Abstract
Objective:
To investigate a new surgical and signal processing technique that provides apical stimulation of the cochlea using a cochlear implant without extending the length of the electrode array.
Patients:
Three adult patients who underwent cochlear implantation using this new technique.
Interventions:
The patients received a cochlear implant. The surgery differed from the standard approach in that a ground electrode was placed in the cochlear helicotrema via an apical cochleostomy rather than in its typical location underneath the temporalis muscle. Clinical fitting was modified such that low frequencies were represented using the apically-placed electrode as a ground.
Main Outcome Measures:
Pitch scaling and speech recognition
Results:
All surgeries were successful with no complications. Pitch scaling demonstrated that use of the apically-placed electrode as a ground lowered the perceived pitch of electric stimulation relative to monopolar stimulation. Speech understanding was improved compared to preoperative scores.
Conclusions:
The new surgical approach and clinical fitting are feasible. A lower pitch is perceived when using the apically-placed electrode as a ground relative to stimulation using an extracochlear ground (i.e. monopolar mode), suggesting that stimulation can be provided more apically without the use of a longer electrode array. Further work is required to determine potential improvements in outcomes and optimal signal processing for the new approach.
Introduction:
The majority of cochlear implant (CI) electrodes are designed to be inserted approximately 360 degrees into the cochlea, leaving more than half of the cochlea unstimulated(1). In the normal hearing ear, the neglected region includes all spiral ganglion cells with characteristic frequencies below ~800 Hz(2). Extending stimulation over the entire cochlea offers many potential advantages, including improved speech understanding(3–5), spectral representation(6), temporal representation(7,8), and sound quality(9,10). One solution to stimulating a greater extent of the cochlea would be to use longer electrode arrays. However, this option has potential disadvantages, including the increased likelihood of damage to cochlear structures as well as difficulty achieving complete electrode insertion (thus defeating the purpose of a longer electrode). Moreover, longer intracochlear electrodes are typically placed along the lateral wall of the scala tympani rather than in a perimodiolar position; the latter may be more desirable for reducing electrical charge levels, reducing spread of excitation, improving battery life, and producing adequate loudness(11,12). Even with the lateral wall design, the longest electrode on the market only reaches approximately 1.75 out of 2.5 turns into the cochlea, leaving approximately the upper (apical) third of the cochlea unstimulated (6).
We have developed a new surgical and signal processing approach designed to stimulate the apical region of the cochlea without requiring longer electrode arrays or sacrificing the potential advantages of perimodiolar arrays. This new approach can be implemented without modification to existing cochlear implants, speech processors, or programming software and using a technique similar to what is employed for placement of a double array for obstructed cochleae. In this manuscript, we describe the surgical and clinical aspects of the new approach as well as preliminary results with the first patients to receive this treatment.
Methods:
Overview:
An existing lead electrode (ECE1 in the Cochlear system) is placed inside the cochlea at/near the cochlear apex to reshape the electric fields within the cochlea. Stimulation between an electrode on the array and the case ground electrode (ECE2) provides standard-of-care monopolar (MP) stimulation. However, when stimulation from the same electrode on the array is grounded to the apically-placed contact (ECE1) it is expected that current will flow up the cochlea in what is effectively a broad bipolar stimulation(13), stimulating a more apical cochlear region and producing a lower pitch percept than when stimulating in MP mode. We designate stimulating from an electrode along the array using the apically-placed ECE1 as #-Apex and using the case electrode (ECE2) as #-MP where “#” indicates the contact number along the array. Using this technique, we expect that we can extend the range of places of stimulation within the cochlea without increasing the insertion depth of the electrode array or sacrificing the ability to use a perimodiolar design.
Subject Demographics:
The demographics of the three subjects (S1-S3) who received the apically-placed ECE1 and a control subject (N102) are described in Table 1.
Table 1.
