Abstract
Objective:
To identify metabolites in pre-surgical blood associated with risk of persistent post-surgical pelvic pain one-year after endometriosis surgery in adolescents and young adult patients.
Design:
Prospective observational study within The Women’s Health Study: From Adolescence to Adulthood, a U.S. based longitudinal cohort of adolescents and women enrolled from 2012–2018.
Setting:
Two tertiary hospitals.
Patients:
Laparoscopically confirmed endometriosis patients (n=180) with blood collected prior to their endometriosis surgery. Of these, 77 patients additionally provided blood samples 5 weeks to 6 months after their surgery. We measured plasma metabolites using liquid chromatography tandem mass spectrometry and a total of 390 known metabolites were included in our analysis.
Intervention(s):
None
Main Outcome Measure(s):
Persistent post-surgical pelvic pain defined as having severe life-impacting pelvic pain one-year after endometriosis surgery.
Results:
The majority of endometriosis patients were rASRM stage I/II (>95%), average age at diagnosis of 18.7 years, with 36% reporting persistent post-surgical pelvic pain. Of the 21 metabolites in pre-surgical blood that were associated with risk of persistent post-surgical pelvic pain, 19 metabolites were associated with increased risk which were mainly lipid metabolites. Only two metabolites were associated with decreased risk, which were pregnenolone sulfate (OR=0.64, 95%CI=0.44–0.92) and fucose (OR=0.69, 95%CI=0.47–0.97). Metabolite set enrichment analysis revealed higher levels of lysophosphatidylethanolamines (FDR=0.01) and lysophosphatidylcholines (FDR=0.01) in pre-surgical blood being associated with increased risk of persistent post-surgical pelvic pain.
Conclusion:
Our results suggest dysregulation of multiple groups of lipid metabolites may play a role in persistence of pelvic pain post-surgery among young endometriosis patients.
Keywords: endometriosis, post-surgical pelvic pain, metabolomics, biomarkers, adolescent
Capsule:
Using a validated metabolomics platform, we identified metabolites in pre-surgical blood associated with risk of persistent post-surgical pelvic pain among young endometriosis patients.
INTRODUCTION
Endometriosis is a gynecologic disease defined by the presence of endometrial-like tissue outside the uterus often presenting with severe pelvic pain and infertility, affecting one in ten reproductive-aged women (1). Endometriosis is typically treated by hormone therapy or removal of lesions by surgery (1). However, treatment response varies among individuals and about one-third of endometriosis patients suffer from persistent pelvic pain unresponsive to conventional treatment (1–3). Unfortunately, existing endometriosis stage definitions based on the revised American Society for Reproductive Medicine (rASRM) classification do not correlate with symptom severity nor treatment response, limiting its ability to inform clinical decisions (4). Currently there are no clinically applicable biomarkers for predicting surgical treatment responses in endometriosis patients.
Metabolites are the downstream products of cellular activities regulated by the genome and modified by environmental factors (5). Investigation of metabolomics have shown promise in discovery of novel biomarkers for multiple chronic diseases such as cardiovascular disease, diabetes, and cancer (6–9). Lipid metabolism has been reported to be dysregulated in endometriosis patients and several human studies report suggestive associations with phosphatidylcholines and sphingolipids (10–15). However, no study has examined the association between pre-surgical blood metabolites with post-surgical outcome among adolescents and young women with endometriosis. Endometriosis diagnosed in adolescents and young adults typically presents with severe pelvic pain and superficial peritoneal lesions, which is distinctly different from endometriosis diagnosed in adults characterized by pelvic pain, infertility, and deep fibrotic lesions (16–19). Thus, discovery of prognostic biomarkers predictive of persistent pelvic pain after endometriosis surgery with will be especially informative to this young population in optimizing clinical care.
Therefore, the objective of this study was to identify individual and groups of metabolites in blood samples collected prior to endometriosis surgery that are associated with persistent post-surgical pelvic pain among adolescents and young adults with laparoscopically confirmed endometriosis, using validated metabolomics assay platforms.
MATERIALS AND METHODS
Study population
The Women’s Health Study: From Adolescence to Adulthood (A2A), is an ongoing, U.S. based longitudinal study of adolescents and women enrolling 1,549 participants from 2012 to 2018, which has been previously described (17, 20, 21). Briefly, endometriosis patients were identified at two tertiary care medical centers. All participants would have been receiving standard care for any existing medical conditions, which could include hormonal therapy for endometriosis-associated pelvic pain. Participants completed an extensive baseline questionnaire assessing lifestyle and reproductive factors, detailed pain characteristics, and medication use at enrollment and are actively followed annually via questionnaires compliant with the World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonization Project (WERF EPHect) (22). Surgically visualized disease was documented using the WERF EPHect Surgical Form, including appearance at laparoscopy of superficial peritoneal lesions, endometrioma, and deep infiltrating disease and endometriosis rASRM score (4). All endometriosis patients included in this study had visualization and removal of endometriotic lesions at surgery (23).
Among participants who consented to a blood draw, blood samples were collected at baseline and 5 weeks to 6 months after their surgery following a standard protocol (24). Collected blood samples were separated into plasma, serum and buffy coat aliquots; and stored at ≤−80°C per WERF EPHect fluids standard operating protocols (with the exception that bloods were centrifuged at 1790 × g for 10 minutes) (25). At the time of blood collection, participants completed a biospecimen questionnaire on which they reported recent hormone use and fasting status.
This study was approved by the Boston Children’s Hospital (BCH) Institutional Review Board on behalf of both BCH and Brigham and Women’s Hospital. All participants provided written consent for participation in the study, with parental consent plus participant assent for participants age <18 years at enrollment.
Pelvic pain assessment
Pelvic pain was assessed at baseline and annually during follow-up using the WERF EPHect endometriosis patient questionnaire, which uses validated measures to capture sufficient information on pain phenotypes, as described previously (22, 26). Life-impacting pelvic pain was chosen as our primary outcome because it reflects a composite across multiple dimensions of pelvic pain, including severity, frequency, and pain perception. This variable was statistically significantly correlated with other validated metrics of pain, such as dysmenorrhea severity and frequency. To assess life-impacting pelvic pain, participants were asked “To what extent has your general pelvic/lower abdominal pain interfered with your normal social activities with work and school in the last 3 months?” and were given options to answer in five ordinal categories (i.e. Not at all, slightly, moderately, quite a bit, extremely). Persistent post-surgical pelvic pain was defined as having moderate to severe life-impacting pelvic pain one-year after surgery, combining those who reported “moderately”, “quite a bit”, or “extremely” to this question.
Covariate information
We collected information on the following covariates: age (years), body mass index (BMI) calculated from self-reported height and weight at enrollment (kg/m2), race/ethnicity (white non-Hispanic, other race/ethnicity), smoking status (never, former, current), analgesic use (never, ever use but not regularly, regular use defined as use of more than once a week), on hormones at blood draw (no, yes), fasting at blood draw (no, yes), history of irritable bowel syndrome diagnosis (no, yes), time from symptom onset to surgical diagnosis (years), rASRM stage calculated from the score documented during laparoscopic surgery (I/II, III/IV), and enrollment site (BCH, BWH). For women aged ≥ 20, BMI was categorized as the following per World Health Organization criteria: underweight (BMI < 18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (BMI 25–29.9 kg/m2), or obese (BMI >30 kg/m2) (27, 28). For participants aged < 20, current BMI was categorized as the following using age and female-specific BMI Z-scores per expert committee recommendations: underweight (Z-score ≤ −2), normal weight (−2 < Z-score <1), overweight (Z-score 1–2), or obese (Z-score > 2) (29).
