Abstract
Background:
Hypoxemia is common during tracheal intubation in intensive care units. To prevent hypoxemia during intubation, 2 methods of delivering oxygen between induction and laryngoscopy have been proposed: bag-mask ventilation and supplemental oxygen delivered by nasal cannula without ventilation (apneic oxygenation). Whether one of these approaches is more effective for preventing hypoxemia during intubation of critically ill patients is unknown.
Methods:
We performed a secondary analysis of data from 138 patients enrolled in 2, consecutive randomized trials of airway management in an academic intensive care unit. A total of 61 patients were randomized to receive bag-mask ventilation in a trial comparing bag-mask ventilation to none, and 77 patients were randomized to receive 100% oxygen at 15 L/min by nasal cannula in a trial comparing apneic oxygenation to none. Using multivariable linear regression accounting for age, body mass index, severity of illness, and oxygen saturation at induction, we compared patients assigned to bag-mask ventilation with those assigned to apneic oxygenation regarding lowest oxygen saturations from induction to 2 min after intubation.
Results:
Patients assigned to bag-mask ventilation and apneic oxygenation were similar at baseline. The median lowest oxygen saturation was 96% (interquartile range [IQR] 89%−100%) in the bag-mask ventilation group and 92% (IQR 84%−99%) in the apneic oxygenation group. After adjustment for prespecified confounders, bag-mask ventilation was associated with a higher lowest oxygen saturation compared to apneic oxygenation (mean difference, 4.2%; 95% confidence interval, 0.7%−7.8%; P = .02). The incidence of severe hypoxemia (oxygen saturation<80%) was 6.6% in the bag-mask ventilation group and 15.6% in the apneic oxygenation group (adjusted odds ratio, 0.33; P = .09).
Conclusions:
This secondary analysis of patients assigned to bag-mask ventilation and apneic oxygenation during 2 clinical trials suggests that bag-mask ventilation is associated with higher oxygen saturation during intubation compared to apneic oxygenation.
Keywords: critical care, endotracheal intubation, respiratory failure, intensive care unit
Introduction
More than one hundred thousand critically ill patients require tracheal intubation each year.1 Intubation of critically ill adults in the intensive care unit is associated with frequent complications such as hypoxemia, peri-procedural cardiac arrest, and death.2–8
Rapid sequence induction and intubation, the most common approach to tracheal intubation in emergency settings, is the administration of a sedative drug immediately followed by a neuromuscular-blocking agent.9–13 The procedure involves an inherent delay between medication administration and laryngoscopy during which the patient progresses from hypopnea to apnea.12,14 The optimal approach to oxygenation and ventilation after medication administration remains uncertain. Providing positive pressure with a bag-mask device during this interval has been reported to reduce the risk of hypoxemia, but has also been hypothesized to increase the risk of aspiration.15,16
The Preventing Hypoxemia with Manual Ventilation during Endotracheal Intubation (PreVent) study was a recent, multicenter randomized trial which demonstrated that bag-mask ventilation reduces hypoxemia compared to no ventilation.17 Although bag-mask ventilation did not appear to increase the incidence of aspiration in PreVent, the trial was underpowered to evaluate this outcome. Further, apneic oxygenation was allowed but not mandated in the PreVent and some have suggested that the results may have been different had all patients in the control group received apneic oxygenation.18,19 For these reasons, many experts continue to recommend avoiding bag-mask ventilation in favor of the apneic oxygenation for emergency tracheal intubation, particularly in settings such as the emergency department and intensive care unit where patients may be higher risk for aspiration.20 Bag-mask ventilation has never been evaluated against a control arm in which all patients received apneic oxygenation.
To determine if bag-mask ventilation is more effective at preventing hypoxemia than apneic oxygenation during tracheal intubation of critically ill adults, we performed a secondary analysis of patients enrolled from a single ICU in the PreVent trial and another recent trial evaluating the use of apneic oxygenation.2,17 We hypothesized that bag-mask ventilation would be associated with higher oxygen saturation during intubation compared to apneic oxygenation.
Methods
Study Design
We performed a secondary analysis of individual patient data from patients enrolled in the PreVent trial17 and the Facilitating Endotracheal intubation by Laryngoscopy technique and apneic Oxygenation Within the intensive care unit (FELLOW) trial.2
Data Sources
The PreVent trial was a multicenter, parallel-group, unblinded, randomized trial comparing bag-mask ventilation from induction to laryngoscopy to no ventilation during tracheal intubation of critically ill adults.17 The FELLOW study was a single-center, randomized, open-label, parallel-group, 2-by-2 factorial trial comparing apneic oxygenation with no apneic oxygenation and video laryngoscopy with direct laryngoscopy among critically ill adults.2
The cohort for this secondary analysis included patients randomized to bag-mask ventilation in the PreVent study and patients randomized to apneic oxygenation in the FELLOW study. To make the patient populations as similar as possible for the primary analysis, we included only patients enrolled in the PreVent study from Vanderbilt University Medical Center, matching the population of the FELLOW study, which was conducted entirely at Vanderbilt University Medical Center. Both trials were conducted by the same research group in the same medical ICU. Both studies shared the same training procedures, data elements, and outcome definitions. Both enrolled all patients 18 years of age or older for whom intubation was required and use of an induction agent was planned unless the participant felt the study intervention was either mandated or contraindicated. In a sensitivity analysis, we compared patients randomized to apneic oxygenation in the FELLOW study to all patients randomized to bag-mask ventilation in the PreVent study regardless of study center. Approximately 2 years passed between the end of enrollment for FELLOW on February 11, 2015 and the beginning of enrollment for PreVent on March 15, 2017.
Eligibility Criteria
Details of the inclusion and exclusion criteria for the original trials have been published previously.2,17,21 Briefly, patients 18 years or older undergoing tracheal intubation in the medical intensive care unit at Vanderbilt University Medical Center were eligible. Patients were excluded if awake intubation was planned, if intubation was needed so emergently that randomization was not possible, or if the attending physician believed another approach to oxygenation or laryngoscopy was necessary for the patient’s safety. Additionally, 7.3% of otherwise eligible patients were excluded from the trial of bag-mask ventilation trial because treating clinicians felt the risk for aspiration precluded safe administration of bag-mask ventilation.
