Abstract
The relationship between ponto-geniculo-occipital (PGO) waves and motor activity during waking and non-rapid eye movement (non-REM) sleep stages was studied in cats treated with the serotonin synthesis inhibitor p-chlorophenylalanine (PCPA). PGO waves appeared in waking after daily treatment with PCPA. The magnitude of the acoustic startle elicited in the absence of prior PGO waves was increased (by a mean of 555%) by the PCPA treatment as compared to that of the pre-drug level. When startle-eliciting stimuli were presented shortly after the occurrence of the PGO wave, the response amplitude was further enhanced as compared to that of the baseline startle. The effect was maximal 50 ms following the peak of the PGO wave (average 192% of the baseline level), with return to the baseline startle level within 200 ms. A similar effect could also be seen with waking eye-movement potentials (EMPs) in drug-naive animals. Over half of the spontaneous PGO waves were found to be preceded or followed by discrete head-body movements. After PCPA, the amplitude of auditory-evoked LGN PGO waves increased during quiet waking (QW) while those in non-REM and REM sleep states did not change. It was concluded that serotonergic systems produce a tonic suppression of startle response and PGO amplitude in waking. PGO spikes in waking are associated with a phasic facilitation of the sensorimotor mechanisms involved in startle.
Keywords: Serotonin, Pons, Geniculate, Startle, Sleep, Ponto-geniculo-occipital wave
INTRODUCTION
PGO waves are high amplitude potentials that originate in the pons and propagate to lateral geniculate nucleus (LGN), cochlear nucleus, auditory and occipital cortices, and other brain regions during the transition from non-rapid-eye-movement (non-REM) sleep to REM sleep and throughout the REM sleep period18,19. The physiological function of these waves is obscure. A close association of PGO waves with phasic muscle pauses and motoneuron hyperpolarization during the transition from non-REM to REM sleep11,25, as well as reduced monosynaptic spinal reflexes17 and enhancement of motoneuron inhibition15 during REM sleep, has been demonstrated. PGO waves, on the other hand, are also associated with general arousal. PGO waves can be elicited by external, notably novel, stimuli, e.g., auditory, somatic, and olfactory, during waking and non-REM sleep16,25. It has thus been hypothesized by Morrison and his colleague16 that PGO waves may represent an alerting response to internal or external events.
If PGO waves are associated with motoneuron inhibition in waking as they are in REM sleep, or with an attenuation of sensory inputs, it would be expected that reflexes would be suppressed when elicited during the occurrence of the PGO waves. If, on the other hand, PGO waves are associated with general arousal of the nervous system, sensorimotor processes may be facilitated. In order to determine the effect of PGO waves on sensorimotor responses during waking and non-REM sleep, animals were treated daily with PCPA to deplete the brain serotonin level and thus ‘release’ spontaneous PGO waves into waking and non-REM sleep7,8,13. We examined the effect of spontaneous PGO waves on the motor response to startle-eliciting auditory stimuli. We also examined the relationship of spontaneous PGO waves with head-body movements.
In a previous study25 we determined the change in amplitude of auditory-evoked PGO wave as a function of sleep-waking state. The evoked PGO amplitude increased monotonically from active waking (AW) to quiet waking (QW) and reached its maximum during the transition from non-REM to REM sleep. We hypothesized then that the increase of the PGO amplitude is due to the reduction in neuronal activity in the raphe nucleus and locus coeruleus in non-REM and REM sleep periods. If this is the case, serotonin depletion would be expected to change the pattern of state-dependent PGO response to auditory stimulation.
MATERIALS AND METHODS
Seven adult cats were used. They were chronically implanted with conventional electrodes for electroencephalography (EEG), electrooculogram (EOG) and neck electromyogram (EMG) for sleep recording as described previously24,25. PGO waves were measured with bipolar electrodes in LGN. Four cats received no drug. Three received daily treatment of PCPA methyl ester HCL (125 mg/kg/day, i.p., Sigma) for 6 days. Startle tests were taken for these 3 cats before and after PCPA treatments. Pre-drug baseline startle amplitude was taken 1 week before the PCPA treatment, and consisted of 12 trials with the startle-eliciting stimulus alone presented 1–2 rain apart during QW and non-REM sleep. Post-drug tests were done between day 4 and day 6, 1 h after PCPA injection, when spontaneous PGO waves occurred during waking and non-REM sleep.
