Abstract
Background:
Beta-2 adrenergic receptor (ß2AR) modulates immune activation and may enhance trastuzumab activity. We assessed the impact of ß2AR gene (ADRB2) expression on the outcomes of patients with HER2-positive early-stage breast cancer enrolled on the NCCTG-N9831 trial.
Patients and Methods:
This is a post-hoc analysis of the NCCTG-N9831 trial, which compared chemotherapy (arm A) versus chemotherapy plus trastuzumab (arms B&C) as adjuvant treatment of patients with HER2-positive early-stage breast cancer, with disease-free survival (DFS) as primary endpoint. Gene expression levels retrieved by DASL assay were used to classify patients as ADRB2-high or ADRB2-low. Hazard ratios (HRs) were calculated by a Cox proportional model adjusted for prognostic variables and ADRB2 expression. Correlations between ADRB2 expression and stromal tumor-infiltrating lymphocyte (TIL) levels were assessed with Pearson coefficient. A multivariable Cox regression model with interaction term was performed to assess the interaction between ADRB2 expression and treatment arm; and ADRB2 expression and a 8-gene signature previously shown to predict trastuzumab benefit.
Results:
Overall, 1,282 patients were included (ADRB2-high [N=944] / ADRB2-low [N=338]). A high expression of ADRB2 was associated with a longer DFS (p=0.01) in the overall population. The addition of trastuzumab to chemotherapy improved DFS only in patients with ADRB2-high tumors (p<0.01). ADRB2 expression was correlated with TIL levels (r=0.24, p<0.001). No association between ADRB2 expression and the 8-gene trastuzumab benefit signature was observed (p=0.32).
Conclusion:
Our findings suggest that a high ADRB2 expression is a favorable prognostic factor and may identify patients with HER2-positive early-stage breast cancer who benefit from adjuvant trastuzumab.
Trial Registration:
Keywords: breast cancer, HER2, beta-2 adrenergic receptor, ADRB2, trastuzumab
Micro Abstract:
Beta-2 adrenergic receptor (ß2AR) modulates T-cell activation and previous studies have associated a high expression of the ß2AR gene (ADRB2) with a favorable prognosis in HER2-positive breast cancer patients. In this post-hoc analysis of the NCCTG-N9831 trial, a high ADRB2 expression was associated with longer disease-free survival and also identified patients who benefited from adjuvant trastuzumab.
Introduction
The development of trastuzumab - a monoclonal antibody that targets the human epidermal growth factor receptor 2 (HER2) - has dramatically improved the outcomes of patients with HER2-positive breast cancer, both in the metastatic and the early-disease settings.1,2 Although more than 90% of patients with HER2-positive early-stage breast cancer remain alive and recurrence-free at 5 years with the standard combination of (neo)adjuvant chemotherapy and HER2 blockade, some of these patients still present with recurrences, and thus the development of prognostic and predictive biomarkers is crucial to support physicians to personalize and refine treatment decisions for this population.3–5
The beta-2 adrenergic receptor (ß2AR) is a G protein-coupled receptor that mediates physiologic processes such as smooth muscle relaxation, chronotropism and inotropism when activated by catecholamines.6 ß2AR is also involved at different stages of inflammatory and immune responses, although its role in these processes is not fully elucidated: whereas some studies show that ß2AR activation leads to the recruitment and differentiation of macrophages, production of pro-inflammatory cytokines, differentiation of immature T cells into T helper lymphocytes, and T cytotoxic lymphocyte activation, other preclinical data suggest that ß2AR stimulation may impair T-cell function whereas the blockade of the ß2AR pathway with ß - blockers promotes immune activation in murine cancer models.7–10 As the induction of an immune response against cells that express HER2 is an important mechanism by which trastuzumab exerts its effects, ß2AR may participate in the immune activation and T cell recruitment observed in the tumoral stroma of some patients after trastuzumab administration, and hence ß2AR may influence trastuzumab activity.11–13
In line with preclinical studies that show a significant cross-talk between G protein-coupled receptors, such as ß2AR, and epidermal growth factor receptors, such as HER2, tumor samples from patients with HER2-positive breast cancer present high expression levels of the ß2AR gene (ADRB2).14–16 Furthermore, in preclinical models of HER2-positive breast cancer, HER2 activation induces the synthesis of catecholamines (ß2AR agonists), whereas ß2AR activation increases HER2 expression on the cell membrane.17 As a consequence, ß2AR activation and ADRB2 expression may render HER2-positive cells more dependent on HER2 signalling.17 In this context, the potential interaction between ß2AR and HER2 in patients with breast cancer warrants further investigation.