Metabolomics measurement
All plasma metabolites were measured at the Broad Institute of MIT (Cambridge, MA) using four complimentary liquid chromatography tandem mass spectrometry (LC-MS) methods as described previously (30–32). We previously reported high reproducibility and stability of these assays, with 92% of metabolites having coefficient of variation (CV) <20% and 90% of metabolites having intraclass correlation coefficients (ICCs) ≥ 0.4 over 1 to 2 years within individuals indicating that a single measurement well represents long-term metabolite levels (33, 34). In total we measured 644 known metabolites. We confirmed metabolite identities using authentic reference standards or reference samples. Quality control (QC) samples, which were blinded to the laboratory, were randomly distributed among the participants’ samples. Overall, in the blinded QC samples, 70% of the known metabolites had CV<25% and 95% had <10% missing metabolite values. We excluded metabolites with CV>25% or missing among QC samples (n=205), missing among >20% of participant samples (n=7) and metabolites measured by multiple platforms (n=42) resulting in a total of 390 metabolites included in our analysis.
Statistical analysis
Within the A2A cohort, there were 297 laparoscopically confirmed endometriosis patients who had blood collected at baseline and completed their baseline questionnaire within 90 days of their surgery. Among these, we excluded patients who had no baseline blood (n=3), had their baseline blood collected after surgery (n=6), did not have superficial peritoneal lesion observed at surgery (n=6), had not completed the questionnaire at one-year post-surgery (n=81), or were missing post-surgical pelvic pain information (n=21), resulting in 180 endometriosis patients included in our analysis with majority (73%) having blood drawn on the day of their endometriosis surgery. There were 81 patients who additionally had blood drawn 5 weeks to 6 months after their endometriosis surgery (median 16.2 weeks). Among these, four patients were missing post-surgical pelvic pain information, resulting in 77 endometriosis patients among whom change in metabolite levels was investigated.
We transformed continuous metabolite values into probit scores to achieve normality and for the results to be comparable across metabolites. For each metabolite missing in <20% of the samples (n=37), we assigned missing values with half the minimum value for that metabolite as this was thought to be due to values being below the limit of detection. We used logistic regression adjusted for age, fasting status at blood draw, and pelvic pain severity at baseline (i.e. not at all/slightly vs moderately/quite a bit/extremely) to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) per one standard deviation (SD) increase in metabolite levels of having severe pelvic pain one year post-surgery. Since the majority of patients (>90%) were on hormones at time of blood draw, we conducted a sensitivity analysis restricting to those who were on hormones at blood draw. We used false discovery rate (FDR) to account for multiple testing. Among endometriosis patients with blood collected at two timepoints (pre-surgery and 5 weeks to 6 months post-surgery), we cross-classified the patients based on the median metabolite levels at the two blood collections and categorized patients at first and second blood collection as low/low (reference), low/high, high/low, or high/high and used logistic regression adjusted for age and fasting status at blood draw to calculate the ORs and CIs for each of the categories. We used metabolite set enrichment analysis (MSEA) (35) to identify groups of biologically similar metabolites that were enriched among the metabolites associated with severe post-surgical pelvic pain. All analyses were performed using the statistical computing language R, version 3.6.3.
RESULTS
Of the 180 laparoscopically confirmed endometriosis patients included in our analysis, 65 (36%) reported persistent post-surgical pelvic pain one-year after their endometriosis surgery (Table 1). Average age at baseline was 18.7 years (SD=4.9) with majority being normal weight and never smokers. Nearly all endometriosis patients were rASRM stage I/II at diagnosis (>95%), which is a typical clinical presentation of endometriosis diagnosed in this young population. Baseline demographic characteristics were similar between patients with and without persistent post-surgical pelvic pain one-year after surgery. Among the four endometriosis patients (2%) with rASRM stage III/IV, only one (25%) reported having persistent post-surgical pelvic pain at one-year after endometriosis surgery whereas among patients with rASRM stage I/II, 36% (64 out of 176) reported having persistent post-surgical pelvic pain.
Table 1.
Characteristics of endometriosis patients by persistent post-surgical pelvic pain at one-year after endometriosis surgery in the A2A (n=180)
| Persistent post-surgical pelvic pain at one-year after surgery | ||
|---|---|---|
| No | Yesa | |
| n=115 | n=65 | |
| Age, years, mean (SD) | 18.4 (4.4) | 18.8 (4.8) |
| Body mass indexb, n (%) | ||
| Underweight | 0 | 0 |
| Normal | 71 (62) | 47 (72) |
| Overweight | 34 (30) | 14 (22) |
| Obese | 10 (9) | 4 (6) |
| Age at menarche, years, mean (SD) | 11.7 (1.4) | 11.8 (1.5) |
| Race, n (%) | ||
| White | 104 (90) | 60 (92) |
| Non-white | 11 (10) | 5 (8) |
| Smoking, n (%) | ||
| Never | 110 (96) | 63 (97) |
| Former | 2 (2) | 1 (2) |
| Current | 2 (2) | 0 |
| Analgesic use, n (%) | ||
| Never | 58 (50) | 27 (42) |
| Ever use but not regularly | 9 (8) | 5 (8) |
| Regular usec | 48 (42) | 33 (51) |
| On hormones at blood draw, n (%) | 108 (94) | 57 (88) |
| Fasting at blood draw, n (%) | ||
| No | 21 (19) | 13 (23) |
| Yes | 70 (63) | 32 (56) |
| Unknown | 20 (18) | 12 (21) |
| Irritable bowel syndrome diagnosis, n (%) | ||
| No | 103 (90) | 52 (80) |
| Yes | 12 (10) | 13 (20) |
| Time since symptom onset to endometriosis diagnosis (years), mean (SD) | 3.6 (2.6) | 3.3 (2.8) |
| ASRM stage, n (%) | ||
| Early (I/II) | 112 (97) | 64 (98) |
| Advanced (III/IV) | 3 (3) | 1 (2) |
| Enrollment site, n (%) | ||
| Boston Children’s Hospital | 91 (79) | 49 (75) |
| Brigham and Women’s Hospital | 24 (21) | 16 (25) |
Abbreviations: A2A, The Women’s Health Study: From Adolescence to Adulthood; SD, standard deviation.
Persistent post-surgical pelvic pain was defined as those who reported having moderate to severe life-impacting pelvic pain at one-year after their endometriosis surgery
For women aged ≥20 years: underweight (BMI < 18.5 kg/m2), normal weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25–29.9 kg/m2), or obese (BMI ≥ 30 kg/m2) according to World Health Organization criteria; For those <20 years, the age- and genderspecific BMI Z-score was calculated, and participants were categorized as underweight (Z-score ≤ −2), normal weight (Z-score >−2 to <1), overweight (Z-score 1–2), or obese (Z-score > 2)
More than once a week
There were 21 metabolites in pre-surgical blood that were associated with risk of persistent post-surgical pelvic pain (p-value < 0.05; Figure 1, Supplemental Table 1). Among the 19 metabolites associated with increased risk with ORs ranging from 1.42 to 1.74, there were 15 lipid-related metabolites, including 7 lysophosphatidylethanolamines (LPEs), 2 lysophosphatidylcholines (LPCs), 3 phosphatidylcholine (PCs), and 3 other lipid derivatives. Only two metabolites were associated with decreased risk, which were fucose (OR=0.69, 95%CI=0.47–0.97) and pregnenolone sulfate (OR=0.64, 95%CI=0.44–0.92). Similar results were observed when we restricted to those who were on hormones at blood draw and after additionally adjusting for pre-diagnosis BMI (Supplemental Table 2).
