Abstract
Objectives
In a population-based study of mild cognitive impairment (MCI), to validate the assessment of social cognition in older adults.
Methods
Cross-sectional study of 902 adults aged 65+ with mean age 76.6 years (SD 8.06). We created a social cognition composite comprising standardized z-scores on the Social Norms Questionnaire and the 10-item Reading the Mind in the Eyes Test. We identified associated factors and compared sensitivity, specificity, and the area under the curve of social cognition, for MCI defined as Clinical Dementia Rating=0.5, to those of other cognitive domains. We calculated the impact of including social cognition on the proportion neuropsychologically classified as MCI.
Results
Better social cognition was associated with younger age, female sex, higher education, better general cognition (MMSE), fewer depressive symptoms, and lower CDR. Adjusting for demographics, associations with MMSE, depressive symptoms, anxiety symptoms, and subjective cognitive complaints remained significant. The sensitivity and specificity of social cognition for CDR = 0.5 were comparable to those of the traditional five cognitive domains. Including social cognition as a sixth domain of cognition resulted in a 5% increase in the proportion classified as MCI.
Conclusions
Brief objective assessment of social cognition may enhance cognitive assessment of older adults.
Keywords: Reading the Mind in the Eyes Test, Social Norms Questionnaire, epidemiology, MYHAT study
INTRODUCTION
Social cognition is the cognitive domain that processes social information, the means by which we make sense of ourselves in relation to others and our environments1. It encompasses multiple cognitive processes2 such as theory of mind (ToM), affective empathy, social perception, and adherence to social norms. These processes underlie the complex and dynamic set of behaviors and mutually shared expectations that enable individuals to successfully interact with one another under different circumstances3. Impaired social cognition is associated with functional dependence on others3 and can have dire social consequences leading to social isolation, increasing risk for morbidity and mortality4.
Disturbances of social cognition are especially prominent in diseases that affect the frontal lobe, such as frontotemporal lobar degeneration (FTLD), autism, schizophrenia, traumatic brain injury, and stroke5,6. Social cognition is now understood to also be impaired in other neurodegenerative disorders including Alzheimer’s disease (AD) 5,6. Thus, the most recent edition of the American Psychiatric Association’s Diagnostic and Statistical Manual for Mental Disorders (DSM-5) introduced social cognition as one of six core domains of neurocognitive function, alongside memory, executive control, complex attention, language, and visuospatial/perceptuomotor function, that can be impaired in the neurocognitive disorders7. It is therefore timely to consider incorporating one or more measures of social cognition into the standard cognitive assessment of older adults, in both clinical and research settings.
We have previously reported population-based norms from older adults on two social cognition measures, the Social Norms Questionnaire [SNQ-22]8,9, assessing social perception, and the 10-question version of the Reading the Mind in the Eyes Test [RMET-10]10–12, measuring ToM. Here, we report on the utility of a social cognition composite measure comprising the SNQ-22 and the RMET-10, including its distribution in a population-based sample of older adults, its association with demographic factors, other cognitive and behavioral measures, the Clinical Dementia Rating (CDR®), its sensitivity and specificity against the CDR, and the effect of including social cognition on estimated prevalence of (cohort proportion classified as) neuropsychologically defined mild cognitive impairment (MCI).
METHODS
Participants
Our study cohort, the Monongahela-Youghiogheny Healthy Aging Team (MYHAT), was accrued by age-stratified random selection from the publicly available voter registration list for a small-town region of southwestern Pennsylvania, USA. The MYHAT study focuses on the epidemiology of mild cognitive impairment (MCI)13. Participants were initially enrolled in the original MYHAT cohort between 2006–2008. The eligibility criteria were a) age 65 years and older, b) residence within the selected geographically defined areas, and c) not in long-term care settings at study entry. The exclusion criteria were d) severe illness, e) vision or hearing impairment severe enough to preclude neuropsychological testing, and f) decisional incapacity13,14. We also excluded individuals who, at study entry, exhibited substantial cognitive impairment by scoring lower than 21/30 on the age-education-corrected Mini-Mental State Examination [MMSE]15,16. The complete evaluation was then administered to 1982 participants. A new sub-cohort of 709 participants, aged of 65–74 between 2016–2019, was added to the study to supplement the original cohort, using the same process and criteria; of them, 703 participants underwent the complete assessment. All participants were invited to undergo annual reassessments which took place in overlapping data collection cycles. All study procedures were approved by the University of Pittsburgh Institutional Research Board for the protection of human subjects; all participants provided written informed consent at study entry.
Assessments
Social Norms Perception:
The Social Norms Questionnaire [SNQ-22]8 measures crystallized knowledge of social norms by asking individuals to identify socially accepted behaviors with yes/no responses in 22 hypothetical vignettes (see Supplemental Digital Content S1).
Theory of Mind:
The Reading the Mind in the Eyes test10, a 10-item version11 [RMET-10], a brief test of ToM, provides grey-scale photographs of the eye areas of 10 people. The test asks participants to select one out of four words that best describes the emotion of the person in each photograph (see Supplemental Digital Content S1).
Each of the above tests represents a different aspect of social cognition, appeared ecologically valid in our study population, and can be administered in 5 minutes.
Neuropsychological Tests:
Tests representing five standard cognitive domains included Attention/Processing Speed (Digit Span, Trail-making Test A), Executive Function (Trail-making Test B, clock drawing, initial letter fluency), Memory (Wechsler Memory Scale-Revised [WMS-R] Logical Memory, immediate and delayed recall; WMS-R Visual Reproduction, immediate and delayed recall; Fuld Object Memory Evaluation), Language (Boston Naming Test, animal fluency, Indiana University Token Test), and Visuospatial Function (Wechsler Adult Intelligence Scale-3 Block Design)17.
Cognitive Domain Composite scores:
For each of the above standard cognitive domains, and for the new social cognition domain comprising the SNQ-22 and the RMET-10, we calculated scores on the component tests as standardized z-scores13; we then calculated the mean of the standardized scores of all the tests in a given domain as that domain’s composite score18.
