Abstract
Objective
BNT162b2 messenger RNA (mRNA) COVID-19 vaccine administered during pregnancy was found to produce a strong maternal immunoglobulin (IgG) response which crosses the placenta to the newborn. Our aim was to evaluate maternal and neonatal SARS-CoV-2 IgG antibody levels at birth, following a COVID-19 booster vaccine during the third trimester.
Study design
A prospective cohort study including women admitted to delivery ward at least 7 days after their BNT162b2 (Pfizer/BioNTech) booster vaccination without a prior clinical COVID-19 infection. SARS-CoV-2 IgG antibodies levels were measured in maternal blood upon admission to delivery and in the umbilical blood within 30 min following delivery. The correlation between antibody titers, feto-maternal characteristics, maternal side effects following vaccination, and time interval from vaccination to delivery were analyzed.
Results
Between September to November 2021, high antibody levels were measured in all 102 women and 93 neonatal blood samples, at a mean ± standard deviation duration of 7.0 ± 2.9 weeks after the third vaccine. We found positive correlation between maternal and neonatal antibodies (r = 0.73, 95% confidence interval [CI] 0.61 to 0.81, p < 0.001), with neonatal titers approximately 1.4 times higher compared to maternal titers. In the multivariable analysis maternal antibody levels dropped by −7.2% (95% CI −12.0 to −2.3%, p = 0.005) for each week that passed since the receipt of the third vaccine dose. In contrary, systemic side effects after the third vaccine were associated with higher maternal antibody levels of 52.0% (95% CI 4.7 to 120.8%, p = 0.028). Also, for each 1 unit increase in maternal body mass index, maternal antibody levels increased by 3.6% (95% CI 0.4 to 6.9%, p = 0.025).
Conclusions
BNT162b2 mRNA COVID-19 booster dose during the third trimester of pregnancy was associated with strong maternal and neonatal responses as reflected by maternal and neonatal SARS-CoV-2 IgG antibody levels measured at birth. These findings support the administration of the COVID-19 booster to pregnant women to restore maternal and neonatal protection during the ongoing pandemic.
Keywords: Antibodies, Booster, COVID-19 vaccination, Pregnancy, Third trimester
Introduction
The messenger RNA (mRNA) COVID-19 vaccines have been found to be highly effective in preventing symptomatic COVID-19, including those at risk for severe disease [1] such as pregnant women [2], [3]. Despite the initially promising results of nationwide vaccination campaigns, many countries are currently experiencing a resurgence of COVID-19 [4], probably due to reduction in the protection of the COVID-19 vaccine after about 6 months from administration [5]. In the past few months, a few health organizations, mainly in Western countries, have authorized the use of a booster dose of COVID-19 vaccine to reestablish protection after it has decreased [6]. On the 19th of August 2021 the Israeli Ministry of Health authorized and recommended the administration of the booster dose to pregnant women who are at least six months after the second vaccine. On the 22 of September 2021 the Food and Drug Administration (FDA) authorized the use of a single booster dose for individuals 18 through 64 years of age at high risk of severe COVID-19 including pregnant women, who are at least six months after completion of the primary vaccinations [7]. Two studies conducted in Israel demonstrated that a third dose of the BNT162b2 mRNA COVID-19 vaccine was effective in protecting individuals against severe COVID-19 related outcomes, compared with those who received only two doses at least 5 months before [4], [8].
Gray et al. found that the mRNA COVID-19 vaccines generate a robust humoral immunity in pregnant women similar to that observed in nonpregnant women [9]. Moreover, a multi-center study found that the mRNA COVID-19 vaccine during the 3rd trimester elicits a strong maternal humoral IgG response that crosses the placenta barrier and approaches maternal titers in the fetus within 15 days following the first dose [10].
An open label randomized study revealed that antibody titers two weeks after a booster dose of an mRNA COVID-19 vaccine were at least as high as the peak titer measurement one month after the primary vaccine series [11]. Flaxman et al. reported data from two randomized controlled trials which discovered that antibody titers 28 days after administration were significantly higher among participants who received a third vaccine dose compared to those who received the second vaccine dose [12]. Also, a robust correlation was seen between antibody titers and efficacy, with higher titers correlating with higher vaccine efficacy [13].
Administration of a third mRNA COVID-19 vaccine dose in pregnancy should restore maternal and neonatal protection after it had decreased. In this study we aim to evaluate maternal and neonatal COVID-19 antibody levels at birth, following the mRNA COVID-19 booster vaccine administration during the third trimester of pregnancy.
Materials and methods
This was a prospective cohort study, conducted between September 2021 to November 2021 at the delivery ward of Carmel Medical Center, Haifa, Israel.
