Abstract
Hereditary transthyretin amyloidosis (ATTRv) is a severe, adult-onset autosomal dominant inherited systemic disease predominantly affecting the peripheral and autonomic nervous system, heart, kidney and the eyes. ATTRv is caused by mutations of the transthyretin (TTR) gene, leading to extracellular deposition of amyloid fibrils in multiple organs including the peripheral nervous system. Typically, the neuropathy associated with ATTRv is characterised by a rapidly progressive and disabling sensorimotor axonal neuropathy with early small-fibre involvement. Carpal tunnel syndrome and cardiac dysfunction frequently coexist as part of the ATTRv phenotype. Although awareness of ATTRv polyneuropathy among neurologists has increased, the rate of misdiagnosis remains high, resulting in significant diagnostic delays and accrued disability. A timely and definitive diagnosis is important, given the emergence of effective therapies which have revolutionised the management of transthyretin amyloidosis. TTR protein stabilisers diflunisal and tafamidis can delay the progression of the disease, if treated early in the course. Additionally, TTR gene silencing medications, patisiran and inotersen, have resulted in up to 80% reduction in TTR production, leading to stabilisation or slight improvement of peripheral neuropathy and cardiac dysfunction, as well as improvement in quality of life and functional outcomes. The considerable therapeutic advances have raised additional challenges, including optimisation of diagnostic techniques and management approaches in ATTRv neuropathy. This review highlights the key advances in the diagnostic techniques, current and emerging management strategies, and biomarker development for disease progression in ATTRv.
Keywords: amyloid, neuropathy
Introduction
Amyloidoses refer to a heterogeneous group of diseases that are pathologically characterised by aggregation of amyloid-fibril proteins deposited extracellularly, resulting in a toxic gain of function.1 In accordance with the official amyloid fibril protein nomenclature list published by the International Society of Amyloidosis, 36 amyloid fibril proteins are known to cause amyloidosis, with diseases classified according to the nature of the amyloid precursor protein.2 Transthyretin (TTR) is a human 56 kDa non-glycosylated amyloidogenic protein. Hereditary transthyretin amyloidosis (ATTRv) is caused by the deposition of variant TTR protein. The main ATTRv phenotypes include polyneuropathy (hereditary transthyretin amyloidosis–polyneuropathy (ATTRv-PN)), hereditary transthyretin amyloidosis–cardiomyopathy (ATTRv-CM), and renal and ocular involvement. Hereditary transthyretin leptomeningeal amyloidosis (ATTRv-LA) is a rare neurological phenotype. ATTRv has been regarded as a rare endemic disorder; however, advances in diagnostic techniques have indicated that ATTRv is more frequent than previously recognised.1 Wild-type transthyretin amyloidosis (ATTRwt) manifests later and is increasingly recognised as the cause of amyloid cardiomyopathy, although it can also rarely cause peripheral neuropathy.3
Advances in understanding of ATTRv pathogenesis has led to development of novel and therapeutic strategies. Aside from liver transplantation, alternative therapeutic options have including TTR tetramer stabilisers, genomic approaches using antisense oligonucleotide and small interfering RNA (siRNA) technologies, as well as novel TTR protein stabilisers and fibril removers. The following review examines the structure and function of TTR, potential pathophysiological mechanisms in ATTRv and the clinical phenotypes associated with ATTRv. Lastly, utility of therapeutic approaches targeting specific pathophysiological mechanisms in ATTRv will be appraised.
Structure and function of TTR
TTR is 55 kDa transport protein that is predominantly synthesised by the liver and secreted into the bloodstream. The main physiological function of TTR is to transport retinol-binding protein–vitamin A complex (holoRBP) and thyroxine (T4). TTR has a tetrameric structure composed of four identical beta-sheet rich subunits with two T4 and four holoRBP-binding sites.4 Studies of TTR knockout (TTR-KO) mice have reported sensorimotor dysfunction and impairment of nerve recovery in response to crush injuries.5 Expression of human TTR in TTR-KO mice rescued the phenotype, reinforcing the importance of TTR in nerve repair.5 A neuroprotective role for TTR in the central nervous system (CNS) has also been reported.6 TTR is synthesised by the choroid plexus, retinal pigment epithelium and pancreas,7 where it may contribute to a variety of physiological functions including transport of thyroid hormones.
Genetics of ATTRv
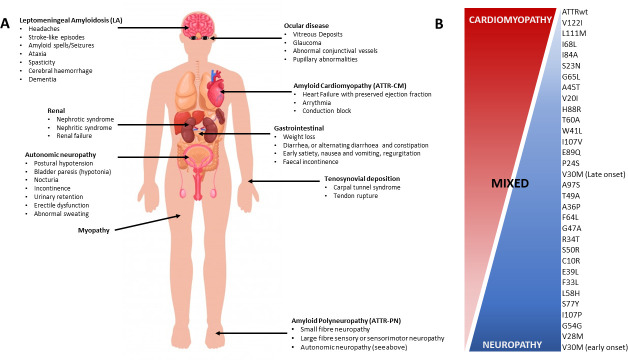
ATTRv encompasses a range of phenotypes (figure 1), with autosomal dominant pattern of inheritance. To date, 140 different TTR mutations have been reported, with the Val30Met (p.Val50Met) variant being the most frequent.8 The Val30Met mutation is responsible for high prevalence of ATTRv in endemic areas, including Portugal (incidence 0.87/100 000, prevalence 22.9/100 000),9 Sweden and Japan.10 The carrier frequency in the Swedish population is high (~1.5%), with evidence of anticipation (~11.7 years).11 The risk of anticipation was higher in males and with maternal transmission. In non-endemic regions, the carrier frequency of the Val30Met variant is lower.7 Carrier frequency markedly varies between racial backgrounds, as underscored by the frequent occurrence of the ATTRv Val122Ile (p.Val142Ile) mutation in Afro-American, West African and Hispanic populations. Distinct founder mutations have been reported in other regions, including the Thr60Ala (p.Thr80Ala) TTR variant in northwest Ireland, and THE Ala97Ser (p.Ala117Ser) variant in China and Taiwan.12 13
Figure 1.
(A) Clinical features and genotype–phenotype correlations. (A) ATTRv a multisystem disease with a variety of clinical features. The most frequent phenotypes include ATTR polyneuropathy (ATTRv-PN), ATTR cardiomyopathy (ATTRv-CM) and ATTR leptomeningeal amyloidosis (ATTRv-LA). (B) To date, 140 mutations in the ATTR gene (TTR) have been reported that lead to ATTRv. The Val30Met is the most frequent mutation, reported globally and in endemic regions. While mutations may give proclivity to specific phenotypes, a poor correlation between phenotype and genotype has been reported. For example, ATTRv Val30Met may be associated with early-onset and late-onset ATTRv-PN, with a paucity of cardiomyopathy in the former. ATTRwt is predominantly associated with ATTR-CM, with relative absence of neuropathy, although carpal tunnel is a common feature. (B) Modified with permission from Castaño and colleagues.111 ATTR, transthyretin amyloidosis; ATTR-PN, transthyretin amyloidosis–polyneuropathy; ATTRv, hereditary transthyretin amyloidosis; ATTRv-CM, hereditary transthyretin amyloidosis–cardiomyopathy; ATTRv-LA, hereditary transthyretin leptomeningeal amyloidosis; ATTRv-PN, hereditary transthyretin amyloidosis–polyneuropathy; ATTRwt, wild-type transthyretin amyloidosis; TTR, transthyretin.
Although different TTR mutations may result in phenotypical overlap, mutations causing predominantly neuropathic, cardiomyopathic or mixed phenotypes have been reported (figure 1). Early-onset Val30Met mutations present with peripheral neuropathy, while Thr60Ala variants often manifest as mixed neuropathic and cardiomyopathic phenotypes. In contrast, the Val122Ile variant presents with late-onset disease with predominant cardiac dysfunction.7 Phenotype heterogeneity has been reported for the same mutations, both across different pedigrees and within familial groups.14 Variability in age of disease onset has been reported, with the best predictor being onset age within the family pedigree. Although ATTRv exhibits an autosomal dominant pattern of inheritance, incomplete penetrance has been described, with different mutations and populations exhibiting varied penetrance rates. While penetrance can be difficult to ascertain for late-onset variants, early-onset phenotypes (Val30Met) have a penetrance of 80% at 50 years and 91% at 70 years in Portuguese, compared with 15% at 50 years in Swedish patients.11 15 The interaction between carrier frequency, age of onset and penetrance determines population prevalence of ATTRv. While the Val30Met carrier frequency in Portugal is low, the high penetrance and early age of onset result in a high population prevalence.9
Clinical spectrum of ATTRv phenotype
ATTRv amyloidosis is a multisystem disease as summarised in figure 1A. Understanding the clinical spectrum of ATTRv will raise suspicion and thereby enable early diagnosis of ATTRv, allowing the institution of effective therapies.
