Abstract
MicroAbstract:
Effective treatment strategies after immunotherapy are greatly needed in NSCLC. The time off platinum chemotherapy (platinum-free-interval) is one predictor used to select patients for platinum re-exposure in other malignancies, but is not known in NSCLC. We analyzed a retrospective cohort of 751 patients who underwent this approach and did not find that the platinum-free-interval predicted better outcomes after platinum re-exposure.
Clinical Practice Points:
Small studies in the era prior to NSCLC immunotherapy have identified a platinum-free-interval of approximately 6 months as predictive of better outcomes with platinum chemotherapy re-treatment. However, in the era of immunotherapy, where patients are on 1st-line therapy and maintenance for a longer period of time, the optimal platinum-free-interval has not been evaluated. Unlike other malignancies, we did not find an optimal platinum-free-interval in this setting that predicts benefit from platinum re-exposure. This may have implications on the design of clinical trials evaluating treatments in the salvage setting as well as treatment decisions in patients after disease progression on chemoimmunotherapy.
Introduction:
Immunotherapy has prolonged the time that NSCLC patients are off platinum-based (PB) chemotherapy. However, the significance of the platinum-free-interval (PFI) is unclear. We evaluated whether an optimal PFI exists in NSCLC for PB re-exposure in contemporary treatment settings.
Methods:
We conducted a retrospective cohort study of patients with metastatic NSCLC treated with 1st-line PB chemotherapy with or without immunotherapy. Using multivariable Cox models stratified by treatment strategies, we evaluated whether salvage PB vs nonPB chemotherapy resulted in superior outcomes and whether this was modulated by the PFI.
Results:
751 patients treated with salvage chemotherapy after PB chemoimmunotherapy were identified in 2 treatment strategy cohorts: 3rd-line after sequential chemotherapy and immunotherapy (Sequential Chemo IO, n=604); 2ndline after chemoimmunotherapy (Concurrent ChemoIO, n=147). An optimal PFI of 5 and 6 months was identified in the Sequential Chemo IO and Concurrent ChemoIO cohorts, but there was no overall survival or progression free survival advantage for PB vs nonPB chemotherapy in long or short PFI groups.
Conclusions:
An optimal PFI was identified in this contemporary NSCLC cohort treated with two common immunotherapy-containing treatment approaches, but PFI threshold did not predict benefit from platinum re-exposure as it has in other malignancies.
Keywords: Non-small cell lung cancer; Immunotherapy; Platinum Chemotherapy; PB, Platinum-based chemotherapy; PFI, Platinum-free-interval
Introduction
Immunotherapy is now central to the standard of care for advanced non-small cell lung cancer (NSCLC). Although a prolonged duration of response occurs in some patients, most patients will ultimately experience disease progression and require subsequent chemotherapy (non-platinum or platinum-based [PB]). Indeed, immunotherapy efficacy may allow a longer time off PB chemotherapy, potentially rendering some patients sensitive to PB chemotherapy upon re-exposure. Thus a need exists to better understand chemotherapy re-exposure strategies and associated treatment outcomes in the immunotherapy era.
While the benefits of PB re-exposure after a 3–6 month platinum-free interval (PFI) is well established in managing patients with advanced small cell lung cancer (SCLC) and ovarian cancer, data defining an optimal PFI in NSCLC are very limited. 1–3 Two small retrospective studies (N= 11 and 22) assessing disease progression after a 6-month PFI demonstrated a favorable median progression free survival (mPFS: 8mo, 5.6 mo) and median overall survival (mOS: 10.4 mo) for PB re-exposure compared to other second-line options.4,5 A larger prospective study (NVALT7) randomized 240 patients with NSCLC and progressive disease ≥ 3 months from completion of PB chemotherapy to pemetrexed with or without carboplatin. The re-introduction of carboplatin resulted in an improvement in mPFS (4.2 mo vs 2.8 mo HR 0.67; p=0.005), but was not associated with an OS improvement. However, analysis of a sub-group of patients with a PFI of 6 months or greater revealed an OS benefit for PB re-exposure (p= 0.001).6
There may also be synergy between PB re-exposure and recent immunotherapy. In a small retrospective Korean study of 73 patients the overall response rate (ORR) was superior for PB over non-PB administered after immunotherapy (66.7% vs. 46.9%, respectively). In addition, the sequence of therapy mattered: an ORR benefit was observed for PB-therapy given after compared to before immunotherapy (66.7% vs 39.5%, p=0.03).7 Additional analyses were limited by the small sample size. Taken together, these limited studies highlight the need to better understand the effects of PFI on subsequent treatment response and ideally refine patient selection for platinum re-exposure in the current era, particularly when immunotherapy may afford longer platinum-free intervals.
Using electronic health record data from a de-identified advanced NSCLC database of mostly community oncology patients at the point of care, we conducted a retrospective cohort study of patients with NSCLC receiving initial chemotherapy with or without immunotherapy followed by subsequent chemotherapy at progression. We assessed the comparative effectiveness of PB versus non-PB chemotherapy at the time of disease progression and estimated the optimal PFI associated with common immunotherapy treatment strategies.