Demographics:
age, sex, race/ancestry (European/non-European), level of education.
Literacy/reading level:
Wechsler Test of Adult Reading [WTAR]19.
Cognitive screen:
Mini-Mental State Examination [MMSE]15. Note that MMSE score reported is from the cycle when social cognitive assessments were administered and not from study baseline.
Depression symptoms:
modified Center for Epidemiological Studies-Depression scale [mCES-D]20,21.
Anxiety symptoms:
Generalized Anxiety Disorder brief scale [GAD-7]22.
Subjective Cognitive Concerns (SCC):
24 standardized items to assess subjective cognitive concerns 23,24.
Dementia Rating:
Clinical Dementia Rating [CDR®]25. The CDR comprises six components: Memory, Orientation, Judgment & Problem Solving, Community Affairs, Home and Hobbies, and Personal Care. Trained interviewers rated each participant at each assessment cycle on each of the CDR functional domains based on independence in cognitively driven everyday functioning17. The CDR explicitly excludes consideration of neuropsychological test performance. The global CDR ratings (stages) of 0, 0.5, and ≥1 indicate normal cognition, mild cognitive impairment (MCI), and at least mild dementia stages, respectively.
Cognitive Classification:
As reported previously13 we also created a separate neuropsychological classification for MCI. Based on normative values for each participant’s age, sex, and education group, we defined MCI as either a) single domain: composite scores in one domain >1.0 SD below the appropriate mean, with all other domains within 1.0 SD of the mean; or b) multiple domains: two or more domain composite scores 1.0 – 2.0 SD below the mean, or no more than one domain >2.0 SD below the mean with other domain(s) 1.0 – 2.0 SD below the mean. These thresholds are consistent with DSM-5 criteria for neurocognitive disorders. We further classified both single-domain MCI and multi-domain MCI into amnestic and non-amnestic subtypes, based on whether or not the memory domain was impaired.
We use two classifications, one purely cognitive and the other purely functional, so as to validate them against each other. Treating social cognition measure as one of our cognitive domains, we could measure its sensitivity and specificity against the global CDR. To avoid circularity we did not attempt to validate our objective social cognition measures against MCI definitions which require objective cognitive data, such as DSM-5 mild neurocognitive disorder7 or the International Working Group criteria for MCI26. We could also determine the effect on our cognitive classification of adding to it a social cognition domain.
Statistical Analyses
Unadjusted associations between the social cognition composite scores and demographic and clinical measures:
We calculated the mean (SD) social cognition composite scores by age, sex, race, and education categories, by categories of the score distributions of the WTAR (≤99, 100 – 108, 109 – 117 and ≥118), MMSE (≤17, 18 – 23, 24 – 27 and ≥28), GAD-7 (0, 1 – 5, 6 – 10 and ≥11), mCES-D (0, 1 – 4, and ≥5), and SCC (0 and ≥1), and by the global CDR stage, and the ratings for each CDR domain. We assessed statistically significant differences in mean social cognition domain scores between categories using two-sample t-tests and analysis of variance (ANOVA). To adjust for multiple comparisons (total of 17 tests), we applied the Bonferroni correction; the threshold for type I error rate was 0.05/17 = 0.003.
Modeling social cognition:
We then fit multiple linear regression models of social cognition domain composite scores on each of the potential covariates adjusting for age, sex, ancestry, education, and original/new cohort. These covariates include global CDR stage, CDR sum-of-boxes, individual CDR domains, MMSE, WTAR, mCES-D, GAD-7, SCC, and the remaining cognitive domain scores (attention, executive function, memory, language, visuospatial function). To adjust for multiple comparisons (total of 18 models), threshold for type I error rate was 0.05/18 = 0.003.
Sensitivity and specificity of social cognition for CDR:
We compared the sensitivity, specificity, and area under the receiver operating characteristic curve (AUC) for all 6 cognitive domains, using their 10th percentile and 50th percentile as thresholds, against the global CDR = 0.5 stage, excluding participants with CDR ≥ 1. Specifically, the outcome was a binary indicator of CDR = 0.5 (CDR = 0 vs. CDR = 0.5); the predictors were each cognitive domain.
Cognitive classification comparison:
We determined an “old” cognitive classification of MCI using the original 5 cognitive domains and a “new” cognitive classification adding the 6th cognitive domain of social cognition, and cross-tabulated the two to determine how many participants changed their cognitive classifications based on impairment in social cognition. We then fit separate logistic models of 1) normal cognition in “old” classification but non-amnestic MCI in “new” classification to normal cognition in both classifications; and 2) normal cognition in “old” classification but non-amnestic MCI in “new” classification to non-amnestic MCI in both classifications on each of the 13 potential characteristics adjusting for age and binary indicator for new vs. old cohort. To adjust for multiple comparisons (total of 13 models), the threshold of type I error rate was 0.05/13 = 0.004.
All statistical analyses were conducted using R4.0.4 statistical software27.
RESULTS
The study sample, combining the original (415 recruited 2006 – 2008) and new (487 recruited 2016 – 2019) cohorts, and restricted to those with complete data on the variables of interest, numbered 902. These participants’ mean (SD) age was 76.6 (8.06) years; 62.4% were women; 93.7% were of European descent; 3.8% had less than high school education.
Table 1 shows average SD above or below the mean social cognitive domain score. On two-sample tests, better social cognition was significantly associated with younger age, female sex, higher education, and European ancestry. On one-way ANOVA using categorized scores, better social cognition was related to higher MMSE, higher WTAR, lower mCES-D, lower global CDR, CDR sum-of-boxes, and CDR functional domains of judgment, memory, community affairs, and home and hobbies. Social cognition was not associated with CDR personal care, CDR orientation, GAD-7 scores, or SCC.
Table 1.
