Abstract
Introduction:
Several guidelines have adopted early integration of palliative intervention (PI) into oncologic care to improve quality of life among patients with advanced malignancies. However, PI utilization patterns and factors associated with its use in metastatic renal cell carcinoma are poorly understood.
Patients and Methods:
Using the National Cancer Database (NCDB), we abstracted patients diagnosed with Stage IV RCC from 2004-2014 and evaluated utilization of PI within this cohort. Socioeconomic and clinical factors were compared for patients receiving and not receiving PI for metastatic RCC. Multivariable logistic regression (MLR) models identified factors that were associated with receipt of PI within overall cohort and treatment-based cohorts.
Results:
We identified 42,014 patients with Stage IV RCC, of which 7,912 patients received PI. From 2004 to 2014, the use of PI minimally increased from 17% to 20% for Stage IV RCC. MLR analysis demonstrated that increased comorbidities, insurance status, higher education status, facility location, care at a comprehensive cancer program or integrated network, sarcomatoid histology, and treatment type significantly increased likelihood of PI use. Various socioeconomic, clinical, and geographical factors that are associated with use of PI based on the treatment received for Stage IV RCC.
Conclusions:
While PI utilization has minimally increased for Stage IV RCC, there are several geographic, socioeconomic, and clinical factors that predict its use among patients with Stage IV RCC in a treatment-specific manner. Taken together, this suggests the need for earlier initiation of PI in a more equitable and systematic fashion among patients with metastatic RCC.
Keywords: palliative care, palliative interventions, metastatic renal cell carcinoma, RCC, kidney cancer
MicroAbstract:
The use of palliative interventions and the determinants of its use for Stage IV RCC are well understood. Using a nationally representative database, we demonstrate that palliative intervention utilization has minimally increased for Stage IV RCC. Several geographic, socioeconomic, and clinical factors predict receipt of palliative care among patients with Stage IV RCC in a treatment-specific manner.
Introduction
Palliative care (PC) offers patients, and often their families, improved quality-of-life (QOL) through the prevention and relief of suffering due to pain and other physical, psychosocial, or spiritual problems related to cancer and cancer treatments. A multidisciplinary team can initiate PC at any state of illness with the aim of improving quality-of-life. Cancer-directed therapies such as surgical, radiation, and systemic treatments along with supportive care interventions such as pain management, emotional and spiritual support can be offered for palliation.1 Furthermore, several studies have demonstrated that PC improves the quality of care at the end of life including decreased depression, pain, and anxiety, while decreasing intensive care utilization and overall cost.2–4 As a result, several guidelines have adopted early integration of PC into oncologic care.5–7
Approximately 90% of kidney cancer cases are due to renal cell carcinoma (RCC). While 30% of patients who undergo extirpative surgery for RCC experience cancer recurrence with distant metastases, approximately one-third present with metastatic RCC at the time of diagnosis.8, 9 These groups of patients would likely benefit from PC, however PC usage in metastatic RCC is poorly understood. The National Cancer Database (NCDB) is the largest dataset that captures PC usage based on interventions like surgery, radiation, systemic therapy, or pain management that are used to prolong a patient’s life or alleviate symptoms and not diagnose or stage RCC. Therefore, this study aims to define the trend in usage of palliative interventions (PI) and factors associated with its use in metastatic RCC.
Material and Methods
Data Collection and Source
The National Cancer Database (NCDB) is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society, which contains information on roughly 70% of incident cancer cases in United States. Data from approximately 1,500 accredited hospitals regarding patient demographics, cancer staging, tumor characteristics, surgical treatment, and patient prognosis are recorded.
Study Population
From 422,258 kidney cancer patients captured in NCDB during 2004-2014, we included only those with AJCC Stage IV (T4NanyM0, TanyNanyM1 RCC (n=50,081). After excluding patients with unknown or missing palliative care status (n=129) and non-RCC histology (n=7,938), the analytic cohort consisted of 42,014 patients with Stage IV RCC who had known PI. The analytic cohort was subdivided based on the treatments including surgery, radiation, systemic therapy, surgery+systemic therapy for RCC.
Study Variables
Receipt of PI was the independent variable. The NCDB codes PI as either no intervention, surgery, radiation, systemic therapy, pain management alone, combination of therapies without pain management, combination of therapies with pain management, or unknown type of palliation delivered. NCDB does not code for formal palliative care consult/services, goals of care discussions, hospice care or psychological/spiritual care. Systemic therapies include chemotherapy, hormonal therapies, targeted therapies or immunotherapy and combined therapy includes surgery or radiation with any systemic therapy. Baseline patient demographic and socioeconomic data included age, sex, race, Charlson-Deyo score (CDS), insurance status, facility type, facility location, distance to facility, income level, and education level. CDS is a weighted score from the sum of scores for each comorbid condition. States located in New England included CT, MA, ME, NH, RI, VT; Middle Atlantic included NJ, NY, PA; South Atlantic included DC, DE, FL, GA, MD, NC, SC, VA, WV; East North Central included IL, IN, MI, OH, WI; East South Central included AL, KY, MS, TN; West North Central included IA, KS, MN, MO, ND, NE, SD; West South Central included AR, LA, OK, TX; Mountain included AZ, CO, ID, MT, NM, NV, UT, WY; and Pacific included AK, CA, HI, OR, WA. Treatment data included tumor stage, year of diagnosis, clinical trial participation, histology, and treatment type.