The test chamber and the generation of auditory stimuli were as previously described25. Briefly, startle-eliciting stimuli, clicks or noise pulses of 115 dB (re 20 μtN/cm2, SPL/A scale), were presented to the animal at 0, 50, 100, 200, or 400 ms after the negative peak of a spontaneous PGO wave, or randomly free of association with the PGO wave (more than 600 ms from any PGO wave). PGO waves were detected by a window discriminator (SA Instrumentation, CA) set for the minimum amplitude of the initial negative deflection. Stimuli were presented once every 1–2 min, following a Latin-square design. A total of 60 trials were presented, 12 trials in each interval condition. Startle responses were measured with a head-mounted accelerometer (Model 8628A50, Kistler Instrument Co., Amherst, NY). The signal output from the accelerometer was amplified, full-wave rectified, averaged over trials, and integrated over 200 ms after the stimulus onset using an IBM PC/XT equipped with CED 1401 signal averaging package (Cambridge Electronic Design Ltd., Cambridge, U.K.). The integrated voltage was taken as the startle amplitude. Startle tests were done during QW and non-REM sleep only. The number of trials presented during QW and non-REM sleep was kept the same across conditions within each animal.
To study the relationship of PGO waves with the startle response in drug-naive cats, the 4 cats that had spontaneous EMPs during waking and non-REM sleep were utilized. Procedures for startle tests were the same as those for the PCPA-treated cats.
The relationship between PGO waves and spontaneous head-body movements after PCPA treatment was also studied in separate trials. Neck EMG level and head-body movements as recorded on the head-mounted accelerometer were averaged with respect to spontaneous PGO waves during QW and non-REM sleep periods. The temporal relationships of PGO waves with discrete head-body movements were measured trial by trial.
State-dependent relationships of the evoked PGO response were studied before and after PCPA treatment. Animals were presented with auditory stimuli (115 db) every 1–2 min during natural waking and sleep states. Evoked PGO responses were recorded and averaged off-line with equal numbers of trials in active waking (AW), QW, non-REM and REM sleep episodes.
For the analysis of the data, the average startle responses or the evoked PGO amplitudes for all conditions were summed for each animal and the score from each condition was then expressed as a proportion (per cent) of that total. This normalizing procedure removed individual differences in overall reactivity so that each animal’s performance was weighted equally in the analysis. Absolute startle amplitudes were used for comparing pre- and post-drug startle responses.
RESULTS
Daily PCPA treatment released waking PGO waves of comparable amplitude to those occurring during transition and REM sleep by day 3–day 4. Non-REM and REM sleep episodes began to reappear after day 4 following an initial insomnia. When a startle-eliciting stimulus was delivered following a spontaneous PGO wave, the amplitude of the startle response was enhanced. As shown in Fig. 1, this effect was maximal at 50 ms (average of 192% of the control level) after the peak of the PGO spike. Startle amplitude returned to the baseline levels when elicited 200 ms or more after the peak of the PGO wave. Analysis of variance with repeated measures for the 3 PCPA-treated animals revealed that there was a significant PGO-stimulus interval effect, F5,10 = 4.04, P < 0.03. Post-hoc analysis revealed that the response amplitude at a 50-ms interval was significantly larger than those of the control trials, trials with 200-ms interval, or trials with 400-ms interval (all P < 0.05, Newman–Keuls). There was no difference among the control, the 100-ms, the 200-ms, and the 400-ms trials.
Fig. 1.
