Abstract
Several low-molecular-weight sulfonates were added to microbial mat slurries to investigate their effects on sulfate reduction. Instantaneous production of sulfide occurred after taurine and cysteate were added to all of the microbial mats tested. The rates of production in the presence of taurine and cysteate were 35 and 24 μM HS− h−1 in a stromatolite mat, 38 and 36 μM HS− h−1 in a salt pond mat, and 27 and 18 μM HS− h−1 in a salt marsh mat, respectively. The traditionally used substrates lactate and acetate stimulated the rate of sulfide production 3 to 10 times more than taurine and cysteate stimulated the rate of sulfide production in all mats, but when ethanol, glycolate, and glutamate were added to stromatolite mat slurries, the resulting increases were similar to the increases observed with taurine and cysteate. Isethionate, sulfosuccinate, and sulfobenzoate were tested only with the stromatolite mat slurry, and these compounds had much smaller effects on sulfide production. Addition of molybdate resulted in a greater inhibitory effect on acetate and lactate utilization than on sulfonate use, suggesting that different metabolic pathways were involved. In all of the mats tested taurine and cysteate were present in the pore water at nanomolar to micromolar concentrations. An enrichment culture from the stromatolite mat was obtained on cysteate in a medium lacking sulfate and incubated anaerobically. The rate of cysteate consumption by this enrichment culture was 1.6 pmol cell−1 h−1. Compared to the results of slurry studies, this rate suggests that organisms with properties similar to the properties of this enrichment culture are a major constituent of the sulfidogenic population. In addition, taurine was consumed at some of highest dilutions obtained from most-probable-number enrichment cultures obtained from stromatolite samples. Based on our comparison of the sulfide production rates found in various mats, low-molecular-weight sulfonates are important sources of C and S in these ecosystems.
The sulfur cycle plays an important role in the geomicrobiology of intertidal and coastal sediments (35, 59). In addition to sulfate, sulfide, and various inorganic intermediates, organosulfur compounds constitute an important sulfur pool as well (5, 50). In the pelagic marine environment, dimethyl sulfide and methanethiol are thought to be biologically important species (38) because of their presumed importance in climate feedback models (11). In addition to these organosulfur compounds, organic polysulfides, which result from chemical reactions of sulfide and low-molecular-weight organic molecules, probably constitute a significant reservoir, especially in the benthic environment (40, 46).
The sulfonates are a group of organosulfur compounds that are commonly found in household and industrial wastewater. Linear alkyl sulfonates are major constituents of detergents (58), and the catabolism of these compounds has been well documented (6, 18) and includes carbon-sulfur bond breakage pathways (39, 44). Recently, it has been shown that a range of low-molecular-weight sulfonates are present in the marine environment. A variety of different marine sediments contain significant amounts of sulfonates, which comprise 20 to 40% of the organosulfur pool (65). Unfortunately, the composition of the sulfonate pool was not characterized further in the study of Vairavamurthy et al. (65). However, several sources of sulfonates have been identified in the marine environment. Sulfonates can be the sole source of sulfur or nitrogen for marine phytoplankton (7), and sulfolipids can be major biomembrane constituents in microscopic algae (56, 57), cyanobacteria (2, 26), diatoms (1), and bacteria (24). Sulfonate-containing exopolymers may be present in benthic diatoms and support the gliding motility of these organisms, like suggestions made for bacteria (25). Taurine (2-aminoethanesulfonate) is present in marine diatoms (34) and zooplankton (9), presumably as a major osmolyte. The intracellular concentration of these compounds typically exceeds 0.2 M, which could easily explain the occurrence of sulfonates in the estuarine environment. Similarly, cysteate (alanine 3-sulfonate) is one of the oxidation products of cysteine residues in proteins (55, 60), and isethionate (2-hydroxyethanesulfonate) has been found in marine algae (31). Clearly, many different sources contribute to the pool of low-molecular-weight sulfonates in the marine environment.
Taurine is one of the several amino acids that are readily bioavailable in marine sediments (48). In addition to being used for anabolic purposes, amino acids are catabolized by microbes. Under anoxic conditions, this occurs through fermentation and, more importantly, sulfate reduction (8, 51, 61). The nitrogen-containing sulfonates taurine and cysteate can also be utilized by sulfate-reducing bacteria (SRB) (27, 28, 42–44). Previously, most workers have focused on using amino acids as electron donors, but recently Lie et al. (44) demonstrated that in an SRB strain isolated from a salt marsh both carbon and the sulfur moiety are catabolized. After cleavage of the carbon-sulfur bond, the sulfite that is presumably generated can be reduced to sulfide. Other low-molecular-weight sulfonates, such as isethionate, also support growth of a variety of SRB (41, 42, 44).
Microbial mats, including microbial mats associated with salt marshes and modern marine stromatolites, are typically dominated by a cyanobacterial community near the surface. High rates of primary production are coupled to high rates of aerobic respiration. High rates of aerobic respiration rapidly deplete O2, and the oxic-anoxic interface is therefore found at a depth of a few millimeters (66). As a result, a significant part of the carbon fixed by the cyanobacteria is oxidized by SRB (36, 68). The maximum sulfate reduction rates (SRR) that have been reported for microbial mats are high and range from 126 to 270 μM h−1 in the salt pond mats of Guerrero Negro (10) and from 13 to 26 μM h−1 in temperate intertidal mats (67). Although the level of local production of organic carbon due to photosynthesis is generally high, sulfate reduction is generally thought to be carbon limited (21). Modern marine stromatolite mats have much lower primary production rates than the rates that have been reported for other microbial mats (10, 36, 69), and so carbon limitation of the SRB should be more pronounced in the former mats.
The aims of this study were to investigate whether low-molecular-weight sulfonates stimulate sulfate reduction in stromatolite mats and, if they do, to establish the importance of these compounds as sources of carbon and sulfur. Furthermore, by comparing the effects of these sulfonates on SRR in a variety of mats, we assessed their importance in biogeochemical cycling of carbon and sulfur.
MATERIALS AND METHODS
Site description and field measurements.
