Abstract
Imaging innovations offer useful techniques applicable to many oncology specialties. Treatment advances in the field of multiple myeloma (MM) have increased the need for accurate diagnosis, particularly in the bone marrow, which is an essential component in myeloma-defining criteria. Modern imaging identifies osteolytic lesions, distinguishes solitary plasmacytoma from MM, and evaluates the presence of extramedullary disease. Furthermore, imaging is increasingly valuable in post-treatment response assessment. Detection of minimal residual disease (MRD) after therapy carries prognostic implications and influences subsequent treatment planning.
Whole-body low-dose Computed Tomography (CT) is now recommended over the conventional skeletal survey, and more sophisticated functional imaging methods, such as 18F-Fluorodeoxyglucose Positron Emission Tomography (FDG-PET)/CT, and diffusion-weighted Magnetic Resonance Imaging (MRI) are proving effective in the assessment and monitoring of MM disease. This review focuses on understanding indications and advantages of these imaging modalities for diagnosing and managing myeloma.
Introduction/Abstract:
Imaging innovations offer useful techniques applicable to many oncology specialties. Treatment advances in the field of multiple myeloma (MM) have increased the need for accurate diagnosis, particularly in the bone marrow, which is an essential component in myeloma-defining criteria. Modern imaging identifies osteolytic lesions, distinguishes solitary plasmacytoma from MM, and evaluates the presence of extramedullary disease. Furthermore, imaging is increasingly valuable in post-treatment response assessment. Detection of minimal residual disease (MRD) after therapy carries prognostic implications and influences subsequent treatment planning.
Whole-body low-dose Computed Tomography (CT) is now recommended over the conventional skeletal survey, and more sophisticated functional imaging methods, such as 18F-Fluorodeoxyglucose Positron Emission Tomography (FDG-PET)/CT, and diffusion-weighted Magnetic Resonance Imaging (MRI) are proving effective in the assessment and monitoring of MM disease. This review focuses on understanding indications and advantages of these imaging modalities for diagnosing and managing myeloma.
Imaging Definition of Myeloma Bone Disease
Osteolytic lesions are a hallmark of MM, occurring in almost all patients during disease. The classic CRAB (Calcium, Renal, Anemia, Bone) feature that defines end-organ damage in myeloma, lytic bone lesions discovered through imaging require treatment.
In 2014, the International Myeloma Working Group (IMWG) updated criteria and established that one or more unequivocal osteolytic lesions (≥ 5 mm in size) on CT (including low-dose CT or from PET/CT) is diagnostic of myeloma, regardless of skeletal radiography findings (1). In comparison, two or more focal bone lesions (each measuring 5mm or greater) seen on MRI, are necessary for MM diagnosis. Both MRI and FDG-PET detect focal lesions, however, only lytic lesions visualized by CT or skeletal survey, qualify as evidence for myeloma bone involvement. Thus, increased focal FDG uptake on PET alone is not enough to define bone disease and osseous destruction on the CT-portion is needed to fulfill criteria. Diffuse osteopenia and vertebral compression fractures in the absence of lytic lesions are no longer considered in the diagnostic classification (1).
From Conventional Skeletal Survey to Whole-Body CT
The conventional skeletal survey encompasses whole-body radiography of the spine, skull, chest, pelvis, humeri and femora, and any symptomatic areas, with a total radiation dose of approximately 1.7–2.4 mSv (2–4). The standard bone imaging method in myeloma for many decades because of its low cost and widespread availability, the skeletal survey suffers inferior sensitivity compared to advanced imaging options in part, because 50–75% trabecular bone destruction must occur before manifesting on X-ray (5). Whole-body low dose CT is more sensitive, providing an enhanced three-dimensional depiction of bone marrow involvement (Figure 1) with doses as low as 3.2–4.8 mSv (6, 7). In a multicenter study by the IMWG, 26% of patients with negative skeletal radiography were positive on CT, and similar observations were reported in smaller cohorts (8–11). Whole-body CT superiorly discriminates spine and pelvic abnormalities better than X-ray, with comparable performance in long bones (9). Additionally, CT can detect extramedullary lesions. From a patient perspective, supine positioning with CT and shorter acquisition times than the skeletal survey is more comfortable and practical. Today, whole-body low dose CT is considered the standard of care in the diagnostic screening of MM, however, if unavailable, the skeletal survey is still acceptable (12).
Figure 1:
CT demonstrates multiple osteolytic lesions in the right distal clavicle, sternum
CT is not as effective for imaging after myeloma therapy, as anatomic changes resulting from treatment appear later or may be absent even when patients are in complete remission. Although there may be marked reduction in osteoclastic activity after therapy, many successfully treated lesions never fully resolve, leading to false-positive readings on morphologic scans. Hence, CT and skeletal radiography are not ideal options for assessing treatment response in MM.
The Role of 18F-FDG-PET/CT imaging in Multiple Myeloma
PET/CT imaging with the radiolabeled glucose analog FDG, evaluates abnormal glucose metabolism to visualize tumors. In patients with plasma cell disorders, FDG-PET/CT recognizes bone lesions and extramedullary disease (Figure 2), with high sensitivity and specificity ranging from 80–100% (13). FDG-PET/CT has demonstrated clinical worth at different stages of the plasma cell disorder spectrum from solitary plasmacytoma, smoldering multiple myeloma and active myeloma.