Previous studies suggest that a high ADRB2 expression may be associated with a favorable prognosis in patients with early-stage breast cancer.14,15 The present study assessed the influence of ADRB2 expression on the outcomes of patients with HER2-positive early-stage breast cancer enrolled on the North Central Cancer Treatment Group (NCCTG) - N9831 trial (NCCTG is now part of the Alliance for Clinical Trials in Oncology [Alliance]).
Methods
Patients
The NCCTG-N9831 trial enrolled 3,505 patients with HER2-positive early-stage breast cancer previously submitted to surgery to receive 4 cycles of doxorubicin and cyclophosphamide q21 days (all patients), followed by either weekly paclitaxel alone (arm A); paclitaxel followed by trastuzumab (arm B); or paclitaxel administered concomitantly with trastuzumab (arm C) (Supplementary Figure 1 and protocol available as Supplementary material). The trial’s main objective was to evaluate the benefit of adding trastuzumab to adjuvant chemotherapy and disease-free survival (DFS) was the primary endpoint.18,19 A statistically and clinically significant DFS improvement yielded by the addition of trastuzumab to adjuvant chemotherapy was demonstrated in the NCCTG-N9831 trial.18–20
The present study is an exploratory post-hoc analysis of the NCCTG-N9831 trial. For the purpose of this study, arms B and C were pooled (arms B&C) in order to represent patients who received adjuvant trastuzumab plus chemotherapy, whereas arm A represents patients who received chemotherapy alone. The Mayo Clinic institutional review board and the Correlative Science Committee of the North American Breast Cancer Group approved this study. Signed informed consent for use of data and samples was obtained from all patients prior to study enrolment.
Gene expression analyses
From the overall 3,505 patients enrolled on the NCCTG-N9831 trial, 1,282 samples (arm A, 433; arm B, 477; arm C, 372) were evaluable for gene expression profiling, and gene expression data were retrieved by DASL assay as previously described (Supplementary Figure 2).21,22 All gene expression analyses were performed centrally at Mayo Clinic Comprehensive Cancer Center, Rochester, USA. The ideal cut-point for ADRB2 expression assessed as a continuous variable for the outcome survival was determined in the overall population per SAS software (=11.903), with levels above this cut-point defined as high (ADRB2-high) and levels below this cut-point as low (ADRB2-low). The cut-point adopted in this study was tested in a previous dataset from patients with HER2-positive early-stage breast cancer as a validation cohort.23
Tumor-infiltrating lymphocytes (TILs)
Histopathologic analysis of the percentage of stromal TILs was performed in a single hematoxylin-eosin–stained section from each tumor using criteria proposed by Denkert et al, Loi et al, Adams et al, and Salgado et al.24–27 TIL levels were defined as the percentage of tumor stroma containing infiltrating lymphocytes that were not in direct contact with tumor cells from an assessment of the entire tumor-containing area of the section. Areas of non-invasive cancer or artefacts were excluded. Histopathologic analyses were conducted centrally by 2 pathologists who were blinded to patient’s treatment, tumor staging and outcome. Patients were classified into 3 categories according to TIL levels (<5%; 5–20%; and >20%) and the correlation between ADRB2 expression and TIL levels was assessed. DFS according to TIL levels was assessed in the overall population and according to ADRB2 expression.
8-Gene Trastuzumab-benefit Signature
A gene expression signature consisting of eight genes associated with the HER2 (ERBB2, c17orf37, GRB7) and the estrogen receptor (ESR1, NAT1, GATA3, CA12, IGF1R) pathways has been shown to predict the magnitude of adjuvant trastuzumab benefit in patients with HER2-positive early breast cancer from NSABP B-31 and NCCTG N9831 trials by categorizing patients into 3 subgroups according to trastuzumab benefit (large, moderate and no-benefit), as previously described.28,29 We investigated the association between ADBR2 expression and the trastuzumab-benefit subgroups according to the 8-gene signature in our population. The interaction between ADBR2 expression and the subgroups that benefit from trastuzumab (large and moderate) per the 8-gene signature was also assessed.