Materials and Methods:
Data Source:
This retrospective observational study utilized Flatiron Health’s nationwide longitudinal, de-identified database derived from electronic health record (EHR) data from approximately 280 US cancer clinics. Data were obtained from the Flatiron Health advanced NSCLC (aNSCLC) cohort of patients treated between 1/1/2011 and 5/1/2019. The de-identified patient-level data in the EHR include structured data (e.g., laboratory values and prescribed drugs) in addition to unstructured data collected via technology-enabled chart abstraction from physician notes and other unstructured documents (e.g., biomarker reports).8,9 Institutional Review Board approval of the study protocol was obtained prior to study conduct and included a waiver of informed consent since patient care was not affected by this retrospective analysis and patient anonymity was maintained. Data provided to third parties were de-identified, and provisions were set up to prevent re-identification to protect patients’ confidentiality.
Study Population:
Subjects were selected for the analytic cohort based on the following eligibility criteria: diagnosis of stage IV NSCLC, administration of 1st-line PB therapy for metastatic disease, and treatment using one of the 2 treatment sequences detailed in Figure 1B. Subjects were not included if the date of disease progression after completion of 1st-line PB chemotherapy was not available or if they did not complete at least 3 administrations of PB chemotherapy without progression. Patients with driver mutations documented at any time in the database were excluded.
Figure 1:
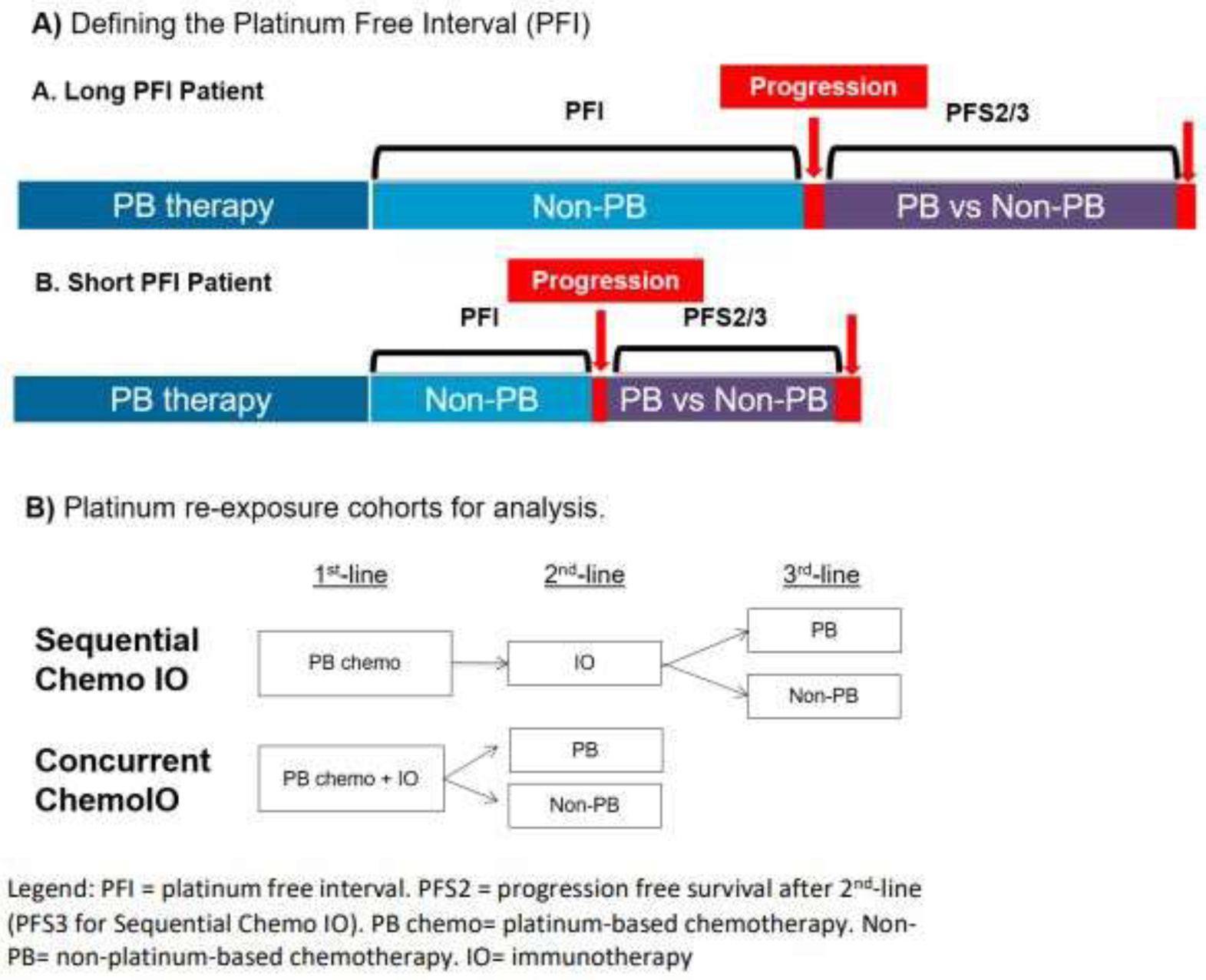
Platinum re-exposure definition and cohorts.