Figure 1. Metabolites associated with risk of persistent post-surgical pelvic pain at one-year after endometriosis surgery in the A2A (n=180).
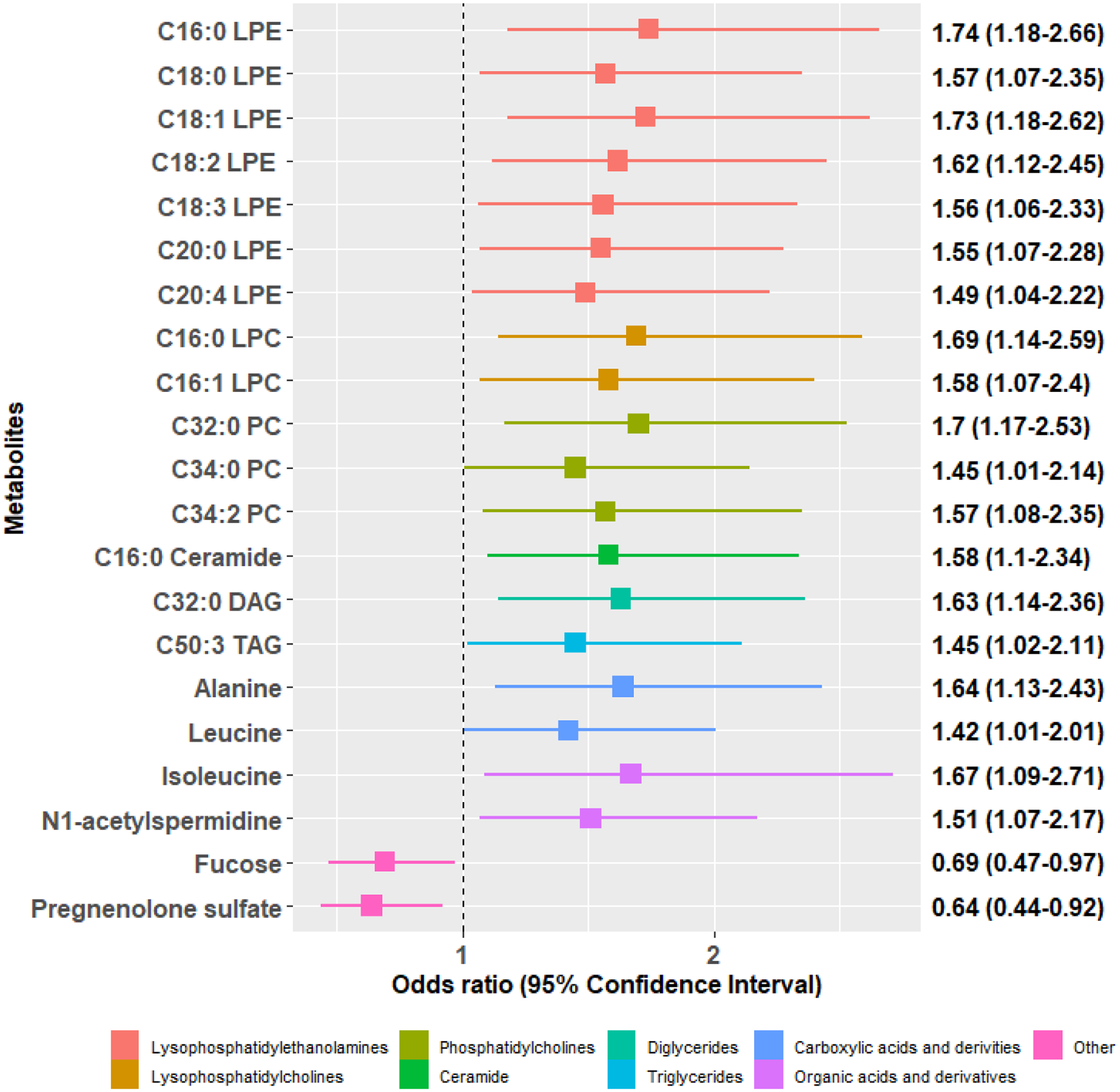
Odds ratios and 95% confidence intervals per 1 standard deviation increase in metabolite levels are presented for the 21 metabolites associated with risk of severe pelvic pain at one-year after surgery (p-value < 0.05). All estimates were adjusted for age, fasting status, and pelvic pain severity at baseline.
We then examined whether change in these 21 blood metabolites before and closely after surgery were associated with risk of persistent post-surgical pelvic pain. Of the 77 endometriosis patients who had blood metabolites measured at two timepoints, 30 (39%) reported persistent post-surgical pelvic pain. Baseline characteristics were similar among those with and without paired pre- and post-surgical blood samples (Supplemental Table 3). While most associations were not significant, metabolites that were elevated at both time points were generally associated with persistent post-surgical pelvic pain in the same direction as in the main analysis. (Table 2, Supplemental Table 4). Compared to those who had persistently low levels before and closely after surgery, endometriosis patients who had persistently high levels of C16:0 LPE, C18:3 LPE, and C32:0 diglycerides (DAG) were associated with increased risk of severe pelvic pain one-year after surgery (OR=7.55, 95%CI=2.05–33.86; OR=15.3, 95%CI=3.17–117.71; and OR=5.28, 95%CI=1.44–23.34 respectively). Patients with persistently high levels of fucose and pregnenolone sulfate were associated with decreased risk of severe pelvic pain one-year after surgery (OR=0.12, 95%CI=0.02–0.48 and OR=0.15, 95%CI=0.04–0.49 respectively).
Table 2.