Pregnant women over 24 weeks of their singleton gestation expected to give birth within three days, who were at least 7 days from their third BNT162b2 (Pfizer/BioNTech) mRNA COVID-19 vaccination and were not known to be previously infected with the virus, were recruited and enrolled consecutively upon admission to the delivery ward. We excluded women who did not receive three doses of the vaccine and those who reported a previous COVID-19 infection. A written and signed informed consent form was obtained from all study participants before enrollment.
A maternal blood sample was obtained following recruitment and an umbilical blood sample was obtained within 30 min following delivery. Both maternal and umbilical blood samples were assessed by SARS-CoV-2 IgG II Quant Abbott@, a two-step chemiluminescent microparticle immunoassay used for the quantitative determination of IgG antibodies to SARS-CoV-2 on Architect and Alinity i systems. The SARS-CoV-2 IgG II Quant assay is designed to detect IgG antibodies, including neutralizing antibodies, to the receptor-binding domain (RBD) of the S1 subunit of the spike protein of SARS-CoV-2 virus in human serum and plasma.
At the time of recruitment, demographic and clinical data were collected including maternal age, body mass index (BMI) at delivery, self-reported ethnicity, history of systemic disease and chronic medications, parity, date and gestational age at the 1st, 2nd and 3rd vaccine, systemic side effects after each vaccine and gestational age at birth. The time interval between the third vaccine and delivery was calculated. Further data were extracted from patients’ charts following delivery including newborn sex and birthweight. The correlation between antibody titers, feto-maternal characteristics, and the time interval from vaccination to delivery were analyzed after the completion of data gathering. Maternal and neonatal IgG antibodies from the current study were compared to antibody levels measured after second trimester 2-dose vaccination in a previous study published by our group [14]. In addition, transplacental SARS-CoV-2 IgG antibody transfer ratios were calculated by dividing neonatal (umbilical cord blood) IgG levels, by the IgG antibody levels in paired maternal blood. Subsequently, correlation between transplacental SARS-CoV-2 IgG antibody transfer ratios and duration from third COVID-19 vaccination to birth were analyzed.
Missing data
Nine of 102 (8.8%) neonates of women who met the inclusion criteria were not included because blood from their umbilical cord was not collected.
Ethical considerations
The study was approved on August 24, 2021 by the Institutional Review Board of Carmel Medical Center (Protocol number CMC-0051–21(.
Statistical analysis
After data collection, the correlation between antibody titers, feto-maternal characteristics, and the time interval from vaccination to delivery were analyzed. Continuous variables are presented as means and standard deviations (SD) or as medians and interquartile ranges (IQR). Categorical variables are presented as percentages. To estimate correlations with antibody levels, we used a univariable linear model (maternal and neonatal separately) with a logarithmic transformation for the antibody level because it is not normally distributed. Correlation coefficients (r) with 95% confidence intervals (CI) for the correlation between the antibody level and duration from receipt of the third vaccine dose are presented. Variables that were found to be statistically significant (2-sided P < 0.05) were entered into a multivariable regression model to estimate adjusted associations with antibody levels. Correlations between the maternal and neonatal antibody levels, between transplacental antibody transfer ratio and duration from third COVID-19 vaccination to birth, and between antibody levels and duration from last vaccine to birth were analyzed using the Spearman correlation. The 95% CI for the correlation coefficient was performed using SAS, version 9.4 (SAS Institute Inc). All other analyses were performed using IBM statistics version 24 (SPSS).
Results
Between September 2021 to November 2021, 102 women were recruited a mean ± SD duration of 7.0 ± 2.9 weeks after the third vaccine. Umbilical blood was not collected in 9 neonates. The first and second vaccination were administered prior to conception in 60 and 36 women, respectively. However, 42 women received the first vaccination after becoming pregnant at a mean ± SD gestational age of 5.7 ± 3.5 weeks, whereas 66 women received their second vaccine after conceiving at a mean ± SD gestational age of 6.7 ± 3.9 weeks. The mean ± SD gestational age at the receipt of the third vaccination was 32.2 ± 3.2 weeks. Furthermore, the mean ± SD time interval from the second to the third vaccine was 28.2 ± 3.7 weeks. Our objective was to recruit women soon after the booster dose was authorized to pregnant women, aiming to evaluate antibodies at birth following third trimester booster vaccination. However, 4 women received the 3rd vaccine at 24–27 weeks of gestation because they delivered prematurely. Nonetheless, they were included in the study analysis because the time from vaccination to delivery was similar to the other participants which were vaccinated in the third trimester.
