Abstract
Background
High speed of COVID-19 vaccination has raised some concerns about the safety of the new vaccines. It is of a great importance to perform a review of the safety and efficacy of the COVID-19 vaccines.
Methods
Two International electronic databases (PubMed, ISI) were searched for clinical trials reporting efficacy and safety of COVID-19 vaccines compared to control group. Pooled risk ratio (RR) for total, systemic and local adverse events following immunization was calculated for different vaccine modalities.
Results
The pooled RRs of total adverse reactions for Inactivated, mRNA, and vector vaccines were 1.46 (95% CI: 1.19–1.78), 2.01 (95% CI: 1.82 – 2.23), and 1.65 (95% CI: 1.31 – 2.32) respectively. The pooled RR for occurrence of systemic adverse reactions following immunization for different vaccine modalities was 1.13 (95% CI: 0.79 – 1.61), 1.53 (95% CI 1.08 – 2.16), 1.58 (95% CI: 1.13 – 1.90), 0.72 (95% CI: 0.34 – 1.55), and 1.62 (95% CI: 1.39 – 1.89) for inactivated vaccine, mRNA, vector, DNA, and protein subunit vaccines respectively. The pooled RR of local adverse event following immunization with inactivated vaccine, mRNA vaccine, vector vaccine, DNA vaccine, and protein subunit vaccine was 2.18 (95% CI: 1.32 – 3.59), 4.96 (95% CI: 4.02 – 6.11), 1.48 (95% CI: 0.88–2.50) 1.04 (95% CI: 0.12–8.75), and 4.09 (95% CI: 2.63–6.35) respectively.
Conclusion
mRNA vaccines are associated with greater risk of adverse events following immunization. However, at the present moment the benefits of all types of vaccines approved by WHO, still outweigh the risks of them and vaccination if available, is highly recommended.
Keywords: COVID-19, SARS-CoV-2, Adverse events, Safety, Efficacy
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The first case of COVID-19 was reported in December 2019 [1]. Since then and until June 15, 2021, more than 175 million COVID-19 cases, including over 3.8 million deaths, were reported all around the world [2]. The COVID-19 pandemic is a global crisis and imposes a devastating health, social and economic burden on worldwide health systems [3], [4]. The COVID-19 pandemic has a remarkable negative impact on people’s lives, both at the individual and social levels. Since the pandemic has started many psychological, health and economic problems and health behaviour changes have resulted [5], [6], [7], [8]. The pandemic is changing rapidly and strategies to maintain clinical preventive services, including immunization is required in order to avoid overloading health systems and their subsequent consequences [9], [10], [11].
Nearly a year after the outbreak of COVID-19, the World Health Organization (WHO) issued the first Emergency Use Listing (EUL) for the first vaccine. The WHO has approved or is currently reviewing the efficacy and safety of more than 100 vaccines of different modalities [12]. Since then, numerous countries have started mass vaccination programs and vaccination is strongly recommended if available. In addition, a previous study suggested that 53%–84% of COVID-19 vaccination coverage is needed to achieve herd immunity [13]. The high speed of COVID-19 vaccination development has raised some concerns about the safety of these new vaccines. Information on COVID-19 vaccines safety is not reported accurately and scientifically by some media, which might increase vaccine hesitation in the public and hinder mass immunization [14].
For this reason, it is of great importance to perform an analysis of the safety and efficacy of the COVID-19 vaccines. In addition, reviewing the safety profile of COVID-19 vaccines will inform the clinicians about potential safety issues and provide the necessary data for decision-makers to evaluate vaccination strategies around the globe. Current evidence about the safety of COVID-19 vaccines mainly comes from the results of clinical trials. Therefore, we performed a meta-analysis of the available clinical trials to determine the risk and clinical features of adverse reactions following immunization of the COVID-19.
2. Methods
The current meta-analysis is conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [15]. The targeted outcome was the pooled risk ratio (RR) to examine the effect of vaccination on occurring adverse events following immunization compared to control or placebo group, total number of healthy individuals to receive vaccination for COVID-19, total number of healthy individuals to receive placebo or control, total number of cases with any adverse events following injection of COVID-19 vaccine, total number of cases with any adverse events following injection of placebo, number of cases with systemic adverse events following vaccination for COVID-19, number of cases with systemic adverse events following receiving placebo, number of cases with local adverse events following vaccination for COVID-19, number of cases with local adverse events following receiving placebo, number of cases with single local (swelling, pain and erythema at the site of injection) and systemic adverse events (fever, fatigue and headache) following vaccination for COVID-19, number of cases with single local (swelling, pain and erythema at the site of injection) and systemic adverse events (fever, fatigue and headache) following receiving placebo. Clinical trial studies - including phase I, II and III - were eligible for inclusion.
2.1. Search strategy
Two International electronic databases (PubMed, ISI) were searched from the first time available to 29 September 2021. The following sets of key terms were used for searching international databases: 1) COVID-19, 2019-nCoV, severe acute respiratory syndrome coronavirus 2, SARS-CoV-2; 2) adverse event, safety; 3) vaccine (Appendix I). No language, time or type of study limitation was applied in the search process. It should be noted that we updated our systematic search on 16 May 2022 to include other recent studies.
2.2. Eligibility criteria
We included studies that provided data on the frequency of occurrence of adverse events following immunization, based on vaccination status (vaccinated or controlled). We included studies where the efficacy and safety of the COVID-19 vaccination is the main outcome. We excluded the studies that have one or more of the following criteria: 1) Studies on any species of SARS other than SARS-CoV-2; 2) observational studies (cross-sectional and cohort studies), case reports, case series, systematic review, meta-analysis, grey literature, 3) Studies with no control group, 4) studies that number of cases with adverse events are not stratified into vaccinated and controlled group; 5) studies on specific groups of population with a disease rather than healthy individuals; 6) full text in any language other than English.
2.3. Screening, data extraction, and quality assessment
Two researchers (HK, HA) screened the titles and abstracts of all records that was retrieved from online databases, independently. Eligible full texts were selected. The full-texts were screened for data of interest mentioned above and if they did not meet any exclusion criteria, data were extracted in the Excel sheet. The extracted data were assessed by a third reviewer. The following data was extracted from included studies: bibliometric information (name of the first author, year of publication), name of the vaccine, type of the study and the phase of the clinical trial, number of total vaccinated and controlled cases, number of cases with adverse events in each group, the mean or median age of patients, percentage of male cases in the study and the most common type of systemic and local adverse events reported. Any Disagreements during the process were resolved by the fourth researcher.
2.4. Statistical analyses
For data analysis, we used meta package in the R statistical software (version 4.1.1) [16]. The RR of adverse events (Total, systemic and local) following immunization was calculated with a 95% confidence interval (CI) for all studies. After that, the pooled risk ratio was estimated using Mantel-Haenszel Method. The random-effects model was used for calculating pooled risk ratio [17]. In this study subgroup analysis was used to report pooled RR for different vaccine modalities (inactivated, mRNA, vector and protein subunit vaccine) separately. The pooled RR of adverse events (Total, systemic and local) following immunization was reported with 95% CI. The forest plot was used to graphically represent the result of conducted subgroup meta-analysis and calculated RR for individual studies. The I2 statistic was used to examine the heterogeneity in the included studies for each subgroup of vaccines. A value of more than 75% was considered as significant heterogeneity. The I2 value represents the percentage of total variation among studies due to heterogeneity. Publication bias was assessed through funnel plot. Any asymety in plot was considered as publication bias.