Exposure:
The exposure of interest was PB chemotherapy without immunotherapy in the 2nd or 3rd line setting, depending on the cohort. This was compared to non-PB chemotherapy without immunotherapy. See Supplemental Table 1 for definition of treatment exposures.
Outcomes:
PFI was defined as the time from completion of PB 1st-line therapy not including non-platinum chemotherapy maintenance until disease progression (Figure 1A, Supplemental Table 2). The progression date provided by Flatiron Health was classified as radiologic, pathologic, clinical progression or pseudoprogression. We defined disease progression in our study as cancer progression based on clinical and radiologic features; we excluded pseudoprogression. Use of this real-world definition of disease progression has compared favorably against RECIST-based progression assessments.10 Progression free survival after second-line (PFS2) or third-line (PFS3) therapy and OS were calculated based on date of last visit, progression or death (Figure 1).
Statistical methods:
Descriptive statistics including mean, median and proportions were used to summarize patient demographics and disease characteristics. Chi-square and Kruskal-Wallis analyses were used to assess differences in baseline characteristics between the cohorts for categorical and continuous variables, respectively. Multivariable Cox proportional hazards models stratified by cohorts were used to evaluate the relationships between PB/nonPB exposure and OS or PFS2/3. Median PFS and OS were estimated using Kaplan-Meier methodology stratified by cohort.
The relationship between PFI and PFS2 (PFS3 in Sequential Chemo IO) was assessed in each cohort first using PFI as a continuous exposure variable and PFS2/3 as the outcome in a Cox model. Next, pre-specified cutoffs of PFI < or ≥ 3, 4, 4.5, 5, 6, 9 and 12 months as binary dichotomous variables were tested in separate univariate Cox models with PFI cutoffs as the exposure and PFS2/3 as the outcome. The HRs from these separate models were plotted against the test cutoff points and each cohort was analyzed using the Joinpoint Regression Program to identify the point of changing trends using the Bayesian information criterion (BIC) method (Supplemental Figure 1). 11
This selected cutoff was then added to the multivariable Cox model investigating the relationship between PB/nonPB exposure and PFS2/3 with the dichotomous PFI cutoff variable also included as an interaction term with PB/nonPB. The coefficient and p value of this interaction term was evaluated within the low and high PFI groups to investigate a differential benefit from PB or nonPB chemotherapy depending on the PFI.
Covariates explored in the model include sex, race, age at diagnosis, smoking status, kidney function, Eastern Cooperative Group Performance Status (ECOG PS) at the time of second- or third-line therapy, practice type (academic vs. community), census region and length of PFS1. Sex, age at diagnosis, race (white vs non-white), and kidney function were used in the multivariate model. Patients with missing values in multivariate analysis were excluded (Table 2). The proportional-hazards assumptions were upheld as tested by the Schoenfeld residual testing on the multivariate and univariate (PFI cutoffs) Cox Proportional Hazards models (Table 2, Figure 3).