Demographic and clinical factors associated with the social cognition domain composite score (unadjusted associations)
| N (%) | Social cognition domain score Mean (SD) | P | ||
|---|---|---|---|---|
| Age | 65–74 | 430 (47.7) | 0.12 (0.70) | <0.001 |
| 75–84 | 298 (33.0) | 0.01 (0.76) | ||
| 85+ | 174 (19.3) | −0.34 (0.79) | ||
| Sex | Male | 339 (37.6) | −0.15 (0.81) | <0.001 |
| Female | 563 (62.4) | 0.08 (0.72) | ||
| Education | ≤ HS | 351 (38.9) | −0.14 (0.82) | <0.001 |
| > HS | 551 (61.1) | 0.08 (0.71) | ||
| Race | Non-European | 57 (6.32) | −0.53 (0.97) | <0.001 |
| European | 845 (93.68) | 0.03 (0.73) | ||
| Clinical Dementia Rating (CDR) | 0 | 756 (83.8) | 0.05 (0.72) | <0.001 |
| 0.5 | 134 (14.9) | −0.27 (0.87) | ||
| ≥ 1 | 12 (1.3) | −0.72 (0.84) | ||
| CDR judgment | 0 | 789 (87.5) | 0.07 (0.71) | <0.001 |
| 0.5 | 94 (10.4) | −0.47 (0.87) | ||
| ≥ 1 | 19 (2.1) | −0.79 (1.02) | ||
| CDR memory | 0 | 754 (83.6) | 0.05 (0.72) | <0.001 |
| 0.5 | 124 (13.7) | −0.24 (0.86) | ||
| ≥ 1 | 24 (2.7) | −0.66 (0.85) | ||
| CDR orientation | 0 | 840 (93.1) | 0.01 (0.75) | 0.046 |
| 0.5 | 46 (5.1) | −0.25 (0.88) | ||
| ≥ 1 | 16 (1.8) | −0.20 (0.76) | ||
| CDR community affairs | 0 | 880 (97.6) | 0.01 (0.74) | <0.001 |
| 0.5 | 12 (1.3) | −0.84 (0.97) | ||
| ≥ 1 | 10 (1.1) | −0.81 (0.83) | ||
| CDR home and hobby | 0 | 858 (95.1) | 0.03 (0.73) | <0.001 |
| 0.5 | 36 (4.0) | −0.64 (0.99) | ||
| ≥ 1 | 8 (0.9) | −0.70 (0.88) | ||
| CDR personal care | 0 | 900 (99.8) | 0.00 (0.76) | 0.35 |
| 0.5 | 0 | / | ||
| ≥ 1 | 2 (0.2) | −0.51 (1.03) | ||
| CDR sum of boxes | 0 | 716 (79.4) | 0.09 (0.69) | <0.001 |
| 0.5–2 | 160 (17.7) | −0.32 (0.88) | ||
| >2 | 26 (2.9) | −0.61 (0.89) | ||
| Mean (SD) | 0.27 (0.85) | / | / | |
| MMSE | ≤ 17 | 5 (0.6) | −0.57 (0.61) | <0.001 |
| 18–23 | 53 (5.9) | −0.54 (1.06) | ||
| 24–27 | 287 (31.8) | −0.19 (0.74) | ||
| ≥ 28 | 557 (61.8) | 0.14 (0.69) | ||
| Mean (SD) | 27.59 (2.33) | / | / | |
| WTAR | ≤ 99 | 255 (28.6) | −0.32 (0.78) | <0.001 |
| 100–108 | 209 (23.5) | −0.01 (0.74) | ||
| 109–117 | 241 (27.0) | 0.14 (0.64) | ||
| ≥ 118 | 186 (20.9) | 0.31 (0.64) | ||
| Mean (SD) | 106.64 (12.50) | / | / | |
| GAD-7 | 0 | 361 (48.0) | 0.03 (0.74) | 0.438 |
| 1–5 | 290 (38.6) | 0.01 (0.76) | ||
| 6–10 | 74 (9.8) | −0.11 (0.83) | ||
| ≥ 11 | 27 (3.6) | −0.09 (0.63) | ||
| Mean (SD) | 2.24 (3.33) | / | / | |
| mCES-D | 0 | 605 (67.5) | 0.06 (0.74) | 0.001 |
| 1–4 | 220 (24.6) | −0.11 (0.75) | ||
| ≥ 5 | 71 (7.9) | −0.18 (0.77) | ||
| Mean (SD) | 1.06 (2.33) | / | / | |
| Subjective Cognitive Complaints | 0 | 359 (39.8) | 0.04 (0.77) | 0.104 |
| ≥ 1 | 543 (60.2) | −0.04 (0.75) | ||
| Mean (SD) | 2.11 (2.76) | / | / |
P-values were derived from two-sample t-test and one-way analysis of variance. The Bonferroni corrected alpha level is 0.05/17 = 0.003. The df for t tests is 900. For F test, the numerator df = number of categories −1, and the denominator df = N – number of categories, where N is the total sample size of each variables (N varies as some variables have missingness).
Abbreviations: MMSE (Mini-Mental Status Examination), WTAR (Wechsler Test of Adult Reading), GAD-7 (Generalized Anxiety Disorder brief scale), mCES-D (modified Center for Epidemiological Studies-Depression scale)
Table 2 shows multiple linear regression models adjusting for age, sex, ancestry, and education. When the predictor is binary (e.g., CDR=0 vs. 0.5), those with CDR=0.5 on average had 0.21 SD lower social cognition domain scores. When the predictor is continuous (e.g., domain scores), 1.0 SD higher memory domain score was associated with 0.16 SD higher social cognition domain score. Better social cognition was significantly associated with global CDR stage (CDR = 0.5 and CDR ≥1 compared to CDR = 0), higher MMSE, lower mCES-D, lower GAD-7, fewer SCC, lower CDR sum-of-boxes. At the CDR = 0.5 level (compared to CDR = 0), social cognition was associated with the functional CDR domains of judgment and home and hobbies. Social cognition was not significantly associated with global CDR stage ≥1; however, it was associated with CDR judgment and memory domains scored as ≥ 1. After correcting for multiple comparisons, the associations of social cognition with mCES-D, GAD-7 and SCC lost significance.