The microbial mats associated with modern marine stromatolites which are found in the Exumas (Bahamas) have been described in detail previously (54, 69). Briefly, these laminated lithified structures typically contain a cyanobacterial community that is dominated by Schizothrix spp. in a 0.5- to 1-mm-thick surface layer which is lithified (layer 1). Under this is a layer which is a few millimeters thick, is soft (unlithified), and contains less biomass (layer 2) and then another lithified layer, which is several millimeters thick and is associated with greater microbial biomass (layer 3). Under this, soft and hard layers alternate. The other microbial mats used in this study were Microcoleus-dominated systems; samples of these mats were obtained from salt ponds (salt contents, 72 and 96 ppt) in Guerrero Negro, Mexico (14), and an intertidal salt marsh in Stonington, Conn. The mats from the salt marsh were similar to the mats found in intertidal areas of the North Sea (66, 68).
The stromatolite mats, which were the mats that were investigated in most detail, were collected in March and August 1998 from a depth of 50 to 75 cm (mean high water) and were processed immediately. Salt pond samples were collected in December 1998 and were stored on ice in the dark before experiments were performed. Salt marsh samples were obtained in September 1998 and February 1999, stored on ice, and processed within 1 h.
Porewater samples were collected in situ with 22-gauge needles or were recovered from sectioned cores in the lab by centrifugation (15,000 × g). The samples were filtered (pore size, 0.2 μm) and frozen until the taurine and cysteate concentrations were measured by high-performance liquid chromatography (see below).
Microelectrode measurements were obtained at each site, as described previously (69), by using needle electrodes for O2 and HS− (Diamond General, Ann Arbor, Mich.; Microscale Measurements, Haren, The Netherlands).
We determined the SRR in discrete layers (69). Samples were separated into layers and kept under air (oxic layers) or an N2 atmosphere (anoxic layers). Triplicate samples of each layer were incubated with 1 μCi of 35SO42− (carrier free; Amersham, Chicago, Ill.) under oxic or anoxic conditions for 6 h. Zinc acetate and freezing were used to stop microbial activity, and after this SRR were determined by using the single-step reduction method (20). Based on 24-h measurements of O2 and HS− concentrations (69), diel SRR were calculated by assuming that 14 h of oxic conditions and 10 h of anoxic conditions occurred in the top 1 mm, 10 h of oxic conditions and 14 h of anoxic conditions occurred in the 1- to 3-mm layer, 8 h of oxic conditions and 16 h of anoxic conditions occurred in the 3- to 5-mm layer, and 24 h of anoxic conditions occurred in the 5- to 10-mm layer.
Most-probable-number (MPN) incubations of SRB were done in the same layers used for sulfate reduction measurements by employing sterilized seawater supplemented with a carbonate-buffered lactate-acetate medium (29, 67). Final scores were obtained after 8 weeks, and population sizes were calculated by using the method of De Man (15).
Taurine and cysteate concentrations in porewater samples were determined by high-performance liquid chromatography by using precolumn derivatization with o-phthalaldehyde and fluorescent detection (49). The detection limit for these sulfonated amino acids was 50 pM. The hydrogen sulfide concentrations in slurry and cell suspension experiments were determined with ion-specific needle electrodes or by the methylene blue method (63).
Laboratory experiments.
Slurry experiments were performed with stromatolite samples by using either bulk sediments (upper 10 mm) or material from layer 3. For mat samples obtained from the salt pond (salt content, 72 ppt) and the salt marsh, the upper 40 mm of the mat was used. Samples were homogenized under an N2 atmosphere, and slurries were prepared by mixing the resulting homogenates with equal volumes of filter-sterilized deoxygenated seawater collected from the respective sampling sites. The slurries (10 to 40 ml) were incubated in small, dark polyvinyl chloride vessels that were sealed without a headspace. After substrate was added, sulfide production was monitored continuously over a 15- to 60-min period with a sulfide needle electrode. The slurries were stirred except when HS− readings were being obtained. The following substrates were tested: acetate, lactate, taurine, and cysteate (all three mat slurries); glutamate (salt marsh and stromatolite mat slurries); and ethanol, glycolate, Schizothrix exopolymer (EPS), isethionate, 2-sulfobenzoate, and sulfosuccinate (stromatolite mat slurries). The initial substrate concentrations in the slurries ranged from 2 to 15 μM (except for EPS, which was added at a concentration of 1.67 μg ml−1), and all substrate additions were replicated at least twice. The effect of adding MoO42− (final concentration, 20 mM) on the sulfide production rates in stromatolite samples was determined in slurries containing acetate, lactate, taurine, and cysteate. All slurry experiments were carried out at the ambient temperature. Taurine use was also tested in two or three of the highest positive MPN enrichment cultures obtained from layers 1 and 3.
An enrichment culture was obtained from a piece of the stromatolite mat (NS8) (69) for which the SRR and MPN were also determined. The inoculum was incubated in an anaerobic basal salt medium (71) from which SO42− was omitted and to which cysteate (10 mM) was added as the sole electron donor and acceptor. Incubation was carried out in Balch tubes under an N2-CO2 (80:20) headspace. The enrichment culture was transferred approximately 20 times and served as the basis for cell suspension experiments.
Cell suspension experiments were carried out with cells in the early stationary phase. A culture was harvested by centrifugation (15,000 × g, 4°C), washed twice, and resuspended in medium without substrate reduced with 18 μM titanous chloride. Cells were incubated in serum bottles sealed with butyl rubber stoppers lined with Teflon. At zero time, each cell suspension received 18, 52, or 154 μM cysteate. Samples were withdrawn every 0.33 to 1 h through the stoppers by using sterile glass syringes, and sulfide and cysteate concentrations were measured. The cell densities in these experiments were determined by acridine orange epifluorescence microscopy (30).
The chemicals used were standard reagent grade. Taurine, cysteic acid, and isethionic acid were purchased from Sigma Chemical Co., St. Louis, Mo.; sulfosuccinate was obtained from Fluka, Milwaukee, Wis.; and 2-sulfobenzoic acid was acquired from Aldrich, Milwaukee, Wis.
RESULTS
Field studies. (i) Stromatolite mats.