Change in plasma metabolite levels before and closely after endometriosis surgery and risk of persistent post-surgical pelvic pain at one-year after endometriosis surgery (n=77) a
| Low/Low | Low/High | High/Low | High/High | Low/High | High/Low | High/High | ||
|---|---|---|---|---|---|---|---|---|
| Metabolite ID (HMDB_ID) | Metabolite name | Reference | OR (95%CI)b | OR (95%CI)b | OR (95%CI)b | P value | P value | P value |
| HMDB0011503 | C16:0 LPE | 1.00 (ref) | 2.01 (0.46,9.66) | 2.03 (0.31,12.68) | 7.55 (2.05,33.86) | 0.36 | 0.44 | <0.01 |
| HMDB0011130 | C18:0 LPE | 1.00 (ref) | 0.72 (0.17,2.89) | 0.75 (0.16,3.29) | 2.71 (0.81,9.79) | 0.65 | 0.71 | 0.11 |
| HMDB0011506 | C18:1 LPE | 1.00 (ref) | 0.27 (0.06,1.09) | 0.52 (0.13,1.94) | 1.13 (0.32,4.09) | 0.08 | 0.34 | 0.85 |
| HMDB0011507* | C18:2 LPE | 1.00 (ref) | 0.45 (0.12,1.54) | 0.57 (0.15,2.08) | 1.6 (0.37,7.26) | 0.21 | 0.4 | 0.53 |
| HMDB0011478 | C18:3 LPE | 1.00 (ref) | 3.4 (0.64,26.67) | 0.44 (0.02,5.49) | 15.3 (3.17,117.71) | 0.18 | 0.53 | <0.01 |
| HMDB0011511 | C20:0 LPE | 1.00 (ref) | 0.55 (0.13,2.07) | 1.31 (0.35,4.94) | 0.91 (0.24,3.32) | 0.39 | 0.69 | 0.89 |
| HMDB0011517 | C20:4 LPE | 1.00 (ref) | 0.44 (0.11,1.64) | 0.34 (0.07,1.34) | 1.29 (0.33,5.17) | 0.23 | 0.13 | 0.72 |
| HMDB0010382 | C16:0 LPC | 1.00 (ref) | 1.97 (0.49,8.45) | 2.23 (0.51,10.24) | 3.15 (0.89,12.5) | 0.34 | 0.28 | 0.09 |
| HMDB0010383 | C16:1 LPC | 1.00 (ref) | 0.58 (0.13,2.41) | 0.88 (0.2,3.75) | 2.03 (0.62,6.95) | 0.47 | 0.87 | 0.25 |
| HMDB0007871* | C32:0 PC | 1.00 (ref) | 1.33 (0.33,5.31) | 2.2 (0.61,8.38) | 1.39 (0.37,5.23) | 0.69 | 0.24 | 0.62 |
| HMDB0007970* | C34:0 PC | 1.00 (ref) | 0.92 (0.21,3.76) | 2.49 (0.66,9.9) | 1.6 (0.47,5.61) | 0.91 | 0.18 | 0.46 |
| HMDB0007973* | C34:2 PC | 1.00 (ref) | 1.7 (0.41,7.88) | 3.2 (0.82,14.48) | 4.35 (0.91,24.06) | 0.47 | 0.11 | 0.07 |
| HMDB0004949 | C16:0 Ceramide (d18:1) | 1.00 (ref) | 0.91 (0.21,3.76) | 4.3 (1.17,17.37) | 1.4 (0.37,5.33) | 0.9 | 0.03 | 0.62 |
| HMDB0007098* | C32:0 DAG | 1.00 (ref) | 3.04 (0.62,16.18) | 8.12 (1.76,45.15) | 5.28 (1.44,23.34) | 0.17 | 0.01 | 0.02 |
| HMDB0005433* | C50:3 TAG | 1.00 (ref) | 0.66 (0.15,2.73) | 0.72 (0.17,2.96) | 2.89 (0.79,11.32) | 0.57 | 0.65 | 0.11 |
| HMDB0000161 | Alanine | 1.00 (ref) | 0.12 (0.01,0.84) | 0.57 (0.11,2.55) | 2.33 (0.72,7.91) | 0.07 | 0.47 | 0.16 |
| HMDB0000687 | Leucine | 1.00 (ref) | 0.87 (0.18,3.74) | 3.5 (0.89,15.04) | 2.16 (0.66,7.56) | 0.85 | 0.08 | 0.21 |
| HMDB0000172 | Isoleucine | 1.00 (ref) | 1.57 (0.38,6.44) | 6.19 (1.42,31.43) | 1.91 (0.56,6.77) | 0.53 | 0.02 | 0.30 |
| HMDB0001276 | N1-acetylspermidine | 1.00 (ref) | 0.56 (0.07,3.19) | 0.41 (0.05,2.19) | 1.7 (0.58,5.17) | 0.54 | 0.33 | 0.34 |
| HMDB0000174 | Fucose | 1.00 (ref) | 2.4 (0.33,21.72) | 1.09 (0.24,5.08) | 0.12 (0.02,0.48) | 0.39 | 0.91 | 0.01 |
| HMDB0000774 | Pregnenolone sulfate | 1.00 (ref) | 0.5 (0.06,3.51) | 0.43 (0.09,1.75) | 0.15 (0.04,0.49) | 0.49 | 0.25 | <0.01 |
Abbreviations: OR, odds ratio; CI, confidence intervals; LPE, lysophosphatidylethanolamines; LPC, lysophosphatidylcholines; PC, phosphatidylcholines; DAG, diglycerides; TAG, triglycerides
representative ID
Based on the median metabolite levels at the two blood collections (pre- and post-surgery), women were categorized at first/second blood collection as low/low, low/high, high/low, or high/high
OR per 1 SD increase in metabolite levels adjusted for age and fasting status
MSEA revealed enrichment of multiple lipid metabolite groups associated with risk of persistent post-surgical pelvic pain (Figure 2, Supplemental Table 5). LPEs (p-value=0.001) and LPCs (p-value=0.001) in pre-surgical blood were significantly associated with increased risk of persistent post-surgical pelvic pain (FDR=0.01). Other lipid metabolite groups, such as ceramides (p-value=0.008) and cholesteryl esters (p-value=0.04), were also enriched among metabolites associated with persistent post-surgical pelvic pain (FDR<0.20).
Figure 2. Metabolite set enrichment analysis (MSEA) results on enriched metabolite groups associated with persistent post-surgical pelvic pain at one-year after endometriosis surgery.