Interventions in Source Trials
Patients randomized to bag-mask ventilation in the PreVent trial received bag-mask ventilation beginning at induction with oxygen flow rates of at least 15 L/min, bag-mask device with an expiratory port valve to generate a positive end-expiratory pressure of 5 to 10 cm of water, and ventilation at 10 breaths/min with the smallest volume required to generate a visible chest rise. The bag-mask device was removed at the initiation of laryngoscopy. Apneic oxygenation was allowed in either group in PreVent, but only about 5% of patients in the bag-mask ventilation group received a nasal cannula for apneic oxygenation in addition to bag-mask ventilation.
In the FELLOW study, the apneic oxygenation group received a nasal cannula delivering 15 L/min flow of oxygen placed in the patient’s nares from induction to intubation. Bag-mask ventilation was allowed in either group for the prevention or treatment of hypoxemia. A total of 38 patients (49.3%) received bag-mask ventilation from induction to laryngoscopy in the apneic oxygenation group. The indication for bag-mask ventilation in the FELLOW trial (prevention or treatment of hypoxemia) was not collected, but the trial was conducted in a period when bag-mask ventilation was largely provided as treatment (not prevention) of hypoxemia.
The intervention in both the PreVent and FELLOW trials focused on the delivery of ventilation and oxygenation. All other decisions, such as preoxygenation device, induction agent, and neuromuscular blockade were left to the discretion of treating clinicians.
Outcomes
The primary outcome for this secondary analysis was the lowest arterial oxygen saturation between induction and 2 min after tracheal intubation. This was the primary outcome in both randomized trials and was collected in each study by an independent observer not involved in the performance of the procedure. The secondary outcome was the proportion of patients with severe hypoxemia, defined as an oxygen saturation (SpO2) less than 80% between induction and 2 min after successful endotracheal tube placement. Additional procedural and clinical outcomes compared between groups included operator-reported aspiration during intubation, peri-procedural cardiac arrest, oxygen saturation less than 90% and 70%, ventilator-free days at day 28, ICU-free days at day 28, and in-hospital death (definitions available in Supplemental Text).
Data Collection
Methods for data collection are described in the original publications.2,17 In brief, a trained observer not participating in the intubation procedure collected data for outcomes before, during, and after the procedure, including both oxygen saturation at the time of induction and lowest oxygen saturation between induction and 2 min following successful intubation, regardless of the number of laryngoscopy attempts. Study personnel collected data from the medical record regarding patients’ age, gender, race, body mass index (BMI), number of ventilator-free days, number of ICU-free days, and in-hospital mortality.
Statistical Analysis
Baseline characteristics of patients in the bag-mask ventilation and apneic oxygenation groups were reported using number and proportion for categorical variables or median and inter-quartile range for continuous variables. Categorical variables were compared with Chi-square tests and continuous variables were compared with Wilcoxon rank-sum tests.
To compare outcomes between groups, we performed multivariable regression analysis to estimate mean differences for continuous outcomes or odds ratios for categorical outcomes, with accompanying 95% confidence intervals. Regression analyses adjusted for potential confounders that were selected for inclusion a priori based on previously published analyses of risk factors for peri-procedural hypoxemia22 and the authors’ clinical perception of variables likely to be associated with both bag mask ventilation or apneic oxygenation and lowest oxygen saturation.
For continuous outcomes, a linear regression model was fit for the dependent variable (eg, lowest oxygen saturation between induction and 2 min following intubation) with independent variables of group assignment (bag-mask ventilation vs apneic oxygenation), the covariates of age, BMI, Acute Physiology and Chronic Health Evaluation (APACHE) II score (using worst values from the 24 h prior to intubation with missing values assumed to be normal),23,24 and oxygen saturation at induction. For the categorical outcomes, a logistic regression model was fit for the dependent variable (eg, severe hypoxemia) and the same independent variables used in the linear model. Complete case analysis was performed. A P-value less than .05 was considered to indicate statistical significance for the primary analysis of the primary outcome. All other analyses were considered exploratory and no adjustments were made for multiple comparisons. Statistical analysis was performed using Stata version 15.1 (StataCorp LLC).
To evaluate whether temporal trends in the primary outcome (longitudinal changes in lowest oxygenation saturation over time) could confound the association between the interventions and the primary outcome, we fit a linear regression model for each trial with lowest oxygen saturation as the dependent variable and order of enrollment in the trial as an independent variable. To further explore whether any potential differences in outcomes between groups could be explained by differences in the patient populations enrolled in the 2 trials, we compared patients in the control groups of each study (the no ventilation group in the study of bag-mask ventilation and the no apneic oxygenation group in the study of apneic oxygenation) with regard to baseline characteristics, receipt of cointerventions (eg, preoxygenation methods), and procedural outcomes.
The clinical trials that generated the data being analyzed were approved by the Institutional Review Board at Vanderbilt University Medical Center and registered at ClinicalTrials.gov. This secondary analysis of deidentified data was determined to qualify as nonhuman subjects research by the Vanderbilt Institutional Review Board (#160158).
Results
Baseline Characteristics
Among the 150 patients randomized in FELLOW, all patients were enrolled in the Vanderbilt University Medical Center medical ICU. Of these, 77 were assigned to apneic oxygenation and included in this analysis. In the PreVent trial 401 patients were randomized, of whom, 124 were enrolled in the Vanderbilt medical ICU. A total of 61 patients randomized in the Vanderbilt medical ICU were assigned to bag-mask ventilation and included in this study. The 2 groups were similar at baseline (Table 1). The median age was 59 (interquartile range [IQR] 44–65) in the bag-mask ventilation group and 60 (IQR 51–68) in the apneic oxygenation group (P = .18). The median BMI for the bag-mask ventilation group was 27.8 (IQR 23.5–33.2), compared to 28.6 (IQR 23.3–32.8) for patients randomized to apneic oxygenation (P = .55). The median APACHE II score was 21 (IQR 15–27) in the bag-mask ventilation group and 22 (IQR 16–27) in the apneic oxygenation group (P = .67). Respiratory failure was the most common indication recorded for intubation in both groups, accounting for 54.1% of intubations in the bag-mask ventilation group compared to 66.2% of the apneic oxygenation group (P = .15). In the previous 6 h, the lowest oxygen saturation, highest FiO2, and use of BiPAP were similar between groups.
Table 1.
