Abstract
Introduction
Since the pandemic of COVID-19 started from December 2019, remarkable numbers of infections and deaths associated with COVID-19 have been recorded worldwide. End-stage kidney disease patients on dialysis are particularly at high risk of infections due to impairments in the innate and adaptive immune systems. Vaccination on dialysis patients (DP) still remains challenging because of the variable response and a low seroconversion rate compared with healthy participants (HP). Therefore, it is urgently necessary to establish a different vaccination strategy for DP, in terms of the dose and administration time.
Methods
Here, we report an observational prospective cohort study in which the immunogenic efficacies of SARS-CoV-2 vaccine BNT162b2 on DP and HP were evaluated by absolute quantification of IgG levels in the blood.
Results
DP showed a delayed seroconversion after two vaccine doses, with a low absolute IgG levels compared to HP. While HP reached complete seroconversion within 10 days from the administration of a second dose, only 76% of DP were seropositive. After the booster dose, DP had a strongly improved seroconversion rate as well as antibody levels, reaching 97% seropositivity and 50 times enhancement on antibody levels.
Discussion/Conclusion
These results prompt to suggest an additional vaccine dose in DP, reducing the interval of time from the second dose. Since limited data are available on immune response in DP overtime after three vaccine doses currently, our study is among the first reports demonstrating the improved seropositivity and IgG levels in DP after the booster vaccine dose.
Keywords: Severe acute respiratory syndrome coronavirus 2, Vaccination, Booster, Dialysis
Introduction
Chronic kidney disease (CKD) constitutes a serious global health problem. According to the Global Burden of Disease study, incidence of CKD reached 9.1% in 2017, resulting in 697.5 million cases worldwide [1]. A marked increase in the mortality rate associated with CKD was also noted, which accounted for 4.6% of global deaths and thereby placed CKD as 12th leading cause of death globally in 2017 [2].
CKD encompasses persistent impairments in renal structures and functions [3]. Patients with CKD may present with a reduced glomerular filtration rate lower than 60 mL/min/1.73 m2 and abnormalities in kidney morphology or urinary/blood composition with variable severity [4, 5]. Progressive declines in renal function eventually result in end-stage kidney disease (ESKD), with 2.5 million patients who require renal replacement therapies, such as kidney transplantation or dialysis, and this number is expected to double by 2030 with huge sanitary costs [6].
Renal failure and dialysis treatment are associated with disorders of the innate and adaptive immune system, contributing to the increase of infection rate [7]. Indeed, infectious disease is the second most common cause of death after cardiovascular disease in patients with CKD [8].
During the global pandemic of COVID-19 due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that has spread across the world from December 2019, a very high mortality rate was associated with dialysis patients (DP) in Europe [9]. Italy was strongly involved in the COVID-19 pandemic, with a dramatic number of infections and deaths. Specifically, a recent report by the Italian National Institute of Health has shown that CKD was among the most frequent comorbidities in COVID-19 death cases, and 2% of dead patients were on dialysis treatment [10]. In the last months, the World Health Organization has approved different SARS-CoV-2 vaccines, classified in three categories on the bases of immunization strategy: adenovirus-vectored vaccine (AZD1222 and Ad26.COV2.S), lipid nanoparticle encapsulating nucleoside-modified messenger RNA vaccine (BNT162b2 and mRNA-1273), and inactivated virus vaccine (BBIBP-CorV and CoronaVac) [11]. The development of several specific vaccines has guaranteed a way to overcome the COVID-19 pandemic, but the impaired immune system determines a low response in DP.
Despite many improvements in defining general guidelines and adapting the dosage schedule, vaccination in CKD patients remains a very tricky chapter of sanitary management, due to the variable response and a low seroconversion rate. The specific case of hepatitis B vaccination is only one of the complex examples of several approaches attempted to obtain active immunization in the last years, and it is still a debate topic for infection prevention in CKD patients [12]. Since it is very useful to investigate the antibody response after a new vaccination, we designed a prospective cohort study to explore the immunogenic efficacy of SARS-CoV-2 vaccines in DP, through the absolute quantification of IgG levels in the blood.