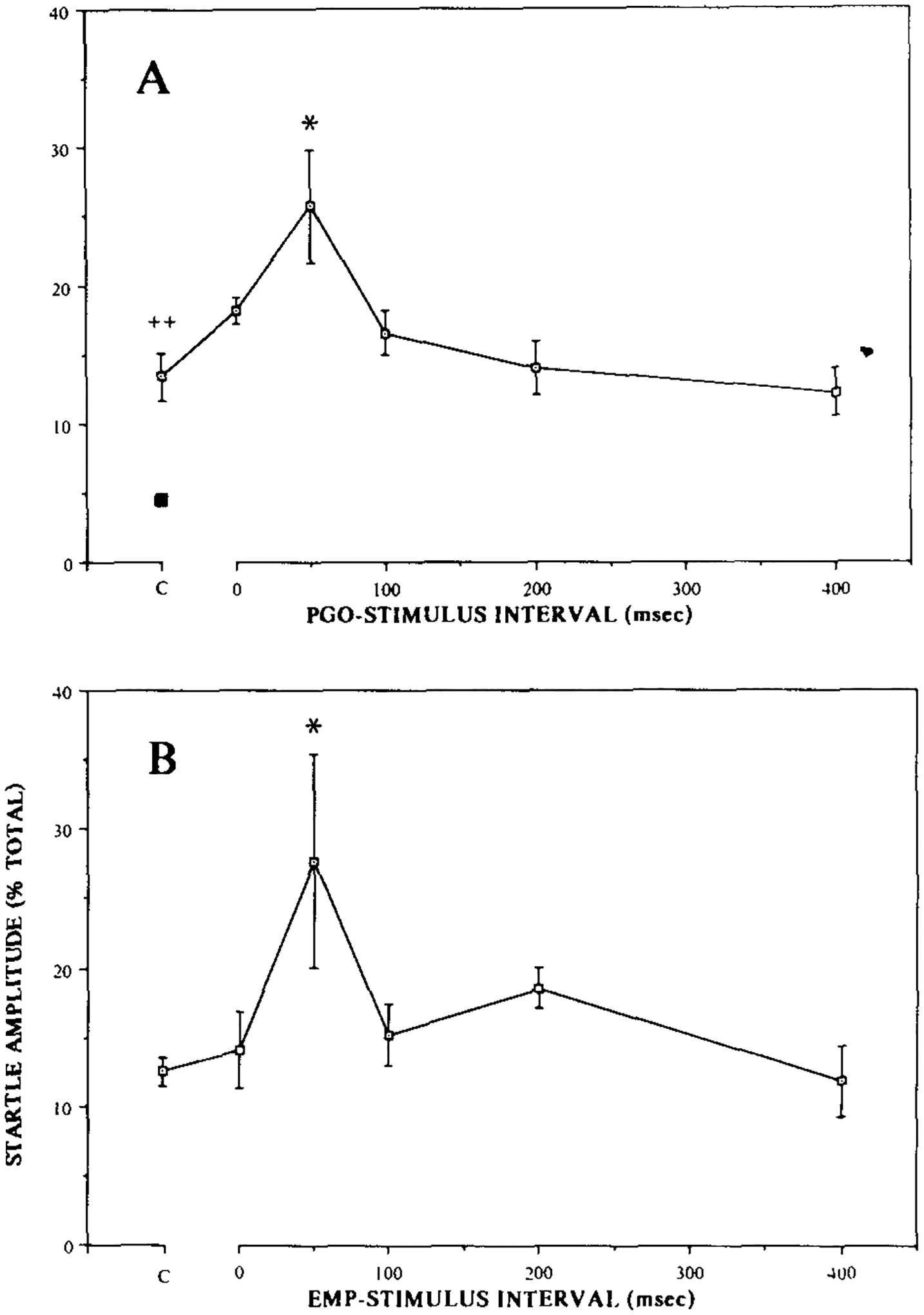
A: spontaneously occurring PGO waves facilitated the subsequently elicited startle response. The facilitation was maximal at 50 ms following the peak of the PGO wave (* P < 0.05, N–K, compared to the control level). The baseline startle amplitude, with no immediate preceding PGO wave, was also increased (555%) by PCPA treatment as compared to the pre-drug level (■) (++ P < 0.004, t-test). Note that the pre-drug baseline startle level is shown here as a percentage of the post-drug level. B: eye-movement potentials also tended to facilitate startle response, and the temporal relationship of facilitation was similar to that produced by PGO waves (* P < 0.05, Bonferroni t-test).
The baseline startle amplitude was also increased significantly by PCPA treatment as compared to that before the drug (Fig. 1). The startle amplitude of the 3 PCPA-treated cats increased by 319%, 712%, and 1252% (average 555%) of the pre-drug startle level (t2 = 15.1, P < 0.004).
