Abstract
Objective
To study whether exposure to childhood emotional, sexual or physical abuse is associated with subsequent multiple sclerosis (MS) development.
Methods
A nationwide, prospective cohort study based on participants in the Norwegian Mother, Father and Child cohort study. Enrolment took place 1999–2008, with follow-up until 31 December 2018. Childhood abuse before age 18 years was obtained from self-completed questionnaires. We identified MS diagnoses through data-linkage with national health registries and hospital records. The Cox model was used to estimate HRs for MS with 95% CIs, adjusting for confounders and mediators.
Results
In this prospective cohort study, 14 477 women were exposed to childhood abuse and 63 520 were unexposed. 300 women developed MS during the follow-up period. 71 of these (24%) reported a history of childhood abuse, compared with 14 406 of 77 697 (19%) women that did not develop MS. Sexual abuse (HR 1.65, 95% CI 1.13 to 2.39) and emotional abuse (HR 1.40, 95% CI 1.03 to 1.90) in childhood were both associated with an increased risk of developing MS. The HR of MS after exposure to physical abuse was 1.31 (95% CI 0.83 to 2.06). The risk of MS was further increased if exposed to two (HR 1.66, 95% CI 1.04 to 2.67) or all three abuse categories (HR 1.93, 95% CI 1.02 to 3.67).
Interpretation
Childhood sexual and emotional abuse were associated with an increased risk of developing MS. The risk was higher when exposed to several abuse categories, indicating a dose–response relationship. Further studies are needed to identify underlying mechanisms.
Keywords: multiple sclerosis; trauma, psychol seque
Key messages.
What is already known on this topic
Trauma in childhood and adolescence can alter the immune system and may increase the risk of autoimmune disorders. Whether stress and adverse events in childhood can have an impact on multiple sclerosis (MS) susceptibility is not known.
What this study adds
Women with exposure to adverse childhood experiences had increased risk of developing MS. This association was most pronounced for sexual abuse and for the combination of several categories of abuse.
How this study might affect research, practice or policy
These results open doors for prevention and insight to disease mechanisms.
Introduction
Trauma and stressful life events have been associated with an increased risk of autoimmune disorders.1 Any impact of stress on multiple sclerosis (MS) is debated,2 but a recent population-based study from Sweden with 2930 MS cases indicated a link between major stressors in adult life, such as loss of a loved one, divorce or personal conflict and subsequent MS disease.3 Adverse childhood experiences such as abuse, neglect and household dysfunction are extreme types of stress, and increase the risk of psychiatric and physical disorders in adulthood,4 including cardiovascular disease, cancer and autoimmune disease.5
Whether adverse events in childhood can have an impact on MS susceptibility is not known. A Danish population-based study found a 13% increased risk of developing MS if exposed to parental divorce,6 but they were unable to adjust for associated lifestyle changes such as smoking and obesity. Few have studied the association between childhood abuse and MS, and these studies were not prospective and arrived at different conclusions.7 8
Some of the most consistent environmental risk factors for MS, including low vitamin D levels, low sun exposure, Epstein-Barr virus infection and obesity seem to have critical periods of susceptibility for MS in childhood and particularly, adolescence.9–11 Exposure to tobacco smoke at a young age may also have an impact.12 13 Better understanding of risk factors and timing of risk exposures, may open doors for prevention and give further insight to disease mechanisms.
Our aim was to investigate whether adverse childhood experiences may contribute to the risk of MS. In this prospective and population-based study, we assessed the association between exposure to childhood emotional, sexual and physical abuse and the risk of developing MS, examining nationwide data from a prospective cohort study in combination with health registries and hospital records.
Methods
Study design and population
We conducted a national, prospective cohort study using the Norwegian Mother, Father and Child cohort (MoBa). The MoBa study included pregnant Norwegian-speaking women from all over Norway in 1999–2008,14 and 41% of the invited women consented to participation. There were no exclusion criteria, and the follow-up is ongoing. The MoBa cohort is linked to The Medical Birth Registry (MBRN), which is a national health registry containing information about all births in Norway. Information in the MBRN is registered by health personnel and registration is mandatory.
We acquired information on childhood adverse experiences and potential confounding and mediating factors at study baseline, which we defined as the year the women were enrolled in the MoBa study. The women completed self-administered questionnaires which included information on demographic and socioeconomic factors (pregnancy weeks 17–20) and history of any previous abuse (pregnancy week 30).