Data Analysis
Baseline demographic and clinical covariates were summarized by receipt of PI with descriptive statistics and compared using a two-sample t-test for continuous predictors and a Chi-square test for categorical predictors. An alpha level of 0.05 was used to determine statistical significance. Trends of PI utilization was shown as a function of year of diagnosis. Univariate and multivariable logistic regression models were used to calculate odd ratios (OR) and 95% CI for PI utilization among the entire cohort and by treatments received. Analyses were conducted using SAS Software (V. 9.4; SAS Institute Inc., SAS Campus Drive, Cary, North Carolina 27513, USA) and GraphPad Prism (V. 7.01; GraphPad Software, La Jolla, California USA).
Results
The analytic cohort consisted of 42,014 patients with Stage IV RCC, of which 18.8% (n = 7,912) received PI. Most Stage IV patients received radiation therapy (50.2%), followed by systemic therapy (17.7%), pain management (8%), and surgery (6.8%) (Supplementary Figure 1). Clinical and socio-demographic data according to PI usage is shown in Table 1. From 2004 to 2014, the use of PI for Stage IV RCC minimally increased from 17% to 20% (Figure 1).
Table 1:
Patient characteristics for palliative care use for Stage IV RCC
| Characteristics | PC | No PC |
|---|---|---|
| N (%) | 7,912 (18.8) | 34,102 (81.2) |
| Age | ||
| Mean (SD) | 64.5 (12.2) | 64.8 (12.3) |
| Median (IQR) | 64 (17) | 65 (18) |
| Sex | ||
| Male (%) | 5,270 (66.6) | 22,774 (66.8) |
| Female (%) | 2,642 (33.4) | 11,328 (33.2) |
| Charlson-Deyo score | ||
| 0 | 5,528 (69.9) | 24,080 (70.6) |
| 1 | 1,682 (21.3) | 7,207 (21.1) |
| 2+ | 702 (8.9) | 2,815 (8.3) |
| Race | ||
| White | 6,888 (87.1) | 29,108 (85.4) |
| Black | 729 (9.2) | 3,549 (10.4) |
| Others | 231 (2.9) | 1,082 (3.2) |
| Unknown | 64 (0.8) | 363 (1.1) |
| Insurance status | ||
| Not insured | 410 (5.2) | 1,715 (5) |
| Private | 2,896 (36.6) | 12,960 (38) |
| Medicaid | 646 (8.2) | 2,501 (7.3) |
| Medicare | 3,707 (46.8) | 15,385 (46.4) |
| Other Government | 142 (1.8) | 431 (1.3) |
| Unknown | 111 (1.4) | 660 (1.9) |
| Facility type | ||
| Community cancer program | 813 (10.3) | 3,489 (10.2) |
| Comprehensive community cancer program | 3,411 (43.1) | 14,143 (41.5) |
| Academic/Research program | 2,908 (36.7) | 13,225 (38.8) |
| Integrated network | 780 (9.9) | 3,245 (9.5) |
| Facility location | ||
| New England | 539 (6.8) | 1,568 (4.6) |
| Middle Atlantic | 1,239 (15.7) | 4,796 (14.1) |
| South Atlantic | 1,562 (19.7) | 7,089 (20.8) |
| East North Central | 1,726 (21.8) | 5,881 (17.2) |
| East South Central | 559 (7.1) | 2,381 (7) |
| West North Central | 818 (10.3) | 3,012 (8.8) |
| West South Central | 589 (7.4) | 3,565 (10.4) |
| Mountain | 339(4.3) | 1,621 (4.8) |
| Pacific | 541 (6.8) | 4,189 (6.8)- |
| Income | ||
| <$38,000 | 1,456 (18.8) | 6,358 (19.1) |
| $38,000-$47,999 | 2,082 (26.9) | 8,434 (25.3) |
| $48,000-$62,999 | 2,172 (28) | 9,226 (27.6) |
| >$63,000 | 2,039 (26.3) | 9,348 (28) |
| Education (% no high school degree) | ||
| ≥ 21% | 1,323 (17.1) | 6,657 (19.9) |
| 13-20% | 2,169 (28) | 9,139 (27.4) |
| 7-12.9% | 2,644 (34.1) | 10,801 (32.4) |
| <7% | 1,617 (20.9) | 6,783 (20.3) |
| Distance to Facility | ||
| ≤ 4.7 miles | 2,254 (28.5) | 9,558 (28) |
| 4.8 – 11.2 miles | 1,972 (24.9) | 8,413 (24.7) |
| 11.3 – 29.5 miles | 2,012 (25.4) | 8,067 (23.7) |
| > 29.5 miles | 1,674 (21.2) | 8,064 (23.6) |
| Histology | ||
| Clear cell | 6,907 (87.3) | 29,499 (86.5) |
| Non-clear Cell | 506 (6.4) | 2,520 (7.4) |
| Sarcomatoid | 499 (6.3) | 2,083 (6.1) |
| Year of Diagnosis | ||
| 2004-2007 | 1,949 (24.6) | 8,883 (26) |
| 2008 - 2010 | 3,021 (38.2) | 13,669 (40.1) |
| 2011 - 2014 | 2,942 (37.2) | 11,550 (33.9) |
| Clinical trial participation | ||
| No | 7,739 (99.8) | 33,630 (99.7) |
| Yes | 13 (0.2) | 111 (0.3) |
| Treatments prior to PC | ||
| None | 1,093 (13,8) | 8,857 (26) |
| Surgery | 274 (3.5) | 4,977 (14.6) |
| Radiation | 1,592 (20.1) | 1,943 (5.7) |
| Systemic therapy | 1,051 (13.3) | 6,850 (20.1) |
| Surgery+Radiation | 476 (6) | 1,009 (3) |
| Surgery+Systemic | 495 (6.3) | 5,516 (16.2) |
| Radiation+Systemic | 1,967 (24.9) | 2,572 (7.5) |
| Surgery+Radiation+Systemic | 862 (10.9) | 1,518 (4.5) |
| Unknown | 102 (1.3) | 860 (2.5) |
Figure 1: Trends in use of palliative interventions among patients with Stage IV RCC.

