Abstract
To gain world-wide control over COVID-19 pandemic, it is necessary to have affordable and accessible vaccine and monoclonal antibody technologies across the globe. In comparison to the western countries, Asian and African countries have less percentage of vaccination done which warrants urgent attention. Global manufacturer production capacities, dependency on advanced nations for the supply of vaccines or the raw material, national economy, limited research facilities, and logistics could be the factors. This review article elaborates the existing therapeutic and prophylactic strategies available for COVID-19, currently adopted vaccine and monoclonal antibody platforms for SARS-CoV-2 along with the approaches to bridge the gap prevailing in the challenges faced by low- and middle-income countries. We believe adoption of yeast-derived P. pastoris technology can help in developing safe, proven, easy to scale-up, and affordable recombinant vaccine or monoclonal antibodies against SARS-CoV-2. This platform has the advantage of not requiring a dedicated or specialized facility making it an affordable option using existing manufacturing facilities, without significant additional capital investments. Besides, the technology platform of multiantigen vaccine approach and monoclonal antibody cocktail will serve as effective weapons to combat the threat posed by the SARS-CoV-2 variants. Successful development of vaccines and monoclonal antibodies using such a technology will lead to self-sufficiency of these nations in terms of availability of vaccines and monoclonal antibodies.
Keywords: SARS-CoV-2, COVID-19, Multiantigen vaccine, Monoclonal antibody cocktail, Pichia pastoris, Biotherapeutics, Prophylactics, Variants of Concern
1. Introduction
1.1. Background
A novel coronavirus now called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) identified in December 2019 in China caught a grip globally to the extent that the World Health Organization (WHO) declared this as a novel coronavirus outbreak and a global pandemic in March 2020 [1]. Pandemic impact is unprecedented on the world economy including healthcare infrastructure and therapeutics. It created a continued demand for healthcare infrastructure and support staff which was a limiting factor in developing countries and the situation remains unchanged even after one year in terms of cases surging at a higher rate on daily basis [2].
Researchers all over the world are relentlessly working on the development of effective antiviral drugs, monoclonal antibodies (mAbs), and vaccines to fight this pandemic. Since the development of such antiviral drugs and mAbs are time consuming processes, a partial success on this front is seen through the development of different vaccines that have currently received Emergency Use Authorization (EUA) and have hit the market. Vaccines are always considered as a prophylactic approach, whereas monoclonal antibodies are both therapeutic as a well as prophylactic. Although vaccination drives across the globe is a step forward to gain control over this dreaded disease, the statistical data reveals a worrying reality. Till the end of May 2022, approximately 79.8% of population in high-income countries have recieved atleast one dose of coronavirus disease 2019 (COVID-19) vaccine, whereas in middle-income countries (includes both lower and upper-middle), this percentage has reached to approximately 71.8. On the other hand, approximately 16.2% of population in low-income countries have recieved one dose of vaccine. This percentage calls for an urgent attention to understand the factors leading to such fewer figures of vaccination in these countries [3]. Global manufacturer production capacities, dependency on advanced nations for the supply of vaccine or the raw material required for vaccine production, national economy, limited research facilities, supply availability, and logistics could be some factors for less percentage of population being vaccinated in these low- and middle- income countries (LMICs). Nevertheless, this clearly highlights the gap in the accessibility and affordability of vaccines by Asian and African countries in comparison to American and European nations [4].
Similarly, there is an urgent need for development of antiviral drugs or other therapeutic agents which are not only effective but also affordable to the developing and/or underdeveloped countries taking into consideration the unavailability of or poor health care facilities [5].
Treatment options for any disease are dictated by the pathogenesis of the disease. Presently, COVID-19 pathogenesis is driven through two main processes. As a result, the current treatment guidelines followed revolves around targeting these two processes. In the initial stages, this disease is seen to progress by the replication of SARS-CoV-2, while in the later stages, a dysregulated immune/inflammatory response to SARS-CoV-2 leads to tissue damage [6]. This is also referred to as cytokine storm. Since there is no concrete solution yet available to fight this dreaded disease, various treatment guidelines suggest the use of available antiviral therapies to gain control on the viral replication in the initial stages followed by immunosuppressive/anti-inflammatory agents to control the cytokine storm. Inclusion of blood thinners is also recommended to prevent blood clotting in certain cases. Other symptomatic treatments include the use of antipyretics for fever and pain, adequate nutrition through food and supplements, and appropriate rehydration [6].
In this review article, we have attempted to elaborate the existing therapeutic and prophylactic strategies available for COVID-19 along with the currently adopted vaccine and monoclonal antibody (mAb) platforms for SARS-CoV-2. This article also proposes the approaches that will bridge the gap prevailing in the challenges faced by LMICs.
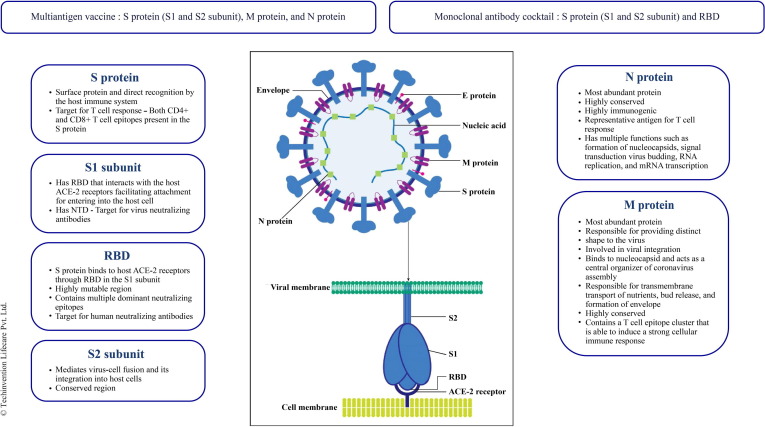
2. Structure of SARS-CoV-2
Coronavirus is a positive sense single stranded RNA (ssRNA) virus whose genome is made up of about 30 kb nucleotides encoding structural as well as several non-structural proteins. The structural proteins include Nucleocapsid (N) protein, Membrane (M) protein, Spike (S) protein, and Envelope (E) protein. The N protein binds to ssRNA and is the most immunogenic protein of the virus. It plays an important role in complex formation in the viral genome [7]. The M protein is the most abundant protein and gives a definite shape to the virus. The S protein is involved in actual binding to the host cell receptor. It is made up of three subunits: ectodomain (actually binds to the host through two subunits S1 & S2), transmembrane anchor, and intracellular tail. This S protein binds to the angiotensin-converting enzyme-2 (ACE-2) receptors to gain entry inside the human cell. The detailed mechanism of the same is described by Wentao Ni and his colleagues [8], [9]. These ACE-2 receptors are also present in the heart, kidneys, liver, lymphocytes, and nerve cells [10]. The E protein is the smallest structural protein which helps in pathogenesis, viral assembly, and release of new virions. In addition, there are 15 non-structural proteins (nsp1 to nsp10 and nsp12 to nsp16) and 8 accessory proteins (3a, 3b, p6, 7a, 7b, 8b, 9b, and ORF 14) that play an important role in viral replication. ORF3a, ORF6, ORF8, ORF7, and ORF9 help the coronavirus-host cell interactions for viral replication, modifying host gene induction, and neutralization of the host's antiviral defence system [11]. Identification of these structural details have opened channels for multiple drug targets. Research driven in the development of therapeutics and prophylactics are targeting these structural and non-structural proteins.
