Abstract
Purpose
Hallermann-Streiff syndrome (HSS) is a rare congenital disorder characterized by dyscephaly, hypotrichosis, microphthalmia, dental anomalies, and cutaneous atrophy. Because of the presence of a characteristic facial appearance, severe visual disturbance, and/or upper airway obstruction, most patients with HSS are diagnosed as having a congenital anomaly as a newborn or early in life. We report a case of HSS that was first recognized when the patient was in her seventh decade of life.
Observations
A 68-year-old woman presented to our department for decreased vision in both eyes (OU). Her ocular medical history included “ocular injections” in her left eye (OS); laser iridotomies OU, cataract surgery OS, and removal of corneal opacity OU; she did not have a remarkable systemic medical history. At the initial visit to our department, her best-corrected visual acuity was 0.5 in her right eye (OD) and 0.1 OS with +4.0-diopter hyperopic correction OU, corneal opacity due to calcification OU, a shallow anterior chamber and iridotrabecular contact OD were observed. During the surgical intervention OD, the surgeon recognized a “blue sclera,” and the physicians initially suspected an underlying systemic malformation. Although mild, she presented with a thin beak-like nose and receding chin. In combination with the ocular features, the proportionate short stature, and a characteristic facial appearance, she was diagnosed with HSS.
Conclusions and importance
Patients with HSS who had no clinically significant cosmetic, visual, and respiratory problems early in life may not be recognized as having HSS. The presence of corneal opacity, short axial length, and a blue sclera recognized by ophthalmologists can lead to the correct diagnosis of this congenital disorder.
Keywords: Oculo-mandibulo-facial syndrome, Blue sclera, Angle closure glaucoma, Microphthalmia, Corneal opacity, Micrognathia
1. Introduction
The Hallermann-Streiff syndrome (HSS), i.e., oculo-mandibulo-facial syndrome, is a rare congenital disorder characterized by dyscephaly, hypotrichosis, microphthalmia, dental anomalies, and cutaneous atrophy restricted mainly to the head and neck.1,2 Other than microphthalmia, the ocular manifestations include nystagmus, strabismus, enopthalmos, lid anomarly, congenital cataract, glaucoma, chorioretinal atrophy, and optic atrophy.3,4 Most patients with HSS were recognized as having a congenital anomaly as a newborn or early in life because of the characteristic facial appearance, severe visual disturbance, and/or upper airway obstruction.2,5, 6, 7, 8, 9, 10, 11 We report a case of HSS that was first recognized when the patient was in her seventh decade of life.
2. Case report
A 68-year-old woman presented to our department for decreased vision in both eyes (OU). Her ocular medical history included “ocular injections” administered several times to treat blurred vision in her left eye (OS) more than 20 years previously, laser iridotomies OU roughly 20 years ago, small-incisional cataract extraction/intraocular lens (IOL) implantation, and removal of a corneal opacity using hydrochloric acid OS 12 years previously and in two procedures using ethylenediaminetetraacetic acid (EDTA) in her right eye (OD) 1 year previously and 1 month previously. Her systemic medical history was unremarkable including respiratory, heart, cranio-maxillo-facial, skeletal, endocrine, auricular abnormalities; she did not use any prescription drugs. At the initial visit to our department, her best-corrected visual acuity (BCVA) was 0.5 OD and 0.1 OS with +4.0-diopter (D) hyperopic correction OU; her intraocular pressures were 14 mmHg OU. Slit-lamp evaluation (Fig. 1a and b) and anterior-segment optical coherence tomography (AS-OCT) (CASIA 2, Tomey Corporation, Nagoya, Japan) (Fig. 1c) showed corneal opacity due to calcification OU and shallow anterior chamber (AC)/iridotrabecular contact (ITC) OD were observed. Fundus examination showed suspected glaucomatous cupping OS. Other than this, no remarkable pathology was observed OU. By optical measurement (OA-2000, Tomey), the lens thickness was 5.08 mm and the axial length was 20.24 mm, but these were unmeasurable due to corneal calcification OS. Small-incisional cataract surgery with +30.00-D IOL implantation combined with goniosynechialysis for treating angle closure OD and EDTA chelation therapy12 for treating corneal opacity OS were performed. Postsurgically, 1.5% levofloxacin (Nipro, Osaka, Japan) and 0.1% betamethasone (Sanbetason, Santen Pharmaceutical, Osaka, Japan) were applied topically four times daily.