Materials and Methods
Study Population
We planned an observational prospective cohort study comparing two groups: ESKD patients on dialysis treatment and healthy participants (HP) volunteers. All the participants enrolled in the study needed to be more than 18 years old, they had to sign a written informed consent, and they had been vaccinated with mRNA vaccine BNT162b2 (Comirnaty, Pfizer). Only patients that had been on dialysis for at least 3 months were included in the study. Individuals developing a SARS-CoV-2 infection during the study, and tested by molecular analysis of nasopharyngeal swabs, were excluded.
A total of 155 DP were enrolled for the study, while 77 HP volunteers belonged to the healthy population. The study protocol was reviewed and approved by the National Ethics Committee Istituto Nazionale Malattie Infettive Lazzaro Spallanzani (Authorization No. 6 of Trials register 2022).
Blood Samples
Seven hemodialysis centers participating in the study provided blood samples and clinical information for all the patients (Dialysis Srl − Avellino, Capodicasa Srl − Avellino, Irpinia Dialisi Srl − Pratola Serra [AV], Padre Pio Srl − Benevento, Neoren Srl − Montesarchio [BN], Sanniomedica Srl − Telese Terme [BN], and Alta Irpinia Srl − Calitri [AV]). These centers are located in the same geographic area (Irpinia and Sannio, the Campania region in South Italy) that have similar environmental and climatic conditions. Blood samples were collected before vaccination and at specific time points, such as within 0–15, 16–21 (second vaccine dose), 22–32, 33–45, 46–60, 61–120, and >120 days post-vaccination (DPV). Only for DP, blood samples were collected also within 30–60 days after a booster vaccine dose (days post-booster, DPB). Samples were centrifuged at 3,000 rpm for 10 min, and the serum was divided in 2 mL aliquots and stored at −80°C until testing.
Absolute Antibody Quantification
IgG levels have been evaluated by using the COVID-19 QuantiGEM SARS-CoV-2 IgG ELISA Kit CE-IVD, developed by Biogem, according to manufacturer instructions. Briefly, serum samples from HP and DP were tested after 1:250 dilution; for samples with optical density above the upper limit of quantitation, higher dilutions were employed (1:500 or 1:1,000). The COVID-19 QuantiGEM SARS-CoV-2 IgG ELISA Kit allows the absolute quantitation of anti-SARS-CoV-2 IgG by the means of a four-parameter logistic (4-PL) calibration curve. The antibody concentration is expressed as both arbitrary units per milliliter (AU/mL) and nanograms per milliliter (ng/mL), as calculated by interpolation with the standard curve. Assay results have been interpreted as per manufacturer instruction: a sample is negative if the antibody titer <0.120 AU/mL, doubtful with a value between 0.120 and 0.170 AU/mL, and positive with a value >0.170 AU/mL.
Statistical Analysis
The statistical analysis was performed using GraphPad Prism version 9.00 (GraphPad Software, La Jolla CA, USA). Four-PL curve with the logarithmic standard concentration was used to evaluate the IgG level. Results are expressed as median and range. Variables not normally distributed were analyzed with Kruskal-Wallis and Dunn's nonparametric tests. A p value <0.05 was considered significant. Spearman's rank-order correlation was applied to evaluate the relationship between the IgG level and characteristics of DP including gender, age, vintage of dialysis, and type of dialysis.
Results
Immune Response after Two Vaccine Doses
In total, 155 DP and 77 HP were analyzed for their antibody levels in response to COVID-19 vaccination with Comirnaty BNT162b2. The characteristics of studied population are summarized in Table 1. The median age of DP was 72 years (CI95: 69–75), 67% were male, and the median days of dialysis per week was 3. In the HP group, median age was 58 years (CI95: 56–60), and 51% were male. DP and HP groups have a significant difference in median ages, which is 72 (CI95: 69–75) versus 58 (CI95: 56–60) years, respectively (Table 1). This is due to the fact that people enrolled in the HP group are working-age healthcare professionals. For this study, we were not able to enroll healthy individuals with matched age compared with DP.
Table 1.
