Abstract
Objectives
This longitudinal study compared emerging plasma biomarkers for neurodegenerative disease between controls, patients with Alzheimer’s disease (AD), Lewy body dementia (LBD), frontotemporal dementia (FTD) and progressive supranuclear palsy (PSP).
Methods
Plasma phosphorylated tau at threonine-181 (p-tau181), amyloid beta (Αβ)42, Aβ40, neurofilament light (NfL) and glial fibrillar acidic protein (GFAP) were measured using highly sensitive single molecule immunoassays (Simoa) in a multicentre cohort of 300 participants (controls=73, amyloid positive mild cognitive impairment (MCI+) and AD dementia=63, LBD=117, FTD=28, PSP=19). LBD participants had known positron emission tomography (PET)-Aβ status.
Results
P-tau181 was elevated in MCI+AD compared with all other groups. Aβ42/40 was lower in MCI+AD compared with controls and FTD. NfL was elevated in all dementias compared with controls while GFAP was elevated in MCI+AD and LBD. Plasma biomarkers could classify between MCI+AD and controls, FTD and PSP with high accuracy but showed limited ability in differentiating MCI+AD from LBD. No differences were detected in the levels of plasma biomarkers when comparing PET-Aβ positive and negative LBD. P-tau181, NfL and GFAP were associated with baseline and longitudinal cognitive decline in a disease specific pattern.
Conclusion
This large study shows the role of plasma biomarkers in differentiating patients with different dementias, and at monitoring longitudinal change. We confirm that p-tau181 is elevated in MCI+AD, versus controls, FTD and PSP, but is less accurate in the classification between MCI+AD and LBD or detecting amyloid brain pathology in LBD. NfL was elevated in all dementia groups, while GFAP was elevated in MCI+AD and LBD.
Keywords: alzheimer's disease, lewy body dementia, frontotemporal dementia, dementia, movement disorders
Introduction
Clinical diagnostics and clinical trials will benefit from scalable non-invasive biomarkers moving beyond positron emission tomography (PET) imaging and cerebrospinal fluid (CSF) analysis to blood based assays.1 Recent years have seen the emergence of many new plasma biomarkers, but head-to-head comparisons, across multiple dementias and over time, are required to assess their potential for differential diagnosis and trials monitoring.2 Here we jointly evaluate a set of biomarkers in Alzheimer’s disease (AD; the most common type of neurodegenerative dementia), Lewy body dementia (LBD; dementia with Lewy bodies (DLB) and Parkinson’s disease dementia (PDD)), frontotemporal dementia (FTD) and progressive supranuclear palsy (PSP).3 Biomarkers are most advanced and validated for AD, including preclinical stages, for diagnosis and in trials of disease-modifying treatments.3 4 For example, biomarkers for AD may use the ‘A/T/N’ classification system referring to levels of amyloid beta (Aβ; A), tau (T) and neurodegeneration/neuronal injury (N). Amyloid is measured using either CSF levels of the 42 and 40 amino acid form of Aβ or using PET with ligands binding to Aβ (PET-Αβ). Tau is measured using CSF levels of phosphorylated tau or PET with ligands binding to tau and neurodegeneration is measured using MRI, fluorodeoxyglucose-PET or CSF total tau.5 6 However, such biomarkers are challenging to apply at scale, or frequently, and also need evidence of their performance and differences across multiple dementias.
Technological advances now allow measurement of such multiple biomarkers in blood plasma with great potential for the development of blood biomarkers for diagnosis and tracking of AD and other neurodegenerative diseases.7 Plasma levels of phosphorylated tau at threonine 181 (p-tau181) at threonine 217 (p-tau217) and at threonine-231 (p-tau231) are promising blood biomarkers for detecting AD pathology.1 4–8 Studies so far have shown high levels of correlation between p-tau181 and p-tau217 with their CSF counterparts and with PET tau.6 9 They are markedly elevated in blood samples of patients with AD when compared with controls and other neurodegenerative conditions, have high accuracy in discriminating between AD and FTD and correlate well with postmortem burden of Αβ and tau neuropathology,2 4–6 9–11 though their ability to distinguish AD from LBD is less clear and only a small number of LBD cases have been included in published studies comparing these biomarkers. They also have a prognostic value to detect preclinical AD and predict both longitudinal decline and conversion from mild cognitive impairment (MCI) to AD.5 7 12–14 In parallel, measurement of the ratio of Aβ42 and Aβ40 in blood using highly sensitive assays is a good proxy for presence of amyloid brain pathology and diagnosis of AD but may be confounded by peripheral amyloid production.15 16 Neurofilament light (NfL), a marker of neuroaxonal damage, in CSF and plasma has been established as a biomarker of neurodegeneration across different types of dementia.17 18 In parallel, neuroinflammation has been linked with the development of dementia and plasma glial fibrillar acidic protein (GFAP), a marker of astrocytic activation, is elevated early in AD and in cognitively normal older adults who are at risk of AD based on brain Αβ load.19 20 Moreover, plasma GFAP was associated with AD pathology in MCI and predicted conversion to AD.21
Less is known about the use of these plasma biomarkers in LBD, whether they can improve the diagnosis of LBD or distinguish the common AD-copathology in LBD.22 LBD is driven by cortical Lewy body pathology but approximately half of patients with LBD have coexisting AD amyloid pathology.23 P-tau181 and p-tau217 may be able to identify LBD cases with coexisting AD pathology as measured with PET tau and CSF Aβ.24 People with non-AD pathologies, including Lewy bodies, show progressively elevated p-tau181 with ageing up until death, while those with AD show increases in plasma p-tau181 very early on in the disease course.4 Evidence is required for the ability of plasma biomarkers for neurodegeneration to distinguish between those with clinical diagnoses of AD, LBD and copathology.
The present study had four primary aims. First, to measure the levels of the plasma biomarkers of neurodegeneration (p-tau181, Aβ42/40 ratio, NfL and GFAP) across a multicentre memory clinical cohort of older participants with PET-Αβ-positive MCI (MCI+) and AD (MCI+AD), LBD, FTD, PSP and controls. Second, to test the ability of these biomarkers to accurately classify a diagnosis of MCI+AD compared with controls, LBD, FTD and PSP. Third to test the ability of plasma biomarkers to detect the presence of AD pathology in LBD and last to examine the relation between plasma biomarkers and baseline cognitive function and subsequent longitudinal decline. We predicted that plasma biomarkers would be differentially elevated among the different types of dementia and that p-tau181 and Αβ42/40 would distinguish AD cases from the different forms of dementia while detecting coexistent ΑD pathology in LBD.
Materials and methods
Participant characteristics
The study included 300 participants from five diagnostic groups (see table 1 for summary characteristics). Participants were above the age of 50. Exclusion criteria included an acute infection, major concurrent psychiatric illness; severe physical illness; a history of other significant neurological illness. Participants with capacity gave their written informed consent to take part in the study. For those who lacked capacity, their participation followed the consultee process in accordance with the UK law.