Hereditary transthyretin amyloidosis–polyneuropathy
ATTRv-PN is the the most common of the amyloid phenotypes and most frequently associated with the Val30Met mutation. Early-onset ATTRv-PN phenotype is common in Portugal and Japan, characterised by onset at <50 years, high penetrance and progressive axonal length‐dependent sensorimotor polyneuropathy.1 16 Small-fibre neuropathy is also frequently reported, characterised by loss of nociception and thermal sensation, burning feet and paresthesias in distal lower extremities.17 18 Autonomic neuropathy (AN) occurs in ~75% of patients with ATTRv, characterised by orthostatic hypotension (lightheadedness, exercise intolerance and non-specific fatigue), gastrointestinal (gastroparesis, recurrent vomiting, diarrhoea or alternating diarrhoea–constipation and faecal incontinence) and genitourinary (urinary retention, nocturia, incomplete emptying and frequency, and erectile dysfunction) symptoms.19 Autonomic symptoms may be disabling, with high morbidity from arrhythmias and sudden death. Malnutrition and weight loss may ensue, which are adverse prognostic factors. Amyloid deposition in the bowel wall may contribute to gastrointestinal symptoms. Cardiac conduction defects appear frequently, requiring pacemaker implantation, along with myocardial thickening.20 Late-onset ATTRv-PN phenotype begins at >50 years of age and occurs in endemic and non-endemic regions. Clinically, it is characterised by progressive axonal sensorimotor neuropathy affecting all sensory modalities, variable penetrance, lack of family history and milder AN symptoms.21–23 Neuropathic pain is more frequent than in early-onset ATTRv,23 particularly with the Thr60Ala variant.24 Upper limb-onset axonal neuropathy has also been reported,25 as have gait ataxia with large-fibre sensory loss, paucity of muscle weakness and generalised areflexia.26 ATTRv-PN may be misdiagnosed as chronic inflammatory polyradiculoneuropathy, with slowing of motor conduction velocities in the demyelinating range, elevated cerebrospinal fluid (CSF) protein, and paucity of autonomic and systemic involvement.27 A pure motor syndrome with absence of AN has also been observed in non-Val30Met variants.28 Amyloid cardiomyopathy and gastrointestinal dysfunction are frequently observed in late-onset ATTRv.16 21
Bilateral carpal tunnel syndrome (CTS) frequently occurs in early-onset and late-onset ATTRv-PN and ATTRwt, commonly preceding other organ involvement by years (Rukavina type).22 Other neuromuscular manifestations include myopathy, exhibiting a limb or axial pattern of muscle weakness, mildly elevated creatine kinase levels, absence of dysphagia and presence of systemic features.18 Spinal canal stenosis is also a feature of transthyretin amyloidosis (ATTR) due to amyloid deposition in the ligamentum flavum, resulting in neurogenic claudication.29
Hereditary transthyretin amyloidosis–cardiomyopathy
ATTRv-CM is evident in ~50% of patients,16 and while frequently associated with Val122Ile (~3% to 4% of African-Americans) and Ile111Met (p.Ile131Met) TTR variants, other mutations are more common in Caucasian, Hispanic and Asian populations.1 16 ATTRv-CM is more common in late-onset (Val30Met) ATTRv, manifesting as restrictive cardiac failure, otherwise known as heart failure with preserved ejection fraction (HFpEF), conduction blocks, ventricular arrhythmias or sudden cardiac death.19 ATTRv-CM is indistinguishable by clinical findings or investigation characteristics from ATTRwt–cardiomyopathy. Low-voltage ECG or pseudo-infarct patterns in right praecordial leads are consistent with ATTR-CM. Myocardial thickening, along with subendocardial late gadolinium enhancement, is a characteristic cardiac MRI features30 Echocardiography demonstrates thickened septal and ventricular walls, impaired diastolic relaxation and reduced global logitudinal strain with an apical sparing pattern.31 Additionally, 99mTc-pyrophosphate (PYP), 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid (DPD) or 99mTc-hydroxymethylene diphosphonate (HMDP) scintigraphy is considered the most sensitive for detecting amyloid deposition in ATTR-CM. Myocardial sympathetic denervation, measured by 123-iodine metaiodobenzylguanidine imaging, is an independent predictor of mortality and represents the importance of autonomic changes in ATTR-CM.32 ATTRv-CM is associated with increased mortality and is the major cause of death in non-endemic regions.33
Leptomeningeal amyloidosis
ATTRv-LA is a rare manifestation of ATTRv, most frequently associated with non-Val30Met mutations (Leu12Pro, Ala25Thr, Gly53Glu, Tyr114Cys, Asp18Gly or Tyr69His). Pathologically, ATTRv-LA is characterised by cerebral amyloid angiopathy and leptomeningeal amyloidosis.14 22 Amyloid deposits may be evident in the media and adventitia of medium-sized and small-sized cortical arteries, arterioles and veins, as well as veins in the subarachnoid space and leptomeninges. In the CNS, the choroid plexus is the main source of amyloid. Ischaemic strokes and intracerebral haemorrhage, along with subarachnoid haemorrhage and hydrocephalus, are common complications. Symptoms include headaches, hearing or visual loss, cerebral haemorrhages, dementia, ataxia, spasticity, transient focal neurological episodes (amyloid spells) and seizures.34 Neuropathy is an inconsistent feature of ATTRv-LA.8 Separately, ATTRv-LA may be evident in patients with the Val30Met mutation, typically 11 years after liver transplantation, due to ongoing variant TTR production by the choroid plexus.35 Age-related cognitive dysfunction was reported in older patients with ATTRv (>50 years) with Val30Met, implying the pathogenic importance of centrally produced TTR variant protein.36 Cranial nerves can be affected, mimicking amyotrophic lateral sclerosis.37
Other organ involvement in ATTRv
Renal disease, manifesting as proteinuria, nephrotic syndrome and progressive renal failure, occurs in one-third of Portuguese patients (Val30Met), most commonly evident in the late-onset phenotype.38 39 Microalbuminuria can be the first abnormality, and 10% develop end-stage renal disease secondary to vascular and glomerular amyloid depositions. Ocular involvement, manifesting as dry eyes and keratoconjunctivitis, vitreous opacity due to amyloid deposition, cataracts, secondary open-angle glaucoma and retinal amyloid angiopathy may be evident in up to 25% of patients with ATTRv typically evident with Val30Met mutations.40 Gastrointestinal symptoms result from both AN and direct deposition of amyloid in the bowel wall, leading to small intestinal bacteria overgrowth (blind-loop phenomenon)41
Wild-type transthyretin amyloidosis
ATTRwt, previously known as senile systemic amyloidosis, is a common disease of ageing, with autopsy studies disclosing that 10%–25% of over 80-year-olds had wild-type amyloid deposition in the myocardium, and 44% in the gastrointestinal tract and subcutaneous tissue.42 A significant burden of amyloid deposition is required to cause symptomatic disease, and hence, histopathological deposition does not necessarily equate to clinical significance. ATTRwt presents between the sixth-eighth decades of life, manifesting as HFpEF, atrial fibrillation and ventricular arrhythmias, occasionally requiring implantation of a cardioverter defibrillator.43 CTS is a common presenting feature,21 as is spinal canal stenosis, while other systemic manifestations are less frequent.19 21
Diagnostic and prognostic biomarkers for ATTR
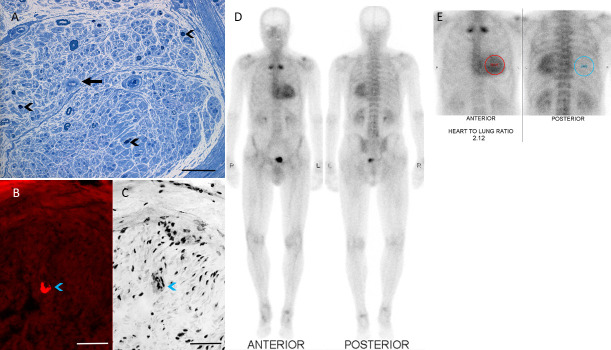
The diagnosis of amyloidosis typically relies on pathological identification of amyloid deposits. Congo red staining with apple green birefringence under polarised light represents the pathognomonic appearance of amyloid (figure 2A–C). While biopsy of a target organ has the highest yield, less invasive approaches, including abdominal fat aspiration or salivary gland biopsy, exhibit sensitivities between 50%–80% and specificity of 90%.19 In clinical practice, diagnostic yields are lower, and if clinical suspicion is high, biopsy of involved tissue should be performed.
Figure 2.
Investigation findings in ATTR. Sural nerve biopsy demonstrating typical features of amyloidosis. (A) Severe reduction in myelinated and unmyelinated nerve fibre density with evidence of active degeneration (black arrowheads); amyloid deposition is observed in endoneural blood vessels, resulting in thickened vessels walls (black arrow), toluidine blue; scale bar=0.05 mm; (B, C) Amyloid deposits in the endoneurial blood vessel wall (blue arrowheads), Congo red-stained frozen sections. (B) Immunofluorescence with Texas red filter and (C) transmitted light. Scale bars=0.1 mm. (D, E) Technetium-99m-pyrophosphate scintigraphy in hereditary transthyretin demonstrating cardiac uptake compared with surrounding tissues with a heart to contralateral lung ratio of 2.1 (normal <1.5). ATTR, transthyretin amyloidosis.
Sural nerve biopsy (figure 2A–C) has been traditionally used for confirming ATTRv-PN and for excluding other aetiologies, with a sensitivity of up to 86%.19 The patchy distribution of amyloid deposits may limit diagnostic utility, potentially requiring repeated nerve biopsies or biopsies of different organs.44 MR neurography,45 peripheral nerve ultrasound46 and whole-body 18F-florbetapir positron emission tomography/MRI imaging47 are promising techniques for detecting early nerve dysfunction and for targeting sites for biopsy when clinical suspicion remains high.
