Abstract
PURPOSE:
To investigate patient-perceived quality of life (QOL) among patients treated with a novel form of breast intraoperative radiation therapy (PB-IORT).
METHODS AND MATERIALS:
Patients treated with PB-IORT as part of a phase II clinical trial from 2013–2020 were identified. Patients were given the European Organization for Research and Treatment of Cancer (EORTC) core 30-item Quality of Life Questionnaire (QLQ-C30) encompassing global health, functionality, and symptomatology at baseline, 1-month, 6-months, 12-months, and 24-months after PB-IORT. Scores were on a 100-point scale with change greater than 10 considered clinically significant. Scores at interval follow-up were compared to baseline using repeated measure modeling with an unstructured covariance matrix.
RESULTS:
The cohort consisted of 303 patients, a majority of which were White (84.2%) with a median age of 64 years (IQR: 52, 76). One month after PB-IORT, a decline from baseline in physical (−2.5, 95% CI: −4.4 – −0.55, p = 0.01), role (−7.6, 95% CI: −11.7 – −3.5, p < .001), and social functioning (−3.0, 95% CI: −5.5 – −0.42, p = 0.02) were observed, which correlated with increased fatigue (8.4, 95% CI: 5.5–11.3, p < 0.001). At 6 months, nearly all QOL measures returned to baseline or improved. There were no statistically or clinically significant differences from baseline in overall global health. All functional and symptom scale differences were less than 10, indicating minimal clinical significance.
CONCLUSIONS:
PB-IORT has minimal negative impact on QOL, further supporting this patient-centered treatment approach for early-stage breast cancer.
Keywords: intraoperative radiation therapy, brachytherapy, breast cancer, quality of life
INTRODUCTION
Breast cancer patients experience physical, emotional, and social distress which inevitably influences their quality of life (QOL) (1, 2). Maintaining QOL is of critical importance to cancer patients (3). Physician and patient awareness regarding the impact of various breast cancer therapy options on QOL is essential, so that patients can make informed decisions and have reasonable expectations of their treatment course.
For patients with early-stage breast cancer, breast conserving therapy (BCT; breast conserving surgery [BCS] plus radiation therapy [RT]) is the preferred treatment option (4). Whole breast irradiation (WBI) has been the standard RT modality, as it is known to reduce ipsilateral breast tumor recurrence (IBTR) rates (5). However, WBI is time consuming and inconvenient to patients, requiring 4–6 weeks of daily treatments (6, 7). Additionally, WBI is also associated with deleterious effects on the skin, chest wall, and heart (6, 8, 9). Given these factors, it is reasonable to expect that patient QOL would be impacted after treatment with WBI. Accelerated partial breast irradiation (APBI), which involves shorter radiation schedules targeted to the at-risk breast tissue, has become an increasingly popular option for adjuvant RT as it provides adequate oncologic outcomes and makes the treatment schedule feasible and more convenient for breast cancer patents (6, 8, 10–12). Intraoperative radiation therapy (IORT) maximizes patient convenience by delivering just one dose of radiation at the time of BCS. However, as with any cancer treatment, IORT has the potential to negatively impact patient QOL.
Investigation into patient-reported QOL after BCT focus on fatigue (7, 13–17), general QOL measures (16–21), and specific breast symptoms as primary endpoints (17–21). Several studies have compared QOL after WBI versus APBI and found that APBI is associated with less fatigue (13, 15, 16) and fewer breast symptoms such as pain, skin discoloration, and asymmetry (16, 18–20). Interestingly, however, many studies show small overall difference in QOL for patients treated with WBI and APBI (16, 18, 19, 21).
At the University of Virginia, we developed a novel form of breast IORT, Precision Breast IORT (PB-IORT) which improves upon conventional breast IORT by incorporating high-dose-rate (HDR) brachytherapy with CT imaging for delivery of a single dose (12.5 Gy) of highly conformal radiation to the lumpectomy cavity at the time of BCS or within 30 days of BCS (6, 10, 22). Observing patient-perceived QOL after this unique treatment option is essential to understanding its long-term patient-centered outcomes and potential for further dissemination. Here, we aim to describe QOL among patients treated with PB-IORT and compare these measures to existing QOL data in other breast cancer RT options.
METHODS
Study Population
The patient cohort for the present study included all patients treated with PB-IORT as part of an ongoing phase II multi-institutional trial (NCT02400658). This project was approved by the University of Virginia Institutional Review Board (IRB#18004). Informed consent was obtained from all participants who were enrolled in the trial at the University of Virginia (UVA), Thomas Jefferson University (TJUH), or Hackensack University Medical Center (HUMC) between 2013–2020. The inclusion criteria for the clinical trial includes age ≥ 45 years, tumor size ≤ 3 cm, node negative disease with invasive or in situ breast carcinoma. Exclusion criteria are ipsilateral breast cancer treated with RT, BRCA gene mutation, breast implants, or treatment with neoadjuvant chemotherapy. Participants can be treated at the time of their BCS (pre-pathology), or after BCS (post-pathology). Post-pathology participants must receive PB-IORT within 30 days of their operation. Patients with invasive disease undergo a sentinel lymph node biopsy prior to the BCS and PB-IORT (UVA) or at the time of BCS (TJUH and HUMC).