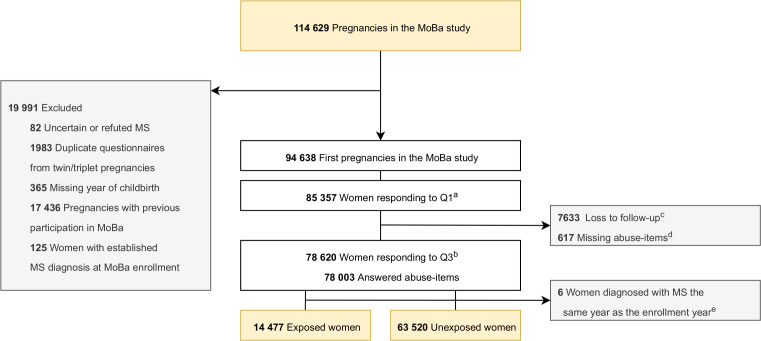
This study is based on version 12 of the MoBa data files, covering 114 629 pregnancies. We excluded duplicate questionnaires due to multiple gestations (n=1983) and recurrent participations in MoBa (n=17 436) to include only one observation per woman (figure 1). We also excluded women with refuted or unvalidated MS diagnosis (n=82), women who did not respond to the questionnaire in pregnancy week 30 including the abuse items, as well as women with missing year of childbirth. Women with an established MS diagnosis at study baseline were excluded to avoid a potential recall bias (n=125). Women who received the MS diagnosis the same year as they were enrolled in the study (observation time=0 years) were not eligible to be included in the time-to-event analysis and thus excluded (n=6).
Figure 1.
Flow chart of included and excluded study participants. aThe first questionnaire (Q1) in the MoBA study was sent out to participants in pregnancy week 18. bThe third questionnaire (Q3) in the MobA study was sent out to participants in pregnancy week 30. cLost to follow-up from pregnancy week 18 to pregnancy week 30. A total of 896 women responded to Q3 without responding to Q1, hence the difference of 6737 from Q1 to Q3. dA total of 617 of the women who responded to Q3 did not answer the abuse items. eThese women were not eligible for the time-to-event analysis since they had 0 observation years. MoBa, The Norwegian mother, father and Child cohort study; MS, multiple sclerosis; Q, Questionnaire.
Outcome measure
Our primary outcome was development of MS. On 31 December 2018, we cross-linked the MoBa cohort with the Norwegian Multiple Sclerosis Registry and Biobank (MSR) and the Norwegian Patient Registry (NPR) to identify all women in MoBa who developed MS after baseline and to ensure validated diagnoses. The MSR had 60% national coverage of MS cases at the time of data-linkage, and we further linked the data to NPR to identify the remaining MS cases. After every consultation in specialist care, registration of diagnoses in NPR is mandatory for health practitioners. The MS diagnosis in NPR have a sensitivity of 97% and a positive predictive value 0.92.15 If the woman was registered in NPR with an MS diagnosis, but not in the MSR, we used hospital records to further validate the diagnosis using the 2017 diagnostic criteria for MS.16 We were able to refute incorrect MS diagnoses from the NPR based on the information from the hospital records. NPR-identified MS cases for whom we did not have access to the hospital records for validation, were excluded.
Exposure
A history of adverse childhood experiences before age 18 years was defined by four abuse items in the pregnancy week 30 questionnaire; humiliation (‘Has anyone over a long period of time systematically tried to subdue, degrade or humiliate you?’), threat (‘Has anyone threatened to hurt you or someone close to you?’), physical abuse (‘Have you been subjected to physical abuse?’) and sexual abuse (‘Have you been forced to do sexual actions?’). We merged the items on humiliation and threat into one category of emotional abuse. Exposure to either emotional, sexual, or physical abuse was defined as responding ‘yes, as a child <18 years’ to the respective category. We considered women who answered ‘no, never’ to the abuse items as non-exposed. The abuse questions in MoBa are adapted from the NorVold Abuse Questionnaire and modified into four screening items. The NorVold Abuse Questionnaire has previously been shown to have good reliability and validity.17
Covariables
MS-specific covariables were assessed from the Norwegian MS Registry and hospital records: Age at MS onset (defined as first clinical symptom), age at MS diagnosis and subtype of MS (relapsing-remitting, primary progressive or unspecified). Other covariables were acquired through the self-completed MoBa questionnaires or through linkage to the MBRN: Age at baseline, birth year, smoking (ever/never), body mass index (BMI) prior to pregnancy (<25/≥25 kg/m2), drop-out before or during high school (completed ≤9 years of elementary school). Adverse socioeconomic status in adulthood was defined as either having low household income (<60% of the study population median income in year of study baseline), being a non-cohabiting mother or short education (≤9 years of school). Depression at study baseline (during pregnancy) was measured by a validated short version of the Hopkins Symptom Checklist 2518 in pregnancy week 30.