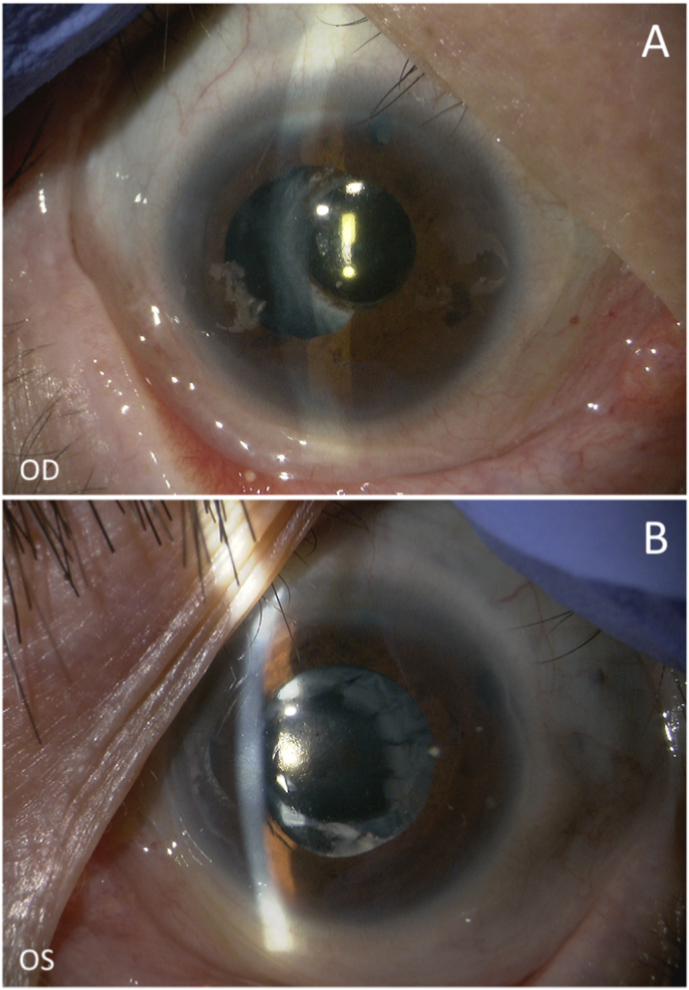
Fig. 1.
(A, B) Slit-lamp photographs, (C) anterior-segment optical coherence tomography, and (D, E) fundus photographs at the initial visit. (A, B) Corneal calcification (arrows) are seen in both eyes (OU), and a shallow anterior chamber is seen in the right eye (OD). (C) Iridotrabecular contact is seen in the temporal and nasal angles OD. (D, E) No remarkable fundus pathology is seen OU. OS, left eye; T, temporal; N, nasal.