Precision-Breast Intraoperative Radiation Therapy (PB-IORT) Technique
The methods for PB-IORT at the University of Virginia have been described in detail (22). In brief, the procedure takes place in an integrated brachytherapy suite with full anesthesia capability, a CT unit that is on rails, and an Iridium 192- high dose rate brachytherapy source. The patient undergoes BCS, a multi-lumen catheter is placed in the lumpectomy cavity and CT images are obtained. A treatment plan is then created and optimized to deliver 12.5 Gy in a single fraction. The catheter is then removed and the incision is closed. At the other two sites, the participant undergoes BCS and catheter placement in the operating room. After recovery, they undergo CT imaging, treatment planning, dose delivery, and catheter removal in the brachytherapy suite.
Study Variables
Participant data was extracted from a prospectively maintained institutional database for the clinical trial. Participant demographic data included age, race, and body mass index (BMI). Tumor variable data collected included tumor size, histology, and receptor status.
Quality of Life Survey
The European Organization for Research and Treatment of Cancer (EORTC) core 30-item Quality of Life Questionnaire (QLQ-C30) version 3.0 was used to assess patient quality of life over their treatment course. The EORTC QLQ-C30 uses five functional scales (Physical, Role, Emotional, Cognitive, and Social), three symptom scales (Fatigue, Nausea and/or Vomiting, Pain), six individual questions addressing dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties, and an overall global health status score (revised global health status) (23). Questions pertaining to functional status and symptoms are rated on a 4-point Likert scale, “not at all”, “a little”, “quite a bit”, and “very much”. Global health questions are rated by patients on a scale of 1 to 7 from “very poor” to “excellent” (24). Overall scoring for each category ranges from 0 to 100, with higher scores indicating more functionality on functional scales and better QOL on global health scales, but higher symptomatology on symptom scales (24).
Data were obtained from participants at baseline, 1-month, 6-months, 12-months, and 24-months after PB-IORT. The EORTC QLQ-C30 surveys were consistently scored using the reported guidelines (24). For this study, clinically significant changes on interval time periods of the 0–100 scale are also based on these guidelines: a change of less than 10 is considered of minimal clinical significance to patients, 10–20 is a moderate effect, and greater than 20 is considered a very large effect on patient QOL (24, 25).
Statistical Analysis
Participant demographics and pathology are summarized as median (interquartile range) for continuous variables or frequency (percentage) for categorical variables. The analytic method used for the EORTC QLQ-C30 scales and subscales included repeated measures modeling with an unstructured covariance matrix. All testing of subsequent time periods (1-, 6-, 12-, and 24-months) was compared to baseline (0 months). Statistical significance was considered an alpha level of p < 0.05 with two-sided hypothesis testing. All analyses were performed using SAS version 9.4 and figures were generated using GraphPad Prism version 9.0.
RESULTS
Participant Characteristics
The study cohort consisted of 303 participants. The median follow-up for the cohort was 24 months. Participant and tumor characteristics are summarized in Table 1. The majority of participants were White (84.2%) and non-Hispanic (96.4%), with a median age of 64 years (IQR: 52, 76). The median BMI of this population was 28.9 kg/m2 (IQR: 20.9, 36.9). The most common tumor pathology was IDC (62.7%), followed by DCIS (29.4%), ILC (6.3%), invasive mucinous carcinoma (1.0%), and mixed IDC and ILC (0.66%). The majority of tumors were ER-positive (94.1%), PR-positive (67.0%), and HER2-negative (68.0%).
Table 1.
Participant demographics and pathology after breast conserving surgery and precision-breast intraoperative radiation therapy
| Patient Characteristics | N (%), n = 303 |
|---|---|
|
| |
| Age (years), median (IQR) | 64 (52, 76) |
| BMI (kg/m2), median (IQR) | 28.9 (20.9, 36.9) |
| Race | |
| White | 255 (84.2) |
| Black or African American | 28 (9.2) |
| Asian | 2 (0.66) |
| Other | 4 (1.3) |
| Unknown | 14 (4.6) |
| Ethnicity | |
| Non-Hispanic | 292 (96.4) |
| Hispanic or Latino | 8 (2.6) |
| Unknown | 3 (1.0) |
| Tumor Size (mm), median (IQR) | 8.0 (1.0, 17.0) |
| Laterality | |
| Left | 136 (44.9) |
| Right | 166 (54.8) |
| Unknown | 1 (0.33) |
| Pathology | |
| Invasive Ductal Carcinoma (IDC) | 190 (62.7) |
| Ductal Carcinoma In Situ (DCIS) | 89 (29.4) |
| Invasive Lobular Carcinoma (ILC) | 19 (6.3) |
| Invasive Mucinous Carcinoma | 3 (1.0) |
| Mixed IDC and ILC | 2 (0.66) |
| Receptor Status | |
| ER-positive | 285 (94.1) |
| ER-negative | 17 (5.6) |
| PR-positive | 203 (67.0) |
| PR-negative | 35 (11.6) |
| PR-unknown | 65 (21.4) |
| HER2-positive | 16 (5.3) |
| HER2-negative | 206 (68.0) |
| HER2-unknown | 81 (26.7) |
| Operation Timing* | |
| Pre-Pathology | 240 (79.2) |
| Post-Pathology | 63 (20.8) |
BMI = body mass index
ER = estrogen receptor
PR = progesterone receptor
HER2 = human epidermal growth factor receptor 2
WBI = whole breast irradiation
Pre-Pathology = BCS and PB-IORT at the same timepoint; Post-Pathology = BCS followed by PB-IORT within 30 days and after pathology is known
Quality of Life After PB-IORT
Model estimated mean scores at each time interval and change from baseline for both global health status and functional subscales can be found in Figure 1 and Table 2. At baseline the model estimated mean patient score for the revised global health status was 82.4 (SEM = 1.2) on a 100-point scale. There were no statistically significant changes over time in the revised global health status. Overall, all QOL scores changes were less than 10 points, indicative of minimal clinical significance (24, 25).