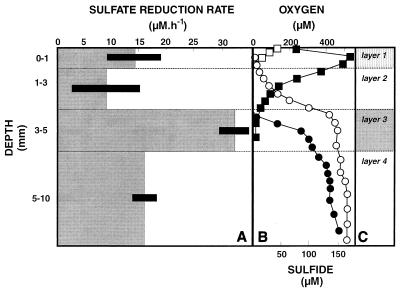
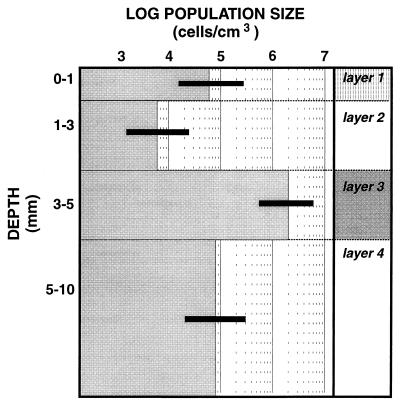
The highest SRR were associated with the lithified layer at a depth of 3 to 5 mm (layer 3), for which a diel SRR of 32 nmol cm−3 h−1 was calculated (Fig. 1). The organic compound-rich cyanobacterial surface layer (layer 1) also had a significant SRR (14 nmol cm−3 h−1) despite the high concentrations of oxygen that were present in that layer during the day (Fig. 1). During the night, however, the depth of O2 penetration was less than 0.6 mm; at this depth HS− was detected, suggesting that SRB played an important role in carbon oxidation in the surface layer. The vertical distribution of SRB determined by the MPN method (Fig. 2) showed that the largest population was in layer 3 at a depth of 3 to 5 mm (2 × 106 cells cm−3), which coincided with the layer that had the highest SRR. Interestingly, the surface layer (layer 1) also contained a significant viable population of SRB (6 × 104 cells cm−3), although it was much smaller than the populations found in deeper layers. However, the cell-specific SRR were much higher at the surface at a depth of 3 to 5 mm. This could have been due to underestimation of the SRB population at the surface due to selectivity of the enumeration medium or, alternatively, to overestimation of the SRR at a depth of 0 to 1 mm. The selectivity of the MPN technique has been well documented (22, 37), and therefore the values determined for populations of SRB probably are underestimates. However, modifying the medium to mimic natural growth conditions, including using a carbonate-buffered medium that contained site water, greatly improved the sensitivity (37).
FIG. 1.
(A and B) Depth profiles for the SRR (A) and the oxygen and sulfide concentrations (B) in a modern marine stromatolite mat (NS8) (69) in Highborne Cay, Bahamas. (C) Approximate locations of layers 1 to 4. Open symbols, values obtained at night (3:30 a.m.); solid symbols, values obtained during the day (12:30 p.m.); squares, O2 concentrations; circles, sulfide concentrations. Measurements were obtained in August 1998. Error bars indicate standard deviations (n = 3).
FIG. 2.
Viable population of SRB as assessed by the MPN method in the presence of lactate plus acetate for the stromatolite mat described in Fig. 1. Error bars indicate the 95% confidence intervals.
Taurine and cysteate were detected in porewater samples obtained from a depth of ca. 8 to 10 mm (Table 1); the concentrations of these two compounds were 0.55 and 0.07 μM, respectively.
TABLE 1.
Concentrations of taurine and cysteate in porewater from different microbial mats
| Source of porewatera | Taurine concn (μM) | Cysteate concn (μM) |
|---|---|---|
| Stromatolite mat (8–10 mm) | 0.545 | 0.065 |
| Salt pond mat (72 ppt) | 0.870 | 0.080 |
| Salt pond mat (96 ppt) | 2.340 | NDb |
| Salt marsh mat (0–5 mm) | 1.605 | 0.280 |
| Salt marsh mat (5–10 mm) | 1.150 | ND |
| Salt marsh mat (10–20 mm) | 0.200 | ND |
| Salt marsh mat (20–30 mm) | ND | ND |
The salt pond porewater was recovered from the upper 15 mm.
ND, not detected.
(ii) Salt pond mats.
The presence of sulfide, as determined with needle electrodes (data not shown), confirmed that an active SRB population was present, as reported previously (10). Cysteate was detected only in the lower-salinity mat (72 ppt) at a concentration of 0.08 μM, but the taurine concentrations were much higher and increased with increasing salinity; the taurine concentrations were 0.87 and 2.34 μM in the mats containing 72 and 96 ppt of salt, respectively (Table 1).
(iii) Salt marsh mats.
The MPN analysis of SRB from salt marsh mats (data not shown) revealed that the population increased with depth in the upper 25 mm of the mat. At a depth of 15 to 25 mm, the population size was 2.3 × 107 cells cm of sediment−3. Porewater samples contained the highest concentrations of both taurine (1.61 μM) and cysteate (0.28 μM) in the surface layer (0 to 10 mm). The cysteate concentration was below the detection limit (50 pM) in deeper layers. The taurine concentrations ranged from 0.2 to 1.6 μM and were similar to the concentrations in stromatolite and salt pond mats (Table 1). The depth profile of taurine indicated that consumption of this sulfonate occurred at a depth of 20 to 30 mm.
Laboratory experiments. (i) Sulfide production in slurries.