3. Results
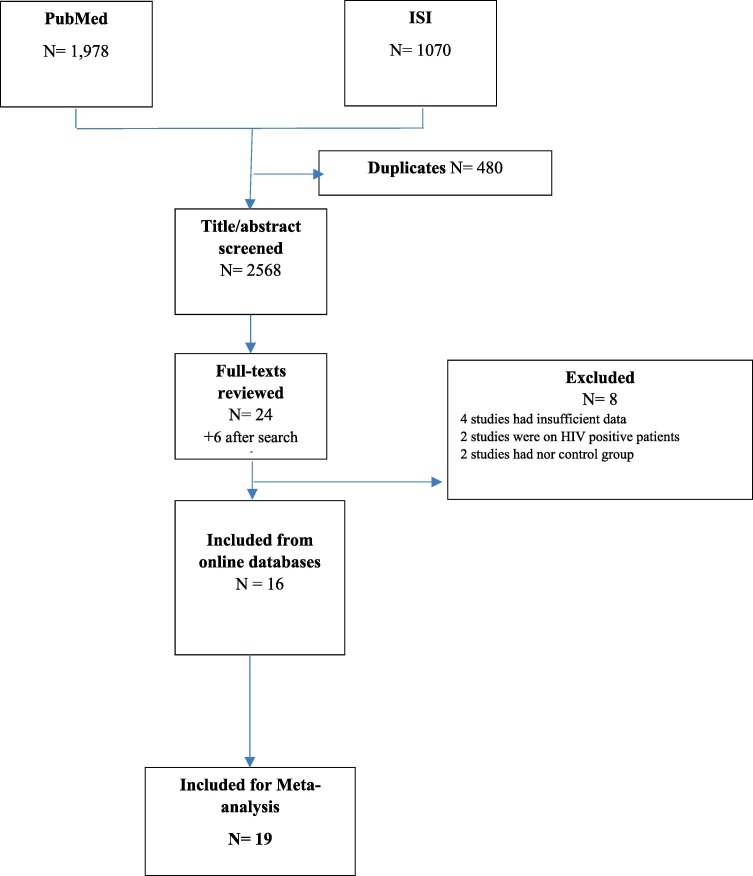
Fig. 1 presents the study selection for the current meta-analysis. In the PubMed and ISI database, 1978 and 1070 records were found by the search strategy reported in methods. After the removal of duplicate studies, 2568 records were retrieved from online databases. Title and/ or abstract of these records were screened for finding eligible studies. In this process, 30 studies were fulfilled the inclusion criteria for full-text review. Elven of these full-texts, were excluded because 1) five studies had insufficient data, 2) four studies had no control (or placebo) group, 3) three studies tested the efficacy of vaccine on HIV positive patients. After Finally, nineteen studies were included in our study [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36]. The characteristics of the selected studies are shown in Table1 .
Fig. 1.
Flow diagram of the study selection.
Table 1.
Characteristics of included studies.
| Author, Year | Name of the Vaccine | Study design | Controlled/vaccinated subjects | Age (year), Male% | Local reactions | Systemic reactions |
|---|---|---|---|---|---|---|
| Xia, 2020 [30] | BBIBP-CorV | Randomized double-blind placebo-controlled phase I/II trials | 80/240 | Mean age: 42.8 Male: 37.5% | Pain itching redness swelling | Fever, coughing diarrhea, fatigue headache, pruritus nausea, vomiting |
| Xia, 2020 [31] | CoronaVac-Wuhan | Randomized double-blind placebo-controlled studies phase I/II trial | 160/480 | Mean age: 45.3 Male: 45.7% | Pain itch redness swelling rash | Fever, fatigue inappetence, nausea, diarrhea, joint pain, Headache, Vomiting |
| Xia, 2020 [29] | BBIBP-CorV | Randomized double-blind placebo-controlled studies phase I/II trial | 160/480 | Age range: 3–18 Male: 51% | Pain itch redness swelling rash | Fever, fatigue inappetence, nausea, diarrhea, joint pain, Headache, Vomiting |
| Zhang, 2020 [32] | CoronaVac | Double-blind randomized placebo-controlled phase I/II clinical trial | 167/576 | Mean age:42.4 Male: 44.6% | Pain swelling redness discoloration | Fatigue, diarrhea fever, muscle pain, headache, nausea, cough, hypersensitivity |
| Wu, 2021 [28] | CoronaVac | Double-blind randomized placebo-controlled phase I/II clinical trial | 73/498 | Mean age: 66.5 Male: 48.9% | Pain swelling erythema pruritus | Fatigue, diarrhea fever, muscle pain headache, nausea cough, hypersensitivity |
| Che, 2020 [19] | Double-blind randomized placebo-controlled phase II clinical trial | 150/600 | Mean age: 39.2Male: 37.7% | Pain Redness Swelling Itch | Fever, Fatigue Diarrhea, Hypersensitivity/urticaria, Cough, Nausea Vomiting Mucosal abnormality | |
| Kremsner, 2021 [22] | CVnCoV | Randomized, double-blind, placebo-controlled phase I | 30/120 | Mean age: 45.8 Male: 42.8% | Pain redness swelling | Headache, fatigue myalgia,,nausea/vomiting, diarrhea |
| Zhu, 2020 [33] | Ad5-vectored | Randomized double-blind placebo-controlled phase II trial | 126/382 | Mean age: 39.7 Male: 50.0% | Pain swelling redness induration itch | Fever, headache, fatigue, vomiting, diarrhea, muscle pain, joint muscle, cough, nausea |
| Folegatti, 2020 [20] | ChAdOx1 | Participant-blinded; multicenter randomized controlled trial phase I/II | 534/543 | Median age: 35 Male: 50.2% | Pain swelling redness itch induration tenderness | Chills, fatigue, fever, feverish, headache, joint pain, malaise, nausea, muscle ache |
| Ramasamy, 2020 [25] | ChAdOx1 | single-blind, randomized, controlled, phase II/III trial | 140/420 | Median age, 18–55 years group: 43.0 56–69 years group: 60.0 70 years and older: 73.0 Male,50.0% | Pain, swelling redness itch induration tenderness | chills, fatigue, fever, feverish, headache, joint pain, malaise, nausea, muscle ache |
| Baden, 2020 [18] | mRNA-1273 | phase III randomized observer-blinded placebo-controlled trial | 15179/15181 | Mean age: 52.4 Male: 52.7% | Pain, Erythema, Swelling, Lymphadenopathy | Fever, Headache, Fatigue, Myalgia, Arthralgia, Chills Nausea, Vomiting |
| Walsh, 2020 [27] | BNT162b1 | placebo-controlled, observer-blinded, randomized dose-escalation,phase I trial | 21/84 | Median age (BNT162b1): younger group:35 group: 69 BNT162b2: younger group: 37 older group: 68 Male: 44.8% | pain redness swelling | fever fatigue chills |
| Mulligan, 2020 [23] | BNT162b1 | placebo-controlled, observer-blinded, randomized, Phase I/II trial | 9/36 | Mean age: 35.4 Male: 51.1% | pain redness swelling | fever, fatigue, chills, headache, vomiting, diarrhea, muscle pain, joint pain |
| Polack, 2020 [24] | BNT162b2 | placebo-controlled, observer-blinded, randomized, Phase III trial | 18846/18860 | Median age: 52 Male: 50.6% | pain redness swelling | fever, fatigue, chills, headache, vomiting, diarrhea, muscle pain, joint pain |
| Richmond, 2020 [26] | SCB-2019 | placebo-controlled, blinded, phase I trial | 32/216 | Mean age: 38.6 Male: 57% | pain redness swelling itching | fever, fatigue, chills, headache, vomiting, diarrhea, myalgia |
| Keech, 2020 [21] | NVX-CoV2373 | randomized, placebo-controlled, phase 1/2 trial | 23/108 | Mean age: 29.5 Male: 40% | pain redness swelling itching | fever, fatigue, chills, headache, vomiting, diarrhea, myalgia |
| Khobragade, 2022 [34] | ZyCoV-D | randomized, placebo-controlled, phase III trial (first dose) | 487/447 | younger population: 12–17 years | pain redness swelling itching | fever, fatigue, headache |
| Khobragade, 2022 [34] | ZyCoV-D | randomized, placebo-controlled, phase III trial (first dose) | 923/924 | older population: greater than60 years | pain swelling | myalgia, headache, fatigue, fever, |
| Masuda, 2022 [35] | NVX-CoV2373 | randomized, placebo-controlled, phase 1/2 trial | 50/150 | Mean age: 52.6 | pain redness swelling itching induration | fever, fatigue, chills, headache, vomiting, diarrhea, myalgia |
| Tabarsi, 2022 [36] | SpikoGen | randomized, placebo-controlled, phase 2 trial | 89/311 | Mean age: 35.69 | pain redness swelling itching induration | fever, fatigue, chills, headache, vomiting, diarrhea, myalgia |
Six of these 19 studies were on inactivated type of vaccine, five on mRNA vaccines, three on vector vaccines, one on DNA vaccines, and four on protein subunit vaccines. The largest group was mRNA vaccines consisting of 3754 subjects receiving inactivated vaccine, with a total of 1134 control cases. 59,986 subjects received mRNA vaccine with 59,222 cases as control. A total of 65,622 subjects (including booster dosage of vaccine) received vaccination for COVID-19 and 61,376 subjects were in control group and received placebo. 1429 subjects received vector vaccine with 916 cases. Protein subunit vaccine was consisted of 1370 subjects in vaccine group and 379 control or placebo cases. Also, 1371 subjects received DNA vaccine and 1410 subjects received placebo.