Objectives
The main objectives of this study were:
To evaluate DFS according to ADRB2 expression.
To evaluate ADRB2 expression as a predictor of trastuzumab benefit.
Subgroup analyses to evaluate patient outcomes according to ADRB2 expression per treatment arm were performed.
Secondary objectives were to assess the correlation between ADRB2 expression and TIL levels, to analyse patient outcomes according to ADRB2 expression per TIL levels and to evaluate the association between ADRB2 expression and the 8-gene trastuzumab-benefit signature subgroups.
Endpoint
As in the NCCTG-N9831 trial, the primary endpoint of this study is DFS, defined as the time between randomization and occurrence of loco-regional or distant recurrence of breast cancer; development of contralateral breast cancer or second primary cancer other than squamous or basal cell carcinoma of the skin, carcinoma in situ of the cervix, or lobular carcinoma in situ of the breast; or death from any cause.18,19
Statistical Analysis
DFS curves were estimated with Kaplan Meier method and p values for comparisons were calculated with log-rank test; Hazard ratios were calculated by a Cox proportional hazard model of multivariable analysis adjusted for age (≤50 vs >50 years); lymph node status (positive vs negative); tumor size (≤2 vs >2cm); histologic grade (3 vs <3); hormone-receptor status (negative vs positive); and ADRB2 expression (high vs. low), with 95% confidence intervals (CI) estimated by chi-square test and p values calculated by Wald test. A multivariable Cox regression model with interaction term was performed to assess the interaction between ADRB2 expression and treatment arm and the interaction between ADRB2 expression and the 8-gene trastuzumab-benefit signature. The correlations between ADRB2 expression and TIL levels were assessed by the Pearson-correlation coefficient. Associations between categorical variables were estimated by chi-square test. The p-values were considered significant if ≤0.05. Statistical analyses were performed with SAS software (version 9.4).
Results
Patient characteristics
A total of 1,282 patients (arm A, N=433; arms B&C, N=849) were included in the present study. Median age was 50 years (22–80); most patients had positive lymph nodes (86.1%), tumors ≤2cm (89.9%), with histologic grade 3 (72.8%) and approximately half had hormone receptor-positive disease (51.6%). In comparison to the 2,223 patients enrolled in the NCCTG-N9831 trial who were not included, high-grade and hormone receptor-negative tumors were more frequent amongst patients included in the present analysis. Nevertheless, the differences in terms of patient outcomes between the three arms for the patients included in this study were similar to those observed in the trial as a whole, as previously reported.21
According to ADRB2 expression levels, 944 patients were classified as ADRB2-high and 338 were classified as ADRB2-low. No significant differences in terms of clinicopathological characteristics were observed between patients with ADRB2-high and those with ADRB2-low tumors (Table 1).
Table 1 –
Characteristics of the patients included in this study.
| Variable | ADRB2-high (N=944) | ADRB2-low (N=338) | Total (N=1,282) | p value |
|---|---|---|---|---|
| Age | 0.6224 | |||
| Median | 49.5 (22–79) | 50.0 (23–80) | 50 (22 – 80) | |
| ≤50 years | 488 (51.7%) | 180 (53.3%) | 668 (52.1%) | |
| >50 years | 456 (48.3%) | 158 (46.7%) | 614 (47.9%) | |
| Lymph Node Status | 0.7235 | |||
| Negative | 133 (14.1%) | 45 (13.3%) | 178 (13.9%) | |
| Positive | 811 (85.9%) | 293 (86.7%) | 1104 (86.1%) | |
| Tumor size | 0.4006 | |||
| ≤2cm | 853 (90.4%) | 300 (88.8%) | 1153 (89.9%) | |
| >2cm | 91 (9.6%) | 38 (11.2%) | 129 (10.1%) | |
| Histologic grade | 0.4675 | |||
| Low/intermediate | 259 (27.7%) | 86 (25.7%) | 345 (27.2%) | |
| High | 675 (72.3%) | 249 (74.3%) | 924 (72.8%) | |
| Missing | 10 | 3 | 13 | |
| Hormone receptor status | 0.3935 | |||
| Negative | 464 (49.2%) | 157 (46.4%) | 621 (48.4%) | |
| Positive | 480 (50.8%) | 181 (53.6%) | 661 (51.6%) | |
| Menopausal status | 0.283 | |||
| Pre | 479 (50.7%) | 183 (54.1%) | 662 (51.6%) | |
| Post | 465 (49.3%) | 155 (45.9%) | 620 (48.4%) | |
| Arm | 0.337 | |||
| Arm A | 326 (34.5%) | 107 (31.7%) | 433 (33.8%) | |
| Arms B&C | 618 (65.5%) | 231 (68.3%) | 849 (66.2%) |
Abbreviations: ADRB2, beta-2 adrenergic receptor gene; cm, centimeters.