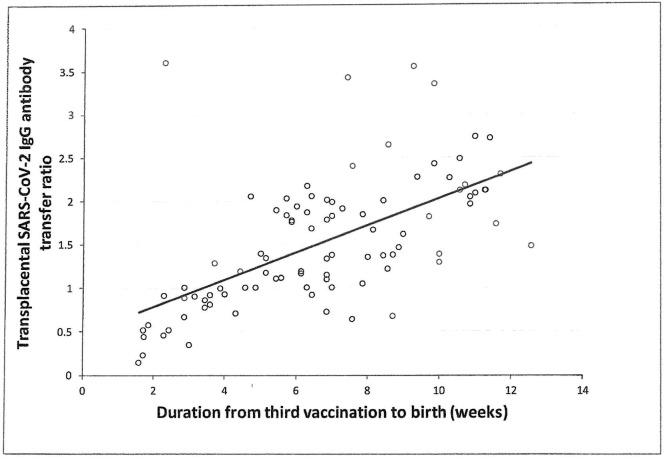
Demographic and clinical characteristics are presented in Table 1 . 100% of maternal and neonate SARS-CoV-2 IgG tests were positive. Median (range) level of IgG antibodies at birth was 13,681 (5718–18095) AU/ml for parturients and 19,221 (8352–26717) AU/ml for neonates. Median (range) maternal and neonatal IgG antibodies at birth following third trimester booster vaccination were higher than antibody levels measured after second trimester 2-dose vaccination (maternal- 1185 [146–21,415] AU/ml; neonatal- 3315 [350–17,643]) in a previous study published by our group (p < 0.001) [14]. We found a median (IQR) transplacental SARS-CoV-2 IgG antibody transfer ratio of 1.37 (0.97–2.01). A positive correlation between transplacental SARS-CoV-2 IgG antibody transfer ratio and duration from last vaccine to birth was found)r = 0.41, 95% CI 0.23 to 0.57, P < 0.001) (Fig. 1 ).
Table 1.
Demographic and clinical characteristics of pregnant women vaccinated with the third BNT162b2 mRNA COVID-19 vaccine.
| All women (n = 102) | |
|---|---|
| All neonates (n = 93) | |
| 33.3 ± 4.6 | Maternal age, mean (SD), years |
| 27.7 (25.7, 31.6) | Body mass index, median (IQR), kg/m2 |
| 29 (28.4) | Background systemic diseasea No. (%) |
| 101 (99.0) | Jewish religion |
| 1 (1.0) | Arab ethnicity |
| 33 (32.4) | Primipara No. (%) |
| 34 (33.3) | Multipara ≥ 2 births No. (%) |
| 2 (1.5) | Grand multipara ≥ 5 births No. (%) |
| 5 (4.9) | Systemic side effects after 1st vaccineb No. (%) |
| 30 (29.4) | Systemic side effects after 2nd vaccineb No. (%) |
| 32.2 ± 3.2 | Gestational age at 3rd vaccine, mean (SD), weeks |
| 28.2 ± 3.7 | Duration from second to third vaccine, mean (SD), weeks |
| 21 (20.6) | Systemic side effects after 3rd vaccineb No. (%) |
| 37 (36.3) | Systemic side effects after any vaccineb No. (%) |
| 38.9 ± 1.6 | Gestational age at birth, mean (SD), weeks |
| 7.0 ± 2.9 | Duration from third vaccine to birth, mean (SD), weeks |
| 13,681 (5718–18095) | Intrapartum maternal SARS-CoV-2 IgG antibodies, median (range), AU/ml |
| 10,198 (8690–11849) | Intrapartum maternal SARS-CoV-2 IgG antibodies, geometric mean (95 % CI), AU/ml |
| 19,221 (8352–26717) | Newborn SARS-CoV-2 IgG antibodies, median (range), AU/ml |
| 13,882 (11591–15398) | Newborn SARS-CoV-2 IgG antibodies, geometric mean (95 % CI), AU/ml |
| 41 (44.1) | Newborn male sex No. (%) |
| 3157 ± 465.0 | Birthweight, mean (SD), grams |
| 4 (43.0) | Neonatal intensive care unit admission No. (%) |
Hypertension (5 women), diabetes (6 women), thyroid disease (12 women), epilepsy (2 women), Crohn’s disease (1 woman), hypercoagluation state (1 woman), fibromyalgia (1 woman), IgG nephropathy and psoriasis (1 woman), complex migranes (1 woman).
General weakness, dizziness, fever, chills, headache, vomiting, general muscle aches, fatigue, general rash.
Fig. 1.
Correlation between transplacental SARS-CoV-2 IgG antibody transfer ratio and duration from third COVID-19 vaccination to birth; r = 0.41, 95% CI 0.23 to 0.57, P < 0.001.