Table 2.
Multiple linear regression of social cognition on each of the 18 potential covariates adjusting for age, sex, education, race, and indicator of new vs. old cohort
| Covariate | CDR level | Coef** (95% CI) | P* |
|---|---|---|---|
| Clinical Dementia Rating (CDR) global stage | CDR = 0.5 (ref: CDR = 0) |
−0.21 (−0.34, −0.07) | <0.001 |
| CDR ≥ 1 (ref: CDR = 0) |
−0.42 (−0.85, 0.01) | 0.056 | |
| CDR Judgment and Problem Solving | CDR Judgment and Problem Solving = 0.5 (ref: CDR Judgment and Problem Solving = 0) |
−0.37 (−0.53, −0.22) | <0.001 |
| CDR Judgment and Problem Solving ≥ 1 (ref: CDR Judgment and Problem Solving = 0) |
−0.63 (−0.95, −0.30) | <0.001 | |
| CDR Memory | CDR Memory = 0.5 (ref: CDR Memory = 0) |
−0.17 (−0.30, −0.03) | 0.018 |
| CDR Memory ≥ 1 (ref: CDR Memory = 0) |
−0.52 (−0.81, −0.23) | <0.001 | |
| CDR Orientation | CDR Orientation = 0.5 (ref: CDR Orientation = 0) |
−0.09 (−0.30, 0.13) | 0.415 |
| CDR Orientation ≥ 1 (ref: CDR Orientation = 0) |
−0.08 (−0.43, 0.28) | 0.665 | |
| CDR Community Affairs | CDR Community Affairs = 0.5 (ref: CDR Community Affairs = 0) |
−0.62 (−1.03, −0.22) | 0.003 |
| CDR Community Affairs ≥ 1 (ref: CDR Community Affairs = 0) |
−0.43 (−0.88, 0.02) | 0.059 | |
| CDR Home and Hobbies | CDR Home and Hobbies = 0.5 (ref: CDR Home and Hobbies = 0) |
−0.47 (−0.71, −0.23) | <0.001 |
| CDR Home and Hobbies ≥ 1 (ref: CDR Home and Hobbies = 0) |
−0.34 (−0.84, 0.16) | 0.183 | |
| CDR Personal Care | CDR Personal Care ≥ 1 (ref: CDR Personal Care = 0) |
−0.24 (−1.23, 0.75) | 0.632 |
| CDR Sum of Boxes | −0.11 (−0.16, −0.05) | <0.001 | |
| MMSE | 0.07 (0.05, 0.09) | <0.001 | |
| WTAR | 0.02 (0.01, 0.02) | <0.001 | |
| GAD-7 | −0.02 (−0.03, −0.004) | 0.015 | |
| mCES-D | −0.03 (−0.05, −0.01) | 0.012 | |
| Subjective Cognitive Complaints | −0.02 (−0.03, −0.001) | 0.037 | |
| Neuropsychological domain composite scores | Attention | 0.16 (0.10, 0.22) | <0.001 |
| Executive | 0.36 (0.29, 0.42) | <0.001 | |
| Language | 0.36 (0.29, 0.43) | 0.001 | |
| Memory | 0.16 (0.11, 0.21) | <0.001 | |
| Visuospatial | 0.26 (0.19, 0.32) | <0.001 | |
The Bonferroni corrected alpha level is 0.05/18= 0.003. The p-values are derived from t test, and the df = N - # of tests, where N is the total sample size of each variables (N varies as some variables have missingness).
Unstandardized coefficient
Abbreviations: MMSE (Mini-mental Status Exam), WTAR (Wechsler Test of Adult Reading), GAD-7 (Generalized Anxiety Disorder brief scale), mCES-D (modified Center for Epidemiological Studies-Depression scale)
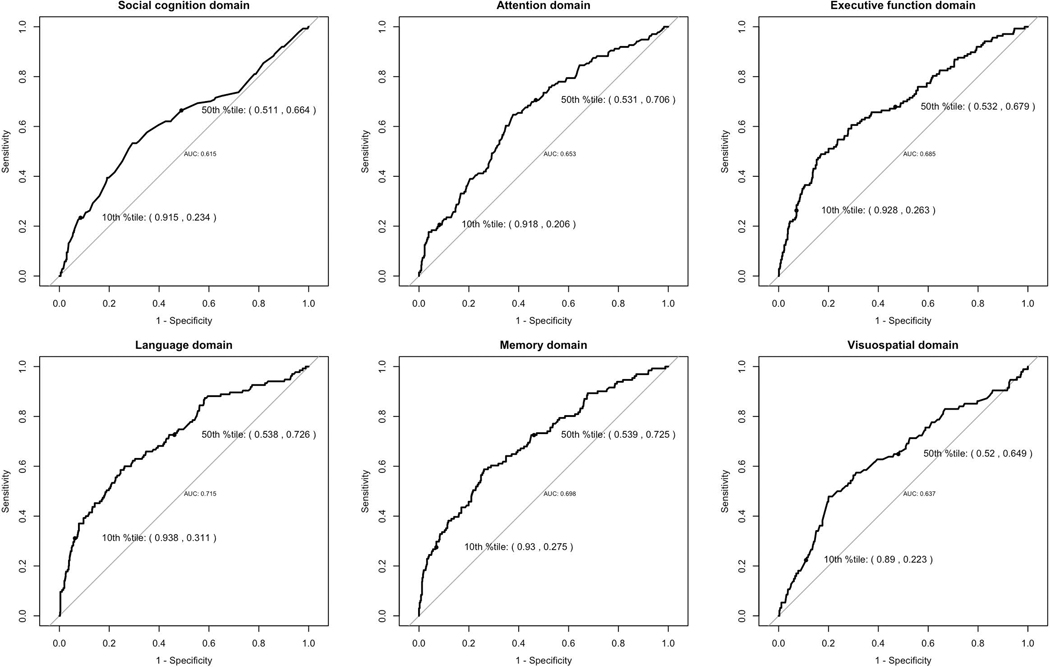
Examining the sensitivity, specificity, and AUC of the various cognitive composites for CDR = 0.5 (Figure 1), all cognitive domains had sensitivities in the 0.2 – 0.3 range at their 10th percentile scores, and in the 0.6 – 0.7 range at their 50th percentile scores, with corresponding specificities in the 0.8 – 0.9 range at the 10th percentile and around 0.5 at their 50th percentile. The AUCs were all in the 0.6 – 0.7 range, with social cognition AUC =0.615.