Dotted lines represent 95% confidence intervals.
We examined factors associated with the utilization of PI in the care of patients with Stage IV RCC. Multivariable logistic regression model adjusting for all available covariates (Table 2) demonstrated that patients with CDS ≥1, non-private insurance, receiving care at comprehensive community cancer program or integrated cancer network program, and higher education status were more likely to have received PI. Additionally, patients receiving care in New England or Middle Atlantic or East North Central or West North Central regions, with sarcomatoid histology, diagnosed within 2011-2014, whose first line of treatment was radiation, systemic therapy, surgery+radiation, radiation+systemic, or surgery+radiation+systemic therapy were more likely to receive PI. Patients were less likely to receive PI if their incomes were >$48,000, received care in the West South Central or Pacific regions, , and received surgery alone and surgery+systemic therapy prior to PI.
Table 2:
Univariable and Multivariable analysis of factors predicting PC use
| Characteristics | Univariate | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age | ||||
| Per year | 0.999 [0.997, 1.001] | 0.16 | 0.999 [0.996, 1.002] | 0.52 |
| Sex | ||||
| Male | Ref | Ref | ||
| Female | 1.01 [0.96, 1.06] | 0.77 | 1.06 [0.996, 1.12] | 0.07 |
| Charlson-Deyo score | ||||
| 0 | Ref | Ref | ||
| 1 | 1.02 [0.96, 1.08] | 0.59 | 1.08 [1.01, 1.15] | 0.03 |
| 2+ | 1.09 [0.99, 1.19] | 0.06 | 1.16 [1.05, 1.28] | <0.01 |
| Race | ||||
| White | Ref | Ref | ||
| Black | 0.87 [0.8, 0.94] | <0.01 | 0.91 [0.82, 1.002] | 0.06 |
| Others | 0.9 [0.78, 1.04] | 0.16 | 1.12 [0.96, 1.32] | 0.16 |
| Unknown | 0.75 [0.57, 0.97] | 0.03 | 0.83 [0.62, 1.11] | 0.20 |
| Insurance status | ||||
| Private | Ref | Ref | ||
| Uninsured | 1.07 [0.95, 1.2] | 0.25 | 1.19 [1.05, 1.36] | <0.01 |
| Medicaid | 1.16 [1.05, 1.27] | <0.01 | 1.18 [1.06, 1.31] | <0.01 |
| Medicare | 1.05 [0.99, 1.11] | 0.09 | 1.09 [1.01, 1.17] | 0.02 |
| Other Government | 1.48 [1.22, 1.79] | <0. 01 | 1.36 [1.09, 1.69] | <0.01 |
| Unknown | 0.75 [0.61, 0.92] | <0.01 | 0.78 [0.62, 0.97] | 0.03 |
| Facility type | ||||
| Academic/Research program | Ref | Ref | ||
| Community cancer program | 1.06 [0.97, 1.16] | 0.19 | 1.03 [0.94, 1.14] | 0.49 |
| Comprehensive community cancer program | 1.1 [1.04, 1.16] | <0.01 | 1.11 [1.05, 1.19] | <0.01 |
| Integrated network | 1.09 [1.001, 1.19] | 0.05 | 1.13 [1.02, 1.25] | 0.02 |
| Facility location | ||||
| South Atlantic | Ref | Ref | ||
| New England | 1.56 [1.39, 1.75] | <0. 01 | 1.57 [1.38, 1.79] | <0.01 |
| Middle Atlantic | 1.17 [1.08, 1.27] | <0. 01 | 1.3 [1.18, 1.43] | <0.01 |
| East North Central | 1.33 [1.23, 1.44] | <0. 01 | 1.32 [1.21, 1.44] | <0.01 |
| East South Central | 1.07 [0.96, 1.19] | 0.25 | 1.03 [0.91, 1.16] | 0.66 |
| West North Central | 1.23 [1.12, 1.36] | <0. 01 | 1.16 [1.04, 1.3] | <0.01 |