Patient Characteristics at Baseline.
| Patient characteristic | Bag-mask ventilation (n = 61) | Apneic oxygenation (n = 77) | P value |
|---|---|---|---|
| Age, median [IQR], years | 59 (44, 65) | 60 (51, 68) | .18 |
| Male sex, No. (%) | 36 (59.0) | 45 (58.4) | .95 |
| White racea, No. (%) | 49 (83.1) | 63 (81.8) | .28 |
| Body mass indexb, median [IQR], kg/m2 | 27.8 (23.5, 33.1) | 28.6 (23.3, 32.8) | .55 |
| APACHE II scorec, median [IQR] | 21 (15, 27) | 22 (16, 27) | .67 |
| Receipt of vasopressors, No. (%) | 9 (14.8) | 11 (14.3) | .94 |
| Active sepsis, No. (%) | 30 (49.2) | 50 (64.9) | .06 |
| Indications for intubationd, No. (%) | |||
| Respiratory failure | 33 (54.1) | 51 (66.2) | .15 |
| Airway protection for decreased level of consciousness | 22 (36.1) | 21 (27.3) | .27 |
| BiPAP in prior 6 h, No. (%) | 16 (26.2) | 26 (33.8) | .34 |
| Highest FiO2 in prior 6 h, median [IQR] | 0.40 (0.21, 0.65) | 0.40 (0.27, 0.6) | .79 |
| Lowest oxygen saturation in prior 6 h, median [IQR], % | 92 (86, 94) | 92 (88, 95) | .64 |
| Neuromuscular blockade, No. (%) | |||
| Any neuromuscular blockade | 59 (97%) | 73 (95%) | .58 |
| Rocuronium | 30 | 47 | |
| Succinylcholine | 29 | 26 | |
| Methods of preoxygenationd, No. (%) | |||
| Bag-mask ventilation | 21 (34.4) | 33 (42.9) | .31 |
| Noninvasive ventilation | 13 (21.3) | 23 (29.9) | .26 |
| High flow nasal cannula | 4 (6.6) | 0 (0) | .02 |
| Nonrebreather | 33 (54.1) | 25 (32.5) | .01 |
| Simple nasal cannula | 0 (0) | 6 (7.8) | .03 |
| Oxygen saturation at inductione, median [IQR], % | 99 (95, 100) | 99 (96, 100) | .99 |
| Oxygen saturation <92% at end of preoxygenation, No. (%) | 35 (17.6) | 13 (16.9) | .89 |
Race was reported by patients or their surrogates and recorded in the electronic health record as a part of routine clinical care. Race was missing for 2 patients (3.3%) in the bag-mask ventilation group.
Information on body mass index at enrollment was missing for 5 patients (3.6%); 3 from the bag-mask ventilation group and 2 from the apneic oxygenation group.
Acute Physiology and Chronic Health Evaluation (APACHE) II score ranges from 0 to 71 with higher scores indicating higher severity of illness.
Facilitating Endotracheal intubation by Laryngoscopy technique and apneic Oxygenation Within the intensive care unit (FELLOW) collected only the highest level of respiratory support provided for preoxygenation. In PreVent, multiple methods of preoxygenation could be provided for each patient.
Oxygen saturation at induction was missing for 3 patients (2.2%); 2 in the bag-mask ventilation group and one patient in the apneic oxygenation group.
All intubations in the apneic oxygenation were performed by pulmonary critical care fellows. A total of 60 intubations in the bag-mask ventilation group were performed by pulmonary critical care fellows and one was performed by a pulmonary critical care attending. All fellow intubations included direct attending supervision. Preoxygenation modality differed significantly between the groups. Preoxygenation with a nonrebreather mask (54% vs 32%, P = .01) and preoxygenation with high flow nasal cannula (7% vs 0%, P = .02) were more common in the bag-mask ventilation group. Conversely, preoxygenation with noninvasive ventilation (21% vs 30%, P = .26), preoxygenation with bag-mask ventilation (34% vs 43%, P = .31), and preoxygenation with simple nasal cannula (0% vs 8%, P = .03) were less common in the bag-mask ventilation group as compared to the apneic oxygenation group. Every patient in both groups received some form of preoxygenation. At the time of induction, the median oxygen saturations were similar between groups (99%, IQR 95%−100% vs 99%, IQR 96%−100%, P = .99).
Primary Outcome
The unadjusted median lowest oxygen saturation was 96% (IQR 89%−100%) in the bag-mask ventilation group and 92% (IQR 84%−99%) in the apneic oxygenation group. In multivariable analysis accounting for prespecified confounders of age, BMI, APACHE II score, and oxygen saturation at induction, the mean difference in lowest oxygen saturation between the bag-mask ventilation group and the apneic oxygenation group was 4.2% (95% CI, 0.7%−7.8%; P = .02) (Figure 1) (Table 2).
Figure 1.