Two weeks after the uncomplicated surgery, the AC deepened (Fig. 2a) and the ITC released (Fig. 2b); fibrin formation and posterior synechia were seen at the pupillary region OD (Fig. 2a). For fibrinolysis,13 tissue plasminogen activator was injected into the AC OD. At this time, the BCVAs were 0.7 OD and 0.1 OS. Nine months after the initial surgery, membrane formation at the pupillary region and pupillary phimosis were seen OD (Fig. 2c). Because the BCVA decreased to 0.4 OD, posterior synechialysis and membranectomy using a vitreous cutter were performed OD. Intraoperatively, the surgeon recognized a blue sclera OD. Postoperatively, the blue sclera was observed in all four quadrants OU (Fig. 3a–h). By reviewing the previous surgical videos, the blue sclera had been present during the initial surgeries OU. AS-OCT clearly depicted the scleral thinning where the sclera appeared blue (Fig. 4a and b). At that time, the physicians suspected an underlying systemic syndrome/malformation. Although mild, the patient presented with a thin beak-like nose and receding chin (i.e., microphthalmia) (Fig. 5a and b). Her height was 143 cm and body weight was 38 kg within normal proportions. She had been pointed out poor teeth alignment. She had no family history of HSS or remarkable visual impairment among her parents, 7 brothers or sisters, and 2 daughters. She used to work making machine parts; she had been no difficulties with schooling or working due to health problems. Combined with the ocular features, proportionate short stature, and characteristic facial appearance, she was diagnosed with HSS. Sixteen months after the initial visit, pupillary phimosis was released OD (Fig. 6a) and the corneal calcification decreased OS (Fig. 6b). The BCVAs were 0.7 OD and 0.06 OS, and the intraocular pressures were 14 mmHg OD and 13 mmHg OS without medication. No fundus pathology other than optic nerve head cupping OS was observed OU.
Fig. 2.
(A, C) Slit-lamp photographs and (B) a AS-OCT image after initial surgery in the right eye (OD). (A, B) Two weeks after the cataract surgery and goniosynechialysis, although the anterior chamber is deepened, fibrin formation and posterior synechia are seen at the pupillary region OD. For fibrinolysis, tissue plasminogen activator was injected the same day. (C) Nine months after the cataract surgery and goniosynechialysis, membrane formation at the pupillary region and pupillary phimosis is seen OD. Posterior synechialysis and membranectomy using a vitreous cutter is planned. T, temporal; N, nasal.
Fig. 3.
(A–D) Slit-lamp photographs of the right (OD) and (E–H) left (OS) eyes. Obvious scleral thinning indicated by blue sclera is seen in all quadrants of both eyes. OD, right eye; OS, left eye. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4.
Anterior-segment optical coherence tomography (AS-OCT) images of the (A) right (OD) and (B) left (OS) eyes. (A, B) The thinned sclera is depicted in the AS-OCT images. OD, right eye; OS, left eye.
Fig. 5.
Facial photographs. (A, B) Micrognathia is seen.
Fig. 6.
(A, B) Slit-lamp photographs 16 months after the initial visit. (A) Pupillary phimosis is relieved in the right eye (OD). (B) Corneal calcification is removed by the ethylenediaminetetraacetic acid chelation therapy OS. OD, right eye; OS, left eye.
3. Discussion and conclusion
Given that HSS is a rare human malformation for which the molecular/genetic basis of disease have not been determined,14 HSS is still diagnosed based on the clinical features. Previously reported patients with HSS were all infants,5,6 children,2,7, 8, 9 or at the oldest, young adolescents.10,11 Thus, the current patient with HSS, first diagnosed in the seventh decade of life, is unique in the literature. Previously, HSS was reported over two or three generations,15,16 while the current case seemed sporadic. François's 7 signs of HSS1 and clinical signs seen in the current case was summarized in Table 1. In the current case, the absence of congenital cataract, mild malformations of the facial bones and teeth alignment, and no marked hypotrichosis and skin atrophy were associated with late diagnosis of HSS. Sporadic nature of disease, and no disabilities with schooling or working likely associated with the late diagnosis, too. The blue sclera is attributed to a variety of mechanisms mostly in genetic syndromes.17 In this case, the surgeons noticed the blue sclera, which led to the diagnosis of HSS. Therefore, we reiterate the importance of the blue sclera in the detection of congenital disorders.
Table 1.
Clinical criteria of HSS seen in the current case.
| François's 7 signs1 | Current case |
|---|---|
| dyscephalia and bird face | present but mild |
| dental abnormalities | present |
| proportionate short stature | present |
| hypotrichosis | absent |
| atrophy of skin especially on nose | present but mild |
| bilateral microphthalmos | present but mild |
| congenital cataract | juvenile cataract |
| – | blue sclera |
| – | corneal opacity |
| – | narrow AC angle |
| prolonged AC inflammation |
AC, anterior chamber.