Table 1.
Summary of participant baseline characteristics and plasma biomarker measurements per diagnostic group
| Control (n=73) | MCI+AD (n=63) | LBD (n=117) | FTD (n=28) | PSP (n=19) | P value | |
| Age (years) | 70.2 (7.79) | 73.9 (7.80) | 75.6 (6.81) | 64.5 (8.62) | 69.0 (5.91) | 1.61e−11 |
| Sex (female) | 30 (42%) | 20 (32%) | 23 (20%) | 12 (43%) | 6 (32%) | 0.012 |
| ACE-R | 94.5 (4.49) | 69.7 (16.5) | 66.4 (16.8) | 71.0 (15.6) | 79.6 (15.3) | <2e−16 |
| P-tau181 (pg/mL) | 2.59 (1.65) | 4.26 (2.00) | 3.49 (2.37) | 2.16 (1.09) | 2.63 (2.03) | 3.24e−05 |
| Aβ42/40 | 0.0646 (0.0145) | 0.0565 (0.00712) | 0.0599 (0.0145) | 0.0699 (0.011) | 0.0671 (0.0237) | 0.05207 |
| NfL (pg/mL) | 20.9 (18.6) | 28.2 (16.8) | 32.3 (25.1) | 38.2 (20.5) | 31.1 (15.0) | <2e−16 |
| GFAP (pg/mL) | 154 (96.5) | 243 (99.9) | 222 (105) | 160 (83.9) | 146 (57.2) | 4.32e−10 |
Data presented as mean (SD). Comparisons were performed using analysis of covariance. Please note that the statistical tests were performed using the log-transformed values.
MCI+AD is the combined group of positron emission tomography Aβ-positive patients with MCI and Alzheimer’s disease (AD), Lewy body dementia is the combined group of dementia with Lewy bodies and patients with Parkinson’s disease dementia.
ACE-R, Addenbrooke’s cognitive examination revised version; FTD, frontotemporal dementia; GFAP, glial fibrillar acidic protein; MCI, mild cognitive impairment; NfL, neurofilament light; pg/ml, picogram/millilitre; PSP, progressive supranuclear palsy; p-tau181, phosphorylated tau at threonine 181; Αβ42/40, the ration between the plasma measurement of amyloid beta (Αβ) 40 and 42.
One hundred and forty-five participants were included from the Neuroimaging of Inflammation in Memory and Related Other Disorders (NIMROD); 76 participants from the Amyloid Imaging for Phenotyping Lewy Body Dementia (AMPLE), 29 from the Clinical Biomarkers for Dementia Research, 27 from the 123I-MIBG in Dementia with Lewy bodies as A marker for Sympathetic denervation (MIDAS) and 23 from the Multimodal Imaging of Lewy Body Disorders study (MILOS). Detailed background, clinical diagnostic criteria, clinical and neuroimaging findings relating to these cohorts have been published previously.23 25–27 The pooled study cohort comprised of a total of 73 non-demented controls, 63 participants in the AD spectrum consisting of 14 PET-Αβ-positive MCI (MCI+) and 49 AD dementia participants who were combined and analysed as a single MCI+AD group as in our previous studies, reflecting different stages on the AD spectrum, 117 LBD (110 DLB and 7 PDD, 59 with PET-Αβ status), 28 patients with FTD and 19 patients with PSP. The FTD group included 10 patients with behavioural variant FTD (bvFTD), 9 patients with non-fluent primary progressive aphasia (nfPPA) and 7 patients with semantic variant PPA (svPPA). Three patients with bvFTD were found to carry the C9orf72 mutation, two bvFTD carried the MAPT mutation, one bvFTD and one nfPPA carried GRN mutation. All FTD subtypes were combined to a single group due to the low number of samples available. Participants were recruited from specialist memory clinics in and around Cambridgeshire and the North of England, the Dementia and Neurodegeneration specialty of the UK Clinical Research Network, the Join Dementia Research platform (www.joindementiaresearch.nihr.ac.uk) as well as from cognitively healthy spouses and partners of participants.
Healthy controls had mini-mental state examination >26 with no acute physical illness, no cognitive complaints and who were independent in function and instrumental activities of daily living. MCI PET-Aβ-positive participants were defined by presence of subjective memory reports, no impairment in activities of daily living and amyloid positivity using Pittsburgh Compound B (PIB) PET using a cutpoint of 19 in the centiloid scale.28 AD participants fulfilled the criteria for AD dementia defined as per the National Institute on Aging-Alzheimer’s Association Criteria.29 Probable DLB was defined by both the 2005 and 2017 consensus criteria and PDD was defined by the Movements Disorders Society clinical diagnosis criteria for PDD.30 31 DLB and PDD were combined in a group representing the LBD group. FTD diagnoses were based on the criteria defined clinically by Rascovsky et al for behavioural variant of FTD and Gorno-Tempini et al 32 33 for PPA variants. Participants with PSP were recruited initially according to the Litvan et al 34 criteria (modified by a relaxation of the falls criterion to falls within 3 years as suggested by the NNIPPS-PSP study group,34 and later reclassified according to the MDS-PSP 2017 criteria for PSP-Richardson’s syndrome.35
Clinical assessments
Participants underwent clinical examination with cognitive and neuropsychiatric assessment at baseline. These were repeated annually for up to 3 years for the NIMROD and MILOS cohorts and at 1 year for the AMPLE and MIDAS cohorts. Cognitive function in this study was measured using the Addenbrooke’s Cognitive Examination Revised version (ACE-R). The ACE-R incorporates five domains of cognitive function (attention/orientation, memory, verbal fluency, language, visuospatial) hand has been shown to capture deficits observed in the neurodegenerative dementias included in this cohort.27 36 37 Longitudinal ACE-R data were available for a total of 185 out of 300 participants (control=52, MCI+AD=50, LBD=52, FTD=15, PSP=16). Nine of the MCI-PET-Aβ-positive participants were clinically diagnosed as converting to AD at subsequent follow-up visits.