Figure 2:
CT (top row) and fused PET/CT (bottom row) demonstrate an intense hypermetabolic focus fusing to an osteolytic lesion at the left proximal clavicle (arrows) that meets the criteria for multiple myeloma.
Like whole-body low dose CT, FDG-PET/CT depicts more lesions than skeletal surveys (14). MM lesions manifest as focal, diffuse, and mixed patterns of FDG uptake and may not correspond to an osteolytic finding on CT, underscoring that only CT lytic lesions (≥1 lesion of ≥ 5 mm in size) satisfy criteria for disease (1, 12). Moreover, CT parameters on PET/CT must be equivalent to stand-alone whole-body low-dose CT to properly assess myeloma bone disease and stability of the spine (15).
The IMWG recommends FDG-PET/CT for the initial assessment of disease burden. With dual metabolic and anatomic capabilities that augment lesion detection within and outside the bone, it serves as a prognostic predictive marker for MM (16–18). Indeed, an increased number and intensity of PET-positive lesions as well as extramedullary tumors found at diagnosis, correlate with lower progression free and overall survival (17, 19). In earlier stages of the disease, i.e. smoldering myeloma, patients with FDG-avid lesions without underlying osteolysis, progress to active MM in a shorter time (20). The IMWG also endorses FDG-PET/CT to distinguish MM from smoldering MM and to evaluate solitary plasmacytoma as a secondary alternative to whole-body MRI.
Additionally, FDG-PET/CT is the preferred imaging technique for therapeutic assessment of MM (Figure 3). Disappearance or decreased FDG uptake less than the surrounding normal tissue at the post-therapy scan indicates complete metabolic resolution and correlates with a highly favorable response to therapy. Effectively targeted focal lesions on FDG-PET resolve sooner than observed with other scans, including CT and MRI, supporting PET/CT use in early detection of treatment response (21).
Figure 3:
Baseline and post-treatment FDG-PET/CT, including maximal intensity projection PET imaging and fused PET/CT demonstrating several regions of bone marrow involvement compatible with multiple myeloma in the proximal clavicle, right posterior rib, left posterior pelvis and bilateral humeri and femurs at baseline scan. Post-treatment FDG-PET/CT shows partial metabolic response.
Identifying residual plasma cell clones within bone is an added strength of the post-treatment PET/CT scan and characterized by metabolically active lesions reduced in size, number, and uptake signaling a partial response. Furthermore, residual tumors on FDG-PET/CT have prognostic significance (16, 22–24). Increased uptake values, extramedullary disease or more than three persistent focal lesions, are associated with poor outcomes with shorter progression-free survival (PFS) of 44 months reported in patients with residual focal lesions on FDG-PET/CT compared to 84 months (24). Similarly, in the French IMAJEM trial, both PFS and overall survival were considerably better in patients with negative FDGPET/CT results before initiation of maintenance therapy (22).
FDG-PET/CT is superior to conventional MRI for treatment follow-up, however, functional MRI sequences through diffusion-weighted imaging improves sensitivity in detecting residual disease compared to FDG-PET/CT (25, 26). Nevertheless, FDG-PET/CT continues to be the reference standard for evaluating treatment response and overall, more specific and accurate for determining remission status. Regardless, consistency in imaging methods used at initial diagnosis and with each stage of follow-up is essential for proper comparison(12).
While FDG-PET/CT has demonstrated substantial impact in patients with MM, it is also important to note limitations. Approximately 10% of patients diagnosed with multiple myeloma bone disease demonstrate no lesions on FDG-PET/CT(27, 28). Rasche et al. reported a false-negative rate of 11% in a large retrospective cohort of 227 patients with MM related to lower expression of the glycolytic enzyme Hexokinase-2 (p<0.001) (27). Thus, FDG-PET/CT is not suitable for therapeutic assessment or evaluation of MRD in this sub-group of symptomatic MM patients without FDG-PET/CT abnormalities at baseline. Conversely, false-positive findings are another FDG-PET/CT concern as increased glucose metabolism is not exclusive to malignant tumors. Inflammation, infection, post-biopsy or postsurgical changes, bone remodeling, fractures, and bone marrow repopulation following chemotherapy, radiation therapy, or growth factors enter the differential for increased radiotracer activity. Distinguishing these factors is even more difficult in earlier stages of disease. More sensitive methods for detecting and measuring tumor burden would improve staging and treatment selection of patients with plasma cell disorders, consequently, there is interest in other unique molecular imaging agents.
Novel Targeted Radiotracers Beyond FDG
Growth in molecular imaging development is exceptionally relevant to MM. Novel PET tracers targeting distinct metabolic pathways, amino-acids, DNA synthesis, and plasma cell receptors have demonstrated interesting preliminarily results for disease detection with corollaries for MRD, especially in patients negative on FDG-PET/CT. Several PET radiotracers beyond FDG, have been investigated in plasma cell disorders, but there is no consensus on their utility in clinical practice.