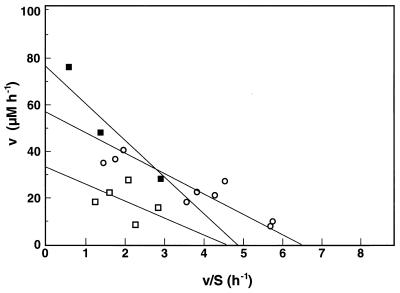
The depth profiles for O2 and HS− concentrations, MPN measurements, and/or SRR measurements suggested that very active populations of SRB were present in the upper parts of all of the mats tested. Therefore, the upper 10 mm of the stromatolite mat and the upper 40 mm of the salt pond and salt marsh mats were used to obtain slurries in which SRB activity was high. Addition of organic carbon increased the SRR, measured as HS− production, in all mat samples (Table 2). Sulfide was produced without a lag in stromatolite slurries, but the salt pond and salt marsh slurries required preincubation for 10 and 60 min, respectively. An experiment in which sulfide was added to autoclaved slurry indicated that a chemical reaction of Fe2+ with HS− occurred. This buffering capacity of the slurry for sulfide may explain the observed lag. In all three mats, HS− production was stimulated the most when acetate and lactate were added. The rates were 15 to 20 times higher than the endogenous production rates when acetate was added and 10 to 15 times higher when lactate was added to the slurries. The rates increased slightly more in salt pond and salt marsh slurries than in stromatolite slurries. Comparable rates were obtained for the stromatolite slurries when slurries containing the top 10 mm and layer 3 were used (data not shown). Addition of taurine and addition of cysteate also stimulated HS− production in all of the slurries (Table 2); the rate of HS− production increased 2- to 4.5-fold in the presence of taurine and 1.5- to 3.5-fold when cysteate was added. In both cases, sulfide was produced without a lag. The relative increases caused by these substrates were higher in stromatolite and salt pond slurries than in salt marsh samples. Isethionate, 2-sulfobenzoate, and sulfosuccinate were tested only with stromatolite slurries, in which the increases in HS− production were small (Table 2). Glutamate added to stromatolite and salt marsh slurries and ethanol and EPS added to stromatolite slurries stimulated HS− production to the same extent that taurine and cysteate stimulated HS− production (Table 2). Also, in the stromatolite mat slurries, additions of substrates at concentrations ranging from 2 to 15 μM resulted in increased rates of HS− production as the substrate concentrations increased. Therefore, these observations were used to determine the kinetic effects of substrates on SRR. Saturation curves for acetate, taurine, and cysteate were linearized by using single-reciprocal (Eadie-Hofstee) plots (19). Kinetic indices describe the rate-limiting activity of the entire SRB community rather than a single enzyme system. The Vmax and apparent half-saturation constant (Km) were 170 μM h−1 and 5.8 μM for acetate (data not shown), 56 μM h−1 and 8.8 μM for taurine, and 33 μM h−1 and 7.2 μM for cysteate, respectively (Fig. 3). In the presence of MoO42−, HS− production was inhibited, albeit not completely; after acetate, lactate, taurine, and cysteate were added, the rates of HS− production decreased 82, 87, 43, and 21%, respectively.
TABLE 2.
SRR in microbial mat slurries prepared from marine stromatolites (Highborne Cay, Bahamas), a salt pond (Guerrero Negro, Mexico), and a salt marsh (Stonington, Conn.) after electron donors were addeda
| Carbon source | SRR (d[S2−]/dt) (μM · h−1)
|
||
|---|---|---|---|
| Stromatolite mat | Salt pond matb | Salt marsh matc | |
| Acetate | 127 (8)d | 201 (17) | 184 (12) |
| Lactate | 79 (11) | 153 (14) | 177 (15) |
| Ethanol | 64 (8) | NTe | NT |
| Glycolate | 38 (5) | NT | NT |
| EPS | 29 (2) | NT | NT |
| Glutamate | 30 (3) | NT | 33 (6) |
| Taurine | 35 (2) | 38 (6) | 27 (4) |
| Cysteate | 24 (3) | 36 (1) | 18 (2) |
| Isethionate | 10 (2) | NT | NT |
| 2-Sulfobenzoate | 13 (2) | NT | NT |
| Sulfosuccinate | 10 (2) | NT | NT |
| Endogenous rate | 8 (1) | 11 (1) | 13 (1) |
With the stromatolite slurries, experiments were replicated three times for acetate, lactate, taurine, and cysteate and twice for the other substrates. The salt pond and salt marsh experiments were replicated twice. In stromatolite slurries, the presence of MoO42− inhibited HS− production 82, 87, 48, and 21% when acetate, lactate, taurine, and cysteate, respectively, were added.
To account for the reaction of HS− with Fe2+, the rates were determined after preincubation for 10 min.
To account for the reaction of HS− with Fe2+, the rates were determined after preincubation for 60 min.
Average (standard deviation).
NT, not tested.
FIG. 3.
Eadie-Hofstee single-reciprocal plot of cysteate (squares) and taurine (circles) consumption kinetics. Solid symbols, cell suspension data; open symbols data from slurry experiments. All measurements were obtained with stromatolite samples (the same samples used for the experiments whose results are shown in Fig. 1 and 2). The slope is defined by −Km.
(ii) Cysteate utilization.
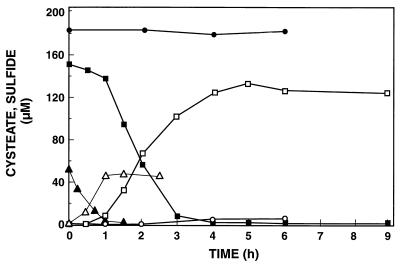
Suspensions of cells from the enrichment culture consumed cysteate immediately when it was added as the sole electron donor. The use of cysteate coincided with production of HS− (Fig. 4); 87 to 93% of the sulfonate sulfur was recovered as HS−. All of the substrate was depleted within hours in cell suspensions that contained 3.5 × 107 to 5.5 × 107 cells ml−1. The maximum specific rate of cysteate consumption was 1.6 pmol cell−1 h−1. The three cysteate concentrations tested (18, 52, and 154 μM) were used in the single-reciprocal (Eadie-Hofstee) plot (Fig. 3). The Vmax and apparent Km for cysteate utilization were 76 μM h−1 and 4.8 μM, respectively.
FIG. 4.
Time course of cysteate consumption and sulfide production in a cell suspension from an enrichment culture from a Bahamian stromatolite. Solid symbols, cysteate; open symbols, sulfide; circles, control (chemical destruction of cysteate when 184 μM cysteate was added); squares and triangles, microbial consumption of 154 and 52 μM cysteate, respectively. The results obtained when 18 μM cysteate was added are not shown.
(iii) Taurine utilization.
Utilization of taurine was tested with the highest positive dilutions of the MPN series of layer 3; no consumption was detected in the 10−7 dilution, but two of three 10−6 dilution preparations consumed taurine when it was added at a final concentration of 100 μM to medium containing 20 mM sulfate. One of two 10−4 dilutions tested from layer 1 also consumed taurine. This implied that at least 10% of the total SRB population in the stromatolite mat could utilize taurine.