3. Current scenario
3.1. Therapeutic and prophylactic strategies under investigation
As of today, there are no specific drugs available that can treat COVID-19. Therefore, the most common therapeutic strategy adopted worldwide and supported with clinical trial data is drug repurposing where existing drugs are investigated for new therapeutic purposes. Drugs that have been selected to treat COVID-19 patients include the ones designed for other diseases such as ebola, influenza, parasites, human immunodeficiency virus (HIV) infections, and immune therapy for some autoimmune and inflammatory diseases. Clinical trials have been conducted where potential antiviral therapy targets were tested, such as blocking viral entry to human cells and inhibiting viral enzymes that were responsible for genome replication. Others focus on the human immune system to boost the innate response and inhibit the inflammatory process to relieve rapid progression of acute lung injuries [12]. WHO Solidarity trial concluded that remdesivir, hydroxychloroquine, lopinavir, and interferon regimens had little or no effect on hospitalized patients with COVID-19, as indicated by overall mortality, initiation of ventilation, and duration of hospital stay [13].
Remdesivir was recommended for use in hospitalized patients who require minimal supplemental oxygen. It is not routinely recommended for patients who require mechanical ventilation due to the lack of data showing benefit in the advanced stage of the disease. However, WHO removed this drug from COVID-19 management drug list [14]. The decision was taken due to the risk of elevation of hepatic enzymes, GI complications, rash, renal impairment, and hypotension seen with this drug. Dexamethasone is strongly recommended in hospitalized patients who require increasing supplemental oxygen [14]. Chakraborty et al. summarized the clinical trial data using different repurposing drugs and have shown that remdesivir, tocilizumab, dexamethasone, and baricitinib have some benefical outcomes. A combination of remdesivir and baricitinib have demonstrated better results as compared to remdesivir alone in COVID-19 patients. Until March 2021, 4952 clinical trials have been registered in ClinicalTrials.gov towards drug and vaccine development for COVID-19 with more than 100 countries particpating in the trials (latest data shows more than 8000 trials). Although, short-term repurposing of the existing drugs may provide a successful outcome for COVID-19 patients, more clinical trials are warranted in search of better therapeutics [15].
Another well accepted therapeutic agents are mAbs. They are designed to mimic or enhance the body’s natural immune response against an infection. Since they are created to specifically target an essential part of the infectious process, mAbs are considered to be of great advantage compared to other treatments. Passive immunization with neutralizing monoclonal antibodies (nMAbs) represents a promising therapeutic approach to reduce SARS-CoV-2 impact on public health worldwide [16]. Several novel humanized or bioengineered mAbs targeting different fragments of the S protein of SARS-CoV-2 are under clinical trials [17]. Adding tocilizumab, a recombinant humanized anti-interleukin-6 receptor (IL-6R) mAb to dexamethasone therapy was found to improve survival among patients who are hospitalized and require oxygen delivery through high flow device or non-invasive ventilation [6]. However, the latest clinical management guidelines issued by WHO inhibits the further use of Tocilizumab in COVID-19 treatment due to associated side effects like upper respiratory tract infections, nasopharyngitis, headache, hypertension, increased alanine aminotransferase (ALT), and injection site reactions [14].
Unlike non-neutralizing antibodies, nMAbs act by neutralizing the biological effects of the antigen which may facilitate better protection [18].
Binding of mAbs to the viral S protein prevents attachment to the host cell, entry of the virus into the host cell and inhibits further viral replication and infection of other host cells [19]. Binding of SARS-CoV-2 to alveolar epithelial cells leads to the production of proinflammatory cytokines and chemokines which eventually triggers the migration of monocytes and macrophages. This process gives rise to a cytokine storm in the body causing sepsis, pneumonitis, inflammatory lung injury, acute respiratory distress syndrome (ARDS), respiratory failure, shock, organ failure, and potential death [20], [21]. Anti-cytokine mAbs could also play a crucial role in controlling this chain of events following the cytokine storm.
Thus, mAbs can act as immunomodulators to modulate the immune response shown by the body or by binding directly to the various proteins present in the virus, thereby becoming a potential candidate in COVID-19 treatment (Table 1 ).
Table 1.
Monoclonal antibodies studied in COVID-19
| Drug class | Molecule | Mechanism of action |
|---|---|---|
| Immunomodulators | Tocilizumab | Inhibits both the membrane interleukin-6 receptors (mIL-6R) and soluble interleukin receptors (sIL-6R), thereby preventing IL-6R activation and hyper-interleukin-6 (IL-6) formation which is known to play a central role in cytokine storm [22] |
| Sarilumab | ||
| Itolizumab | Binds to domain 1 of cluster of differentiation 6 (CD6), a receptor present on effector T cells which blocks co-stimulation pathway leading to the inhibition of proliferation of naive T cells as well as proinflammatory cytokines such as interleukin-17A (IL-17A), tumor necrosis factor-alpha (TNF-α), IL-6, interferon-gamma (IFN-γ), and interleukin-2 (IL-2) [23] | |
| Siltuximab | Binds to IL-6, resulting in the inhibition of IL-6R activation and hyper IL-6 formation [22] | |
| Infliximab | Binds to TNF-α and supresses hyper immune response [24] | |
| Lenzilumab | Directly binds granulocyte-macrophage colony-stimulating factor (GM-CSF), blocks intracellular signaling and reduces hyperinflammation [25] | |
| Emapalumab | Acts by blocking the binding of IFNγ to cell surface receptors and activation of inflammatory signals [26] | |
| Canakinumab | Specifically inhibits interleukin-1 beta (IL-1β), a pro-inflammatory cytokine that mediates immune responses during infection and inflammation [27] | |
| Anakinra | Interleukin-1 (IL-1) receptor antagonist that inhibits the activity of the proinflammatory cytokine IL-1, specifically interleukin-1 alpha (IL-1α) and IL-1β [28] | |
| Adalimumab | Binds to TNF-α and prevents subsequent release of inflammatory cytokines [22] | |
| Bevacizumab | Binds to vascular endothelial growth factor (VEGF) to inhibit pulmonary edema caused by VEGF overexpression [22] | |
| Meplazumab | Binds to cluster of differentiation 147 (CD147) on the host cells which is used by the S protein of SARS-CoV-2 for gaining entry [29], [30] | |
| nMAbs | Bamlanivimab plus Etesevimab | Binds to the receptor-binding domain (RBD) of the S protein and blocks its attachment to the human ACE-2 receptors [31] |
| Sotrovimab | Targets an epitope in the RBD of the S protein that is conserved between SARS-CoV and SARS-CoV-2 [31] | |
| Tixagevimab plus Cilgavimab | Binds to the nonoverlapping epitopes of the S protein RBD of SARS-CoV-2 [31] | |
| Bebtelovimab | Binds to the S protein of SARS-CoV-2 [31] | |
| Casirivimab plus Imdevimab | Binds to the nonoverlapping epitopes of the S protein RBD of SARS-CoV-2 [16] | |
| Regdanvimab | Binds to the RBD of the S protein of SARS-CoV-2 to block the interactions with the host ACE-2 receptors [32] |
According to latest NIH COVID-19 treatment guidelines, Bamlanivimab plus Etesevimab or Casirivimab plus Imdevimab is recommended in outpatients with mild-to-moderate COVID-19 who are at high risk for disease progression [6].
3.2. Convalescent plasma therapy
The use of convalescent plasma (CP) therapy for the treatment of various diseases is a proven concept. This concept is also actively used by collecting convalescent plasma from COVID-19 recovered individuals to passively transfer antibodies to COVID-19 patients. Multiple studies have been initiated to use this technique in treating COVID-19 infections [33], [34], [35], [36]. Reviews summarizing various studies to evaluate the effectiveness of CP therapy in COVID-19 patients and also describing the pros and cons of this therapy are available in the literature [37], [38]. According to this, the clinical efficiency and zero mortality are the main advantage of CP therapy seen in COVID-19 patients. This strategy can be utilized as both therapeutic and prophylactic way of managing COVID-19. Along with the target antibodies, CP therapy can transfer from donor plasma to recipient the immunomodulatory effects in the form of anti-inflammatory cytokines and antibodies by blocking complement activation, inflammatory cytokines, and autoantibodies [39]. Constraints like adverse reactions, immunological reactions, and risk of transfusion associated reinfection can be minimized by testing plasma compatibility. Non-availability of standardized transfusion dose of CP, high infusion volumes, time of administration (usually should be administered before humoral immunity is developed), and mutations in the virus causing waning of antibodies are other limitations [38]. CP therapy may be an effective therapeutic option until the availability of therapeutic and/or prophylactic agents for COVID-19, with some early promising evidence on safety, viral clearance, and reduction in mortality. However, large multicentre clinical trials are required for establishing a stronger evidence regarding the effectiveness of CP therapy along with the optimal doses and time of treatment initiation [40].