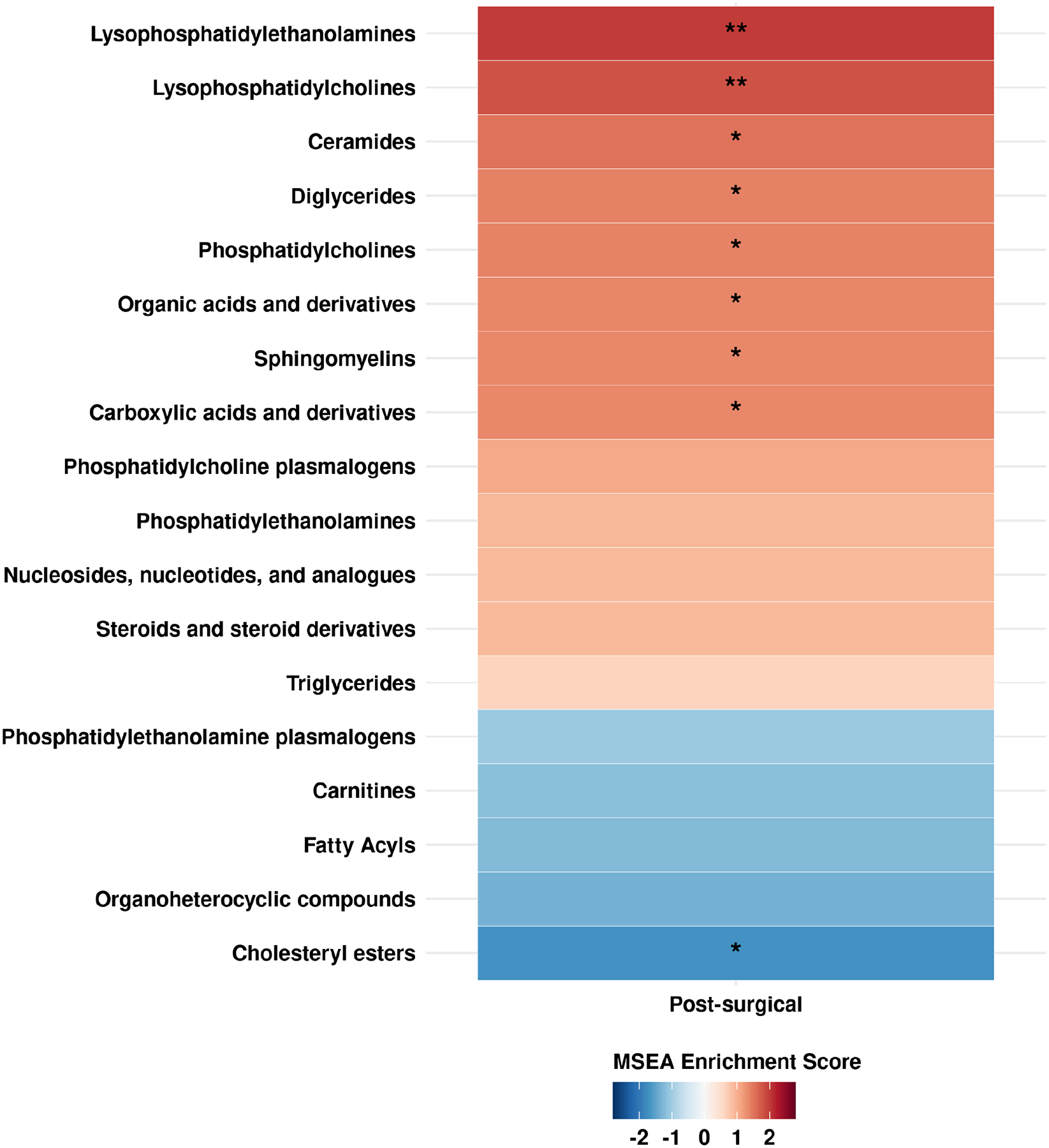
Red shades represent positive coefficients while blue shades represent negative coefficients. Significance of the association is overlaid on the heat map as follows: ** FDR<0.05, * FDR<0.20.
DISCUSSION
Using a validated, reproducible metabolomics platform, we conducted the first comprehensive analysis examining the prognostic value of pre-surgical blood metabolomics among young endometriosis patients who commonly present with severe pelvic pain symptoms with superficial peritoneal lesions. Our prospective analysis identified higher levels of multiple lipid metabolites, including LPEs and LPCs, in pre-surgical blood being associated with increased risk of persistent post-surgical pelvic pain.
Our study focused on young endometriosis patients which is an understudied population, often presenting with severe pelvic pain and superficial peritoneal lesions (17, 18). While some studies reported that adolescent endometriosis may be a more severe, progressive type of disease (36–38), 36% of our endometriosis patients reported severe pelvic pain one-year after surgery, which is similar to prior studies reporting about 30% of endometriosis patients have persistent pain unresponsive to conventional treatment (3, 39, 40).
Several cross-sectional studies have compared blood metabolites in women with and without endometriosis, reporting increased blood levels of lipid metabolites (e.g. phosphatidylcholines, sphingomyelins) as well as amino acids (e.g. valine, leucine) and decreased levels of isoleucine and tryptophan in endometriosis patients compared to controls (12, 14, 15, 41–46), however did not address associations with persistent post-surgical pelvic pain among endometriosis patients. While there were few studies reporting metabolites of chronic pain (47–49), only one study reported urinary metabolites in relation to pelvic pain (50) which did not focus on endometriosis patients. Thus, evidence on metabolites associated with persistent post-surgical pelvic pain in endometriosis patients is limited.
In this study, classes of lysophospholipids, LPEs and LPCs, were significantly associated with increased risk of post-surgical pelvic pain in endometriosis patients, which may include patients with centralized pain (51). Interestingly, two prior studies reported LPCs being associated with fibromyalgia and multisite musculoskeletal pain (48, 49), which are both conditions known for increased sensitivity to pain due to central sensitization (52). Mechanistic linkages between lysophospholids (LPLs) and modulation of pain and inflammation are complex as LPLs are as both immediate precursors in the synthesis of lysophosphatidic acids (LPAs) and byproducts of phospholipase A2 activity upstream of prostaglandin (PG) biosynthesis (53–55). In mouse models, LPAs have been shown to initiate neuropathic pain through lysophosphatidic acid receptor 1 (LPA1) signaling and that intrathecally administered LPC can serve as upstream substrate of this activity (56, 57). LPC and LPE are also generated by phospholipase A2 catalyzed hydrolysis of phospholipids and are therefore directly associated with liberation of the free fatty acid precursor to eicosanoid biosynthesis, arachidonic acid (54). Arachidonic acid can be converted to prostaglandins by cyclooxygenases (COX) (58), and upregulation of prostaglandin synthesis has been reported to contribute to the growth and survival of endometriotic lesions (59, 60). In addition, overexpression of COX2 may play a key role in endometriosis development (61) and is associated with increased endometriosis-associated pain and recurrence (62, 63). One study reported that overexpression of COX2 was positively correlated with intensity of dysmenorrhea and acyclic pelvic pain (62). Another study reported overexpression of NF-kB, which in part mediates COX2 expression, was significantly associated with post-surgical endometriosis lesion recurrence (63). Furthermore, multiple inflammatory mediators, including IL-1β, IL-6, TNF-α, have been reported to contribute to endometriosis-associated pain (64). These inflammatory mediators upregulate expression of COX2 and prostaglandin synthesis (65). Since LPLs are also byproducts of prostaglandin synthesis, it is possible that elevation of LPEs and LPCs are reflecting the upregulation of prostaglandin synthesis triggered by increased pro-inflammatory mediators. Thus, our observation further supports that upregulation of prostaglandin synthesis may be an important pathway in persistent post-surgical pelvic pain among endometriosis patients.
Interestingly, higher levels of plasma pregnenolone sulfate and fucose were suggestively associated with lower risk of persistent post-surgical pelvic pain. Pregnenolone is a precursor to progesterone, which is thought to have a critical role in endometriosis development and progression (66, 67). Endometriosis mouse model studies show that progesterone resistant endometrial tissue has increased invasiveness and vasculature, suggesting this may be a key step in endometriosis development (68). Fucose is an essential sugar involved in glycosylation reactions (69). Lower levels of plasma fucose may indicate acceleration of fucosylation, or fucose being linked to proteins and lipids, which has been reported in pathological conditions including inflammation and signaling events by the Notch receptor family (70, 71). Elevated level of plasma fucose has been reported in endometriosis cases compared to controls (72) suggesting it may be an important metabolite in endometriosis but no evaluation with persistent post-surgical pelvic pain was assessed.
Although studies report about one third of endometriosis patients suffer from persistent pelvic pain despite surgical treatment (2, 3), currently there are no established predictors of treatment response after endometriosis surgery. While it is expected that patients with severe pelvic pain are at greater risk of persistent post-surgical pelvic pain, prior literature report severity of pelvic pain at time of surgery do not correlate with symptom recurrence (73). Our results suggest there are specific blood metabolomic profiles that may be predictive of post-surgical outcomes. Furthermore, underlying mechanisms of symptom progression in endometriosis patients are not fully understood. Central sensitization may be one underlying pathophysiology(74, 75), although results from our study showing upregulation of prostaglandin pathways provide insight into additional potential biological pathways involved in persistent pelvic pain among endometriosis patients.