Figure 1.
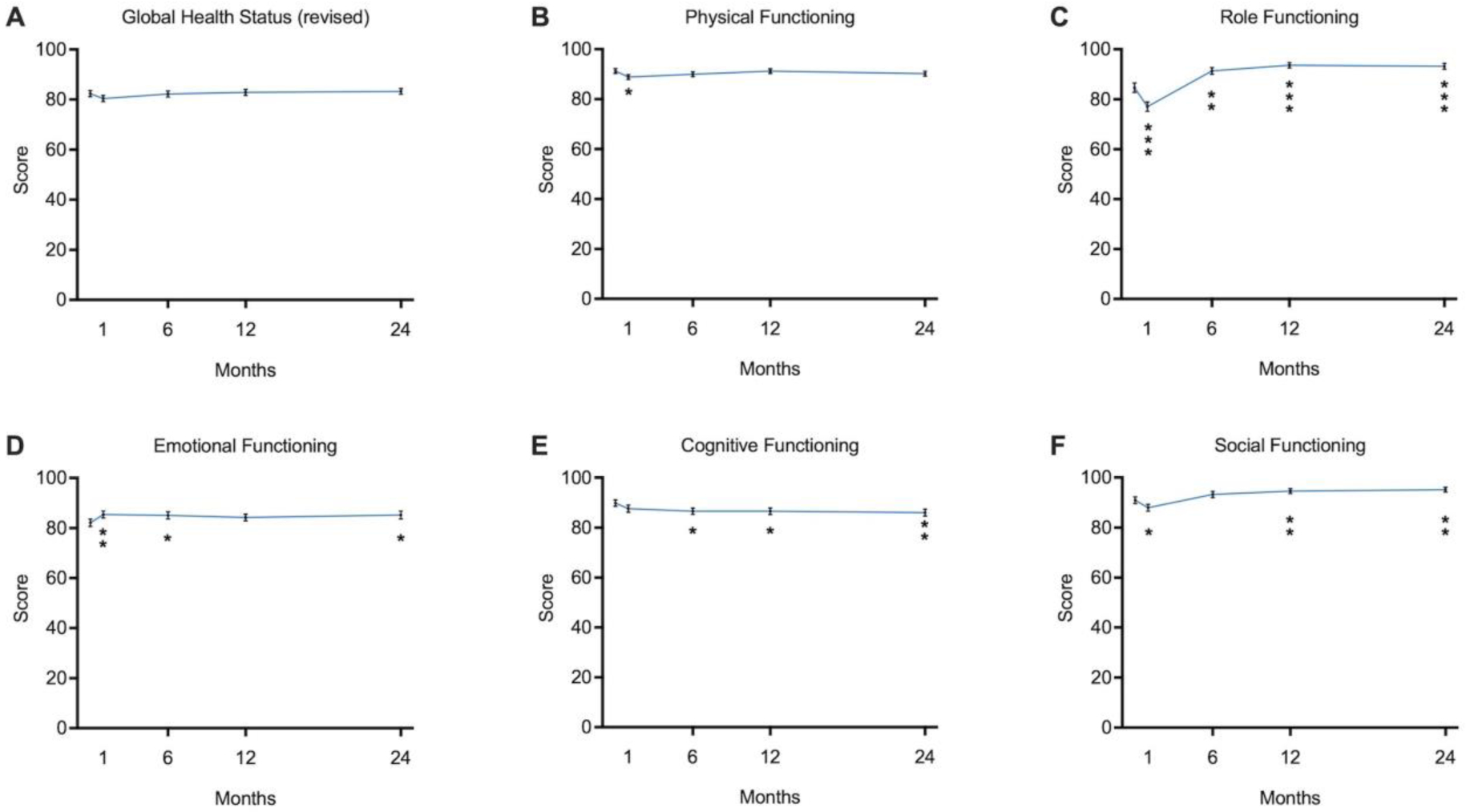
Participant model estimated mean survey scores for quality of life functional subscales at each time interval; significance determined by difference from baseline (0 months) compared to subsequent time interval. * = p < 0.05, ** = p < 0.01, *** = p < 0.001
Table 2.
Participant model estimated mean survey scores for quality of life functional subscales at each time interval and change over time.