Four drug-naive animals had spontaneous EMPs during waking and non-REM sleep. These EMPs were similar in waveform, but smaller in amplitude, to the PGO waves that occur during REM sleep or after PCPA treatments. These EMPs also tended to enhance acoustic startle response, as shown in Fig. 1B, and the time-course of effect was similar to that for the PGO waves, maximal at 50 ms following the peak (P < 0.05, Bonferroni t-test). The effect was, however, more variable both within and between animals than that produced by the PGO waves in the PCPA-treated animals.
Spontaneous PGO waves in the PCPA-treated animals were often associated with minute head-body movements resembling orienting responses. More than half of the time the individual PGO waves were preceded or followed by discrete head-body movements within 600 ms of the PGO wave in the 3 PCPA-treated animals examined. Among trials with PGO associated movements, head-body movements preceded the onset of PGO waves 62% of the time and coincided with or followed the PGO waves 38% of the time. The interval between the head-body movement and the PGO wave onset, however, varied from trial to trial and across states. Figure 2 shows the averaged PGO wave and the accelerometer output of head-body movement of one of the PCPA-treated cats during quiet waking and non-REM sleep. Of those trials in which the movement onset preceded the PGO wave, the average lead time was 140.0, 147.9, and 152.8 ms for the 3 animals. The lead time of the head-body movement over the PGO spike decreased during non-REM sleep to 75.6 and 97.0 ms, respectively for the latter 2 animals which were observed in sleep.
Fig. 2.
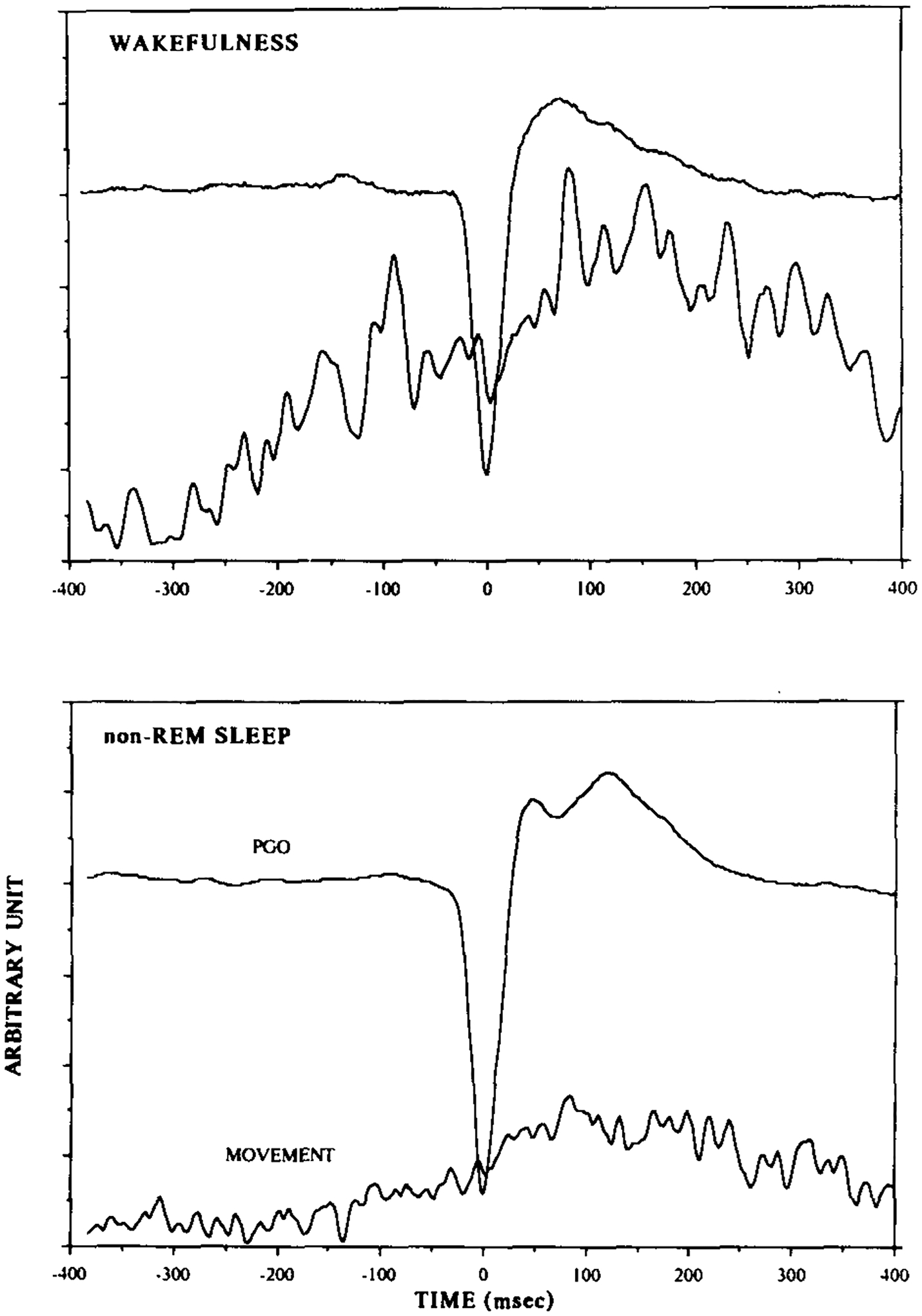