DISCUSSION
Taurine and cysteate are two of the low-molecular-weight sulfonates which are rapidly degraded when they are added to anoxic microbial mat slurries that are producing HS− concomitantly. All microbial mats investigated in this study had high SRR. The use of low-molecular-weight sulfonates observed in the slurry experiments was confirmed with an enrichment culture obtained from the stromatolite mat grown with cysteate. This corroborated previous reports that both taurine and cysteate are enrichment substrates that are used by aerobic (23, 39, 64) and anaerobic bacteria (12, 16), including SRB (41, 43, 44). The enrichment culture rapidly converted cysteate stoichiometrically to HS− in the absence of sulfate and thus must have depended on C-S bond cleavage. Some, but not all, of the MPN enrichment cultures, which were selected by using acetate and lactate as electron donors, were also capable of taurine utilization.
Low-molecular-weight sulfonates are dominant organosulfur species in terrestrial and marine environments (3, 48, 65). Taurine and cysteate (at nanomolar to micromolar concentrations) were detected in the porewater of a variety of microbial mats, and the concentrations of taurine were higher when the salinity was greater. This finding supports the hypothesis that taurine may be used as an osmolyte by a variety of marine organisms (9, 13, 33, 34, 47, 53). The observed pool sizes of taurine and cysteate may have been small, but the rapid increase in HS− production under anoxic conditions suggests that the in situ turnover rates were high. Tight coupling of production and consumption of organic carbon is typical for microbial mats (66). Traditional substrates, such as lactate, acetate, and ethanol, clearly stimulated SRR more than the sulfonates tested stimulated SRR. However, in stromatolite slurries the rates observed when glutamate, glycolate, and EPS were added were the same as the rates observed when taurine and cysteate were added in this system. Interestingly and surprisingly, Schizothrix EPS stimulated sulfate reduction more than it stimulated aerobic respiration (69). Although the composition of this EPS is not known, it is conceivable that sulfonated residues are part of the polymer matrix. The sulfur moiety can be a significant part (up to 14% of the total dry weight) of cyanobacterial EPS (17) and is believed to be associated with sulfate esters. However, due to the use of rigorous digestion techniques in certain assays, sulfonates could account for part of the EPS sulfur as well.
The compositions of the SRB populations in sediments change with depth (37, 67). The changes are determined in part by the available substrates (21). At the surface of a mat the substrates are directly derived from cyanobacteria (e.g., glycolate), but at depth they are produced by fermentative organisms (which produce ethanol, lactate, etc.). Similarly, different populations of SRB are expected when different mats are compared, depending on physicochemical parameters, such as temperature, light, and substrate availability. The SRR in the stromatolite mat is 10 to 20 times lower than the SRR reported for salt pond mats (10). Despite this difference in SRR, the salt pond slurries had only slightly higher HS− production rates than the stromatolite slurries had when traditional substrates were added and similar rates when taurine and cysteate were added. A possible explanation for this is that the SRB in the stromatolite mats are more severely substrate limited, perhaps due to diffusion barriers (lithified layers) that are present (32). Alternatively, the stromatolite mats may contain different types of SRB, which, given the very distinct physicochemical parameters, would not be too surprising. Interestingly, despite the pronounced differences in SRR, SRB population sizes, etc., all mat slurries consumed low-molecular-weight sulfonates.
Molybdate is a specific inhibitor of sulfate reduction (52), and its effect on SRB in slurries when acetate and lactate were added was as expected (82 and 87% inhibition, respectively). However, the inhibitory effect of MoO42− was less pronounced when a sulfonate was added; HS− production with taurine was inhibited 43%, and HS− production with cysteate was inhibited 21%. A similar effect was noticed by Lie et al. (45) when pure cultures of a SRB were amended with MoO42− and either isethionate or cysteate. Molybdate competes with sulfate for active sites of ATP sulfurylase. If indeed C-S cleavage of sulfonates results in SO3− instead of SO42−, as suggested previously (39, 41, 43, 44), production of APS is not required, and thus, no inhibitory effects of MoO42− are expected. The fact that some inhibition was observed could be attributed to the presence of both SO42− and ATP sulfurylase in the slurry, which should have resulted in some ATP depletion when MoO42− was added (62) and, perhaps, a lower rate of overall HS− production. Alternatively, as mentioned above, a significant number of novel S-reducing organisms, which are less sensitive to MoO42−, could be present in the stromatolite mat. Clearly, the true inhibitory effect of molybdate needs to be reevaluated, so the use of molybdate in environmental studies does not lead to misinterpretations, as has been suggested previously (45, 52, 67).
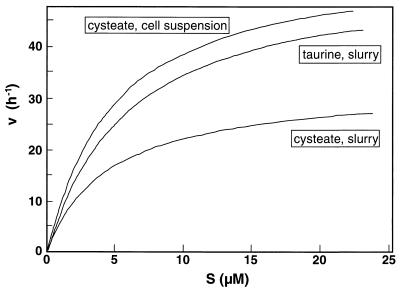
The use of low-molecular-weight sulfonates in slurries and cell suspensions revealed remarkably similar substrate affinities (defined as the initial slope of the v-s graph, or Vmax/Km) (Fig. 5). This indicates that organisms similar to the enrichment culture organisms consume sulfonate in situ. When the maximum rate observed in the slurry is divided by the highest MPN dilution of SRB that used taurine, the theoretical cell-specific consumption rates for taurine and cysteate are 35 and 24 pmol cell−1 h−1, respectively. Compared to the maximum cell-specific rate observed in the cell suspension (1.6 pmol cell−1 h−1), these rates are 1 order of magnitude higher, which is not atypical for slurry experiments (8). We should emphasize that the size of the taurine-utilizing fraction of SRB used for this calculation is only a rough approximation. Clearly, the size of the population of sulfidogenic organisms that use low-molecular-weight sulfonates needs to be assessed more carefully. Lie et al. (44) observed that H2 utilization by Desulfovibrio desulfuricans IC1 occurs at a lower H2 concentration (3 ppmv) in the presence of isethionate as the electron acceptor than in the presence of either sulfate or sulfite (H2 thresholds, 15 and 8 ppmv, respectively). Similar mechanisms could contribute to higher rates of HS− production in slurries in which the presence of H2 is conceivable.
FIG. 5.