PET-Aβ in LBD
Imaging was performed at baseline. Details of the MRI and PET acquisition and analysis have been published previously.23 25 In summary, PET-Aβ imaging data were available for 59 participants with LBD. For the NIMROD and MILOS cohort 550 MBq of [11C] PIB PET imaging was carried out using a GE Advance PET scanner (GE Healthcare) or a GE Discovery 690 PET/CT, with attenuation correction provided by a transmission scan or a low dose CT scan, with 550 MBq of PIB injected as a bolus and imaging performed for 30 min starting at 40 min post injection. Participants were considered PET-Aβ-positive using a cutpoint of 19 on the centiloid scale.28
For the AMPLE cohort imaging was performed using a Siemens Biograph-40 PET-CT scanner. Participants were given a 370 MBq intravenous injection of 18F-florbetapir (Amyvid). PET imaging was carried out for 15 min, commencing 30–50 min after injection. Attenuation correction was performed using CT scan data. Amyloid PET images were visually rated as positive or negative based on the manufacturer’s criteria by a panel of five raters.23
Sample collection and processing
Blood samples were obtained by venepuncture and collected in EDTA tubes. They were centrifuged to isolate plasma, aliquoted and stored at −70°C until further analyses. Plasma assays were conducted at the UK Dementia Research Institute biomarker laboratory. Plasma samples were thawed on wet ice, centrifuged at 500× g for 5 min at 4°C. Calibrators (neat) and samples (plasma: 1:4 dilution) were measured in duplicates. The plasma assays measured were the Quanterix Simoa Human Neurology 4-Plex E assay (measuring Aβ40, Aβ42, GFAP and NfL) and the Quanterix Simoa p-tau181 measuring p-tau181 of the human tau protein. Assays were performed using the Simoa-HD1 according to the manufacturer’s protocol (Quanterix Corp, Billerica, Massachusetts, USA) (Rissin et al). All samples were analysed at the same time using the same batch of reagents. A four-parameter logistic curve fit data reduction method was used to generate a calibration curve. Two control samples of known concentration of the protein of interest (high-control and low-control) were included as quality control. The mean coefficient of variation percentage for p-tau181 was 6.29, for NfL 4.08, for Aβ42 3.06, for Aβ40 2.55 and for GFAP 4.12.
Statistical analyses
Statistical analyses were performed in R V.4.0.3, R Foundation for Statistical Computing, Vienna, Austria (https://www.R-project.org/). Figures were generated using the R package ggplot2. Baseline demographics were compared using analysis of variance and χ2 test. The plasma biomarker levels were not normally distributed and were log10 transformed which allowed linear model testing. The Αβ42 and Aβ40 analytes were combined to derive the Aβ42/Α40 ratio. To compare baseline plasma biomarkers across groups, we ran an analysis of covariance model to test for the main effect of diagnostic group, accounting for age and sex as covariates using the R package lme4. Post-hoc pairwise comparisons were carried out using the Bonferroni correction method for each biomarker tested.
Classification analyses were performed using receiver operating characteristic curve (ROC) analyses to estimate the diagnostic ability of the age adjusted levels of the plasma biomarkers using the R package cutpointr (https://github.com/thie1e/cutpointr) calculating a cut-off score using the Youden’s index and reporting the area under the curve (AUC), sensitivity (sens) and specificity (spec) for each comparison. The R package pROC was used for visualisation of the ROC curves. The classification analyses focused on the ability of the plasma biomarkers to discriminate between MCI+AD and controls, LBD, FTD and PSP.
An analysis of covariance model was used when comparing plasma biomarkers between LBD PET-Αβ-positive and PET-Aβ-negative LBD with age and sex as covariates. Associations between plasma biomarkers and baseline ACE-R were tested in each dementia diagnostic group separately using linear regression models with age and sex as covariates. Longitudinal cognitive decline was measured using a linear mixed effects model applied across the longitudinal cognitive scores to estimate the rate of annual decline (slope) in each participant as previously described in detail.38 For each participant, we included ACE-R scores at baseline, 1-year and 2-year follow-up visits where available. The model included the estimation of a random intercept and slope, with time (years) as independent variable and ACE-R scores as dependent variable. The individuals’ estimated rate of annual cognitive decline (slope) was then associated with the plasma biomarkers using a linear regression model, with slope as the dependent variable, the plasma biomarker as the independent variable with age and sex as covariates. Associations between longitudinal cognitive decline and plasma biomarkers were tested in each dementia diagnostic group separately to examine disease specific effects.
Results
Participant characteristics
Baseline demographics, cognitive scores and plasma biomarkers levels are shown in table 1. One measurement of GFAP in a control participant was considered an outlier as it exceeded three SD of the mean and therefore this participant was removed from further analyses (GFAP >6000 pg/mL). Age (p=1.6e−11) and sex (p=0.012) were differentially distributed among the groups, in keeping with the known epidemiological characteristics of each neurodegenerative condition. In regression models including diagnosis as a covariate, age was significantly associated with p-tau181 (β=0.007, p=3.24e−05), NfL (β=0.01, p<2e−16) and GFAP (β=0.009, p=4.32e−10) but not with Αβ42/40 (β=0.001, p=0.052). Sex was not associated with significant differences in the levels of the four plasma biomarkers tested. Table 2 summarises the baseline characteristics and biomarker levels of the LBD group stratified per PET Aβ status.
Table 2.
Summary of the baseline characteristics and plasma biomarker levels of the Lewy body dementia subgroup that had positron emission tomography-Αβ data available. Data are presented as mean (SD). Comparisons were performed using t-test for age, χ2 for sex and a general linear model adjusting for age and sex for ACE-R and the plasma markers
| PET-ΑßNegative LBD (n=30) | PET-Αß-Positive LBD (n=29) | P value | |
| Age (years) | 73.9 (6.25) | 75.2 (6.75) | 0.467 |
| Sex (female) | 5 (17 %) | 6 (21 %) | 0.691 |
| ACE-R | 74.1 (14.7) | 71.3 (11.9) | 0.602 |
| P-tau181 (pg/mL) | 3.33 (3.49) | 3.30 (1.85) | 0.476 |
| Aβ42/40 | 0.0596 (0.013) | 0.0613 (0.0208) | 0.601 |
| NfL (pg/mL) | 25.5 (13.4) | 35.8 (35.8) | 0.248 |
| GFAP (pg/mL) | 179 (62.0) | 219 (85.9) | 0.181 |
ACE-R, Addenbrooke’s Cognitive Examination Revised version; Aβ, amyloid beta; GFAP, glial fibrillar acidic protein; NfL, neurofilament light; p-tau, phosphorylated tau.
Plasma biomarkers per diagnostic group
P-tau181
Analysis of covariance revealed significant differences in the levels of p-tau181 between diagnostic groups (F=9.059, p=6.62e−07) after adjusting for age and sex. Post-hoc comparisons using the Bonferroni correction method found higher levels of p-tau181 in the MCI+AD group when compared with all other groups: MCI+AD versus controls (F=5.55, p=6.35e−7), MCI+AD versus LBD (F=3.47, p=6.05e−3), MCI+AD versus FTD (F=4.09, p=5.57e−4) and MCI+AD versus PSP (F=3.56, p=4.36e−3). See figure 1A for schematic representation of results.
Figure 1.