DFS according to ADRB2 expression – overall population
In the overall population (N=1,282), DFS was significantly longer in patients with ADRB2-high tumors when compared to those with ADRB2-low tumors (HR 0.77; 95% CI 0.63–0.96; p=0.01) (Figure 1).
Figure 1 –
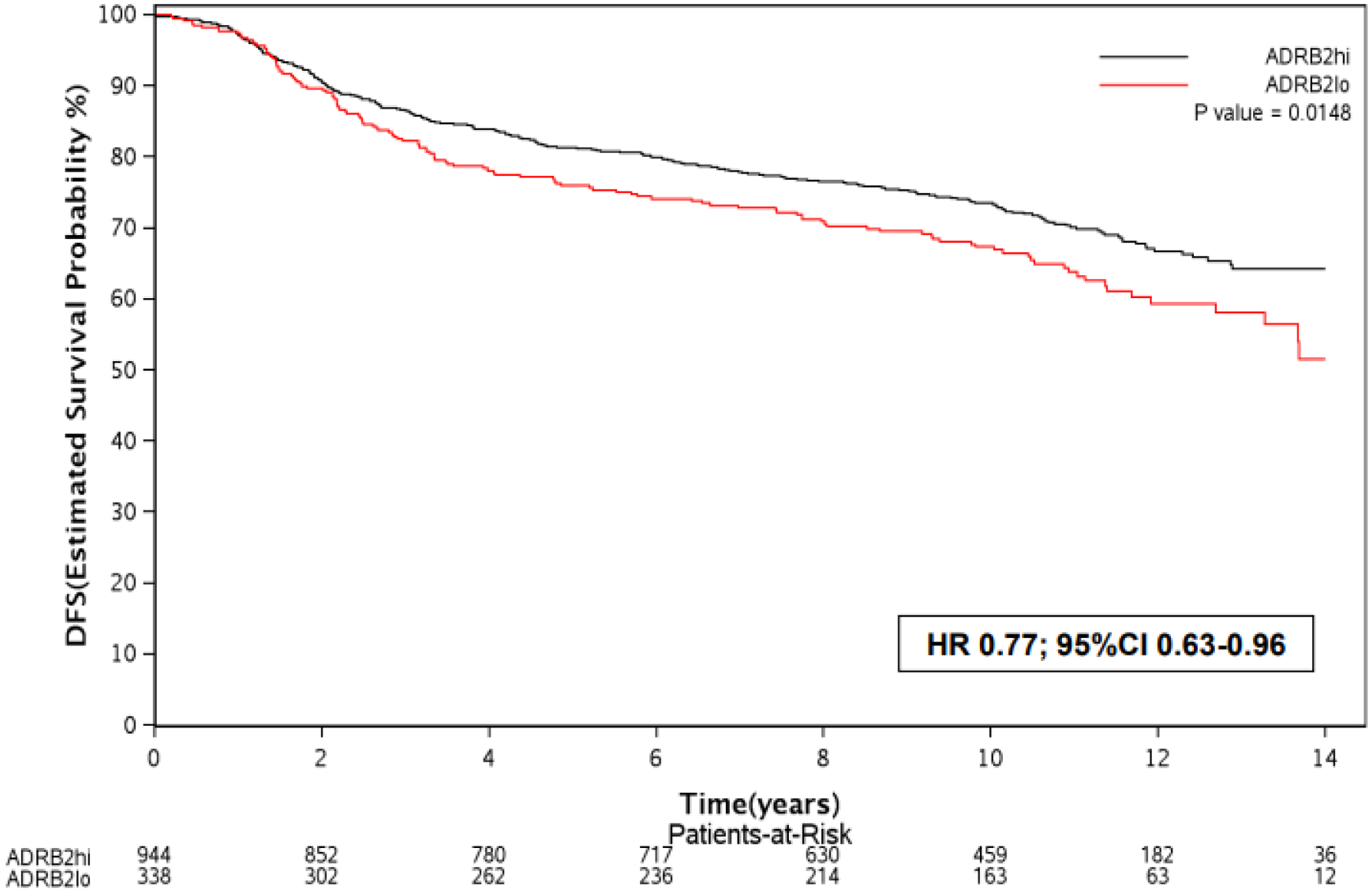
Kaplan Meier curves for DFS according to ADRB2 expression (high vs. low) in the overall population.