Most of the studies (nine studies) reported the result of the clinical trials phase I and II together. Three studies reported the results of phase I clinical trial, three studies were phase II and III each and one study reported the results of a phases II/III clinical trial. Among the included studies, seven studies were conducted in China [19], [28], [29], [30], [31], [32], [33]; three studies in the United States [18], [23], [27]; two in the United Kingdom [20], [25]; one in Iran [36], one in Japan [35], one in India [34], one in Europe [22] and two in Australia [21], [26]. One study was from multiple centers including the United States, Brazil, Argentina, Germany, Turkey and South Africa [24].
Among the 16 included studies, 10 studies reported the incidence of total adverse reactions, 10 reported the incidence of systemic and local adverse reactions, and 15 studies reported the incidence of single adverse reactions including pain, swelling, erythema, fatigue, headache and fever.
3.1. Total adverse events
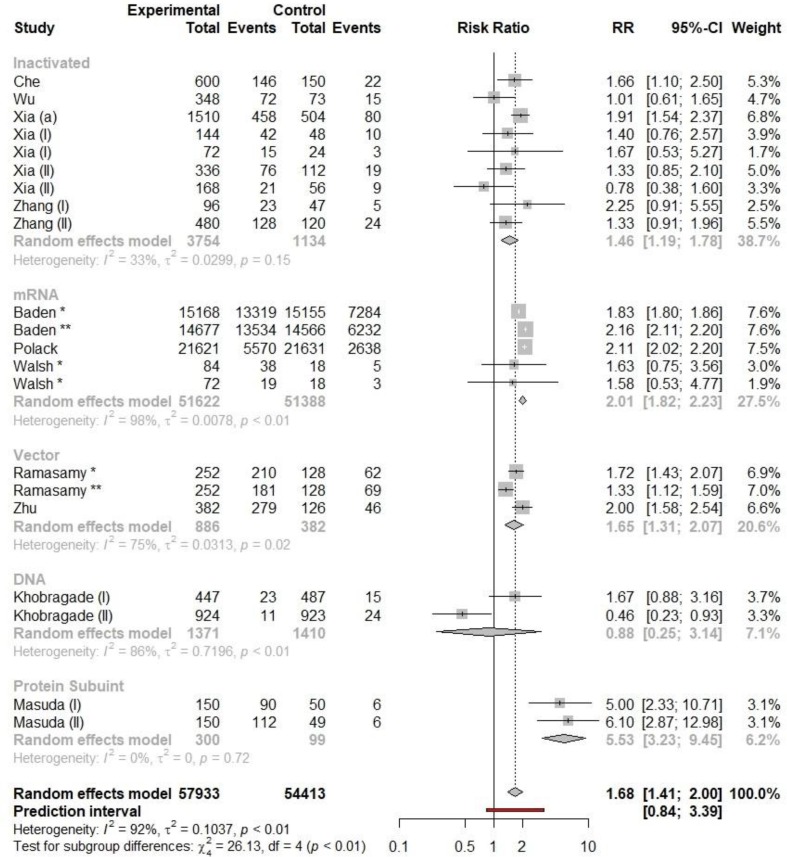
The pooled meta-analysis of RR of any adverse reactions following immunization for different vaccine modalities which contained 19 clinical trials is shown in Fig. 2 . The pooled RRs of total adverse reactions for Inactivated, mRNA and vector vaccines were 1.46 (95% CI: 1.19–1.78), 2.01 (95% CI: 1.82 – 2.23) and 1.65 (95% CI: 1.31 – 2.32) respectively. The RR of occurrence of any adverse reaction was least for inactivated vaccine and highest for mRNA vaccines (see Fig. 3 and Fig. 4 ).
Fig. 2.
The forest plot of pooled RRs of any adverse reaction following immunization for different types of vaccines.
Fig. 3.
The forest plot of pooled RRs of systemic adverse reaction following immunization for different types of vaccines.
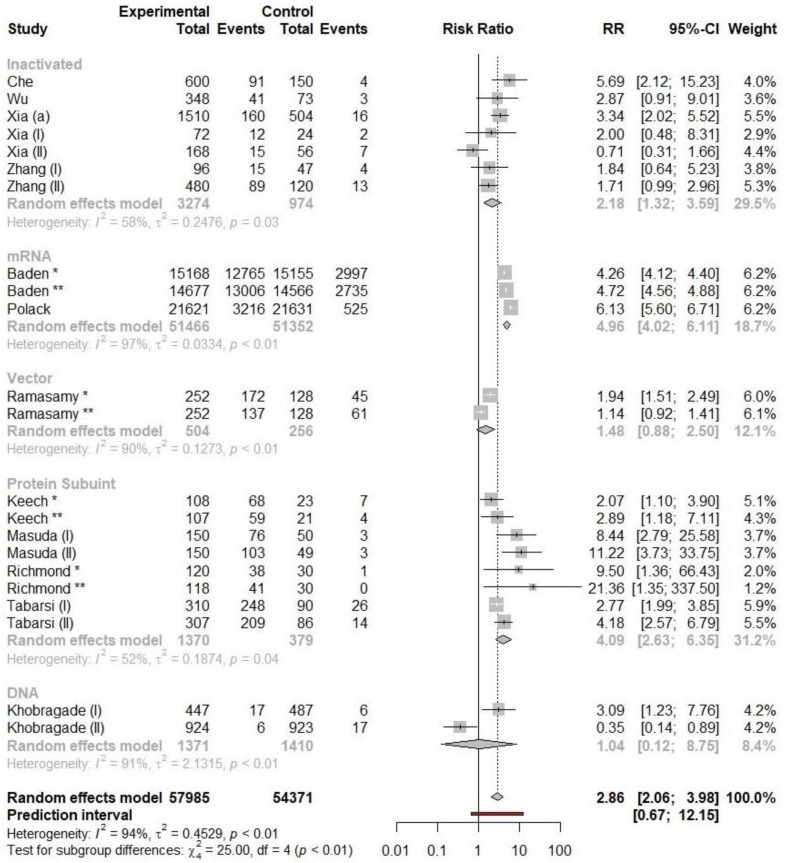
Fig. 4.
The forest plot of pooled RRs of local adverse reaction following immunization for different types of vaccines.
3.2. Systemic adverse reaction
The pooled RR for occurrence of systemic adverse reactions following immunization for different vaccine modalities was 1.13 (95% CI: 0.79 – 1.61), 1.53 (95% CI 1.08 – 2.16), 1.58 (95% CI: 1.13 – 1.90), 0.72 (95% CI: 0.34 – 1.55), and 1.62 (95% CI: 1.39 – 1.89) for inactivated vaccine, mRNA, vector, DNA, and protein subunit vaccines respectively. The RR of occurrence of systemic adverse reactions following inactivated vaccine was not statistically significant. The most common types of systemic reactions were fever, fatigue and headache and the most common type of local adverse events were pain, redness (erythema) and swelling at the site of injection. We separately calculated the pooled RR of occurrence of any of these reactions for different types of vaccines. Pooled RRs of single systemic adverse reactions to the inactivated vaccines was 1.23 (95% CI: 0.77–1.96), 0.85 (95% CI: 0.55–1.29) and 0.97 (95% CI: 0.45–2.07) for fever, fatigue and headache respectively. which were not significantly different from that of the control group. Risk of single systemic adverse reactions to the mRNA vaccines, such as fever (RR = 8.35, 95% CI: 3.57–19.51), headache (RR = 1.89, 95% CI: 1.20–2.62) and fatigue (RR = 1.95, 95% CI: 1.52; 2.51), was significantly higher than that in the control group. Pooled RRs of systemic adverse reactions to viral-vector vaccines, including fatigue (RR = 1.90, 95% CI: 0.55–6.55) and headache (RR = 0.99, 95% CI: 0.48–2.05) were not significantly higher than that in the control group.