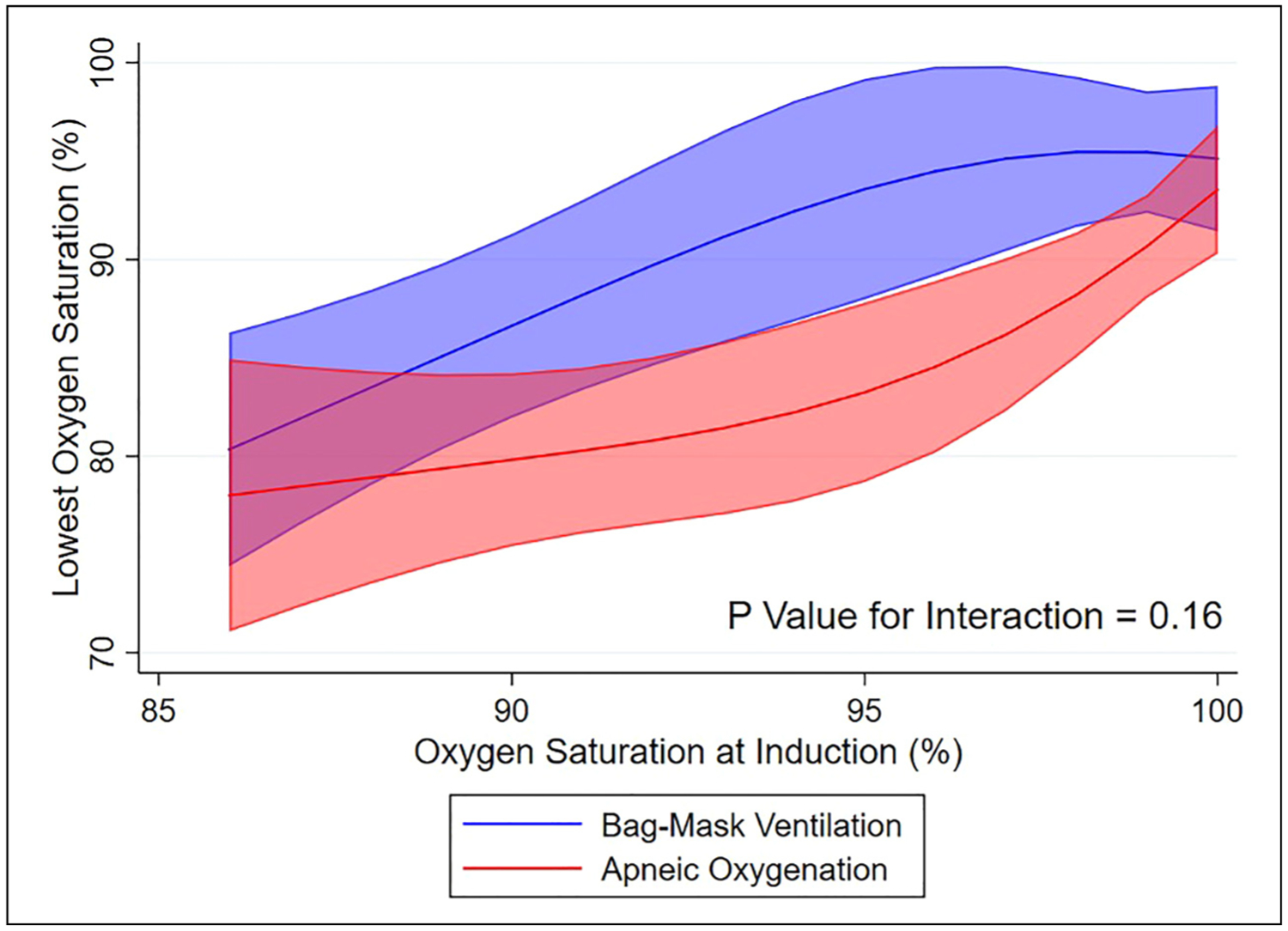
Lowest oxygen saturation for bag-mask ventilation versus apneic oxygenation. Left panel: A scatterplot of the unadjusted lowest oxygen saturations for all patients included in the bag-mask ventilation and apneic oxygenation groups in the primary analysis. Right panel: the adjusted mean lowest arterial oxygen saturation and 95% confidence interval for patients in the bag-mask ventilation and apneic oxygenation. The adjusted mean lowest oxygen saturation is adjusted to the median of the remaining model covariates: age of 60 years, BMI of 28.4 kg/m2, and APACHE II score of 21.
Abbreviations: BMV, bag-mask ventilation; AO, apneic oxygenation; APACHE II, Acute Physiology and Chronic Health Evaluation; BMI, body mass index.
Table 2.
Multivariable Regression Models for the Primary and Secondary Outcomes.
| Lowest oxygen saturation | Adjusted mean difference | 95% Confidence intervals | P value |
|---|---|---|---|
| Bag-mask ventilation, versus apneic oxygenation | 4.23 | 0.67 to 7.79 | .02 |
| Age, years | 0.02 | −0.10 to 0.14 | .75 |
| BMI | −0.20 | −0.40 to 0.00 | .05 |
| APACHE II score | 0.02 | −0.21 to 0.26 | .86 |
| Oxygen saturation at induction, (%) | 1.13 | 0.77 to 1.49 | <.001 |
| Severe hypoxemia | Adjusted odds ratio | 95% Confidence intervals | P value |
| Bag-mask ventilation, versus apneic oxygenation | 0.33 | 0.09 to 1.18 | .09 |
| Age, years | 0.98 | 0.94 to 1.02 | .35 |
| BMI | 1.03 | 0.98 to 1.09 | .28 |
| APACHE II score | 0.95 | 0.88 to 1.03 | .20 |
| Oxygen saturation at induction, (%) | 0.88 | 0.80 to 0.98 | .02 |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation; BMI, body mass index.
The coefficient describes the increase in lowest oxygen saturation (%) or change in odds ratio associated with bag-mask ventilation compared to apneic oxygenation, an increase in age of 1 year, an increase in BMI of 1 kg/m2, an increase in APACHE II score of 1 point, and an increase in oxygen saturation at induction of 1%.
In a post hoc sensitivity analysis adjusting for preoxygenation modality, in addition to prespecified confounders, the mean difference in lowest oxygen saturation between groups was 3.8% (95% CI, 0.07%−7.5%, P = .046) (Supplemental Appendix Table S1).
Oxygen saturation at induction did not significantly modify the association between bag-mask ventilation versus apneic oxygenation and lowest oxygen saturation (P-value for interaction = .16). The adjusted mean lowest oxygen saturation was higher for bag-mask ventilation than for apneic oxygenation across the full range of observed oxygen saturations at induction (Figure 2).
Figure 2.