Characteristics of the study population
| DP | HP | |
|---|---|---|
| Individuals, N | 155 | 77 |
| Age, years, median (range) | 72 (69–75) | 58 (56–60) |
| Male, % | 67 | 51 |
| Day of dialysis per week, median | 3 | – |
| Dialysis vintage, median year (range) | 3 (2–6) | – |
| Dialysis vintage ≤4 years | 99 | – |
| Dialysis vintage >4 years | 56 | – |
| Type of dialysis | ||
| BIC-HD | 115 | – |
| Online HDF | 40 | – |
| Cause of kidney failure, N (%) | ||
| Primary and secondary glomerulopathies | 23 (14.8) | – |
| Diabetes and metabolic diseases | 43 (27.7) | – |
| Polycystic kidney and hereditary diseases | 21 (13.5) | – |
| Interstitial nephropathies | 5 (3.2) | – |
| Pyelonephritis | 4 (2.6) | – |
| Nephroangiosclerosis | 29 (18.7) | – |
| Unknown | 30 (19.4) | – |
BIC-HD, standard bicarbonate hemodialysis; HDF, hemodiafiltration.
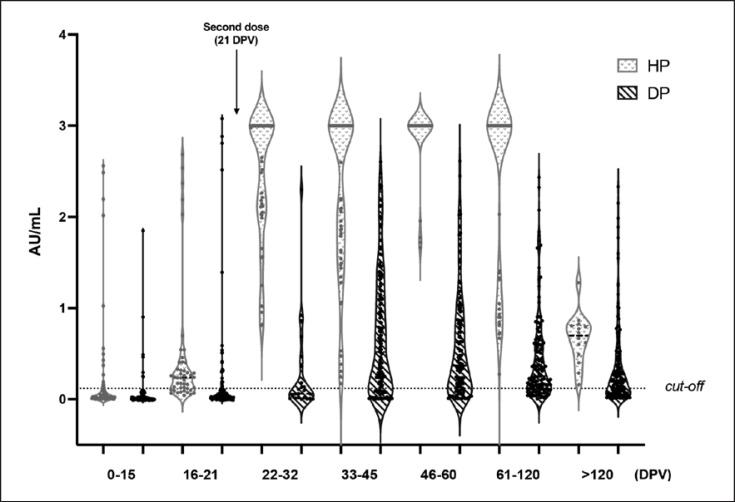
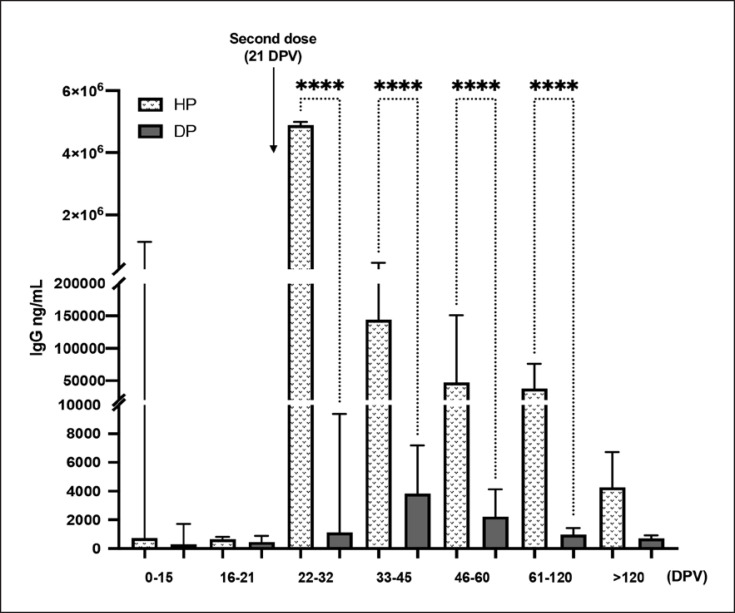
The distribution of the anti-SARS-CoV-2 antibody IgG in terms of AU/mL after vaccination at different time points is shown in Figure 1 as the violin plot, in both populations (HP and DP). The HP group showed a significant increase in IgG levels within 10 days from the administration of the second dose (22–32 DPV). The seroconversion rate (Table 2) reached 100% at this time point, and IgG persisted up to >120 DPV, although their levels declined overtime. Furthermore, quantification of the IgG level in terms of ng/mL, performed only on seroconverted subjects, reveals that the antibody level reaches 4,882,623 ng/mL (CI95: 1,177,973–5,000,000 ng/mL) at 22–32 DPV to 4,257 ng/mL (CI95: 1,318–6,703 ng/mL) at >120 DPV in HP (Fig. 2).