Statistical analysis
For the time-to-event analysis, the observation period began at enrolment in MoBa (online supplemental figure 1). Time was measured in years from start of the observation period until year of MS diagnosis or end of study period (31 December 2018).
jnnp-2021-328700supp001.pdf (273.5KB, pdf)
We used Cox proportional-hazards models to measure the risk of MS after exposure to childhood abuse, estimating HRs and 95% CIs. Confidence intervals not including 1 were considered statistically significant. In addition to examining any childhood abuse, we separately examined the HRs for subtypes of abuse (emotional, sexual, physical) and severity of abuse (exposure to one, two or three subtypes). The models were stratified by the women’s birth year in groups and adjusted in a two-step approach for (1) possible confounders and (2) possible confounders and mediators.
We considered birth year and childhood social status19 as possible confounders and used early drop-out from school as a proxy for the latter. Birth year was taken into account as the incidence of child maltreatment probably has decreased during the last decades prior to inclusion in MoBa.20 Possible mediators were smoking, high BMI, and adverse socioeconomic status as an adult—factors associated with both childhood abuse4 21 and MS.11 22–24
The statistical models were checked for the proportional hazard assumption both by visual inspection and statistical test of the Schoenfeld residuals. We included birth year as a stratification factor in the Cox model, but no other variables violated the proportional hazard assumption.
Statistical analyses were performed using IBM SPSS Statistics V.26 and Stata V.16 (StataCorp).
Sensitivity analysis
Adolescents with preclinical MS disease activity may theoretically be affected in ways that increase the susceptibility of being exposed to abuse. To limit the possibility of reverse causality, we performed a sensitivity analysis excluding women that might have been in the prodromal phase of MS when exposed to abuse, that is, women with their first clinical symptom of MS before and including age 22 years (within 5 years after the end of the exposure window) (n=15) (online supplemental figure 2A).
To ensure that the exclusion of women that already had MS at the time of enrolment did not affect our results, we performed a sensitivity analysis comprising all women with MS in MoBa, both prevalent and incident cases (online supplemental figure 2B). In this sensitivity analysis, the observation period was calculated from age 18 years.
Results
We included 77 997 women from the MoBa cohort in our study and they contributed with a total of 1 010 926 person-years at risk (mean follow-up 13 years, IQR 11–15). A total of 14 477 women (19%) were exposed to adverse childhood experiences and 63 520 (81%) were unexposed (table 1). The women exposed to childhood abuse more often had a history of smoking, were overweight and had more depression at study baseline. During follow-up, 300 women developed MS of whom 71 (24%) reported a history of childhood abuse, compared with 14 406 (19%) among the 77 697 women who did not develop MS.
Table 1.
Background characteristics of the study population exposed and unexposed to childhood abuse
| Exposed n=14 477 |
Unexposed n=63 520 |
|
| Age at study baseline;* mean (SD) | 29 (5) | 30 (5) |
| Missing; n (%) | 0 (0) | 1 (<1) |
| Observation years;† median (IQR) | 13 (4) | 13 (4) |
| Adverse socioeconomic status‡; n (%) | 2349 (16) | 5787 (9) |
| Missing; n (%) | 187 (1) | 686 (1) |
| Low household income; n (%) | 1578 (11) | 3942 (6) |
| Maternal short education; n (%) | 514 (4) | 1036 (2) |
| Non-cohabiting mother; n (%) | 582 (4) | 1192 (2) |
| Ever smoker; n (%) | 8785 (61) | 30 745 (48) |
| Missing; n (%) | 207 (1) | 933 (2) |
| BMI ≥25; n (%) | 4963 (34) | 18 717 (30) |
| Missing; n (%) | 561 (4) | 2140 (3) |
| Depression at study baseline (pregnancy); n (%) | 2573 (18) | 4732 (8) |
| Missing; n (%) | 105 (<1) | 447 (<1) |
| Age at end of study§; mean (SD) | 42 (6) | 43 (5) |
| Missing; n (%) | 0 (0) | 1 (<1) |
| Age at MS diagnosis; mean (SD) | 36 (6) | 36 (5) |
| Missing; n (%) | 0 (0) | 0 (0) |
| Age at MS onset; mean (SD) | 33 (7) | 33 (6) |
| Missing; n (%) | 0 (0) | 0 (0) |
| Type of MS; n (%) | ||
| RRMS | 71 (100) | 219 (95) |
| PPMS | 0 (0) | 4 (2) |
| Uncertain | 0 (0) | 6 (3) |
*Study baseline is the year the women were enrolled in the MoBa study, and when the information on exposure were acquired.