3.3. Local adverse reaction
The RR of local adverse reactions following immunization with mRNA vaccines was considerably higher than other types of vaccines. The RR of local adverse events following immunization with inactivated vaccine, mRNA vaccine, vector vaccine, DNA, and protein subunit vaccine was 2.18 (95% CI: 1.32 – 3.59), 4.96 (95% CI: 4.02 – 6.11), 1.48 (95% CI: 0.88–2.50), 1.04 (95% CI: 0.12–8.75), and 4.09 (95% CI: 2.63–6.35), respectively. Pooled RRs of individual local adverse reactions to the inactivated vaccines were calculated to be 0.86 (95% CI: 0.33–2.20) and 0.62 (95% CI: 0.33–1.18) for swelling and pain respectively which were not statistically significant. However, the RR of occurrence of pain in the site of injection was calculated to be 2.52 (95% CI: 1.53–4.14); the only significant adverse event following inactivated COVID-19 vaccine injection. In addition, incidence of single local adverse events to the mRNA vaccines, such as pain (RR = 5.39, 95% CI: 4.26–6.82), erythema (RR = 7.65, 95% CI: 3.94–14.86), swelling (RR = 10.6, 95% CI: 4.52–24.88) also higher than that of control group. Pooled RRs of local adverse reactions to viral-vector vaccines such as pain (RR = 3.29, 95% CI: 0.91–11.77), erythema (RR = 1.33, 95% CI: 0.67–2.65), swelling (RR = 2.22, 95% CI: 0.33–14.88), were not significantly higher than that in the control group. The results are shown in Table 2 .
Table 2.
Incidence of local and systematic adverse reactions among vaccination group versus control group.
| Type of Vaccine | Reactions/Total |
RR | 95% CI | I2 | |
|---|---|---|---|---|---|
| Vaccination | Control | ||||
| Local adverse reactions (any) to different vaccine types | |||||
| Pain | |||||
| Inactivated | 391/3754 | 42/1134 | 2.52 | 1.53; 4.14 | 55.5 |
| mRNA | 31924/38065 | 5946/37519 | 5.39 | 4.26; 6.82 | 92.7 |
| Vector | 573/1429 | 209/916 | 3.29 | 0.91; 11.77 | 94.8 |
| Protein Subunit | 517/832 | 40/220 | 3.3 | 2.08; 5.3 | 45.1 |
| DNA | 16/1371 | 12/1410 | 1.33 | 0.4; 4.38 | 57.3 |
| Swelling | |||||
| Inactivated | 21/3154 | 5/984 | 0.86 | 0.33; 2.20 | 0.0 |
| mRNA | 3843/38065 | 149/37519 | 10.6 | 4.52; 24.88 | 88.7 |
| Vector | 37/1429 | 13/916 | 2.22 | 0.33; 14.88 | 53.2 |
| Protein Subunit | 148/832 | 5/220 | 7.07 | 3.05; 16.37 | 0.0 |
| DNA | 2/1371 | 5/1410 | 0.62 | 0.04; 9.16 | 50.6 |
| Erythema | |||||
| Inactivated | 30/3754 | 12/1134 | 0.62 | 0.33; 1.18 | 0.0 |
| mRNA | 2126/37650 | 194/37457 | 7.65 | 3.94; 14.86 | 93.3 |
| Vector | 22/1429 | 18/916 | 1.33 | 0.67; 2.65 | 0.0 |
| Protein Subunit | 36/832 | 6/220 | 1.47 | 0.65; 3.35 | 0.0 |
| DNA | 2/1371 | 4/1371 | 0.76 | 0.02; 34.5 | 69.5 |
| Systemic adverse reactions (any) to different vaccine types | |||||
| Fever | |||||
| Inactivated | 206/3754 | 46/1134 | 1.2292 | 0.7696; 1.9632 | 0.0 |
| mRNA | 3175/38365 | 128/37591 | 8.3464 | 3.57; 19.51 | 94.9 |
| Protein Subunit | 16/832 | 1/220 | 2.98 | 0.56; 15.85 | 0.0 |
| DNA | 3/1371 | 0/1410 | 2.16 | 0.28; 16.71 | 0.0 |
| Fatigue | |||||
| Inactivated | 88/3754 | 12/1134 | 0.85 | 0.55; 1.29 | 0.0 |
| mRNA | 2126/38365 | 194/37591 | 1.95 | 1.52; 2.51 | 99.0 |
| Vector | 22/1429 | 18/916 | 1.90 | 0.55; 6.55 | 94.7 |
| Protein Subunit | 356/832 | 58/220 | 1.65 | 1.31; 2.08 | 0.0 |
| DNA | 2/1371 | 4/1410 | 0.30 | 0.06; 1.44 | 0.0 |
| Headache | |||||
| Inactivated | 30/3154 | 12/984 | 0.97 | 0.45; 2.07 | 0.0 |
| mRNA | 2126/38365 | 194/37591 | 1.89 | 1.20; 2.62 | 98.9 |
| Vector | 22/1429 | 18/916 | 0.99 | 0.48; 2.05 | 95.2 |
| Protein | 277/832 | 53/220 | 1.36 | 1.06; 1.75 | 0.0 |
| DNA | 5/1371 | 5/1410 | 1.31 | 0.35; 4.95 | 0.0 |
RR risk ratios, CI confidence interval.
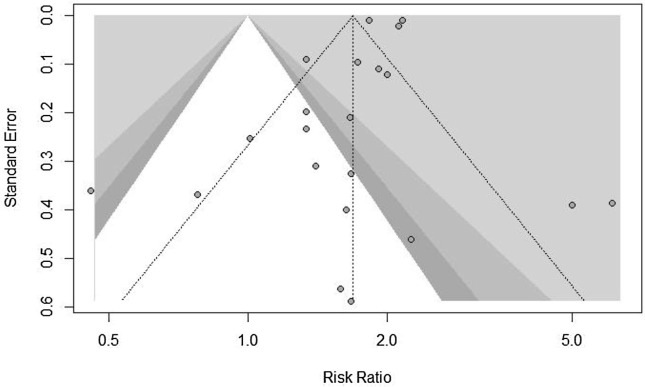
A high level of heterogeneity was observed for the group of mRNA vaccines in the meta-analyses of total adverse reactions (I2 = 98%), systemic adverse reactions (I2 = 100%), and local adverse reactions (I2 = 97%). Fig. 5 also shows the funnel plot of included studies. The plot is symmetric which is considered as negative publication bias.
Fig. 5.
the funnel plot of included stydies.
4. Discussion
In the current meta-analysis on the adverse events following COVID-19 immunization, we found different vaccine modalities are different regarding the level of adverse events contributing to immunization. mRNA vaccines are associated with a greater risk of any adverse events and local adverse events following immunization. Protein subunit vaccines are associated with the greatest risk of systemic adverse events followed by mRNA vaccines. In contrast, inactivated vaccines have the least RR for any adverse events or systemic adverse events following immunization. In addition, fever, fatigue and headache were the most reported systemic adverse events. Pain, swelling and, redness at the site of injection were the most reported local adverse events.
Numerous people around the world are getting vaccinated for COVID-19. This mass vaccination has led to numerous concerns in the public about the safety of these new vaccines. In response to these concerns, immunization safety surveillance systems, such as the US Vaccine Adverse Event Reporting System (VAERS) and China National adverse events following immunization Information System (CNAEFIS) [37], were launched at national or international levels to ensure effective monitoring of adverse events after COVID-19 vaccination.