Skin biopsy may be of utility in early diagnosis of neuropathy, disclosing reduced intraepidermal nerve fibre density and Congo red positive amyloid deposits.48 Confocal corneal microscopy is an emerging technique for detecting small-fibre neuropathy and represents a non-invasive alternative. Corneal nerve fibre length (CNFL) is shorter in ATTRv and correlates with autonomic, clinical and neurophysiological measures of large-fibre neuropathy.49 Additionally, CNFL has no floor effect when compared with neurophysiological testing and intraepidermal nerve fibre density measurements.
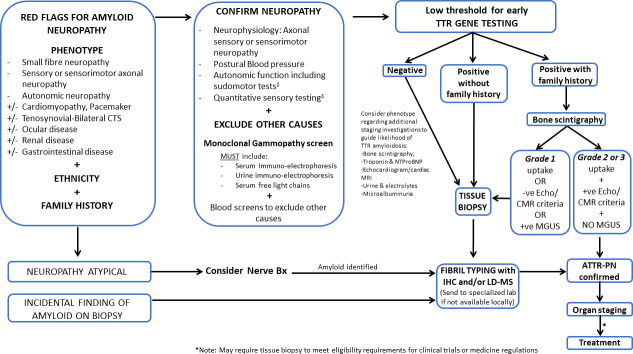
Identification of a TTR gene mutation is strongly suggestive of ATTRv if the neurological phenotype and investigations are concordant, including positive cardiac uptake on 99 m Tc-PYP, 99 m Tc-DPD or HMDP scintigraphy with negative monoclonal gammopathy and general neuropathy screen (figure 3).19 TTR mutations can be excluded or established on genetic testing, as sequence analysis identifies >99% of pathogenic variants, with updated lists of TTR mutations and phenotypes reported in an online database (http://www.amyloidosismutations.com/mut-attr.php). Diagnostic practices may vary across countries, and pathological diagnosis remains the gold standard. Genetic testing is required to differentiate between ATTRwt and ATTRv.
Figure 3.
Diagnostic algorithm for ATTR polyneuropathy. Tissue biopsy may include a target organ such as nerve, heart, bone marrow or skin, or an ‘off-target’ biopsy such as salivary gland or abdominal fat pad aspirate. $Depending on availability; IHC and LD-MS are important for amyloid fibril typing. Bone scintigraphy may be semiquantitatively graded relative to rib uptake with the following grades: grade 0, no uptake and normal bone scan; grade 1, uptake less than rib uptake; grade 2, uptake equal to rib uptake; and grade 3, uptake greater than rib uptake with mild or absent rib uptake abnormalities. Echo and CMR are important investigations for detecting cardiac amyloid disease. ATTR, transthyretin amyloidosis; ATTR-PN, transthyretin amyloidosis–polyneuropathy; CMR, cardiac MRI; CTS, carpal tunnel syndrome; Echo, echocardiogram; IHC, immunohistochemistry; LD-MS, laser dissection–mass spectrometry; MGUS, monoclonal gammopathy of uncertain significance; TTR, transthyretin.
Once amyloid deposition is confirmed histologically, amyloid protein typing is essential to guide management. Immunohistochemical stains can be used, although amyloid precursor protein is identified in only 30% of cases.50 Mass spectrometry of tissue specimens, derived by laser microdissection, can accurately type the amyloid precursor protein with a sensitivity of 80% in cases where immunohistochemical staining was unrevealing.50
99 m Tc-PYP and 99 m Tc-DPD scintigraphy (figure 2D, E) demonstrate ATTR amyloid deposition in the myocardium with high sensitivity, specificity and positive predictive value (>99%) when there is no concurrent monoclonal gammopathy on serum and urine electrophoresis or immunofixation, and in the absence of serum-free light chains.51 Consequently, non-biopsy diagnosis of ATTR-CM amyloidosis is now possible when there is considerable cardiac bone scintigraphy uptake (grade 2 or 3) and absence of monoclonal protein (figure 3). In cases where pathological TTR mutations coexist with monoclonal gammopathy of uncertain significance, neuropathy could result from either ATTRv or light-chain amyloidosis (AL). Pathological confirmation with fibril typing is essential, preferably by mass spectrometry (figure 3).51
Plasma neurofilament light chain (NfL) is emerging as a useful prognostic biomarker in ATTR-PN, demonstrating a fourfold increase and correlating with disease severity.52 52 53 53 54 54 NfL may discriminate between asymptomatic Val30Met mutation carriers and ATTR-PN in early disease.53 A significant reduction of NfL levels was reported in patients with ATTR-PN treated with gene silencing therapies, correlating with an improvement in the neuropathy impairment score.54
Diagnostic approach for ATTR neuropathy
A putative diagnostic algorithm for ATTRv-PN is depicted in figure 3. In all patients presenting with polyneuropathy, particularly with small fibre or AN, we recommend a review of amyloid red flag symptoms, such as the presence of bilateral CTS, cardiomyopathy or pacemaker insertion, along with consideration of ethnicity and family history (figure 3). It is essential to consider amyloidosis in these scenarios to shorten diagnostic delays and prevent misdiagnosis. As ATTRv is now treatable, we suggest a low threshold for early TTR gene testing (figure 3).
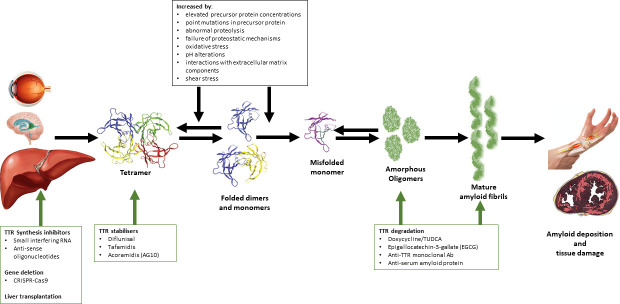
Figure 4.
Mechanisms by which TTR mutants exert pathogenesis and therapeutic strategies for ATTRv. TTR is predominantly produced by the liver. The conversion of TTR tetramer into insoluble amyloid fibrils is a multistep dynamic process, with dissociation of TTR tetramers into misfolded monomers being the rate-limiting step for the formation of amyloid fibrils. TTR gene mutations destabilise quaternary and tertiary TTR structures and induce thermodynamic instability, resulting in the formation of misfolded monomers. Amyloid fibril formation occurs by nucleation-dependent polymerisation and is influenced by a variety of physiological factors. Therapeutic strategies aimed at different stages of amyloid formation have shown efficacy in ATTRv. ATTRv, hereditary transthyretin amyloidosis; TTR, transthyretin.
If a TTR gene variant is detected in cases with no family history, a tissue diagnosis is required. Practice varies across countries and with different phenotypical expression, but may include abdominal fat pad aspirate, skin, salivary gland, nerve or other target organ biopsies. Amyloid protein typing by immunohistochemistry or preferably laser dissection–mass spectrometry is critical, particularly in the setting of a coincidental paraprotein (figure 3). Occasionlly, amyloid is identified incidentally on a diagnostic nerve biospy. In this circumstance, typing of the amyloid fibril is critical, as while more commonly AL, ATTRv is a possibility and should prompt gene testing. If local laboratories are not able to confidently type the fibril, we recommend that tissue be referred to a specialised laboratory for fibril typing. Early gene testing may help to guide typing and improve time to diagnosis.