Abbreviations: ADRB2, beta-2 adrenergic receptor gene; DFS, disease-free survival; hi, high; lo, low.
In multivariable analysis adjusted for clinicopathological factors, age >50 years, lymph-node positive status and tumor size >2cm were associated with a worse prognosis, whereas hormone-receptor positive status was a favorable prognostic factor (Table 2).
Table 2 –
Multivariable Cox regression model assessing the impact of clinicopathological variables in terms of DFS in the overall population.
| Variable | Number of patients | DFS events | HR | 95% CI lower | 95% CI higher | p value |
|---|---|---|---|---|---|---|
| ADRB2 expression (high vs low) | 1269 | 393 | 0.77 | 0.63 | 0.96 | 0.02 |
| Age (>50 years vs ≤50 years) | 1269 | 393 | 1.22 | 1.00 | 1.49 | 0.05 |
| Lymph node (positive vs negative) | 1269 | 393 | 1.56 | 1.11 | 2.19 | 0.01 |
| Tumour size (>2cm vs ≤2cm) | 1269 | 393 | 1.49 | 1.11 | 1.99 | <0.01 |
| Histologic grade (high vs not high) | 1269 | 393 | 1.02 | 0.81 | 1.28 | 0.87 |
| Hormone receptor (positive vs negative) | 1269 | 393 | 0.77 | 0.63 | 0.95 | 0.01 |
Abbreviations: ADRB2, beta-2 adrenergic receptor gene; CI, confidence interval; cm, centimeters; HR, hazard ratio.
DFS according to ADRB2 expression – per treatment arm
In patients from arm A (N=433), no significant difference in terms of DFS was observed between patients with ADRB2-high tumors versus those with ADRB2-low tumors (HR 0.93; 95% CI, 0.65–1.32; p=0.68). In patients from arms B&C (N=849), DFS was significantly longer in those with ADRB2-high tumors when compared to those with ADRB2-low tumors (HR 0.69; 95% CI, 0.53–0.91; p<0.01) (Supplementary Table 1).
ADRB2 expression cut-point validation
Using the same cut-point for ADRB2 expression from this study in a publicly available dataset from patients with HER2-positive early-stage breast cancer (N=175), we observed a trend for a longer DFS in the ADRB2-high expression group (HR 0.55; 95% CI, 0.30–1.01; Supplementary Figure 3).23
Trastuzumab benefit according to ADRB2 expression
In patients with ADRB2-high tumors (N=944), the benefit of adding trastuzumab to chemotherapy was observed: DFS was significantly longer in arms B&C when compared to arm A (HR 0.65; 95% CI, 0.51–0.82; p<0.01) (Figure 2 A). In patients with ADRB2-low tumors (N=338), no significant DFS difference was observed between arms B&C versus arm A (HR 0.87; 95% CI, 0.60–1.26; p=0.47) (Figure 2 B). Patients from arms B&C with ADRB2-high tumors had a significantly longer DFS in comparison to the other subgroups (p<0.01) (Figure 2 C), however no significant interaction between ADRB2 expression and treatment arm was observed for the outcome DFS (p=0.19).