| Quality of Life Measure | Month | Model Mean Score | SE of the Mean | Estimated Difference from 0 months | SE | 95% Confidence Interval | P-value | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Global Health Status Revised* | 0 | 82.4 | 1.2 | |||||
| 1 | 80.4 | 1.2 | −2.0 | 1.2 | −4.3 | 0.35 | 0.10 | |
| 6 | 82.2 | 1.3 | −0.14 | 1.5 | −3.0 | 2.8 | 0.93 | |
| 12 | 82.9 | 1.2 | 0.48 | 1.4 | −2.2 | 3.2 | 0.72 | |
| 24 | 83.2 | 1.1 | 0.83 | 1.2 | −1.5 | 3.2 | 0.49 | |
|
| ||||||||
| Physical Functioning | 0 | 91.3 | 0.94 | |||||
| 1 | 88.8 | 1.0 | −2.5 | 0.97 | −4.4 | −0.55 | 0.01 | |
| 6 | 90.0 | 1.0 | −1.3 | 0.96 | −3.2 | 0.57 | 0.17 | |
| 12 | 91.3 | 0.92 | −0.03 | 0.80 | −1.6 | 1.6 | 0.97 | |
| 24 | 90.3 | 0.97 | −1.04 | 0.98 | −3.0 | 0.88 | 0.29 | |
|
| ||||||||
| Role Functioning | 0 | 84.6 | 1.9 | |||||
| 1 | 77.1 | 1.9 | −7.6 | 2.1 | −11.7 | −3.5 | <.001 | |
| 6 | 91.3 | 1.4 | 6.7 | 2.1 | 2.5 | 10.9 | 0.002 | |
| 12 | 93.7 | 1.1 | 9.0 | 2.0 | 5.1 | 12.9 | <.001 | |
| 24 | 93.2 | 1.2 | 8.6 | 2.1 | 4.4 | 12.7 | <.001 | |
|
| ||||||||
| Emotional Functioning | 0 | 82.1 | 1.5 | |||||
| 1 | 85.4 | 1.4 | 3.3 | 1.2 | 0.99 | 5.6 | 0.005 | |
| 6 | 85.0 | 1.4 | 2.9 | 1.4 | 0.18 | 5.7 | 0.04 | |
| 12 | 84.3 | 1.3 | 2.1 | 1.4 | −0.55 | 4.8 | 0.12 | |
| 24 | 85.2 | 1.6 | 3.1 | 1.4 | 0.28 | 5.9 | 0.03 | |
|
| ||||||||
| Cognitive Functioning | 0 | 89.8 | 1.3 | |||||
| 1 | 87.6 | 1.5 | −2.2 | 1.2 | −4.6 | 0.17 | 0.07 | |
| 6 | 86.6 | 1.3 | −3.3 | 1.3 | −5.8 | −0.77 | 0.01 | |
| 12 | 86.6 | 1.3 | −3.3 | 1.3 | −5.8 | −0.73 | 0.01 | |
| 24 | 86.0 | 1.4 | −3.8 | 1.2 | −6.3 | −1.4 | 0.002 | |
|
| ||||||||
| Social Functioning | 0 | 90.9 | 1.3 | |||||
| 1 | 88.0 | 1.4 | −3.0 | 1.3 | −5.5 | −0.42 | 0.02 | |
| 6 | 93.3 | 1.2 | 2.4 | 1.5 | −0.55 | 5.3 | 0.11 | |
| 12 | 94.6 | 0.98 | 3.7 | 1.3 | 1.0 | 6.3 | 0.007 | |
| 24 | 95.2 | 0.96 | 4.2 | 1.5 | 1.3 | 7.1 | 0.005 | |
Global Health Status revised is the most updated measure from The European Organization for Research and Treatment of Cancer (EORTC) core 30-item Quality of Life Questionnaire (QLQ-C30) version 3.0
Physical functioning was high at baseline, with a model estimated mean score of 91.3 (SEM = 0.94). One- month postoperatively, the physical functioning score declined to 88.8, a statistically significant decrease compared to baseline but of minimal clinical significance (−2.5, 95% CI: −4.4 – −0.55, p = 0.01) (Figure 1, Table 2). At 6, 12, and 24-months after PB-IORT, there were no statistical or clinically significant changes, and patient reported physical functioning returned to baseline. Role functioning, which encompasses a patient’s ability to do their daily activities including work, childcare, and participating in leisure activities, had a model estimated mean baseline score of 84.6 (SEM = 1.9). Role functioning was significantly decreased after 1-month to 77.1 (p < .001). Despite declining role functioning in the first month, patients reported a significantly improved role functioning status at 6-months to 91.3 (p = 0.002), 12-months to 93.7 (p < .001), and 24-months to 93.2 (p < .001) when compared to baseline. The greatest difference on the point scale was between baseline and 12-months (9.1), which approaches moderate clinical significance. Model estimated mean emotional functioning score at 0-months was 82.1 (SEM = 1.9). Emotional functioning was statistically significantly improved from baseline at 1-month to 85.4 (p = 0.005), 6-months to 85.0 (p = 0.04), and 24-months to 85.2 (p = 0.03) compared to baseline. The model estimated mean baseline score for cognitive functioning was 89.8 (SEM = 1.5) and steadily declined over the 24-month period after treatment, with significant differences from baseline seen at 6-months, 12-months and 24-months (all p < 0.05). The model estimated mean score for social functioning at baseline was 90.9 (SEM = 1.3). Patients reported a significant decrease in social functioning after 1-month to 88.0 (p = 0.02). This resolved and returned to baseline by 6-months, and then significantly increased compared to baseline at 12-months (3.7, 95% CI: 1.0–6.3, p = 0.007) and 24-months (4.2, 95% CI: 1.3–7.1, p = 0.005) (Figure 1, Table 2).