Michaelis-Menten kinetics for taurine utilization (slurry) and cysteate utilization (slurry and cell suspension). The kinetic indices Vmax and Km were 56 h−1 and 8.8 μM, 33 h−1 and 7.2 μM, and 76 h−1 and 15.8 μM for taurine, for cysteate in slurries, and for cysteate in a cell suspension, respectively. The corresponding affinities (Vmax/Km) were 6.36, 4.58, and 4.80 h−1 μM−1, respectively. The Vmax and Km for acetate in slurry experiments (data not shown) were 170 h−1 and 5.8 μM, respectively, while the affinity was 29.31 h−1 μM−1.
Amino acids are important substrates for bacteria in marine sediments, and several amino acids support SRB growth (8, 27). The importance of sulfonate-containing amino acids for SRB metabolism seems clear, especially when the HS− production rates on taurine and cysteate are compared with the HS− production rates on glutamate. In addition to anaerobic consumption of sulfonates, aerobic degradation was observed as well; in stromatolite slurries, a rapid change in the oxygen concentration over time (d[O2]/dt) was observed, and the rates were approximately one-third the rates observed when acetate or lactate was added (69, 70). Similarly, methanesulfonate, another low-molecular-weight sulfonate and a chemical oxidation product of dimethyl sulfide, was also degraded aerobically by marine methylotrophs (4).
We concluded that low-molecular-weight sulfonates contribute significantly to the substrates that support SRB growth in microbial mats. The fact that the relative stimulation of the SRR was much greater in stromatolites could indicate either that the substrate limitation in these mats was greater or that a substantially different community of SRB was present or both. The observation that relatively high concentrations of O2 are present in the layers which exhibit high SRR for at least part of a diel cycle (69) and the observation that MoO42− has a limited inhibitory effect indicate that a more detailed study of the physiological diversity and the molecular diversity of SRB is necessary.
ACKNOWLEDGMENTS
This study was supported by NSF grant OCE 9619314 and by NASA collaborative agreement NCC2-1067.
Alan Decho kindly provided Schizothrix slime. The technical assistance of and fruitful discussions with Shelley Hoeft, Tonna-Marie Surgeon, Pamela Reid, Brad Bebout, John Thompson, and Tom Lie are much appreciated.
Footnotes
This is RIBS contribution number 03.
REFERENCES
- 1.Anderson R, Kates M, Volcani B E. Identification of the sulfolipids in the non-photosynthetic diatom Nitzschia alba (Algae) Biochim Biophys Acta. 1978;528:89–106. doi: 10.1016/0005-2760(78)90055-3. [DOI] [PubMed] [Google Scholar]
- 2.Archer S D, McDonald K A, Jackman A P. Effect of light irradiance on the production of sulfolipids from Anabaena 7120 in a fed-batch photobioreactor. Appl Biochem Biotechnol. 1997;67:139–152. doi: 10.1007/BF02787848. [DOI] [PubMed] [Google Scholar]
- 3.Autry A R, Fitzgerald J W. Sulfonate S: a major form of forest soil organic sulfur. Biol Fertil Soils. 1990;10:50–56. [Google Scholar]
- 4.Baker S C, Kelly D P, Murrell J C. Microbial degradation of methanesulphonic acid: a missing link in the biogeochemical sulphur cycle. Nature. 1991;350:627–628. [Google Scholar]
- 5.Bates T E, Carpenter R. Organo-sulfur compounds in sediments in the Puget Sound. Geochim Cosmochim Acta. 1979;43:1209–1221. [Google Scholar]
- 6.Berna J L, Moreno A, Ferrer J. The behavior of LAS in the environment. J Chem Technol Biotechnol. 1991;50:387–398. [Google Scholar]
- 7.Biedlingmaier S, Körst H-P, Schmidt A. Utilization of sulfonic acids as the only sulfur source for growth of photosynthetic organisms. Planta. 1986;169:518–523. doi: 10.1007/BF00392101. [DOI] [PubMed] [Google Scholar]
- 8.Burdige D J. The effect of sediment slurrying on microbial processes, and the role of amino acids as substrates for sulfate reduction in anoxic sediments. Biogeochemistry. 1989;8:1–23. [Google Scholar]
- 9.Burton R S, Feldman M W. Changes in free amino acid concentrations during osmotic response in the intertidal copepod Tigriopus californicus. Comp Biochem Physiol. 1982;73:441–445. [Google Scholar]
- 10.Canfield D E, DesMarais D J. Aerobic sulfate reduction in microbial mats. Science. 1991;251:1471–1473. doi: 10.1126/science.11538266. [DOI] [PubMed] [Google Scholar]
- 11.Charlson R J, Lovelock J E, Andreae M O, Warren S G. Oceanic phytoplankton, atmospheric sulfur, cloud albedo and climate. Nature. 1987;326:655–661. [Google Scholar]
- 12.Chien C-C, Leadbetter E R, Godchaux W., III Sulfonate-sulfur can be assimilated for fermentative growth. FEMS Microbiol Lett. 1995;129:189–194. [Google Scholar]
- 13.Costa C J, Pierce S K, Warren M K. The intracellular mechanism of salinity tolerance in polychaetes: volume regulation by isolated Glycera dibranchiata red coelomocytes. Biol Bull. 1980;159:626–638. [Google Scholar]
- 14.D’Amelio E D, Cohen Y, DesMarais D J. Comparative functional ultrastructure of two hypersaline submerged cyanobacterial mats: Guerrero Negro, Baja California Sur, and Solar Lake, Sinai, Egypt. In: Cohen Y, Rosenberg E, editors. Microbial mats: physiological ecology of benthic microbial communities. Washington, D.C: American Society for Microbiology; 1989. pp. 97–113. [Google Scholar]
- 15.De Man J. The probability of most probable numbers. Eur J Appl Microbiol. 1975;1:67–78. [Google Scholar]