Trials have studied choline, a component of cell membranes, which is abundant in proliferating cells (often associated with cancers) and involved in membrane metabolism and growth. Labeled with Carbon-11 (11C-Choline) or Fluorine-18 (18F-fluorocholine), sensitivity is reported greater than FDG in myeloma (29). 18F-Choline-PET/CT revealed significantly more lesions than FDG-PET/CT in patients with relapsed or refractory MM, and interestingly, the majority of lesions missed by FDG were in the axial skeleton, including the skull vault (30). Unfortunately, physiologic distribution of Choline-PET is increased in normal bone marrow, potentially masking lesions, which poses diagnostic challenges. 18Ffluorothymidine, a pyrimidine deoxy-nucleoside involved with DNA synthesis and cell proliferation has also been studied in MM with related physiologic issues (31). Other attempts to image myeloma in smaller pilot studies have explored 11C-methionine, a marker of amino-acid metabolism with high affinity for myeloma bone deposits correlating with bone marrow involvement and clinical parameters (32, 33).
A more promising tracer, 68Ga-Pentixafor, is a labelled peptide targeting CXCR4, a G-proteincoupled chemokine receptor, often concentrated on myeloma cells (34). Two thirds of MM patients carry tumors with elevated CXCR4 surface expression which correlates with disease progression and poor outcome (35). In a small MM patient series, 68Ga-Pentixafor PET/CT had higher positive rates compared to FDG-PET/CT (36, 37). Notably, this new PET ligand has favorable qualities for therapeutic labelling with ß-emitting isotopes, such as 177-Lutetium or 90-Yttrium, and could offer directed treatment potential(38, 39).
An emerging field in imaging, immunoPET focuses on antibody-based PET radiotracers for diagnostic and therapeutic opportunities. Daratumumab is an FDA-approved monoclonal antibody for MM therapy that targets CD38, a transmembrane glycoprotein richly expressed on MM cells. Conjugating with Copper-64 (64Cu) and Zirconium-89 (89Zr) PET radioisotopes led to recent Phase I first-in-human studies with 89Zr-DFO-Daratumumab-PET/CT and 64Cu-Daratumumab-PET/CT producing encouraging results identifying myelomatous disease (40, 41). Daratumumab-directed imaging opens possibilities for appropriately selecting patients who may benefit from the antibody treatment and ultimately, predict effectiveness of therapy.
B cell maturation antigen (BCMA) is another novel treatment target for multiple myeloma (MM) with highly selective expression on malignant plasma cells. Multiple BCMA-targeted therapeutics, including antibody-drug conjugates (ADC), chimeric antigen receptor (CAR)-T cells, and bispecific T cell engagers (BiTE), have achieved remarkable clinical responses in patients with relapsed and refractory MM. In a pre-clinical study (42), ultra-small gadolinium containing nanoparticles targeting antibodies against BCMA improved signal-to-noise for MM detection by MRI, demonstrating feasibility for pursuing BCMA through molecular imaging modalities. New radiolabeled tracers enhance understanding of MM disease and stimulate further progress in management.
Whole body MRI
Whole body (WB) MRI has been recognized by worldwide scientific panels as the most sensitive imaging modality for interrogating bone marrow for myeloma, especially in early disease. Noninvasive and without ionizing radiation, this leading edge technology offers versatile applications in plasma cell disorders. Consensus recommendations from the IMWG advocate for the use of WB MRI in various stages of the disease. For example, when a pre-myeloma condition such as MGUS (monoclonal gammopathy of unknow significance), is suspected and whole body CT is inconclusive, WB MRI should be performed to exclude focal bone lesions. Evaluation of solitary bone plasmacytoma is another indication. Further on the disease spectrum, suspicion for smoldering or overt multiple myeloma with a negative or inconclusive whole body CT warrants extended work-up with WB MRI. Monitoring treatment response is not currently endorsed, but MRI is favored to assess relapse after a negative or equivocal CT (12).
MRI employs powerful magnetic fields to align protons throughout the body to create images derived from intrinsic fat and water properties that result in detailed soft tissue morphology. The standard WB MRI procedure (Table 1) captures the spine on sagittal plane and the skull, ribs, pelvis and proximal long bones are imaged in the coronal or transverse planes to detect myeloma in bone marrow and extramedullary sites. While WB MRI is the preferred modality for myeloma diagnosis, the IMWG has acknowledged that axial MRI, which only covers the spine and pelvis, is acceptable in places where WB MRI access is limited (12). The majority of myeloma lesions are found in the vertebral bodies (49%) and skull (35%), and approximately 10% of patients are noted to have lesions only outside the axial skeleton (43). The proximal femurs and humerus are where most non-axial lesions are located, so WB MRI is preferred for a comprehensive evaluation.
Table 1.