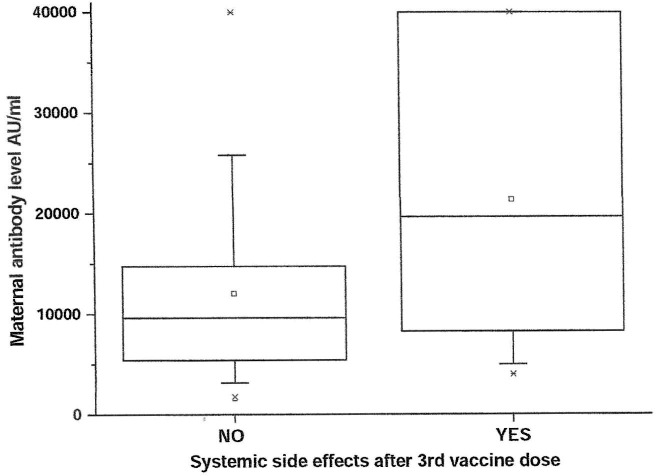
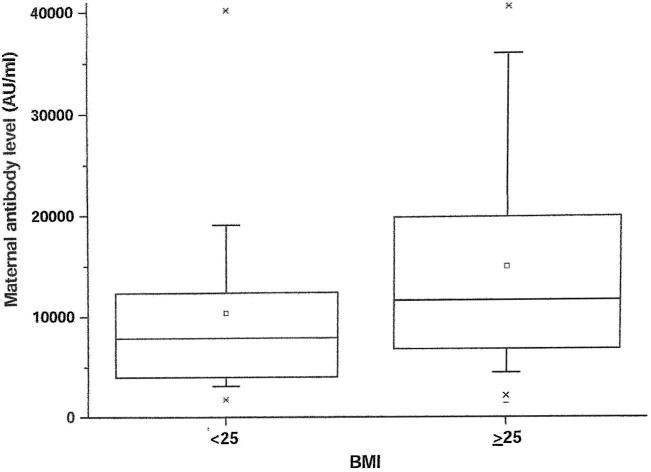
Univariable analysis demonstrated positive correlation between maternal SARS-CoV-2 IgG antibody titer levels at delivery and systemic side effects after the 3rd vaccine dose or BMI, and negative correlation with duration from the 3rd vaccine to birth. Systemic side effects after the 3rd vaccine were associated with higher maternal antibody levels of 71.9% (95% CI 16.8, 153.0%, p = 0.03). Maternal SARS-CoV-2 IgG antibody levels in women with side effects after the 3rd vaccine were higher than women without systemic side effects after the 3rd vaccine (median [IQR] 19,641 [7864–40000] vs. 9622 [5361–14776] AU/ml; P = 0.005) (Fig. 2 ). Also, for each unit increase in BMI, maternal antibody levels increased by 4.4% (95% CI 1.0 to 7.9%, p = 0.01). Maternal SARS-CoV-2 IgG antibody levels in overweight/obese women (BMI ≥ 25 kg/m2) were higher than normal weight women (BMI < 25 kg/m2) (median [IQR] 11,506 [6664–19399] vs. 7861 [3661–11981] AU/ml; P = 0.033) (Fig. 3 ). Furthermore, for each week that passed since the 3rd vaccine, maternal antibody levels dropped by 8.0% (95% CI −13.0 to −3.0%, p = 0.003) (Fig. 4 ). No correlation was found between maternal SARS-CoV-2 IgG antibody levels and maternal age, any maternal background systemic disease or parity.
Fig. 2.
Box plot presenting the difference between maternal SARS-CoV-2 IgG antibody levels of women with or without systemic side effects after the 3rd vaccine; The boxes represent the data between the 25 percentile to the 75 percentile, the line in the middle of the boxes represents the median.
Fig. 3.
Box plot presenting the difference between maternal SARS-CoV-2 IgG antibody levels of overweight/obese women (BMI ≥ 25 kg/m2) and normal weight women (BMI < 25 kg/m2); The boxes represent the data between the 25 percentile to the 75 percentile, the line in the middle of the boxes represents the median.
Fig. 4.
Correlation between the time interval from the 3rd COVID-19 vaccination and maternal SARS-CoV-2 IgG antibodies; r = -0.31, 95% CI −0.47 to −0.12, P = 0.002.