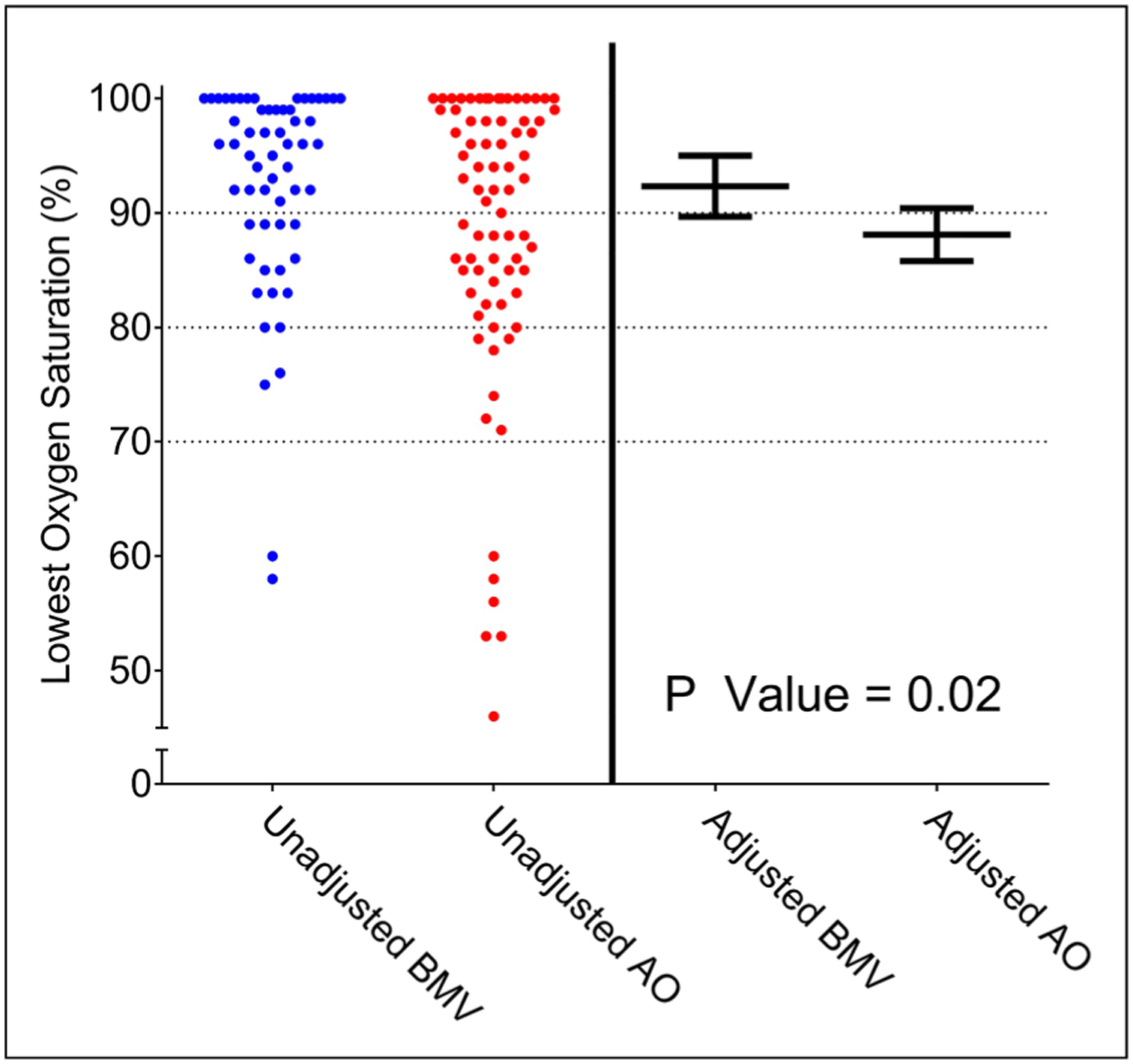
Heterogeneity of treatment effect by oxygen saturation at induction. The adjusted mean and 95% confidence interval for lowest arterial oxygen saturation is displayed for patients who received bag-mask ventilation (blue) and apneic oxygenation (red) across a range of oxygen saturations at induction. This partial effect plot represents how oxygen saturation at induction potentially modifies the effect of bag-mask ventilation on lowest oxygen saturation. Predictions are adjusted to the median of the remaining model covariates: age of 60 years, BMI of 28.4 kg/m2, and APACHE II score of 21.
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation; BMI, body mass index.
In a prespecified sensitivity analysis comparing all patients randomized to bag-mask ventilation in the PreVent study, regardless of center, to all patients randomized to apneic oxygenation in the FELLOW study, the adjusted mean difference in lowest oxygen saturation between groups was 2.9% (95% CI, 0.2–5.6, P = .04) (Supplemental Tables S2–S4).
Secondary Outcome
A total of 4 patients (6.6%) in the bag-mask ventilation group experienced a lowest oxygen saturation <80% (severe hypoxemia), compared to 12 patients (15.6%) in the apneic oxygenation group. In multivariable analysis adjusting for prespecified potential confounders, the adjusted odds ratio for severe hypoxemia with bag-mask ventilation compared to apneic oxygenation was 0.33 (95% CI, 0.09–1.18; P = .09) (Table 3).
Table 3.
Outcomes of Tracheal Intubation.
| Outcomes | Bag-mask ventilation (n = 61) | Apneic oxygenation (n = 77) | Adjusted mean difference or odds ratiob (95% confidence intervals) |
|---|---|---|---|
| Primary outcome | |||
| Lowest oxygen saturationa, median [IQR], % | 96 (89, 100) | 92 (84, 99) | 4.23 (0.67 to 7.79) |
| Secondary outcome | |||
| Lowest oxygen saturation <80%, No. (%) | 4 (6.6) | 12 (15.6) | 0.33 (0.09 to 1.18) |
| Exploratory procedural outcome | |||
| Lowest oxygen saturation <90%, No. (%) | 17 (27.9) | 34 (44.2) | 0.34 (0.13 to 0.88) |
| Lowest oxygen saturation <70%, No. (%) | 2 (3.3) | 6 (7.8) | 0.38 (0.06 to 2.31) |
| Successful intubation on first laryngoscopy attempt, No. (%) | 53 (85.9) | 52 (67.5) | 3.50 (1.34 to 9.17) |
| Time from induction to successful intubation, median [IQR], seconds | 107 (85, 130) | 132 (88, 205) | −58 (−120.1 to 3.0) |
| Operator-reported aspiration, No. (%) | 1 (1.6) | 0 (0.0) | c |
| Peri-procedural cardiac arrest, No. (%) | 2 (3.3) | 3 (3.9) | 0.55 (0.05 to 5.84) |
| Exploratory clinical outcomes | |||
| Ventilator-free days, median [IQR] | 19 (0, 26) | 17 (0, 26) | −1.15 (−5.10 to 2.81) |
| ICU-free days, median [IQR] | 17 (0, 24) | 18 (0, 25) | −0.71 (−4.30 to 2.89) |
| Died before hospital discharge, No. (%) | 23 (37.7) | 27 (35.1) | 1.20 (0.53 to 2.72) |
Lowest oxygen saturation was missing for 3 patients (2.2%); 2 in the bag-mask ventilation group and one patient in the apneic oxygenation group.
Multivariable regression model includes age (years), body mass index (kg/m2), Acute Physiology and Chronic Health Evaluation II score, and oxygen saturation (%) at induction as covariates.
Unable to calculate given low numbers of patients with values in one or both groups.