The model estimated mean score for fatigue at baseline was 18.3 (SEM = 1.5), and only significantly increased from baseline after the first month to 26.7 (8.4, 95% CI: 5.5–11.3, p < 0.001) (Figure 2, Table 3). This same trend was true for nausea and/or vomiting which increased from a baseline score of 3.0 to 5.4 after the first month (2.4, 95% CI: 0.59–4.2, p = 0.01). By 6 months after treatment, fatigue, nausea and vomiting scores had returned to baseline and were not statistically different from their respective baselines. Concern for financial difficulties was also significantly increased after 1-month from a score of 8.4 to 12.0 (3.6, 95% CI: 0.55–6.7, p = 0.02). These financial concerns resolved at subsequent time intervals. Reports of dyspnea initially decreased from baseline to 1-month (−0.22, 95% CI: −11.7 – −3.5, p < 0.001) and then were significantly increased from baseline at each subsequent time interval (p ≤ 0.002). There were no significant changes in patient reported insomnia, appetite loss, constipation, or diarrhea across all four follow-up timepoints (Figure 2, Table 3).
Figure 2.

Participant model estimated mean survey scores for quality of life symptom subscales at each time interval; significance determined by difference from baseline (0 months) compared to subsequent time interval. * = p < 0.05, ** = p < 0.01, *** = p < 0.001
Table 3.
Participant model estimated mean survey scores for quality of life symptom subscales at each time interval and change over time.
| Quality of Life Measure | Month | Model Mean Score | SE of the Mean | Estimated Difference from 0 months | SE | 95% Confidence Interval | P-value | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Fatigue | 0 | 18.3 | 1.5 | |||||
| 1 | 26.7 | 1.6 | 8.4 | 1.5 | 5.5 | 11.3 | <.001 | |
| 6 | 20.1 | 1.4 | 1.9 | 1.6 | −1.3 | 5.0 | 0.25 | |
| 12 | 17.7 | 1.4 | −0.63 | 1.6 | −3.7 | 2.5 | 0.69 | |
| 24 | 17.2 | 1. | −1.1 | 1.3 | −3.7 | 1.6 | 0.44 | |
|
| ||||||||
| Nausea and/or Vomiting | 0 | 3.0 | 0.79 | |||||
| 1 | 5.4 | 0.91 | 2.4 | 0.92 | 0.59 | 4.2 | 0.01 | |
| 6 | 4.5 | 0.79 | 1.6 | 0.88 | −0.19 | 3.3 | 0.08 | |
| 12 | 3.1 | 0.72 | 0.09 | 0.80 | −1.5 | 1.7 | 0.91 | |
| 24 | 2.9 | 0.82 | −0.04 | 0.83 | −1.7 | 1.6 | 0.97 | |
|
| ||||||||
| Pain | 0 | 19.2 | 1.7 | |||||
| 1 | 20.8 | 1.5 | 1.6 | 1.6 | −1.5 | 4.6 | 0.32 | |
| 6 | 16.6 | 1.5 | −2.7 | 1.8 | −6.3 | 0.96 | 0.15 | |
| 12 | 14.9 | 1.4 | −4.4 | 1.9 | −8.0 | −0.72 | 0.02 | |
| 24 | 13.4 | 1.6 | −5.9 | 2.0 | −9.9 | −1.9 | 0.004 | |
|
| ||||||||
| Insomnia | 0 | 26.5 | 2.1 | |||||
| 1 | 24.8 | 2.1 | −1.6 | 2.1 | −5.9 | 2.6 | 0.44 | |
| 6 | 26.5 | 2.0 | 0.02 | 2.3 | −4.5 | 4.6 | 0.99 | |
| 12 | 24.8 | 2.0 | −1.6 | 2.3 | −6.3 | 3.0 | 0.49 | |
| 24 | 24.6 | 2.1 | −1.8 | 2.2 | −6.2 | 2.5 | 0.40 | |
|
| ||||||||
| Appetite Loss | 0 | 6.7 | 1.2 | |||||
| 1 | 9.1 | 1.4 | 2.4 | 1.5 | −0.63 | 5.4 | 0.12 | |
| 6 | 8.1 | 1.5 | 1.4 | 1.6 | −1.8 | 4.6 | 0.39 | |
| 12 | 6.1 | 1.2 | −0.66 | 1.4 | −3.4 | 2.1 | 0.64 | |
| 24 | 4.4 | 1.0 | −2.4 | 1.4 | −5.0 | 0.32 | 0.08 | |
|
| ||||||||
| Financial Difficulties | 0 | 8.4 | 1.5 | |||||
| 1 | 12.0 | 1.8 | 3.6 | 1.6 | 0.55 | 6.7 | 0.02 | |
| 6 | 10.4 | 1.6 | 2.0 | 1.8 | −1.5 | 5.5 | 0.26 | |
| 12 | 7.4 | 1.4 | −1.0 | 1.8 | −4.7 | 2.6 | 0.57 | |
| 24 | 7.4 | 1.5 | −0.96 | 1.7 | −4.3 | 2.4 | 0.57 | |
|
| ||||||||
| Diarrhea | 0 | 9.5 | 1.5 | |||||
| 1 | 8.2 | 1.5 | −1.3 | 1.5 | −4.3 | 1.7 | 0.40 | |
| 6 | 7.6 | 1.3 | −2.0 | 1.3 | −4.6 | 0.65 | 0.14 | |
| 12 | 7.7 | 1.2 | −1.8 | 1.3 | −4.4 | 0.74 | 0.16 | |
| 24 | 8.6 | 1.4 | −0.92 | 1.4 | −3.8 | 1.9 | 0.53 | |
|
| ||||||||
| Dyspnea | 0 | 6.2 | 1.3 | |||||
| 1 | 6.0 | 1.0 | −0.22 | 1.3 | −11.7 | −3.5 | <.001 | |
| 6 | 9.2 | 1.4 | 3.0 | 1.4 | 2.5 | 10.9 | 0.002 | |
| 12 | 9.0 | 1.4 | 2.9 | 1.5 | 5.1 | 12.9 | <.001 | |
| 24 | 8.3 | 1.2 | 2.1 | 1.3 | 4.4 | 12.7 | <.001 | |
|
| ||||||||
| Constipation | 0 | 10.5 | 1.6 | |||||
| 1 | 12.8 | 1.5 | 2.3 | 1.6 | −0.93 | 5.6 | 0.16 | |
| 6 | 11.6 | 1.7 | 1.0 | 1.7 | −2.2 | 4.3 | 0.54 | |
| 12 | 9.0 | 1.4 | −1.5 | 1.6 | −4.7 | 1.6 | 0.34 | |
| 24 | 9.5 | 1.3 | −1.1 | 1.7 | −4.4 | 2.2 | 0.52 | |
All functional and symptom scale estimated changes across the two-year observation period were less than 10, indicating a minimal clinical difference (24, 25).