Figure 2 –
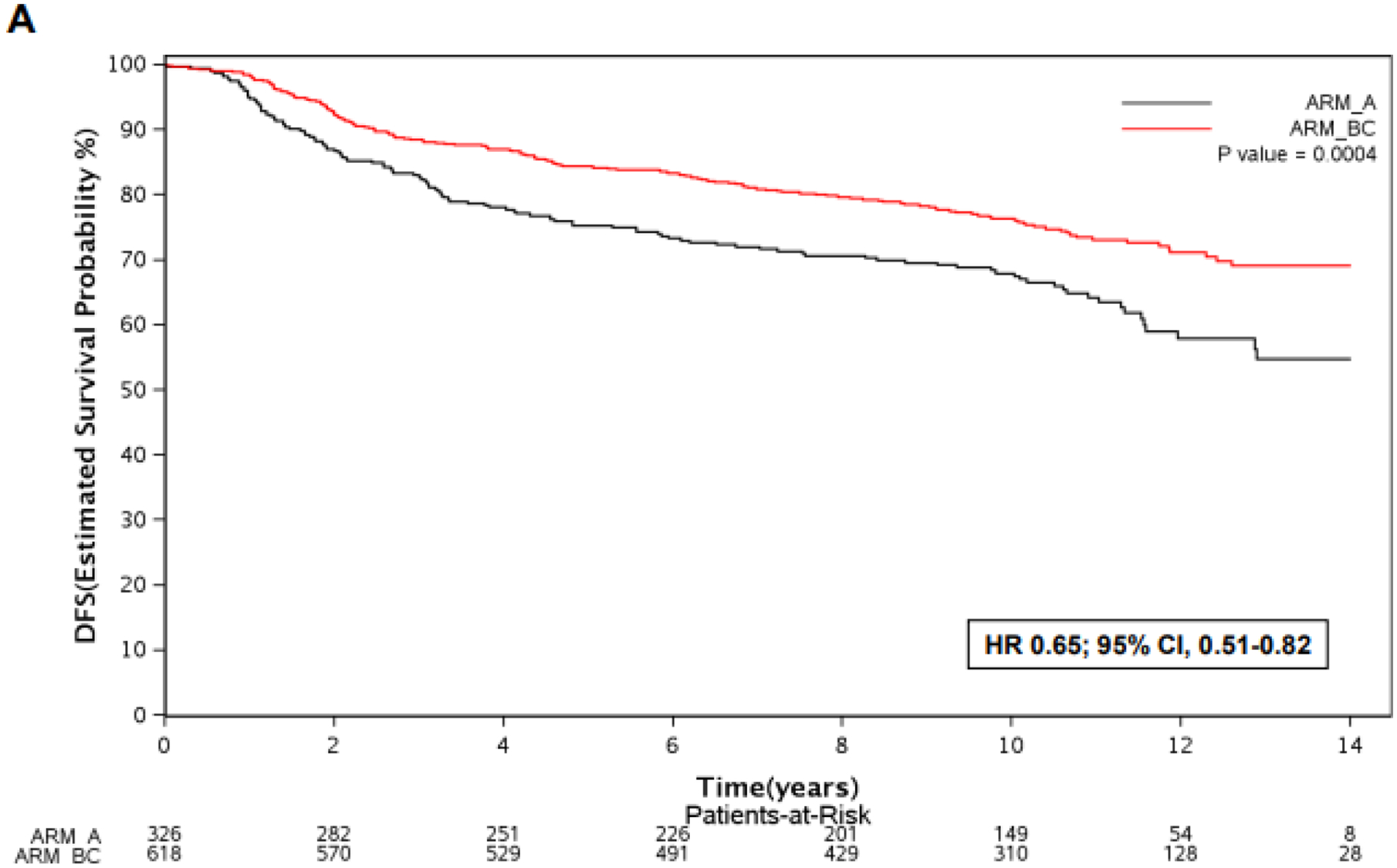
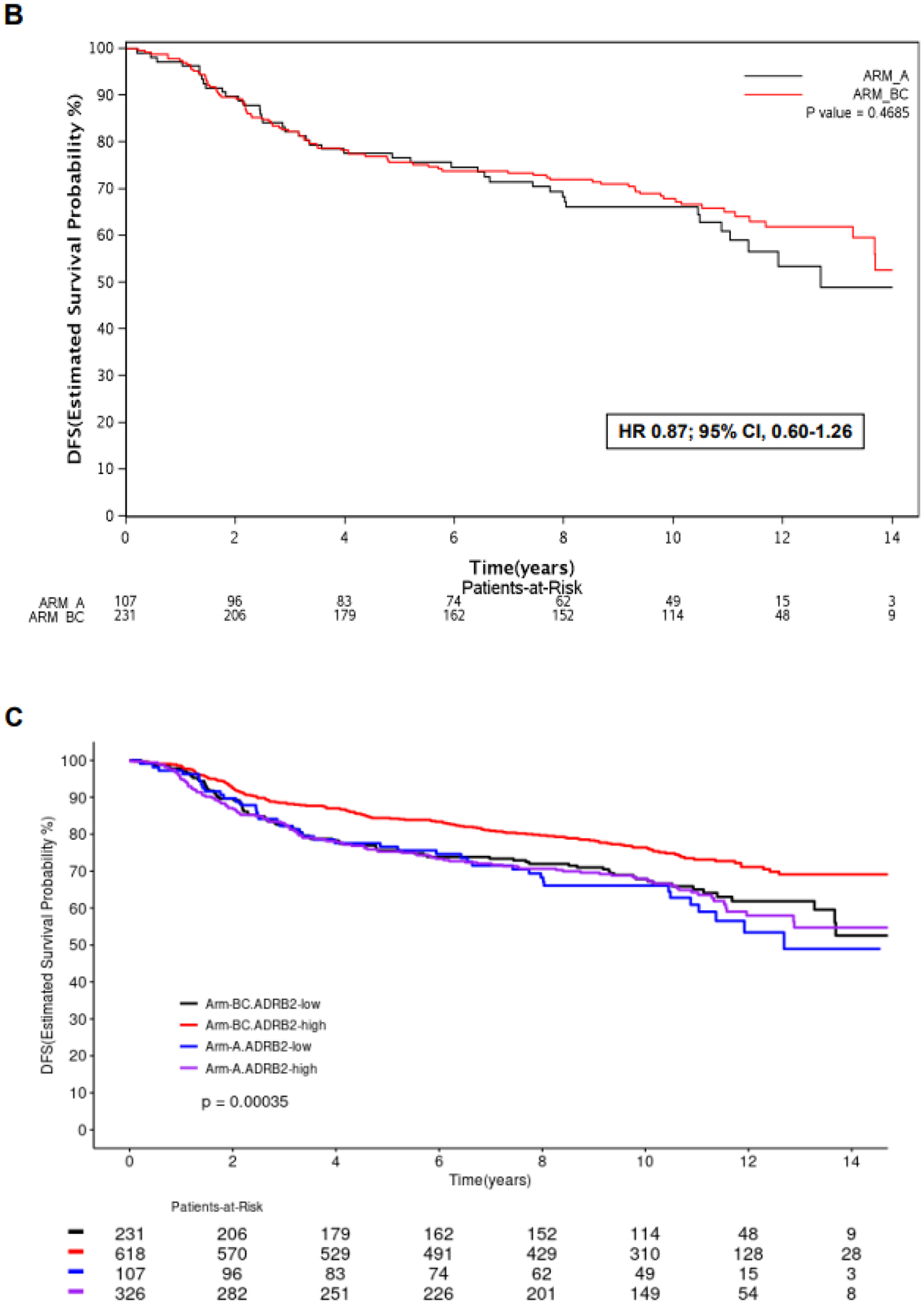
Kaplan Meier curves for DFS according to treatment arm (B&C vs. A) in patients with ADRB2-high tumors (A), ADRB2-low tumors (B), and DFS according to ADRB2 expression and treatment arm (C).
Abbreviations: ADRB2, beta-2 adrenergic receptor gene; DFS, disease-free survival; hi, high; lo, low; HR, hazard ratio.
ADRB2 expression and TILs
Out of the 1,282 patients included in this study, 566 had tumor samples evaluable for stromal TIL level assessment, out of whom 222 had TILs <5%; 222 had TILs between 5% and 20%; and 122 had TILs >20% (Supplementary Figure 2). ADRB2 expression was significantly correlated with TIL levels (r=0.24; p<0.01) (Figure 3).