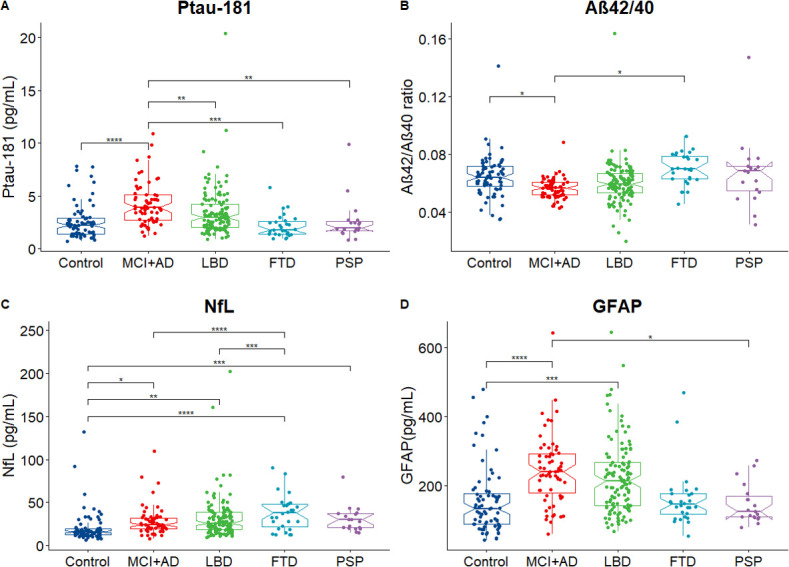
Levels of plasma biomarkers across diagnostic groups. Analysis of covariance revealed a significant effect of diagnosis for all four markers. (A) P-tau181 was elevated in the MCI+AD group when compared with controls, LBD, FTD and PSP. (B) The ratio between Aβ42/40 was lower in MCI+AD group when compared with controls. (C) NfL was elevated across all diagnostic groups when compared with controls and was higher in FTD when compared with LBD and MCI+AD. (D) GFAP was elevated in AD and LBD when compared with controls and in MCI+AD compared with PSP. The Notch graphs display the 95% CIs around the median. Pairwise post-hoc comparisons using the Bonferroni correction are visualised with ****p<00 001, ***p<0001, **p < 0.01, *p < 0.05. Aβ, amyloid beta; AD, Alzheimer’s disease; FTD, frontotemporal dementia; GFAP, glial fibrillar acidic protein; LBD, Lewy body dementia; MCI, mild cognitive impairment; NfL, neurofilament light; PSP, progressive supranuclear palsy; p-tau, phosphorylated tau.
Αβ42/40
There was a significant effect of diagnosis when comparing the levels of Aβ42/40 among the diagnostic groups (F=3.152, p=0.015). Bonferroni post-hoc comparisons revealed that the Αβ42/40 ratio was lower in the MCI+AD group compared with controls (F=2.84, p=0.0479) and compared with the FTD group (F=2.88, p=0.04) (see figure 1B).
NfL
There was a significant effect of diagnosis when comparing levels of NfL among the diagnostic groups (F=14, 9, p=1.38e−10). Bonferroni post-hoc comparisons showed that all dementia groups had higher levels of NfL when compared with controls: controls versus MCI+AD (F=−2.98, p=3.10e−2), versus LBD (F=−3.72, p=2.40e−3), versus FTD (F=−6.90, p=3.31e−10) and versus PSP (F=−4.48, p=1.08e−4). Post-hoc comparisons also showed that patients with FTD had higher NfL levels when compared with MCI+AD (F=4.51, p=9.30e−5) and LBD (F=4.47 p=1.11e−4) (see figure 1C).
GFAP
Levels of plasma GFAP were elevated among diagnostic groups when compared with controls (F=0.08, p=1.18e−07). Post-hoc comparisons showed that GFAP was higher in MCI+AD when compared with controls (F=−5.78, p=1.91e−7) and higher in LBD when compared with controls (F=−4.417, p=1.43e−4), while MCI+AD participants also had higher GFAP when compared with PSP (F=3.19, p=1.57e−2) (see figure 1D).
Classification analyses
P-tau181
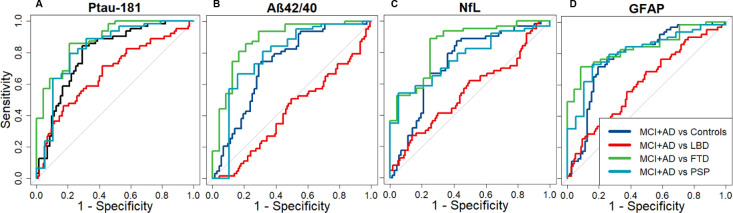
An age adjusted cut-off score of 0.43 in the log10 converted levels of p-tau181 could classify MCI+AD from controls with an AUC of 0.80 (sens: 0.84, spec: 0.70). A log10 p-tau181 cut-off score of 0.67 could classify MCI+AD from LBD with an AUC of 0.67 (sens: 0.58, spec: 0.71), a score of 0.65 MCI+AD from FTD with an AUC of 0.88 (sens: 0.85, spec: 0.79) and a score of 0.69 could classify MCI+AD from PSP with AUC of 0.83 (sens: 0.79, spec: 0.79) (see figure 2A)
Figure 2.
Classification using area under the curve (AUC). (A) P-tau181 could classify MCI+AD from controls with an AUC of 0.80 (sensitivity: 0.84, specificity: 0.70), MCI+AD from LBD with an AUC of 0.67 (sensitivity: 0.58, specificity: 0.71), MCI+AD from FTD with an AUC of 0.88 (sensitivity: 0.85, specificity: 0.79) and MCI+AD from PSP with an AUC of 0.83 (sensitivity: 0.79, specificity: 0.79). (B) The Aβ42/40 ratio could classify MCI+AD from controls with an AUC of 0.72 (sensitivity: 0.75, specificity: 0.69), MCI+AD from LBD with an AUC of 0.42 (sensitivity: 0.50, specificity: 0.51), MCI+AD from FTD with an AUC of 0.88 (sensitivity: 0.86, specificity: 0.79) and MCI+AD from PSP with an AUC of 0.78 (sensitivity: 0.67, specificity: 0.84). (C) NfL could classify MCI+AD from controls with an AUC of 0.73 (sensitivity: 0.87, specificity: 0.58), MCI+AD from LBD with an AUC of 0.55 (sensitivity: 0.27, specificity: 0.86), MCI+AD from FTD with an AUC of 0.85 (sensitivity: 0.89, specificity: 0.75) and MCI+AD from PSP with an AUC of 0.77 (sensitivity: 0.54, specificity: 0.95). (D) GFAP could classify MCI+AD from controls with an AUC of 0.78 (sensitivity: 0.71, specificity: 0.81), MCI+AD from LBD with an AUC of 0.61 (sensitivity: 0.68, specificity: 0.50), MCI+AD from FTD with an AUC of 0.84 (sensitivity: 0.716, specificity: 0.92) and MCI+AD from PSP with an AUC of 0.81(sensitivity: 0.73, specificity: 0.84). Aβ, amyloid beta; AD, Alzheimer’s disease; FTD, frontotemporal dementia; GFAP, glial fibrillar acidic protein; LBD, Lewy body dementia; MCI, mild cognitive impairment with positive PET amyloid scan; NfL, neurofilament light; PSP, progressive supranuclear palsy; p-tau, phosphorylated tau.
