI089978) and previously received investigator-initiated research grants from Cubist, Pfizer, and Astellas.
REFERENCES
- 1.Frederiksen MS, et al. Changing epidemiology of pediatric Staphylococcus aureus bacteremia in Denmark from 1971 through 2000. The Pediatric Infectious Disease Journal 2007; 26: 398–405. [DOI] [PubMed] [Google Scholar]
- 2.Gaynes RP, et al. Nosocomial infections among neonates in high-risk nurseries in the United States. National Nosocomial Infections Surveillance System. Pediatrics 1996; 98: 357–361. [PubMed] [Google Scholar]
- 3.Richards MJ, et al. Nosocomial infections in pediatric intensive care units in the United States. National Nosocomial Infections Surveillance System. Pediatrics 1999; 103: e39. [DOI] [PubMed] [Google Scholar]
- 4.Watson RS, et al. The epidemiology of severe sepsis in children in the United States. American Journal of Respiratory and Critical Care Medicine 2003; 167: 695–701. [DOI] [PubMed] [Google Scholar]
- 5.Wisplinghoff H, et al. Nosocomial bloodstream infections in pediatric patients in United States hospitals: epidemiology, clinical features and susceptibilities. The Pediatric Infectious Disease Journal 2003; 22: 686–691. [DOI] [PubMed] [Google Scholar]
- 6.Styers D, et al. Laboratory-based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Annals of Clinical Microbiology and Antimicrobials 2006; 5: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wisplinghoff H, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clinical Infectious Diseases 2004; 39: 309–317. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus – Minnesota and North Dakota, 1997–1999. JAMA 1999; 282: 1123–1125. [PubMed] [Google Scholar]
- 9.Andrade ES, et al. Community-acquired versus healthcare-associated Staphylococcus aureus bacteraemia among children in a tropical region. Infectious Diseases (London, England) 2017; 49: 549–551. [DOI] [PubMed] [Google Scholar]
- 10.Fortunov RM, et al. Community-acquired Staphylococcus aureus infections in term and near-term previously healthy neonates. Pediatrics 2006; 118: 874–881. [DOI] [PubMed] [Google Scholar]
- 11.Purcell K, Fergie J. Epidemic of community-acquired methicillin-resistant Staphylococcus aureus infections: a 14-year study at Driscoll Children's Hospital. Archives of Pediatrics and Adolescent Medicine 2005; 159: 980–985. [DOI] [PubMed] [Google Scholar]
- 12.Fowler VG Jr., et al. Role of echocardiography in evaluation of patients with Staphylococcus aureus bacteremia: experience in 103 patients. Journal of the American College of Cardiology 1997; 30: 1072–1078. [DOI] [PubMed] [Google Scholar]
- 13.Bendig EA, et al. The impact of the central venous catheter on the diagnosis of infectious endocarditis using Duke criteria in children with Staphylococcus aureus bacteremia. The Pediatric Infectious Disease Journal 2008; 27: 636–639. [DOI] [PubMed] [Google Scholar]
- 14.Denniston S, Riordan FA. Staphylococcus aureus bacteraemia in children and neonates: a 10 year retrospective review. The Journal of Infection 2006; 53: 387–393. [DOI] [PubMed] [Google Scholar]
- 15.Ferrieri P, et al. Unique features of infective endocarditis in childhood. Pediatrics 2002; 109: 931–943. [DOI] [PubMed] [Google Scholar]
- 16.Hajjeh RA, et al. Toxic shock syndrome in the United States: surveillance update, 1979 1996. Emerging Infectious Diseases 1999; 5: 807–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hakim H, Mylotte JM, Faden H. Morbidity and mortality of Staphylococcal bacteremia in children. American Journal of Infection Control 2007; 35: 102–105. [DOI] [PubMed] [Google Scholar]
- 18.Ross AC, et al. Frequency and risk factors for deep focus of infection in children with Staphylococcus aureus bacteremia. The Pediatric Infectious Disease Journal 2008; 27: 396–399. [DOI] [PubMed] [Google Scholar]
- 19.Stockheim JA, et al. Are the Duke criteria superior to the Beth Israel criteria for the diagnosis of infective endocarditis in children? Clinical Infectious Diseases 1998; 27: 1451–1456. [DOI] [PubMed] [Google Scholar]
- 20.Suryati BA, Watson M. Staphylococcus aureus bacteraemia in children: a 5-year retrospective review. Journal of Paediatrics and Child Health 2002; 38: 290–294. [DOI] [PubMed] [Google Scholar]
- 21.Valente AM, et al. Frequency of infective endocarditis among infants and children with Staphylococcus aureus bacteremia. Pediatrics 2005; 115: e15–e19. [DOI] [PubMed] [Google Scholar]
- 22.Cobos-Carrascosa E, et al. Staphylococcus aureus bacteremia in children: changes during eighteen years. The Pediatric Infectious Disease Journal 2015; 34: 1329–1334. [DOI] [PubMed] [Google Scholar]
- 23.Klieger SB, et al. Staphylococcus aureus bacteremia in hospitalized children: incidence and outcomes. Infection Control and Hospital Epidemiology 2015; 36: 603–605. [DOI] [PubMed] [Google Scholar]
- 24.McMullan BJ, et al. Epidemiology and mortality of Staphylococcus aureus bacteremia in Australian and New Zealand children. JAMA Pediatrics 2016; 170: 979–986. [DOI] [PubMed] [Google Scholar]
- 25.Darouiche RO, et al. Bacterial spinal epidural abscess. Review of 43 cases and literature survey. Medicine (Baltimore) 1992; 71: 369–385. [PubMed] [Google Scholar]
- 26.Nadji G, et al. Comparison of clinical and morphological characteristics of Staphylococcus aureus endocarditis with endocarditis caused by other pathogens. Heart 2005; 91: 932–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dantes R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Internal Medicine 2013; 173: 1970–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pai S, Enoch DA, Aliyu SH. Bacteremia in children: epidemiology, clinical diagnosis and antibiotic treatment. Expert Review of Anti-Infective Therapy 2015; 13: 1073–1088. [DOI] [PubMed] [Google Scholar]
- 29.Miles F, et al. Review of Staphylococcus aureus infections requiring admission to a paediatric intensive care unit. Archives of Disease in Childhood 2005; 90: 1274–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez G, et al. Community-acquired Staphylococcus aureus bacteremia in children: a cohort study for 2010–2014. Archivos Argentinos de Pediatría 2016; 114: 508–513. [DOI] [PubMed] [Google Scholar]
- 31.Yaw LK, Robinson JO, Ho KM. A comparison of long-term outcomes after methicillin-resistant and methicillin-sensitive Staphylococcus aureus bacteraemia: an observational cohort study. The Lancet Infectious Diseases 2014; 14: 967–975. [DOI] [PubMed] [Google Scholar]
- 32.Chuang YY, Huang YC, Lin TY. Toxic shock syndrome in children: epidemiology, pathogenesis, and management. Paediatric Drugs 2005; 7: 11–25. [DOI] [PubMed] [Google Scholar]
- 33.Lenz R, et al. The distinct category of healthcare associated bloodstream infections. BMC Infectious Diseases 2012; 12: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]