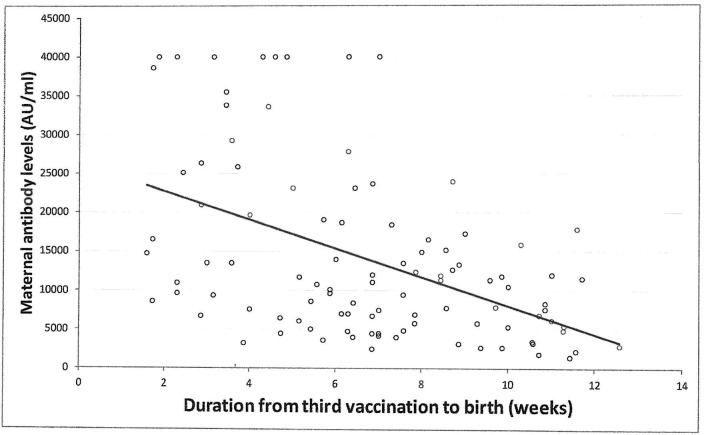
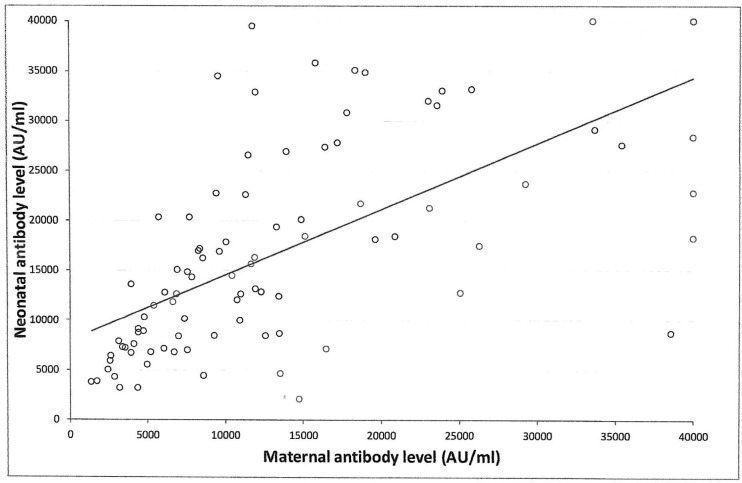
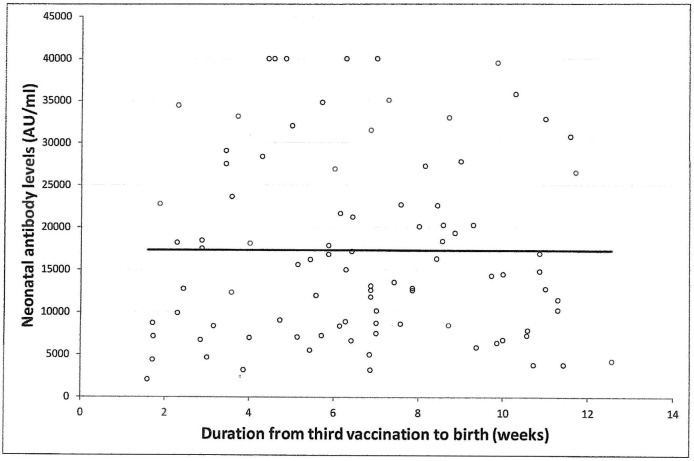
Univariable analysis demonstrated positive correlation between neonatal SARS-CoV-2 IgG antibody titers at delivery with maternal antibodies (r = 0.73, 95% CI 0.61 to 0.81; p < 0.001) (Fig. 5 ), and maternal systemic side effects after the 3rd vaccine, and maternal BMI. For each one percent increase in maternal antibody level, neonatal antibody levels increased by 0.6% (95% CI 0.5 to 0.8%, p < 0.001). Additionally, systemic side effects after the 3rd vaccine were associated with higher neonatal antibody levels of 61.1% (95% CI 14.7 to 126.6%, p = 0.01). Also, for each unit increase in BMI, neonatal antibody levels increased by 3.2% (95% CI 0.1 to 6.2%, p = 0.03). No significant correlation was found between neonatal SARS-CoV-2 IgG antibody levels and any maternal background systemic disease, parity, gestational age at 3rd vaccine, gestational age at birth, duration from 3rd vaccine to birth (Fig. 6 ), newborn sex or birthweight, and neonatal intensive care unit admission.
Fig. 5.
Correlation between maternal SARS-CoV-2 IgG antibody levels and newborn SARS-CoV-2 IgG antibody levels at birth after BNT162b2 mRNA COVID-19 third vaccine dose during third trimester of pregnancy; r = 0.73, 95% CI 0.61 to 0.81; P < 0.001.
Fig. 6.
Correlation between the time interval from the 3rd COVID-19 vaccination and neonatal SARS-CoV-2 IgG antibodies; r = -0.04, 95% CI −0.24 to 0.16, P = 0.709.
Multivariable analysis revealed an inverse correlation between maternal titers at delivery with the time interval from the 3rd vaccination. For each week that passed since receipt of the 3rd vaccine dose, maternal antibody levels dropped by −7.2% (95% CI −12.0 to −2.3%, p = 0.005). Furthermore, systemic side effects after the 3rd vaccine were associated with higher maternal antibody levels of 52.0% (95% CI 4.7 to 120.8%, p = 0.028). Moreover, for each 1 unit increase in the mother’s BMI, maternal antibody levels increased by 3.6% (95% CI 0.4 to 6.9%, p = 0.007).
In the multivariable analysis only maternal antibody titers remained significantly correlated with neonatal antibody titers. For each one percent increase in maternal antibody level, neonatal antibody levels increased by 0.6% (95% 0.5 to 0.7%, p < 0.001).