Spontaneously occurring PGO waves were often accompanied by discrete head-body movements resembling orienting reflexes and measurable by the accelerometer. This is the average of the PGO waves and the accelerometer output of head-body movements of a PCPA-treated cat over 300 randomly selected trials during QW and non-REM sleep states. Auditory stimuli were not presented in this experiment. The magnitude of head-body movements peaked right after the PGO wave, and the lead time of head-body movements over the PGO wave tended to be greater in waking than in non-REM sleep.
The state-dependent amplitude function of auditory evoked PGO spikes changed after PCPA treatments. As shown in Fig. 3, the PGO spike amplitude before PCPA treatment increased from AW to QW and to non-REM, and peaked during the transition from non-REM sleep to REM sleep. Following PCPA, the amplitude of the evoked PGO spike did not change relative to baseline in AW, non-REM or REM sleep. The evoked PGO response during QW, however, increased 63% over the pre-drug level. The repeated measures ANOVA of drug treatment by state revealed a significant main effect of state (F3,6 = 9.7, P < 0.01), and a significant interaction between drug treatment and state (F3,6 = 5.9, P < 0.04). Tests for simple main effects further showed that the PGO spike amplitude was enhanced by PCPA only in QW (Fl,6 = 31, P < 0.002).
Fig. 3.
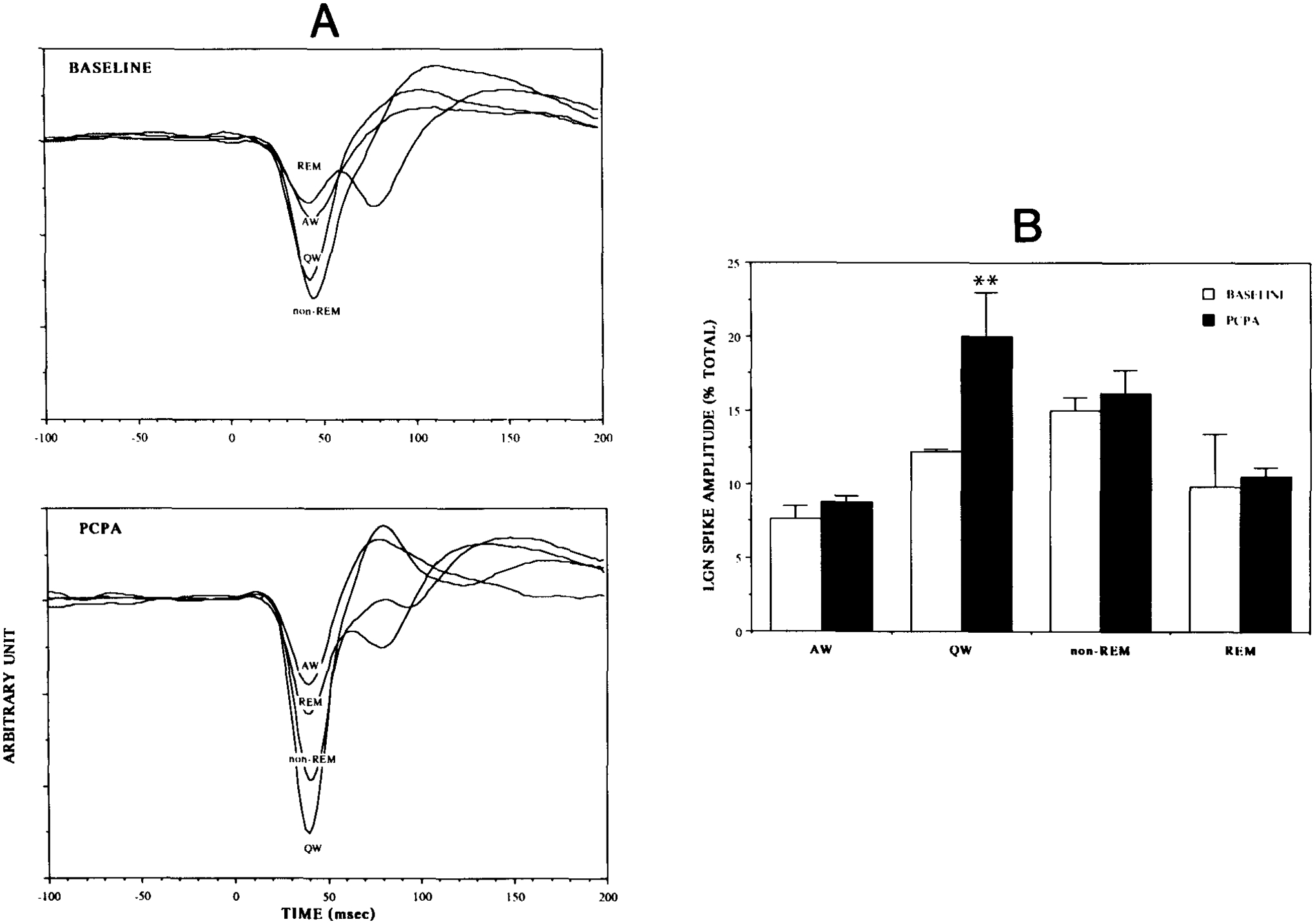
The amplitude of the auditory-evoked PGO wave was normally greatest in non-REM sleep and the transition to REM sleep, smaller during QW, and smallest during REM sleep and active waking. This relationship changed after PCPA treatment. A is the average of the auditory-evoked PGO responses of a cat before and after PCPA treatments. B compares the average evoked PGO spike amplitude over 3 cats before and after PCPA treatments. The evoked PGO amplitude during QW increased 63% over the pre-drug level (** P < 0.002, simple main effect ANOVA), while those in other states did not change significantly.