Whole Body MRI Examination Sequences
| SEQUENCE | MRI PARAMETERS | STANDARD OR OPTIONAL |
|---|---|---|
| T1-weighted fast spin-echo | Whole spine, Sagittal, slice thickness 4–5 mm | Standard |
| T2 STIR ( Short tau inversion recovery) or fat suppressed T2-weighted | Whole spine, Sagittal, slice thickness 4–5 mm | Standard |
| T1-weighted, gradient echo Dixon technique | Whole body (vertex to knees), axial or coronal, Fat and water image reconstruction, slice thickness 5 mm | Standard |
| Diffusion-weighted Imaging (DWI), STIR fat suppression | Whole body (vertex to knees), axial, slice thickness 5 mm, 2 b- values (50–100 sec/mm2 and 800–900 sec/mm2), ADC calculations | Standard Optional: 3 b-value imaging (additional 500–600 sec/mm2), 3-dimensional maximum intensity projection reconstructions of highest b-value images |
| T2-weighted fast spin-echo without fat suppression | Whole body (vertex to knees), axial, 5 mm | Optional |
Predominantly an osseous cancer, myeloma lesions are classified on MRI into 5 marrow infiltration patterns: normal, focal, diffuse, combined focal and diffuse, and a variegated, “salt and pepper” presentation. A normal appearance is seen in 28% of patients and characterized by high signal on T1-weighted and intermediate signal intensity on T2-weighted spin-echo images as well as low signal in fat-saturated sequences (44). Focal myeloma lesions have low signal intensity on T1-weighted images and intermediate to high signal intensity on T2-weighted fat suppressed or short tau inversion recovery (STIR) images, high signal intensity on DWI and are present in 30% of patients. The IMWG defines focal lesions as 5mm or greater in size and two or more lesions on MRI as a biomarker of malignancy(1, 12). A diffuse pattern has decreased signal on T1 fast spin-echo or Dixon in-phase and fat only images and increased signal on T2- weighted images and high b-value DW images. The signal intensity of an intervertebral disc or normal muscle should be used as reference particularly when high tumor burden is suspected(12). The diffuse pattern has not yet been considered a defined pattern of multiple myeloma and follow-up should be obtained in 3–6 months to document signs of progression in MRI. In 11% of patients, the combined pattern is marked by decreased signal on T1-weighted spin-echo with additional focal regions that are easier to distinguish on gradient echo and fat saturated sequences. The “salt and pepper” pattern is the least common and found in 3%. The bone marrow displays an inhomogeneous patchy arrangement on T1-weighted spin-echo, gradient echo and T2-weighted spin-echo images without hyperintense signal on fat saturated views. Histologically, this results from adipose tissue fragments mixed in with normal bone marrow and a small volume of abnormal plasma cells. Variegated and normal patterns usually indicate low tumor involvement. Studies demonstrate that these patterns are associated with prognostic implications. An increasing number of focal lesions and diffuse infiltration pattern correlate with worse survival and shorter time to progression (23, 45–47). Extramedullary lesions are uncommon, occurring in 3.4% of patients and often related to an unfavorable course (48).
WB MRI sensitivity for lesion detection is high but specificity is low. A recent systematic review reported superior sensitivity with MRI ranging from 68–100% compared to 47–100% for FDG-PET/CT but lower specificity at 37–83% for MRI while PET/CT ranged from 62–85.7% (49)(Figure 4). Several factors contribute to MRI’s low specificity, including challenges in response evaluation after therapy because signal intensity changes in the marrow can lag up to 12 months. Limited anatomic sequences to keep scan times down also add to the high false positive rate (49). Therefore, FDG-PET/CT is recommended for response assessment in addition to extramedullary disease detection.
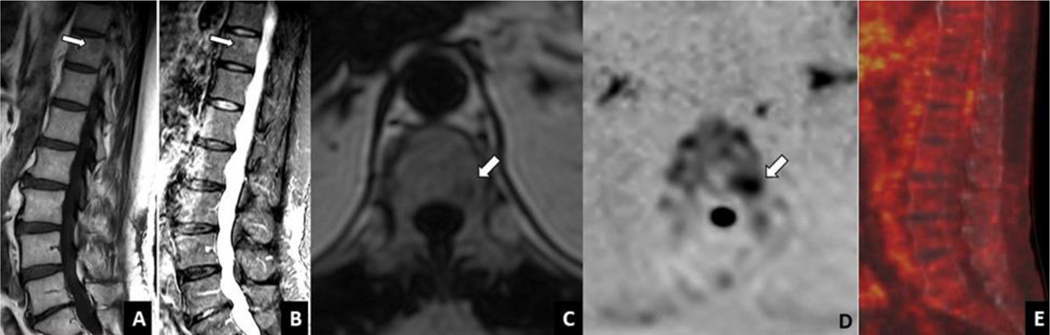
Figure 4A-E:
Whole body MRI spine images show a focal lesion in the T11 vertebral body with T1 hypointensity and T2 intermediate to high intensity in sagittal T1 weighted fast spin echo (A) and STIR (B) images, respectively (arrows). Lesion is hypointense in the T1 weighted fat reconstruction image using Dixon technique (C) and demonstrates increased signal on axial DW imaging (inverted scale, B val ue 900 sec/mm2)(D).There is no corresponding focal, increased metabolic activity in the T11 vertebral body on PET/CT(E). STIR= short tau inversion recovery; DW= diffusion weighted
Diffusion Weighted Imaging
To improve WB MRI specificity, diffusion weighted imaging (DWI) has been promoted as a solution to this issue through its functional capabilities examining marrow cellularity. Developed within the last 20 years, DWI focuses on water molecule movements and interactions within various compartments in the body. Sensitizing gradients, known as b-values, are deployed with consequent signal emission from tissue (50). Lesions with densely packed tumor cells, as found in myeloma, will have restricted water movement and high signal compared to normal tissue (Figure 5). Molecules surrounded by less cells will produce lower signal. In practice, high b-values are more useful, however, low and sometimes intermediate levels are also obtained, which add more time in acquisition. Signal level grading on high b-value images are determined by comparing adjacent muscle intensity as reference. Lesions with hyperintense signal are typically suspicious for myeloma tumor, but other benign causes such as infection, bone infarcts, or marrow edema caused by fractures can present similarly. False negative assessment can occur due to body movement and background bone marrow hyperplasia. DWI is now regularly incorporated in WB MRI and endorsed by IMWG and the Myeloma Response Assessment and Diagnosis System (MY-RADs) imaging guideline (1, 12, 51).