DISCUSSION
In this study we summarized the QOL after treatment with a novel form of IORT for breast cancer therapy. Participants treated with PB-IORT were found to have overall high QOL and low symptomatology at baseline. One month after PB-IORT, there was a minimal decline in global QOL, physical functioning, role functioning, and social functioning which appropriately correlated with an increase in fatigue. At the 6-month follow up, nearly all QOL measures returned to baseline or improved with the exception of cognitive functioning. These findings demonstrate that PB-IORT has minimal negative impact on QOL, and further supports this patient-centered treatment approach for women with early-stage breast cancer.
Global health status is a non-specific measure of a patient’s overall outlook on their wellbeing. Patients treated with PB-IORT had a high baseline global health status and this did not significantly change over the course of 24-months after treatment. High global health status scores have been reported after treatment with APBI (16, 18–20). Corica et al. found a similar pattern in a sub-study of post-pathology patients from the TARGIT-A trial where, using the EORTC QLQ-C30, the mean global QOL score minimally declined directly after treatment and returned to baseline by one year. We could reason that PB-IORT participants may have a more favorable outlook on their treatment given they self-selected into the study versus randomization. In addition, a majority (79.2%) in our study were not in the post-pathology cohort, and therefore received surgery and radiation at a single timepoint and did not have to undergo a second procedure for radiation.
Eighty-percent of breast cancer patients who undergo WBI experience some level of fatigue, with 40% reporting persistent fatigue long after their treatment has ended (7, 26). In the present analysis, patient reported fatigue had a statistically significant increase at 1-month compared to baseline, but the change was of minimal clinical significance. Fatigue was not statistically or clinically significant by 6-months after treatment. This is consistent with other studies examining fatigue after APBI, in which fatigue increases shortly after treatment but resolves and is overall quite low in comparison to patients undergoing WBI (13, 15, 16, 20, 21). We have not yet directly compared PB-IORT patients to those treated with WBI. However, our findings of low overall fatigue after PB-IORT are consistent with other forms of APBI and support the decreased fatigue in APBI patients in comparison to WBI.
Cancer treatments directly impacts patients’ physical, emotional, and social wellbeing (26). Not surprisingly, our PB-IORT patient population experienced significant declines in physical, role, and social functioning after the first month from treatment, which was directly correlated with an increase in fatigue. All three functional scales increased with decreasing fatigue at each subsequent timepoint. Role functioning was significantly higher than baseline at all time periods after treatment. Of all of the functional scales, role functioning improved the most from baseline with changes approaching moderate clinical significance (10–20 point difference). Persistently high role functioning scores after treatment with PB-IORT suggests that it is unlikely to cause appreciable disruption in a patient’s usual daily activities.
A longitudinal QOL study from the Group Europèen de Curiethèreapie European Society for Radiotherapy and Oncology (GEC-ESTRO) trial compared patients who received daily WBI over several weeks to those who received APBI with pulsed-dose-rate brachytherapy or HDR over 4–5 days (16). This study showed a similar trend as in our study, with declining role functioning immediately after treatment which improved to above baseline by 3-months after treatment (16).
The financial impact of breast cancer on patients and their families is substantial with greater than one third reporting a worse financial situation after their diagnosis and treatment (27). Among PB-IORT patients, financial difficulties were only statistically significantly increased from baseline after 1-month but were overall quite low. Several inferences can be drawn from this finding. First, the financial burden of out-of-pocket medical expenses is between $500 to over $10,000 for the majority of breast cancer patients (27). Second, we also found that PB-IORT patients reported an increase in fatigue and a decrease in role functioning one month after treatment which returned to at or above baseline by 6-month follow up. Fatigue that hinders daily functions is associated with prolonging return to work and unemployment (7, 14, 28) Third, the travel time and associated cost with travel and parking could be a contributor to the increase in financial difficulties after 1-month. The QOL study from the GEC-ESTRO trial showed a low mean score for financial difficulties after radiation (13.5, SD 23.4), which was significantly lower than their WBI group immediately after radiation and at 3-month follow up (16). Further investigation into the financial perceptions of PB-IORT and WBI will better elucidate this difference.