Fig. 1.
Violin plot showing the semiquantitative IgG levels measured in DP and HP at the indicated time points. Violin plot lines: median and interquartile ranges; Y-axis dotted line: 0.120 corresponding to the positivity cutoff. AU, arbitrary units.
Table 2.
Seroconversion rate in HP and DP in response to mRNA vaccine BNT162b2 at different DPV
| DPV |
|||||||
|---|---|---|---|---|---|---|---|
| 0–15 | 16–21 | 22–32 | 33–45 | 46–60 | 61–120 | >120 | |
| Seroconversion in HP, % | 25 | 74 | 100 | 100 | 100 | 100 | 100 |
| Seroconversion in DP, % | 9 | 18 | 39 | 75 | 76 | 74 | 63 |
Fig. 2.
Bar plot summarizing the absolute IgG Levels measured in DP and HP. Data are reported as median values (ng/mL) ± CI95 as obtained by interpolation with the standard curve. ****p < 0.0001.
In contrast, less than 40% of DP was seropositive at 22–32 DPV. The median IgG level was 1,116 ng/mL (CI95: 307.5–9,366 ng/mL), which is approximately 50-fold lower with respect to the values in the HP group at the same time point. Within 20 days after the second dose (33–45 DPV), the seroconversion rate in the DP group increased to 75%. The seropositivity remained almost unchanged during the follow-up period up to 61–120 DPV and decreased to 63% at >120 DPV time point. Maximum IgG levels in DP was 3,830 ng/mL at 33–45 DPV but remained very low overtime and continuously decreased until 714.6 ng/mL (CI95: 527.1–919.2 ng/mL) after more than 4 months from the first dose.
Immune Response after the Third Dose
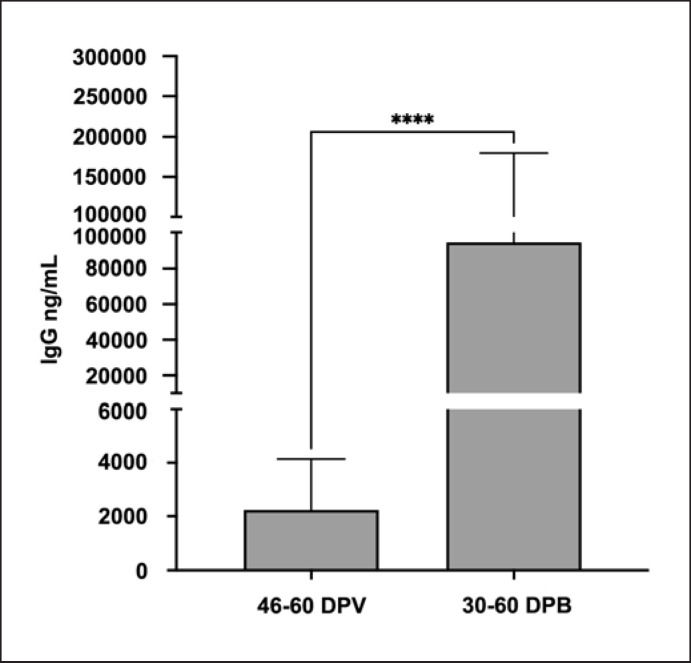
The booster vaccine dose induced a strong seroconversion in DP. In details, after 30–60 days from the third vaccination dose, 97% of patients showed an IgG level over the minimal threshold, with a median value of 94,350 ng/mL (CI95: 40,809–179,738 ng/mL). This result is comparable to the percentage of seroconversion in HP after two vaccine doses (100% HP vs. 97% DP), indicating that in DP, an additional vaccine dose is essential to have the almost complete seroconversion. Moreover, the antibody level in DP showed a 50-fold increase after the booster dose compared to the second dose, when the median value of IgG was lower, reaching 2,232 ng/mL (CI95: 1,160–4,139 ng/mL) (Fig. 3). Although DP was able to reach a satisfying percentage of seroconversion after three vaccine doses, the antibody level was still low, precisely 40 times lower than HP after two doses (4.88 mg HP 22–32 DPV vs. 0.94 mg DP 30–60 days post-booster).