Roules et al. reported stromal corneal opacities in six patients with HSS, all of whom had undergone surgery for congenital cataract.7 The Brown-McLean syndrome is another corneal pathology characterized by peripheral corneal edema involving the stroma and epithelium.10 Thus, corneal calcifications in eyes with/without a previous cataract surgery is unique in the literature. The mention of ocular injections administered several times might have been a steroid injection for iridocyclitis; thus, the chronic inflammation was likely to be associated with the corneal opacity in the current case. A previous laser iridotomy OU and cataract surgery OS also were likely to be associated with the inflammation. The current case also suggested the possible usefulness of EDTA chelation to remove corneal calcifications in patients with HSS.
In pediatric cases of HSS, four eyes of two cases developed exudative retinal detachments after lensectomy and anterior vitrectomy at ages of 2–4 months.6 Scleral abnormalities that impede transscleral intraocular fluid outflow and resulting congestion of the choroidal vein might be associated with this pathology.6 In the current case, no retinal pathology developed postoperatively. However, AC inflammation was sustained after the combined cataract and glaucoma surgeries. Inflammation after cataract surgery was suspected to be associated with hypersensitivity to the cataractous lens substances in patients with HSS.11 Accordingly, we speculated that eyes with HSS are prone to inflammation by mechanisms that are not fully recognized. Previously, as causative factors of glaucoma in HSS, iridocyclitis after cataract surgery and/or malformation of the AC angle have been reported.11,18 In addition, the current case clearly demonstrated that angle closure due to a short axial length also was associated with glaucoma development in HSS, especially when the cataract surgery was not performed when the patient was young.
Patients with HSS with no clinically significant cosmetic, visual, and respiratory problems during early life may not be recognized as having HSS. The anomalies of HSS are seen in multiple organs/tissues and required treatments change with aging, therefore, timely diagnosis and subsequent planning of multidisciplinary treatment approaches at each stage are critical.19 The presence of a corneal opacity, short axial length, and a blue sclera recognized by ophthalmologists can lead to the correct diagnosis of this congenital disorder.
Statement of ethics
This study adhered to the tenets of the Declaration of Helsinki. The institutional review board of Shimane University Hospital did not require an ethics committee review process to report this case.
Patient consent
The patient provided written informed consent for publication of this case report and any accompanying pictures.
Funding sources
No financial support was provided.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Author contributions
YT, SI, AI, and MT treated the subject and collected the clinical data. AS and MT wrote the manuscript, and YT, SI, and AI revised the manuscript. All authors approved the final version of the manuscript. The authors agree to be responsible for all aspects of this work.
Data availability statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Declaration of competing interest
The authors have no conflicts of interest associated with this report.
Acknowledgements
None.