†Observation years in the time-to-event analysis are calculated from enrollment in MoBa.
‡Adverse socioeconomic status is one of the following: non-cohabiting mother, short education <9 years or low household income (<60% of study population median in the given enrolment year).
§Age in 2018 among participants who did not experience the event (censored).
BMI, body mass index; MoBa, The Norwegian mother, father and Child cohort study; MS, multiple sclerosis; PPMS, primary progressive MS; RRMS, relapsing remitting MS.
The MS incidence rates were 41, 49 and 40 per 100 000 person-years for women exposed to emotional, sexual, and physical abuse, respectively, and 28 per 100 000 person-years in women unexposed to childhood abuse (table 2).
Table 2.
Incidence rates and HRs for Multiple Sclerosis among women exposed to childhood abuse
| Exposure | N (%) total cohort |
N (%) women with MS |
Person Time 100 000 Years |
IR* (95% CI) | Unadjusted HR (95% CI) |
HR† (95% CI) | HR‡ (95% CI) |
| No childhood abuse | 63 520 (81) | 229 (76) | 8.2 | 28 (25 to 32) | Ref | Ref | Ref |
| Any childhood abuse | 14 477 (19) | 71 (24) | 1.9 | 38 (30 to 48) | 1.36 (1.04 to 1.78) | 1.34 (1.03 to 1.76) | 1.31 (0.99 to 1.72) |
| Emotional abuse | 10 702 (14) | 56 (20) | 1.4 | 41 (31 to 53) | 1.46 (1.09 to 1.95) | 1.43 (1.06 to 1.93) | 1.40 (1.03 to 1.90) |
| Emotional abuse: Humiliation | 9414 (13) | 48 (17) | 1.2 | 40 (30 to 53) | 1.42 (1.04 to 1.94) | 1.39 (1.01 to 1.90) | 1.37 (0.99 to 1.89) |
| Emotional abuse: Threat | 3406 (5) | 20 (8) | 0.4 | 46 (30 to 71) | 1.64 (1.04 to 2.58) | 1.59 (1.00 to 2.52) | 1.42 (0.86 to 2.29) |
| Sexual abuse | 5416 (8) | 34 (13) | 0.7 | 49 (35 to 68) | 1.74 (1.21 to 2.49) | 1.75 (1.21 to 2.51) | 1.65 (1.13 to 2.39) |
| Physical abuse | 4287 (6) | 22 (9) | 0.6 | 40 (26 to 61) | 1.42 (0.92 to 2.20) | 1.41 (0.91 to 2.19) | 1.31 (0.83 to 2.06) |
| No of abuse categories (Ref: 0) |
|||||||
| 1 | 9947 (13) | 40 (13) | 1.3 | 31 (23 to 43) | 1.12 (0.80 to 1.56) | 1.09 (0.78 to 1.54) | 1.11 (0.79 to 1.56) |
| 2 | 3132 (4) | 21 (7) | 0.4 | 52 (34 to 80) | 1.85 (1.19 to 2.90) | 1.87 (1.19 to 2.92) | 1.66 (1.04 to 2.67) |
| 3 | 1398 (2) | 10 (3) | 0.2 | 56 (30 to 104) | 1.99 (1.06 to 3.75) | 2.00 (1.05 to 3.77) | 1.93 (1.02 to 3.67) |
*Incidence rates per 100 000 person-years. The incidence rate is lower for ‘any childhood abuse’ than for the separate subcategories of abuse because of longer person-time (more individuals under observation in the total abuse group). IR = ‘Number of new cases’/‘Total person-time at risk’.
†HRs adjusted for school drop-out (≤9 years elementary school). Birth year was included as a stratification factor in the Cox model.
‡HRs adjusted for adverse socioeconomic factors (≤9 years elementary school, non-cohabiting mother or low household income), smoking (ever vs never) and BMI ≥25 before study baseline). Birth year was included as a stratification factor in the Cox model, but no other covariable violated the proportional hazard assumption.
BMI, body mass index; IR, incidence rate; MS, multiple sclerosis.
We found an association between exposure to emotional or sexual abuse and subsequent MS development after adjustment for potential confounders and when accounting for possible mediators, HR 1.40 (95% CI 1.03 to 1.90) and HR 1.65 (95% CI 1.13 to 2.39), respectively (table 2). In the fully adjusted analyses, the HR for MS after exposure to physical abuse was 1.31 (95% CI 0.83 to 2.06) and the HR for MS after exposure to any type of childhood abuse was 1.31 (95% CI 0.99 to 1.72).