Fig. 3.
Bar plot showing the IgG levels in DP after second and third doses of vaccine. Data are reported as median values (ng/mL) ± CI95. ****p < 0.0001.
Correlation Analysis between Patients' Characteristics and Antibody Titer
Spearman's correlation analyses have been performed to evaluate the influence of sex, age, dialysis vintage, and dialysis type on IgG levels in DP. The results are summarized in Table 3. Sex seems to show a weak negative correlation with antibody response to vaccination within the first 21 days (rho = −0.208, p < 0.05). Patients' age shows a low to moderately negative correlation (rho = −0.254, p < 0.01 at 33–45 DPV; rho = −0.277, p < 0.01 at >120 DPV) with anti-SARS-CoV-2 IgG level. Dialysis vintage is moderately associated with a lower antibody response at 22–32 DPV (rho = −0.433, p < 0.05) suggesting an impact of this parameter on the immune response rate, although this effect decreased over time. In contrast, no correlation between the IgG level and dialysis's type was observed.
Table 3.
Spearman correlation analyses for sex, age, dialysis vintage, and dialysis type on IgG levels in DP
| 0–15 (DPV) | 16–21 (DPV) | 22–32 (DPV) | 33–45 (DPV) | 46–60 (DPV) | 61–120 (DPV) | >120 (DPV) | 30–60 (DPB) | |
|---|---|---|---|---|---|---|---|---|
| Sex (male vs. female) | –0.200 | –0.208* | 0.239 | –0.096 | –0.065 | 0.004 | 0.062 | –0.057 |
| Age (≤60 vs. > 60) | 0.017 | –0.037 | –0.207 | –0.254** | –0.180 | –0.188* | –0.277** | –0.118 |
| Dialysis vintage (≤4 years vs. > 4 years) | 0.035 | 0.059 | –0.433* | 0.032 | 0.059 | –0.182* | –0.013 | 0.027 |
| Dialysis type (BIC-HD vs. HDF) | –0.151 | 0.086 | 0.058 | –0.035 | –0.078 | –0.055 | –0.073 | –0.006 |
Correlation coefficient (rho) at different time points are shown. BIC-HD, standard bicarbonate hemodialysis; HDF, hemodiafiltration; DPB, days postbooster. * p value <0.05. ** p value <0.01.
Discussion/Conclusion
In this study, IgG anti-SARS-CoV-2 was quantified to compare the efficacy of BNT162b2 vaccine in DP and HP populations. Our data clearly demonstrate that the humoral response in DP was delayed and weaker with respect to the one observed in HP at the same time points. These results agree with those recently obtained from other research groups [13, 14, 15, 16]. It is worth noting that the DP group reached only 76% of seropositivity after two doses, while the HP group showed 100% of seroconversion within 10 days from the administration of the second dose. Obtained results suggest only a slightly to moderately negative correlation between age and dialysis vintage in the DP group. These data suggest that the weak immune response to vaccination in DP, both in terms of seroconversion frequency and the absolute IgG level, is mainly due to disorders of the innate and adaptive immune systems, which are usually related to renal pathologies [7]. However, the difference in age between the two populations analyzed (72 vs. 58 years), which is per se a study limitation, could partially account for the difference in IgG levels observed [17].
The additional third dose induced an almost complete seroconversion in DP, reaching 97% of individuals, demonstrating that it is required to achieve a sufficient response in these patients. Similar results have already been shown in a recent paper by a research group in France [18], where the booster dose has been administered to fragile people earlier than in other European countries, like Italy. Only in October 2021, the Italian Ministry of Health has recommended the administration of booster dose after 6 months from the conclusion of primary vaccine cycle, for fragile people of every age, included DP [19]. Starting from January 2022, the interval of time has been progressively reduced to 4 months [20]. Furthermore, very recently many governments worldwide are deciding to anticipate the booster dose also for healthy individuals, due to the spread of the Omicron variant [21, 22] that is more contagious than the previous ones. To our knowledge, this is the first study showing data that have been collected in Italy.