In an individual with typical neuropathy and biopsy-proven family history of ATTRv, gene testing should be performed as an initial investigation (figure 3). If a TTR variant is identified, a 99m-Tc-DPD or 99m-Tc-PYP bone scan may be performed as the next diagnostic investigation. ATTR may be diagnosed without the need for tissue biopsy, if grade 2 or 3 scintigraphic uptake abnormalities are identified and if echocardiographic/cardiac MRI criteria are satisfied, and a paraprotein is not identified on complete monoclonal gammopathy testing (serum and urine electrophoresis, immunofixation and serum-free light chains). For many clinical trials and medicine regulators, biopsy confirmation may be required. If bone scintigraphy, echocardiogram or cardiac MRI criteria are not met, tissue biopsy is required for diagnosis (figure 3). TTR gene mutations may coexist with other neuropathy-causing diseases, especially in older patients, such as diabetes mellitus, and in this circumstance, a nerve biospy should be considered to confirm that the neuropathy is secondary to amyloidosis. It should be stressed that additional diagnostic approaches have been propsoed and are discussed in detail elsewhere.55
Pathogenesis of ATTRv
TTR amyloid fibril formation
The conversion of TTR tetramer into insoluble amyloid fibrils is a multistep dynamic process (figure 4). Dissociation of TTR tetramer into misfolded monomers appears to be a rate-limiting step for formation of amyloid fibrils and other aggregate morphologies.1 TTR gene mutations destabilise quaternary and tertiary TTR structures and induce thermodynamic instability, thereby favouring formation of misfolded monomers.4 16 These destabilising effects impact the cell’s efficiency at degrading and secreting mutant TTR, thereby modulating disease severity. However, it must be noted that TTR amyloid deposition can occur without destabilising mutations, as is observed in ATTRwt. Further, wild-type TTR is frequently identified in amyloid deposits in patients with ATTRv, in particular in late-onset ATTRV30M cases and in cardiac tissues after liver transplantation.56 57
Amyloid fibril formation occurs by nucleation-dependent polymerisation, whereby formation of the oligomeric high-energy quaternary structures is required prior to aggregation of misfolded monomers.14 Subsequently, deposition of TTR monomers proceeds by spontaneous downhill polymerisation, and C-terminal TTR protein fragments produced by trypsin proteolysis are likely to promote TTR aggregation.58 59 The formation of amyloid fibrils is influenced by several factors including temperature, pH, presence of cosolvent precursor protein concentration, abnormal proteolysis and proteostatic mechanisms, oxidative stress, shear stress, pH alterations and interactions with extracellular matrix components, such as sulfated glycosaminoglycans.60 61
Toxicity of amyloid fibrils
Amyloid fibrils may cause tissue damage by compression, obstruction, cellular toxicity or disturbance of local blood circulation.16 Vascular deposition may be a pathological feature of systemic amyloidosis, resulting in atypical amyloid presentations, such as jaw claudication and myocardial ischaemia in the absence of atherosclerotic disease. Cellular toxicity is mediated by low-molecular-weight oligomers and protofibrils,1 by activation of the receptor for advanced glycation end products, resulting in endoplasmic reticulum stress, caspase-dependent apoptosis and activation of extracellular signal-regulated kinases with disruption of cellular differentiation.16 Induction of calcium influx and increased oxidative stress represent additional pathogenic mechanisms by which oligomers and protofibrils exert toxic effects. Furthermore, direct fibril ultrastructural damage has been identified by a recent electron microscopic study which demonstrated amyloid fibril maturation from prefibrillar and rounded structures arising within the amorphous extracellular material. Fibril maturation resulted in distortion of the surrounding Schwann cell basement and cytosolic membranes, leading to atrophy of Schwann cells.62
Disease-modifying therapies for ATTRv
Strategies for treating ATTRv are directed at reducing the production and deposition of the pathogenic TTR.1 The following therapeutic approaches have shown efficacy: (1) targeting of TTR production, (2) stabilisation of TTR tetramers and (3) degradation of TTR amyloid deposits. Online supplemental table 1 provides a summary of clinical trials in ATTRv.
jnnp-2021-327909supp001.pdf (138.8KB, pdf)
Liver transplantation
Liver transplantation is effective at ameliorating the course of ATTRv63 by replacing the mutated TTR gene with wild type, resulting in marked reduction of variant TTR serum concentrations and regression of amyloid deposition.64 The survival rates of 5 and 10 years are 85% and 73%, respectively,65 with greater effects evident in Val30Met ATTR-PN. Early-onset disease, shorter disease duration, better nutritional status prior to transplantation and improved perioperative management have been associated with improved survival.65 66 No significant effects were reported in late-onset disease ATTRv, although women with late-onset disease had a longer survival.66 The outcome after liver transplantation is better for live compared with cadaveric donor grafts.67
Liver transplantation is associated with improvement or stabilisation in peripheral neuropathy and AN.16 20 63 65 While liver transplantation is an accepted therapeutic option, limitations include procedure-related morbidity and mortality (~10%), requirement for donors and long-term immunosuppression, along with lack of equivalent efficacy for non-Val30Met ATTRv. Liver transplantation fails to reduce TTR production in the choroid plexus and retinal pigment cells, potentially leading to development of oculoleptomeningeal disease.68 Domino-liver transplantation was used to overcome organ shortage, but recipients developed polyneuropathy about 10 years after intervention.69 Nowadays, liver transplantation could be considered in patients refractory or intolerant to other pharmacological options, or when other treatment options are unavailable.
TTR tetramer stabilisers
Diflunisal
Diflunisal is an oral non-steroidal anti-inflammatory drug that inhibits amyloid fibril formation by binding to the T4 binding sites of TTR protein.14 Diflunisal led to stabilisation of variant TTR tetramer and reduction of protein aggregation.70 A phase III trial demonstrated efficacy of diflunisal (250 mg two times per day) in ATTRv-PN (Val30Met and non-Val30Met mutations), with sustained slowing of neuropathy progression and improvement in quality of life.71
A long-term open-label study demonstrated stabilisation in peripheral neuropathy, although the alteration in rate of decline was not significant.14 Separately, cardiac dysfunction was reduced by diflunisal. Significant side effects were reported, including gastrointestinal symptoms and transitory renal function impairment.72
Tafamidis
Tafamidis is an oral TTR–tetramer protein stabiliser which reduces TTR–variant protein aggregation by associating with the T4 binding site.73 While tafamidis failed to meet the coprimary endpoints (Neuropathy Impairment Score–Lower Limbs (NIS-LL) and Norfolk Quality of Life–Diabetic Neuropathy (QOL-DN) total scores),74 a greater proportion of tafamidis-treated patients were classified as NIS-LL responders and reported preserved total qulaity of life (TQOL), suggesting clinical efficacy. An open-label extension study disclosed stabilisation of NIS-LL and TQOL scores,74 with greater preservation of neurological function in patients initiated at earlier stages of disease progression. The clinical benefit was associated with stabilisation of plasma TTR levels. Efficacy and safety of tafamidis was also reported in a small cohort of Japanese patients with ATTRv (Val30Met and non-Val30Met mutations).75 A long-term extension study in mild ATTR-PN reported a sustained delay in neuropathy progression and long-term preservation of nutritional status.76 No clinical effects were evident in patients with ATTRv with advanced disease.77 Tafamidis reduced mortality risk in early (91%) and late (82%) affected patients compared with untreated patients.78 Greater baseline neurological dysfunction appears to be an independent predictor of disease progression, and early treatment was associated with slower rates of disease progression.79 The European medicine agency approved tafamidis in 2011 for stage 1 ATTRv, although 30%–40% of the patients were considered non-responders.
A recent multicentre phase III trial established efficacy of tafamidis in ATTR-CM,80 with lower mortality, cardiovascular-related hospitalisations, improved quality of life and functional capacity. A subsequent long-term study reaffirmed the clinical effectiveness.81 Tafamidis was approved in 2020 for treating ATTRwt and ATTRv-related cardiomyopathy.
Other TTR stabilisers
Acoramidis
AG10 is a novel and selective oral TTR stabiliser developed for treatment of ATTR.82 Recent studies demonstrated the safety and biological efficacy of AG10.82 83 Currently, phase III trials are assessing the effectiveness of AG10 in ATTR-CM (NCT03860935) and ATTR-PN (NCT04882735).
Green tea extract, which contains the catechin epigallocatechin-3-gallate (EGCG), is an inhibitor of amyloid fibril formation and disrupts fibril aggregation.84 Orally administered EGCG was associated with stabilisation of cardiac function in ATTRwt amyloidosis.85 Utility of EGCG in ATTRv neuropathy remains unresolved, although high CNS bioavailability makes this an interesting target for potential multidrug therapies. Tolcapone, a TTR stabiliser that effectively crosses the blood–brain barrier, is being investigated as a potential treatment for ATTRv-LA.86
TTR fibril removal
Combination therapy with doxycycline, which interferes with formation of TTR amyloid fibrils,87 and tauroursodeoxycholic acid (TUDCA), a biliary acid that reduces non-fibrillary TTR aggregation, was reported to exert synergistic effect in the TTR mouse model (Val30Met).88 Stabilisation of neurological and cardiac function over 12 months was reported by combining doxycycline (200 mg/day) and TUDCA (250 mg three times per day).89 Monoclonal antibodies developed against serum amyloid P (SAP) (constituent that contributes to aggregate stability) and TTR (including PRX004) have disclosed mixed results. While initial murine and human studies of anti-SAP led to clearance of amyloid tissue deposits, ongoing clinical trials have been suspended.90–92 A recent small phase I trial of PRX004 demonstrated safety and tolerability of anti-TTR treatment while reducing progression of neuropathy and cardiac disease (https://ir.prothena.com/static-files/ee65ab75-2e8b-4eae-989b-3de7c7bd9149). Phase II studies of anti-TTR monoclonal antibodies are ongoing.
Gene therapies
Gene silencing therapies using antisense oligonucleotides (ASOs) or siRNAs have emerged as safe and effective strategies for treating ATTR. The two main gene silencing compounds that have undergone extensive clinical assessment in ATTR include inotersen (ASO) and patisiran (siRNA) and have been approved for treating stage 1 and 2 ATTRv.
Antisense oligonucleotides
Inotersen is a short ASO that selectively binds to the complementary RNA, preventing RNA translation and protein expression. Preclinical studies established that TTR-specific ASOs suppress production of TTR mRNA by hepatocytes, with reduction of serum TTR protein levels in transgenic mouse models (Ile84Ser).93 Subsequently, an ASO (ISIS-TTRRX) binding to the 3’ non-translated portion of the human TTR mRNA was developed and shown to significantly decrease liver mRNA and plasma TTR protein levels in animal models.94 A first in human phase I clinical trial established the safety and biological efficacy of ISIS-TTRRX.94
The pivotal phase III (NEURO-TTR) study established efficacy of inotersen (300 mg/week) in adult-onset patients with stage 1 (ambulatory) and stage 2 (ambulatory with assistance) ATTRv-PN.95 The decline in modified NIS+7 (mNIS+7) and Norfolk QOL-DN were significantly reduced by inotersen, and effectiveness was independent of disease stage, TTR mutation or presence of cardiomyopathy. Thrombocytopenia and glomerulonephritis were evident in 3% of patients. An open-label extension study confirmed clinical effectiveness of inotersen, including in patients switching from placebo,96 97 associated with significant reductions in serum TTR levels.95 97
Additional analysis of the NEURO-TTR trial revealed stabilisation or improvement in sensory, motor and autonomic symptoms.98 Analysis of modified NIS+7 components and lower limb function revealed effects on muscles weakness, sensation and heat pain perception as well as lower limb reflexes.99 Improvement in lower limb function and summated mNIS+7 neurophysiological subscores (five nerves), particularly ulnar nerve motor amplitudes, were reported.