The only functional scale that persistently declined after PB-IORT was cognitive functioning. Although statistically significant, changes from baseline were of only minimal clinical significance. Given that the majority (94.1%) of PB-IORT patients had tumors with estrogen-receptor positivity, it is possible that the use of adjuvant endocrine therapy is a confounding factor when looking at cognitive functioning. Recent studies have shown an association between prolonged endocrine therapy use and declining patient-perceived cognition (29, 30). Despite mild declining cognition in our study, emotional functionality was persistently improved from baseline at every timepoint, a finding which is consistent with other QOL studies of patients undergoing APBI (12, 16, 31).
The present study is limited by certain factors which are inherent to using survey data. First, all patients did not take every survey at each time-point. It is reassuring that the majority of patients completed five surveys through 24-month follow up, but we must consider that patients who did not complete as many follow-ups have unaccounted differences that, if included, could impact the conclusions drawn from this study. Fortunately, only 9% of our cohort missed one of the surveys. Second, QOL is patient-reported data and therefore susceptible to subjectivity. QOL can be difficult to measure because it is nuanced and inconsistent, with various studies using different surveys thus limiting the ability to compare studies directly. Furthermore, even if the same survey is used, interpretation of scores can be variable (32, 33). We chose to use the EORTC QLQ-C30 because of its known validity and widespread use among cancer patients (23, 24), including in the TARGIT-A and GEC-ESTRO clinical trials (16, 18, 19). Also, given that BCS was performed at the same time or in close temporal proximity to PB-IORT, it is difficult to discern which therapy contributes to decline in QOL. A study using the EORTC QLQ-C30 survey examined change in QOL from baseline to immediately after breast surgery, but prior to adjuvant therapy (34). These patients had a similar decline in functional scales with an increase in fatigue, suggesting that surgery alone may attribute to these changes (34). Examining patient and procedural factors associated with worse QOL is beyond the scope of this study but would be important to explore in order to identify patients at higher risk for poor QOL. We are currently collecting QOL data at similar timepoints for our WBI patients and will be able to directly compare their QOL outcomes to the PB-IORT population.
CONCLUSION
PB-IORT offers greater specificity of radiotherapy, optimizes patient convenience, and minimizes toxicity (6, 10, 35), while also preserving QOL. Innovative treatment options that have a low impact on patient QOL and offer minimal disruption to everyday functionality are essential to breast cancer therapy.
FUNDING
This project was supported by the National Institutes of Health T32CA163177 grant to Courtney M. Lattimore, MD, Max O. Meneveau, MD MS, and Gabriella C. Squeo, MD and 1R01CA214594-01A1 to Shayna L. Showalter, MD, Timothy N. Showalter, MD MPH and Gina R. Petroni, PhD. A research grant from Varian Medical Systems was awarded to Timothy N. Showalter, MD MPH. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
DISCLOSURES
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article. None of the other authors have any other disclosures or conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Paraskevi T Quality of life outcomes in patients with breast cancer. Oncol Rev 2012; 6(1):e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Perry S, Kowalski TL, Chang CH. Quality of life assessment in women with breast cancer: benefits, acceptability and utilization. Health Qual Life Outcomes 2007; 5:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Shrestha A, Martin C, Burton M, Walters S, Collins K, Wyld L. Quality of life versus length of life considerations in cancer patients: A systematic literature review. Psychooncology 2019; 28(7):1367–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Consensus statement: treatment of early-stage breast cancer. National Institutes of Health Consensus Development Panel. J Natl Cancer Inst Monogr 1992;(11):1–5. [PubMed] [Google Scholar]
- [5].Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002; 347(16):1233–1241. [DOI] [PubMed] [Google Scholar]
- [6].Dutta SW, Showalter SL, Showalter TN, Libby B, Trifiletti DM. Intraoperative radiation therapy for breast cancer patients: current perspectives. Breast Cancer (Dove Med Press) 2017; 9:257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Forster T, Jäkel C, Akbaba S, et al. Fatigue following radiotherapy of low-risk early breast cancer - a randomized controlled trial of intraoperative electron radiotherapy versus standard hypofractionated whole-breast radiotherapy: the COSMOPOLITAN trial (NCT03838419). Radiat Oncol 2020; 15(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bennion NR, Baine M, Granatowicz A, Wahl AO. Accelerated partial breast radiotherapy: a review of the literature and future directions. Gland Surg 2018; 7(6):596–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dutta SW, Mehaffey JH, Sanders JC, et al. Implementation of an HDR brachytherapy-based breast IORT program: Initial experiences. Brachytherapy 2019; 18(3):285–291. [DOI] [PubMed] [Google Scholar]
- [10].Hassinger TE, Showalter TN, Schroen AT, et al. Utility of CT imaging in a novel form of high-dose-rate intraoperative breast radiation therapy. Journal of medical imaging and radiation oncology 2018; 62(6):835–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Vaidya JS, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014; 383(9917):603–613. [DOI] [PubMed] [Google Scholar]