DISCUSSION
The possibility that the PGO wave is an indication of arousal of the organism, during which time sensorimotor responses may be facilitated, was tested in the present study, using the acoustic startle response. Following PCPA treatments, the animals showed spontaneous PGO spikes during waking and non-REM sleep, as well as during REM sleep, as has been reported7,8,13. These spontaneously occurring PGO waves facilitated the acoustic startle response, with maximal facilitation at 50 ms after the peak of the PGO wave. Startle responses were similarly facilitated by the eye-movement potentials in drug-naive cats, suggesting a common arousing function of the spontaneous PGO waves (EMPs) and the PGO waves released by the serotonin depletion.
PCPA alone also potentiated the baseline startle amplitude by 300–1250% during periods without PGO activity. Tonic facilitating effects of serotonin depletion on startle response have also been reported in the rat1,3,4,9,10,12,23. The present results demonstrate for the first time in cats the facilitating effects of PCPA on the acoustic startle response.
PCPA has been shown to be a specific depletor of brain serotonin with little effect on other brain amines14. Brain-stem serotonin level in the cat has been shown to be depleted by about 90% following 3–6 days of PCPA administration8. The PCPA effects on startle and PGO waves which we report here are consistent with evidence for the involvement of serotonin in these phenomena4,8,10,12,13,23. The tonic facilitation of startle after PCPA treatments is very likely to be due to its blocking of a tonic ‘inhibitory’ effect. Serotonin, when infused into the lateral ventricle in rats, has been shown to suppress the acoustic startle5. This effect is likely to be mediated via an action on descending non-serotonergic brainstem motor control systems19.