Aβ42/40
An age adjusted cut-off score of 0.46 in the log10 converted Αβ42/40 ratio could classify MCI+AD from controls with an AUC of 0.72 (sens: 0.75, spec: 0.69). Similarly a cut-off score of 0.34 could classify MCI+AD from LBD with an AUC of 0.42 (sens: 0.50, spec: 0.51), a score of 0.64 MCI+AD from FTD with an AUC of 0.88 (sens: 0.86, spec: 0.79) and a score of 0.77 MCI+AD from PSP with an AUC of 0.78 (sens: 0.67, spec: 0.84) (see figure 2B).
NfL
An age adjusted cut-off score of 0.39 in the log10 converted levels of NFL could classify MCI+AD from controls with an AUC of 0.73 (sens: 0.87, spec: 0.58). Similarly a cut-off score of 0.41 could classify MCI+AD from LBD with an AUC of 0.55 (sens: 0.27, spec: 0.86), a score of 0.58 MCI+AD from FTD with an AUC of 0.85 (sens: 0.89, spec: 0.75) and a score of 0.81 MCI+AD from PSP with an AUC of 0.77 (sens: 0.54, spec: 0.95) (see figure 2C).
GFAP
An age adjusted cut-off score of 0.52 in the log10 converted levels of NfL could classify MCI+AD from controls with an AUC of 0.78 (sens: 0.71, spec: 0.81). Similarly a cut-off score of 0.33 could classify MCI+AD from LBD with an AUC of 0.61 (sens: 0.68, spec: 0.50), a score of 0.71 MCI+AD from FTD with an AUC of 0.84 (sens: 0.71, spec: 0.92) and a score of 0.75 MCI+AD from PSP with an AUC of 0.81 (sens: 0.73, spec: 0.84) (see figure 2D).
Plasma biomarkers in PET-Aβ-positive LBD
There were no significant differences in the levels of the four plasma biomarkers when comparing patients with PET-Aβ-positive LBD with patients with PET-Aβ-negative (p-tau181 p=0.249, Aβ42/40 p=0.855, NFL p=0.338, GFAP p=0.129; figure 3)
Figure 3.
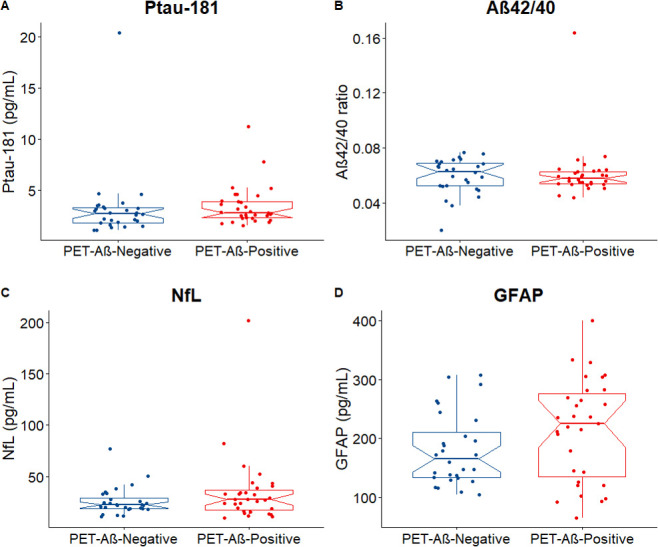
Plasma biomarkers in PET-Αβ-negative versus PET-Aβ-positive LBD cases. No differences were detected when comparing the plasma levels of p-tau181(A), Αβ42/40(B), NfL(C) and GFAP(D) among PET-Αβ-negative(n=30) and PET-Aβ-positive(n=29) LBD cases. The notches display the 95% CI around the median. Aβ, amyloid beta; GFAP, glial fibrillar acidic protein; LBD, Lewy body dementia; NfL, neurofilament light; PET, positron emission tomography; p-tau, phosphorylated tau.
Association between plasma biomarkers and baseline cognition
P-tau181
No associations were found between baseline ACE-R scores and p-tau181 in MCI+AD (p=0.696), LBD (p=0.491) and FTD (p=0.491) after adjusting for the effects of age and sex. P-tau181 was associated with baseline ACE-R scores in PSP (β=−29.17, p=0.026) (see online supplemental figure 1A).
jnnp-2021-327788supp001.pdf (216.2KB, pdf)
Aβ42/40
No associations were found between baseline ACE-R scores and p-tau181 in MCI+AD (p=0.783), LBD (p=0.465), FTD (p=0.544) and PSP (p=0.750) after adjusting for the effects of age and sex (see online supplemental figure 1B).
NfL
NfL was associated with baseline ACE-R in the MCI+AD group (β=−26.42, p=0.045). No associations were found between baseline ACE-R scores and NFL in LBD (p=0.07), FTD (p=0.510) and PSP (p=0.662) after adjusting for the effects of age and sex (see online supplemental figure 1C).
GFAP
No associations were found between baseline ACE-R scores and GFAP in MCI+AD (p=0.136), LBD (p=0.107), FTD (p=0.471) after adjusting for the effects of age and sex. GFAP was associated with baseline ACE-R scores in PSP (β=−73.64, p=0.019) (see online supplemental figure 1D).
Association between plasma biomarkers and longitudinal cognitive decline
P-tau181
P-tau181 was associated with ACE-R slopes in the MCI+AD group (β=−7.4, p=0.040) after adjusting for the effects of age and sex. No associations were found between ACE-R slopes and p-tau181 in LBD (p=0.382), FTD (p=0.585) and PSP (p=0.205) (see online supplemental figure 2A).
Aβ42/40
No associations were found between baseline ACE-R scores and Αβ42/40 in MCI+AD (p=0.348), LBD (p=0.263), FTD (p=0.532) and PSP (p=0.132) after adjusting for the effects of age and gender (see online supplemental figure 2B).
NfL
No associations were found between ACE-R slopes and NFL in MCI+AD (p=0.123), LBD (p=0.553), FTD (p=0.308) and PSP (p=0.257) after adjusting for the effects of age and sex (see online supplemental figure 2C).
GFAP
GFAP was associated with ACE-R slopes in the MCI+AD group (β=−9.75, p=0.016) after adjusting for the effects of age and sex. No associations were found between ACE-R slopes and GFAP in LBD (p=0.450), FTD (p=0.842) and PSP (p=0.605) (see online supplemental figure 2D).
Discussion
We show the differential distribution of plasma biomarkers for neurodegeneration in a multicentre clinical cohort comprising patients with PET-Αβ-positive MCI, AD, DLB and Parkinson’s dementia, FTD and PSP. We confirmed that patients in the AD spectrum have high levels of plasma p-tau181 and low Aβ42/40 ratio compared with the other dementias.1 39 However, in addition, there are elevated levels of GFAP in both LBD and AD. Patients with FTD and PSP have high NfL but no significant increase in the other biomarkers.