Discussion
In this prospective study, maternal booster vaccine dose in the third trimester and at least 6 months after the second mRNA COVID-19 vaccination, was found to provide high SARS-CoV-2 IgG antibody levels in both the mother and neonate. Maternal antibody titers were positively correlated with the presence of systemic side effects after the third vaccine and maternal BMI but negatively correlated with the duration of time between the booster vaccine and delivery. There was a positive correlation between neonatal and maternal antibody titers. We found a median transplacental SARS-CoV-2 IgG antibody transfer ratio of 1.4. Furthermore, a positive correlation between transplacental SARS-CoV-2 IgG antibody transfer ratio and duration from last vaccine to birth was demonstrated.
Our results are in line with previous studies demonstrating good maternal antibody response and placental transmission of SARS-CoV-2 IgG antibodies after third trimester mRNA COVID-19 vaccination [2], [3], [10], [15], [16], [17]. In a previous study by our group, we found that second trimester mRNA COVID-19 vaccination elicits high SARS-CoV-2 IgG antibody levels up to delivery, suggesting sufficient maternal and neonatal COVID-19 protection [14]. Interestingly, as in the current study, maternal antibody titers were negatively correlated to the time passed since vaccination. Yang et al. [18]. found that maternal anti-spike IgG levels were detectable at delivery, regardless of timing of vaccination, among fully vaccinated women, although early third-trimester vaccination was associated with the highest anti-spike IgG levels in maternal and umbilical cord blood. In their study, 20 pregnant women received a booster vaccine dose in the third trimester, with maternal anti-spike IgG levels greater than third-trimester vaccination in both maternal and neonatal blood. Similarly, in the current study we found higher absolute median maternal and neonatal antibody levels after maternal third trimester booster vaccine, compared to second trimester vaccinations [14]. However, this may be a result of either the proximity of the booster vaccination to delivery or the higher humoral response after a third vaccination dose.
Our findings are also in accordance with the efficacy of a third versus second vaccination dose in non-pregnant women that has been shown to be of high potency [11], [12], [13]. Choi et al. demonstrated that antibody titers two weeks after a booster dose vaccine were at least as high as the peak titer measurement one month after the primary vaccine series [11]. Flaxman et al. describes that antibody titers 28 days after administration were significantly higher among participants who received a third vaccine dose compared to those who received the second vaccine dose [12].
Interestingly, we found that both presence of systemic side effects of the third dose of vaccination and BMI were positively correlated with antibody levels. Women who suffered from systemic side effects such as fever, chills, and fatigue eventually presented higher antibody titers. Adverse side effects have been previously shown to correlate with a higher vaccine response, especially when the side effects are systemic (e.g., fever) [19], [20], yet the data is limited.
Contrary to our findings, other researchers did not find that higher BMI influences vaccine serological response [21], [22], [23]. Our study and these previous studies did not evaluate vaccine efficacy. Therefore, and especially while pregnancy itself affects women’s BMI; the clinical significance of this association requires further investigation.
Our finding of a median transplacental SARS-CoV-2 IgG antibody transfer ratio of 1.4 is similar to the 1.3 reported by Poon et al. [24] following confirmed SARS-CoV-2 infection during the third trimester of pregnancy. However, Nir et al. demonstrated a median transfer ratio of 0.7 following vaccination with the second BNT162b2 COVID-19 vaccine in the third trimester [25]. Nonetheless, we described a transfer ratio of 2.6 in our previous publication of women who received the second BNT162b2 COVID-19 vaccine during the second trimester [14]. The ratio difference between vaccination in the second and third trimester may be explained by a reduction of SARS-CoV-2 specific antibody transfer in the third trimester due to altered SARS-CoV-2 antibody glycosylation profiles [26]. In accordance with our study, Dustin et al. reported that a higher transfer ratio was associated with increasing duration between onset of maternal infection and time of delivery [27]. These findings might suggest a benefit in COVID-19 vaccination earlier in pregnancy to enhance neonatal protection.
In conjunction with previous studies that examined the administration of the mRNA COVID-19 booster vaccination to non-pregnant individuals [11], [12], [13], our findings support vaccination with the booster dose to pregnant women, to restore maternal and neonatal protection during the pandemic. Higher titers in neonates may imply longer postnatal protection, yet this assumption must be further studied. As side effects may increase vaccine hesitancy, the suggestion of positive correlation between the presence of systemic side effects and vaccine efficacy, may improve vaccine acceptance rates.
All women in this study got their third vaccine dose in the third trimester; therefore assessment of maternal and neonatal antibody levels following booster vaccination earlier in pregnancy was beyond the reach of the current study. The exact timing and schedule of COVID-19 vaccines before and during pregnancy, in order to ensure sustained maternal protection throughout pregnancy as well as sufficient neonatal protection, needs further evaluation.