There was a close temporal relationship between head-body movements and the occurrence of PGO spikes. These movements were maximal right after the peak of the PGO wave (Fig. 2). The somatic motor activity that we found preceding and following the spontaneous PGO spike has not been previously described. It indicates that the PGO spike is not the trigger of this motor arousal, but rather marks the middle of a period of phasically enhanced motor activity. Thus, both the motor and the PGO activity are likely to have a common underlying trigger. The PGO-associated motor activation may explain our finding that the maximum facilitating effect of PGO waves on the startle response occurred 50 ms after the PGO peak. Thus the same mechanisms that produce a generalized facilitation of motor activity at the time of PGO spikes may also facilitate the startle response.
PGO-associated motoneuron hyperpolarization has been reported during the transition from non-REM sleep to REM sleep11 and during REM sleep15. However, waking PGO waves may be associated with motoneuron depolarization rather than the hyperpolarization seen in REM sleep2. In a previous paper we reported that the motor changes accompanying elicited PGO spikes were a function of both the amplitude of the eliciting stimulus and the time after elicitation25. Both motor inhibition and excitation were seen, the latter being most marked after intense, startle-eliciting stimuli. Motor inhibition was most marked during non-REM sleep and the transition states, while motor excitation was maximal in waking.
The descending pathways responsible for the phasic motor facilitation may include those originating in the nucleus reticularis pontis caudalis (RPC). Lesion studies have shown that the RPC is required for acoustic startle6. Reticulospinal units in this area respond only to auditory stimuli that exceed the startle threshold, at a latency appropriate for mediation of the startle behavior24. These same cells also fire phasically during waking movements and during the myoclonic twitches correlated with the PGO bursts of REM sleep20–22. It is therefore conceivable that a similar RPC activation in conjunction with the PGO spikes of waking and in the PCPA-treated animal, mediates the PGO facilitation of startle. It is also possible that motoneuron activation by non-RPC pathways may contribute to the motor facilitation. Additional PGO facilitation of the acoustic startle response might occur at the cochlear nucleus and nucleus of the lateral lemniscus, regions that show PGO-related potentials18.
In summary, the present experiments demonstrate that serotonin produces a tonic inhibition of the startle response. PGO spikes, released by serotonin depletion, mark the middle of periods of motor activation, and are associated with a further phasic facilitation of the startle response.
Acknowledgements.
This research was supported by the Medical Research Service of the Veterans Administration and PHS Grants MH 43811 and NS 14610.
REFERENCES
- 1.Carlton EL. and Advokat C, Attenuated habituation due to parachlorophenylalanine, Pharmacol. Biochem. Behav, 1 (1973) 657–663. [DOI] [PubMed] [Google Scholar]
- 2.Chase MH and Morales FR, Subthreshold excitatory activity and motoneuron discharge during REM periods of active sleep, Science, 221 (1983) 1195–1198. [DOI] [PubMed] [Google Scholar]
- 3.Conner RL, Stolk JM, Barchas JD and Levine S, Parachlorophenylalanine and habituation to repetitive auditory startle stimuli in rats, Physiol. Behav, 5 (1970) 1215–1219. [DOI] [PubMed] [Google Scholar]
- 4.Davis M and Sheard MH, Habituation and sensitization of the rat startle response: effects of raphe lesions, Physiol. Behav, 12 (1974) 425–431. [DOI] [PubMed] [Google Scholar]
- 5.Davis M, Astrachan DI and Kaas E, Excitatory and inhibitory effects of serotonin on sensorimotor reactivity measured with acoustic startle, Science, 209 (1980) 521–523. [DOI] [PubMed] [Google Scholar]