The safety and efficacy of inotersen was also reported in ATTR-CM.100 Stabilisation of cardiac disease, as measured by left ventricular wall thickness and mass, as well as 6 min walk test and echocardiographic global longitudinal strain, was reported in patients with moderate-to-severe restrictive cardiomyopathy. An open-label trial is under way to assess tolerability and efficacy of inotersen in ATTR-CM (NCT03702829). As only a small fraction (<1%) of ASO cross the blood–brain barrier, inotersen is unlikely to have utility in leptomeningeal amyloidosis.
In order to improve the safety and dosing profile of inotersen, the ASO has been ligand-conjugated to allow hepatic receptor uptake.101 A phase I study (NCT03728634) has confirmed improved potency and safety of eplontersen (AKCEA-TTR-LRx), with no serious adverse events noted.101 Phase III studies are now under way in both ATTRv-PN (NCT04136184) and ATTR-CM (NCT04136171).
Small interfering RNAs
Gene expression can be modulated by siRNA (21–23 nucleotide long double-stranded RNA molecules) which target and cleaves complementary mRNAs. The siRNAs are synthetically produced and introduced into the cell, leading to ‘knockdown’ of target gene expression. Patisiran is a TTR-specific siRNA which has exhibited efficacy in preclinical and clinical studies. Targeting of the 3′ untranslated region of TTR mRNA in primates resulted in 90% reduction of TTR at day 14 postinfusion and >70% reduction at day 28.102 A phase I study in mild–moderate ATTR-PN resulted in a dose-dependent reduction in serum TTR protein levels.102 The duration of supression was greater with higher doses, while TTR knockdown observed in humans was identical to that seen in non-human primates.
The APOLLO trial established clinical efficacy of patisiran (0.3 mg/kg infused three times per week) in ATTRv.103 Improvement in mNIS7+ scores at 18 months was reported, with clincial effects evident 9 months after treatment initiation. The clinical benefits were evident irrespective of the degree of peripheral neuropathy and correlated with TTR protein reduction. Improvements in AN, quality of life, gait speed, nutritional status and cardiac function were also seen. Patisiran was safe with mild–moderate infusion-related reactions reported.
An open-label extension study reported sustained long-term clinical effectiveness of patisiran.104 Specifically, improvement in mNIS7+ was maintained at 12 months, as were improvements in AN, quality of life, nutritional status and overall disability. Switching from placebo to patisiran also resulted in an improvement or stabilisation of clinical parameters. No long-term safety issues were identified with patisiran treatment. The clinical benefits were evident across all measures of quality of life, including physical functioning, activities of daily living, and autonomic and small-fibre neuropathy symptoms, as well as pain perception.105
A second-generation siRNA (vutrisiran) is under investigation for treatement of ATTRv. Like patisiran, it contains an siRNA that targets a highly conserved mRNA sequence across all known TTR variants (including wild-type TTR).106 Subcutaneously administered vutrisiran (5–300 mg) led to a dose-dependent reduction in TTR, with mean TTR reduction varying between 57% and 97%.106 The reduction in TTR protein levels was maintained for ≥90 days post dose. The effectiveness of vutrisiran in ATTRv-PN and ATTR-CM is currently being investigated. Like ASOs, siRNAs do not efficiently cross the blood–brain barrier and hence are unlikely to have therapeutic utility in ATTR-LA.
Gene editing
An alternative to mRNA target-based gene silencing strategies is the use of clustered, regularly interspaced short palindromic repeats and associated Cas9 endonuclease (CRISPR–Cas9) system to achieve in vivo gene editing. Given that ATTR is monogenic, and knockdown of TTR has limited physiological effects, CRISPR–Cas9-mediated in vivo gene editing would seem ideal for treatment of ATTRv. Additionally, TTR is largely produced by the liver for which established targeting systems (lipid nanoparticles (LNPs)) are available. NTLA-2001 (a novel CRISPR–Cas9-based gene-editing therapy) consists of a single-guide RNA (sgRNA) targeting human TTR with a human codon-optimised mRNA sequence of Streptococcus pyogenes (Spy)–Cas9 protein.107 The proprietary LNP enables targeted delivery of NTLA-2001 to hepatocytes by endocytosis through low-density lipoprotein receptors,108 and once in the cell, the Spy–Cas9 mRNA is translated to a Spy–Cas9 endonuclease, resulting in the formation of the Cas9–sgRNA ribonucleoprotein complex that enters into the nucleus and removes the mutant TTR gene.109 Intravenous infusion of NTLA-2001 was shown to reduce TTR protein levels by 95% after a single infusion in animal studies.107
A first-in-human study demonstrated a dose-dependent reduction of serum TTR protein levels in ATTRv-PN, after a single infusion of NTLA-2001.109 No serious adverse events were recorded, although three patients reported mild infusion related side effects. Further trials of CRISPR–Cas9-based therapeutic approaches are warranted to determine clinical efficacy and safety.
Summary
This review outlines the recent advances in understanding of the pathogenesis, clinical phenotypes, diagnosis and treatment of ATTRv. Novel treatment strategies are revolutionising patient outcomes, underscoring the importance of early diagnosis. The ideal timing of treatment initiation, use of combination therapies, potential long-term complications of TTR depletion, and the treatment of sequestered leptomeningeal and occular sites are issues that need to be further addressed. Recognition of the heterogeneous clinical presentations of amyloidosis and diagnostic strategies is essential for all clinicians, with the potential to greatly impact patient outcomes. It is likely that novel gene-editing therapies will herald a new era for treating protein misfolding diseases. Moreover, epigenetic factors and mitochondrial polymorphism are additional areas that may be approached to modify disease outcomes.110
Footnotes
Twitter: @jnnp_bmj
Contributors: AC and SV planned the review and wrote the initial draft. PJD, MdC, MK, MMR and MCK provided critical review, edited the manuscript and approved the final version.
Funding: SV gratefully acknowledges funding support from the National Health and Medical Research Council (NHMRC) of Australia (project grant numbers 510233, 1024915 and 1055778; program grant number 1132524; dementia research team grant number 1095127; and Partnership Project number 1153439) and the Motor Neuron Disease Research Institute of Australia. MCK was supported by a NHMRC Practitioner Fellowship (number 1156093).
Competing interests: Author MMR has consulted for Alnylam, IONIS and AKCEA.
Provenance and peer review: Commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study does not involve human participants.