- 16.Denger K, Laue H, Cook A M. Anaerobic taurine oxidation: a novel reaction by nitrate-reducing Alcaligenes sp. Microbiology. 1997;143:1919–1924. doi: 10.1099/00221287-143-6-1919. [DOI] [PubMed] [Google Scholar]
- 17.De Philippis R, Vincenzini M. Exocellular polysaccharides from cyanobacteria and their possible applications. FEMS Microbiol Rev. 1998;22:151–175. [Google Scholar]
- 18.Federle T W, Ventullo R M. Mineralization of surfactants by the microbiota of submerged plant detritus. Appl Environ Microbiol. 1990;56:333–339. doi: 10.1128/aem.56.2.333-339.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fersht A. Enzyme structure and mechanism. W. H. New York, N.Y: Freeman & Co.; 1985. [Google Scholar]
- 20.Fossing H, Jørgensen B B. Measurement of bacterial sulfate reduction in sediments: evaluation of a single-step chromium reduction method. Biogeochemistry. 1989;8:205–222. [Google Scholar]
- 21.Fründ C, Cohen Y. Diurnal cycles of sulfate reduction under oxic conditions in microbial mats. Appl Environ Microbiol. 1992;58:70–77. doi: 10.1128/aem.58.1.70-77.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibson G R, Parkes R J, Herbert R A. Evaluation of viable counting procedures for the enumeration of sulfate-reducing bacteria in estuarine sediments. J Microbiol Methods. 1987;7:201–210. [Google Scholar]
- 23.Giehl T J, Qodronfleh M W, Wilkinson B J. Transport, nutritional and metabolic studies of taurine in staphylococci. J Gen Microbiol. 1987;133:849–856. doi: 10.1099/00221287-133-4-849. [DOI] [PubMed] [Google Scholar]
- 24.Godchaux W, III, Leadbetter E R. Unusual sulfonolipids are characteristic of Cytophaga-Flexibacter group. J Bacteriol. 1983;153:1238–1246. doi: 10.1128/jb.153.3.1238-1246.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godchaux W, III, Leadbetter E R. Sulfonolipids of gliding bacteria: structures of the N-acylaminosulfonates. J Biol Chem. 1984;259:621–623. [PubMed] [Google Scholar]
- 26.Guler S, Seeliger A, Hartel H, Renger G, Benning C. A null mutant of Synechococcus sp. PCC7942 deficient in the sulfolipid sulfoquinovosyl dialkylglycerol. J Biol Chem. 1996;271:7501–7507. doi: 10.1074/jbc.271.13.7501. [DOI] [PubMed] [Google Scholar]
- 27.Hansen L S, Holmer M, Blackburn T H. Mineralization of organic nitrogen and carbon (fish food) added to anoxic sediment in microcosms: role of sulphate reduction. Mar Ecol Prog Ser. 1993;102:199–204. [Google Scholar]
- 28.Hansen L S, Blackburn T H. Amino acid degradation by sulfate-reducing bacteria. Limnol Oceanogr. 1995;40:502–510. [Google Scholar]
- 29.Hines M E, Visscher P T, Devereux R. Sulfur cycling. In: Newell S Y, et al., editors. Manual of environmental microbiology. Aquatic environments. Washington, D.C: American Society for Microbiology; 1996. pp. 324–334. [Google Scholar]
- 30.Hobbie J E, Daley R J, Jasper S. Use of Nuclepore filters for counting bacteria by fluoerescence microscopy. Appl Environ Microbiol. 1977;33:1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holst P B, Nielsen S E, Anthoni U, Bisht K S, Christophersen C, Gupta S, Parmar V S, Nielsen P H, Sahoo D B, Singh A. Isethionate in certain red algae. J Appl Phycol. 1994;6:443–446. [Google Scholar]
- 32.Huettel M, Ziebis W, Foster S. Flow-induced uptake of particulate matter in permeable sediments. Limnol Oceanogr. 1996;41:309–322. [Google Scholar]
- 33.Huxtable R J. Physiological aspects of taurine. Physiol Rev. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 34.Jackson A E, Ayer S W, Laycock M V. The effect of salinity on growth and amino acid composition in the marine diatom Nitzschia pungens. Can J Bot. 1992;70:2198–2201. [Google Scholar]
- 35.Jørgensen B B. Mineralization of organic matter in the sea bed—the role of sulphate reduction. Nature. 1982;296:643–645. [Google Scholar]
- 36.Jørgensen B B, Cohen Y. Solar Lake (Sinai). 5. The sulfur cycle of the benthic cyanobacterial mats. Limnol Oceanogr. 1977;22:657–666. [Google Scholar]
- 37.Jørgensen B B, Bak F. Pathways and microbiology of thiosulfate transformations and sulfate reduction in a marine sediment (Kattegat, Denmark) Appl Environ Microbiol. 1991;57:847–856. doi: 10.1128/aem.57.3.847-856.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiene R P. Microbial sources and sinks for methylated sulfur compounds in the marine environment. In: Murrell J C, Kelly D P, editors. Microbial growth on C1 compounds. Andover, United Kingdom: Intercept Ltd.; 1993. pp. 15–33. [Google Scholar]
- 39.Kondo H, Niki H, Takahashi S, Ishimoto M. Enzymatic oxidation of isethionate to sulfoacetaldehyde in bacterial extract. J Biochem. 1977;81:1911–1916. doi: 10.1093/oxfordjournals.jbchem.a131653. [DOI] [PubMed] [Google Scholar]
- 40.LaLonde R T, Ferrara L M, Hayes M P. Low-temperature, polysulfide reactions of conjugated ene carbonyls: a reaction model of S-heterocycles. Org Geochem. 1987;11:563–571. [Google Scholar]
- 41.Laue H, Denger K, Cook A M. Taurine reduction in anaerobic respiration of Bilophila wadsworthia RZATAU. Appl Environ Microbiol. 1997;63:2016–2021. doi: 10.1128/aem.63.5.2016-2021.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laue H, Denger K, Cook A M. Fermentation of cysteate by a sulfate-reducing bacterium. Arch Microbiol. 1997;168:210–214. doi: 10.1007/s002030050502. [DOI] [PubMed] [Google Scholar]
- 43.Lie T J, Pitta T, Leadbetter E R, Godchaux III W, Leadbetter J R. Sulfonates: novel electron acceptors in anaerobic respiration. Arch Microbiol. 1996;166:204–210. doi: 10.1007/s002030050376. [DOI] [PubMed] [Google Scholar]