| West South Central | 0.75 [0.68, 0.83] | <0.01 | 0.78 [0.69, 0.87] | <0.01 |
| Mountain | 0.95 [0.83, 1.08] | 0.43 | 0.89 [0.77, 1.02] | 0.10 |
| Pacific | 0.59 [0.53, 0.65] | <0.01 | 0.55 [0.49, 0.62] | <0.01 |
| Income | ||||
| <$38,000 | Ref | Ref | ||
| $38,000-$47,999 | 1.08 [1.001, 1.16] | 0.05 | 0.94 [0.86, 1.02] | 0.16 |
| $48,000-$62,999 | 1.03 [0.96, 1.11] | 0.46 | 0.85 [0.77, 0.94] | <0.01 |
| >$63,000 | 0.95 [0.88, 1.03] | 0.20 | 0.76 [0.68, 0.85] | <0.01 |
| Education (% no high school degree) | ||||
| ≥ 21% | Ref | Ref | ||
| 13-20% | 1.19 [1.11, 1.29] | <0.01 | 1.12 [1.03, 1.23] | <0.01 |
| 7-12.9% | 1.23 [1.14, 1.33] | <0.01 | 1.17 [1.06, 1.29] | <0.01 |
| <7% | 1.2 [1.11, 1.3] | <0.01 | 1.26 [1.12, 1.42] | <0.01 |
| Distance to Facility | ||||
| ≤ 4.7 miles | Ref | Ref | ||
| 4.8 – 11.2 miles | 0.99 [0.93, 1.06] | 0.86 | 1.02 [0.95, 1.1] | 0.55 |
| 11.3 – 29.5 miles | 1.06 [0.99, 1.13] | 0.10 | 1.06 [0.98, 1.15] | 0.13 |
| > 29.5 miles | 0.88 [0.82, 0.94] | <0. 01 | 0.95 [0.88, 1.04] | 0.26 |
| Histology | ||||
| Clear cell | Ref | Ref | ||
| Non-clear Cell | 0.86 [0.78, 0.95] | <0.01 | 1.11 [0.99, 1.24] | 0.08 |
| Sarcomatoid | 1.02 [0.92, 1.13] | 0.66 | 1.17 [1.04, 1.31] | <0.01 |
| Year of Diagnosis | ||||
| 2004-2007 | Ref | - | Ref | - |
| 2008 - 2010 | 0.997 [0.93, 1.07] | 0.92 | 1.06 [0.98, 1.14] | 0.15 |
| 2011 - 2014 | 1.13 [1.06, 1.2] | <0.01 | 1.21 [1.13, 1.3] | <0.01 |
| Clinical trial participation | ||||
| No | Ref | Ref | ||
| Yes | 0.51 [0.29, 0.91] | 0.02 | 0.76 [0.41, 1.4] | 0.379 |
| Treatments | ||||
| None | Ref | Ref | ||
| Surgery | 0.45 [0.39, 0.51] | <0.01 | 0.46 [0.4, 0.53] | <0.01 |
| Radiation | 6.64 [6.06, 7.27] | <0.01 | 7 [6.36, 7.7] | <0.01 |
| Systemic therapy | 1.24 [1.14, 1.36] | <0.01 | 1.25 [1.14, 1.38] | <0.01 |
| Surgery + Radiation | 3.82 [3.37, 4.34] | <0.01 | 4 [3.5, 4.57] | <0.01 |
| Surgery + Systemic | 0.73 [0.65, 0.81] | <0.01 | 0.75 [0.66, 0.84] | <0.01 |
| Radiation + Systemic | 6.2 [5.69, 6.75] | <0.01 | 6.36 [5.8, 6.97] | <0.01 |
| Surgery + Radiation + Systemic | 4.6 [4.14, 5.11] | <0.01 | 4.87 [4.34, 5.45] | <0.01 |
| Unknown | 0.96 [0.78, 1.19] | 0.72 | 0.97 [0.78, 1.21] | 0.81 |
Given the strong association between type of treatment and palliative intervention (Supplementary Figure 2), we performed an additional analysis to identify factors associated with PI utilization among patients treated with surgery only, radiation only, systemic therapy only, or surgery+systemic therapy (Figure 2, Supplementary Table 1–4). A multivariable logistic regression model was created for each treatment and each model was adjusted for all available covariates. Among patients who received surgery, sarcomatoid histology were associated with increased likelihood of receiving PI (Figure 2, Supplementary Table 1).
Figure 2: Forest plot based on multivariable logistic regression model predicting receipt of palliative intervention by treatment type.