- 6.Davis M, Gendelman DS, Tischler M and Gendelman PM, A primary acoustic startle circuit: lesion and stimulation studies, J. Neurosci, 2 (1982) 791–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delorme F, Froment JL and Jouvet M, Suppression du sommeil par la p-chlorméthamphétamine et la p-chlorophénylalanine, C.R. Soc. Biol, 160 (1966) 2347–2351. [PubMed] [Google Scholar]
- 8.Dement WC, Mitler MM and Henriksen SJ, Sleep changes during chronic administration of parachlorophenylalanine, Rev. Can. Biol, 31 (Suppl.) (1972) 239–246. [PubMed] [Google Scholar]
- 9.Fechter LD, Central serotonin involvement in the elaboration of the startle reaction in rats, Pharmacol. Biochem. Behav, 2 (1974) 161–171. [DOI] [PubMed] [Google Scholar]
- 10.Geyer MA, Puerto A, Menkes DB, Segal DS and Mandell AJ, Behavioral studies following lesions of the mesolimbic and mesostriatal serotonergic pathways, Brain Research, 106 (1976) 257–270. [DOI] [PubMed] [Google Scholar]
- 11.Glenn L and Dement WC, Motoneuron properties during electromyogram pauses in sleep, Brain Research, 243 (1982) 11–23. [DOI] [PubMed] [Google Scholar]
- 12.Hole K, Johnson GE and Berge O-G, 5,7-Dihydroxytryptamine lesions of the ascending 5-hydroxytryptamine pathways: habituation, motor activity and agonistic behavior, Pharmacol. Biochem. Behav, 7 (1977) 205–210. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs BL, Henriksen SJ and Dement WC, Neurochemical bases of the PGO wave, Brain Research, 48 (1972) 406–411. [DOI] [PubMed] [Google Scholar]
- 14.Koe BK and Weissman A, p-Chlorophenylalanine: a specific depictor of brain serotonin, J. Pharmacol. Exp. Ther, 154 (1966) 499–516. [PubMed] [Google Scholar]
- 15.Morales ER., Lopez E, Stafford-Segert I, Soja PJ and Chase MH, A puzzle of motor control during active sleep in the cat: the enhancement of motoneuron inhibition that occurs in conjunction with pontogeniculooccipital waves, Sleep Res, 18 (1989) 20. [Google Scholar]
- 16.Morrison AR, Relationships between phenomena of paradoxical sleep and their counterparts in wakefulness, Acta Neurobiol. Exp, 39 (1979) 567–583. [PubMed] [Google Scholar]
- 17.Pivik RT, Metz J and Rechtschaffen A, Spinal reflexes and lateral geniculate nucleus activity during sleep: quantitative relationships, Exp. Neurol, 77 (1982) 142–162. [DOI] [PubMed] [Google Scholar]
- 18.Roffwarg H, Adrien J, Marks G and Farber J, Central and peripheral REM sleep activity in the auditory system of the cat, Sleep Res., 8 (1979) 35. [Google Scholar]
- 19.Siegel JM, Brainstem mechanisms generating REM sleep. In Kryger MH, Roth R and Dement WC (Eds.), Principles and Practice of Sleep Medicine, W.B. Saunders Co., Philadelphia, PA, 1989, pp. 104–120. [Google Scholar]
- 20.Siegel JM and McGinty DJ, Pontine reticular formation neurons: relationship of discharge to motor activity, Science, 196 (1977) 678–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siegel JM and Tomaszewski KS, Behavioral organization of reticular formation: studies in the unrestrained cat, I. Cells related to axial, limb, eye, and other movements, J. Neurophysiol, 50 (1983) 696–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siegel JM and Tomaszewski KS, Behavioral organization of reticular formation: studies in the unrestrained cat, II. Cells related to facial movements, J. Neurophysiol, 50 (1983) 717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walters JK, Davis MH and Sheard MH, Tryptophan-free diet: effects on the acoustic startle reflex in rats, Psychopharmacology, 62 (1979) 103–109. [DOI] [PubMed] [Google Scholar]
- 24.Wu M-E, Suzuki SS and Siegel JM, Anatomical distribution and response patterns of reticular neurons active in relation to acoustic startle, Brain Research, 457 (1988) 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu M-F, Mallick BN and Siegel JM, Lateral geniculate spikes, muscle atonia and startle response elicited by auditory stimuli as a function of stimulus parameters and arousal state, Brain Research, 499 (1989) 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]