References
- 1. Sekijima Y. Transthyretin (ATTR) amyloidosis: clinical spectrum, molecular pathogenesis and disease-modifying treatments. J Neurol Neurosurg Psychiatry 2015;86:1036–43. 10.1136/jnnp-2014-308724 [DOI] [PubMed] [Google Scholar]
- 2. Benson MD, Buxbaum JN, Eisenberg DS, et al. Amyloid nomenclature 2020: update and recommendations by the International Society of amyloidosis (Isa) Nomenclature Committee. Amyloid 2020;27:217–22. 10.1080/13506129.2020.1835263 [DOI] [PubMed] [Google Scholar]
- 3. Gertz MA, Dispenzieri A. Systemic amyloidosis recognition, prognosis, and therapy: a systematic review. JAMA 2020;324:79–89. 10.1001/jama.2020.5493 [DOI] [PubMed] [Google Scholar]
- 4. Blake CC, Geisow MJ, Swan ID, et al. Strjcture of human plasma prealbumin at 2-5 A resolution. A preliminary report on the polypeptide chain conformation, quaternary structure and thyroxine binding. J Mol Biol 1974;88:1–12. 10.1016/0022-2836(74)90291-5 [DOI] [PubMed] [Google Scholar]
- 5. Fleming CE, Saraiva MJ, Sousa MM. Transthyretin enhances nerve regeneration. J Neurochem 2007;103:831–9. 10.1111/j.1471-4159.2007.04828.x [DOI] [PubMed] [Google Scholar]
- 6. Brouillette J, Quirion R. Transthyretin: a key gene involved in the maintenance of memory capacities during aging. Neurobiol Aging 2008;29:1721–32. 10.1016/j.neurobiolaging.2007.04.007 [DOI] [PubMed] [Google Scholar]
- 7. Minnella AM, Rissotto R, Antoniazzi E, et al. Ocular involvement in hereditary amyloidosis. Genes 2021;12. 10.3390/genes12070955. [Epub ahead of print: 22 06 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaku M, Berk JL. Neuropathy associated with systemic amyloidosis. Semin Neurol 2019;39:578–88. 10.1055/s-0039-1688994 [DOI] [PubMed] [Google Scholar]
- 9. Inês M, Coelho T, Conceição I, et al. Epidemiology of transthyretin familial amyloid polyneuropathy in Portugal: a nationwide study. Neuroepidemiology 2018;51:177–82. 10.1159/000490553 [DOI] [PubMed] [Google Scholar]
- 10. Holmgren G, Costa PM, Andersson C, et al. Geographical distribution of TTR Met30 carriers in northern Sweden: discrepancy between carrier frequency and prevalence rate. J Med Genet 1994;31:351–4. 10.1136/jmg.31.5.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gorram F, Olsson M, Alarcon F, et al. New data on the genetic profile and penetrance of hereditary Val30Met transthyretin amyloidosis in Sweden. Amyloid 2021;28:84–90. 10.1080/13506129.2020.1841623 [DOI] [PubMed] [Google Scholar]
- 12. Reilly MM, Staunton H, Harding AE. Familial amyloid polyneuropathy (TTR Ala 60) in North West ireland: a clinical, genetic, and epidemiological study. J Neurol Neurosurg Psychiatry 1995;59:45–9. 10.1136/jnnp.59.1.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hsieh S-T. Amyloid neuropathy with transthyretin mutations: overview and unique Ala97Ser in Taiwan. Acta Neurol Taiwan 2011;20:155–60. [PubMed] [Google Scholar]
- 14. Sekijima Y, Tojo K, Morita H, et al. Safety and efficacy of long-term diflunisal administration in hereditary transthyretin (ATTR) amyloidosis. Amyloid 2015;22:79–83. 10.3109/13506129.2014.997872 [DOI] [PubMed] [Google Scholar]
- 15. Planté-Bordeneuve V, Carayol J, Ferreira A, et al. Genetic study of transthyretin amyloid neuropathies: carrier risks among French and Portuguese families. J Med Genet 2003;40:e120. 10.1136/jmg.40.11.e120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Finsterer J, Iglseder S, Wanschitz J, et al. Hereditary transthyretin-related amyloidosis. Acta Neurol Scand 2019;139:92–105. 10.1111/ane.13035 [DOI] [PubMed] [Google Scholar]
- 17. Koike H, Misu K, Sugiura M, et al. Pathology of early- vs late-onset TTR Met30 familial amyloid polyneuropathy. Neurology 2004;63:129–38. 10.1212/01.wnl.0000132966.36437.12 [DOI] [PubMed] [Google Scholar]
- 18. Pinto MV, Dyck PJB, Liewluck T. Neuromuscular amyloidosis: unmasking the master of disguise. Muscle Nerve 2021;64:23–36. 10.1002/mus.27150 [DOI] [PubMed] [Google Scholar]
- 19. Kapoor M, Rossor AM, Laura M, et al. Clinical presentation, diagnosis and treatment of TTR amyloidosis. J Neuromuscul Dis 2019;6:189–99. 10.3233/JND-180371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamamoto S, Wilczek HE, Nowak G, et al. Liver transplantation for familial amyloidotic polyneuropathy (FAP): a single-center experience over 16 years. Am J Transplant 2007;7:2597–604. 10.1111/j.1600-6143.2007.01969.x [DOI] [PubMed] [Google Scholar]
- 21. Koike H, Tanaka F, Hashimoto R, et al. Natural history of transthyretin Val30Met familial amyloid polyneuropathy: analysis of late-onset cases from non-endemic areas. J Neurol Neurosurg Psychiatry 2012;83:152–8. 10.1136/jnnp-2011-301299 [DOI] [PubMed] [Google Scholar]
- 22. Sekijima Y, Ueda M, Koike H, et al. Diagnosis and management of transthyretin familial amyloid polyneuropathy in Japan: red-flag symptom clusters and treatment algorithm. Orphanet J Rare Dis 2018;13:6. 10.1186/s13023-017-0726-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Conceição I, De Carvalho M. Clinical variability in type I familial amyloid polyneuropathy (Val30Met): comparison between late- and early-onset cases in Portugal. Muscle Nerve 2007;35:116–8. 10.1002/mus.20644 [DOI] [PubMed] [Google Scholar]
- 24. Carr AS, Pelayo-Negro AL, Evans MR, et al. A study of the neuropathy associated with transthyretin amyloidosis (ATTR) in the UK. J Neurol Neurosurg Psychiatry 2016;87:620–7. 10.1136/jnnp-2015-310907 [DOI] [PubMed] [Google Scholar]
- 25. Théaudin M, Lozeron P, Algalarrondo V, et al. Upper limb onset of hereditary transthyretin amyloidosis is common in non-endemic areas. Eur J Neurol 2019;26:497–e436. 10.1111/ene.13845 [DOI] [PubMed] [Google Scholar]
- 26. Dohrn MF, Röcken C, De Bleecker JL, et al. Diagnostic hallmarks and pitfalls in late-onset progressive transthyretin-related amyloid-neuropathy. J Neurol 2013;260:3093–108. 10.1007/s00415-013-7124-7 [DOI] [PubMed] [Google Scholar]
- 27. Cortese A, Vegezzi E, Lozza A, et al. Diagnostic challenges in hereditary transthyretin amyloidosis with polyneuropathy: avoiding misdiagnosis of a treatable hereditary neuropathy. J Neurol Neurosurg Psychiatry 2017;88:457–8. 10.1136/jnnp-2016-315262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Salvi F, Pastorelli F, Plasmati R, et al. Genotypic and phenotypic correlation in an Italian population of hereditary amyloidosis TTR-related (HA-TTR): clinical and neurophysiological AIDS to diagnosis and some reflections on misdiagnosis. Amyloid 2012;19 Suppl 1:58–60. 10.3109/13506129.2012.682187 [DOI] [PubMed] [Google Scholar]
- 29. Carr AS, Shah S, Choi D, et al. Spinal stenosis in familial transthyretin amyloidosis. J Neuromuscul Dis 2019;6:267–70. 10.3233/JND-180348 [DOI] [PubMed] [Google Scholar]
- 30. Martinez-Naharro A, Treibel TA, Abdel-Gadir A, et al. Magnetic Resonance in Transthyretin Cardiac Amyloidosis. J Am Coll Cardiol 2017;70:466–77. 10.1016/j.jacc.2017.05.053 [DOI] [PubMed] [Google Scholar]
- 31. Phelan D, Collier P, Thavendiranathan P, et al. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 2012;98:1442–8. 10.1136/heartjnl-2012-302353 [DOI] [PubMed] [Google Scholar]
- 32. Coutinho MCA, Cortez-Dias N, Cantinho G, et al. Reduced myocardial 123-iodine metaiodobenzylguanidine uptake: a prognostic marker in familial amyloid polyneuropathy. Circ Cardiovasc Imaging 2013;6:627–36. 10.1161/CIRCIMAGING.112.000367 [DOI] [PubMed] [Google Scholar]
- 33. Klaassen SHC, Tromp J, Nienhuis HLA, et al. Frequency of and prognostic significance of cardiac involvement at presentation in hereditary Transthyretin-Derived amyloidosis and the value of N-terminal pro-B-type natriuretic peptide. Am J Cardiol 2018;121:107–12. 10.1016/j.amjcard.2017.09.029 [DOI] [PubMed] [Google Scholar]
- 34. McColgan P, Viegas S, Gandhi S, et al. Oculoleptomeningeal amyloidosis associated with transthyretin Leu12Pro in an African patient. J Neurol 2015;262:228–34. 10.1007/s00415-014-7594-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maia LF, Magalhães R, Freitas J, et al. Cns involvement in V30M transthyretin amyloidosis: clinical, neuropathological and biochemical findings. J Neurol Neurosurg Psychiatry 2015;86:159–67. 10.1136/jnnp-2014-308107 [DOI] [PubMed] [Google Scholar]
- 36. Martins da Silva A, Cavaco S, Fernandes J, et al. Age-Dependent cognitive dysfunction in untreated hereditary transthyretin amyloidosis. J Neurol 2018;265:299–307. 10.1007/s00415-017-8668-8 [DOI] [PubMed] [Google Scholar]
- 37. Goyal NA, Mozaffar T. Tongue atrophy and fasciculations in transthyretin familial amyloid neuropathy: an ALS mimicker. Neurol Genet 2015;1:e18. 10.1212/NXG.0000000000000018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ferraro PM, D’Ambrosio V, Di Paolantonio A, et al. Renal involvement in hereditary transthyretin amyloidosis: an Italian single-centre experience. Brain Sci 2021;11:980. 10.3390/brainsci11080980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lobato L, Rocha A. Transthyretin amyloidosis and the kidney. Clin J Am Soc Nephrol 2012;7:1337–46. 10.2215/CJN.08720811 [DOI] [PubMed] [Google Scholar]