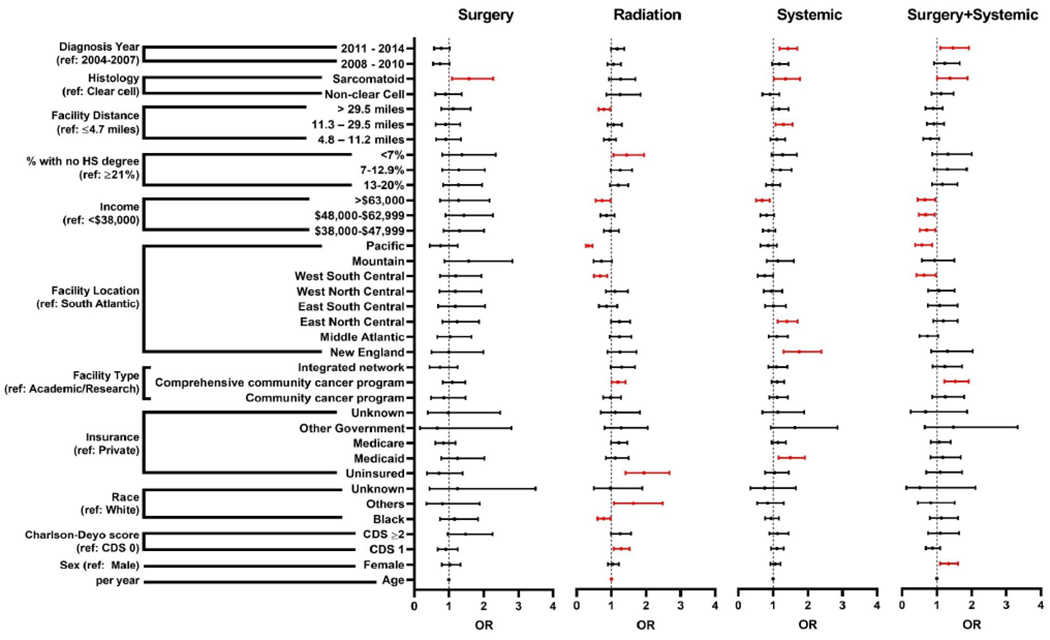
Odds ratios (OR) with 95% confidence intervals are shown. Red intervals represent statistically significant OR (p<0.05).
Among patients who received radiation therapy, CDS 1, other race, no insurance, care at comprehensive community cancer program, and higher education status were more likely to receive PI (Figure 2, Supplementary Table 2). In contrast, Black race, being treated in West South Central or Pacific regions, and travel distance to facility >29.5 miles were associated with decreased likelihood of PI use.
Patients who received systemic therapy were more likely to receive PI if they had Medicaid, received treatment in New England or East North Central region, were 11.3-29.5 miles from facility, had sarcomatoid histology, were diagnosed in 2011-2014 (Figure 2, Supplementary Table 3). Within this cohort, patients with incomes >$63,000 were less likely to receive palliative interventions.
Finally, female patients, treatment at a comprehensive community cancer program, sarcomatoid histology, and contemporary diagnosis were associated with increased likelihood of PI utilization among patients who had received both surgery and systemic therapy for Stage IV RCC (Figure 2, Supplementary Table 4). Patients receiving treatment in the Pacific regions, and with incomes of $38,000-$62,999 were less likely to receive PI.
Discussion
PI utilization has been shown to improve patient QOL, while decreasing hospital usage and cost for various malignancies.3, 4, 10 However, trends in utilization of PI for Stage IV RCC have yet to be evaluated and factors that influence its usage are not well established. We demonstrate that use of PI has minimally increased from 17 to 20% for Stage IV RCC. Factors influencing the use of PI varied within the overall cohort and based on the type of treatments patients received for metastatic RCC. We demonstrate the odds of PI use are approximately 7, 6.4, 4.9, and 4 times higher for patients who received radiation therapy, radiation+systemic therapy, surgery+radiation+systemic therapy, and surgery+radiation, respectively, for Stage IV RCC compared to no therapy.
Recent work from Lec et al. also evaluated PI among patients diagnosed with Stage IV bladder, prostate, and kidney cancer using the NCDB.11 Lec et al. demonstrate that private insurance, survival 6-24 months and >24 months, other or Black race, age 65-79 and ≥80 were associated with decreased likelihood of receiving PI for metastatic RCC. Key differences between this work and ours exist. Our multivariable models include additional variables such as education, facility location, facility distance, histology, and prior treatments that can significantly influence multivariable models. We also establish a temporal trend of PI utilization among Stage III/ IV RCC patients and identify factors for receipt of PC based on prior treatment types.
In an era where the armamentarium against advanced and metastatic RCC continues to expand, the prevalence of patients living with recurrent or metastatic disease is likely to increase as well. Thus, the need for PI will continue to rise as well. Other genitourinary malignancies, such as bladder and prostate cancer, have also seen a limited rise in PC.12–14 Several professional societies, such as American Society of Clinical Oncology (ASCO), the National Comprehensive Cancer Network (NCCN), and the Kidney Cancer Association (KCA), have introduced guidelines for the early integration of PI into patient care. In fact, concurrent PC consultation at the time of outpatient urological visits was associated increased rates of hospice use without changes to QOL or satisfaction among patients with metastatic prostate, bladder, or kidney cancers.15 Additionally, inpatient PC consultation for metastatic prostate cancer was associated with increased use of do-not-resuscitate orders, increased transfer to hospice, and decreased cost of treatment.16 Furthermore, the Project ENABLE II randomized controlled trial showed that patients who received nurse-led PC within 8 weeks of an advanced cancer diagnosis had higher QOL and mood without any difference in overall survival.17 This underscores the need for establishing an integrated approach for the initiation of PC concurrently with oncologic treatment and further provider/patient education regarding its benefits.