Figure 5A-F:
Lateral skull radiograph shows multiple lytic calvarial lesions in a patient with multiple myeloma (A, arrows). Whole body MRI shows diffusion restriction in these lesions in axial DW imaging (inverted scale, B value 900 sec/mm2) (B) and ADC map (C) (arrows). Anteroposterior pelvis radiograph shows no focal lytic lesion at the right posterior iliac bone in the same patient (D). Whole body MRI demonstrates diffusion restricted focal lesion in axial DW image (E) and ADC map (F) at the left posterior iliac bone (arrows). DW= diffusion-weighted; ADC= apparent diffusion coefficient
The apparent diffusion coefficient (ADC) is a quantitative method of measuring water diffusion after the application of b-values and is inversely correlated to cell density. ADC can be affected by patient motion, scanner specifications and susceptibility effects. Accurate ADC measurements rely on the presence of a water signal and can be complicated to interpret in the bone marrow. Low water content, lipid cells, vascularity and extracellular space can influence signal intensity and ADC values. ADC values are helpful in distinguishing normal, focal and diffuse myeloma patterns and in newly diagnosed myeloma patients, baseline measurements have been found to predict progression free survival and overall survival (52–54). As a tool for monitoring treatment, mean ADC values have shown significant change after therapy and may be helpful in predicting response (55). Another investigation demonstrated strong performance in assessing progressive disease, but could improve on complete and objective response measures (56). In comparison to FDG-PET/CT, ADC and maximum standardized uptake values (SUVmax) correlated well between myeloma treatment responders and non-responders in a recent study, bolstering its helpfulness in post-therapy applications (57).
Despite many advantages, there are some limitations with DWI. Caution in interpretation of DWI is advised due to several confounding aspects that could lead to inaccurate interpretations such as T2 shine-through, respiratory motion artifacts and dispersed tumor. For instance, liquid transformation of cured focal lesions may result in hyperintense signal on high-b value sequences due to T2 shine-through.
Therefore, state-of-the-art imaging is beneficial in evaluating myeloma disease presenting as osteolytic lesions, focal or diffuse bone marrow lesions or extramedullary tumors. Accordingly, CT is the best modality to recognize destructive bone lesions necessary for diagnosis. FDG-PET/CT pursues glycolytic tumor cell properties to localize disease and prevails as the reference standard to measure response assessment to therapy. Lastly, WB MRI with DWI provides unparalleled bone marrow and soft tissue depiction through strong, non-ionizing magnetic forces for diagnosis of multiple myeloma. Continued imaging developments through novel PET radiotracers, DW MRI sequences and CT advances are encouraging steps to further improve clinical management of plasma cell disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–48. [DOI] [PubMed] [Google Scholar]
- 2.Dimopoulos M, Terpos E, Comenzo RL, Tosi P, Beksac M, Sezer O, et al. International myeloma working group consensus statement and guidelines regarding the current role of imaging techniques in the diagnosis and monitoring of multiple Myeloma. Leukemia. 2009;23(9):1545–56. [DOI] [PubMed] [Google Scholar]
- 3.Terpos E, Kleber M, Engelhardt M, Zweegman S, Gay F, Kastritis E, et al. European Myeloma Network guidelines for the management of multiple myeloma-related complications. Haematologica. 2015;100(10):1254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ormond Filho AG, Carneiro BC, Pastore D, Silva IP, Yamashita SR, Consolo FD, et al. Whole-Body Imaging of Multiple Myeloma: Diagnostic Criteria. Radiographics. 2019;39(4):1077–97. [DOI] [PubMed] [Google Scholar]
- 5.Edelstyn GA, Gillespie PJ, Grebbell FS. The radiological demonstration of osseous metastases. Experimental observations. Clin Radiol. 1967;18(2):158–62. [DOI] [PubMed] [Google Scholar]
- 6.Ippolito D, Besostri V, Bonaffini PA, Rossini F, Di Lelio A, Sironi S. Diagnostic value of whole-body low-dose computed tomography (WBLDCT) in bone lesions detection in patients with multiple myeloma (MM). Eur J Radiol. 2013;82(12):2322–7. [DOI] [PubMed] [Google Scholar]
- 7.Horger M, Claussen CD, Bross-Bach U, Vonthein R, Trabold T, Heuschmid M, et al. Whole-body low-dose multidetector row-CT in the diagnosis of multiple myeloma: an alternative to conventional radiography. Eur J Radiol. 2005;54(2):289–97. [DOI] [PubMed] [Google Scholar]