Table 2:
Multivariate analysis of the effect the platinum free interval on PFS (A) and OS (B) after re-exposure to platinum vs. non-platinum chemotherapy
| A) Progression Free Survival | ||||||
| Sequential Chemo IO | Concurrent ChemoIO | |||||
| Chemo ➔ IO ➔ Chemo N=546 | ChemoIO ➔ Chemo N=137 | |||||
| Variable | HR | 95% CI | p | HR | 95% CI | p |
| PB vs. non-PB | 1.36 | 0.97–1.9 | 0.08 | 1.37 | 0.69–2.8 | 0.37 |
| PFI ≥ 5 mo vs. < 5 mo | 0.76 | 0.58–1.0 | 0.04 | |||
| PFI ≥6 mo vs. <6 mo | 1.7 | 0.81–3.5 | 0.16 | |||
| Sex, male vs. female | 1.0 | 0.8–1.3 | 0.9 | 1.12 | 0.64–2 | 0.69 |
| Age diagnosis ≥70 vs. <70 yrs old | 0.88 | 0.7–1.1 | 0.3 | 0.86 | 0.5–1.6 | 0.62 |
| Race, white vs. non-white | 0.99 | 0.78–1.3 | 0.95 | 1.07 | 0.6 –1.95 | 0.8 |
| Kidney function | 0.96 | 0.8 –1.2 | 0.7 | 1.1 | 0.5 –2.6 | 0.85 |
| Among PFI long | PFI ≥ 5 mo | PFI ≥6 mo | ||||
| PB vs. non-PB | 0.8 | 0.6–1.1 | 0.2 | 0.6 | 0.2–1.8 | 0.4 |
| Among PFI short | PFI < 5 mo | PFI <6 mo | ||||
| PB vs. non-PB | 1.36 | 0.97–1.9 | 0.08 | 1.37 | 0.7 –2.8 | 0.37 |
| Among PB | ||||||
| PFI long vs short | 0.44 | 0.3–0.66 | 0.000 | 0.8 | 0.3–2.1 | 0.6 |
| Among non-PB | ||||||
| PFI long vs short | 0.76 | 0.6–0.99 | 0.043 | 1.7 | 0.8–4 | 0.2 |
| B) Overall Survival | ||||||
| Sequential Chemo IO | Concurrent ChemoIO | |||||
| Chemo ➔ IO ➔ Chemo N=546 | ChemoIO ➔ Chemo N=137 | |||||
| Variable | HR | 95% CI | p | HR | 95% CI | p |
| PB vs. non-PB | 0.88 | 0.65–1.2 | 0.4 | 1.17 | 0.65–2.1 | 0.6 |
| PFI ≥ 5 mo vs. < 5 mo | 0.64 | 0.5–0.8 | 0.000 | |||
| PFI ≥6 mo vs. <6 mo | 0.5 | 0.21–1.2 | 0.11 | |||
| Sex, male vs. female | 1.23 | 1.01–1.5 | 0.038 | 1.17 | 0.71–1.9 | 0.5 |
| Age diagnosis ≥70 vs. <70 yrs old | 1.2 | 0.99–1.5 | 0.067 | 1.1 | 0.67–1.9 | 0.7 |
| Race, white vs. non-white | 1.16 | 0.94–1.4 | 0.16 | 1.01 | 0.6–1.7 | 0.97 |
| Kidney function | 0.77 | 0.6–1 | 0.051 | 1.1 | 0.5–2.4 | 0.84 |
| Among PFI long | PFI ≥ 5 mo | PFI ≥6 mo | ||||
| PB vs. non-PB | 0.8 | 0.58–1.2 | 0.19 | 0.73 | 0.2–2.6 | 0.64 |
| Among PFI short | PFI < 5 mo | PFI <6 mo | ||||
| PB vs. non-PB | 0.88 | 0.65–1.2 | 0.4 | 1.17 | 0.65–2.1 | 0.6 |
| Among PB | ||||||
| PFI long vs short | 0.59 | 0.4–0.86 | 0.007 | 0.3 | 0.1–0.95 | 0.04 |
| Among non-PB | ||||||
| PFI long vs short | 0.64 | 0.5–0.8 | 0.000 | 0.5 | 0.21–1.2 | 0.11 |
Legend: Chemo = chemotherapy, IO= immunotherapy, ChemoIO= combination chemoimmunotherapy, yrs= years, HR= hazard ratio, CI= confidence interval. Kidney function was assessed by creatinine within 30 days of start of therapy. Subjects with missing values were excluded from the multivariate analysis (Sequential Chemo IO: 58, Concurrent ChemoIO: 10 patients excluded).
Figure 3.
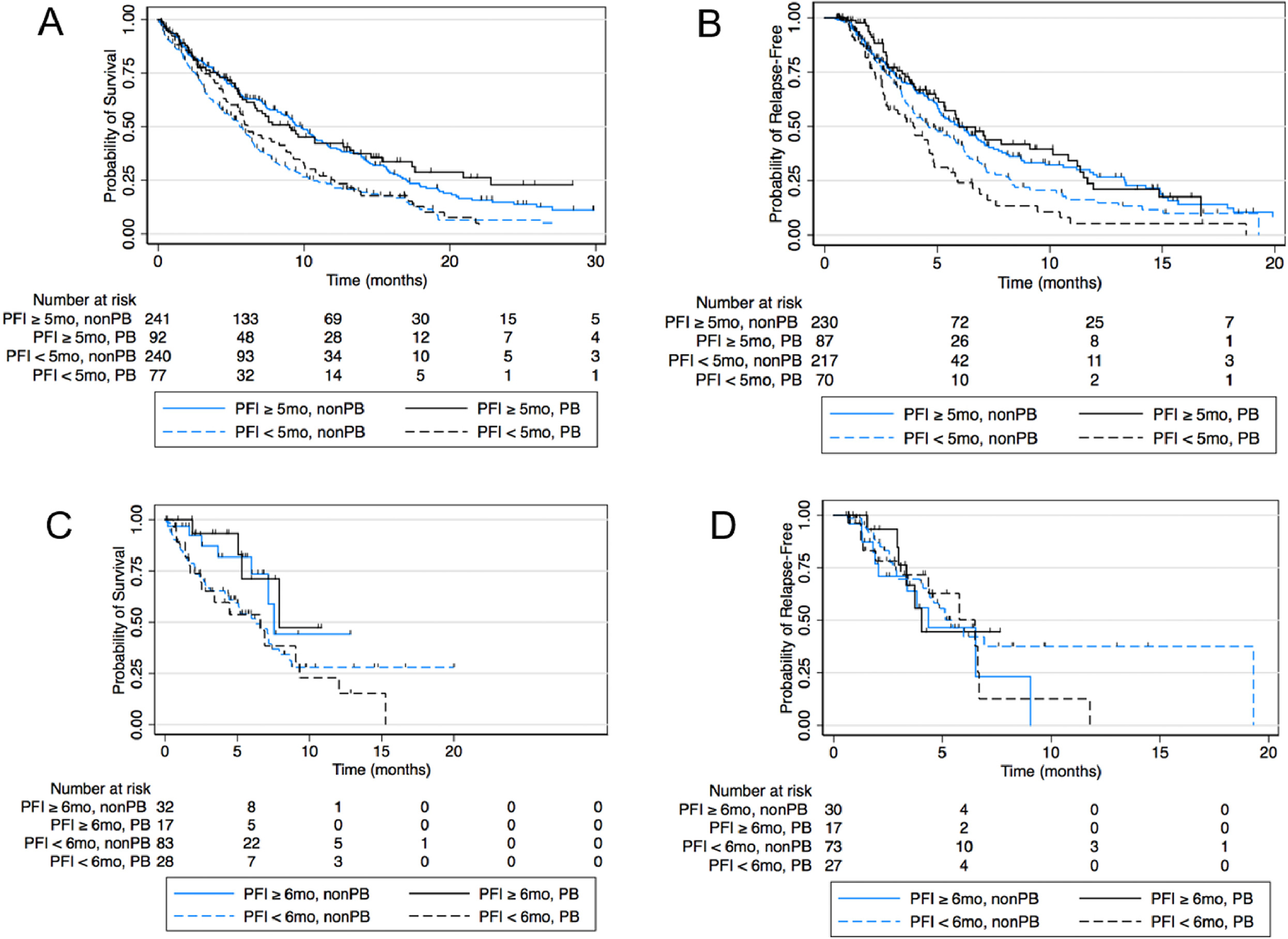
Unadjusted Kaplan Meier curves by PFI cutoffs and plantinum exposure for (A) Sequential ChemoIO OS (B) Sequential ChemoIO PFS (C) Concurrent ChemoIO OS (D) Concurrent ChemoIO PFS.