While research in these various therapeutic strategies is still in progress, prophylactic strategy like vaccines is of utmost importance to gain control on this fast-spreading disease. World has shown considerable advancement in vaccine development and based on the clinical trial data, few of the successful candidates have also received EUA [41]. Table 2 provides a list of the COVID-19 vaccines commercially available at the time of writing this manuscript.
Table 2.
Commercially available COVID-19 vaccines
| Vaccine | Vaccine form | Vaccine platform | Institute | Target antigens |
|---|---|---|---|---|
| EpiVacCorona | Protein subunit | Peptide-based protein subunit | Vektor State Research Center of Virology and Biotechnology in Russia | Multiple epitopes [42], [43] |
| mRNA-1273 | RNA | Prefusion stabilized S protein mRNA encapsulated in lipid nanoparticles (LNP) | Moderna / National Institute of Allergy and Infectious Diseases | Stabilized S protein [42], [43] |
| BNT-162b2 | RNA | Modified nucleoside mRNA LNP formulation |
Pfizer / BioNtech | RBD [42], [43] |
| Covaxin | Inactivated | Whole-virion inactivated | Bharat Biotech | Whole virus [42], [43] |
| Coronavac | Inactivated | Beta-propiolactone inactivated alum adjuvant | Sinovac | Whole virus [42], [43] |
| BBIB-P-CorV | Inactivated | β-propiolactone inactivated aluminium hydroxide-adjuvanted whole-virion SARS-CoV-2 | Sinopharm | Whole virus [42], [44] |
| WIBP-CorV | Inactivated | Chemically inactivated whole virus vaccines | Sinopharm | Whole virus [42], [44] |
| AZD1222 Covishield |
Non-replicating viral vector | Chimpanzee adenovirus vector displaying S protein on its surface | Oxford / AstraZeneca | S protein [42], [43] |
| Ad5-nCov trade-named Convidecia | Non-replicating viral vector | Adenovirus type 5 (Ad5) expressing S protein | CanSino | S protein [42], [43] |
| Sputnik-V | Non-replicating viral vector | Recombinant adenovirus type 26 (rAd26) + Recombinant adenovirus type 5 (rAd5) expressing S protein | Gamaleya Research Institute | S protein [42], [43] |
| AD26.COV2.S | Non-replicating viral vector | Adenovirus type 26 (Ad26) expressing Spike protein | Johnson & Johnson (J&J) | S protein [42], [43] |
| Covivac | Inactivated | β-propiolactone-inactivated whole-virion | Chumakov Centre, a branch of the Russian Academy of Sciences | Whole virus [45] |
| ZF2001, trade-named RBD-Dimer | Protein subunit | Adjuvanted recombinant protein | Anhui Zhifei Longcom in collaboration with the Institute of Microbiology at the Chinese Academy of Sciences | RBD dimer [42], [43], [46] |
| ZyCoV-D | DNA | Plasmid DNA | Zydus Cadila | Plasmid DNA encoding SARS-CoV-2 S protein and IgE signal peptide [46] |
3.3. Prevailing vaccine platforms
According to the WHO news published on its website, till December 2020, there were over 200 vaccine candidates for COVID-19 being developed. From these, at least 52 candidates were in human trials [47]. A striking feature of the COVID-19 vaccine development process is the range of technology platforms that are being evaluated. It includes nucleic acid (DNA and RNA), virus-like particles (VLPs), peptide, viral vector (replicating and non-replicating), recombinant protein, live attenuated virus, and inactivated virus approaches [48]. Live attenuated virus and inactivated virus can be clubbed under the whole microbe approach. Approaches like nucleic acid (DNA and RNA) can be a genetic approach, whereas VLPs are a subunit approach [46].
In vaccine development, it is essential to understand the basis of each identified approach. According to Gavi, the Vaccine Alliance, whole virus vaccines utilize a weakened (attenuated) or deactivated form of the pathogen. This causes a disease to trigger protective immunity to it [49]. Live attenuated vaccines use a weakened form of the virus that can still grow and replicate, but does not cause illness. Inactivated vaccines contain viruses whose genetic material has been destroyed and hence cannot infect cells and replicate. It can still trigger an immune response. Whole vaccine approach as mentioned above in Table 2 for Bharat Biotech, Sinopharm, and Sinovac use inactivated pathogens and therefore cannot infect cells and replicate, but can trigger an immune response. The RNA vaccine consists of messenger RNA (mRNA) that codes for a specific part of the SARS-CoV-2. Most of the available vaccines target the S protein. This mRNA, when inside the human body, instructs the cells to produce antigens (S protein) which are then detected by immune cells to trigger a response. Examples include Pfizer-BioNTech and Moderna. The killer T cells destroy the infected cells, while the B cells and helper T cells support antibody production. Non-replicating viral vector vaccines introduce a safe, modified version of the virus or the vector to deliver genetic code for the antigen. Once the cells are infected, they trigger an immune response. Oxford-AstraZeneca and Sputnik V are the examples that produce S protein vector [49], [50]. Protein subunit vaccines contain fragments of protein which are identified through studies to produce a strong and effective immune response. This approach of restricting access to the pathogen reduces the risk of side effects. Such vaccines are also easy to produce in a cost-effective manner and more stable than those containing whole viruses or bacteria [51]. DNA vaccine delivers a gene or its fragment encoding immunogenic antigen to the host cell by using DNA plasmids as a vector to induce both humoral and cell-mediated immune responses efficiently [52]. Compared to live attenuated vaccines, DNA vaccines can induce broad immune response with efficient large-scale, low-cost, production, and high storage stability [53]. ZyCoV-D by Zydus Cadila is an example of this. Antiviral vaccines developed from surface proteins called as VLPs is another technology in vaccine development. Production of VLPs in the cells and further conversion into a stable vaccine is a multi-stage process [54].
Though all the approaches are justified, they have their own merits and demerits as seen in Table 3 .
Table 3.
Merits and demerits of various vaccine approaches
| Type of vaccine | Target antigen | Merits | Demerits |
|---|---|---|---|
| Live attenuated | Whole virus | ||
| Inactivated whole virus | Whole virus |
|
|
| Subunit | S protein | ||
| VLPs | S protein |
|
|
| Viral vector (both replicating and non-replicating) | S protein | ||
| DNA | S protein | ||
| RNA | S protein |
4. Current issues and challenges
4.1. Mutations in SARS-CoV-2
The SARS-CoV-2 genome has undergone several mutations from the time it was first identified in Wuhan, China [62]. Of particular clinical relevance is the mutation in the S protein, especially within the N-terminal domain (NTD) and the RBD which are targets of potent virus neutralizing antibodies. There is a growing concern that the new variants may impair the efficacy of current vaccines or monoclonal antibodies [19].
The S protein mutation outside of the RBD (D614G) emerged early during the pandemic and became the dominant circulating variant globally by June 2020 [62]. Since then, several variants have been identified with some designated as 'Variants of Concern (VOCs)' namely B.1.1.7 (Alpha variant - first detected in UK, September 2020), B.1.351 (Beta variant - first detected in South Africa, December 2020), and P.1 (Gamma variant - first reported in Brazil, January 2021) [62], [63]. They all share the D614G mutation in addition to other novel mutations of the S protein, including 2 other mutations in the RBD which are of particular concern. They are N501Y which increases affinity for the ACE-2 receptor, and E484K which is considered an escape mutation as it potentially reduces antibody neutralization sensitivity, thereby evading the immune system [62].