The risk of MS was further increased in women exposed to two (HR 1.66, 95% CI 1.04 to 2.67), or all three categories of childhood abuse (HR 1.93, 95% CI 1.02 to 3.67).
Sensitivity analyses
We found similar or stronger associations between childhood abuse and MS in the sensitivity analysis after excluding women that could have been in a prodromal phase of MS when experiencing abuse (online supplemental table 1). The HR was 1.77 (95% CI 1.22 to 2.57) for sexual abuse and 1.40 (95% CI 1.01 to 1.95) for emotional abuse.
The association between childhood emotional and sexual abuse and MS persisted when including women that already had an MS diagnosis at baseline (online supplemental table 2).
Missing data
A total of 7633 of 85 357 (9%) women who answered the questionnaire in pregnancy week 18 did not answer the questionnaire in week 30 that included the abuse items. Their baseline characteristics were similar to our included participants (online supplemental table 3). A total of 617 of 78 620 (0.8%) women who answered the questionnaire in week 30 did not complete the abuse items. These women had more often an adverse socioeconomic status (online supplemental table 3).
Discussion
In this nationwide, prospective cohort study, women who were exposed to childhood sexual or emotional abuse had an increased risk of developing MS. There was a similar tendency for exposure to physical abuse. The risk estimates were higher when exposed to several abuse categories, indicating a dose–response relationship.
Our results are supported by previously published retrospective studies.7 25 The increased risk of MS after exposure to childhood sexual and emotional abuse may have a biological explanation. Childhood abuse can cause dysregulation of the hypothalamic–pituitary–adrenal axis,26 lead to oxidative stress27 and induce a proinflammatory state decades into adulthood.28 Psychological stress has been shown to disrupt the blood–brain barrier29 and cause epigenetic changes that may increase the risk of neurodegenerative disorders, including MS.30 Neonatal emotional and physical stress increased the susceptibility and severity of MS-like disease in mice, due to downregulation of adrenergic receptors in innate immune cells.31
We found a higher risk of MS in women exposed to more than one type of abuse. A similar dose–response association has been observed between the risk of adult autoimmune disease hospitalisations and the number of childhood adverse events.5
This is the first fully prospective study that has assessed the association between childhood adverse events and subsequent MS. Previous studies on adverse events have mainly focused on adulthood and have found that most events happened during the last 1–5 years before MS onset.3 32 33
The nationwide cohort design, long follow-up and the inclusion of thoroughly validated MS cases through data-linkage with national health registries contribute to a high validity of our study. Sensitivity analyses minimised the possibility that our findings can be explained by reverse causality. We were able to adjust for important confounders and mediators, including childhood social status, adult socioeconomic factors, smoking and obesity. These environmental factors are associated with both exposure to childhood abuse4 21 and the risk of developing MS.11 22–24 The risk estimates for MS after exposure to emotional and sexual abuse slightly decreased after adjusting for these factors, but the associations remained significant. This suggests that childhood abuse may have an independent effect on MS susceptibility.
The sensitivity analysis showed consistent results also when including prevalent cases of MS, although the HR estimates were slightly reduced. The group of women with established MS did not report higher occurrence of childhood abuse than women with future MS as one might have expected from a recall bias perspective.
Women exposed to childhood abuse had higher depression rates when included during pregnancy. Retrospective reports of childhood trauma may be biased by current mood.34 However, some suggest such bias is minor.35 A more plausible explanation may be that exposure to childhood abuse gives increased risk of depression, in particular depression during pregnancy.36
Limitations
External validity represents a potential limitation of our study as we studied pregnant women and only 41% of the invited women consented to participation. Women with low socioeconomic status are underrepresented in the MoBa-cohort,37 and women who skipped the abuse items in the questionnaire had lower socioeconomic status than the included population. Further, these findings may not be generalisable to men or non-white individuals.
As in all observational studies, residual confounding may be another limitation. We had detailed information on behavioural risk factors in adulthood such as smoking and obesity, but childhood abuse may be associated with other environmental factors such as diet, nutrition, physical exercise, and parental smoking, which could be independent risk factors for MS.
We used a screening questionnaire to assess the three main categories of abuse. Childhood abuse tends to be under-reported rather than over-reported in adulthood.35 This could influence our prevalence rates but not affect exposure–outcome associations.
We did not have information on death or emigration which may bias observation time. Among Norwegian women in the age group 20–49 years,38 0.003% emigrate39 and 0.0005% die40 each year. Thus, these events should have minimal effects on our results.