Results:
Baseline Characteristics by Cohort
Baseline characteristics for the PB vs. nonPB chemotherapy cohorts are detailed in Table 1. Patients who received nonPB chemotherapy instead of PB chemotherapy were younger (60% <70 years old) in the Sequential Chemo IO cohort, but there was no difference in age between the treatment groups in the Concurrent ChemoIO cohort. Sex was well balanced in the Sequential Chemo IO cohort while the PB group had slightly more males (65.9%) compared to the nonPB group (48.5%) in the Concurrent ChemoIO cohort (p=0.053). White vs. non-white race was well balanced between cohorts. Almost all (~90%) patients had a history of smoking. ECOG performance status was well balanced between groups in both cohorts, but there were significant missing data (18.2 – 28.7%) (Table 1).
Table 1:
Baseline Characteristics by Cohort
| Sequential Chemo IO | Concurrent ChemoIO | |||||
|---|---|---|---|---|---|---|
| Chemo ➔ IO ➔ Chemo | ChemoIO ➔ Chemo | |||||
| Variable | nonPB | PB | p | nonPB | PB | p |
| Male, n(%) | 237 (53.0%) | 84 (53.5%) | 0.917 | 50 (48.5%) | 29 (65.9%) | 0.053 |
| Age diagnosis ≥70, n(%) | 178 (39.8%) | 44 (28.0%) | 0.008 | 35 (34.0%) | 21 (47.7%) | 0.116 |
| Race, white, n(%) | 309 (69.1%) | 106 (67.5%) | 0.708 | 72 (69.9%) | 28 (63.6%) | 0.456 |
| Year of advanced diagnosis, n(%) | ||||||
| 2011–2015 | 221 (49.4%) | 83 (52.9%) | 0.315 | 2 (1.9%) | 0 (0.0%) | 0.612 |
| 2016 | 134 (30.0%) | 51 (32.5%) | 0 (0.0%) | 0 (0.0%) | ||
| 2017 | 71 (15.9%) | 20 (12.7%) | 49 (47.6%) | 20 (45.5%) | ||
| 2018 | 21 (4.7%) | 3 (1.9%) | 52 (50.5%) | 24 (54.5%) | ||
| Census Region | ||||||
| Northeast | 84 (20.8%) | 35 (24.1%) | 0.478 | 37 (38.1%) | 13 (31.0%) | 0.438 |
| Midwest | 86 (21.3%) | 24 (16.6%) | 12 (12.4%) | 4 (9.5%) | ||
| South | 169 (41.8%) | 58 (40.0%) | 32 (33.0%) | 20 (47.6%) | ||
| West | 65 (16.1%) | 28 (19.3%) | 16 (16.5%) | 5 (11.9%) | ||
| Payer | ||||||
| Commercial | 170 (38.9%) | 53 (34.2%) | 0.245 | 46 (45.1%) | 21 (48.8%) | 0.562 |
| Government | 137 (31.4%) | 60 (38.7%) | 30 (29.4%) | 9 (20.9%) | ||
| Other | 130 (29.7%) | 42 (27.1%) | 26 (25.5%) | 13 (30.2%) | ||
| Community based practice, n(%) | 410 (91.7%) | 146 (93.0%) | 0.612 | 98 (95.1%) | 42 (95.5%) | 0.936 |
| Non-squamous Histology, n(%) | 308 (68.9%) | 106 (67.5%) | 0.747 | 91 (88.3%) | 38 (86.4%) | 0.737 |
| Stage at diagnosis | ||||||
| I-III | 120 (26.8%) | 51 (32.5%) | 0.263 | 22 (22.0%) | 4 (9.3%) | 0.115 |
| IV | 319 (71.4%) | 105 (66.9%) | 76 (76.0%) | 39 (90.7%) | ||
| Missing | 8 (1.8%) | 1 (0.6%) | 2 (2.0%) | 0 (0.0%) | ||
| History of smoking, n(%) | 402 (89.9%) | 142 (90.4%) | 0.853 | 95 (92.2%) | 40 (90.9%) | 0.788 |
| PD-L1 category | ||||||
| <1% | 75 (16.8%) | 29 (18.5%) | 0.139 | 37 (35.9%) | 14 (31.8%) | 0.455 |
| 1–49% | 57 (12.8%) | 19 (12.1%) | 33 (32.0%) | 10 (22.7%) | ||
| ≥50% | 23 (5.1%) | 16 (10.2%) | 16 (15.5%) | 10 (22.7%) | ||
| Missing | 292 (65.3%) | 93 (59.2%) | 17 (16.5%) | 10 (22.7%) | ||
| PFS1, mean (SD) ‡ | 7.99 (5.9) | 10.56 (9.5) | 0.004 | 5.21 (2.6) | 5.60 (2.6) | 0.39 |
| ECOG performance status at time of nonPB vs. PB chemotherapy | ||||||