Figure 1.
Sensitivity, Specificity, and AUC of cognitive domain scores on CDR=0.5 versus CDR=0. In each figure, the specificity and sensitivity (Sp, Se) are labeled for the 10% percentile and 50% percentile of each domain score.
Cross-tabulating the “old” (5-domain) MCI cognitive classification with the “new” (6-domain) classification including social cognition (Table 3), 43 individuals who were normal under the 5-domain classification (9.0% of the previously normal, 4.8% of the total sample) were MCI under the 6-domain classification, while 34 individuals changed classification from non-amnestic single domain MCI to non-amnestic multi-domain MCI.
Table 3.
Cross tabulation of “old” (5-domain) and “new: (6-domain) cognitive classifications, N=891*
| New Cognitive Classification (6 domains) | |||||||
|---|---|---|---|---|---|---|---|
| Severe Cognitive Impairment | Amnestic MCI focal | Amnestic MCI multiple | Non-amnestic MCI focal | Non-amnestic MCI multiple | Normal Cognition | ||
| Old Cognitive Classification (5 domains) | Severe Cognitive impairment | 54 | 0 | 0 | 0 | 0 | 0 |
| Amnestic MCI focal | 0 | 24 | 5 | 0 | 0 | 0 | |
| Amnestic MCI multiple | 3 | 0 | 48 | 0 | 0 | 0 | |
| Non-amnestic MCI focal | 0 | 0 | 0 | 139 | 34 | 0 | |
| Non-amnestic MCI multiple | 4 | 0 | 0 | 0 | 87 | 0 | |
| Normal Cognition | 0 | 0 | 0 | 43 | 0 | 450 | |
11 out of 902 participants had missing data in two or more cognitive domains.
In logistic regression models (Table 4), compared to the 260 individuals with 5-domain MCI, the 43 individuals with 6-domain MCI had significantly higher MMSE scores. Compared to the 450 individuals who were normal under both classifications, these 43 individuals were significantly more likely to be impaired in CDR home and hobbies, and to have higher GAD-7 scores (and lower WTAR scores. The sample sizes for the two regression models are not large, and none of the above results remained significant after multiple comparison adjustment.
Table 4.
Association between individual-level characteristics and MCI reclassifycation from old (5-domain) to new (6-domain) MCI definition
| Main covariate* | Y = Normal to MCI (n = 43) vs. MCI to MCI (n =260) | Y = Normal to MCI (n = 43) vs. Normal to Normal (n = 450) | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Female (ref: male) | 0.65 (0.33,1.27) | 0.202 | 0.88 (0.47,1.68) | 0.698 |
| Education > High School (ref: <=High School) | 1.64 (0.80,3.60) | 0.191 | 1.57 (0.79,3.37) | 0.220 |
| Race = White (ref: non-white) | 2.01 (0.65,8.85) | 0.281 | 0.27 (0.07,1.26) | 0.059 |
| CDR > 0 (ref: CDR = 0) | 0.88 (0.76,0.98) | 0.999 | 2.10 (0.75,5.05) | 0.122 |
| CDR Memory > 0 (ref: CDR = 0) | 0.83 (0.30,2.02) | 0.707 | 2.10 (0.75,5.05) | 0.122 |
| CDR Orientation > 0 (ref: CDR = 0) | 1.21 (0.18,4.90) | 0.812 | 2.52 (0.37,10.38) | 0.252 |
| CDR Home and Hobbies > 0 (ref: CDR = 0) | 1.62 (0.34,5.72) | 0.488 | 9.86 (1.80,50.11) | 0.005 |
| CDR Sum of Boxes | 0.68 (0.23,1.35) | 0.370 | 1.79 (0.64,4.10) | 0.203 |
| MMSE | 1.33 (1.09,1.66) | 0.009 | 0.94 (0.78,1.16) | 0.547 |
| WTAR | 1.02 (0.99,1.05) | 0.132 | 0.97 (0.94,1.00) | 0.032 |
| GAD7 | 1.02 (0.93,1.12) | 0.619 | 1.12 (1.02,1.23) | 0.013 |
| mCES-D | 1.00 (0.88,1.13) | 0.986 | 1.11 (0.99,1.24) | 0.057 |
| Subjective Cognitive Complaints | 0.99 (0.86,1.11) | 0.843 | 1.11 (0.98,1.24) | 0.091 |
Adjusting covariates in each of the 13 logistic regression models include age and new vs. old cohort indicator.The p-values are derived from Z test.
Abbreviations: MMSE (Mini-mental Status Exam), WTAR (Wechsler Test of Adult Reading), GAD-7 (Generalized Anxiety Disorder brief scale), mCES-D (modified Center for Epidemiological Studies-Depression scale)
DISCUSSION
Social cognition has been established as a distinct cognitive process separable from general cognitive abilities based on both behavioral and neurobiological evidence.3,28 It is affected in various neurocognitive disorders including FTLD, AD, Parkinson’s disease, dementia with Lewy bodies, and dementia due to vascular disease,5,6 and therefore included as core cognitive domains in DSM-57.