Additional Outcomes
In multivariable analysis adjusting for prespecified confounders, a lowest oxygen saturation <90% was significantly less common in the bag-mask ventilation group compared to the apneic oxygenation group (adjusted odds ratio 0.34; 95% CI, 0.13–0.88) (Table 3). Two patients (3.3%) in the bag-mask ventilation group experienced a lowest oxygen saturation of less than 70%, compared to 6 patients in the apneic oxygenation group (7.8%) (adjusted odds ratio, 0.38; 95% CI, 0.06–2.31). One patient in the bag-mask ventilation group and no patients in the apneic oxygenation group experienced operator-reported aspiration. Two patients in the bag mask ventilation group (3.3%) and 3 patients in the apneic oxygenation group (3.9%) experienced peri-procedural cardiac arrest (adjusted odds ratio, 0.55; 95% CI, 0.05–5.84).
Patients in the bag-mask ventilation group experienced a median of 19 ventilator-free days (IQR 0–26) compared to a median of 17 in the apneic oxygenation group (IQR 0–26) (adjusted mean difference, −1.15; 95% CI, −5.10 to 2.81). Twenty-three patients in the bag-mask ventilation group (37.7%) died before discharge from the hospital, compared to 27 patients in the apneic oxygenation group (35.1%) (adjusted odds ratio, 1.20; 95% CI, 0.53 to 2.72).
Trial Similarity Assessment
There was no relationship between the order of enrollment and the lowest oxygen saturation in either study (Supplemental Figures S1 and S2). Comparison of the control groups in the 2 studies (the no bag-mask ventilation group and no apneic oxygenation group) revealed similar patient populations and similar differences in preoxygenation devices as seen in the intervention groups (Supplemental Table S5).
Discussion
In this secondary analysis of data from 2 randomized trials, bag-mask ventilation between induction and laryngoscopy was independently associated with higher oxygen saturation during tracheal intubation of critically ill adults than apneic oxygenation.
Hypoxemia occurs commonly during tracheal intubation of critically ill adults and is associated with cardiac arrest and death.2–7,25,26 Bag-mask ventilation between induction and laryngoscopy has been shown to prevent hypoxemia, but many experts continue to recommended against routine bag-mask ventilation, recommending it only as a rescue technique to treat hypoxemia. A common rationale to avoid bag-mask ventilation during rapid sequence induction and intubation is a hypothesized increase in the risk of aspiration.10,12,27,28 Delivering supplemental oxygen to the nasopharynx without ventilation (apneic oxygenation) has been proposed as an alternative method to prevent hypoxemia without increasing the risk of aspiration. During apnea, oxygen-rich gas may be drawn down from the nasopharynx to the alveolar space by diffusion and a pressure gradient from the atmosphere to the alveolar space.29 Trials in the operating room have suggested that apneic oxygenation via a nasopharyngeal catheter prolongs the period of time a patient can be apneic without experiencing hypoxemia.30–33 Unlike in the operating room, trials examining apneic oxygenation during the intubation of critically ill adults in the intensive care unit2,34,35 or emergency departments36 have not demonstrated a clear effect on hypoxemia.37,38
Until recently, there has been little evidence on the safety of bag-mask ventilation during the tracheal intubation of critically ill adults. The PreVent study17 was the first trial evaluating bag-mask ventilation during the intubation of critically ill adults and found that bag-mask ventilation increased lowest oxygen saturation and reduced the incidence of severe hypoxemia during tracheal intubation, without an apparent effect on the incidence of aspiration. However, most patients in the control group of PreVent did not receive apneic oxygenation following initiation of laryngoscopy, and it has remained unclear if providing apneic oxygenation to all patients would have been as effective as bag-mask ventilation.
No prior trials have directly compared bag-mask ventilation to apneic oxygenation during tracheal intubation of critically ill adults.37,38 Our analysis in this study used patient-level data from the PreVent trial of bag-mask ventilation and the FELLOW trial of apneic oxygenation2 to directly compare the effects of bag-mask ventilation and apneic oxygenation on oxygen saturation. After adjusting for potential confounders, we found a difference in lowest oxygen saturation between groups of 4.2% in favor of bag-mask ventilation over apneic oxygenation. This difference is similar to the difference between bag-mask ventilation and no ventilation in the PreVent trial, where apneic oxygenation was provided to a minority of patients. Results were similar in several sensitivity analyses, including an analysis adjusting for preoxygenation device.
In the absence of a direct comparison as part of a clinical trial, this analysis represents unique and innovative method to compare 2 interventions from similar clinical trials. Statistical methods for indirect adjusted estimates are used in network meta-analyses to estimate the relative effect of 2 interventions that have both been studied against a common comparator. Because these indirect comparison methods lack individual patient data, they leverage other methods to account for differences between trials in patient characteristics and trial design.39 Indeed, these methods have previously been used to compare methods of preoxygenation during emergency tracheal intubation.38 However, because the PreVent and FELLOW trials enrolled large number of patients from the same study unit, close together in time, using the same group of operators, they provide a unique opportunity for indirect comparison of the interventions in these 2 trials. Further, while the baseline characteristics of the 2 groups, and the characteristics and outcomes of the 2 control arms from trials suggested that the trial populations were similar, access to patient level data in this analysis allowed for the application of robust methods to account for potential differences in confounders between groups.
The current analysis has several strengths. It employed prospectively collected data from 2 prior randomized trials of airway management outside of the operating room in which allocation of the interventions of interest were controlled by study group assignment. Both trials occurred in the same ICU, were designed by the same investigators, used largely the same methods, and enrolled patient populations that were similar between the 2 studies overall. No temporal relationship was detected between order of enrollment and lowest oxygen saturation in either study, and the outcomes of the control arms in both studies were similar, arguing against temporal changes in lowest oxygen saturation during intubation over time. In both studies, data on the primary outcome of lowest oxygen saturation was captured by an independent observer rather than self-report.