- 40. Reynolds MM, Veverka KK, Gertz MA, et al. Ocular manifestations of familial transthyretin amyloidosis. Am J Ophthalmol 2017;183:156–62. 10.1016/j.ajo.2017.09.001 [DOI] [PubMed] [Google Scholar]
- 41. Obici L, Suhr OB. Diagnosis and treatment of gastrointestinal dysfunction in hereditary TTR amyloidosis. Clin Auton Res 2019;29:55–63. 10.1007/s10286-019-00628-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ruberg FL, Grogan M, Hanna M, et al. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 2019;73:2872–91. 10.1016/j.jacc.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lane T, Fontana M, Martinez-Naharro A, et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation 2019;140:16–26. 10.1161/CIRCULATIONAHA.118.038169 [DOI] [PubMed] [Google Scholar]
- 44. Simmons Z, Specht CS. The neuromuscular manifestations of amyloidosis. J Clin Neuromuscul Dis 2010;11:145–57. 10.1097/CND.0b013e3181d05994 [DOI] [PubMed] [Google Scholar]
- 45. Kollmer J, Sahm F, Hegenbart U, et al. Sural nerve injury in familial amyloid polyneuropathy: Mr neurography vs clinicopathologic tools. Neurology 2017;89:475–84. 10.1212/WNL.0000000000004178 [DOI] [PubMed] [Google Scholar]
- 46. Salvalaggio A, Coraci D, Cacciavillani M, et al. Nerve ultrasound in hereditary transthyretin amyloidosis: red flags and possible progression biomarkers. J Neurol 2021;268:189–98. 10.1007/s00415-020-10127-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shouman K, Broski SM, Muchtar E, et al. Novel imaging techniques using 18 F-florbetapir PET/MRI can guide fascicular nerve biopsy in amyloid multiple mononeuropathy. Muscle Nerve 2021;63:104–8. 10.1002/mus.27100 [DOI] [PubMed] [Google Scholar]
- 48. Zeidman LA. Advances in the management of small fiber neuropathy. Neurol Clin 2021;39:113–31. 10.1016/j.ncl.2020.09.006 [DOI] [PubMed] [Google Scholar]
- 49. Rousseau A, Cauquil C, Dupas B, et al. Potential role of in vivo confocal microscopy for imaging corneal nerves in transthyretin familial amyloid polyneuropathy. JAMA Ophthalmol 2016;134:983–9. 10.1001/jamaophthalmol.2016.1889 [DOI] [PubMed] [Google Scholar]
- 50. Rezk T, Gilbertson JA, Mangione PP, et al. The complementary role of histology and proteomics for diagnosis and typing of systemic amyloidosis. J Pathol Clin Res 2019;5:145–53. 10.1002/cjp2.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016;133:2404–12. 10.1161/CIRCULATIONAHA.116.021612 [DOI] [PubMed] [Google Scholar]
- 52. Kapoor M, Foiani M, Heslegrave A, et al. Plasma neurofilament light chain concentration is increased and correlates with the severity of neuropathy in hereditary transthyretin amyloidosis. J Peripher Nerv Syst 2019;24:314–9. 10.1111/jns.12350 [DOI] [PubMed] [Google Scholar]
- 53. Maia LF, Maceski A, Conceição I, et al. Plasma neurofilament light chain: an early biomarker for hereditary ATTR amyloid polyneuropathy. Amyloid 2020;27:97–102. 10.1080/13506129.2019.1708716 [DOI] [PubMed] [Google Scholar]
- 54. Ticau S, Sridharan GV, Tsour S, et al. Neurofilament light chain as a biomarker of hereditary Transthyretin-Mediated amyloidosis. Neurology 2021;96:e412–22. 10.1212/WNL.0000000000011090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Adams D, Ando Y, Beirão JM, et al. Expert consensus recommendations to improve diagnosis of ATTR amyloidosis with polyneuropathy. J Neurol 2021;268:2109–22. 10.1007/s00415-019-09688-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Koike H, Ando Y, Ueda M, et al. Distinct characteristics of amyloid deposits in early- and late-onset transthyretin Val30Met familial amyloid polyneuropathy. J Neurol Sci 2009;287:178–84. 10.1016/j.jns.2009.07.028 [DOI] [PubMed] [Google Scholar]
- 57. Yazaki M, Mitsuhashi S, Tokuda T, et al. Progressive wild-type transthyretin deposition after liver transplantation preferentially occurs onto myocardium in FAP patients. Am J Transplant 2007;7:235–42. 10.1111/j.1600-6143.2006.01585.x [DOI] [PubMed] [Google Scholar]
- 58. Hurshman AR, White JT, Powers ET, et al. Transthyretin aggregation under partially denaturing conditions is a downhill polymerization. Biochemistry 2004;43:7365–81. 10.1021/bi049621l [DOI] [PubMed] [Google Scholar]
- 59. Mangione PP, Porcari R, Gillmore JD, et al. Proteolytic cleavage of Ser52Pro variant transthyretin triggers its amyloid fibrillogenesis. Proc Natl Acad Sci U S A 2014;111:1539–44. 10.1073/pnas.1317488111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Owen MC, Gnutt D, Gao M, et al. Effects of in vivo conditions on amyloid aggregation. Chem Soc Rev 2019;48:3946–96. 10.1039/c8cs00034d [DOI] [PubMed] [Google Scholar]
- 61. Dobson CM, Knowles TPJ, Vendruscolo M. The amyloid phenomenon and its significance in biology and medicine. Cold Spring Harb Perspect Biol 2020;12. 10.1101/cshperspect.a033878. [Epub ahead of print: 03 02 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Koike H, Nishi R, Ikeda S, et al. The morphology of amyloid fibrils and their impact on tissue damage in hereditary transthyretin amyloidosis: an ultrastructural study. J Neurol Sci 2018;394:99–106. 10.1016/j.jns.2018.09.011 [DOI] [PubMed] [Google Scholar]
- 63. Holmgren G, Ericzon BG, Groth CG, et al. Clinical improvement and amyloid regression after liver transplantation in hereditary transthyretin amyloidosis. Lancet 1993;341:1113–6. 10.1016/0140-6736(93)93127-m [DOI] [PubMed] [Google Scholar]
- 64. Tsuchiya A, Yazaki M, Kametani F, et al. Marked regression of abdominal fat amyloid in patients with familial amyloid polyneuropathy during long-term follow-up after liver transplantation. Liver Transpl 2008;14:563–70. 10.1002/lt.21395 [DOI] [PubMed] [Google Scholar]
- 65. Wilczek HE, Larsson M, Ericzon B-G, et al. Long-term data from the familial amyloidotic polyneuropathy world transplant registry (FAPWTR). Amyloid 2011;18 Suppl 1:193–5. 10.3109/13506129.2011.574354072 [DOI] [PubMed] [Google Scholar]
- 66. Okamoto S, Wixner J, Obayashi K, et al. Liver transplantation for familial amyloidotic polyneuropathy: impact on Swedish patients' survival. Liver Transpl 2009;15:1229–35. 10.1002/lt.21817 [DOI] [PubMed] [Google Scholar]
- 67. Suhr OB, Larsson M, Ericzon B-G, et al. Survival after transplantation in patients with mutations other than Val30Met: extracts from the FAP world transplant registry. Transplantation 2016;100:373–81. 10.1097/TP.0000000000001021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ando Y, Terazaki H, Nakamura M, et al. A different amyloid formation mechanism: de novo oculoleptomeningeal amyloid deposits after liver transplantation. Transplantation 2004;77:345–9. 10.1097/01.TP.0000111516.60013.E6 [DOI] [PubMed] [Google Scholar]
- 69. Conceição I, Evangelista T, Castro J, et al. Acquired amyloid neuropathy in a Portuguese patient after domino liver transplantation. Muscle Nerve 2010;42:836–9. 10.1002/mus.21806 [DOI] [PubMed] [Google Scholar]
- 70. Sekijima Y, Dendle MA, Kelly JW. Orally administered diflunisal stabilizes transthyretin against dissociation required for amyloidogenesis. Amyloid 2006;13:236–49. 10.1080/13506120600960882 [DOI] [PubMed] [Google Scholar]
- 71. Berk JL, Suhr OB, Obici L, et al. Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA 2013;310:2658–67. 10.1001/jama.2013.283815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ibrahim M, Saint Croix GR, Lacy S, et al. The use of diflunisal for transthyretin cardiac amyloidosis: a review. Heart Fail Rev 2021;387. 10.1007/s10741-021-10143-4 [DOI] [PubMed] [Google Scholar]
- 73. Merlini G, Planté-Bordeneuve V, Judge DP, et al. Effects of tafamidis on transthyretin stabilization and clinical outcomes in patients with non-Val30Met transthyretin amyloidosis. J Cardiovasc Transl Res 2013;6:1011–20. 10.1007/s12265-013-9512-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Coelho T, Maia LF, da Silva AM, et al. Long-Term effects of tafamidis for the treatment of transthyretin familial amyloid polyneuropathy. J Neurol 2013;260:2802–14. 10.1007/s00415-013-7051-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ando Y, Sekijima Y, Obayashi K, et al. Effects of tafamidis treatment on transthyretin (TTR) stabilization, efficacy, and safety in Japanese patients with familial amyloid polyneuropathy (TTR-FAP) with Val30Met and non-Val30Met: a phase III, open-label study. J Neurol Sci 2016;362:266–71. 10.1016/j.jns.2016.01.046 [DOI] [PubMed] [Google Scholar]