In clinical settings, impaired social cognition is typically identified by family reports of changes in patients’ behavior, judgment, and “personality,” such as disinhibition and loss of empathy, rather than by standardized tests administered to the patient. Well-known standardized informant questionnaires, such as the IQCODE29, lack social cognition items. While patients do not typically view these behavior changes in themselves as deficits, or spontaneously complain of them to health care providers, they will often acknowledge them as present if asked30. A standardized functional domain corresponding to social cognition, along the lines suggested by DSM-5, would be a useful addition to our assessment toolbox, particularly for patients in whom memory loss is not the first neurocognitive deficit to appear.
In a population-based cohort of older adults, we standardized and combined two tests of social cognition into a single composite score to examine its distribution and associated factors. We found better social cognition among participants likely to be younger (including those in our “new” cohort) and female, consistent with previous studies10,31, and among those with higher education and reading levels. They also scored higher on the MMSE and had fewer depressive and anxiety symptoms and subjective cognitive complaints. As previously described, the close association between social cognition and general cognitive abilities likely reflects its relationship with processing speed, executive function, and working memory32–34. Social cognitive deficits have been reported in major depressive disorder, but reports on its association with anxiety are mixed.
Participants with better social cognition had lower Clinical Dementia Ratings both globally and in several functional domains. Notably, we found associations with CDR sum-of-boxes and global CDR = 0.5 (MCI) but not with global CDR ≥1 (dementia). Social cognition was associated with the functional domains of judgment and home and hobbies at the CDR=0.5 level, and with the functional domains of judgment and memory at the CDR=1 level.
Considering neuropsychologically defined MCI, the inclusion of social cognition as a sixth domain of cognition resulted in about 5% of the sample becoming newly classified as MCI. These individuals differed from those already classified as MCI only in having higher MMSE scores; but they differed from those still classified as normal in having more anxiety symptoms, lower reading levels, and impairment in the CDR domain of home and hobbies. One of few previous population-based studies assessing social cognition is the LIFE-Adult study in Leipzig35, which used DSM-5 diagnostic criteria for mild neurocognitive disorder (equivalent to MCI). Note the MYHAT purely neuropsychological definition of MCI, unlike DSM-5, does not take into account subjective concerns, functional independence, or the presence of other mental disorders. Further, the average age of LIFE-Adult study cohort was around 70 years, including 44% women, compared to about 77 years in MYHAT with 62% women. With those caveats, among MYHAT participants free of dementia, the prevalence of MCI (with the 6-domain classification) was double (45.8 % vs 22%) the prevalence of miNCD observed in LIFE-Adult. However, in MYHAT, 11.3% of individuals with MCI (compared to 11.7% in LIFE-Adult) and 5.2% (compared to 2.6% in LIFE-Adult) of all individuals without dementia, had single-domain MCI due to social cognition impairment alone. These values are remarkably close, given the differences in age range, sex ratio, and diagnostic criteria between the two studies. In the Australian PATH study36 an algorithm to generate DSM-5 diagnoses increased by 19% the proportion classified as MCI, with the new cases marked by poorer social cognition.
Increasing the estimated prevalence of MCI (as per the cognitive classification) is only beneficial if it reflects identifying otherwise undetected cases of true MCI. To avoid circularity, we used the purely functional definition of MCI reflected in a global CDR rating of 0.5. We found that social cognition had sensitivity, specificity for CDR that was comparable to those of the five conventional cognitive domains of attention, executive functions, language, memory, and visuospatial function. While our AUC values in the 0.6 – 0.7 range are lower than others in the literature, earlier studies involved some circularity by using MCI definitions that included objective cognitive impairment; further, they did not include tests of social cognition37,38. Even so, in those studies, especially in older age groups, AUCs of various neuropsychological tests ranged from 0.53 to 0.84, with AUCs less than 0.7 in 9 out of 14 tests38.
The cognitive domain of social cognition, as we have defined it, has face validity in that its component measures in our study clearly assess awareness of social norms and affective empathy. Regarding convergent validity, we found social cognition was associated with the other cognitive domains, and with the CDR, and with the CDR functional domain of judgment. Further, the proportions of our cohort with social cognition impairment were comparable to those in the Leipzig study. However, we are unable to meaningfully ascertain criterion validity because we lack an external “gold standard” for assessing its validity in an older population-based sample. The CDR, our standard functional measure of MCI and dementia, having been designed primarily to rate individuals on the Alzheimer’s disease spectrum, is memory-centric and does not adequately assess social cognition.
Subjective concerns about social cognition would be useful to articulate in future clinical research. Previous population-based studies 35,36 which operationalized DSM-5 criteria did not include subjective concerns about social cognition. Our own self-reported subjective concerns questionnaire does not include social cognition items, and neither does the self-report Memory Assessment Questionnaire (MAC-C)39 used in the Australian study. There appear to be no standard self-report questions40, and also no intuitively obvious questions that clinicians routinely ask patients, about self-perceived difficulties with social cognition. However, the Dysexecutive Questionnaire (DEX)40 regarding perceived difficulties with executive function, and the Frontal Systems Behavior Scale (FrSBe)41 do include some items which could be classified under social cognition. In our study, individuals with intact social cognition reported fewer subjective cognitive concerns. Subjective concerns reflect meta-cognition, i.e., awareness of one’s own cognitive deficits, while social cognition at least in part indicates awareness of others’ perspectives and feelings. In a study of patients with Huntington’s disease, in which social cognition is impaired, individuals with moderate disease had poor awareness of their memory deficits while those with mild disease had better awareness of the same42. We did conduct a post-hoc exploratory analysis (data not shown) to determine whether social cognition moderated the association between subjective cognitive concerns and objective memory deficits but found no significant effect.