This study has several strengths. It is a prospective study, including 102 pregnant women and their neonates, emphasizing on the effects of booster vaccination in pregnant women and the resulting levels of antibodies in the umbilical cord. Furthermore, all women received the same vaccine, and none was previously diagnosed with COVID-19 infection.
The study also has several limitations. This is a single center study with an ethnically homogeneous population and all women received the BNT162b2 mRNA COVID-19 vaccine thus the results might not be applicable to other vaccines or settings. We presumed that women did not have a natural infection based on history alone, therefore some may have been asymptomatic or with a mild illness and without a confirmed diagnosis. The antibody test used in our laboratory also recognizes IgG to nucleocapsid protein, hence it is feasible that occult natural infection lead to an increased immune response. Another limitation of our study was that maternal SARS-CoV-2 IgG antibodies were not evaluated prior to the third vaccination. Therefore, it is possible the results may reflect higher antibody levels preceding the third dose, resulting in higher post-vaccination antibodies. However, all of the women received the third mRNA COVID-19 Pfizer vaccine at least five months after their second mRNA COVID-19 Pfizer vaccination so it may be assumed the baseline antibody levels were relatively low.
Conclusion
In this cohort study, receipt of the BNT162b2 mRNA COVID-19 third vaccine dose during the third trimester of pregnancy was associated with strong maternal and neonatal humoral responses as reflected by maternal and neonatal SARS-CoV-2 IgG antibody levels measured at birth. These findings support the administration of the mRNA COVID-19 booster dose to pregnant individuals to restore maternal and neonatal protection during the pandemic.
CRediT authorship contribution statement
Nir Kugelman: Conceptualization, Data curation, Project administration, Investigation, Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Chen Nahshon: Project administration, Data curation, Writing – original draft, Writing – review & editing. Pninit Shaked-Mishan: Methodology, Project administration, Resources, Writing – review & editing. Nadav Cohen: Data curation, Investigation, Project administration. Maayan Lahav Sher: Data curation, Investigation, Project administration. Hanin Barsha: Data curation, Investigation, Project administration. Eiman Shalabna: Data curation, Investigation, Project administration. Avi Zolotarevsky: Data curation, Investigation, Project administration. Ofer Lavie: Formal analysis, Supervision, Methodology, Writing – review & editing. Reuven Kedar: Data curation, Investigation, Project administration. Shlomit Riskin-Mashiah: Conceptualization, Data curation, Project administration, Investigation, Methodology, Formal analysis, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We wish to thank Ms. Nili Stein from the Department of Community Medicine and Epidemiology, the Lady Davis Carmel Medical Center, for her aid in the calculations and analysis of the statistical section of the study.
Funding source
No external funding for this manuscript.
Financial disclosure
The authors have no financial relationships relevant to this article to disclose.
References
- 1.Pilishvili T., Gierke R., Fleming-Dutra K.E., Farrar J.L., Mohr N.M., Talan D.A., et al. Effectiveness of mRNA Covid-19 Vaccine among U.S. Health Care Personnel. N Engl J Med. 2021;385(25):e90. doi: 10.1056/NEJMoa2106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dagan N., Barda N., Biron-Shental T., Makov-Assif M., Key C., Kohane I.S., et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med. 2021;27(10):1693–1695. doi: 10.1038/s41591-021-01490-8. [DOI] [PubMed] [Google Scholar]
- 3.Goldshtein I., Nevo D., Steinberg D.M., Rotem R.S., Gorfine M., Chodick G., et al. Association Between BNT162b2 Vaccination and Incidence of SARS-CoV-2 Infection in Pregnant Women. JAMA. 2021;326(8):728. doi: 10.1001/jama.2021.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barda N., Dagan N., Cohen C., Hernán M.A., Lipsitch M., Kohane I.S., et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398(10316):2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.García-Botella A., García-Lledó A., Gómez-Pavón J., González del Castillo J., Hernández-Sampelayo T., Martín-Delgado M.C., et al. Booster or additional vaccination doses in patients vaccinated against COVID-19. Rev Esp Quimioter. 2022;35(2):105–114. doi: 10.37201/req/149.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.FDA Authorizes Booster Dose of Pfizer-BioNTech COVID-19 Vaccine for Certain Populations | FDA n.d. https://www.fda.gov/news-events/press-announcements/fda-authorizes-booster-dose-pfizer-biontech-covid-19-vaccine-certain-populations (accessed November 23, 2021).