- 76. Waddington Cruz M, Amass L, Keohane D, et al. Early intervention with tafamidis provides long-term (5.5-year) delay of neurologic progression in transthyretin hereditary amyloid polyneuropathy. Amyloid 2016;23:178–83. 10.1080/13506129.2016.1207163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lozeron P, Théaudin M, Mincheva Z, et al. Effect on disability and safety of Tafamidis in late onset of Met30 transthyretin familial amyloid polyneuropathy. Eur J Neurol 2013;20:1539–45. 10.1111/ene.12225 [DOI] [PubMed] [Google Scholar]
- 78. Coelho T, Inês M, Conceição I, et al. Natural history and survival in stage 1 Val30Met transthyretin familial amyloid polyneuropathy. Neurology 2018;91:e1999–2009. 10.1212/WNL.0000000000006543 [DOI] [PubMed] [Google Scholar]
- 79. Amass L, Li H, Gundapaneni BK, et al. Influence of baseline neurologic severity on disease progression and the associated disease-modifying effects of tafamidis in patients with transthyretin amyloid polyneuropathy. Orphanet J Rare Dis 2018;13:225. 10.1186/s13023-018-0947-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018;379:1007–16. 10.1056/NEJMoa1805689 [DOI] [PubMed] [Google Scholar]
- 81. Damy T, Garcia-Pavia P, Hanna M, et al. Efficacy and safety of tafamidis doses in the Tafamidis in transthyretin cardiomyopathy clinical trial (ATTR-ACT) and long-term extension study. Eur J Heart Fail 2021;23:277–85. 10.1002/ejhf.2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fox JC, Hellawell JL, Rao S, et al. First-In-Human study of AG10, a novel, oral, specific, selective, and potent transthyretin stabilizer for the treatment of transthyretin amyloidosis: a phase 1 safety, tolerability, pharmacokinetic, and pharmacodynamic study in healthy adult volunteers. Clin Pharmacol Drug Dev 2020;9:115–29. 10.1002/cpdd.700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Judge DP, Heitner SB, Falk RH, et al. Transthyretin Stabilization by AG10 in Symptomatic Transthyretin Amyloid Cardiomyopathy. J Am Coll Cardiol 2019;74:285–95. 10.1016/j.jacc.2019.03.012 [DOI] [PubMed] [Google Scholar]
- 84. Bieschke J, Russ J, Friedrich RP, et al. Egcg remodels mature alpha-synuclein and amyloid-beta fibrils and reduces cellular toxicity. Proc Natl Acad Sci U S A 2010;107:7710–5. 10.1073/pnas.0910723107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. aus dem Siepen F, Bauer R, Aurich M, et al. Green tea extract as a treatment for patients with wild-type transthyretin amyloidosis: an observational study. Drug Des Devel Ther 2015;9:6319–25. 10.2147/DDDT.S96893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sousa L, Coelho T, Taipa R. Cns involvement in hereditary transthyretin amyloidosis. Neurology 2021;97:1111–9. 10.1212/WNL.0000000000012965 [DOI] [PubMed] [Google Scholar]
- 87. Cardoso I, Merlini G, Saraiva MJ. 4'-Iodo-4'-Deoxydoxorubicin and tetracyclines disrupt transthyretin amyloid fibrils in vitro producing noncytotoxic species: screening for TTR fibril disrupters. Faseb J 2003;17:803–9. 10.1096/fj.02-0764com [DOI] [PubMed] [Google Scholar]
- 88. Cardoso I, Martins D, Ribeiro T, et al. Synergy of combined doxycycline/TUDCA treatment in lowering transthyretin deposition and associated biomarkers: studies in FAP mouse models. J Transl Med 2010;8:74. 10.1186/1479-5876-8-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Obici L, Cortese A, Lozza A, et al. Doxycycline plus tauroursodeoxycholic acid for transthyretin amyloidosis: a phase II study. Amyloid 2012;19 Suppl 1:34–6. 10.3109/13506129.2012.678508 [DOI] [PubMed] [Google Scholar]
- 90. Benbrahim M, Norman K, Sanchorawala V, et al. A review of novel agents and clinical considerations in patients with ATTR cardiac amyloidosis. J Cardiovasc Pharmacol 2021;77:544–8. 10.1097/FJC.0000000000001004 [DOI] [PubMed] [Google Scholar]
- 91. Richards DB, Cookson LM, Berges AC, et al. Therapeutic clearance of amyloid by antibodies to serum amyloid P component. N Engl J Med 2015;373:1106–14. 10.1056/NEJMoa1504942 [DOI] [PubMed] [Google Scholar]
- 92. Ino H, Doi Y, Liefaard L, et al. Evaluation of the safety, tolerability, pharmacokinetics, and pharmacodynamics of a single intravenous dose of Miridesap in healthy Japanese subjects. Clin Pharmacol Drug Dev 2019;8:612–8. 10.1002/cpdd.631 [DOI] [PubMed] [Google Scholar]
- 93. Benson MD, Kluve-Beckerman B, Zeldenrust SR, et al. Targeted suppression of an amyloidogenic transthyretin with antisense oligonucleotides. Muscle Nerve 2006;33:609–18. 10.1002/mus.20503 [DOI] [PubMed] [Google Scholar]
- 94. Ackermann EJ, Guo S, Booten S, et al. Clinical development of an antisense therapy for the treatment of transthyretin-associated polyneuropathy. Amyloid 2012;19 Suppl 1:43–4. 10.3109/13506129.2012.673140 [DOI] [PubMed] [Google Scholar]
- 95. Benson MD, Waddington-Cruz M, Berk JL, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med 2018;379:22–31. 10.1056/NEJMoa1716793 [DOI] [PubMed] [Google Scholar]
- 96. Coelho T, Yarlas A, Waddington-Cruz M, et al. Inotersen preserves or improves quality of life in hereditary transthyretin amyloidosis. J Neurol 2020;267:1070–9. 10.1007/s00415-019-09671-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Brannagan TH, Wang AK, Coelho T, et al. Early data on long-term efficacy and safety of inotersen in patients with hereditary transthyretin amyloidosis: a 2-year update from the open-label extension of the NEURO-TTR trial. Eur J Neurol 2020;27:1374–81. 10.1111/ene.14285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Dyck PJB, Coelho T, Waddington Cruz M, et al. Neuropathy symptom and change: Inotersen treatment of hereditary transthyretin amyloidosis. Muscle Nerve 2020;62:509–15. 10.1002/mus.27023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dyck PJB, Kincaid JC, Wiesman JF, et al. mNIS+7 and lower limb function in inotersen treatment of hereditary transthyretin-mediated amyloidosis. Muscle Nerve 2020;62:502–8. 10.1002/mus.27022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Benson MD, Dasgupta NR, Rissing SM, et al. Safety and efficacy of a TTR specific antisense oligonucleotide in patients with transthyretin amyloid cardiomyopathy. Amyloid 2017;24:219–25. 10.1080/13506129.2017.1374946 [DOI] [PubMed] [Google Scholar]
- 101. Viney NJ, Guo S, Tai L-J, et al. Ligand conjugated antisense oligonucleotide for the treatment of transthyretin amyloidosis: preclinical and phase 1 data. ESC Heart Fail 2021;8:652–61. 10.1002/ehf2.13154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Coelho T, Adams D, Silva A, et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med 2013;369:819–29. 10.1056/NEJMoa1208760 [DOI] [PubMed] [Google Scholar]
- 103. Adams D, Gonzalez-Duarte A, O'Riordan WD, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med 2018;379:11–21. 10.1056/NEJMoa1716153 [DOI] [PubMed] [Google Scholar]
- 104. Adams D, Polydefkis M, González-Duarte A, et al. Long-Term safety and efficacy of patisiran for hereditary transthyretin-mediated amyloidosis with polyneuropathy: 12-month results of an open-label extension study. Lancet Neurol 2021;20:49–59. 10.1016/S1474-4422(20)30368-9 [DOI] [PubMed] [Google Scholar]
- 105. Obici L, Berk JL, González-Duarte A, et al. Quality of life outcomes in Apollo, the phase 3 trial of the RNAi therapeutic patisiran in patients with hereditary transthyretin-mediated amyloidosis. Amyloid 2020;27:153–62. 10.1080/13506129.2020.1730790 [DOI] [PubMed] [Google Scholar]
- 106. Habtemariam BA, Karsten V, Attarwala H, et al. Single-Dose pharmacokinetics and pharmacodynamics of transthyretin targeting N-acetylgalactosamine-Small interfering ribonucleic acid conjugate, Vutrisiran, in healthy subjects. Clin Pharmacol Ther 2021;109:372–82. 10.1002/cpt.1974 [DOI] [PubMed] [Google Scholar]
- 107. Finn JD, Smith AR, Patel MC, et al. A Single Administration of CRISPR/Cas9 lipid nanoparticles achieves robust and persistent in vivo genome editing. Cell Rep 2018;22:2227–35. 10.1016/j.celrep.2018.02.014 [DOI] [PubMed] [Google Scholar]
- 108. Akinc A, Maier MA, Manoharan M, et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat Nanotechnol 2019;14:1084–7. 10.1038/s41565-019-0591-y [DOI] [PubMed] [Google Scholar]
- 109. Gillmore JD, Gane E, Taubel J, et al. Crispr-Cas9 in vivo gene editing for transthyretin amyloidosis. N Engl J Med 2021;385:493–502. 10.1056/NEJMoa2107454 [DOI] [PubMed] [Google Scholar]
- 110. Bonaïti B, Olsson M, Hellman U, et al. Ttr familial amyloid polyneuropathy: does a mitochondrial polymorphism entirely explain the parent-of-origin difference in penetrance? Eur J Hum Genet 2010;18:948–52. 10.1038/ejhg.2010.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Castaño A, Drachman BM, Judge D, et al. Natural history and therapy of TTR-cardiac amyloidosis: emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev 2015;20:163–78. 10.1007/s10741-014-9462-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2021-327909supp001.pdf (138.8KB, pdf)