Our observed associations of social cognition with education and literacy, and with race/ancestry should be interpreted cautiously. The impact of education could merely reflect its relevance to cognitive test performance in general but might also reflect the socialization components of education. Further, as both SNQ-22 and RMET-10 rely on participants’ vocabulary and use culturally based stimuli, the critique has been made that the test’s results are influenced by social class and culture. The SNQ-22 is based on social norms of the prevailing culture8 and current generation of older adults, limiting our ability to apply the test across generational and national/ethnic subgroups. Further, when interpreting RMET-10 scores, the “Other-Race Effect” is relevant; people consistently display worse recognition memory for other-race faces compared to same-race faces43. Taken together, social cognition measures should be used judiciously in ethnically diverse populations. Further follow-up of this cohort will enable us to determine whether impaired social cognition predicts subsequent incident dementia and decline in other cognitive domains.
Our large, population-based cohort provides sufficient statistical power with minimal selection bias. All data reported were obtained directly from participants. Due in part to the demographic characteristics of older adults in our study region, only 6% of our study participants were of non-European ancestry. Therefore, our findings should be replicated in other cohorts with greater minority representation. As this was a cross-sectional study, inferences regarding the directions of the associations and trajectory of social cognition must await prospective follow-up of the cohort. Meanwhile, given the lack of self-report questions that reliably identify potential deficits or changes in social cognition, it appears a worthwhile consideration for standard assessments to routinely include brief objective assessments of this domain.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank all MYHAT study members for their participation and all MYHAT study staff for their efforts.
DISCLOSURE/CONFLICT OF INTEREST
The word reported here was supported in part by research grant # R37AG023657 from the National Institute on Aging, National Institutes of Health, US Department of Health and Human Services.
Dr. Ganguli reports grants from National Institute on Aging during the conduct of the study. Dr. Lee, Ms. Jia, Dr. Snitz, and Dr. Chang report no conflicts with any product mentioned or concept discussed in this article.
The data in this paper has never been presented or published.
REFERENCES
- 1.Fiske ST. Social cognition and social perception. Annu Rev Psychol. 1993;44:155–94. doi: 10.1146/annurev.ps.44.020193.001103 [DOI] [PubMed] [Google Scholar]
- 2.Forbes CE, Grafman J. The role of the human prefrontal cortex in social cognition and moral judgment. Annu Rev Neurosci. 2010;33:299–324. doi: 10.1146/annurev-neuro-060909-153230 [DOI] [PubMed] [Google Scholar]
- 3.Cosentino S, Zahodne LB, Brandt J, et al. Social cognition in Alzheimer’s disease: a separate construct contributing to dependence. Alzheimers Dement. Nov 2014;10(6):818–26. doi: 10.1016/j.jalz.2013.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cacioppo S, Capitanio JP, Cacioppo JT. Toward a neurology of loneliness. Psychol Bull. Nov 2014;140(6):1464–504. doi: 10.1037/a0037618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henry JD, von Hippel W, Molenberghs P, Lee T, Sachdev PS. Clinical assessment of social cognitive function in neurological disorders. Nature Reviews Neurology. 2016/01/01 2016;12(1):28–39. doi: 10.1038/nrneurol.2015.229 [DOI] [PubMed] [Google Scholar]
- 6.Shany-Ur T, Rankin KP. Personality and social cognition in neurodegenerative disease. Curr Opin Neurol. Dec 2011;24(6):550–5. doi: 10.1097/WCO.0b013e32834cd42a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diagnostic and statistical manual of mental disorders: DSM-5™, 5th ed. Diagnostic and statistical manual of mental disorders: DSM-5™, 5th ed. American Psychiatric Publishing, Inc.; 2013:xliv, 947-xliv, 947. [Google Scholar]
- 8.Kramer JH, Mungas D, Possin KL, et al. NIH EXAMINER: conceptualization and development of an executive function battery. J Int Neuropsychol Soc. Jan 2014;20(1):11–9. doi: 10.1017/s1355617713001094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganguli M, Sun Z, McDade E, et al. That’s Inappropriate! Social Norms in an Older Population-based Cohort. Alzheimer Dis Assoc Disord. Apr-Jun 2018;32(2):150–155. doi: 10.1097/wad.0000000000000224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test Revised Version: A Study with Normal Adults, and Adults with Asperger Syndrome or High-functioning Autism. Journal of Child Psychology and Psychiatry. 2001/02/01 2001;42(2):241–251. doi: 10.1111/1469-7610.00715 [DOI] [PubMed] [Google Scholar]
- 11.Olderbak S, Wilhelm O, Olaru G, Geiger M, Brenneman MW, Roberts RD. A psychometric analysis of the reading the mind in the eyes test: toward a brief form for research and applied settings. Front Psychol. 2015;6:1503. doi: 10.3389/fpsyg.2015.01503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S, Jacobsen EP, Jia Y, Snitz BE, Chang C-CH, Ganguli M. Reading the Mind in the Eyes: A Population-Based Study of Social Cognition in Older Adults. The American Journal of Geriatric Psychiatry. 2020/12/01/2020; doi: 10.1016/j.jagp.2020.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganguli M, Chang CC, Snitz BE, Saxton JA, Vanderbilt J, Lee CW. Prevalence of mild cognitive impairment by multiple classifications: The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) project. Am J Geriatr Psychiatry. Aug 2010;18(8):674–83. doi: 10.1097/JGP.0b013e3181cdee4f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganguli M, Snitz B, Vander Bilt J, Chang CC. How much do depressive symptoms affect cognition at the population level? The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) study. Int J Geriatr Psychiatry. Nov 2009;24(11):1277–84. doi: 10.1002/gps.2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. Nov 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 16.Mungas D, Marshall SC, Weldon M, Haan M, Reed BR. Age and education correction of Mini-Mental State Examination for English and Spanish-speaking elderly. Neurology. Mar 1996;46(3):700–6. doi: 10.1212/wnl.46.3.700 [DOI] [PubMed] [Google Scholar]