Subject Demographics
| Subject | Sex | Age at Implant | Implated Ear | Electrode | Duration of Deafness | Etiology | Onset | Pre- implantation description of HL |
|---|---|---|---|---|---|---|---|---|
| S1 | M | 79 | R | 632 | 8–10 yrs | Unknown | Progressive | Moderate sloping to profound SNHL from 125–2000Hz. No response from 6000–8000Hz. |
| S2 | F | 49 | R | 632 | initial loss 10 years with progression in the last 2–3 years | Unknown | Progressive | Profound mixed hearing loss from 125–2000 Hz. Rising to moderately severe at 6000 Hz, sloping to severe at 8000 Hz. |
| S3 | M | 56 | R | 632 | 7 yrs RE; 1 yr LE | Unknown | Sudden | Severe to profound SNHL in the right ear |
| N102 | F | 61 | R | Freedom CI24RE | ~13 years | R:Unknown; L: Head Trauma/ Lymes Disease | Progressive | Moderate sloping to severe rising to moderately-severe SNHL. |
Surgery:
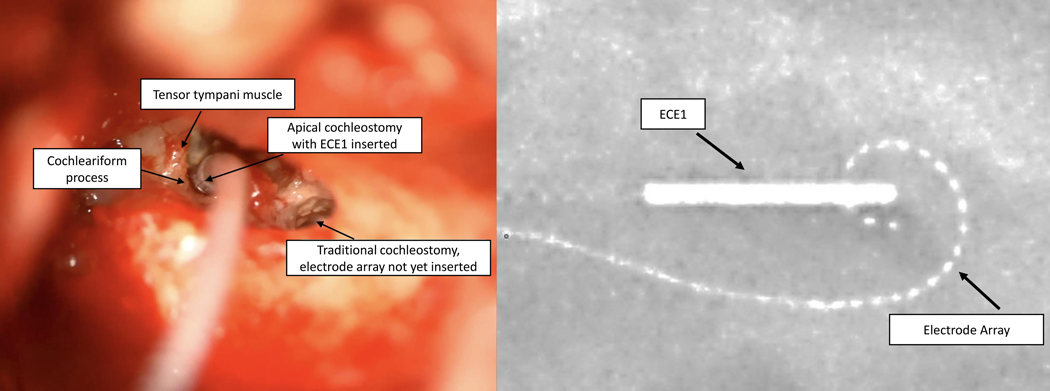
The surgical procedure used the same approach as the standard transmastoid/transfacial recess CI procedure to insert the electrode array but instead of placing the free ground electrode (ECE1) under the temporalis muscle, it was placed in a separate apical cochleostomy and secured with a small piece of periosteum. This was achieved by removing the incus bar and the incus, opening the facial recess (posterior tympanotomy from the top down), giving access to the cochlear apex which lies just medial to the tensor tympani muscle and anterior to the cochleariform process and oval window. The apical cochleostomy is drilled to the endosteum. The endosteum is opened with a small rasp and the electrode placed. The procedure and resulting placement are illustrated in Figure 1. Note that while only FDA-approved devices are used in this study, placement of ECE1 in the cochlea is considered “off-label”.
Figure 1:
ECE1 placed via an apical cochleostomy (left panel). Intraoperative x-ray of the inserted electrode array and ECE1 in place (right panel).
Signal Processing:
The clinical fitting software from Cochlear Ltd. (Custom Sound) allows selection of the stimulating and ground electrode on a per-channel basis, allowing individual channels to be stimulated in #-MP or #-Apex modes. Maps were implemented such that the lowest frequency channel (channel 22, representing 188 to 313 Hz) provided stimulation on 22-Apex. The second lowest frequency channel (21, representing 313 to 438 Hz) provided stimulation on 22-MP. Each subsequent channel was programmed to the next most apical electrode in #-MP mode. As Custom Sound only allows 22 channels, stimulation on electrode 1 was deactivated. The default frequency allocation table was used (188 – 7938 Hz). We refer to this map as “ACE-Apex”. On average, clinical settings of c-level for 22-Apex were 12 Nucleus Current units higher than 22-MP. This increment was never enough to encounter compliance issues or require the increase of the pulse width. Other than selecting the stimulation mode and electrode for each channel, ACE-Apex was fit using standard clinical practice.
Speech Testing:
Speech perception was evaluated at 3-month and 6-month follow-up clinical appointments. Speech perception was measured for CNC words, CNC phonemes, AzBio sentences in quiet, and AzBio sentences in +10 dB SNR noise using two maps: ACE-Apex, and ACE-Ch22–0. The latter is identical to ACE-Apex, except that the C-level in channel 22 is set to 0. Outside of testing, subjects had no experience with the ACE-Ch22–0 map. One list was evaluated for each condition.