- 8.Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Kalkstein N., et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray K.J., Bordt E.A., Atyeo C., Deriso E., Akinwunmi B., Young N., et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;225(3):303.e1–303.e17. doi: 10.1016/j.ajog.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beharier O., Plitman Mayo R., Raz T., Nahum Sacks K., Schreiber L., Suissa-Cohen Y., et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J Clin Invest. 2021;131 doi: 10.1172/JCI150319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi A., Koch M., Wu K., Chu L., Ma LingZhi, Hill A., et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat Med. 2021;27(11):2025–2031. doi: 10.1038/s41591-021-01527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flaxman A., Marchevsky N.G., Jenkin D., Aboagye J., Aley P.K., Angus B., et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002) Lancet. 2021;398:981–990. doi: 10.1016/S0140-6736(21)01699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Earle K.A., Ambrosino D.M., Fiore-Gartland A., Goldblatt D., Gilbert P.B., Siber G.R., et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39(32):4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kugelman N., Nahshon C., Shaked-Mishan P., Cohen N., Sher M.L., Gruber M., et al. Maternal and Neonatal SARS-CoV-2 Immunoglobulin G Antibody Levels at Delivery After Receipt of the BNT162b2 Messenger RNA COVID-19 Vaccine During the Second Trimester of Pregnancy. JAMA Pediatr. 2022;176(3):290. doi: 10.1001/jamapediatrics.2021.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rottenstreich A., Zarbiv G., Oiknine-Djian E., Zigron R., Wolf D.G., Porat S. Efficient Maternofetal Transplacental Transfer of Anti- Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Spike Antibodies After Antenatal SARS-CoV-2 BNT162b2 Messenger RNA Vaccination. Clin Infect Dis. 2021;73:1909–1912. doi: 10.1093/cid/ciab266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mithal L.B., Otero S., Shanes E.D., Goldstein J.A., Miller E.S. Cord blood antibodies following maternal coronavirus disease 2019 vaccination during pregnancy. Am J Obstet Gynecol. 2021;225:192–194. doi: 10.1016/j.ajog.2021.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zdanowski W., Waśniewski T. Evaluation of SARS-CoV-2 Spike Protein Antibody Titers in Cord Blood after COVID-19 Vaccination during Pregnancy in Polish Healthcare Workers: Preliminary Results. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9060675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y.J., Murphy E.A., Singh S., Sukhu A.C., Wolfe I., Adurty S., et al. (COVID-19) Vaccination, History of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection, and a Vaccine Booster Dose With Maternal and Umbilical Cord Antibody Levels at Delivery. Obstet Gynecol. 2022;139(3):373–380. doi: 10.1097/AOG.0000000000004693. [DOI] [PubMed] [Google Scholar]
- 19.Naaber P., Tserel L., Kangro K., Sepp E., Jürjenson V., Adamson A., et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur. 2021;10 doi: 10.1016/j.lanepe.2021.100208. 100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goel R.R., Apostolidis S.A., Painter M.M., Mathew D., Pattekar A., Kuthuru O., et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S.W., Moon J.-Y., Lee S.-K., Lee H., Moon S., Chung S.J., et al. Anti-SARS-CoV-2 Spike Protein RBD Antibody Levels After Receiving a Second Dose of ChAdOx1 nCov-19 (AZD1222) Vaccine in Healthcare Workers: Lack of Association With Age, Sex, Obesity, and Adverse Reactions. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.779212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozgocer T., Dagli Ş.N., Ceylan M.R., Disli F., Ucar C., Yildiz S. Analysis of long-term antibody response in COVID-19 patients by symptoms grade, gender, age, BMI, and medication. J Med Virol. 2022;94:1412–1418. doi: 10.1002/jmv.27452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pellini R., Venuti A., Pimpinelli F., Abril E., Blandino G., Campo F., et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. EClinicalMedicine. 2021;36 doi: 10.1016/j.eclinm.2021.100928. 100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poon L.C., Leung B.W., Ma T., Yu F.N.Y., Kong C.W., Lo T.K., et al. Relationship between viral load, infection-to-delivery interval and mother-to-child transfer of anti-SARS-CoV-2 antibodies. Ultrasound Obstet Gynecol. 2021;57:974–978. doi: 10.1002/uog.23639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nir O., Schwartz A., Toussia-Cohen S., Leibovitch L., Strauss T., Asraf K., et al. Maternal-neonatal transfer of SARS-CoV-2 immunoglobulin G antibodies among parturient women treated with BNT162b2 messenger RNA vaccine during pregnancy. Am J Obstet Gynecol MFM. 2022;4 doi: 10.1016/j.ajogmf.2021.100492. 100492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atyeo C., Pullen K.M., Bordt E.A., Fischinger S., Burke J., Michell A., et al. Compromised SARS-CoV-2-specific placental antibody transfer. Cell. 2021;184(3):628–642.e10. doi: 10.1016/j.cell.2020.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flannery D.D., Gouma S., Dhudasia M.B., Mukhopadhyay S., Pfeifer M.R., Woodford E.C., et al. Assessment of Maternal and Neonatal Cord Blood SARS-CoV-2 Antibodies and Placental Transfer Ratios. JAMA Pediatr. 2021;175(6):594. doi: 10.1001/jamapediatrics.2021.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]