| 0 | 84 (18.8%) | 42 (26.8%) | 0.132 | 26 (25.2%) | 17 (38.6%) | 0.268 |
| 1 | 184 (41.2%) | 56 (35.7%) | 43 (41.7%) | 12 (27.3%) | ||
| 2–4 | 55 (12.3%) | 14 (8.9%) | 13 (12.6%) | 7 (15.9%) | ||
| Missing | 124 (27.7%) | 45 (28.7%) | 21 (20.4%) | 8 (18.2%) | ||
| Creatinine, mean (SD)‡ | 0.99 (0.7) | 0.95 (0.3) | 0.668 | 0.88 (0.3) | 0.90 (0.3) | 0.40 |
Legend: Creatinine (mg/dL) is within 30-days prior to PB on nonPB chemotherapy re-exposure.
Kruskal-wallis p value for a continuous variable. PB: platinum-based chemotherapy, nonPB: non-platinum based chemotherapy, SD: standard deviation, ECOG: Eastern Co-operative Group
Most patients (69–88%) had non-squamous NSCLC. PD-L1 levels, when known, were balanced between both cohorts; however, there were significant missing data in the Sequential Chemo IO cohort (~60%) as one would expect given the time period when these patients were treated. PD-L1 was infrequently tested prior to approval of PDL1-based recommendations for pembrolizumab use 12. As expected, the majority of patients treated with Sequential Chemo IO initiated treatment between 2011 and 2015, whereas patients receiving Concurrent ChemoIO initiated treatment after 2017, when 1st line concurrent chemoimmunotherapy became available 13. The mean creatinine levels were well balanced between the PB and nonPB groups. Most patients were primarily treated in the community (92–96%) vs. academic centers.
Survival outcomes after platinum vs. non-platinum chemotherapy re-exposure
Overall survival (OS) and progression free survival (PFS) were calculated starting from the dates of PB vs. nonPB re-exposure. Median follow up time from PB or non-PB exposure in the Sequential Chemo IO and Concurrent ChemoIO cohorts was 16.8 and 5.7 months, respectively. In both the Sequential Chemo IO and Concurrent ChemoIO cohorts, the mOS overall was 7.2 months. In the absence of analysis of PFI, PB chemotherapy was not associated with improved mOS in the Sequential Chemo IO cohort (mOS 7.6 vs 7.2 mo; HR 0.9, p=0.3) or Concurrent ChemoIO (mOS 7.9 vs 7.2 mo; HR 0.7, p=0.9). Similarly, there was no PFS advantage to PB chemotherapy over nonPB chemotherapy in either cohort (Sequential Chemo IO: mPFS 4.9 vs 5.5 mo, HR 1.1, 95% CI 0.86 – 1.4, p=0.5; Concurrent ChemoIO: mPFS 5.8 vs 5.2 mo, HR 1.1, 95% CI 0.64 – 1.95, p= 0.7).
Identification of the optimal platinum free interval prior to platinum re-exposure
First, the association between PFI and PFS2/3 was analyzed using continuous variables. In the Sequential Chemo IO cohort, PFI was significantly associated with PFS3 (HR 0.98, 95% CI 0.96–0.99, p=0.003). In the Concurrent ChemoIO cohort there was no association between PFI and PFS2 (HR 1.01, 95% CI 0.92 – 1.12, p=0.834). Next, the association between PFI and PFS2/3 was analyzed using a Cox Regression model of PFS2/3 as the outcome and pre-specified PFI cutoffs as the exposure. Cox regression HRs at different time points were entered into the Joinpoint analysis software which identified an optimal PFI of 5 months in the Sequential Chemo IO cohort and 6 months in the Concurrent ChemoIO cohort (Supplemental Figure 1).