P-tau181 only modestly discriminated between MCI+AD and LBD (AUC 0.67) compared with better discrimination of MCI+AD versus controls (AUC 0.80), FTD (AUC 0.88) and PSP (AUC 0.83). Similarly, Αβ42/40, NfL and GFAP showed good performance in classifying MCI+AD from FTD and PSP but showed limited ability to classify MCI+AD from LBD. The large cohort of well characterised LBD is of particular interest, with biomarker confirmation of the diagnosis through Dopamine Transporter scan (DAT) scan or MIBG in most cases and PET-Aβ scans in half.23 26 30 There were no significant differences when comparing the distribution of biomarkers in PET-Αβ-positive LBD compared with PET-Aβ-negative LBD suggesting the limited potential of these plasma biomarkers to identify coexisting AD pathology in LBD, at least with regard to brain amyloid. The p-tau181 and GFAP were associated with baseline cognitive function in PSP, NfL was associated with baseline cognition in the MCI+AD group. Furthermore, the p-tau181 and GFAP were associated with longitudinal cognitive decline in the MCI+AD group suggesting that these may have a potential of prognostic value.
Our findings are in keeping with previous work showing good performance of p-tau181 in discriminating AD from FTD and PSP4–6 and we show that the Aβ42/40 ratio, NfL and GFAP perform equally well in such comparisons. Using a large LBD cohort we have however shown that these plasma biomarkers are not as accurate in discriminating between MCI+AD and LBD in contrast to previous work that used smaller LBD cohorts (17 PDD and 6 DLB6 and 17 LBD4). Moreover our findings of no differences when comparing the levels of plasma biomarkers between PET-Αβ-negative to PET-Aβ-positive LBD are in contrast with previous work suggesting that p-tau181 discriminates LBD cases with and without AD pathology; however that study used CSF Aβ42/40 and PET-tau instead of PET-Αβ.24 There can be several factors affecting our results. We observed high variability in both PET-Aβ-positive and PET-Aβ-negative groups which could be related to technical factors such as PET ligand selection and sensitivity. One of our LBD cohorts included PET PIB with positivity defined using the centiloid method while our other LBD cohort used 18F-florbetapir with visual rating to define positivity. Considering our sample size it is possible that our study was not powered to detect small effect sizes with high variability. There can also however be underlying neurobiological explanations for not detecting differences between PET-Aβ-negative and PET-Aβ-positive LBD cases. For example, the levels of Aβ pathology in the PET-Αβ-negative cases may have not been sufficient to reach the positivity threshold but may still have affected the levels of p-tau181. Alternatively the presence of α-synuclein pathology may be associated with increase in p-tau independent of the presence of Αβ40
The elevated GFAP in AD and LBD is in keeping with previous work of elevated serum and CSF GFAP in these conditions41 42 and contribute to the body of evidence pointing towards the involvement of neuroinflammation in neurodegenerative disorders.19 43 44 Further research needs to explore the pathophysiology of elevated plasma GFAP and whether it reflects neuroinflammation in specific brain regions or disease stages as, for example, microglial activation in DLB has been linked with early stages of the disease.43 44 Interestingly we have not observed elevated levels in of GFAP in FTD and PSP while these have been linked with neuroinflammation using in vivo PET imaging studies.38 45 A recent study by Katisko et al reported elevated levels of GFAP in serum and whole blood samples of FTD compared with patients with primary psychiatric disorders (PPD) and healthy controls and our analyses in plasma samples were not able to replicate these findings.46 It is unclear if the lack of replication is related to our study using plasma samples compared with whole blood or serum used in the study of Katisko et al or the age and sample size differences (107 FTD vs 44 PPD serum samples and 10 FTD vs 18 controls whole blood) while the patients with PPD were on average 10 years younger than the patients with FTD.46 A study by the Genetic FTD Initiative cohort showed that raised plasma GFAP appears to be unique to progranulin-associated FTD (GRN mutation carriers).47
Our findings of p-tau181 and GFAP associations with longitudinal decline in ACE-R scores in MCI+AD are in keeping with previous studies showing that these biomarkers have a prognostic value and it will be interesting for future work to test whether they could also have a potential as markers of treatment response.48 49 It is difficult to establish the reason as to whether the changes were more pronounced in the MCI+AD group, whether this is a disease-specific finding or associated with limitations in statistical power considering the number of cases available with longitudinal data across the disease groups and the baseline differences. Future studies will also need to replicate our findings with respect to PSP considering the low number of cases available in our cohort.
Our study has several strengths. We tested the clinical utility of the plasma biomarkers in a pooled multicentre clinical cohort of older participants and thus our findings are representative of such clinical populations while we used a cohort with four different types of neurodegenerative disorders. There are however limitations to our study. We had baseline sex and age differences due in part to including neurodegenerative dementias with different epidemiological characteristics which may confound our analyses. We did not have CSF, PET or postmortem confirmation of diagnosis for all our participants and therefore it is likely some of the clinical diagnoses arise from mixed pathologies or alternative pathologies.22 50 51 In addition, we had a single biomarker assessment at baseline, so we cannot determine how change in biomarkers over time varies between the different disorders. We had small available number of samples from patients with PSP and FTD while our FTD cohort comprised of mixed pathologies including cases with bvFTD, nfPPA and svPPA with genetic and sporadic cases. Future studies need to validate our findings of elevated plasma markers of neurodegeneration in LBD, FTD and PSP and test the levels of p-tau217 and p-tau231, the association between plasma biomarkers, CSF and neuroimaging.
Collectively our findings suggest that plasma biomarkers in neurodegenerative disorders have a strong potential for diagnosis and monitoring considering their accessibility, convenience, low cost and reproducibility.52 We have shown their potential utility in a representative cohort recruited from UK National Health Service memory clinics and showed that each specific neurodegenerative disorder has a characteristic pattern that may help clinicians in determining the subtype of diagnosis. Our results highlight the importance of the development of biomarkers for non-AD dementias to complement the current panel of plasma markers in order to better characterise patients with dementia and the different subtypes as this is likely to be of importance if these are used for clinical trials especially with the development of anti-Aβ therapies.53
Acknowledgments
We thank our participant volunteers for their participation in this study and the research nurses of the Cambridge Centre for Parkinson-plus for their invaluable support in data acquisition. We thank the East Anglia Dementias and Neurodegenerative Diseases Research Network (DeNDRoN) for help with subject recruitment.
Footnotes
Twitter: @ChouliarasL, @M_Malpetti, @PaulCDonaghy, @jpmkane, @ElijahMak, @ajh1269, @LiSu2099450, @CambridgeFTD, @ProfJTOBrien
Contributors: Conceptualisation and design: LC, AT, JBR, JTO. Data analysis: LC, MM, AJH. Methodology: LC; PD, MM, EM, AJH, HZ. Interpretation: LC, AT, MM, AJH, HZ, LS, JBR, JTO. Data collection: LC, AT, MM, PD, JK, GS, MAP-S, LS. First draft: LC, AT, MM, HZ, JBR, JTO. Supervision: AT, JBR, JTO. Guarantors: LC, JBR, JTO
Funding: This study was funded by the Cambridge Centre for Parkinson-Plus, the National Institute for Health Research (NIHR) Biomedical Research Centre at Cambridge University Hospitals NHS Foundation Trust and the University of Cambridge (BRC-1215–20014) and the NIHR Newcastle Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. The UK Dementia Research Institute, receives its funding from UK DRI, funded by the UK Medical Research Council, Alzheimer’s Society and Alzheimer’s Research UK. JBR is supported by the Wellcome Trust (220258). HZ is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018–02532), the European Research Council (#681712), Swedish State Support for Clinical Research (#ALFGBG-720931), the Alzheimer Drug Discovery Foundation (ADDF), USA (#2 01 809–2016862), the UK Dementia Research Institute at UCL, the Wellcome Trust and an anonymous donor. The MILOS study was funded by the Alzheimer's Research UK and the Lewy Body Society.