Adverse reactions following immunization may be any undesirable sign or symptom of disease and laboratory findings [38], [39] including local and systemic adverse reactions. Vaccines that present perfect efficacy against specific pathogens and induce limited or no adverse reactions are the ideal vaccines. However, practically and in clinical settings, there is a chance that all vaccines induce some undesirable side effects which do not necessarily have a causal relationship with vaccination. The exact mechanism of adverse reactions following immunization is not clear in most cases and may be due to the component of the vaccines such as adjuvant, stabilizers or preservatives [40]. Adverse events following immunization for COVID-19 are mostly mild, similar to other previous vaccines. Severe adverse events (SAEs) are rare, and usually were reported in some cases as hypersensitivity, acute allergic reactions, urticaria and anaphylactic shock [41]. Acute allergic reactions following immunization might be due to vaccine antigen, preservatives and stabilizers in the vaccine formulation or residual nonhuman protein. Yet local adverse reactions may be commonly associated with IgE-mediated reactions and the active antigen in the vaccine [42].
Vaccine-induced anaphylaxis shock is a rare adverse event, and for most known and previous vaccines occurs in approximately one case per million injections [43]. However, the incidence of Vaccine-induced anaphylaxis with the COVID-19 mRNA vaccines (including Pfizer-BioNtech and Moderna) was reported to be approximately ten times higher than that reported in previous vaccines [41]. The mechanism of this adverse reaction is still to be studied. Although it is suggested that some people are at a higher risk for non-IgE related mast-cell activation or complement activation due to some inactive components or products of the vaccine manufacturing process of the vaccines such as lipid or the polyethylene glycol (PEG)-lipid component [41].
The mRNA vaccines use a lipid-based nanoparticle carrier system that prevents the rapid degradation of mRNA in vivo delivery. This carrier system is further stabilized by a PEG 2000 lipid conjugate that supplies a hydrophilic layer which prolongs the half-life [44], [45]. The Pfizer–BioNtech and Moderna vaccines are the first mRNA vaccines to receive an Emergency Use Authorization (EUA). therefore, there is no prior experience to explain the mechanism of allergic reactions associated with mRNA vaccines.
Very few and sporadic cases of Bell’s palsy following immunization with mRNA vaccines (Pfizer-BioNtech and Moderna) were also reported in clinical trials [18], [46]. However, there is no evidence that these cases are causally related to mRNA vaccination and follow-up for long-term safety of mRNA vaccines is needed [47], [48]. Nevertheless, RNA-based vaccines have the greatest efficacy among all types of vaccine modalities until now. The efficacy had reached greater than 94%, due to their effective presentation of SARS-CoV-2 antigens to the immune system and strong immunogenicity [18], [24]. Besides, RNA-based vaccines may be more effective against mutant strains of SARS-CoV-2. As many of the adverse events following immunization with mRNA vaccines are mild and transient, RNA vaccines should be considered an excellent option to protect against COVID-19.
Based on the current literature, several systematic review and meta-analysis studies, regarding the efficacy and safety of COVID-19 vaccines, have been recently conducted and pulished. A meta-analysis of 12 clinical trials have reported that the most common adverse event was fatigue and heache, similar to our study [49]. The findings of other studies show similar results and reported heacache, fatigue, and myalgia as the most common systemic adverse events after injection and pain, erythema, and swelling at the injection site as the most common local adverse event [50], [51], [52]. It is also reported that the amount of reactiogencity and adverse events is higher in particpinats who were injected with RNA-based vaccines compared to other types [50]. It should also be noted that none of these studies reported severe adverse events due to COVID-19 vaccination. Hence, as the current literatue and findings of this study suggest, mild and short adverse events such as pain, erythema, swelling, heacache, fever, fatigue, myalgia, and in some cases diarhea and arthralgia can be expreicned due to the onset of active immune responses, however, these responses are generally mild and are usually experienced for short durations. Hence, based on the high reported efficacy of vaccination in reducing the risk of hospitalization, ICU admission, ventilation, and death, vaccination should be continued and sudden onset of the aforementioned adverse events should be expected [49], [50], [52], [53].
Our study has some limitations and the result of this study should be interpreted with caution. First, heterogeneity in some vaccine subgroups was high, although we tried to decrease it by doing subgroup analyses. Second, subgroup analysis was not feasible for some single adverse events. Third, the sample size for viral-vector vaccines and protein subunit vaccines was very lower than mRNA and inactivated vaccines. Last but not least, we only included safety analysis in this study and we did not include efficacy analysis, and efficacy is as important as safety (if not more important) in making decisions about the right vaccine modality. The safety profiles of COVID-19 vaccines, even those currently in use, are still incomplete and studies about the safety of vaccines are still ongoing. Besides, the safety and efficacy of COVID-19 vaccines in certain subgroups of population, such as children, pregnant women, and people with underlying conditions, are remained to be studied.
Safety issues noted in mass vaccination may impend the global immunization program. However, the benefits of all types of vaccines studied in this article, still outweigh the risks at the present moment. Government agencies and decision-makers should continue to encourage vaccination and reduce public vaccine hesitancy.
5. Conclusion
In conclusion, mRNA vaccines are associated with a greater risk of adverse events following vaccination, but at the same time greater efficacy these vaccines showed in clinical trials. At the present moment the benefits of all types of vaccines approved by WHO, still outweigh the risks of them and vaccination if available is highly recommended.
6. Authors’ contributions
HK, and HA drafted the manuscript, statistical analysis, and interpretation of data. All authors contributed intellectually to the data interpretation and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Zhu N.a., Zhang D., Wang W., Li X., Yang B.o., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in china, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Weekly epidemiological update—9 March 2021.https://www.who.int/publications/m/item/weekly-epidemiological-update---10-march-2021. Accessed 15 Mar 2021.
- 3.Pak A., et al. Economic Consequences of the COVID-19 Outbreak: the Need for Epidemic Preparedness. Front Public Health. 2020;8:241. doi: 10.3389/fpubh.2020.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang A.Y., Cullen M.R., Harrington R.A., Barry M. The impact of novel coronavirus COVID-19 on noncommunicable disease patients and health systems: a review. J Intern Med. 2021;289(4):450–462. doi: 10.1111/joim.13184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ansari M., et al. Discrimination in COVID-19 vaccination programs - a possible risk for mental health. Asian J. Psychiatr. 2021;63 doi: 10.1016/j.ajp.2021.102758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tabatabaee, M., et al., Loneliness in the presence of others: A mixed-method study of social networks of caregivers of patients with severe mental disorders. International Journal of Social Psychiatry, 2022: p. 00207640221077580. [DOI] [PubMed]
- 7.Shoaee, S., et al., Experiences from the management of COVID-19 pandemic in a nursing home in Iran (March-April, 2020). J Diabetes Metab Disord, 2022: p. 1-5. [DOI] [PMC free article] [PubMed]
- 8.Sofi-Mahmudi A., Masinaei M., Shamsoddin E., Tovani-Palone M.R., Heydari M.-H., Shoaee S., Ghasemi E., Azadnajafabad S., Roshani S., Rezaei N., Rashidi M.-M., Kalantar Mehrjardi R., Hajebi A.A., Larijani B., Farzadfar F. Global, regional, and national burden and quality of care index (QCI) of lip and oral cavity cancer: a systematic analysis of the Global Burden of Disease Study 1990–2017. BMC Oral Health. 2021;21(1) doi: 10.1186/s12903-021-01918-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sultana J., Mazzaglia G., Luxi N., Cancellieri A., Capuano A., Ferrajolo C., de Waure C., Ferlazzo G., Trifirò G. Potential effects of vaccinations on the prevention of COVID-19: rationale, clinical evidence, risks, and public health considerations. Expert Rev. Vaccines. 2020;19(10):919–936. doi: 10.1080/14760584.2020.1825951. [DOI] [PubMed] [Google Scholar]
- 10.Zacchigna S., Marcello A., Banks L. Spotlight on COVID-19: from biology to therapy and prevention. Febs j. 2020;287(17):3606–3608. doi: 10.1111/febs.15530. [DOI] [PubMed] [Google Scholar]
- 11.Bielecki M., et al. Air travel and COVID-19 prevention in the pandemic and peri-pandemic period: a narrative review. Travel Med. Infect. Dis. 2021;39 doi: 10.1016/j.tmaid.2020.101915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.BIO, S., Status of COVID-19 Vaccines within WHO EUL/PQ evaluation process. 2021.