Our study has several strengths. We had prospectively collected blood samples on 180 endometriosis patients prior to endometriosis surgery with detailed pelvic pain symptom information one-year after surgery. We also had serial blood samples allowing to examine change in metabolite levels before and closely after surgery in relation to severe pelvic pain one-year after surgery. We had detailed information on potential confounders that we were able to account for in our analyses. Furthermore, we used validated metabolomics assays with high reproducibility and stability and were able to comprehensively examine over 300 metabolites simultaneously. All patients received standard of care for endometriosis, with the majority receiving hormonal treatment after surgery. Our observed association could have been mediated by a change in type of medical interventions that occurred after surgery. However, since the vast majority of our study population were on hormonal treatment at one-year follow-up, we were not able to quantify the potential mediation effect in our dataset (76). Less than half of patients had post-surgical metabolite data available for evaluation, which may result in potential selection bias. However, baseline characteristics were similar among those with and without paired pre- and post-surgical blood samples, and therefore the potential influence of selection bias on the results is minimal. Another limitation of this observational study is the potential for unmeasured confounders (i.e. factors that were not accounted for in these analyses that preceded and were correlated with both pre-surgical plasma metabolite levels and post-surgical pelvic pain). While we observed little evidence of confounding by the factors that were considered, replication of these findings in independent datasets is necessary and may reveal residual confounders. Generalizability is limited primarily to white, young endometriosis patients with superficial peritoneal lesions / rASRM stage I/II who presented with pelvic pain. Superficial peritoneal lesions are the predominant clinical presentation of endometriosis patients overall (17–19), and the majority of patients regardless of age at diagnosis report onset of pelvic pain symptoms during adolescence (77). However, our findings may not be applicable to those diagnosed later in adulthood, those presenting with infertility, or those with endometriomas, deep endometriosis, or rASRM stage III/IV disease. Further research is need to investigate similarities and differences in molecular profiles of endometriosis patients diagnosed in adolescents and adulthood.
CONCLUSION
In summary, among adolescents and young adults with laparoscopically confirmed endometriosis, we report the first prospective study examining metabolites and metabolomic profiles in pre-surgical blood associated with risk of persistent post-surgical pelvic pain. While validation in independent datasets is warranted, our results support that plasma metabolites may be promising biomarkers to differentiate endometriosis patients who may experience poor surgical outcome, detecting signals beyond clinical characteristics and self-reported pelvic pain severity at the time of surgery. Furthermore, these results provide important insight that dysregulation of lipid metabolism may play a role in persistence of pelvic pain post-surgery among endometriosis patients. Future research investigating longitudinal change in these plasma biomarkers and its correlation with pelvic pain severity is needed to discover novel biomarkers that could be used clinically for monitoring recurrence or progression of the disease.
Supplementary Material
Acknowledgments:
The authors would like to thank the participants of the Women’s Health Study: From Adolescence to Adulthood (A2A) for their valuable contributions and all staff members of the Boston Center for Endometriosis.
Funding:
Financial support for establishment of and data collection within the A2A cohort were provided by the J. Willard and Alice S. Marriott Foundation; and support for assay costs was provided by the Peery family. N.S., A.F.V, S.A.M, M.R.L., K.L.T. has received funding from Marriott Family Foundations. S.A.M and K.L.T. were supported by NICHD R01 HD094842.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: M.R.L. has received royalties from UpToDate and Wolters Kluwer, consultancy from Next Gen Jane. S.A.M. serves as an advisory board member for AbbVie and a single working group service for Roche; neither are related to this study. All authors report no conflict of interest.
REFERENCES
- 1.Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med 2020;382:1244–56. [DOI] [PubMed] [Google Scholar]
- 2.Caumo W, Deitos A, Carvalho S, Leite J, Carvalho F, Dussán-Sarria JA et al. Motor Cortex Excitability and BDNF Levels in Chronic Musculoskeletal Pain According to Structural Pathology. Front Hum Neurosci 2016;10:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbott J, Hawe J, Hunter D, Holmes M, Finn P, Garry R. Laparoscopic excision of endometriosis: a randomized, placebo-controlled trial. Fertil Steril 2004;82:878–84. [DOI] [PubMed] [Google Scholar]
- 4.Becker CM, Laufer MR, Stratton P, Hummelshoj L, Missmer SA, Zondervan KT et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project: I. Surgical phenotype data collection in endometriosis research. Fertil Steril 2014;102:1213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerszten RE, Wang TJ. The search for new cardiovascular biomarkers. Nature 2008;451:949–52. [DOI] [PubMed] [Google Scholar]
- 6.Mayers JR, Wu C, Clish CB, Kraft P, Torrence ME, Fiske BP et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med 2014;20:1193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papandreou C, Bullo M, Ruiz-Canela M, Dennis C, Deik A, Wang D et al. Plasma metabolites predict both insulin resistance and incident type 2 diabetes: a metabolomics approach within the Prevencion con Dieta Mediterranea (PREDIMED) study. The American journal of clinical nutrition 2019;109:626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paynter NP, Balasubramanian R, Giulianini F, Wang DD, Tinker LF, Gopal S et al. Metabolic Predictors of Incident Coronary Heart Disease in Women. Circulation 2018;137:841–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clish CB. Metabolomics: an emerging but powerful tool for precision medicine. Cold Spring Harbor molecular case studies 2015;1:a000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cordeiro FB, Cataldi TR, Perkel KJ, do Vale Teixeira da Costa L, Rochetti RC, Stevanato J et al. Lipidomics analysis of follicular fluid by ESI-MS reveals potential biomarkers for ovarian endometriosis. J Assist Reprod Genet 2015;32:1817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Gao Y, Guan L, Zhang H, Sun J, Gong X et al. Discovery of Phosphatidic Acid, Phosphatidylcholine, and Phosphatidylserine as Biomarkers for Early Diagnosis of Endometriosis. Front Physiol 2018;9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Letsiou S, Peterse DP, Fassbender A, Hendriks MM, van den Broek NJ, Berger R et al. Endometriosis is associated with aberrant metabolite profiles in plasma. Fertil Steril 2017;107:699–706.e6. [DOI] [PubMed] [Google Scholar]