VOCs can be associated with changes in both morbidity and mortality. Poor clinical outcomes might be attributed to higher viral loads in infected individuals, altered transmission dynamics, or suppression of the host immune response. New SARS-CoV-2 VOCs will continue to emerge as the pandemic progresses. The concerns over these variants include increased disease severity and transmissibility, reduction in the efficacy of vaccines, increased rates of reinfections due to immune escape, and exacerbation of already crippling outbreaks which would ultimately prolong the pandemic [64], [65], [66]. Data published on the CDC website for various characteristics of VOCs shows that they exhibit increased transmissibility, reduced susceptibility to available monoclonal antibodies, and reduced neutralization by convalescent and post-vaccination sera [67].
Recently, India has seen a sharp rise in new coronavirus infections with more than 180,000 cases a day recorded in mid-April 2021, up from 10,000 cases a day in early February which has been attributed to a new more transmissible variant, namely the B.1.617 variant (Delta) which contains two mutations, E484Q and L452R, known to be associated with increased infectivity and immune escape [68]. This B.1.617 variant has now been designated as a 'Variant of Concern (VOC)' by WHO [69].
This highlights the need for undertaking alternative therapeutic and prophylactic strategies. An article by Vashishtha et al. highlights the importance of type and number of antigens targeted in vaccine or monoclonal antibody success [70]. Currently available vaccines and monoclonal antibodies focus on targeting the S protein and RBD respectively. This is a one-sided approach to target the virus. Mechanism and technology for multidirectional attack at various viral domains could be a strategy that can take care of the newer variants.
4.2. Accessibility and affordability
Several companies augmented their vaccine development programs and achieved partial success. Across the globe, vaccination campaigns are running on priority. Yet, the statistics show disparity in percentage of population that is vaccinated in various continents. Approach of repurposing of drugs is also yet to find a promising candidate for COVID-19. Considering this, it is important to understand the road blocks in a fight against this pandemic. An insight into this segment can help in designing additional and alternative strategies.
Besides, given the demand for vaccines, several countries including the United States and Europe have indicated that vaccines will be initially provided to their citizens [71]. This approach of developed countries to protect their own nationals have created to some extent an ignorance towards developing nations or poor countries. Hence, there is an urgent need to develop safe and affordable vaccines for LMICs of Asia, Africa, and Latin America which rely on proven technologies such as recombinant protein–based vaccines to facilitate its transfer for emerging market vaccine manufacturers. This aligns with the vision of Dr. Seth Berkeley, CEO of Gavi, the Vaccine Alliance, who emphasized on prioritizing a COVID-19 vaccine specifically for these countries [4], [72].
Further, there is also a very limited use of mAbs in these nations. The prime challenge could be the delays in regulatory filing and approval of these products as well as high cost due to its complex manufacturing process [73]. These factors ultimately affect the reach of these mAbs to underprivileged countries [74]. According to the news by International Aids Vaccine Initiative (IAVI), 80% of the sales of mAbs are in the U.S., Canada, and Europe, while 85% of the world’s population lives in LMICs. Hence, addressing this inequity by identifying ways to expand the global access to mAbs through timely and sustainable means is important [75]. IAVI has also recently published a global call to action, commissioned by Wellcome, that highlights the lack of equitable access to mAbs in LMICs and proposes a set of actions that could solve the access problem, including a call for global health players to form innovative partnerships to address the need for affordable mAbs worldwide [76]. It will be imperative for leaders across the globe to support this call to action by IAVI and Wellcome to ensure global access to innovative antibody-based solutions for COVID-19 and other diseases [76].
5. Risk mitigating approaches
5.1. Prophylactic approach
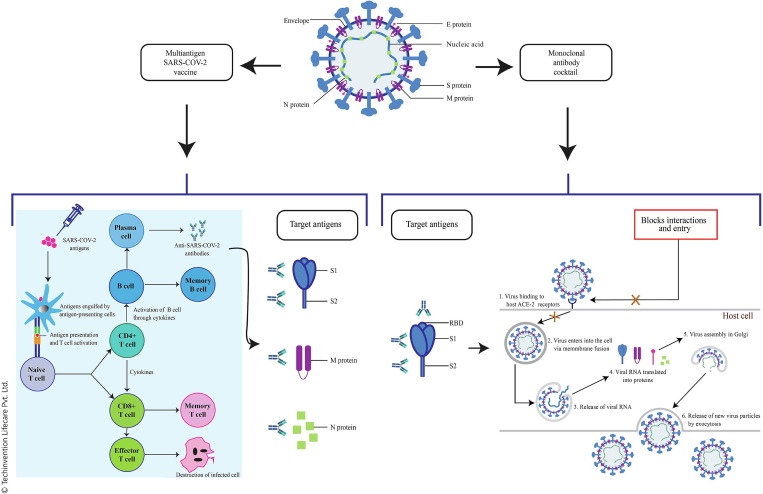
Wang et al. have strongly emphasized on new composition updated vaccines that can prevent infection taking into consideration the threat posed by the ever-emerging SARS-CoV-2 variants. The currently authorized vaccines target only the S protein and do not do involve the other structural proteins which play a critcal role in host cell responses and T cell memory. These non-S proteins are grossly overlooked in the vaccine development. A universal vaccine capable of inducing durable cross-reactive viral-neutralizing antibodies along with broad T cell immunity is required to combat these variants [77]. The complex genetic makeup and high mutation rate of SARS-CoV-2 warants the strategic development of a vaccine which targets all the structural proteins simultaneously [78]. Numerous reports have suggested S protein (S1 and S2 subunits), M protein, and N protein of SARS-CoV-2 as the most suitable targets in infectious stages [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89].
In this review, we propose a multiantigen SARS-CoV-2 vaccine comprising the S1 and S2 subunits, M protein, and N protein to address the issue of mutation. The rationale for targeting each of these proteins is given in Fig. 1 .
Fig. 1.
Rationale for targeting different regions of SARS-CoV-2 for multiantigen vaccine design and monoclonal antibody cocktail
5.2. Therapeutic approach
As shown in Table 2, most of the mAbs that have received EUA target the RBD which is present in the S1 subunit of the S protein. Mutations present within the RBD region of these highly transmissible variants raises concerns that this therapy might prove to be less effective if the mutations lie within the epitopes targeted by mAbs [90]. It would therefore be of great importance to develop mAbs that retain neutralizing activity against these variants that have mutations in the S protein [90]. Identifying antibodies that would target highly conserved epitopes within the S protein which the virus cannot readily mutate can be the ideal approach [90]. Strategies like broad-neutralizing antibodies targeting a conserved region on the S protein of SARS-CoV-2 or a cocktail strategy combining two or more antibodies might be effective in treating COVID-19 and future infections [91]. The continuous emergence of antibody-resistant SARS-CoV-2 variants that limit the therapeutic usefulness of mAbs can be mitigated by the use of antibody combinations that target distinct neutralizing epitopes [92].
Another approach for the development of therapeutic antibodies against SARS-CoV-2 could be targeting the NTD within the S1 subunit which work by restraining S conformational changes from the pre-fusion to post-fusion stages [93].
The S2 protein of human coronaviruses (CoVs) can also be potentially targeted to develop broad-spectrum prophylactic and therapeutic antibodies for preventing and treating virus infection. Different from the RBD specific neutralizing antibodies, the ones targeting S2 have broad-spectrum neutralizing activity due to the presence of relatively conserved sequences in S2 region among different CoVs in the same group. SARS-CoV-2 S2-specific antibodies have not been reported so far. Thus, efforts are necessary to develop such mAbs with potent and broad-spectrum neutralizing ability [93].