- 13.Cai C., Peng Y., Shen E., Huang Q., Chen Y., Liu P., Guo C., Feng Z., Gao L.e., Zhang X., Gao Y., Liu Y., Han Y., Zeng S., Shen H. A comprehensive analysis of the efficacy and safety of COVID-19 vaccines. Mol. Ther. 2021;29(9):2794–2805. doi: 10.1016/j.ymthe.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen M., Li Y., Chen J., Wen Z., Feng F., Zou H., Fu C., Chen L., Shu Y., Sun C. An online survey of the attitude and willingness of Chinese adults to receive COVID-19 vaccination. Hum Vaccin Immunother. 2021;17(7):2279–2288. doi: 10.1080/21645515.2020.1853449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwarzer G., Carpenter J.R., Rücker G. Meta-analysis with R. 2015;Vol. 4784:Springer. [Google Scholar]
- 17.DerSimonian R., Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemporary clinical trials. 2007;28(2):105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Che Y., et al. Randomized, double-blinded and placebo-controlled phase II trial of an inactivated SARS-CoV-2 vaccine in healthy adults. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., Bellamy D., Bibi S., Bittaye M., Clutterbuck E.A., Dold C., Faust S.N., Finn A., Flaxman A.L., Hallis B., Heath P., Jenkin D., Lazarus R., Makinson R., Minassian A.M., Pollock K.M., Ramasamy M., Robinson H., Snape M., Tarrant R., Voysey M., Green C., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Pollard A.J., Aboagye J., Adams K., Ali A., Allen E., Allison J.L., Anslow R., Arbe-Barnes E.H., Babbage G., Baillie K., Baker M., Baker N., Baker P., Baleanu I., Ballaminut J., Barnes E., Barrett J., Bates L., Batten A., Beadon K., Beckley R., Berrie E., Berry L., Beveridge A., Bewley K.R., Bijker E.M., Bingham T., Blackwell L., Blundell C.L., Bolam E., Boland E., Borthwick N., Bower T., Boyd A., Brenner T., Bright P.D., Brown-O'Sullivan C., Brunt E., Burbage J., Burge S., Buttigieg K.R., Byard N., Cabera Puig I., Calvert A., Camara S., Cao M., Cappuccini F., Carr M., Carroll M.W., Carter V., Cathie K., Challis R.J., Charlton S., Chelysheva I., Cho J.-S., Cicconi P., Cifuentes L., Clark H., Clark E., Cole T., Colin-Jones R., Conlon C.P., Cook A., Coombes N.S., Cooper R., Cosgrove C.A., Coy K., Crocker W.E.M., Cunningham C.J., Damratoski B.E., Dando L., Datoo M.S., Davies H., De Graaf H., Demissie T., Di Maso C., Dietrich I., Dong T., Donnellan F.R., Douglas N., Downing C., Drake J., Drake-Brockman R., Drury R.E., Dunachie S.J., Edwards N.J., Edwards F.D.L., Edwards C.J., Elias S.C., Elmore M.J., Emary K.R.W., English M.R., Fagerbrink S., Felle S., Feng S., Field S., Fixmer C., Fletcher C., Ford K.J., Fowler J., Fox P., Francis E., Frater J., Furze J., Fuskova M., Galiza E., Gbesemete D., Gilbride C., Godwin K., Gorini G., Goulston L., Grabau C., Gracie L., Gray Z., Guthrie L.B., Hackett M., Halwe S., Hamilton E., Hamlyn J., Hanumunthadu B., Harding I., Harris S.A., Harris A., Harrison D., Harrison C., Hart T.C., Haskell L., Hawkins S., Head I., Henry J.A., Hill J., Hodgson S.H.C., Hou M.M., Howe E., Howell N., Hutlin C., Ikram S., Isitt C., Iveson P., Jackson S., Jackson F., James S.W., Jenkins M., Jones E., Jones K., Jones C.E., Jones B., Kailath R., Karampatsas K., Keen J., Kelly S., Kelly D., Kerr D., Kerridge S., Khan L., Khan U., Killen A., Kinch J., King T.B., King L., King J., Kingham-Page L., Klenerman P., Knapper F., Knight J.C., Knott D., Koleva S., Kupke A., Larkworthy C.W., Larwood J.P.J., Laskey A., Lawrie A.M., Lee A., Ngan Lee K.Y., Lees E.A., Legge H., Lelliott A., Lemm N.-M., Lias A.M., Linder A., Lipworth S., Liu X., Liu S., Lopez Ramon R., Lwin M., Mabesa F., Madhavan M., Mallett G., Mansatta K., Marcal I., Marinou S., Marlow E., Marshall J.L., Martin J., McEwan J., McInroy L., Meddaugh G., Mentzer A.J., Mirtorabi N., Moore M., Moran E., Morey E., Morgan V., Morris S.J., Morrison H., Morshead G., Morter R., Mujadidi Y.F., Muller J., Munera-Huertas T., Munro C., Munro A., Murphy S., Munster V.J., Mweu P., Noé A., Nugent F.L., Nuthall E., O'Brien K., O'Connor D., Oguti B., Oliver J.L., Oliveira C., O'Reilly P.J., Osborn M., Osborne P., Owen C., Owens D., Owino N., Pacurar M., Parker K., Parracho H., Patrick-Smith M., Payne V., Pearce J., Peng Y., Peralta Alvarez M.P., Perring J., Pfafferott K., Pipini D., Plested E., Pluess-Hall H., Pollock K., Poulton I., Presland L., Provstgaard-Morys S., Pulido D., Radia K., Ramos Lopez F., Rand J., Ratcliffe H., Rawlinson T., Rhead S., Riddell A., Ritchie A.J., Roberts H., Robson J., Roche S., Rohde C., Rollier C.S., Romani R., Rudiansyah I., Saich S., Sajjad S., Salvador S., Sanchez Riera L., Sanders H., Sanders K., Sapaun S., Sayce C., Schofield E., Screaton G., Selby B., Semple C., Sharpe H.R., Shaik I., Shea A., Shelton H., Silk S., Silva-Reyes L., Skelly D.T., Smee H., Smith C.C., Smith D.J., Song R., Spencer A.J., Stafford E., Steele A., Stefanova E., Stockdale L., Szigeti A., Tahiri-Alaoui A., Tait M., Talbot H., Tanner R., Taylor I.J., Taylor V., Te Water Naude R., Thakur N., Themistocleous Y., Themistocleous A., Thomas M., Thomas T.M., Thompson A., Thomson-Hill S., Tomlins J., Tonks S., Towner J., Tran N., Tree J.A., Truby A., Turkentine K., Turner C., Turner N., Turner S., Tuthill T., Ulaszewska M., Varughese R., Van Doremalen N., Veighey K., Verheul M.K., Vichos I., Vitale E., Walker L., Watson M.E.E., Welham B., Wheat J., White C., White R., Worth A.T., Wright D., Wright S., Yao X.L., Yau Y. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keech C., Albert G., Cho I., Robertson A., Reed P., Neal S., Plested J.S., Zhu M., Cloney-Clark S., Zhou H., Smith G., Patel N., Frieman M.B., Haupt R.E., Logue J., McGrath M., Weston S., Piedra P.A., Desai C., Callahan K., Lewis M., Price-Abbott P., Formica N., Shinde V., Fries L., Lickliter J.D., Griffin P., Wilkinson B., Glenn G.M. Phase 1–2 Trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N. Engl. J. Med. 2020;383(24):2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kremsner P.G., Mann P., Kroidl A., Leroux-Roels I., Schindler C., Gabor J.J., Schunk M., Leroux-Roels G., Bosch J.J., Fendel R., Kreidenweiss A., Velavan T.P., Fotin-Mleczek M., Mueller S.O., Quintini G., Schönborn‑Kellenberger O., Vahrenhorst D., Verstraeten T., Alves de Mesquita M., Walz L., Wolz O., Oostvogels L., De Boever F., Desimpel A., Esen M., Fischer I., Flügge J., Geisenberger O., Geldmacher C., Held K., Hoffmann L., Hölscher M., Huber K., Jacobs B., Joye J., Kirschke J., Klopp N., Koehne E., Köhler C., Lalremruata A., Lamsfus-Calle C., Linh L.T.K., Maes C., Metaxa D., Molnar M.-L., Mueller M., Müller-Schöner G., Quindel M., Rappe S., Schultze-Naumburg L., Schumacher C., Schuster S., Thiel V., Vejda S., Waerlop G., Westenberg C., Wons K., Zeder A. Safety and immunogenicity of an mRNA-lipid nanoparticle vaccine candidate against SARS-CoV-2: A phase 1 randomized clinical trial. Wien Klin. Wochenschr. 