References
- 1.Francois J. A new syndrome; dyscephalia with bird face and dental anomalies, nanism, hypotrichosis, cutaneous atrophy, microphthalmia, and congenital cataract. AMA Arch Ophthalmol. 1958;60:842–862. [PubMed] [Google Scholar]
- 2.Gopakumar M., Hedge A.M. Atypical Hallerman-Streif syndrome: a case report. J Clin Pediatr Dent. 2005;30:73–76. doi: 10.17796/jcpd.30.1.91036513g7u55705. [DOI] [PubMed] [Google Scholar]
- 3.Bardelli A.M., Lasorella G., Barberi L., Vanni M. Ocular manifestations in Kniest syndrome, Smith-Lemli-Opitz syndrome, Hallermann-Streiff-François syndrome, Rubinstein-Taybi syndrome and median cleft face syndrome. Ophthalmic Paediatr Genet. 1985;6:343–347. [PubMed] [Google Scholar]
- 4.Nucci P., de Conciliis C., Sacchi M., Serafino M. Hallermann-Streiff syndrome with severe bilateral enophthalmos and radiological evidence of silent brain syndrome: a new congenital silent brain syndrome? Clin Ophthalmol. 2011;5:907–911. doi: 10.2147/OPTH.S21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato M., Terasaki H., Amano E., Okamoto Y., Miyake Y. Ultrasound biomicroscopic findings in hallerman-streiff syndrome. Jpn J Ophthalmol. 2002;46:451–454. doi: 10.1016/s0021-5155(02)00530-0. [DOI] [PubMed] [Google Scholar]
- 6.Nishina S., Suzuki Y., Azuma N. Exudative retinal detachment following cataract surgery in Hallermann-Streiff syndrome. Graefes Arch Clin Exp Ophthalmol. 2008;246:453–455. doi: 10.1007/s00417-007-0741-z. [DOI] [PubMed] [Google Scholar]
- 7.Roulez F.M., Schuil J., Meire F.M. Corneal opacities in the Hallermann-Streiff syndrome. Ophthalmic Genet. 2008;29:61–66. doi: 10.1080/13816810802027101. [DOI] [PubMed] [Google Scholar]
- 8.Pasyanthi B., Mendonca T., Sachdeva V., Kekunnaya R. Ophthalmologic manifestations of Hallermann-Streiff-Francois syndrome: report of four cases. Eye. 2016;30:1268–1271. doi: 10.1038/eye.2016.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Numabe H., Kosaki K. Prevalence of Hallermann-Streiff syndrome in a Japanese pediatric population. Pediatr Int. 2021;63:474–475. doi: 10.1111/ped.14434. [DOI] [PubMed] [Google Scholar]
- 10.Mohebbi M., Shadravan M., Pour E.K., Ameli K., Badiei S. Brown-McLean syndrome in a patient with hallermann-streiff syndrome. Kor J Ophthalmol. 2016;30:76–77. doi: 10.3341/kjo.2016.30.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen W., Dai M., Su Y., Zhang Q., Li H. Hallermann-Streiff syndrome with uncommon ocular features, ultrasound biomicroscopy and optical coherence tomography findings: a case report. Medicine (Baltim) 2019;98 doi: 10.1097/MD.0000000000018272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shirakami T., Takai Y., Mochiji M., Tanito M. Ethylenediaminetetraacetic acid chelation for band keratopathy before ab interno glaucoma surgery. Open J Ophthalmol. 2019;9:165–167. [Google Scholar]
- 13.Tanito M., Sugihara K., Tsutsui A., Hara K., Manabe K., Matsuoka Y. Midterm results of microhook ab interno trabeculotomy in initial 560 eyes with glaucoma. J Clin Med. 2021;10 doi: 10.3390/jcm10040814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Innes A.M., Lynch D.C. Fifty years of recognizable patterns of human malformation: insights and opportunities. Am J Med Genet. 2021;185:2653–2669. doi: 10.1002/ajmg.a.62240. [DOI] [PubMed] [Google Scholar]
- 15.Gerinec A., Spissáková B., Chynoranský M. [The Hallermann-Streiff syndrome in 2 generations] Cesk Oftalmol. 1989;45:326–333. [PubMed] [Google Scholar]
- 16.Epée E., Beleho D., Bitang A.T., Njami V.A., Bengondo C., Ebana Mvogo C. A familial study of Hallermann-Streiff-François syndrome. Int Med Case Rep J. 2017;10:193–201. doi: 10.2147/IMCRJ.S114115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks J.K. A review of syndromes associated with blue sclera, with inclusion of malformations of the head and neck. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;126:252–263. doi: 10.1016/j.oooo.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Hopkins D.J., Horan E.C. Glaucoma in the hallermann-streiff syndrome. Br J Ophthalmol. 1970;54:416–422. doi: 10.1136/bjo.54.6.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godzieba A., Smektała T., Dowgierd K., Sporniak-Tutak K., Kowalski J. Diagnosis, early care, and treatment of hallermann-streiff syndrome: a review of the literature. Pediatr Ann. 2021;50:e227–e231. doi: 10.3928/19382359-20210415-01. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.