- 8.Wolf MB, Murray F, Kilk K, Hillengass J, Delorme S, Heiss C, et al. Sensitivity of whole-body CT and MRI versus projection radiography in the detection of osteolyses in patients with monoclonal plasma cell disease. Eur J Radiol. 2014;83(7):1222–30. [DOI] [PubMed] [Google Scholar]
- 9.Hinge M, Andersen KT, Lund T, Jorgensen HB, Holdgaard PC, Ormstrup TE, et al. Baseline bone involvement in multiple myeloma - a prospective comparison of conventional X-ray, low-dose computed tomography, and 18flourodeoxyglucose positron emission tomography in previously untreated patients. Haematologica. 2016;101(10):e415–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Princewill K, Kyere S, Awan O, Mulligan M. Multiple myeloma lesion detection with whole body CT versus radiographic skeletal survey. Cancer Invest. 2013;31(3):206–11. [DOI] [PubMed] [Google Scholar]
- 11.Hillengass J, Moulopoulos LA, Delorme S, Koutoulidis V, Mosebach J, Hielscher T, et al. Whole-body computed tomography versus conventional skeletal survey in patients with multiple myeloma: a study of the International Myeloma Working Group. Blood Cancer J. 2017;7(8):e599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hillengass J, Usmani S, Rajkumar SV, Durie BGM, Mateos MV, Lonial S, et al. International myeloma working group consensus recommendations on imaging in monoclonal plasma cell disorders. Lancet Oncol. 2019;20(6):e302–e12. [DOI] [PubMed] [Google Scholar]
- 13.Lu YY, Chen JH, Lin WY, Liang JA, Wang HY, Tsai SC, et al. FDG PET or PET/CT for detecting intramedullary and extramedullary lesions in multiple Myeloma: a systematic review and meta-analysis. Clin Nucl Med. 2012;37(9):833–7. [DOI] [PubMed] [Google Scholar]
- 14.Zamagni E, Nanni C, Patriarca F, Englaro E, Castellucci P, Geatti O, et al. A prospective comparison of 18F-fluorodeoxyglucose positron emission tomography-computed tomography, magnetic resonance imaging and whole-body planar radiographs in the assessment of bone disease in newly diagnosed multiple myeloma. Haematologica. 2007;92(1):50–5. [DOI] [PubMed] [Google Scholar]
- 15.Moulopoulos LA, Koutoulidis V, Hillengass J, Zamagni E, Aquerreta JD, Roche CL, et al. Recommendations for acquisition, interpretation and reporting of whole body low dose CT in patients with multiple myeloma and other plasma cell disorders: a report of the IMWG Bone Working Group. Blood Cancer J. 2018;8(10):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartel TB, Haessler J, Brown TL, Shaughnessy JD Jr., van Rhee F, Anaissie E, et al. F18fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood. 2009;114(10):2068–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zamagni E, Patriarca F, Nanni C, Zannetti B, Englaro E, Pezzi A, et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood. 2011;118(23):5989–95. [DOI] [PubMed] [Google Scholar]
- 18.Davies FE, Rosenthal A, Rasche L, Petty NM, McDonald JE, Ntambi JA, et al. Treatment to suppression of focal lesions on positron emission tomography-computed tomography is a therapeutic goal in newly diagnosed multiple myeloma. Haematologica. 2018;103(6):1047–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sevcikova S, Minarik J, Stork M, Jelinek T, Pour L, Hajek R. Extramedullary disease in multiple myeloma - controversies and future directions. Blood Rev. 2019;36:32–9. [DOI] [PubMed] [Google Scholar]
- 20.Zamagni E, Nanni C, Gay F, Pezzi A, Patriarca F, Bello M, et al. 18F-FDG PET/CT focal, but not osteolytic, lesions predict the progression of smoldering myeloma to active disease. Leukemia. 2016;30(2):417–22. [DOI] [PubMed] [Google Scholar]
- 21.Hillengass J, Landgren O. Challenges and opportunities of novel imaging techniques in monoclonal plasma cell disorders: imaging “early myeloma”. Leuk Lymphoma. 2013;54(7):1355–63. 22. [DOI] [PubMed] [Google Scholar]
- 22.Moreau P, Attal M, Caillot D, Macro M, Karlin L, Garderet L, et al. Prospective Evaluation of Magnetic Resonance Imaging and [(18)F]Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography at Diagnosis and Before Maintenance Therapy in Symptomatic Patients With Multiple Myeloma Included in the IFM/DFCI 2009 Trial: Results of the IMAJEM Study. J Clin Oncol. 2017;35(25):2911–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker R, Barlogie B, Haessler J, Tricot G, Anaissie E, Shaughnessy JD, Jr., et al. Magnetic resonance imaging in multiple myeloma: diagnostic and clinical implications. J Clin Oncol. 2007;25(9):1121–8. [DOI] [PubMed] [Google Scholar]
- 24.Zamagni E, Nanni C, Mancuso K, Tacchetti P, Pezzi A, Pantani L, et al. PET/CT Improves the Definition of Complete Response and Allows to Detect Otherwise Unidentifiable Skeletal Progression in Multiple Myeloma. Clin Cancer Res. 2015;21(19):4384–90. [DOI] [PubMed] [Google Scholar]