Modulation of OS benefit of platinum vs non-platinum chemotherapy re-exposure based on length of platinum free interval
There was no significant PFI modulation of PB vs non-PB OS benefit in the Sequential Chemo IO cohort. In a multivariable model evaluating the interaction of PB vs non-PB chemotherapy and PFI group (≥ 5 months, n= 317 vs <5 months n=287), median OS was not different for PB vs nonPB chemotherapy in either PFI group (Long PFI: 9 vs 9.8 mo, HR 0.8, 95% CI 0.58 – 1.2, p =0.19. Short PFI: 6.1 vs 5.6 mo, HR 0.9, 95% CI 0.65 – 1.2, p=0.4, Table 2B). Similarly, in the Concurrent ChemoIO cohort there was no clinically significant PFI modulation of OS for PB vs nonPB chemotherapy (Long PFI: HR 0.73, 95% CI 0.2–2.6, p=0.64,. Short PFI: HR 1.17, 95% CI 0.65 – 2.1, p=0.6, Table 2B). These results are consistent whether confounders are included or excluded from the model. Median OS is not able to be accurately estimated from Kaplan Meier curves in the Concurrent ChemoIO cohort due to limited follow up time (Figure 3).
Long PFI (≥ 5 months) is associated with improved OS regardless of receipt of PB or non-PB chemotherapy. In the Sequential Chemo IO cohort there was an OS benefit for long PFI compared to short among the PB and non-PB groups. In the Concurrent ChemoIO cohort this benefit was only statistically significant among those that received PB chemotherapy (Table 2B).
Modulation of PFS benefit of platinum vs non-platinum chemotherapy re-exposure based on length of platinum free interval
In the Sequential Chemo IO cohort, there was no PFI modulation of PFS for PB vs nonPB chemotherapy (Table 2A). In a multivariable model there was no modulation of PFS3 benefit for PB over non-PB by PFI group (Long PFI: 6 vs 6.2, HR 0.8, 95% CI 0.6 – 1.1, p<0.2. Short PFI: 3.9 vs 4.6 mo, HR 1.36, 95% CI 0.97–1.9, p= 0.08). Results were similar in the Concurrent ChemoIO cohort for PFS2 (Long PFI: HR 0.6, 95% CI 0.2 −1.8, p=0.4. Short PFI: HR 1.37, 95% CI 0.7 – 2.8, p=0.37). There was also no difference seen in the models excluding potential confounders. Median PFS2 is not able to be accurately estimated from Kaplan Meier curves in the Concurrent ChemoIO cohort due to limited follow up time (Figure 3).
Long PFI (≥ 6 months) is associated with improved PFS3 in the Sequential Chemo IO cohort regardless of receipt of PB vs non-PB chemotherapy. Long PFI was not associated with improved PFS2 in the Concurrent ChemoIO cohort in either treatment group (Table 2A).
Discussion:
In this retrospective analysis of patients with advanced NSCLC treated with PB chemotherapy and immunotherapy, we investigated the role of PFI in predicting outcomes after subsequent treatment with PB or non-PB chemotherapy regimens. We identified a PFI cutoff of 5–6 months, depending on the NSCLC treatment sequence, that predicted improved OS and PFS, but did not predict benefit for PB over non-PB chemotherapy as it has in other malignancies. This PFI is in the range seen in other diseases such as SCLC (3 months) and consistent with previous smaller studies conducted in earlier NSCLC cohorts treated with chemotherapy alone.
In the larger Sequential Chemo IO cohort multivariable model there was no OS or PFS benefit for PB over non-PB chemotherapy regardless of PFI. We did observe that patients above the 5-month PFI threshold identified had a significantly better PFS and OS regardless of which subsequent therapy they received (Figure 3, Table 2). This is likely due to the underlying biology of disease that is stable for 5 months or longer without platinum based chemotherapy being less aggressive rather than an effect of treatment.
In the smaller, but more contemporary, Concurrent ChemoIO cohort there was no OS or PFS benefit for PB over non-PB chemotherapy regardless of PFI. We did observe that patients above the 6-month PFI threshold compared to below had improved OS among patients who received PB chemotherapy, but did not reach statistical significance among patients who received subsequent non-PB chemotherapy. A PFS benefit for long PFI was not seen. This may be due to the smaller numbers in this cohort or limited follow up time leading to immature survival curves.
Several potentially important confounders were not included in our analysis due to missing data. Although ECOG status at the time of PB re-exposure was not included in this analysis due to missing data in 27% of patients, there was no statistical difference in the proportions of patients within each ECOG performance score stratum (Chi squared p = 0.132) so there was no evidence to suggest that only patients with excellent performance status were being selected for PB chemotherapy re-exposure. Patients selected for platinum re-exposure did have a longer PFS1 following the original platinum regimen compared to nonPB chemotherapy (PFS1 10.6 mo vs 8 mo, respectively) and therefore may have been selected based on their excellent response to platinum chemotherapy in the first-line. However, patients did not appear to be selected for PB chemotherapy in the Sequential Chemo IO group based on PFI since those who received PB chemotherapy were split between a PFI of <5 months and PFI of ≥ 5 month groups (70 vs 87 patients, respectively).