Our data suggest that a significant proportion of Stage IV RCC patients do not receiving PI, which is likely due to patient, physician, and infrastructural barriers. Patient-related factors such as decreased awareness of PC, misperceptions of palliative therapies, and unclear goals-of-care all remain barriers for expanding PC use.18, 19 Likewise, physicians may be unskilled or uncomfortable with initiating PC, are driven by referral patterns, and are likely interested in managing long-term patients.18, 20, 21 Our data corroborates this, as patients who received surgery were less likely to receive palliative interventions. Patient selection may influence which patients are offered surgery, as sarcomatoid histology was associated with likelihood of receiving PI.
Compounding these problems are infrastructural issues such as critical shortage of PC providers, regionalization of care, and distinct criteria for initiating PC.22, 23 Our data support this as noted by regional differences in use of palliative interventions in the overall cohort and treatment-based cohort. Our findings are further corroborated by work from Morrison et al. which demonstrated higher prevalence of PC care teams in New England and East North Central and lower prevalence of PC care teams mainly in states in the West South Central region.2 In 2011, the American College of Surgeons’ Commission on Cancer, which runs the NCDB, integrated palliative care into the standards for accreditation and may have consequently led to changes in coding practices. While temporal trends do not show an increase in PI use after 2011, multivariable analysis highlighted that diagnosis from 2011-2014 was associated with increased likelihood of PC use in the overall cohort, systemic therapy cohort, and surgery+systemic therapy cohort.
Our work suggests that demographic and socioeconomic factors may also contribute to utilization of palliative interventions. In accordance with Roeland et al., our data also demonstrate that females are more likely to receive PI.24 Racial disparities also existed, with Blacks being less likely to receive palliative radiation therapy while other races were more likely to receive palliative radiation therapy. Racial disparities among prostate and kidney cancers have also been shown to contribute to reduced PI utilization. Work from Abdollah et al. highlighted that Black patients with metastatic prostate cancer were less likely to have diagnostic and therapeutic interventions, while having higher rates of aggressive and high-intensity end-of-life care compared to their white counterparts.12 Lec et al. showed that minorities were less likely to receive PI for prostate and kidney cancer.11 While literature on racial disparities in PC delivery is well-established and robust, our work further establishes that Black patients receiving radiation therapy for Stage IV RCC are less likely to receive palliative treatment compared to their white counterparts. Taken together, these findings indicate potential racial distrust and/or cultural biases towards palliation, or how palliative services are offered,25, 26 and highlight the need to establish culturally competent pathways to reach vulnerable and historically underserved/excluded patient populations more effectively.
Our work has several important implications. First, it adds to the collective literature supporting a heightened recognition of palliative therapies in urologic oncology.14, 15, 18, 27, 28 National organizations, such as KCA, ASCO, NCCN, have advocated for early adoption of palliative treatment in the management of patients with kidney cancers. We feel that establishing a streamlined multidisciplinary pathway for patients with ≥T3N1Mx-1 disease is of utmost importance as these patients are likely need additional systemic therapies and will have multiple sequelae during the disease course. During the disease course, palliation intensity can be altered to address the patient’s needs. Initiating a team-led conversation about goals-of-care, QOL measures, and symptom management can improve patient-reported outcomes.29 This also provides an educational opportunity to address disparities in usage of palliative services among different subspecialities and empower a team of clinicians who can provide different levels of supportive care. Furthermore, it provides an opportunity to formally teach residents and fellows in subspecialities, such as urology or urologic oncology, how to initiate primary palliative care measures and how to establish the appropriate dialogue with patients to aid in bridging the gap prior to consulting a PC specialist. Our work also highlights the growing need for secondary and tertiary PC overall and in specific regions within the US, especially as therapeutics/interventions are prolonging life expectancy in patients with advanced malignancies.30 Initiatives to train advanced nurse practitioners and physicians to fill this gap is of utmost importance. As the role of PC interventions are to increase QOL while decreasing symptom burden, reduced higher level of care and cost can be motivating outcomes for healthcare systems. Therefore, earlier adoption of supportive care can help mitigate resource utilization while providing improved QOL for patients in need.
The findings of our study should be interpreted considering several limitations. First, the data abstracted from the NCDB only allows for a retrospective analysis. As such, granular data to identify the overall population at risk and the subset of patients that would most likely benefit from palliative treatments are difficult to ascertain. While our work identifies several factors that contribute to PI use among patients who received multiple lines of therapies for metastatic RCC, further work is necessary to identify the true extent of the population that would benefit from PC intervention. Next, the NCDB reports the type of PI patients received but does not report when these interventions were performed during the disease course or the associated costs with these interventions. Subsequent palliative interventions after the first course therapy are not captured in the NCDB. Additionally, we do not have granular data on what patient-level decisions, such as advanced directives, do-not-resuscitate status, and psychosocial/spiritual counseling, and adjunctive therapies were made with PI. Third, while the NCDB provides the largest database that reports on PI use, variations in coding could impact how well PI are coded. Finally, as survival is a surrogate for many oncologic studies, patient-reported outcomes on QOL, depression/mood, and psychosocial needs are more established surrogates for PI given that this cohort has worse outcomes a priori. Therefore, we plan to assess this in future studies using validated surveys and qualitative methods that have been established in other genitourinary malignancies like prostate and bladder cancer.