It is also notable that percentage of seropositive in the HP group was stable overtime, while in the DP group, there was a gradual and remarkable reduction in IgG levels, with a seroconversion rate downward from 75% at 33–45 DPV to 63% after more than 4 months from the first dose. These data suggest that, once again, vaccination in DP needs a different schedule, in terms of the dose and administration time, in comparison with healthy population.
Although cell-mediated immunity and correlations with specific clinical parameters were not evaluated, the observed trend of humoral immune response of DP denotes that two doses are not sufficient to guarantee them the protection from severe COVID-19 disease. Thus this information could prompt to reduce the interval of time between the second and third doses for DP. In the history of virology and vaccination, it is well known that CKD patients are more affected by viral infections and do not show a suitable immune response to vaccination. In addition to the classic example of the hepatitis B vaccination, there are other examples of recommended vaccines for CKD patients that have been adapted in dose and time of administration, such as influenza, pneumococcal, and tetanus-diphtheria vaccines [23]. Thus, it will be useful also for SARS-CoV-2 vaccines to define the more suitable schedule for DP. No data on immune response of HP group after booster dose were collected for comparative analysis with the DP group, even if it is clear from our data that the antibody levels in DP after the booster dose are lower than HP after two doses, expecting that the HP group can reach again its high levels of antibodies after the third dose, as suggested by Munro et al. [24] who analyzed the trend of immune response in healthy people after the booster. To conclude, a third dose of SARS-CoV-2 vaccine substantially increased antibody levels in DP. This study confirms that an additional dose is essential to guarantee immune response in the majority of DP, reaching significant antibody levels and the development of potentially neutralizing antibodies. This information suggests that the booster dose must be administered in a shorter interval of time from the conclusion of the primary vaccine cycle because two doses are not sufficient to give an immune protection in all DP. Another possibility could be to adopt a personalized schedule of vaccination, for DP that do not respond to the first two doses, on the basis of a specific follow-up overtime.
Statement of Ethics
This study protocol was conducted in accordance with the Declaration of Helsinki and approved by the National Ethics Committee Istituto Nazionale Malattie Infettive Lazzaro Spallanzani (Authorization No. 6 of Trials register 2022). Written informed consent was obtained from all the participants to the study.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
Biogem, Biology and Molecular Genetics Institute, was the promoter of the study.
Author Contributions
Alessandra Fucci, Simona Giacobbe, and Egildo Luca D'Andrea performed the experiments and statistical analyses. Marianna Scrima, Luigi Amedeo Chiuchiolo, Ermanno Salvatore, Roberta Renzulli, Ludovico La Peccerella, Giuseppe Marra, Marco Liuzzi, Domenico Santoro, Enrico Zulli, Romolo Gentile, and Gennaro Clemente enrolled the subjects in the study and collected the blood samples. Ilaria Guerriero and Yoko Suzumoto wrote the manuscript. Alessandra Fucci, Simona Giacobbe, Maria Luisa Nolli, Anna Iervolino, and Giovambattista Capasso revised the manuscript. Giovambattista Capasso designed and supervised the work. All the authors read and agreed with the final version of manuscript.
Data Availability Statement
The data that support the findings of this study are not publicly available due to their containing information that could compromise the privacy of research participants but available from the corresponding authors (I.G., ilaria.guerriero@biogem.it and Y.S., yoko.suzumoto@biogem.it) upon reasonable request.
Acknowledgment
We are grateful to the patients and volunteers for their contributions to this work.