2021;133(17-18):931–941. doi: 10.1007/s00508-021-01922-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Raabe V., Bailey R., Swanson K.A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.-Y., Türeci Ö., Tompkins K.R., Walsh E.E., Frenck R., Falsey A.R., Dormitzer P.R., Gruber W.C., Şahin U., Jansen K.U. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586(7830):589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 24.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., Voysey M., Aley P.K., Angus B., Babbage G., Belij-Rammerstorfer S., Berry L., Bibi S., Bittaye M., Cathie K., Chappell H., Charlton S., Cicconi P., Clutterbuck E.A., Colin-Jones R., Dold C., Emary K.R.W., Fedosyuk S., Fuskova M., Gbesemete D., Green C., Hallis B., Hou M.M., Jenkin D., Joe C.C.D., Kelly E.J., Kerridge S., Lawrie A.M., Lelliott A., Lwin M.N., Makinson R., Marchevsky N.G., Mujadidi Y., Munro A.P.S., Pacurar M., Plested E., Rand J., Rawlinson T., Rhead S., Robinson H., Ritchie A.J., Ross-Russell A.L., Saich S., Singh N., Smith C.C., Snape M.D., Song R., Tarrant R., Themistocleous Y., Thomas K.M., Villafana T.L., Warren S.C., Watson M.E.E., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Faust S.N., Pollard A.J., Aboagye J., Adams K., Ali A., Allen E.R., Allen L., Allison J.L., Andritsou F., Anslow R., Arbe-Barnes E.H., Baker M., Baker N., Baker P., Baleanu I., Barker D., Barnes E., Barrett J.R., Barrett K., Bates L., Batten A., Beadon K., Beckley R., Bellamy D., Berg A., Bermejo L., Berrie E., Beveridge A., Bewley K., Bijker E.M., Birch G., Blackwell L., Bletchly H., Blundell C.L., Blundell S.R., Bolam E., Boland E., Bormans D., Borthwick N., Boukas K., Bower T., Bowring F., Boyd A., Brenner T., Brown P., Brown-O'Sullivan C., Bruce S., Brunt E., Burbage J., Burgoyne J., Buttigieg K.R., Byard N., Cabera Puig I., Camara S., Cao M., Cappuccini F., Carr M., Carroll M.W., Cashen P., Cavey A., Chadwick J., Challis R., Chapman D., Charles D., Chelysheva I., Cho J.-S., Cifuentes L., Clark E., Collins S., Conlon C.P., Coombes N.S., Cooper R., Cooper C., Crocker W.E.M., Crosbie S., Cullen D., Cunningham C., Cuthbertson F., Datoo B.E., Dando L., Datoo M.S., Datta C., Davies H., Davies S., Davis E.J., Davis J., Dearlove D., Demissie T., Di Marco S., Di Maso C., DiTirro D., Docksey C., Dong T., Donnellan F.R., Douglas N., Downing C., Drake J., Drake-Brockman R., Drury R.E., Dunachie S.J., Edwards C.J., Edwards N.J., El Muhanna O., Elias S.C., Elliott R.S., Elmore M.J., English M.R., Felle S., Feng S., Ferreira Da Silva C., Field S., Fisher R., Fixmer C., Ford K.J., Fowler J., Francis E., Frater J., Furze J., Galian-Rubio P., Galloway C., Garlant H., Gavrila M., Gibbons F., Gibbons K., Gilbride C., Gill H., Godwin K., Gordon-Quayle K., Gorini G., Goulston L., Grabau C., Gracie L., Graham N., Greenwood N., Griffiths O., Gupta G., Hamilton E., Hanumunthadu B., Harris S.A., Harris T., Harrison D., Hart T.C., Hartnell B., Haskell L., Hawkins S., Henry J.A., Hermosin Herrera M., Hill D., Hill J., Hodges G., Hodgson S.H.C., Horton K.L., Howe E., Howell N., Howes J., Huang B., Humphreys J., Humphries H.E., Iveson P., Jackson F., Jackson S., Jauregui S., Jeffers H., Jones B., Jones C.E., Jones E., Jones K., Joshi A., Kailath R., Keen J., Kelly D.M., Kelly S., Kelly D., Kerr D., Khan L., Khozoee B., Killen A., Kinch J., King L.D.W., King T.B., Kingham L., Klenerman P., Knight J.C., Knott D., Koleva S., Lang G., Larkworthy C.W., Larwood J.P.J., Law R., Lee A., Lee K.Y.N., Lees E.A., Leung S., Li Y., Lias A.M., Linder A., Lipworth S., Liu S., Liu X., Lloyd S., Loew L., Lopez Ramon R., Madhavan M., Mainwaring D.O., Mallett G., Mansatta K., Marinou S., Marius P., Marlow E., Marriott P., Marshall J.L., Martin J., Masters S., McEwan J., McGlashan J.L., McInroy L., McRobert N., Megson C., Mentzer A.J., Mirtorabi N., Mitton C., Moore M., Moran M., Morey E., Morgans R., Morris S.J., Morrison H.M., Morshead G., Morter R., Moya N.A., Mukhopadhyay E., Muller J., Munro C., Murphy S., Mweu P., Noé A., Nugent F.L., O'Brien K., O'Connor D., Oguti B., Olchawski V., Oliveira C., O'Reilly P.J., Osborne P., Owen L., Owino N., Papageorgiou P., Parracho H., Parsons K., Patel B., Patrick-Smith M., Peng Y., Penn E.J., Peralta-Alvarez M.P., Perring J., Petropoulos C., Phillips D.J., Pipini D., Pollard S., Poulton I., Pratt D., Presland L., Proud P.C., Provstgaard-Morys S., Pueschel S., Pulido D., Rabara R., Radia K., Rajapaska D., Ramos Lopez F., Ratcliffe H., Rayhan S., Rees B., Reyes Pabon E., Roberts H., Robertson I., Roche S., Rollier C.S., Romani R., Rose Z., Rudiansyah I., Sabheha S., Salvador S., Sanders H., Sanders K., Satti I., Sayce C., Schmid A.B., Schofield E., Screaton G., Sedik C., Seddiqi S., Segireddy R.R., Selby B., Shaik I., Sharpe H.R., Shaw R., Shea A., Silk S., Silva-Reyes L., Skelly D.T., Smith D.J., Smith D.C., Smith N., Spencer A.J., Spoors L., Stafford E., Stamford I., Stockdale L., Stockley D., Stockwell L.V., Stokes M., Strickland L.H., Stuart A., Sulaiman S., Summerton E., Swash Z., Szigeti A., Tahiri-Alaoui A., Tanner R., Taylor I., Taylor K., Taylor U., te Water Naude R., Themistocleous A., Thomas M., Thomas T.M., Thompson A., Thompson K., Thornton-Jones V., Tinh L., Tomic A., Tonks S., Towner J., Tran N., Tree J.A., Truby A., Turner C., Turner R., Ulaszewska M., Varughese R., Verbart D., Verheul M.K., Vichos I., Walker L., Wand M.E., Watkins B., Welch J., West A.J., White C., White R., Williams P., Woodyer M., Worth A.T., Wright D., Wrin T., Yao X.L., Zbarcea D.-A., Zizi D. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. The Lancet. 2020;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richmond P., Hatchuel L., Dong M., Ma B., Hu B., Smolenov I., Li P., Liang P., Han H.H., Liang J., Clemens R. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397(10275):682–694. doi: 10.1016/S0140-6736(21)00241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh E.E., Frenck R.W., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., Swanson K.A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.-Y., Türeci Ö., Tompkins K.R., Lyke K.E., Raabe V., Dormitzer P.R., Jansen K.U., Şahin U., Gruber W.C. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N. Engl. J. Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Z., Hu Y., Xu M., Chen Z., Yang W., Jiang Z., Li M., Jin H., Cui G., Chen P., Wang L., Zhao G., Ding Y., Zhao Y., Yin W. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(6):803–812. doi: 10.1016/S1473-3099(20)30987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia S., Duan K., Zhang Y., Zhao D., Zhang H., Xie Z., Li X., Peng C., Zhang Y., Zhang W., Yang Y., Chen W., Gao X., You W., Wang X., Wang Z., Shi Z., Wang Y., Yang X., Zhang L., Huang L., Wang Q., Lu J., Yang Y., Guo J., Zhou W., Wan X., Wu C., Wang W., Huang S., Du J., Meng Z., Pan A.n., Yuan Z., Shen S., Guo W., Yang X. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324(10):951. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia S., Zhang Y., Wang Y., Wang H., Yang Y., Gao G.F., Tan W., Wu G., Xu M., Lou Z., Huang W., Xu W., Huang B., Wang H., Wang W., Zhang W., Li N.a., Xie Z., Ding L., You W., Zhao Y., Yang X., Liu Y., Wang Q., Huang L., Yang Y., Xu G., Luo B., Wang W., Liu P., Guo W., Yang X. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021;21(1):39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia S., Zhang Y., Wang Y., Wang H., Yang Y., Gao G.F., Tan W., Wu G., Xu M., Lou Z., Huang W., Xu W., Huang B., Wang W., Zhang W., Li N.a., Xie Z., Zhu X., Ding L., You W., Zhao Y., Zhao J., Huang L., Shi X., Yang Y., Xu G., Wang W., Liu P., Ma M., Qiao Y., Zhao S., Chai J., Li Q., Fu H., Xu Y., Zheng X., Guo W., Yang X. Safety and immunogenicity of an inactivated COVID-19 vaccine, BBIBP-CorV, in people younger than 18 years: a randomised, double-blind, controlled, phase 1/2 trial. Lancet Infect. Dis. 2022;22(2):196–208. doi: 10.1016/S1473-3099(21)00462-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., Han W., Chen Z., Tang R., Yin W., Chen X., Hu Y., Liu X., Jiang C., Li J., Yang M., Song Y., Wang X., Gao Q., Zhu F. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu F.-C., Guan X.-H., Li Y.-H., Huang J.-Y., Jiang T., Hou L.-H., Li J.-X., Yang B.-F., Wang L., Wang W.-J., Wu S.-P., Wang Z., Wu X.-H., Xu J.-J., Zhang Z., Jia S.-Y., Wang B.-S., Hu Y.i., Liu J.-J., Zhang J., Qian X.-A., Li Q., Pan H.-X., Jiang H.-D., Deng P., Gou J.-B., Wang X.-W., Wang X.-H., Chen W. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khobragade A., Bhate S., Ramaiah V., Deshpande S., Giri K., Phophle H., Supe P., Godara I., Revanna R., Nagarkar R., Sanmukhani J., Dey A., Rajanathan T.M.C., Kansagra K., Koradia P. Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): the interim efficacy results of a phase 3, randomised, double-blind, placebo-controlled study in India. Lancet. 2022;399(10332):1313–1321. doi: 10.1016/S0140-6736(22)00151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masuda T., Murakami K., Sugiura K., Sakui S., Schuring R.P., Mori M. Safety and immunogenicity of NVX-CoV2373 (TAK-019) vaccine in healthy Japanese adults: Interim report of a phase I/II randomized controlled trial. Vaccine. 2022;40(24):3380–3388. doi: 10.1016/j.vaccine.2022.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tabarsi P., Anjidani N., Shahpari R., Mardani M., Sabzvari A., Yazdani B., Roshanzamir K., Bayatani B., Taheri A., Petrovsky N., Li L., Barati S. Safety and immunogenicity of SpikoGen®, an advax-cpg55.2-adjuvanted sars-cov-2 spike protein vaccine: a phase 2 randomized placebo-controlled trial in both seropositive and seronegative populations. Clin. Microbiol. Infect. 2022 doi: 10.1016/j.cmi.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimabukuro T.T., Nguyen M., Martin D., DeStefano F. Safety monitoring in the vaccine adverse event reporting system (VAERS) Vaccine. 2015;33(36):4398–4405. doi: 10.1016/j.vaccine.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO. Immunization safety surveillance: guidelines for immunization programme managers on surveillance of adverse events following immunization. 3rd edn.https://iris.wpro.who.int/handle/10665.1/12620. Accessed 4 Mar 2021.
- 39.Rostam-Abadi Y., et al. Public health risks associated with methadone in Iran: a systematic review and meta-analysis. Int. J. Drug Policy. 2022;100 doi: 10.1016/j.drugpo.2021.103529. [DOI] [PubMed] [Google Scholar]
- 40.Padda, I.S. and M. Parmar, COVID (SARS-COV-2) Vaccine, in StatPearls. 2021, StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.: Treasure Island (FL). [PubMed]
- 41.Longo D.L., Castells M.C., Phillips E.J. Maintaining safety with SARS-CoV-2 vaccines. N. Engl. J. Med. 2021;384(7):643–649. doi: 10.1056/NEJMra2035343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stone C.A., Liu Y., Relling M.V., Krantz M.S., Pratt A.L., Abreo A., Hemler J.A., Phillips E.J. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J. Allergy Clin. Immunol. Pract. 2019;7(5):1533–1540.e8. doi: 10.1016/j.jaip.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stone C.A., Rukasin C.R.F., Beachkofsky T.M., Phillips E.J. Immune-mediated adverse reactions to vaccines. Br. J. Clin. Pharmacol. 2019;85(12):2694–2706. doi: 10.1111/bcp.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hou X., Zaks T., Langer R., Dong Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021;6(12):1078–1094. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reichmuth A.M., Oberli M.A., Jaklenec A., Langer R., Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016;7(5):319–334. doi: 10.4155/tde-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.CDC. Interim clinical considerations for use of mRNA COVID-19 vaccines currently authorized in the United States. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html. Accessed 4 Mar 2021.
- 48.Ansari M., et al. Buprenorphine abuse and health risks in Iran: A systematic review. Drug Alcohol Depend. 2021;226 doi: 10.1016/j.drugalcdep.2021.108871. [DOI] [PubMed] [Google Scholar]
- 49.Haas J.W., Bender F.L., Ballou S., Kelley J.M., Wilhelm M., Miller F.G., Rief W., Kaptchuk T.J. Frequency of Adverse Events in the Placebo Arms of COVID-19 Vaccine Trials: A Systematic Review and Meta-analysis. JAMA Network Open. 2022;5(1):e2143955. doi: 10.1001/jamanetworkopen.2021.43955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pormohammad A., Zarei M., Ghorbani S., Mohammadi M., Razizadeh M.H., Turner D.L., Turner R.J. Efficacy and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Vaccines (Basel) 2021;9(5):467. doi: 10.3390/vaccines9050467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen J., Cai Y., Chen Y., Williams A.P., Gao Y., Zeng J. Nervous and Muscular Adverse Events after COVID-19 Vaccination: A Systematic Review and Meta-Analysis of Clinical Trials. Vaccines (Basel) 2021;9(8):939. doi: 10.3390/vaccines9080939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Q., Qin C., Liu M., Liu J. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis. Infect. Dis. Poverty. 2021;10(1) doi: 10.1186/s40249-021-00915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen M., Yuan Y., Zhou Y., Deng Z., Zhao J., Feng F., Zou H., Sun C. Safety of SARS-CoV-2 vaccines: a systematic review and meta-analysis of randomized controlled trials. Infect Dis. Poverty. 2021;10(1) doi: 10.1186/s40249-021-00878-5. [DOI] [PMC free article] [PubMed] [Google Scholar]