The current analysis also has several important limitations. Although patients were assigned to bag-mask ventilation or apneic oxygenation by the trial protocol, the data derived from 2 separate trials conducted sequentially. Despite adjustment, differences in patient or operator characteristics over time might confound the observed effects of bag-mask ventilation and apneic oxygenation on lowest oxygen saturation. Patients assigned to bag-mask ventilation were less likely to receive preoxygenation with positive pressure ventilation—although accounting for preoxygenation modality did not appear to affect the findings. This study can only examine bag-mask ventilation and apneic oxygenation as they were delivered in the original trials. Results may have been different if ventilation had been provided via noninvasive ventilation or if apneic oxygenation had been provided at higher flow rates via by high flow nasal cannula rather than 15 L/min. Because the studies included in this analysis enrolled patients solely from an intensive care unit in an academic medical center, it is unclear whether these results generalize to patients undergoing tracheal intubation in other settings where characteristics of the patients and the experience of the proceduralists and supporting teams may be different. Further, the small size of the study limits the ability to evaluate subgroup differences or interactions, and observed differences in lowest oxygen saturation may be explained, at least in part, by other differences in the intubation procedure such as the observed difference in first pass success. Finally, a significant proportion of patients in apneic oxygenation group received bag-mask ventilation, but this is thought to represent treatment of hypoxemia (“rescue”), not prevention of hypoxemia (the intervention in PreVent), and if anything, this type of contamination would be expected to bias towards the null and reduce any observed differences between groups.
In conclusion, this secondary analysis of patient-level data from 2 randomized trials found that oxygen saturation during tracheal intubation was significantly higher for patients assigned to bag-mask ventilation compared with patients assigned to apneic oxygenation. Future randomized trials should directly compare bag-mask ventilation to apneic oxygenation during tracheal intubation to definitively assess the effects on oxygen saturation and aspiration.
Supplementary Material
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: J.D.C. was supported in part by the NIH (K23HL153584). M.W.S. was supported in part by the NHLBI (K23HL143053). D.W.R. was supported in part by the NIH/NHLBI (T32HL105346-07). T.W.R. was supported in part by the NIH (R34HL105869). Data collection used the Research Electronic Data Capture (REDCap) tool developed and maintained with Vanderbilt Institute for Clinical and Translational Research grant support (UL1 TR000445 from NCATS/NIH). The funding institutions had no role in (1) conception, design, or conduct of the study, (2) collection, management, analysis, interpretation, or presentation of the data, or (3) preparation, review, or approval of the manuscript.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Pfuntner A, Wier LM, Stocks C. Most frequent procedures performed in U.S. Hospitals, 2011: statistical brief #165. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality; (US: ); 2013, 1–10. http://www.ncbi.nlm.nih.gov/books/NBK174682/. Accessed June 4, 2018. [Google Scholar]
- 2.Semler MW, Janz DR, Lentz RJ, et al. Randomized trial of apneic oxygenation during endotracheal intubation of the critically Ill. Am J Respir Crit Care Med. 2016;193(3):273–280. doi: 10.1164/rccm.201507-1294OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semler MW, Janz DR, Russell DW, et al. A multicenter, randomized trial of ramped position versus sniffing position during endotracheal intubation of critically Ill adults. Chest. 2017;152(4): 712–722. doi: 10.1016/j.chest.2017.03.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janz DR, Semler MW, Lentz RJ, et al. Randomized trial of video laryngoscopy for endotracheal intubation of critically ill adults. Crit Care Med. 2016;44(11):1980–1987. doi: 10.1097/CCM.0000000000001841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janz DR, Semler MW, Joffe AM, et al. A multicenter randomized trial of a checklist for endotracheal intubation of critically Ill adults. Chest. 2018;153(4):816–824. doi: 10.1016/j.chest.2017.08.1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simpson GD, Ross MJ, McKeown DW, Ray DC. Tracheal intubation in the critically ill: a multi-centre national study of practice and complications. Br J Anaesth. 2012;108(5):792–799. doi: 10.1093/bja/aer504 [DOI] [PubMed] [Google Scholar]
- 7.Martin LD, Mhyre JM, Shanks AM, Tremper KK, Kheterpal S. 3,423 emergency tracheal intubations at a university hospital: Airway outcomes and complications. Anesthesiology. 2011;114(1):42–48. doi: 10.1097/ALN.0b013e318201c415 [DOI] [PubMed] [Google Scholar]
- 8.Russotto V, Myatra SN, Laffey JG, et al. Intubation practices and adverse peri-intubation events in critically Ill patients From 29 countries. JAMA. 2021;325(12):1164–1172. doi: 10.1001/jama.2021.1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Murphy-Lavoie H, Bugas C, Martinez J, Preston C. Complications of emergency intubation with and without paralysis. Am J Emerg Med. 1999;17(2):141–143. doi: 10.1016/S0735-6757(99)90046-3 [DOI] [PubMed] [Google Scholar]
- 10.El-Orbany M, Connolly LA. Rapid sequence induction and intubation: current controversy. Anesth Analg. 2010;110(5):1318–1325. doi: 10.1213/ANE.0b013e3181d5ae47 [DOI] [PubMed] [Google Scholar]
- 11.Jaber S, Amraoui J, Lefrant J, et al. Clinical practice and risk factors for immediate complications of endotracheal intubation in the intensive care unit: a prospective, multiple-center study*. Crit Care Med. 2006;34(9):2355–2361. doi: 10.1097/01.CCM.0000233879.58720.87 [DOI] [PubMed] [Google Scholar]
- 12.Brown CA. The Walls Manual of Emergency Airway Management. 5th. Wolters Kluwer Health; 2017. http://proxy.library.vanderbilt.edu/login?url=http://ovidsp.ovid.com/ovidweb.cgi?T=JS&NEWS=n&CSC=Y&PAGE=booktext&D=books&AN=01996178$&XPATH=/PG(0)&EPUB=Y. Accessed May 4, 2018. [Google Scholar]
- 13.Brown CAIII, Bair AE, Pallin DJ, Walls RM. Techniques, success, and adverse events of emergency department adult intubations. Ann Emerg Med. 2015;65(4):363–370. e1. doi: 10.1016/j.annemergmed.2014.10.036 [DOI] [PubMed] [Google Scholar]
- 14.Stept WJ, Safar P. Rapid induction-intubation for prevention of gastric-content aspiration. Anesth Analg. 1970;49(4):633–636. [PubMed] [Google Scholar]
- 15.Baillard C, Fosse J-P, Sebbane M, et al. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am J Respir Crit Care Med. 2006;174(2):171–177. doi: 10.1164/rccm.200509-1507OC [DOI] [PubMed] [Google Scholar]
- 16.Salem MRMD. Anesthetic management of patients With “A full stomach” A critical review. Anesth Analg. 1970;49(1):47–55. [PubMed] [Google Scholar]
- 17.Casey JD, Janz DR, Russell DW, et al. Bag-Mask ventilation during tracheal intubation of critically Ill adults. N Engl J Med. 2019;380(9):811–821. doi: 10.1056/NEJMoa1812405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swaminathan A PreVent: Bag-Mask Ventilation Prior to Intubation. REBELEM. Published March 21, 2019. https://rebelem.com/prevent-bag-mask-ventilation-prior-to-intubation/. Accessed June 7, 2019.