The procedure was repeated with an additional subject (N102) who had ECE1 implanted in the standard location and a map with the default frequency allocation. Data with this subject was collected to provide insight into the magnitude of the effect of removing the frequency information represented by channel 22 (188 – 313 Hz) when not represented by #-Apex stimulation.
Pitch Scaling:
Pitch scaling was conducted 3-months post activation for S2 and S3 to determine if #-Apex stimulation provided a lower pitch than #-MP stimulation. A single stimulus was played in each trial. The subject was asked to rate how “high” the sound was by clicking on a line on a computer screen. Stimuli consisted of equally-loud single-channel pulse trains on electrodes 18, 19, 20, 21, or 22 in #-MP or #-Apex configurations. An additional stimulus consisted of a pulse train presented on the apical electrode and grounded to the case (Apex-MP). The stimuli were presented in a randomized order. The procedure was repeated until each stimulus was pitch scaled ten times. The pitch scaling experiment was conducted using the NIC4 research interface(14) which allows bypassing the CI sound processor to provide direct control over the implant, including stimulation in MP-Apex mode. S1 was unavailable for pitch scaling evaluation due to logistical issues related to Covid-19 restrictions.
Results:
Surgery:
All surgeries were successful with no complications or adverse events with intraoperative confirmation of correct placement based on x-ray (see Figure 1) and Transimpedance Matrices (TIM; Supplemental Digital Content).
Speech Testing:
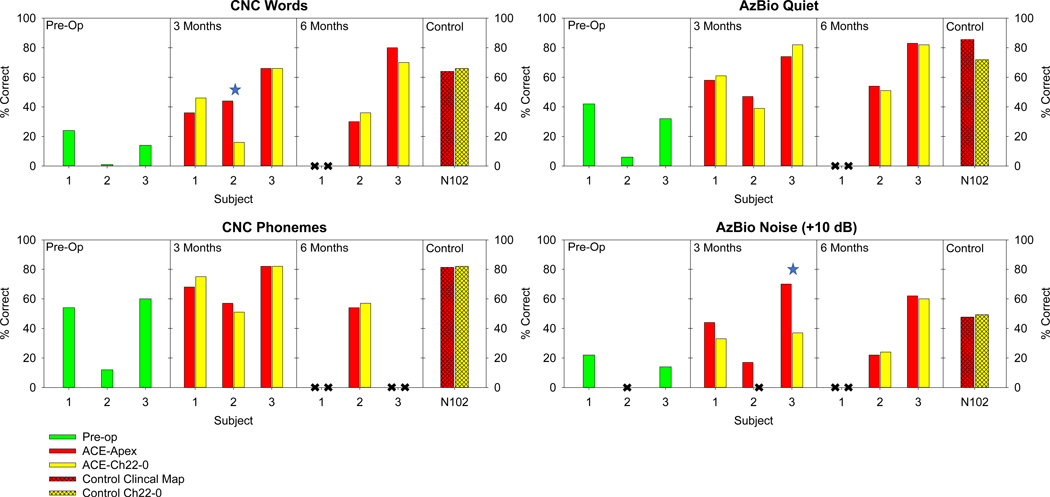
For all 3 apical-electrode subjects, speech recognition scores were better with the implant than pre-operatively using hearing aids, indicating a successful intervention. Performance with the two maps (ACE-Apex and ACE-Ch22–0) was generally similar (±10 percentage points) for each subject on each of the four speech tests. The only two differences that were significant at the individual level based on binomial 95% confidence intervals favored the ACE-Apex map (Subject 2, CNC words, 3 months, and Subject 3, AzBio in noise, 3 months).For N102 with ECE1 placed under the temporalis muscle, performance was similar for both maps (within 2 percentage points) for CNC words, CNC Phonemes, and AzBio Noise. A not statistically difference of 14 percentage points was observed when removing channel 22 from N102’s map.
Results are presented in Figure 2.