In this review, we suggest a mAb cocktail targeting the RBD, S1 subunit as well the S2 subunit to address the mutational drift currently seen with SARS-CoV-2. The rationale for targeting each of these regions is given in Fig. 1.
Antibody cocktail therapy consisting of neutralizing antibodies targeting different epitopes on the S protein can prove to be useful in improving the overall efficacy of each individual therapeutic in neutralization, inhibitory activity, and/or protection against SARS-COV-2 infection with or without mutations [93]. The synergyism and complementarity of each mAb in a cocktail makes it a preferred choice for treating infectious diseases [94].
6. Bridging the gap
Thus, considering the merits and demerits of common vaccine and mAb approaches undertaken for COVID-19 drug discovery development and the challenges in manufacturing, accessibility, logistics, and cost of vaccine and monoclonal antibody production, it is necessary to focus on more supple, rapid, scalable, transferable, and low-cost development and manufacturing technologies. This could possibly reduce the dependency of LMICs on other developed nations for vaccines and other therapeutics for COVID-19. In addition, it is essential to advance in an approach that will overcome the issue of continuous mutation in the virus that is making available vaccines and mAbs ineffective.
7. Alternative approaches to vaccine technology
An article by Kis et al. describes the traditional vaccine platforms and assesses the risk and feasibility of alternative vaccine development platform that can produce a wide range of vaccines [95]. The commonly practiced vaccine platforms and expression technologies include peptide vaccines, recombinant protein expression in mammalian cells, recombinant protein expression in Escherichia coli (E. coli), budded VLPs, recombinant protein expression in avian embryos and related cell lines, or exosome-based vaccines. However, the complexity and cost of production involved with these host organisms is high, and hence alternative expression technologies that enable faster and low-cost process development with easy scale-up becomes inevitable. Article also describes humanized, high-yield yeast recombinant protein vaccines, insect cell baculovirus ADDomer vaccines, Generalized Modules for Membrane Antigens (GMMA) vaccines, and RNA vaccines as these alternative expression strategies based on their low technological complexity, scalability, flexibility, and cost for producing a range of vaccines with better thermostability. Using these technologies in vaccine production to fight the COVID-19 pandemic will ensure meeting the vaccine demands in LMICs [95]. An article by Makenga et al. mentions about availability of GMMA technology in Africa, whereas Hotez et al. mentions about recombinant protein-based COVID-19 vaccine developed using Pichia pastoris (P. pastoris) to cater the needs of LMICs [4], [96]. This COVID-19 vaccine developed using P. pastoris is suitable for technology transfer to emerging market vaccine manufacturers (Developing Countries Vaccine Manufacturers' Network) having expertise in fermentation technology [4].
We suggest P. pastoris technology for the development of multiantigen vaccine and mAb cocktail against SARS-CoV-2. Yeast is a well-known host organism for the production of multiple recombinant subunit vaccines such as Hepatitis B, Influenza B, and Human papillomavirus (HPV) [97], [98]. In comparison to the prokaryote E. coli, P. pastoris exhibits proper protein folding, disulfide bridge formation, post-translational modifications, secretory cleavage, and also allows robust production with low-cost and full scalability [98]. It is a methylotrophic yeast (can use methanol as sole carbon and energy source) that can produce high yields of recombinant proteins [99]. Since yeast comes under the category of GRAS (Generally Recognized As Safe), high scalability, robustness, and cost-effective production can be adopted easily [100].
8. P. pastoris technology in vaccine development
P. pastoris is a safe and high yielding host system for the expression of S1 subunit from the S protein of SARS-CoV-2. S1 subunit is the main antigenic component among all the structural components that is responsible for host immune responses, neutralizing antibodies, and protective immunity against viral infection [8], [93], [98]. Recombinant P. pastoris clone development procedure including target gene synthesis and cloning using published S1 gene sequence (GenBank: QHD43416.1) followed by competent cell transformation and expression validation would be a robust methodology for this technology [101]. Successful clone expression and validation followed by large scale production of vaccine will be a cost-effective approach. Using this same approach, we recommend to designing individual recombinant clones of SARS-CoV-2 S1 subunit, S2 subunit, M protein, and N protein further formulated to develop a multiantigen vaccine using a suitable adjuvant. This multiantigen vaccine approach against COVID-19 would be a breakthrough in overcoming the burning issue of viral mutation. Fig. 2 elaborates our multiantigen vaccine approach.
Fig. 2.
Multiantigen vaccine and monoclonal antibody cocktail approach
9. P. pastoris technology in monoclonal antibody development
Due to the advantages of P. pastoris highlighted above, the technology can also be used for the development of of fully humanized mAbs. Fig. 2 highlights our mAb cocktail approach.
The mAb clones developed using phage display technology can be subcloned in a suitable vector for expression in P. pastoris. The stable cell line can then be utilized for the bioproduction of mAbs against COVID-19. Phage preparation can be achieved through RNA extraction from blood samples of COVID-19 survivors followed by conversion into cDNA for insertion into a cloning vector. Cloning vector can then be inserted into competent E. coli cells to generate a library of transformed cells. Further selection of the required clones can be done using a process called as biopanning which was successfully used for the isolation of Middle East respiratory syndrome coronavirus (MERS-CoV) nucleoprotein antibodies [102], [103].
10. Conclusion
Globally, though we manage to develop and deploy various treatments for COVID-19 including vaccines, mAbs, and drugs, the main uncertainty lies in how mutations of SARS-CoV-2 will affect its effectiveness. Additionally, the production and distribution of a huge number of vaccine doses across nations is another constraint. Researchers are making progress in characterizing the new coronavirus variants as well as in the drug development process, but many questions still remain unanswered. The disturbing dependency of LMICs on developed nations for COVID-19 treatment emphasizes on the need to adopt and invest more in those technologies which are affordable and also readily available in these countries. We strongly believe that our multiantigen and mAb cocktail approach will serve as effective weapons in the arsenal to combat the threat posed by the SARS-CoV-2 variants. We also believe adoption of P. pastoris technology can help in developing safe, proven, easy to scale-up, and affordable recombinant vaccine or mAbs against SARS-CoV-2. This platform has the advantage of not requiring a dedicated or specialized facility, making it an affordable option using existing manufacturing facilities without significant additional capital investments. Successful development of vaccines and mAbs using this technology will lead to self-sufficiency of these nations in terms of availability of vaccines and mAbs.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors would like to acknowledge Dr. Arati Ranade from Jehangir Clinical Development Centre, Pune for her support in technical writing of this manuscript. Authors would also like to thank Mr. Manish Manghani from Ashira Group, Mumbai, and Mr. Kaustubh Amare from Techinvention Lifecare Pvt. Ltd., Mumbai, for their support in creating and editing the images of the manuscript.
References
- 1.WHO Director-General's opening remarks at the media briefing on COVID-19 –11 March 2020, https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020 [accessed 12 April 2021].
- 2.Singh A., Deedwania P., Vinay K., Chowdhury A.R., Khanna P. Is India’s Health Care Infrastructure Sufficient for Handling COVID 19 Pandemic? Int Arch Public Health Community Med. 2020;4:041. doi: 10.23937/2643-4512/1710041. [DOI] [Google Scholar]
- 3.Coronavirus (COVID-19) Vaccinations, https://ourworldindata.org/covid-vaccinations [accessed 12 April 2021].
- 4.Hotez P.J., Botazzi M.E. Developing a low-cost and accessible COVID-19 vaccine for global health. PLoS Negl Trop Dis. 2020;14(7):e0008548. doi: 10.1371/journal.pntd.0008548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maxwell D., Sanders K.C., Sabot O., Hachem A., Llanos-Cuentas A., Olotu A., et al. COVID-19 Therapeutics for Low- and Middle-Income Countries: A Review of Candidate Agents with Potential for Near-Term Use and Impact. Am J Trop Med Hyg. 2021;105(3):584–595. doi: 10.4269/ajtmh.21-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.COVID-19 Treatment Guidelines, https://www.covid19treatmentguidelines.nih.gov/therapeutic-management/ [accessed 27 May 2021].