We lacked information on chronicity of abuse. Exposure to abuse as a one-time incident could have different impact compared with repetitive abuse. Nevertheless, our finding of a dose–response relationship probably represents higher level of abuse severity. We do not know the age at abuse, and there may exist vulnerable periods during childhood and adolescence for MS development. We had no information on potential protective mechanisms such as social network, caregivers, family/friends or therapeutic interventions. Future studies may be strengthened through more nuanced exposure assessment.
In conclusion, children exposed to adverse experiences had an increased risk of developing MS later in life, independent of known environmental risk factors for MS. The risk increased with number of abuse categories in a dose–response manner. The underlying mechanisms behind this association should be investigated further.
Acknowledgments
The Norwegian Mother, Father and Child Cohort study is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research. We acknowledge Heidi Ø. Flemmen MD (Department of Neurology, Telemark Hospital Trust, Skien, Norway); Åslaug R. Lorentzen MD PhD (Department of Neurology, Sørlandet Hospital, Kristiansand, Norway); Cecilia S. Simonsen MD (Department of Neurology, Vestre Viken Hospital Trust, Drammen, Norway); Johannes Sverre Willumsen MD (Department of Neurology, Molde Hospital, Molde, Norway); Nina Øksendal MD (Department of Neurology, Nordland Hospital Trust, Bodø, Norway); Barbara Ratajczak-Tretel MD (Department of Neurology, Østfold Hospital, Østfold, Norway); Britt Bruland CNS (Department of Neurology, Førde Hospital, Førde, Norway) for contributing with data extraction and validation of MS diagnoses. We are grateful to all the participating families in Norway who take part in this ongoing cohort study. The results from this study were presented as an oral presentation at the ECTRIMS 2021 Conference.
Footnotes
Twitter: @CeliusElisabeth
Contributors: KE: conception and design of the study, acquisition and analysis of data, drafting the manuscript. KE acts as the guarantor of the study and takes full responsibility for the work. K-MM, CFT, TR, NEG and M-HB: conception and design of the study, acquisition and analysis of data. ØT, JA, MA, AB, EGC, MC, AKD, TH, SS and SW: acquisition and analysis of data. All authors revised the manuscript and approved the final draft.
Funding: KE has governmental funding (doctoral scholarship) from the Western Norway Regional Health Authority (Grant F-12503). Neuro-SysMed is funded by the Norwegian Research Council grant 288164.
Competing interests: KE has received unrestricted research grant from Novartis. ØT has received speaker honoraria from and served on scientific advisory boards for Biogen, Sanofi-Aventis, Merck and Novartis. AB has received unrestricted research from Novartis. EGC has received honoraria for lecturing and advice from Biogen, Bristol Meyers Squibb, Janssen, Novartis, Merck, Roche and Sanofi, and her department has received grants from Novartis and Sanofi. AKD has received project funding from Pfizer. TH has received speaker honoraria from Biogen, Merck, Novartis, Roche, Bristol Myers Squibb, and Sanofi, and has participated in clinical trials organised by Biogen, Merck, and Roche. K-MM has received unrestricted research grants to his institution; scientific advisory board and speaker honoraria from Biogen, Merck, Novartis, Roche and Sanofi, and has participated in clinical trials organised by Biogen, Merck, Novartis, Roche and Sanofi. CFT has served on scientific advisory board for Astra Zeneca. SW has received honoraria from Biogen, Novartis and Sanofi. NEG has received honoraria from UCB, Ra, Argenx, Roche, Merck, Immunovant, Alexion. M-HB has received personal honoraria for lecturing from Teva, Lilly, Eisai and Novartis, and consultancy honoraria and unrestricted research support from Novartis. Institutional contract research fees from Sanofi.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. Enquiries regarding access to data from MoBa and the MBRN can be directed to the Norwegian Institute of Public Health. Data from the MSR are accessible for researchers by application (http://norskmsregister.no).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The establishment of MoBa and initial data collection was based on a license from the Norwegian Data Protection Agency and approval from the Regional Committees for Medical and Health Research Ethics (REK). The MoBa cohort is regulated by the Norwegian Health Registry Act.Ethics approval for the current study was obtained from REK (reference 2016/906). Written informed consent for use of information in research and for data linkage was acquired during enrolment in MoBa and MSR.