- 13.Dutta M, Anitha M, Smith PB, Chiaro CR, Maan M, Chaudhury K et al. Metabolomics Reveals Altered Lipid Metabolism in a Mouse Model of Endometriosis. Journal of proteome research 2016;15:2626–33. [DOI] [PubMed] [Google Scholar]
- 14.Vouk K, Ribic-Pucelj M, Adamski J, Rizner TL. Altered levels of acylcarnitines, phosphatidylcholines, and sphingomyelins in peritoneal fluid from ovarian endometriosis patients. The Journal of steroid biochemistry and molecular biology 2016;159:60–9. [DOI] [PubMed] [Google Scholar]
- 15.Vouk K, Hevir N, Ribic-Pucelj M, Haarpaintner G, Scherb H, Osredkar J et al. Discovery of phosphatidylcholines and sphingomyelins as biomarkers for ovarian endometriosis. Human reproduction (Oxford, England) 2012;27:2955–65. [DOI] [PubMed] [Google Scholar]
- 16.Shafrir AL, Farland LV, Shah DK, Harris HR, Kvaskoff M, Zondervan K et al. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol 2018;51:1–15. [DOI] [PubMed] [Google Scholar]
- 17.DiVasta AD, Vitonis AF, Laufer MR, Missmer SA. Spectrum of symptoms in women diagnosed with endometriosis during adolescence vs adulthood. Am J Obstet Gynecol 2018;218:324 e1–e11. [DOI] [PubMed] [Google Scholar]
- 18.Shah DK, Missmer SA. Scientific investigation of endometriosis among adolescents. J Pediatr Adolesc Gynecol 2011;24:S18–9. [DOI] [PubMed] [Google Scholar]
- 19.Shim JY, Laufer MR. Adolescent Endometriosis: An Update. J Pediatr Adolesc Gynecol 2020;33:112–9. [DOI] [PubMed] [Google Scholar]
- 20.Miller JA, Missmer SA, Vitonis AF, Sarda V, Laufer MR, DiVasta AD. Prevalence of migraines in adolescents with endometriosis. Fertil Steril 2018;109:685–90. [DOI] [PubMed] [Google Scholar]
- 21.Sasamoto N, Farland LV, Vitonis AF, Harris HR, DiVasta AD, Laufer MR et al. In utero and early life exposures in relation to endometriosis in adolescents and young adults. European J Obstet & Gyn and Reprod Biol 2020;252:393–8. [DOI] [PubMed] [Google Scholar]
- 22.Vitonis AF, Vincent K, Rahmioglu N, Fassbender A, Buck Louis GM, Hummelshoj L et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonization Project: II. Clinical and covariate phenotype data collection in endometriosis research. Fertil Steril 2014;102:122332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor HS, Adamson GD, Diamond MP, Goldstein SR, Horne AW, Missmer SA et al. An evidence-based approach to assessing surgical versus clinical diagnosis of symptomatic endometriosis. Int J Gynaecol Obstet 2018;142:131–42. [DOI] [PubMed] [Google Scholar]
- 24.Rahmioglu N, Fassbender A, Vitonis AF, Tworoger SS, Hummelshoj L, D’Hooghe TM et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonization Project: III. Fluid biospecimen collection, processing, and storage in endometriosis research. Fertil Steril 2014;102:1233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fassbender A, Rahmioglu N, Vitonis AF, Vigano P, Giudice LC, D’Hooghe TM et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project: IV. Tissue collection, processing, and storage in endometriosis research. Fertil Steril 2014;102:1244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasamoto N, DePari M, Vitonis AF, Laufer MR, Missmer SA, Shafrir AL et al. Evaluation of CA125 in relation to pain symptoms among adolescents and young adult women with and without surgically-confirmed endometriosis. PLoS One 2020;15:e0238043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eveleth PB. Physical Status: The Use and Interpretation of Anthropometry. Report of a WHO Expert Committee. American Journal of Human Biology 1996;8:786–7. [Google Scholar]
- 28.WHO. World Health Organization. BMI classification. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html. In. Vol. 2017. [Google Scholar]
- 29.Barlow SE, Expert C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007;120 Suppl 4:S164–92. [DOI] [PubMed] [Google Scholar]
- 30.Paynter NP, Balasubramanian R, Giulianini F, Wang DD, Tinker LF, Gopal S et al. Metabolic predictors of incident coronary heart disease in women. Circulation 2018;137:841–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’sullivan JF, Morningstar JE, Yang Q, Zheng B, Gao Y, Jeanfavre S et al. Dimethylguanidino valeric acid is a marker of liver fat and predicts diabetes. The Journal of clinical investigation 2017;127:4394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mascanfroni ID, Takenaka MC, Yeste A, Patel B, Wu Y, Kenison JE et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nature medicine 2015;21:638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Townsend MK, Bao Y, Poole EM, Bertrand KA, Kraft P, Wolpin BM et al. Impact of Pre-analytic Blood Sample Collection Factors on Metabolomics. Cancer Epidemiol Biomarkers Prev 2016;25:823–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Townsend MK, Clish CB, Kraft P, Wu C, Souza AL, Deik AA et al. Reproducibility of metabolomic profiles among men and women in 2 large cohort studies. Clin Chem 2013;59:1657–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia J, Wishart DS. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis. Curr Protoc Bioinformatics 2016;55:14 0 1–0 91. [DOI] [PubMed] [Google Scholar]
- 36.Brosens I, Gordts S, Benagiano G. Endometriosis in adolescents is a hidden, progressive and severe disease that deserves attention, not just compassion. Human reproduction (Oxford, England) 2013;28:2026–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stuparich MA, Donnellan NM, Sanfilippo JS. Endometriosis in the Adolescent Patient. Semin Reprod Med 2017;35:102–9. [DOI] [PubMed] [Google Scholar]
- 38.Benagiano G, Guo SW, Puttemans P, Gordts S, Brosens I. Progress in the diagnosis and management of adolescent endometriosis: an opinion. Reprod Biomed Online 2018;36:102–14. [DOI] [PubMed] [Google Scholar]
- 39.Song X-C, Yu X, Luo M, Yu Q, Zhu L. Clinical Characteristics and Postoperative Symptoms of 85 Adolescents with Endometriosis. Journal of Pediatric and Adolescent Gynecology 2020;33:519–23. [DOI] [PubMed] [Google Scholar]
- 40.Abbott JA, Hawe J, Clayton RD, Garry R. The effects and effectiveness of laparoscopic excision of endometriosis: a prospective study with 2–5 year follow-up. Human reproduction (Oxford, England) 2003;18:1922–7. [DOI] [PubMed] [Google Scholar]
- 41.Dutta M, Joshi M, Srivastava S, Lodh I, Chakravarty B, Chaudhury K. A metabonomics approach as a means for identification of potential biomarkers for early diagnosis of endometriosis. Mol Biosyst 2012;8:3281–7. [DOI] [PubMed] [Google Scholar]
- 42.Vicente-Muñoz S, Morcillo I, Puchades-Carrasco L, Payá V, Pellicer A, Pineda-Lucena A. Nuclear magnetic resonance metabolomic profiling of urine provides a noninvasive alternative to the identification of biomarkers associated with endometriosis. Fertil Steril 2015;104:1202–9. [DOI] [PubMed] [Google Scholar]
- 43.Maignien C, Santulli P, Kateb F, Caradeuc C, Marcellin L, Pocate-Cheriet K et al. Endometriosis phenotypes are associated with specific serum metabolic profiles determined by proton-nuclear magnetic resonance. Reprod Biomed Online 2020;41:640–52. [DOI] [PubMed] [Google Scholar]