- 7.Shah V.K., Firmal P., Alam A., Ganguly D., Chattopadhyay S. Overview of Immune Response During SARS-CoV-2 Infection: Lessons From the Past. Front Immunol. 2020;11:1949. doi: 10.3389/fimmu.2020.01949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ni W., Yang X., Yang D., Bao J., Li R., Xiao Y., et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24(1):422. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mokhtari T., Hassani F., Ghaffari N., Ebrahimi B., Yarahmadi A., Hassanzadeh G. COVID-19 and multiorgan failure: A narrative review on potential mechanisms. J Mol Histol. 2020;51(6):613–628. doi: 10.1007/s10735-020-09915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belete T.M. A review on Promising vaccine development progress for COVID-19 disease. Vacunas. 2020;21(2):121–128. doi: 10.1016/j.vacun.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shih H.I., Wu C.J., Tu Y.F., Chi C.Y. Fighting COVID-19: A quick review of diagnoses, therapies, and vaccines. Biomed J. 2020;43(4):341–354. doi: 10.1016/j.bj.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan H., Peto R., Henao-Restrepo A.M., Preziosi M.P., Sathiyamoorthy V., Abdool Karim Q., et al. WHO Solidarity Trial Consortium Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med. 2021;384(6):497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical management of COVID-19, https://apps.who.int/iris/bitstream/handle/10665/332196/WHO-2019-nCoV-clinical-2020.5-eng.pdf [accessed 27 May 2021].
- 15.Chakraborty C., Sharma A.R., Bhattacharya M., Agoramoorthy G., Lee S.S. The Drug Repurposing for COVID-19 Clinical Trials Provide Very Effective Therapeutic Combinations: Lessons Learned From Major Clinical Studies. Front Pharmacol. 2021;12:704205. doi: 10.3389/fphar.2021.704205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaworski J.P. Neutralizing monoclonal antibodies for COVID-19 treatment and prevention. Biomed J. 2021;44(1):7–17. doi: 10.1016/j.bj.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renn A., Fu Y., Hu X., Hall M.D., Simeonov A. Fruitful Neutralizing Antibody Pipeline Brings Hope To Defeat SARS-Cov-2. Trends Pharmacol Sci. 2020;41(11):815–829. doi: 10.1016/j.tips.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bootz A., Karbach A., Spindler J., Kropff B., Reuter N., Sticht H., et al. Protective capacity of neutralizing and non-neutralizing antibodies against glycoprotein B of cytomegalovirus. PLoS Pathog. 2017;13(8):e1006601. doi: 10.1371/journal.ppat.1006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho D., Wang P., Liu L., Iketani S., Luo Y., Guo Y., et al. Increased Resistance of SARS-CoV-2 Variants B.1.351 and B.1.1.7 to Antibody Neutralization. Res Sq [Preprint] 2021;29 doi: 10.21203/rs.3.rs-155394/v1. [DOI] [Google Scholar]
- 20.Tang Yv, Liu J., Zhang D., Xu Z., Ji J., Wen C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deb P., Molla M.M.A., Saif-Ur-Rahman K.M. An update to monoclonal antibody as therapeutic option against COVID-19. Biosaf Health. 2021;3(2):87–91. doi: 10.1016/j.bsheal.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chastain D.B., Stitt T.M., Ly P.T., Henao-Martínez A.F., Franco-Paredes C., Osae S.P. Countermeasures to Coronavirus Disease 2019: Are Immunomodulators Rational Treatment Options-A Critical Review of the Evidence. Open Forum Infect Dis. 2020;7(7):ofaa219. doi: 10.1093/ofid/ofaa219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loganathan S., Athalye S.N., Joshi S.R. Itolizumab, an anti-CD6 monoclonal antibody, as a potential treatment for COVID-19 complications. Expert Opin Biol Ther. 2020;20(9):1025–1031. doi: 10.1080/14712598.2020.1798399. [DOI] [PubMed] [Google Scholar]
- 24.Tandon S., Aggarwal A., Jain S., Shukla S., Chaudhary S. Perspective on the Role of Antibodies and Potential Therapeutic Drugs to Combat COVID-19. Protein J. 2020;39(6):631–643. doi: 10.1007/s10930-020-09921-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Temesgen Z., Assi M., Shweta F.N.U., Vergidis P., Rizza S.A., Bauer P.R., et al. GM-CSF Neutralization With Lenzilumab in Severe COVID-19 Pneumonia: A Case-Cohort Study. Mayo Clin Proc. 2020;95(11):2382–2394. doi: 10.1016/j.mayocp.2020.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magro G. COVID-19: Review on latest available drugs and therapies against SARS-CoV-2. Coagulation and inflammation cross-talking. Virus Res. 2020;286:198070. doi: 10.1016/j.virusres.2020.198070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landi L., Ravaglia C., Russo E., Cataleta P., Fusari M., Boschi A., et al. Blockage of interleukin-1β with canakinumab in patients with Covid-19. Sci Rep. 2020;10(1):21775. doi: 10.1038/s41598-020-78492-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franzetti M., Forastieri A., Borsa N., Pandolfo A., Molteni C., Borghesi L., et al. IL-1 Receptor Antagonist Anakinra in the Treatment of COVID-19 Acute Respiratory Distress Syndrome: A Retrospective, Observational Study. J Immunol. 2021;206(7):1569–1575. doi: 10.4049/jimmunol.2001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scavone C., Mascolo A., Rafaniello C., Sportiello L., Trama U., Zoccoli A., et al. Therapeutic strategies to fight COVID-19: Which is the status artis? Br J Pharmacol. 2022;179(10):2128–2148. doi: 10.1111/bph.15452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar R., Gupta N., Kodan P., Mittal A., Soneja M., Wig N. Battling COVID-19: using old weapons for a new enemy. Trop Dis Travel Med Vaccines. 2020;6:6. doi: 10.1186/s40794-020-00107-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anti-SARS-CoV-2 Monoclonal Antibodies, https://www.covid19treatmentguidelines.nih.gov/therapies/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/ [accessed 6 June 2022].
- 32.Tuccori M., Ferraro S., Convertino I., Cappello E., Valdiserra G., Blandizzi C., et al. Anti-SARS-CoV-2 neutralizing monoclonal antibodies: clinical pipeline. MAbs. 2020;12(1):1854149. doi: 10.1080/19420862.2020.1854149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agarwal A., Mukherjee A., Kumar G., Chatterjee P., Bhatnagar T., Malhotra P., PLACID Trial Collaborators Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simonovich V.A., Burgos Pratx L.D., Scibona P., Beruto M.V., Vallone M.G., Vázquez C., et al. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N Engl J Med. 2021;384(7):619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Libster R., Pérez Marc G., Wappner D., Coviello S., Bianchi A., Braem V., et al. Fundación INFANT–COVID-19 Group Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. N Engl J Med. 2021;384(7):610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joyner M.J., Wright R.S., Fairweather D., Senefeld J.W., Bruno K.A., Klassen S.A., et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791–4797. doi: 10.1172/JCI140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajendran K., Krishnasamy N., Rangarajan J., Rathinam J., Natarajan M., Ramachandran A. Convalescent plasma transfusion for the treatment of COVID-19: Systematic review. J Med Virol. 2020;92(9):1475–1483. doi: 10.1002/jmv.25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagoba B., Gavkare A., Jamadar N., Mumbre S., Selkar S. Positive aspects, negative aspects and limitations of plasma therapy with special reference to COVID-19. J Infect Public Health. 2020;13(12):1818–1822. doi: 10.1016/j.jiph.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lünemann J.D., Nimmerjahn F., Dalakas M.C. Intravenous immunoglobulin in neurology--mode of action and clinical efficacy. Nat Rev Neurol. 2015;11(2):80–89. doi: 10.1038/nrneurol.2014.253. [DOI] [PubMed] [Google Scholar]
- 40.Sarkar S., Soni K.D., Khanna P. Convalescent plasma is a clutch at straws in COVID-19 management! A systematic review and meta-analysis. J Med Virol. 2021;93(2):1111–1118. doi: 10.1002/jmv.26408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emergency Use Authorization, https://www.fda.gov.ph/list-of-fda-issued-emergency-use-authorization/ [accessed 27 April 2021].