References
- 1. Song H, Fang F, Tomasson G, et al. Association of stress-related disorders with subsequent autoimmune disease. JAMA 2018;319:2388–400. 10.1001/jama.2018.7028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Briones-Buixassa L, Milà R, Mª Aragonès J, et al. Stress and multiple sclerosis: a systematic review considering potential Moderating and mediating factors and methods of assessing stress. Health Psychol Open 2015;2:205510291561227. 10.1177/2055102915612271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jiang X, Olsson T, Hillert J, et al. Stressful life events are associated with the risk of multiple sclerosis. Eur J Neurol 2020;27:2539–48. 10.1111/ene.14458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. Am J Prev Med 1998;14:245–58. 10.1016/s0749-3797(98)00017-8 [DOI] [PubMed] [Google Scholar]
- 5. Dube SR, Fairweather D, Pearson WS, et al. Cumulative childhood stress and autoimmune diseases in adults. Psychosom Med 2009;71:243–50. 10.1097/PSY.0b013e3181907888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nielsen NM, Pedersen BV, Stenager E, et al. Stressful life-events in childhood and risk of multiple sclerosis: a Danish nationwide cohort study. Mult Scler 2014;20:1609–15. 10.1177/1352458514528761 [DOI] [PubMed] [Google Scholar]
- 7. Spitzer C, Bouchain M, Winkler LY, et al. Childhood trauma in multiple sclerosis: a case-control study. Psychosom Med 2012;74:312–8. 10.1097/PSY.0b013e31824c2013 [DOI] [PubMed] [Google Scholar]
- 8. Riise T, Mohr DC, Munger KL, et al. Stress and the risk of multiple sclerosis. Neurology 2011;76:1866–71. 10.1212/WNL.0b013e31821d74c5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Handel AE, Giovannoni G, Ebers GC, et al. Environmental factors and their timing in adult-onset multiple sclerosis. Nat Rev Neurol 2010;6:156–66. 10.1038/nrneurol.2010.1 [DOI] [PubMed] [Google Scholar]
- 10. Cortese M, Riise T, Bjørnevik K, et al. Timing of use of cod liver oil, a vitamin D source, and multiple sclerosis risk: the EnvIMS study. Mult Scler 2015;21:1856–64. 10.1177/1352458515578770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Høglund RAA, Meyer HE, Stigum H, et al. Association of body mass index in adolescence and young adulthood and long-term risk of multiple sclerosis: a population-based study. Neurology 2021;97:e2253–61. 10.1212/WNL.0000000000012957 [DOI] [PubMed] [Google Scholar]
- 12. Mikaeloff Y, Caridade G, Tardieu M, et al. Parental smoking at home and the risk of childhood-onset multiple sclerosis in children. Brain 2007;130:2589–95. 10.1093/brain/awm198 [DOI] [PubMed] [Google Scholar]
- 13. Salzer J, Hallmans G, Nyström M, et al. Smoking as a risk factor for multiple sclerosis. Mult Scler 2013;19:1022–7. 10.1177/1352458512470862 [DOI] [PubMed] [Google Scholar]
- 14. Magnus P, Birke C, Vejrup K, et al. Cohort profile update: the Norwegian mother and child cohort study (MobA). Int J Epidemiol 2016;45:382–8. 10.1093/ije/dyw029 [DOI] [PubMed] [Google Scholar]
- 15. Benjaminsen E, Myhr K-M, Grytten N, et al. Validation of the multiple sclerosis diagnosis in the Norwegian patient registry. Brain Behav 2019;9:e01422. 10.1002/brb3.1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018;17:162–73. 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 17. Swahnberg IMK, Wijma B. The NorVold abuse questionnaire (NorAQ): validation of new measures of emotional, physical, and sexual abuse, and abuse in the health care system among women. Eur J Public Health 2003;13:361–6. 10.1093/eurpub/13.4.361 [DOI] [PubMed] [Google Scholar]
- 18. Tambs K, Moum T. How well can a few questionnaire items indicate anxiety and depression? Acta Psychiatr Scand 1993;87:364–7. 10.1111/j.1600-0447.1993.tb03388.x [DOI] [PubMed] [Google Scholar]
- 19. Briggs FBS, Acuña BS, Shen L, et al. Adverse socioeconomic position during the life course is associated with multiple sclerosis. J Epidemiol Community Health 2014;68:622–9. 10.1136/jech-2013-203184 [DOI] [PubMed] [Google Scholar]
- 20. Gilbert R, Fluke J, O'Donnell M, et al. Child maltreatment: variation in trends and policies in six developed countries. Lancet 2012;379:758–72. 10.1016/S0140-6736(11)61087-8 [DOI] [PubMed] [Google Scholar]
- 21. Houtepen LC, Heron J, Suderman MJ, et al. Associations of adverse childhood experiences with educational attainment and adolescent health and the role of family and socioeconomic factors: a prospective cohort study in the UK. PLoS Med 2020;17:e1003031. 10.1371/journal.pmed.1003031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Degelman ML, Herman KM. Smoking and multiple sclerosis: a systematic review and meta-analysis using the Bradford Hill criteria for causation. Mult Scler Relat Disord 2017;17:207–16. 10.1016/j.msard.2017.07.020 [DOI] [PubMed] [Google Scholar]