Figure 2:
Percent correct on four speech tests: CNC words (top left), CNC Phonemes (bottom left), AzBio in quiet (top right), and AzBio in +10 dB noise (bottom right) are presented for the three subjects pre-op and at 3- and 6-month intervals. Individual significant differences as evaluated with a binomial 95% confidence interval are labeled with a blue star. Missing data is indicated by a black X. Additionally, performance is presented for a control subject (N102) for whom ECE1 is implanted in the standard location presented. Speech scores were presented for N102 with her clinical map and with the c-level of channel 22 set to 0.
Pitch Scaling:
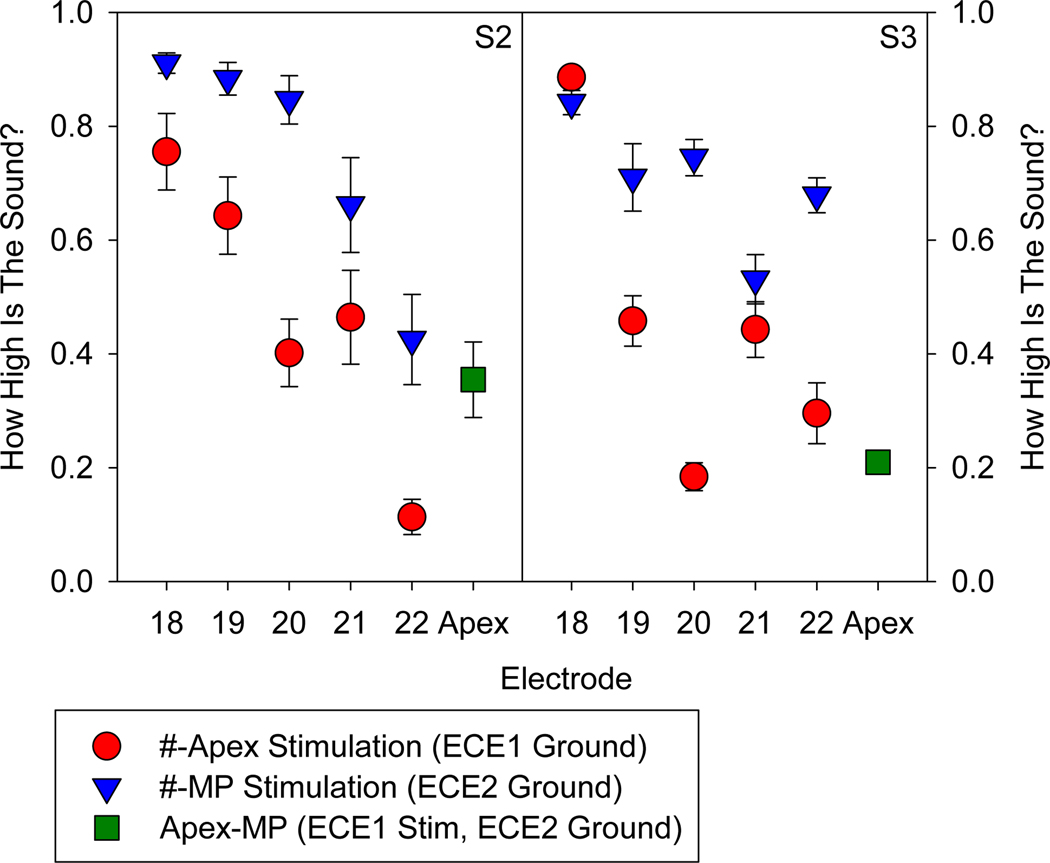
Pitch scaling results are plotted in Figure 3 for S2 and S3. Except for S3 electrode 18, stimulation from each intra-cochlear electrode in #-Apex mode (red circles) was scaled as lower than when in #-MP mode (blue triangles). A binomial test describes the probability of this happening by chance (i.e. that apical grounding does not produce a lower pitch percept than case grounding) as 0.0215. Additionally, stimulation from the apex using the case ground (Apex-MP) was perceived as lower in pitch than other electrodes in #-MP mode.
Figure 3:
Pitch scaling data for single channel stimulation on electrodes 18–22 when grounded to apical contact (red circles), or case (blue triangles). Data from stimulation in MP mode with the apical contact is also presented (green square).