- 17.Ganguli M, Bilt JV, Lee CW, et al. Cognitive test performance predicts change in functional status at the population level: the MYHAT Project. J Int Neuropsychol Soc. Sep 2010;16(5):761–70. doi: 10.1017/s1355617710000561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganguli M, Snitz BE, Lee CW, Vanderbilt J, Saxton JA, Chang CC. Age and education effects and norms on a cognitive test battery from a population-based cohort: the Monongahela-Youghiogheny Healthy Aging Team. Aging Ment Health. Jan 2010;14(1):100–7. doi: 10.1080/13607860903071014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wechsler D. Wechsler Test of Adult Reading (WTAR). The Psychological Corporation; 2001. [Google Scholar]
- 20.Radloff LS. The CES-D Scale:A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 21.Ganguli M, Gilby J, Seaberg E, Belle S. Depressive Symptoms and Associated Factors in a Rural Elderly Population: The MoVIES Project. The American Journal of Geriatric Psychiatry. 1995/03/01/ 1995;3(2):144–160. doi: 10.1097/00019442-199500320-00006 [DOI] [PubMed] [Google Scholar]
- 22.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. May 22 2006;166(10):1092–7. doi: 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 23.Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. Jul 13 2004;63(1):115–21. doi: 10.1212/01.wnl.0000132523.27540.81 [DOI] [PubMed] [Google Scholar]
- 24.Snitz BE, Yu L, Crane PK, Chang CC, Hughes TF, Ganguli M. Subjective cognitive complaints of older adults at the population level: an item response theory analysis. Alzheimer Dis Assoc Disord. Oct–Dec 2012;26(4):344–51. doi: 10.1097/WAD.0b013e3182420bdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. Nov 1993;43(11):2412–4. doi: 10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- 26.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. Sep 2004;256(3):240–6. doi: 10.1111/j.1365-2796.2004.01380.x [DOI] [PubMed] [Google Scholar]
- 27.R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2020. https://www.R-project.org/ [Google Scholar]
- 28.Gur RC, Gur RE. Social cognition as an RDoC domain. Am J Med Genet B Neuropsychiatr Genet. Jan 2016;171b(1):132–41. doi: 10.1002/ajmg.b.32394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. Feb 1994;24(1):145–53. doi: 10.1017/s003329170002691x [DOI] [PubMed] [Google Scholar]
- 30.Shany-Ur T, Lin N, Rosen HJ, Sollberger M, Miller BL, Rankin KP. Self-awareness in neurodegenerative disease relies on neural structures mediating reward-driven attention. Brain. Aug 2014;137(Pt 8):2368–81. doi: 10.1093/brain/awu161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henry JD, Phillips LH, Ruffman T, Bailey PE. A meta-analytic review of age differences in theory of mind. Psychology and Aging. 2013;28(3):826–839. doi: 10.1037/a0030677 [DOI] [PubMed] [Google Scholar]
- 32.Moran JM, Jolly E, Mitchell JP. Social-Cognitive Deficits in Normal Aging. The Journal of Neuroscience. 2012;32(16):5553–5561. doi: 10.1523/jneurosci.5511-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandoz M, Démonet JF, Fossard M. Theory of mind and cognitive processes in aging and Alzheimer type dementia: a systematic review. Aging Ment Health. Sep 2014;18(7):815–27. doi: 10.1080/13607863.2014.899974 [DOI] [PubMed] [Google Scholar]
- 34.Kessels RPC, Waanders-Oude Elferink M, van Tilborg I. Social cognition and social functioning in patients with amnestic mild cognitive impairment or Alzheimer’s dementia. J Neuropsychol. Jun 2021;15(2):186–203. doi: 10.1111/jnp.12223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luck T, Then FS, Schroeter ML, et al. Prevalence of DSM-5 Mild Neurocognitive Disorder in Dementia-Free Older Adults: Results of the Population-Based LIFE-Adult-Study. Am J Geriatr Psychiatry. Apr 2017;25(4):328–339. doi: 10.1016/j.jagp.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 36.Eramudugolla R, Mortby ME, Sachdev P, Meslin C, Kumar R, Anstey KJ. Evaluation of a research diagnostic algorithm for DSM-5 neurocognitive disorders in a population-based cohort of older adults. Alzheimers Res Ther. Mar 4 2017;9(1):15. doi: 10.1186/s13195-017-0246-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serna A, Contador I, Bermejo-Pareja F, et al. Accuracy of a Brief Neuropsychological Battery for the Diagnosis of Dementia and Mild Cognitive Impairment: An Analysis of the NEDICES Cohort. J Alzheimers Dis. 2015;48(1):163–73. doi: 10.3233/jad-150086 [DOI] [PubMed] [Google Scholar]
- 38.Takuya Yagi DI, Daisuke Sugiyama, Iwasawa Satoko, Tabuchi Hajime, Konishi Mika, Araki Machiko, Saitoh Naho, Nihei Yoshihiro, Mimura Masaru, Suzuki Norihiro. Diagnostic accuracy of neuropsychological tests for classification of dementia. Neurology Asia. 2016;21(1):47–54. [Google Scholar]
- 39.Crook TH 3rd, Feher EP, Larrabee GJ Assessment of memory complaint in age-associated memory impairment: the MAC-Q. Int Psychogeriatr. Fall 1992;4(2):165–76. doi: 10.1017/s1041610292000991 [DOI] [PubMed] [Google Scholar]
- 40.Wilson BA, Alderman N, Burgess PW, Emslie H, Evans JJ. BADS: Behavioural assessment of the dysexecutive syndrome. Pearson; 1996. [Google Scholar]
- 41.Grace J. Frontal systems behavior scale: professional manual. Psychological Assessment Resources. 2001; [Google Scholar]
- 42.Cleret de Langavant L, Fénelon G, Benisty S, Boissé MF, Jacquemot C, Bachoud-Lévi AC. Awareness of memory deficits in early stage Huntington’s disease. PLoS One. 2013;8(4):e61676. doi: 10.1371/journal.pone.0061676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindsay DS, Jack PC, Christian MA. Other-race face perception. Journal of Applied Psychology. 1991;76(4):587–589. doi: 10.1037/0021-9010.76.4.587 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.