Conclusions
We find that use of PI in Stage IV RCC has minimally increased from 17% to 20% from 2004 to 2014. Multiple socioeconomic and clinical factors are independent predictors for their use among patients with Stage IV RCC. Patients who received radiation therapy, radiation+systemic therapy, surgery+radiation+systemic therapy, and surgery+radiation have significantly increased likelihood of receiving PI. We establish multiple factors that are independent predictors for PI use among patients who have received different lines of therapies for Stage IV RCC. Taken together, this work supports expanding the metrics used to capture PI usage within administrative databases, identifies a subgroup of RCC patients who could benefit from earlier and increased PC utilization, illustrates the need for enhanced primary PC education for providers treating patients with advanced RCC, and supports adopting multidisciplinary efforts to deliver secondary and tertiary PC to RCC patients.
Supplementary Material
Highlights.
Palliative interventions for Stage IV RCC are poorly understood.
Palliative intervention use has minimally increased for metastatic RCC.
Several factors affect palliative intervention use for metastatic RCC.
Treatment-specific factors influence palliative intervention use for metastatic RCC
Clinical Practice Points.
Palliative interventions (PI) for several malignancies have several benefits and consequently, several guidelines have adopted early integration of PI in oncologic care. However, the use of PI for Stage IV RCC is not well understood. Results of this study demonstrate that despite these guidelines, the use of PI for Stage IV RCC has minimally increased. PI use is influenced by several clinical, demographic, and socioeconomic factors. Considering the evolving need to initiate PI early in oncologic care, this study maybe used to inform efforts to establish pathways for increasing PI use for Stage IV RCC.
Acknowledgement
The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Funding
This work is supported by a grant from the National Cancer Institute (P30CA072720).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
Tina M. Mayer has acted as a paid consultant for ICON Medical and AstraZeneca for work performed outside of the current study. Biren Saraiya has acted as a paid member of the Advisory Board for Eisai Corporation and Sanofi Inc for work performed outside of the current study.
References
- 1.Hugar LA, Wulff-Burchfield EM, Winzelberg GS, Jacobs BL, Davies BJ. Incorporating palliative care principles to improve patient care and quality of life in urologic oncology. Nat Rev Urol. 2021. 10.1038/s41585-021-00491-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrison RS, Dietrich J, Ladwig S, et al. Palliative care consultation teams cut hospital costs for Medicaid beneficiaries. Health Aff (Millwood). 2011;30:454–463. 10.1377/hlthaff.2010.0929 [DOI] [PubMed] [Google Scholar]
- 3.Khandelwal N, Kross EK, Engelberg RA, Coe NB, Long AC, Curtis JR. Estimating the effect of palliative care interventions and advance care planning on ICU utilization: a systematic review. Critical care medicine. 2015;43:1102–1111. 10.1097/CCM.0000000000000852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. 10.1056/NEJMoa1000678 [DOI] [PubMed] [Google Scholar]
- 5.Association AU. Clinical Ethics for Urologists. Vol 20112008. [Google Scholar]
- 6.Dunn GP, Martensen R, Weissman D. Surgical Palliative Care: A Resident’s Guide. Chicago: American College of Surgeons; 2009:278. [Google Scholar]
- 7.Alliance WPC. Global Atlas of Palliative Care. 2nd ed. London, UK: World Health Organization; 2020. [Google Scholar]
- 8.Zisman A, Pantuck AJ, Wieder J, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 2002;20:4559–4566. 10.1200/JCO.2002.05.111 [DOI] [PubMed] [Google Scholar]
- 9.Nerich V, Hugues M, Paillard MJ, et al. Clinical impact of targeted therapies in patients with metastatic clear-cell renal cell carcinoma. Onco Targets Ther. 2014;7:365–374. 10.2147/OTT.S56370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higginson IJ, Evans CJ. What is the evidence that palliative care teams improve outcomes for cancer patients and their families? Cancer J. 2010;16:423–435. 10.1097/PPO.0b013e3181f684e5 [DOI] [PubMed] [Google Scholar]
- 11.Lec PM, Lenis AT, Brisbane W, et al. Trends in palliative care interventions among patients with advanced bladder, prostate, or kidney cancer: A retrospective cohort study. Urol Oncol. 2020;38:854 e851–854 e859. 10.1016/j.urolonc.2020.04.029 [DOI] [PubMed] [Google Scholar]