Despite its retrospective nature, our study does have the largest sample size of any examining PB chemotherapy re-exposure in the immunotherapy era. In addition, most patients were treated in the community and not in academic cancer centers so our observed results may be generalized to most patients receiving lung cancer treatment in real world practice. While we did not identify a PFI that predicts benefit from platinum re-exposure the PFI identified does delineate two groups with likely different tumor biology. This could be helpful in the design of clinical trials evaluating salvage therapies after Sequential Chemo IO and Concurrent ChemoIO strategies. For example, if one treatment arm contained more patients with short PFI then they may be expected to do worse regardless of the efficacy of the treatment. While randomization can help account for these unmeasured confounders, in small (n<100) clinical trials evaluating new therapies randomization may not adequately control for this difference. While we did not see a benefit for PB over non-PB chemotherapy in OS or PFS, the patient experience on PB chemotherapies may be superior to some of the salvage options available (e.g docetaxel based regimens). The toxicity profile of platinum-based chemotherapies can avoid hair loss and have a lower risk of neuropathy. Therefore, PB re-exposure may still be a viable option for some patients and we did not find that survival results were inferior with this approach.
In summary, we found that in NSCLC PFI is not predictive of benefit of PB re-exposure as it is in SCLC. The PFI threshold identified does delineate potentially different tumor biology that may be useful in analyzing clinical trials evaluating salvage therapies after chemoimmunotherapy. Ultimately, this study is hypothesis generating and should be further evaluated in a larger dataset, in particular for the Concurrent ChemoIO cohort, with longer follow up time.
Supplementary Material
Funding
This work was supported by a NIH Cancer Epidemiology T32 grant (CA009679).
Footnotes
Conflict of interest
Melina E. Marmarelis reports researching funding from Eli Lilly (Inst), Trizell (Inst), AstraZeneca (Inst); consulting role with Astra Zeneca, Novocure, Boehringer Ingelheim; stock in Gilead Sciences, Portola Pharmaceuticals, Merck, Bluebird Bio, Johnson & Johnson, Pfizer; previous medical writing support from Novartis. Charu Aggarwal reports serving as an advisory board member for AZ, Bluprint, Celgene, Eli Lilly, Merck, Daichi Sankyo and research funding to institution from Merck, Macrogenics, Novartis and AZ. Corey J. Langer reports research funding (all institutional) from Merck, Trizell, Eli Lilly, Takeda, Astra Zeneca, Johnson and Johnson; consultative/advisory roles with Merck, BI, AZ, Genentech/Roche, Takeda, Amgen, Gilead, Regeneron, Novocure, Foundation Medicine, and Pfizer; prior medical writing support from Takeda, Merck and Novartis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Huisman C, Postmus PE, Giaccone G, Smit EF. Second-line chemotherapy and its evaluation in small cell lung cancer. Cancer Treat Rev. 1999;25(4):199–206. [DOI] [PubMed] [Google Scholar]
- 2.Giaccone G Clinical perspectives on platinum resistance. Drugs. 2000;59 Suppl 4:9–17; discussion 37–18. [DOI] [PubMed] [Google Scholar]
- 3.Markman M, Hoskins W. Responses to salvage chemotherapy in ovarian cancer: a critical need for precise definitions of the treated population. J Clin Oncol. 1992;10(4):513–514. [DOI] [PubMed] [Google Scholar]
- 4.Khan K, Hanna GG, Campbell L, et al. Re-challenge chemotherapy with gemcitabine plus carboplatin in patients with non-small cell lung cancer. Chin J Cancer. 2013;32(10):539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Souza PH, Rodrigues AN, Dienstmann R, et al. Outcomes of advanced stage non-small cell lung cancer (NSCLC) patients reexposed to platinum-based chemotherapy upon progression. Journal of Clinical Oncology. 2006;24(18_suppl):17136–17136. [Google Scholar]
- 6.Smit EF, Burgers SA, Biesma B, et al. Randomized Phase II and Pharmacogenetic Study of Pemetrexed Compared With Pemetrexed Plus Carboplatin in Pretreated Patients With Advanced Non–Small-Cell Lung Cancer. Journal of Clinical Oncology. 2009;27(12):2038–2045. [DOI] [PubMed] [Google Scholar]
- 7.Park SE, Lee SH, Ahn JS, Ahn MJ, Park K, Sun JM. Increased Response Rates to Salvage Chemotherapy Administered after PD-1/PD-L1 Inhibitors in Patients with Non-Small Cell Lung Cancer. J Thorac Oncol. 2018;13(1):106–111. [DOI] [PubMed] [Google Scholar]
- 8.Ma X, Long L, Moon S, Adamson BJS, Baxi SS. Comparison of Population Characteristics in Real-World Clinical Oncology Databases in the US: Flatiron Health, SEER, and NPCR. medRxiv. 2020:2020.2003.2016.20037143. [Google Scholar]
- 9.Birnbaum B NN, Seidl-Rathkpof K, Agrawal M, Estevez M, Estola E, Haimson J, He L, Larson P, Richardson P. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. Computers and Society. 2020;arXiv:2011.09765[cx.CY]. [Google Scholar]
- 10.Huang Bartlett C, Mardekian J, Cotter MJ, et al. Concordance of real-world versus conventional progression-free survival from a phase 3 trial of endocrine therapy as first-line treatment for metastatic breast cancer. PLoS One. 2020;15(4):e0227256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. [DOI] [PubMed] [Google Scholar]
- 12.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375(19):1823–1833. [DOI] [PubMed] [Google Scholar]
- 13.Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