Competing interests: Competing interests unrelated to this work, JBR serves as an associate editor to Brain and is a non-remunerated trustee of the Guarantors of Brain, Darwin College and the PSP Association (UK). He provides consultancy to Asceneuron, Biogen, UCB and has research grants from AZ-Medimmune, Janssen, Lilly as industry partners in the Dementias Platform UK. Unrelated to this work, JTOB has received honoraria for work as DSMB chair or member for TauRx, Axon, Eisai, has acted as a consultant for Roche, and has received research support from Alliance Medical and Merck. HZ has served at scientific advisory boards and/or as a consultant for Alector, Eisai, Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, Nervgen, AZTherapies, CogRx and Red Abbey Labs, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure and Biogen, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). Avid Radiopharmaceuticals, a wholly owned subsidiary of Eli Lilly and Company, enabled use of the 18F-florbetapir by providing tracer and funding scanner time, but was not involved in data analysis or interpretation.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data are available upon reasonable request. Anonymised data may be shared by request to the senior author from a qualified investigator for non-commercial use (data sharing may be subject to restrictions according to consent and data protection legislation).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by the respective NIHR National Research Ethic Service Committees (East of England (13/EE/0104), North East-Newcastle & North Tyneside 13/NE/0064). Participants gave informed consent to participate in the study before taking part.
References
- 1. Hansson O. Biomarkers for neurodegenerative diseases. Nat Med 2021;27:954–63. 10.1038/s41591-021-01382-x [DOI] [PubMed] [Google Scholar]
- 2. Alcolea D, Delaby C, Muñoz L, et al. Use of plasma biomarkers for AT(N) classification of neurodegenerative dementias. J Neurol Neurosurg Psychiatry 2021;92:1206–14. 10.1136/jnnp-2021-326603 [DOI] [PubMed] [Google Scholar]
- 3. 2020 Alzheimer’s disease facts and figures. Alzheimer's & Dementia 2020;16:391–460. 10.1002/alz.12068 [DOI] [PubMed] [Google Scholar]
- 4. Lantero Rodriguez J, Karikari TK, Suárez-Calvet M, et al. Plasma p-tau181 accurately predicts Alzheimer's disease pathology at least 8 years prior to post-mortem and improves the clinical characterisation of cognitive decline. Acta Neuropathol 2020;140:267–78. 10.1007/s00401-020-02195-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karikari TK, Pascoal TA, Ashton NJ, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol 2020;19:422–33. 10.1016/S1474-4422(20)30071-5 [DOI] [PubMed] [Google Scholar]
- 6. Janelidze S, Mattsson N, Palmqvist S, et al. Plasma p-Tau181 in Alzheimer's disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer's dementia. Nat Med 2020;26:379–86. 10.1038/s41591-020-0755-1 [DOI] [PubMed] [Google Scholar]
- 7. Palmqvist S, Tideman P, Cullen N, et al. Prediction of future Alzheimer’s disease dementia using plasma phospho-tau combined with other accessible measures. Nat Med 2021;27:1034–42. 10.1038/s41591-021-01348-z [DOI] [PubMed] [Google Scholar]
- 8. Ashton NJ, Pascoal TA, Karikari TK, et al. Plasma p-tau231: a new biomarker for incipient Alzheimer's disease pathology. Acta Neuropathol 2021;141:709–24. 10.1007/s00401-021-02275-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative accuracy of plasma Phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA 2020;324:772–81. 10.1001/jama.2020.12134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer's disease and frontotemporal lobar degeneration. Nat Med 2020;26:387–97. 10.1038/s41591-020-0762-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mattsson-Carlgren N, Janelidze S, Palmqvist S, et al. Longitudinal plasma p-tau217 is increased in early stages of Alzheimer's disease. Brain 2020;143:3234–41. 10.1093/brain/awaa286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karikari TK, Benedet AL, Ashton NJ, et al. Diagnostic performance and prediction of clinical progression of plasma phospho-tau181 in the Alzheimer's disease neuroimaging initiative. Mol Psychiatry 2021;26:429–42. 10.1038/s41380-020-00923-z [DOI] [PubMed] [Google Scholar]
- 13. Tissot C, L. Benedet A, Therriault J, et al. Plasma pTau181 predicts cortical brain atrophy in aging and Alzheimer’s disease. Alzheimers Res Ther 2021;13:69. 10.1186/s13195-021-00802-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pereira JB, Janelidze S, Stomrud E, et al. Plasma markers predict changes in amyloid, tau, atrophy and cognition in non-demented subjects. Brain 2021;144:2826–36. 10.1093/brain/awab163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakamura A, Kaneko N, Villemagne VL, et al. High performance plasma amyloid-β biomarkers for Alzheimer's disease. Nature 2018;554:249–54. 10.1038/nature25456 [DOI] [PubMed] [Google Scholar]
- 16. Palmqvist S, Janelidze S, Stomrud E, et al. Performance of fully automated plasma assays as screening tests for Alzheimer disease-related β-amyloid status. JAMA Neurol 2019;76:1060–9. 10.1001/jamaneurol.2019.1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bridel C, van Wieringen WN, Zetterberg H, et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol 2019;76:1035–48. 10.1001/jamaneurol.2019.1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ashton NJ, Janelidze S, Al Khleifat A, et al. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat Commun 2021;12:3400. 10.1038/s41467-021-23620-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chatterjee P, Pedrini S, Stoops E, et al. Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer’s disease. Transl Psychiatry 2021;11:1–10. 10.1038/s41398-020-01137-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Elahi FM, Casaletto KB, La Joie R, et al. Plasma biomarkers of astrocytic and neuronal dysfunction in early- and late-onset Alzheimer's disease. Alzheimers Dement 2020;;16:681–95. Apr. 10.1016/j.jalz.2019.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cicognola C, Janelidze S, Hertze J, et al. Plasma glial fibrillary acidic protein detects Alzheimer pathology and predicts future conversion to Alzheimer dementia in patients with mild cognitive impairment. Alzheimers Res Ther 2021;13:68. 10.1186/s13195-021-00804-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thomas AJ, Mahin-Babaei F, Saidi M, et al. Improving the identification of dementia with Lewy bodies in the context of an Alzheimer’s-type dementia. Alzheimers Res Ther 2018;10:27. 10.1186/s13195-018-0356-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Donaghy PC, Firbank MJ, Thomas AJ, et al. Clinical and imaging correlates of amyloid deposition in dementia with Lewy bodies. Mov Disord 2018;33:1130–8. 10.1002/mds.27403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hall S, Janelidze S, Londos E, et al. Plasma phospho-tau identifies Alzheimer's Co-Pathology in patients with Lewy body disease. Mov Disord 2021;36:767–71. 10.1002/mds.28370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bevan-Jones WR, Surendranathan A, Passamonti L, et al. Neuroimaging of inflammation in memory and related other disorders (NIMROD) study protocol: a deep phenotyping cohort study of the role of brain inflammation in dementia, depression and other neurological illnesses. BMJ Open 2017;7:e013187. 10.1136/bmjopen-2016-013187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kane JPM, Roberts G, Petrides GS, et al. 123I-MIBG scintigraphy utility and cut-off value in a clinically representative dementia cohort. Parkinsonism Relat Disord 2019;62:79–84. 10.1016/j.parkreldis.2019.01.024 [DOI] [PubMed] [Google Scholar]