- 19.PreVent RCT - Bag-Mask Ventilation During RSI? JournalFeed. 2021. https://journalfeed.org/article-a-day/2019/prevent-rct-bag-mask-ventilation-during-rsi. Accessed July 21, 2021.
- 20.Brown C, Sakles JC. Rapid Sequence Intubation for Adults outside the Operating Room. 2019. https://www.uptodate.com/contents/rapid-sequence-intubation-for-adults-outside-the-operating-room?sectionName=Adjunct%20strategies&topicRef=8365&anchor=H3397071694&source=see_link#H3397071694. Accessed February 17, 2019.
- 21.Casey JD, Janz DR, Russell DW, et al. Manual ventilation to prevent hypoxaemia during endotracheal intubation of critically ill adults: protocol and statistical analysis plan for a multicentre randomised trial. BMJ Open. 2018;8(8):e022139. doi: 10.1136/bmjopen-2018-022139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKown AC, Casey JD, Russell DW, et al. Risk factors for and prediction of hypoxemia during tracheal intubation of critically Ill adults. Ann Am Thorac Soc. Published online August. 2018;15(11):1320–1327. doi: 10.1513/AnnalsATS.201802-118OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knaus W, Draper E, Wagner D, Zimmerman J. APACHE II: a severity of disease classification system. CCM. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 24.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Chest. 1991;100(6):1619–1636. doi: 10.1378/chest.100.6.1619 [DOI] [PubMed] [Google Scholar]
- 25.Mort TC. The incidence and risk factors for cardiac arrest during emergency tracheal intubation: a justification for incorporating the ASA guidelines in the remote location. J Clin Anesth. 2004;16-(7):508–516. doi: 10.1016/j.jclinane.2004.01.007 [DOI] [PubMed] [Google Scholar]
- 26.Kaiser SK. Cardiac arrest and mortality related to intubation procedure in critically Ill adult patients: a multicenter cohort study. J Emerg Med. 2018;54(6):903–904. doi: 10.1016/j.jemermed.2018.04.048 [DOI] [PubMed] [Google Scholar]
- 27.Adnet F, Borron SW, Lapostolle F. The safety of rapid sequence induction. Anesthes. 2002;96(2):517–517. [DOI] [PubMed] [Google Scholar]
- 28.Sakles JC. Maintenance of oxygenation during rapid sequence intubation in the emergency department. Acad Emerg Med. 2017;24(11):1395–1404. doi: 10.1111/acem.13271 [DOI] [PubMed] [Google Scholar]
- 29.Weingart SD, Levitan RM. Preoxygenation and prevention of desaturation during emergency airway management. Ann Emerg Med. 2012;59(3):165–175. e1. doi: 10.1016/j.annemergmed.2011.10.002 [DOI] [PubMed] [Google Scholar]
- 30.Teller LE, Alexander CM, Frumin MJ, Gross JB. Pharyngeal insufflation of oxygen prevents arterial desaturation during apnea. Anesthesiology. 1988;69(6):980–982. [DOI] [PubMed] [Google Scholar]
- 31.Taha SK, Siddik-Sayyid SM, El-Khatib MF, Dagher CM, Hakki MA, Baraka AS. Nasopharyngeal oxygen insufflation following pre-oxygenation using the four deep breath technique. Anaesthesia. 2006;61(5):427–430. doi: 10.1111/j.1365-2044.2006.04610.x [DOI] [PubMed] [Google Scholar]
- 32.Baraka AS, Taha SK, Siddik-Sayyid SM, et al. Supplementation of pre-oxygenation in morbidly obese patients using nasopharyngeal oxygen insufflation. Anaesthesia. 2007;62(8):769–773. doi: 10.1111/j.1365-2044.2007.05104.x [DOI] [PubMed] [Google Scholar]
- 33.Ramachandran SK, Cosnowski A, Shanks A, Turner CR. Apneic oxygenation during prolonged laryngoscopy in obese patients: a randomized, controlled trial of nasal oxygen administration. J Clin Anesth. 2010;22(3):164–168. doi: 10.1016/j.jclinane.2009.05.006 [DOI] [PubMed] [Google Scholar]
- 34.Vourc’h M, Asfar P, Volteau C, et al. High-flow nasal cannula oxygen during endotracheal intubation in hypoxemic patients: a randomized controlled clinical trial. Intensive Care Med. 2015;41-(9):1538–1548. doi: 10.1007/s00134-015-3796-z [DOI] [PubMed] [Google Scholar]
- 35.Guitton C, Ehrmann S, Volteau C, et al. Nasal high-flow preoxygenation for endotracheal intubation in the critically ill patient: a randomized clinical trial. Intensive Care Med. Published online January. 2019;45(4):447–458. doi: 10.1007/s00134-019-05529-w [DOI] [PubMed] [Google Scholar]
- 36.Caputo N, Azan B, Domingues R, et al. Emergency department use of apneic oxygenation versus usual care during rapid sequence intubation: a randomized controlled trial (The ENDAO trial). Acad Emerg Med. 2017;24(11):1387–1394. doi: 10.1111/acem.13274 [DOI] [PubMed] [Google Scholar]
- 37.Chaudhuri D, Granton D, Wang DX, et al. Moderate certainty evidence suggests the Use of high-flow nasal cannula does Not decrease hypoxia when compared With conventional oxygen therapy in the peri-intubation period: results of a systematic review and meta-analysis. Crit Care Med. 2020;48(4):571–578. doi: 10.1097/CCM.0000000000004217 [DOI] [PubMed] [Google Scholar]
- 38.Fong KM, Au SY, Ng GWY. Preoxygenation before intubation in adult patients with acute hypoxemic respiratory failure: a network meta-analysis of randomized trials. Crit Care. 2019;23(1):1–12. doi: 10.1186/s13054-019-2596-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–691. doi: 10.1016/s0895-4356(97)00049-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