- 44.Murgia F, Angioni S, D’Alterio MN, Pirarba S, Noto A, Santoru ML et al. Metabolic Profile of Patients with Severe Endometriosis: a Prospective Experimental Study. Reprod Sci 2021;28:728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loy SL, Zhou J, Cui L, Tan TY, Ee TX, Chern BSM et al. Discovery and validation of peritoneal endometriosis biomarkers in peritoneal fluid and serum. Reprod Biomed Online 2021. [DOI] [PubMed] [Google Scholar]
- 46.Murgia F, Angioni S, D’Alterio MN, Pirarba S, Noto A, Santoru ML et al. Metabolic Profile of Patients with Severe Endometriosis: a Prospective Experimental Study. Reprod Sci 2021;28:728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teckchandani S, Nagana Gowda GA, Raftery D, Curatolo M. Metabolomics in chronic pain research. Eur J Pain 2021;25:313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu M, Xie Z, Costello CA, Zhang W, Chen L, Qi D et al. Metabolomic analysis coupled with extreme phenotype sampling identified that lysophosphatidylcholines are associated with multisite musculoskeletal pain. Pain 2021;162:600–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caboni P, Liori B, Kumar A, Santoru ML, Asthana S, Pieroni E et al. Metabolomics analysis and modeling suggest a lysophosphocholines-PAF receptor interaction in fibromyalgia. PLoS One 2014;9:e107626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parker KS, Crowley JR, Stephens-Shields AJ, van Bokhoven A, Lucia MS, Lai HH et al. Urinary Metabolomics Identifies a Molecular Correlate of Interstitial Cystitis/Bladder Pain Syndrome in a Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network Cohort. EBioMedicine 2016;7:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coxon L, Horne AW, Vincent K. Pathophysiology of endometriosis-associated pain: A review of pelvic and central nervous system mechanisms. Best Pract Res Clin Obstet Gynaecol 2018;51:53–67. [DOI] [PubMed] [Google Scholar]
- 52.Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. PAIN 2011;152:S2–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aoki J, Inoue A, Okudaira S. Two pathways for lysophosphatidic acid production. Biochim Biophys Acta 2008;1781:513–8. [DOI] [PubMed] [Google Scholar]
- 54.Balsinde J, Winstead MV, Dennis EA. Phospholipase A(2) regulation of arachidonic acid mobilization. FEBS Lett 2002;531:2–6. [DOI] [PubMed] [Google Scholar]
- 55.Nakanaga K, Hama K, Aoki J. Autotaxin--an LPA producing enzyme with diverse functions. J Biochem 2010;148:13–24. [DOI] [PubMed] [Google Scholar]
- 56.Inoue M, Xie W, Matsushita Y, Chun J, Aoki J, Ueda H. Lysophosphatidylcholine induces neuropathic pain through an action of autotaxin to generate lysophosphatidic acid. Neuroscience 2008;152:296–8. [DOI] [PubMed] [Google Scholar]
- 57.Inoue M, Rashid MH, Fujita R, Contos JJ, Chun J, Ueda H. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat Med 2004;10:712–8. [DOI] [PubMed] [Google Scholar]
- 58.Balsinde J, Winstead MV, Dennis EA. Phospholipase A2 regulation of arachidonic acid mobilization. FEBS Letters 2002;531:2–6. [DOI] [PubMed] [Google Scholar]
- 59.Laschke MW, Elitzsch A, Scheuer C, Vollmar B, Menger MD. Selective cyclo-oxygenase-2 inhibition induces regression of autologous endometrial grafts by down-regulation of vascular endothelial growth factor-mediated angiogenesis and stimulation of caspase-3-dependent apoptosis. Fertil Steril 2007;87:163–71. [DOI] [PubMed] [Google Scholar]
- 60.Olivares C, Bilotas M, Buquet R, Borghi M, Sueldo C, Tesone M et al. Effects of a selective cyclooxygenase-2 inhibitor on endometrial epithelial cells from patients with endometriosis. Human reproduction (Oxford, England) 2008;23:2701–8. [DOI] [PubMed] [Google Scholar]
- 61.Lai Z-Z, Yang H-L, Ha S-Y, Chang K-K, Mei J, Zhou W-J et al. Cyclooxygenase-2 in Endometriosis. Int J Biol Sci 2019;15:2783–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuan L, Shen F, Lu Y, Liu X, Guo SW. Cyclooxygenase-2 overexpression in ovarian endometriomas is associated with higher risk of recurrence. Fertil Steril 2009;91:1303–6. [DOI] [PubMed] [Google Scholar]
- 63.Shen F, Wang Y, Lu Y, Yuan L, Liu X, Guo SW. Immunoreactivity of progesterone receptor isoform B and nuclear factor kappa-B as biomarkers for recurrence of ovarian endometriomas. Am J Obstet Gynecol 2008;199:486.e1–.e10. [DOI] [PubMed] [Google Scholar]
- 64.Machairiotis N, Vasilakaki S, Thomakos N. Inflammatory Mediators and Pain in Endometriosis: A Systematic Review. Biomedicines 2021;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Díaz-Muñoz MD, Osma-García IC, Cacheiro-Llaguno C, Fresno M, Iñiguez MA. Coordinated upregulation of cyclooxygenase-2 and microsomal prostaglandin E synthase 1 transcription by nuclear factor kappa B and early growth response-1 in macrophages. Cell Signal 2010;22:1427–36. [DOI] [PubMed] [Google Scholar]
- 66.Marquardt RM, Kim TH, Shin JH, Jeong JW. Progesterone and Estrogen Signaling in the Endometrium: What Goes Wrong in Endometriosis? Int J Mol Sci 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand 2017;96:623–32. [DOI] [PubMed] [Google Scholar]
- 68.Bruner-Tran KL, Ding T, Osteen KG. Dioxin and endometrial progesterone resistance. Semin Reprod Med 2010;28:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schneider M, Al-Shareffi E, Haltiwanger RS. Biological functions of fucose in mammals. Glycobiology 2017;27:601–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miyoshi E, Moriwaki K, Nakagawa T. Biological function of fucosylation in cancer biology. J Biochem 2008;143:725–9. [DOI] [PubMed] [Google Scholar]
- 71.Becker DJ, Lowe JB. Fucose: biosynthesis and biological function in mammals. Glycobiology 2003;13:41r–53r. [DOI] [PubMed] [Google Scholar]
- 72.Vicente-Muñoz S, Morcillo I, Puchades-Carrasco L, Payá V, Pellicer A, Pineda-Lucena A. Pathophysiologic processes have an impact on the plasma metabolomic signature of endometriosis patients. Fertil Steril 2016;106:1733–41.e1. [DOI] [PubMed] [Google Scholar]
- 73.Vignali M, Bianchi S, Candiani M, Spadaccini G, Oggioni G, Busacca M. Surgical treatment of deep endometriosis and risk of recurrence. J Minimally Inv Gyn 2005;12:508–13. [DOI] [PubMed] [Google Scholar]
- 74.Li T, Mamillapalli R, Ding S, Chang H, Liu ZW, Gao XB et al. Endometriosis alters brain electrophysiology, gene expression and increases pain sensitization, anxiety, and depression in female mice. Biol Reprod 2018;99:349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.As-Sanie S, Kim J, Schmidt-Wilcke T, Sundgren PC, Clauw DJ, Napadow V et al. Functional Connectivity is Associated With Altered Brain Chemistry in Women With Endometriosis-Associated Chronic Pelvic Pain. J Pain 2016;17:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farland LV, Correia KFB, Dodge LE, Modest AM, Williams PL, Smith LH et al. The importance of mediation in reproductive health studies. Human reproduction (Oxford, England) 2020;35:1262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nnoaham KE, Hummelshoj L, Webster P, d’Hooghe T, de Cicco Nardone F, de Cicco Nardone C et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril 2011;96:366–73.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