- 27. Prats-Sedano MA, Savulich G, Surendranathan A, et al. The revised Addenbrooke's cognitive examination can facilitate differentiation of dementia with Lewy bodies from Alzheimer's disease. Int J Geriatr Psychiatry 2021;36:831–8. 10.1002/gps.5483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jack CR, Wiste HJ, Weigand SD, et al. Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimers Dement 2017;13:205–16. 10.1016/j.jalz.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–9. 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 2017;89:1455. 10.1212/WNL.0000000000004058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Movement Disorders 2007;22:1689–707. 10.1002/mds.21507 [DOI] [PubMed] [Google Scholar]
- 32. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134:2456–77. 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76:1006–14. 10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP International workshop. Neurology 1996;47:1–9. 10.1212/wnl.47.1.1 [DOI] [PubMed] [Google Scholar]
- 35. Höglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder Society criteria. Mov Disord 2017;32:853–64. 10.1002/mds.26987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mioshi E, Dawson K, Mitchell J, et al. The Addenbrooke's cognitive examination revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry 2006;21:1078–85. 10.1002/gps.1610 [DOI] [PubMed] [Google Scholar]
- 37. Rittman T, Ghosh BC, McColgan P, et al. The Addenbrooke's cognitive examination for the differential diagnosis and longitudinal assessment of patients with parkinsonian disorders. J Neurol Neurosurg Psychiatry 2013;84:544–51. 10.1136/jnnp-2012-303618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Malpetti M, Passamonti L, Jones PS, et al. Neuroinflammation predicts disease progression in progressive supranuclear palsy. J Neurol Neurosurg Psychiatry 2021;92:769–75. 10.1136/jnnp-2020-325549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ashton NJ, Hye A, Rajkumar AP, et al. An update on blood-based biomarkers for non-Alzheimer neurodegenerative disorders. Nat Rev Neurol 2020;16:265–84. 10.1038/s41582-020-0348-0 [DOI] [PubMed] [Google Scholar]
- 40. Vergallo A, Bun R-S, Toschi N, et al. Association of cerebrospinal fluid α-synuclein with total and phospho-tau181 protein concentrations and brain amyloid load in cognitively normal subjective memory complainers stratified by Alzheimer's disease biomarkers. Alzheimers Dement 2018;14:1623–31. 10.1016/j.jalz.2018.06.3053 [DOI] [PubMed] [Google Scholar]
- 41. Ishiki A, Kamada M, Kawamura Y, et al. Glial fibrillar acidic protein in the cerebrospinal fluid of Alzheimer's disease, dementia with Lewy bodies, and frontotemporal lobar degeneration. J Neurochem 2016;136:258–61. 10.1111/jnc.13399 [DOI] [PubMed] [Google Scholar]
- 42. Oeckl P, Halbgebauer S, Anderl-Straub S, et al. Glial fibrillary acidic protein in serum is increased in Alzheimer's disease and correlates with cognitive impairment. J Alzheimers Dis 2019;67:481–8. 10.3233/JAD-180325 [DOI] [PubMed] [Google Scholar]
- 43. Surendranathan A, Rowe JB, O'Brien JT. Neuroinflammation in Lewy body dementia. Parkinsonism Relat Disord 2015;21:1398–406. 10.1016/j.parkreldis.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 44. Surendranathan A, Su L, Mak E, et al. Early microglial activation and peripheral inflammation in dementia with Lewy bodies. Brain 2018;141:3415–27. 10.1093/brain/awy265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Malpetti M, Rittman T, Jones PS, et al. In vivo PET imaging of neuroinflammation in familial frontotemporal dementia. J Neurol Neurosurg Psychiatry 2021;92:319–22. 10.1136/jnnp-2020-323698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Katisko K, Cajanus A, Huber N, et al. Gfap as a biomarker in frontotemporal dementia and primary psychiatric disorders: diagnostic and prognostic performance. J Neurol Neurosurg Psychiatry 2021;92:1305–12. 10.1136/jnnp-2021-326487 [DOI] [PubMed] [Google Scholar]
- 47. Heller C, Foiani MS, Moore K, et al. Plasma glial fibrillary acidic protein is raised in progranulin-associated frontotemporal dementia. J Neurol Neurosurg Psychiatry 2020;91:263–70. 10.1136/jnnp-2019-321954 [DOI] [PubMed] [Google Scholar]
- 48. Cullen NC, Leuzy A, Janelidze S, et al. Plasma biomarkers of Alzheimer's disease improve prediction of cognitive decline in cognitively unimpaired elderly populations. Nat Commun 2021;12:3555. 10.1038/s41467-021-23746-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xiao Z, Wu X, Wu W, et al. Plasma biomarker profiles and the correlation with cognitive function across the clinical spectrum of Alzheimer's disease. Alzheimers Res Ther 2021;13:123. 10.1186/s13195-021-00864-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jellinger KA, Attems J. Neuropathological evaluation of mixed dementia. J Neurol Sci 2007;257:80–7. 10.1016/j.jns.2007.01.045 [DOI] [PubMed] [Google Scholar]
- 51. Schneider JA, Arvanitakis Z, Bang W, et al. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007;69:2197–204. 10.1212/01.wnl.0000271090.28148.24 [DOI] [PubMed] [Google Scholar]
- 52. Zetterberg H, Blennow K. Moving fluid biomarkers for Alzheimer's disease from research tools to routine clinical diagnostics. Mol Neurodegener 2021;16:10. 10.1186/s13024-021-00430-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Alexander GC, Emerson S, Kesselheim AS. Evaluation of Aducanumab for Alzheimer disease: scientific evidence and regulatory review involving efficacy, safety, and futility. JAMA 2021;325:1717–8. 10.1001/jama.2021.3854 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2021-327788supp001.pdf (216.2KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data are available upon reasonable request. Anonymised data may be shared by request to the senior author from a qualified investigator for non-commercial use (data sharing may be subject to restrictions according to consent and data protection legislation).