- 42.Tregoning J.S., Brown E.S., Cheeseman H.M., Flight K.E., Higham S.L., Lemm N.M., et al. Vaccines for COVID-19. Clin Exp Immunol. 2020;202(2):162–192. doi: 10.1111/cei.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung J.Y., Thone M.N., Kwon Y.J. COVID-19 vaccines: The status and perspectives in delivery points of view. Adv Drug Deliv Rev. 2021;170:1–25. doi: 10.1016/j.addr.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xia S., Zhang Y., Wang Y., Wang H., Yang Y., Gao G.F., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kozlovskaya L.I., Piniaeva A.N., Ignatyev G.M., Gordeychuk I.V., Volok V.P., Rogova Y.V., et al. Long-term humoral immunogenicity, safety and protective efficacy of inactivated vaccine against COVID-19 (CoviVac) in preclinical studies. Emerg Microbes Infect. 2021;10(1):1790–1806. doi: 10.1080/22221751.2021.1971569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.AboulFotouh K., Cui Z., Williams R.O. 3rd Next-Generation COVID-19 Vaccines Should Take Efficiency of Distribution into Consideration. AAPS PharmSciTech. 2021;22(3):126. doi: 10.1208/s12249-021-01974-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.The different Types of COVID-19 Vaccines, https://www.who.int/news-room/feature-stories/detail/the-race-for-a-covid-19-vaccine-explained [accessed 14 April 2021].
- 48.Thanh Le T., Andreadakis Z., Kumar A., Gomez Román R., Tollefsen S., Saville M., et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19(5):305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 49.What are whole virus vaccines and how could they be used against COVID-19?, https://www.gavi.org/vaccineswork/what-are-whole-virus-vaccines-and-how-could-they-be-used-against-covid-19 [accessed 14 April 2021].
- 50.The four types of COVID-19 vaccine – a snapshot, https://www.healthcareitnews.com/news/emea/four-types-covid-19-vaccine-snapshot [accessed 14 April 2021].
- 51.What are protein subunit vaccines and how could they be used against COVID-19?, https://www.gavi.org/vaccineswork/what-are-protein-subunit-vaccines-and-how-could-they-be-used-against-covid-19 [accessed 27 April 2021].
- 52.Silveira M.M., Moreira G.M.S.G., Mendonça M. DNA vaccines against COVID-19: Perspectives and challenges. Life Sci. 2021;267:118919. doi: 10.1016/j.lfs.2020.118919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silveira M.M., Oliveira T.L., Schuch R.A., McBride A.J.A., Dellagostin O.A., Hartwig D.D. DNA vaccines against leptospirosis: A literature review. Vaccine. 2017;35(42):5559–5567. doi: 10.1016/j.vaccine.2017.08.067. [DOI] [PubMed] [Google Scholar]
- 54.Calina D., Docea A.O., Petrakis D., Egorov A.M., Ishmukhametov A.A., Gabibov A.G., et al. Towards effective COVID‑19 vaccines: Updates, perspectives and challenges (Review) Int J Mol Med. 2020;46(1):3–16. doi: 10.3892/ijmm.2020.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haque A., Pant A.B. Efforts at COVID-19 Vaccine Development: Challenges and Successes. Vaccines (Basel) 2020;8(4):739. doi: 10.3390/vaccines8040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khuroo M.S., Khuroo M., Khuroo M.S., Sofi A.A., Khuroo N.S. COVID-19 Vaccines: A Race Against Time in the Middle of Death and Devastation. J Clin Exp Hepatol. 2020;10(6):610–621. doi: 10.1016/j.jceh.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rawat K., Kumari P., Saha L. COVID-19 vaccine: A recent update in pipeline vaccines, their design and development strategies. Eur J Pharmacol. 2021;892:173751. doi: 10.1016/j.ejphar.2020.173751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brisse M., Vrba S.M., Kirk N., Liang Y., Ly H. Emerging Concepts and Technologies in Vaccine Development. Front Immunol. 2020;11:583077. doi: 10.3389/fimmu.2020.583077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghorbani A., Zare F., Sazegari S., Afsharifar A., Eskandari M.H., Pormohammad A. Development of a novel platform of virus-like particle (VLP)-based vaccine against COVID-19 by exposing epitopes: an immunoinformatics approach. New Microbes New Infect. 2020;38:100786. doi: 10.1016/j.nmni.2020.100786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Funk C.D., Laferrière C, Ardakani A. A Snapshot of the Global Race for Vaccines Targeting SARS-CoV-2 and the COVID-19 Pandemic. Front Pharmacol. 2020;11:937. doi: 10.3389/fphar.2020.00937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karpiński T.M., Ożarowski M., Seremak-Mrozikiewicz A., Wolski H., Wlodkowic D. The 2020 race towards SARS-CoV-2 specific vaccines. Theranostics. 2021;11(4):1690–1702. doi: 10.7150/thno.53691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rauseo A.M., O’Halloran J.A. What Are the Clinical Implications of the SARS-CoV-2 Variants: 5 Things Every Cardiologist Should Know. JACC Basic Transl Sci. 2021;6(3):305–308. doi: 10.1016/j.jacbts.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu J., Peng P., Wang K., Fang L., Luo F.Y., Jin A.S., et al. Emerging SARS-CoV-2 variants reduce neutralization sensitivity to convalescent sera and monoclonal antibodies. Cell Mol Immunol. 2021;18(4):1061–1063. doi: 10.1038/s41423-021-00648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Darby A.C., Hiscox J.A. Covid-19: variants and vaccination. BMJ. 2021;372:n771. doi: 10.1136/bmj.n771. [DOI] [PubMed] [Google Scholar]
- 65.Shastri J., Parikh S., Aggarwal V., Agrawal S., Chaterjee N., Shah R., et al. Severe SARS-CoV-2 Breakthrough Reinfection With Delta Variant After Recovery From Breakthrough Infection by Alpha Variant in a Fully Vaccinated Health Worker. Front Med (Lausanne) 2021;8:737007. doi: 10.3389/fmed.2021.737007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grubaugh N.D., Hodcroft E.B., Fauver J.R., Phelan A.L., Cevik M. Phelan AL, Cevik M. Public health actions to control new SARS-CoV-2 variants. Cell. 2021;184(5):1127–1132. doi: 10.1016/j.cell.2021.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.SARS-CoV-2 Variant Classifications and Definitions, https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/variant-surveillance/variant-info.html#Concern [accessed 7 May 2021].
- 68.Chatterjee P. Covid-19: India authorises Sputnik V vaccine as cases soar to more than 180 000 a day. BMJ 2021;373:n978. 10.1136/bmj.n978. [DOI] [PubMed]
- 69.Vaidyanathan G. Coronavirus variants are spreading in India - what scientists know so far. Nature. 2021;593(7859):321–322. doi: 10.1038/d41586-021-01274-7. [DOI] [PubMed] [Google Scholar]
- 70.Vashishtha V.M., Kumar P. Development of SARS-CoV-2 vaccines: challenges, risks, and the way forward. Hum Vaccin Immunother. 2021;17(6):1635–1649. doi: 10.1080/21645515.2020.1845524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharma O., Sultan A.A., Ding H., Triggle C.R. A Review of the Progress and Challenges of Developing a Vaccine for COVID-19. Front Immnol. 2020;11:585354. doi: 10.3389/fimmu.2020.585354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berkley S. COVAX: more than a beautiful idea. Lancet. 2021;398(10298):388. doi: 10.1016/S0140-6736(21)01544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ledford H. Antibody therapies could be a bridge to a coronavirus vaccine - but will the world benefit? Nature. 2020;584(7821):333–334. doi: 10.1038/d41586-020-02360-y. [DOI] [PubMed] [Google Scholar]
- 74.DeFrancesco L. COVID-19 antibodies on trial [published correction appears in Nat Biotechnol. 2021 Feb;39(2):246] Nat Biotechnol. 2020;38(11):1242–1252. doi: 10.1038/s41587-020-0732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Expanding access to monoclonal antibody-based products: A global call to action, https://www.iavi.org/news-resources/expanding-access-to-monoclonal-antibody-based-products-a-global-call-to-action [accessed 24 Feb 2021].