- 25.Pawlyn C, Fowkes L, Otero S, Jones JR, Boyd KD, Davies FE, et al. Whole-body diffusion-weighted MRI: a new gold standard for assessing disease burden in patients with multiple myeloma? Leukemia. 2016;30(6):1446–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasche L, Alapat D, Kumar M, Gershner G, McDonald J, Wardell CP, et al. Combination of flow cytometry and functional imaging for monitoring of residual disease in myeloma. Leukemia. 2019;33(7):1713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rasche L, Angtuaco E, McDonald JE, Buros A, Stein C, Pawlyn C, et al. Low expression of hexokinase-2 is associated with false-negative FDG-positron emission tomography in multiple myeloma. Blood. 2017;130(1):30–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abe Y, Ikeda S, Kitadate A, Narita K, Kobayashi H, Miura D, et al. Low hexokinase-2 expressionassociated false-negative (18)F-FDG PET/CT as a potential prognostic predictor in patients with multiple myeloma. Eur J Nucl Med Mol Imaging. 2019;46(6):1345–50. [DOI] [PubMed] [Google Scholar]
- 29.Nanni C, Zamagni E, Cavo M, Rubello D, Tacchetti P, Pettinato C, et al. 11C-choline vs. 18F-FDG PET/CT in assessing bone involvement in patients with multiple myeloma. World J Surg Oncol. 2007;5:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cassou-Mounat T, Balogova S, Nataf V, Calzada M, Huchet V, Kerrou K, et al. 18F-fluorocholine versus 18F-fluorodeoxyglucose for PET/CT imaging in patients with suspected relapsing or progressive multiple myeloma: a pilot study. Eur J Nucl Med Mol Imaging. 2016;43(11):1995–2004. [DOI] [PubMed] [Google Scholar]
- 31.Agool A, Schot BW, Jager PL, Vellenga E. 18F-FLT PET in hematologic disorders: a novel technique to analyze the bone marrow compartment. J Nucl Med. 2006;47(10):1592–8. [PubMed] [Google Scholar]
- 32.Lapa C, Knop S, Schreder M, Rudelius M, Knott M, Jorg G, et al. 11C-Methionine-PET in Multiple Myeloma: Correlation with Clinical Parameters and Bone Marrow Involvement. Theranostics. 2016;6(2):254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luckerath K, Lapa C, Albert C, Herrmann K, Jorg G, Samnick S, et al. 11C-Methionine-PET: a novel and sensitive tool for monitoring of early response to treatment in multiple myeloma. Oncotarget. 2015;6(10):8418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Philipp-Abbrederis K, Herrmann K, Knop S, Schottelius M, Eiber M, Luckerath K, et al. In vivo molecular imaging of chemokine receptor CXCR4 expression in patients with advanced multiple myeloma. EMBO Mol Med. 2015;7(4):477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vande Broek I, Leleu X, Schots R, Facon T, Vanderkerken K, Van Camp B, et al. Clinical significance of chemokine receptor (CCR1, CCR2 and CXCR4) expression in human myeloma cells: the association with disease activity and survival. Haematologica. 2006;91(2):200–6. [PubMed] [Google Scholar]
- 36.Pan Q, Cao X, Luo Y, Li J, Feng J, Li F. Chemokine receptor-4 targeted PET/CT with (68)GaPentixafor in assessment of newly diagnosed multiple myeloma: comparison to (18)F-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2020;47(3):537–46. [DOI] [PubMed] [Google Scholar]
- 37.Lapa C, Schreder M, Schirbel A, Samnick S, Kortum KM, Herrmann K, et al. [(68)Ga]PentixaforPET/CT for imaging of chemokine receptor CXCR4 expression in multiple myeloma - Comparison to [(18)F]FDG and laboratory values. Theranostics. 2017;7(1):205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herrmann K, Schottelius M, Lapa C, Osl T, Poschenrieder A, Hanscheid H, et al. First-in-Human Experience of CXCR4-Directed Endoradiotherapy with 177Lu- and 90Y-Labeled Pentixather in AdvancedStage Multiple Myeloma with Extensive Intra- and Extramedullary Disease. J Nucl Med. 2016;57(2):248–51. [DOI] [PubMed] [Google Scholar]
- 39.Lapa C, Herrmann K, Schirbel A, Hanscheid H, Luckerath K, Schottelius M, et al. CXCR4-directed endoradiotherapy induces high response rates in extramedullary relapsed Multiple Myeloma. Theranostics. 2017;7(6):1589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ulaner GA, Sobol NB, O’Donoghue JA, Kirov AS, Riedl CC, Min R, et al. CD38-targeted ImmunoPET of Multiple Myeloma: From Xenograft Models to First-in-Human Imaging. Radiology. 2020;295(3):606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krishnan A, Adhikarla V, Poku EK, Palmer J, Chaudhry A, Biglang-Awa VE, et al. Identifying CD38+ cells in patients with multiple myeloma: first-in-human imaging using copper-64-labeled daratumumab. Blood Adv. 2020;4(20):5194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Detappe A, Reidy M, Yu Y, Mathieu C, Nguyen HV, Coroller TP, et al. Antibody-targeting of ultra-small nanoparticles enhances imaging sensitivity and enables longitudinal tracking of multiple myeloma. Nanoscale. 2019;11(43):20485–96. [DOI] [PubMed] [Google Scholar]