- 23. Bjørnevik K, Riise T, Cortese M, et al. Level of education and multiple sclerosis risk after adjustment for known risk factors: the EnvIMS study. Mult Scler 2016;22:104–11. 10.1177/1352458515579444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hedström AK, Hillert J, Olsson T, et al. Factors affecting the risk of relapsing-onset and progressive-onset multiple sclerosis. J Neurol Neurosurg Psychiatry 2021;92:1096–102. 10.1136/jnnp-2020-325688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shaw MT, Pawlak NO, Frontario A, et al. Adverse childhood experiences are linked to age of onset and reading recognition in multiple sclerosis. Front Neurol 2017;8:242. 10.3389/fneur.2017.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heim C, Newport DJ, Mletzko T, et al. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology 2008;33:693–710. 10.1016/j.psyneuen.2008.03.008 [DOI] [PubMed] [Google Scholar]
- 27. Karanikas E, Daskalakis NP, Agorastos A. Oxidative dysregulation in early life stress and posttraumatic stress disorder: a comprehensive review. Brain Sci 2021;11. 10.3390/brainsci11060723. [Epub ahead of print: 29 05 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baumeister D, Akhtar R, Ciufolini S, et al. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol Psychiatry 2016;21:642–9. 10.1038/mp.2015.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Esposito P, Gheorghe D, Kandere K, et al. Acute stress increases permeability of the blood-brain-barrier through activation of brain mast cells. Brain Res 2001;888:117–27. 10.1016/S0006-8993(00)03026-2 [DOI] [PubMed] [Google Scholar]
- 30. Babenko O, Kovalchuk I, Metz GA. Epigenetic programming of neurodegenerative diseases by an adverse environment. Brain Res 2012;1444:96–111. 10.1016/j.brainres.2012.01.038 [DOI] [PubMed] [Google Scholar]
- 31. Khaw YM, Majid D, Oh S, et al. Early-life-trauma triggers interferon-β resistance and neurodegeneration in a multiple sclerosis model via downregulated β1-adrenergic signaling. Nat Commun 2021;12:105. 10.1038/s41467-020-20302-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Warren S, Greenhill S, Warren KG. Emotional stress and the development of multiple sclerosis: case-control evidence of a relationship. J Chronic Dis 1982;35:821–31. 10.1016/0021-9681(82)90047-9 [DOI] [PubMed] [Google Scholar]
- 33. Saul A, Ponsonby A-L, Lucas RM, et al. Stressful life events and the risk of initial central nervous system demyelination. Mult Scler 2017;23:1000–7. 10.1177/1352458516667566 [DOI] [PubMed] [Google Scholar]
- 34. Gaddy MA, Ingram RE. A meta-analytic review of mood-congruent implicit memory in depressed mood. Clin Psychol Rev 2014;34:402–16. 10.1016/j.cpr.2014.06.001 [DOI] [PubMed] [Google Scholar]
- 35. Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. J Child Psychol Psychiatry 2004;45:260–73. 10.1111/j.1469-7610.2004.00218.x [DOI] [PubMed] [Google Scholar]
- 36. Eid K, Torkildsen Øivind Fredvik, Aarseth J, et al. Perinatal depression and anxiety in women with multiple sclerosis: a population-based cohort study. Neurology 2021;96:e2789–800. 10.1212/WNL.0000000000012062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nilsen RM, Vollset SE, Gjessing HK, et al. Self-Selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol 2009;23:597–608. 10.1111/j.1365-3016.2009.01062.x [DOI] [PubMed] [Google Scholar]
- 38. Norway S. 05196: population, by sex, age and citizenship 1977-2021: statistics Norway, 2021. Available: https://www.ssb.no/en/statbank/table/05196/ [Accessed 18 Aug 2021].
- 39. Norway S. 09203: immigration, emigration and net migration, by sex and age 2001-2020: statistics Norway, 2021. Available: https://www.ssb.no/en/statbank/table/09203 [Accessed 18 Aug 2021].
- 40. Norway S. 08462: deaths, by sex and 10-year age groups (C) 1974-2020: statistics Norway, 2021. Available: https://www.ssb.no/en/statbank/table/08426/ [Accessed 18 Aug 2021].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2021-328700supp001.pdf (273.5KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. Enquiries regarding access to data from MoBa and the MBRN can be directed to the Norwegian Institute of Public Health. Data from the MSR are accessible for researchers by application (http://norskmsregister.no).