- 76.IAVI, Merck KGaA, Darmstadt, Germany, and Serum Institute of India Join Forces to Develop Monoclonal Antibodies for COVID-19 and Ensure Prompt and Equitable Global Access; https://www.iavi.org/news-resources/press-releases/2020/iavi-merck-serum-institute-of-india-join-forces-to-develop-monoclonal-antibodies-for-covid-19 [accessed 24 February 2021].
- 77.Wang CY, Hwang K.P., Kuo HK, Kuo B.S., Liu H, Hou K.L., et al. UB-612, a Multitope Universal Vaccine Eliciting a Balanced B and T Cell Immunity against SARS-CoV-2 Variants of Concern. medRxiv. 2022;11:22272364. doi: 10.1101/2022.04.11.22272364. [DOI] [Google Scholar]
- 78.Singh A., Thakur M., Sharma L.K., Chandra K. Designing a multi-epitope peptide based vaccine against SARS-CoV-2. Sci Rep. 2020;10(1):16219. doi: 10.1038/s41598-020-73371-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu F., Xiang R., Deng X., Wang L., Yu Z., Tian S., et al. Receptor-binding domain-specific human neutralizing monoclonal antibodies against SARS-CoV and SARS-CoV-2. Signal Transduct Target Ther. 2020;5(1):212. doi: 10.1038/s41392-020-00318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Naqvi A.A.T., Fatima K., Mohammad T., Fatima U., Singh I.K., Singh A., et al. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim Biophys Acta Mol Basis Dis. 2020;1866(10):165878. doi: 10.1016/j.bbadis.2020.165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Asghari A., Naseri M., Safari H., Saboory E., Parsamanesh N. The Novel Insight of SARS-CoV-2 Molecular Biology and Pathogenesis and Therapeutic Options. DNA Cell Biol. 2020;39(10):1741–1753. doi: 10.1089/dna.2020.5703. [DOI] [PubMed] [Google Scholar]
- 82.Dhama K., Khan S., Tiwari R., Sircar S., Bhat S., Malik Y.S., et al. Coronavirus Disease 2019-COVID-19. Clin Microbiol Rev. 2020;33(4):e00028–20. doi: 10.1128/CMR.00028-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang J., Zeng H., Gu J., Li H., Zheng L., Zou Q. Progress and Prospects on Vaccine Development against SARS-CoV-2. Vaccines (Basel) 2020;8(2):153. doi: 10.3390/vaccines8020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dutta N.K., Mazumdar K., Gordy J.T. The Nucleocapsid Protein of SARS-CoV-2: a Target for Vaccine Development. J Virol. 2020;94(13):e00647–20. doi: 10.1128/JVI.00647-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dai L, Gao G.F. Viral targets for vaccines against COVID-19. Nat Rev Immunol. 2021;21(2):73–82. doi: 10.1038/s41577-020-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Littler D.R., MacLachlan B.J., Watson G.M., Vivian J.P., Gully B.S. A pocket guide on how to structure SARS-CoV-2 drugs and therapies. Biochem Soc Trans. 2020;48(6):2625–2641. doi: 10.1042/BST20200396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alanagreh L., Alzoughool F., Atoum M. The Human Coronavirus Disease COVID-19: Its Origin, Characteristics, and Insights into Potential Drugs and Its Mechanisms. Pathogens. 2020;9(5):331. doi: 10.3390/pathogens9050331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang Y., Yang C., Xu X.F., Xu W., Liu S.W. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41(9):1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tada T., Dcosta B.M., Zhou H., Vaill A., Kazmierski W., Landau N.R. Decreased neutralization of SARS-CoV-2 global variants by therapeutic anti-spike protein monoclonal antibodies. bioRxiv [Preprint] 2021;02:18.431897. doi: 10.1101/2021.02.18.431897. [DOI] [Google Scholar]
- 91.Ho M. Perspectives on the development of neutralizing antibodies against SARS-CoV-2. Antib Ther. 2020;3(2):109–114. doi: 10.1093/abt/tbaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weisblum Y., Schmidt F., Zhang F., DaSilva J., Poston D., Lorenzi J.C., et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. Elife. 2020;9:e61312. doi: 10.7554/eLife.61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jiang S., Zhang X., Du L. Therapeutic antibodies and fusion inhibitors targeting the spike protein of SARS-CoV-2. Expert Opin Ther Targets. 2021;25(6):415–421. doi: 10.1080/14728222.2020.1820482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ning L., Abagna H.B., Jiang Q., Liu S., Huang J. Development and application of therapeutic antibodies against COVID-19. Int J Biol Sci. 2021;17(6):1486–1496. doi: 10.7150/ijbs.5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kis Z., Shattock R., Shah N., Kontoravdi C. Emerging Technologies for Low-Cost, Rapid Vaccine Manufacture. Biotechnol J. 2019;14(1):e1800376. doi: 10.1002/biot.201800376. [DOI] [PubMed] [Google Scholar]
- 96.Makenga G., Bonoli S., Montomoli E., Carrier T., Auerbach J. Vaccine Production in Africa: A Feasible Business Model for Capacity Building and Sustainable New Vaccine Introduction. Front Public Health. 2019;7:56. doi: 10.3389/fpubh.2019.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kumar R., Kumar P. Yeast-based vaccines: New perspective in vaccine development and application. FEMS Yeast Res. 2019;19(2):foz007. doi: 10.1093/femsyr/foz007. [DOI] [PubMed] [Google Scholar]
- 98.Lee J., Liu Z., Chen W.H., Wei J., Kundu R., Adhikari R. Process development and scale-up optimization of the SARS-CoV-2 receptor binding domain-based vaccine candidate, RBD219-N1C1. Appl Microbiol Biotechnol. 2021;105(10):4153–4165. doi: 10.1007/s00253-021-11281-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Karbalaei M., Rezaee S.A., Farsiani H. Pichia pastoris: A highly successful expression system for optimal synthesis of heterologous proteins. J Cell Physiol. 2020;235(9):5867–5881. doi: 10.1002/jcp.29583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kaur S.P., Gupta V. COVID-19 Vaccine: A comprehensive status report. Virus Res. 2020;288:198114. doi: 10.1016/j.virusres.2020.198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.surface glycoprotein [Severe acute respiratory syndrome coronavirus 2], https://www.ncbi.nlm.nih.gov/protein/1791269090 [accessed 19 April 2021].
- 102.Smith G.P. Phage Display: Simple Evolution in a Petri Dish (Nobel Lecture) Angew Chem Int Ed Engl. 2019;58(41):14428–14437. doi: 10.1002/anie.201908308. [DOI] [PubMed] [Google Scholar]
- 103.Lim C.C., Woo P.C.Y., Lim T.S. Development of a Phage Display Panning Strategy Utilizing Crude Antigens: Isolation of MERS-CoV Nucleoprotein human antibodies. Sci Rep. 2019;9(1):6088. doi: 10.1038/s41598-019-42628-6. [DOI] [PMC free article] [PubMed] [Google Scholar]