- 43.Bäuerle T, Hillengass J, Fechtner K, Zechmann CM, Grenacher L, Moehler TM, et al. Multiple Myeloma and Monoclonal Gammopathy of Undetermined Significance: Importance of Whole-Body versus Spinal MR Imaging. Radiology. 2009;252(2):477–85. [DOI] [PubMed] [Google Scholar]
- 44.Baur-Melnyk A, Buhmann S, Dürr HR, Reiser M. Role of MRI for the diagnosis and prognosis of multiple myeloma. European Journal of Radiology. 2005;55(1):56–63. [DOI] [PubMed] [Google Scholar]
- 45.Hillengass J, Fechtner K, Weber M-A, Bäuerle T, Ayyaz S, Heiss C, et al. Prognostic significance of focal lesions in whole-body magnetic resonance imaging in patients with asymptomatic multiple myeloma. J Clin Oncol. 2010;28(9):1606–10. [DOI] [PubMed] [Google Scholar]
- 46.Mai EK, Hielscher T, Kloth JK, Merz M, Shah S, Hillengass M, et al. Association between magnetic resonance imaging patterns and baseline disease features in multiple myeloma: analyzing surrogates of tumour mass and biology. Eur Radiol. 2016;26(11):3939–48. [DOI] [PubMed] [Google Scholar]
- 47.Moulopoulos LA, Dimopoulos MA, Kastritis E, Christoulas D, Gkotzamanidou M, Roussou M, et al. Diffuse pattern of bone marrow involvement on magnetic resonance imaging is associated with high risk cytogenetics and poor outcome in newly diagnosed, symptomatic patients with multiple myeloma: a single center experience on 228 patients. Am J Hematol. 2012;87(9):861–4. [DOI] [PubMed] [Google Scholar]
- 48.Usmani SZ, Heuck C, Mitchell A, Szymonifka J, Nair B, Hoering A, et al. Extramedullary disease portends poor prognosis in multiple myeloma and is over-represented in high-risk disease even in the era of novel agents. Haematologica. 2012;97(11):1761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gariani J, Westerland O, Natas S, Verma H, Cook G, Goh V. Comparison of whole body magnetic resonance imaging (WBMRI) to whole body computed tomography (WBCT) or (18)F-fluorodeoxyglucose positron emission tomography/CT ((18)F-FDG PET/CT) in patients with myeloma: Systematic review of diagnostic performance. Crit Rev Oncol Hematol. 2018;124:66–72. [DOI] [PubMed] [Google Scholar]
- 50.Hynes JP, Hughes N, Cunningham P, Kavanagh EC, Eustace SJ. Whole-body MRI of bone marrow: A review. Journal of Magnetic Resonance Imaging. 2019;50(6):1687–701. [DOI] [PubMed] [Google Scholar]
- 51.Messiou C, Hillengass J, Delorme S, Lecouvet FE, Moulopoulos LA, Collins DJ, et al. Guidelines for Acquisition, Interpretation, and Reporting of Whole-Body MRI in Myeloma: Myeloma Response Assessment and Diagnosis System (MY-RADS). Radiology. 2019;291(1):5–13. [DOI] [PubMed] [Google Scholar]
- 52.Zhang L, Wang Q, Wu X, Zhao A, Feng J, Zhang H, et al. Baseline bone marrow ADC value of diffusion-weighted MRI: a potential independent predictor for progression and death in patients with newly diagnosed multiple myeloma. Eur Radiol. 2020. [DOI] [PubMed] [Google Scholar]
- 53.Koutoulidis V, Fontara S, Terpos E, Zagouri F, Matsaridis D, Christoulas D, et al. Quantitative Diffusion-weighted Imaging of the Bone Marrow: An Adjunct Tool for the Diagnosis of a Diffuse MR Imaging Pattern in Patients with Multiple Myeloma. Radiology. 2016;282(2):484–93. [DOI] [PubMed] [Google Scholar]
- 54.Padhani AR, van Ree K, Collins DJ, D’Sa S, Makris A. Assessing the relation between bone marrow signal intensity and apparent diffusion coefficient in diffusion-weighted MRI. AJR Am J Roentgenol. 2013;200(1):163–70. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Xiong X, Fu Z, Dai H, Yao F, Liu D, et al. Whole-body diffusion-weighted MRI for evaluation of response in multiple myeloma patients following bortezomib-based therapy: A large single-center cohort study. European Journal of Radiology. 2019;120:108695. [DOI] [PubMed] [Google Scholar]
- 56.Park HY, Kim KW, Yoon MA, Lee MH, Chae EJ, Lee JH, et al. Role of whole-body MRI for treatment response assessment in multiple myeloma: comparison between clinical response and imaging response. Cancer Imaging. 2020;20(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paternain A, García-Velloso MJ, Rosales JJ, Ezponda A, Soriano I, Elorz M, et al. The utility of ADC value in diffusion-weighted whole-body MRI in the follow-up of patients with multiple myeloma. Correlation study with 18F-FDG PET-CT. European Journal of Radiology. 2020;133:109403. [DOI] [PubMed] [Google Scholar]