References
- 1.Collaboration GBDCKD Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395((10225)):709–33. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cockwell P, Fisher LA. The global burden of chronic kidney disease. Lancet. 2020;395((10225)):662–4. doi: 10.1016/S0140-6736(19)32977-0. [DOI] [PubMed] [Google Scholar]
- 3.Romagnani P, Remuzzi G, Glassock R, Levin A, Jager KJ, Tonelli M, et al. Chronic kidney disease. Nat Rev Dis Primers. 2017;3:17088. doi: 10.1038/nrdp.2017.88. [DOI] [PubMed] [Google Scholar]
- 4.Winearls CG, Glassock RJ. Dissecting and refining the staging of chronic kidney disease. Kidney Int. 2009;75((10)):1009–14. doi: 10.1038/ki.2009.49. [DOI] [PubMed] [Google Scholar]
- 5.Versino E, Piccoli GB. Chronic kidney disease: the complex history of the organization of long-term care and bioethics. Why now, more than ever, action is needed. Int J Environ Res Public Health. 2019;16((5)) doi: 10.3390/ijerph16050785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385((9981)):1975–82. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 7.Kato S, Chmielewski M, Honda H, Pecoits-Filho R, Matsuo S, Yuzawa Y, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3((5)):1526–33. doi: 10.2215/CJN.00950208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy S, Chitturi C, Yee J. Vaccination in chronic kidney disease. Adv Chronic Kidney Dis. 2019;26((1)):72–8. doi: 10.1053/j.ackd.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Jager KJ, Kramer A, Chesnaye NC, Couchoud C, Sanchez-Alvarez JE, Garneata L, et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98((6)):1540–8. doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. https://www.epicentro.iss.it/en/coronavirus/bollettino/Report-COVID-2019_21_july_2021.pdf .
- 11. https://covid19.trackvaccines.org .
- 12.Fabrizi F, Cerutti R, Dixit V, Ridruejo E. Hepatitis B virus vaccine and chronic kidney disease. The advances. Nefrologia. 2021 Mar-Apr;41((2)):115–22. doi: 10.1016/j.nefro.2020.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Yanay NB, Freiman S, Shapira M, Wishahi S, Hamze M, Elhaj M, et al. Experience with SARS-CoV-2 BNT162b2 mRNA vaccine in dialysis patients. Kidney Int. 2021;99((6)):1496–8. doi: 10.1016/j.kint.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Billany RE, Selvaskandan H, Adenwalla SF, Hull KL, March DS, Burton JO, et al. Seroprevalence of antibody to S1 spike protein following vaccination against COVID-19 in patients receiving hemodialysis: a call to arms. Kidney Int. 2021;99((6)):1492–4. doi: 10.1016/j.kint.2021.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Attias P, Sakhi H, Rieu P, Soorkia A, Assayag D, Bouhroum S, et al. Antibody response to the BNT162b2 vaccine in maintenance hemodialysis patients. Kidney Int. 2021;99((6)):1490–2. doi: 10.1016/j.kint.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torreggiani M, Blanchi S, Fois A, Fessi H, Piccoli GB. Neutralizing SARS-CoV-2 antibody response in dialysis patients after the first dose of the BNT162b2 mRNA COVID-19 vaccine: the war is far from being won. Kidney Int. 2021;99((6)):1494–6. doi: 10.1016/j.kint.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller L, Andree M, Moskorz W, Drexler I, Walotka L, Grothmann R, et al. Age-dependent immune response to the biontech/Pfizer BNT162b2 coronavirus disease 2019 vaccination. Clin Infect Dis. 2021;73((11)):2065–72. doi: 10.1093/cid/ciab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bensouna I, Caudwell V, Kubab S, Acquaviva S, Pardon A, Vittoz N, et al. SARS-CoV-2 antibody response after a third dose of the BNT162b2 vaccine in patients receiving maintenance hemodialysis or peritoneal dialysis. Am J Kidney Dis. 2022;79((2)):185–92.e1. doi: 10.1053/j.ajkd.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. https://www.salute.gov.it/portale/nuovocoronavirus/homeNuovoCoronavirus.jsp?lingua=english .
- 20. https://www.salute.gov.it/portale/nuovocoronavirus/dettaglioFaqNuovoCoronavirus.jsp?lingua=italiano&id=255 .
- 21.Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398((10317)):2126–8. doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burki TK. Omicron variant and booster COVID-19 vaccines. Lancet Respir Med. 2021;10((2)):e17. doi: 10.1016/S2213-2600(21)00559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haddiya I. Current knowledge of vaccinations in chronic kidney disease patients. Int J Nephrol Renovasc Dis. 2020;13:179–85. doi: 10.2147/IJNRD.S231142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munro APS, Janani L, Cornelius V, Aley PK, Babbage G, Baxter D, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398((10318)):2258–76. doi: 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not publicly available due to their containing information that could compromise the privacy of research participants but available from the corresponding authors (I.G., ilaria.guerriero@biogem.it and Y.S., yoko.suzumoto@biogem.it) upon reasonable request.