- 44.Lie T J, Leadbetter J R, Leadbetter E R. Metabolism of sulfonic acids and other organosulfur compounds by sulfate reducing bacteria. Geomicrobiol J. 1998;15:135–149. [Google Scholar]
- 45.Lie, T. J., W. Godchaux III, and E. R. Leadbetter. Sulfidigenesis from sulfonates: sulfonates as terminal electron acceptors for growth of sulfite-reducing bacteria (Desulfitobacterium spp.) and sulfate-reducing bacteria. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 46.Luther G W, III, Church T M, Scudlark J R, Cosman M. Inorganic and organic sulfur cycling in salt-marsh porewaters. Science. 1986;232:746–749. doi: 10.1126/science.232.4751.746. [DOI] [PubMed] [Google Scholar]
- 47.Manahan D T, Nourizadeh S. Requirements for specific amino acids and proteins by oyster larvae (Crassostrea gigas) J Shellfish Res. 1988;8:322–323. [Google Scholar]
- 48.Mayer L M, Schick L L, Sawyer T, Plante C J, Jumars P A, Self R L. Bioavaialble amino acids in sediments: a biomimetic kinetic-based approach. Limnol Oceanogr. 1985;40:511–520. [Google Scholar]
- 49.Mopper K, Delmas D. Trace determination of biological thiols by liquid chromatography and precolumn fluorometric labeling with o-phtalaldehyde. Anal Chem. 1984;56:2557–2560. doi: 10.1021/ac00277a064. [DOI] [PubMed] [Google Scholar]
- 50.Mopper K, Taylor B F. Biogeochemical cycling of sulfur: thiols in coastal marine sediments. In: Sohn M L, editor. Organic marine geochemistry. Washington, D.C: American Chemical Society; 1986. pp. 323–339. [Google Scholar]
- 51.Nanninga H J, Gottschal J C. Amino acid fermentation and hydrogen transfer in mixed cultures. FEMS Microbiol Ecol. 1985;31:261–269. [Google Scholar]
- 52.Oremland R S, Capone D G. Use of “specific” inhibitors in biogeochemistry and microbial ecology. Adv Microb Ecol. 1988;10:285–383. [Google Scholar]
- 53.Pierce S K, Rowand-Faux L M, O’Brien S M. Different salinity mechanism in Atlantic and Chesapeake Bay conspecific oysters: glycine betaine and amino acid pool variations. Mar Biol. 1992;113:115–117. [Google Scholar]
- 54.Reid R P, Macintyre I G, Steineck R S, Brown K M, Miller T E. Stromatolites in the Exuma Cays: uncommonly common. Facies. 1995;33:1–18. [Google Scholar]
- 55.Roy A B, Trudinger P A. The biochemistry of inorganic compounds of sulphur. Cambridge, United Kingdom: Cambridge University Press; 1970. [Google Scholar]
- 56.Saidha T, Schiff J A. The role of mitichondria in sulfolipid biosynthesis by Euglena chloroplasts. Biochim Biophys Acta. 1989;1001:263–273. [Google Scholar]
- 57.Sandman G, Boger P. Formation and degradation of photosynthetic membranes determined by 35S (sulfur isotope) labeled sulfolipid (Scenedesmus acutus, algae) Plant Sci Lett. 1982;24:347–352. [Google Scholar]
- 58.Schmidt W W, Lillienthal W, Raney K H, Dubey S T. A novel dianionic surfactant from the reaction of C14-alkenylsuccinic acid with sodium isethionate. J Am Oil Chem Soc. 1994;71:695–703. [Google Scholar]
- 59.Sørensen J, Jørgensen B B. Early diagenesis in sediments from Danish coastal waters: microbial activity and Mn-Fe-S geochemistry. Geochim Cosmochim Acta. 1987;51:1583–1590. [Google Scholar]
- 60.Spindle M, Stadler R, Tanner H. Amino acid analysis of feedstuffs: determination of methionine and cysteine after oxidation with performic acid and hydrolysis. J Agric Food Chem. 1984;32:1366–1371. [Google Scholar]
- 61.Stams A J M, Hansen T A, Skyring G W. Utilization of amino acids as energy substrates by two marine Desulfovibrio strains. FEMS Microbiol Ecol. 1985;31:11–15. [Google Scholar]
- 62.Taylor B F, Oremland R S. Depletion of adenosine triphosphate in Desulfovibrio by oxyanions of group VI elements. Curr Microbiol. 1979;3:101–103. [Google Scholar]
- 63.Trüper H G, Schlegel H G. Sulphur metabolism in Thiorhodaceae. I. Quantitative measurements of growing cells of Chromatium okenii. Antonie Leeuwenhoek J Microbiol Serol. 1964;30:225–238. doi: 10.1007/BF02046728. [DOI] [PubMed] [Google Scholar]
- 64.Uria-Nickelsen M R, Leadbetter E R, Godchaux W., III Sulphonate utilization by enteric bacteria. J Gen Microbiol. 1993;139:203–208. doi: 10.1099/00221287-139-2-203. [DOI] [PubMed] [Google Scholar]
- 65.Vairavamurthy A, Zhou W, Eglinton T, Manowitz B. Sulfonates: a novel class of organic sulfur compounds in marine sediments. Geochim Cosmochim Acta. 1994;58:4681–4687. [Google Scholar]
- 66.Van Gemerden H. Microbial mats: a joint venture. Mar Geol. 1993;113:3–25. [Google Scholar]
- 67.Visscher P T, Prins R A, van Gemerden H. Rates of sulfate reduction and thiosulfate consumption in a marine microbial mat. FEMS Microbiol Ecol. 1992;86:383–394. [Google Scholar]
- 68.Visscher P T, van Gemerden H. Sulfur cycling in laminated marine ecosystems. In: Oremland R S, editor. Biogeochemistry of global change: radiatively active trace gases. New York, N.Y: Chapman and Hall; 1993. pp. 672–693. [Google Scholar]
- 69.Visscher P T, Reid R P, Bebout B M, Hoeft S E, Macintyre I G, Thompson J A., Jr Formation of lithified micritic laminae in modern marine stromatolites (Bahamas): the role of sulfur cycling. Am Minerol. 1998;83:1482–1493. [Google Scholar]
- 70.Visscher, P. T. 1998. Unpublished data.
- 71.Widdel F, Bak F. Gram-negative mesophilic sulfate-reducing bacteria. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. A handbook on the biology of bacteria: ecophysiology, isolation, identification, applications. 2nd ed. New York, N.Y: Springer-Verlag; 1992. pp. 3353–3378. [Google Scholar]