- [12].Belkacémi Y, Chauvet M-P, Giard S, et al. Partial breast irradiation as sole therapy for low risk breast carcinoma: Early toxicity, cosmesis and quality of life results of a MammoSite brachytherapy phase II study. Radiotherapy and Oncology 2009; 90(1):23–29. [DOI] [PubMed] [Google Scholar]
- [13].Albuquerque K, Tell D, Lobo P, Millbrandt L, Mathews HL, Janusek LW. Impact of partial versus whole breast radiation therapy on fatigue, perceived stress, quality of life and natural killer cell activity in women with breast cancer. BMC Cancer 2012; 12:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ekenga CC, Pérez M, Margenthaler JA, Jeffe DB. Early-stage breast cancer and employment participation after 2 years of follow-up: A comparison with age-matched controls. Cancer 2018; 124(9):2026–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Taunk NK, Haffty BG, Chen S, et al. Comparison of radiation-induced fatigue across 3 different radiotherapeutic methods for early stage breast cancer. Cancer 2011; 117(18):4116–4124. [DOI] [PubMed] [Google Scholar]
- [16].Schäfer R, Strnad V, Polgár C, et al. Quality-of-life results for accelerated partial breast irradiation with interstitial brachytherapy versus whole-breast irradiation in early breast cancer after breast-conserving surgery (GEC-ESTRO): 5-year results of a randomised, phase 3 trial. Lancet Oncol 2018; 19(6):834–844. [DOI] [PubMed] [Google Scholar]
- [17].Sosin M, Gupta SS, Wang JS, et al. A Prospective Analysis of Quality of Life and Toxicity Outcomes in Treating Early Breast Cancer With Breast Conservation Therapy and Intraoperative Radiation Therapy. Front Oncol 2018; 8:545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Corica T, Nowak AK, Saunders CM, et al. Cosmesis and Breast-Related Quality of Life Outcomes After Intraoperative Radiation Therapy for Early Breast Cancer: A Substudy of the TARGIT-A Trial. Int J Radiat Oncol Biol Phys 2016; 96(1):55–64. [DOI] [PubMed] [Google Scholar]
- [19].Welzel G, Boch A, Sperk E, et al. Radiation-related quality of life parameters after targeted intraoperative radiotherapy versus whole breast radiotherapy in patients with breast cancer: results from the randomized phase III trial TARGIT-A. Radiat Oncol 2013; 8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Meattini I, Saieva C, Miccinesi G, et al. Accelerated partial breast irradiation using intensity modulated radiotherapy versus whole breast irradiation: Health-related quality of life final analysis from the Florence phase 3 trial. Eur J Cancer 2017; 76:17–26. [DOI] [PubMed] [Google Scholar]
- [21].Pérez M, Schootman M, Hall LE, Jeffe DB. Accelerated partial breast irradiation compared with whole breast radiation therapy: a breast cancer cohort study measuring change in radiation side-effects severity and quality of life. Breast Cancer Res Treat 2017; 162(2):329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Showalter SL, Petroni G, Trifiletti DM, et al. A Novel Form of Breast Intraoperative Radiation Therapy With CT-Guided High-Dose-Rate Brachytherapy: Results of a Prospective Phase 1 Clinical Trial. Int J Radiat Oncol Biol Phys 2016; 96(1):46–54. [DOI] [PubMed] [Google Scholar]
- [23].Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85(5):365–376. [DOI] [PubMed] [Google Scholar]
- [24].Fayers PM, N.K. A, K. B, M. G, D. C, A. B. The EORTC QLQ-C30 Scoring Manual (3rd Edition). In: Group TEQoL, ed. Brussels: European Organisation for Research and Treatment of Cancer; 2001. [Google Scholar]
- [25].Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998; 16(1):139–144. [DOI] [PubMed] [Google Scholar]
- [26].Berger AM, Mooney K, Alvarez-Perez A, et al. Cancer-Related Fatigue, Version 2.2015. J Natl Compr Canc Netw 2015; 13(8):1012–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jagsi R, Ward KC, Abrahamse PH, et al. Unmet need for clinician engagement regarding financial toxicity after diagnosis of breast cancer. Cancer 2018; 124(18):3668–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Balak F, Roelen CA, Koopmans PC, Ten Berge EE, Groothoff JW. Return to work after early-stage breast cancer: a cohort study into the effects of treatment and cancer-related symptoms. J Occup Rehabil 2008; 18(3):267–272. [DOI] [PubMed] [Google Scholar]
- [29].Boele FW, Schilder CM, de Roode ML, Deijen JB, Schagen SB. Cognitive functioning during long-term tamoxifen treatment in postmenopausal women with breast cancer. Menopause 2015; 22(1):17–25. [DOI] [PubMed] [Google Scholar]
- [30].Ganz PA. Understanding the impact of breast cancer adjuvant endocrine therapy on cognitive function: a work in progress. Br J Cancer 2016; 114(9):953–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Garsa AA, Ferraro DJ, DeWees TA, et al. A prospective longitudinal clinical trial evaluating quality of life after breast-conserving surgery and high-dose-rate interstitial brachytherapy for early-stage breast cancer. Int J Radiat Oncol Biol Phys 2013; 87(5):1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Buiting HM, Olthuis G. Importance of Quality-of-Life Measurement Throughout the Disease Course. JAMA Network Open 2020; 3(3):e200388–e200388. [DOI] [PubMed] [Google Scholar]
- [33].Westerman MJ, Hak T, Sprangers MA, Groen HJ, van der Wal G, The AM. Listen to their answers! Response behaviour in the measurement of physical and role functioning. Qual Life Res 2008; 17(4):549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rotonda C, Guillemin F, Bonnetain F, Velten M, Conroy T. Factors associated with fatigue after surgery in women with early-stage invasive breast cancer. Oncologist 2013; 18(4):467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Meneveau MO, Petroni GR, Varhegyi NE, et al. Toxicity and cosmetic outcomes after treatment with a novel form of breast IORT. Brachytherapy 2020; 19(5):679–684. [DOI] [PMC free article] [PubMed] [Google Scholar]