Discussion:
A new approach for providing apical stimulation without longer electrodes is described. In this approach, a ground electrode (ECE1) which is normally placed under the temporalis muscle is instead inserted into the cochlear apex via a separate cochleostomy. Without modifications to clinical software, signal processing can be implemented using the apically-placed electrode.
Results demonstrate that the surgery is safe, without complications, and outcomes with the new approach are consistent with standard outcomes. As stimulation using the apical ground (#-Apex) provides a lower in pitch than stimulation using the case ground (#-MP), it can be concluded that we can extend the effective range of place of stimulation without a longer electrode array. This phase of the intervention successfully demonstrates feasibility.
The benefits on speech understanding have yet to be demonstrated. The results are limited by both a small sample size as well as a within-subject control condition (removing of the one apical channel) that is unlikely to have a large effect on speech understanding. It is therefore important to expand the study to a larger population as well as consider modifying the sound coding strategy to better utilize the apically-placed electrode.
Supplementary Material
Acknowledgements:
We gratefully acknowledge assistance from Christopher Long, PhD (Cochlear Americas). Manuscript preparation was supported by a contract from Cochlear Ltd. (PI: Roland), and by grants from NIH-NIDCD (R21-DC019743, PIs: Landsberger and Roland, and R01-DC03937, PI: Svirsky).
Footnotes
No Conflicts of Interest
References:
- 1.Landsberger DM, Padilla M, Srinivasan AG. Reducing current spread using current focusing in cochlear implant users. Hear Res 2012;284:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stakhovskaya O, Sridhar D, Bonham BH et al. Frequency map for the human cochlear spiral ganglion: implications for cochlear implants. J Assoc Res Otolaryngol 2007;8:220–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchman CA, Dillon MT, King ER et al. Influence of Cochlear Implant Insertion Depth on Performance: A Prospective Randomized Trial. Otol Neurotol 2014. [DOI] [PubMed] [Google Scholar]
- 4.Buchner A, Illg A, Majdani O et al. Investigation of the effect of cochlear implant electrode length on speech comprehension in quiet and noise compared with the results with users of electro-acoustic-stimulation, a retrospective analysis. PloS one 2017;12:e0174900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canfarotta MW, Dillon MT, Buchman CA et al. Long-Term Influence of Electrode Array Length on Speech Recognition in Cochlear Implant Users. The Laryngoscope 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canfarotta MW, Dillon MT, Buss E et al. Frequency-to-Place Mismatch: Characterizing Variability and the Influence on Speech Perception Outcomes in Cochlear Implant Recipients. Ear Hear 2020;41:1349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Middlebrooks JC, Snyder RL. Selective electrical stimulation of the auditory nerve activates a pathway specialized for high temporal acuity. J Neurosci 2010;30:1937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stahl P, Macherey O, Meunier S et al. Rate discrimination at low pulse rates in normal-hearing and cochlear implant listeners: Influence of intracochlear stimulation site. J Acoust Soc Am 2016;139:1578. [DOI] [PubMed] [Google Scholar]
- 9.Landsberger DM, Vermeire K, Claes A et al. Qualities of Single Electrode Stimulation as a Function of Rate and Place of Stimulation with a Cochlear Implant. Ear Hear 2016;37:e149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nogueira W, Litvak LM, Saoji AA et al. Design and Evaluation of a Cochlear Implant Strategy Based on a “Phantom” Channel. PloS one 2015;10:e0120148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibson P, Boyd P. Optimal electrode design: Straight versus perimodiolar. European annals of otorhinolaryngology, head and neck diseases 2016;133 Suppl 1:S63–5. [DOI] [PubMed] [Google Scholar]
- 12.Lee JY, Hong SH, Moon IJ et al. Effect of Cochlear Implant Electrode Array Design on Electrophysiological and Psychophysical Measures: Lateral Wall versus Perimodiolar Types. J Audiol Otol 2019;23:145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatterjee M, Galvin JJ 3rd, Fu QJ et al. Effects of stimulation mode, level and location on forward-masked excitation patterns in cochlear implant patients. J Assoc Res Otolaryngol 2006;7:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Litovsky RY, Goupell MJ, Kan A et al. Use of Research Interfaces for Psychophysical Studies With Cochlear-Implant Users. Trends in hearing 2017;21:2331216517736464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.