- 12.Abdollah F, Sammon JD, Majumder K, et al. Racial Disparities in End-of-Life Care Among Patients With Prostate Cancer: A Population-Based Study. J Natl Compr Canc Netw. 2015;13:1131–1138. 10.6004/jnccn.2015.0138 [DOI] [PubMed] [Google Scholar]
- 13.Mazzone E, Knipper S, Mistretta FA, et al. Trends and Social Barriers for Inpatient Palliative Care in Patients With Metastatic Bladder Cancer Receiving Critical Care Therapies. J Natl Compr Canc Netw. 2019;17:1344–1352. 10.6004/jnccn.2019.7319 [DOI] [PubMed] [Google Scholar]
- 14.Hugar LA, Lopa SH, Yabes JG, et al. Palliative care use amongst patients with bladder cancer. BJU Int. 2019;123:968–975. 10.1111/bju.14708 [DOI] [PubMed] [Google Scholar]
- 15.Huen K, Huang C, Liu H, et al. Outcomes of an Integrated Urology-Palliative Care Clinic for Patients With Advanced Urological Cancers: Maintenance of Quality of Life and Satisfaction and High Rate of Hospice Utilization Through End of Life. Am J Hosp Palliat Care. 2019;36:801–806. 10.1177/1049909119833663 [DOI] [PubMed] [Google Scholar]
- 16.Mistry NA, Raza SJ, Siddiqui SA. Analysis of Inpatient Palliative Care Consultations for Patients With Metastatic Prostate Cancer. Am J Hosp Palliat Care. 2020;37:136–141. 10.1177/1049909119864576 [DOI] [PubMed] [Google Scholar]
- 17.Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302:741–749. 302/7/741 [pii] 10.1001/jama.2009.1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar P, Casarett D, Corcoran A, et al. Utilization of supportive and palliative care services among oncology outpatients at one academic cancer center: determinants of use and barriers to access. J Palliat Med. 2012;15:923–930. 10.1089/jpm.2011.0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szekendi MK, Vaughn J, Lal A, Ouchi K, Williams MV. The Prevalence of Inpatients at 33 U.S. Hospitals Appropriate for and Receiving Referral to Palliative Care. J Palliat Med. 2016;19:360–372. 10.1089/jpm.2015.0236 [DOI] [PubMed] [Google Scholar]
- 20.Wancata LM, Hinshaw DB, Suwanabol PA. Palliative Care and Surgical Training: Are We Being Trained to Be Unprepared? Ann Surg. 2017;265:32–33. 10.1097/SLA.0000000000001779 [DOI] [PubMed] [Google Scholar]
- 21.Suwanabol PA, Kanters AE, Reichstein AC, et al. Characterizing the Role of U.S. Surgeons in the Provision of Palliative Care: A Systematic Review and Mixed-Methods Meta-Synthesis. J Pain Symptom Manage. 2018;55:1196–1215 e1195. 10.1016/j.jpainsymman.2017.11.031 [DOI] [PubMed] [Google Scholar]
- 22.Spetz J, Dudley N, Trupin L, Rogers M, Meier DE, Dumanovsky T. Few Hospital Palliative Care Programs Meet National Staffing Recommendations. Health Aff (Millwood). 2016;35:1690–1697. 10.1377/hlthaff.2016.0113 [DOI] [PubMed] [Google Scholar]
- 23.Harrison KL, Dzeng E, Ritchie CS, et al. Addressing Palliative Care Clinician Burnout in Organizations: A Workforce Necessity, an Ethical Imperative. J Pain Symptom Manage. 2017;53:1091–1096. 10.1016/j.jpainsymman.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roeland EJ, Triplett DP, Matsuno RK, et al. Patterns of Palliative Care Consultation Among Elderly Patients With Cancer. J Natl Compr Canc Netw. 2016;14:439–445. 10.6004/jnccn.2016.0050 [DOI] [PubMed] [Google Scholar]
- 25.Ornstein KA, Roth DL, Huang J, et al. Evaluation of Racial Disparities in Hospice Use and End-of-Life Treatment Intensity in the REGARDS Cohort. JAMA Netw Open. 2020;3:e2014639. 10.1001/jamanetworkopen.2020.14639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhodes RL, Batchelor K, Lee SC, Halm EA. Barriers to end-of-life care for African Americans from the providers’ perspective: opportunity for intervention development. Am J Hosp Palliat Care. 2015;32:137–143. 10.1177/1049909113507127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabow MW, Benner C, Shepard N, Meng MV. Concurrent urologic and palliative care after cystectomy for treatment of muscle-invasive bladder cancer. Urol Oncol. 2015;33:267 e223–269. 10.1016/j.urolonc.2015.02.012 [DOI] [PubMed] [Google Scholar]
- 28.Sinclair CT, Kalender-Rich JL, Griebling TL, Porter-Williamson K. Palliative Care of Urologic Patients at End of Life. Clin Geriatr Med. 2015;31:667–678. 10.1016/j.cger.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 29.Demme RA, Singer EA, Greenlaw J, Quill TE. Ethical issues in palliative care. Anesthesiol Clin. 2006;24:129–144.S0889-8537(05)00110-0 [pii] 10.1016/j.atc.2005.11.004 [DOI] [PubMed] [Google Scholar]
- 30.von Gunten CF. Secondary and tertiary palliative care in US hospitals. JAMA. 2002;287:875–881. 10.1001/jama.287.7.875 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