Figure 3 –
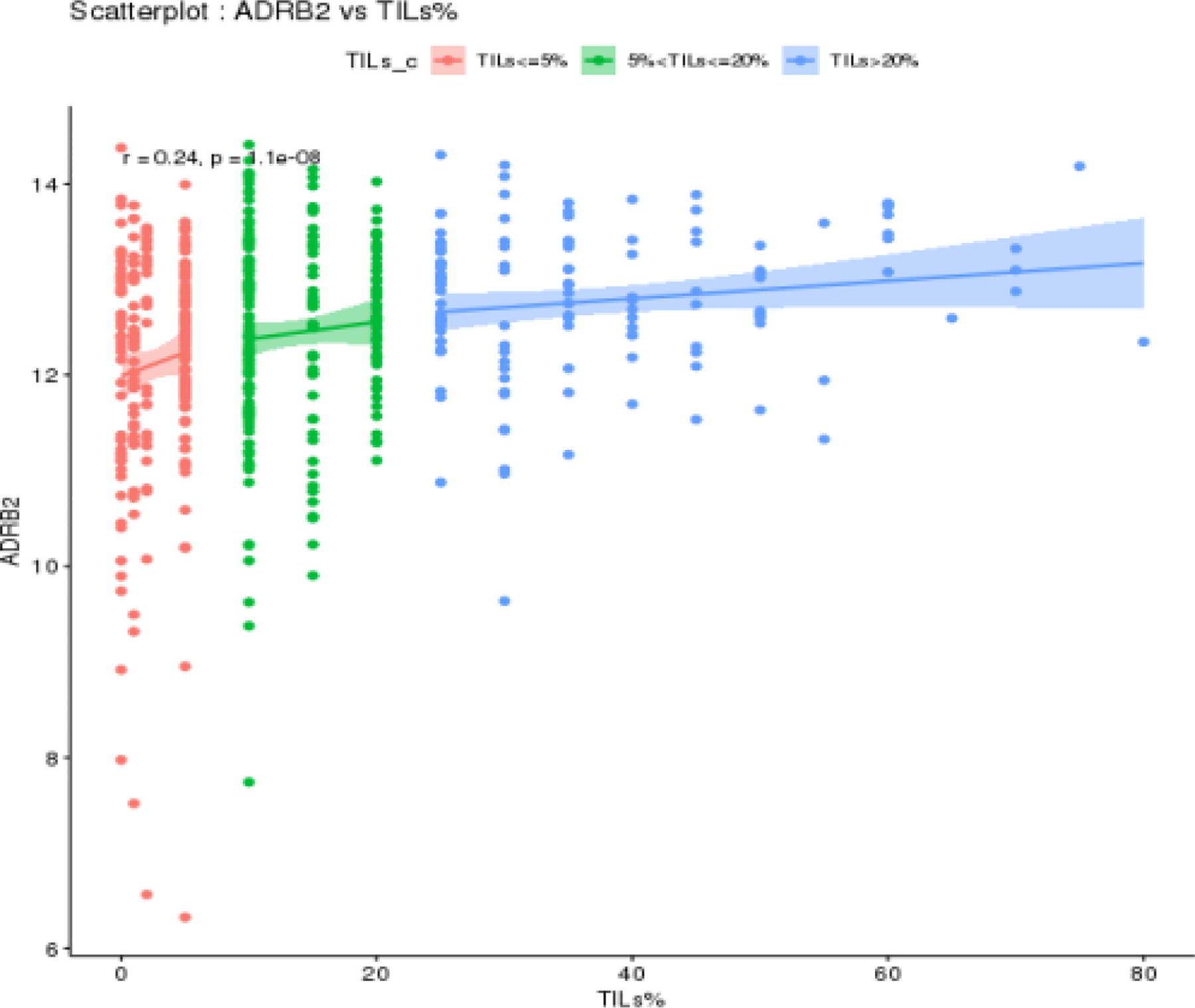
Scatterplot illustrating the correlation between ADRB2 expression and TIL levels (>20%, 5–20% and <5%) in patients who had samples evaluable for gene expression analyses and TIL levels assessment (N=566).
Abbreviations: ADRB2, beta-2 adrenergic receptor gene; DFS, disease-free survival; TILs, tumor-infiltrating lymphocytes.
In patients with ADRB2-high tumors (N=409), DFS was significantly longer in those whose tumors had TILs >20% when compared to those with TILs <5% (p=0.03), whereas no significant DFS difference was observed between patients whose tumors had TIL levels between 5–20% versus those with TILs <5% (p=0.60).
In patients with ADRB2-low tumors (N=157), no significant difference in terms of DFS was observed according to TIL levels (p=0.12) (Figures 4 A–B). Multivariable analyses adjusted for clinicopathological factors including TILs were performed in the 566 patients who had evaluable samples for TIL levels assessment (Supplementary Table 2).
Figure 4 –
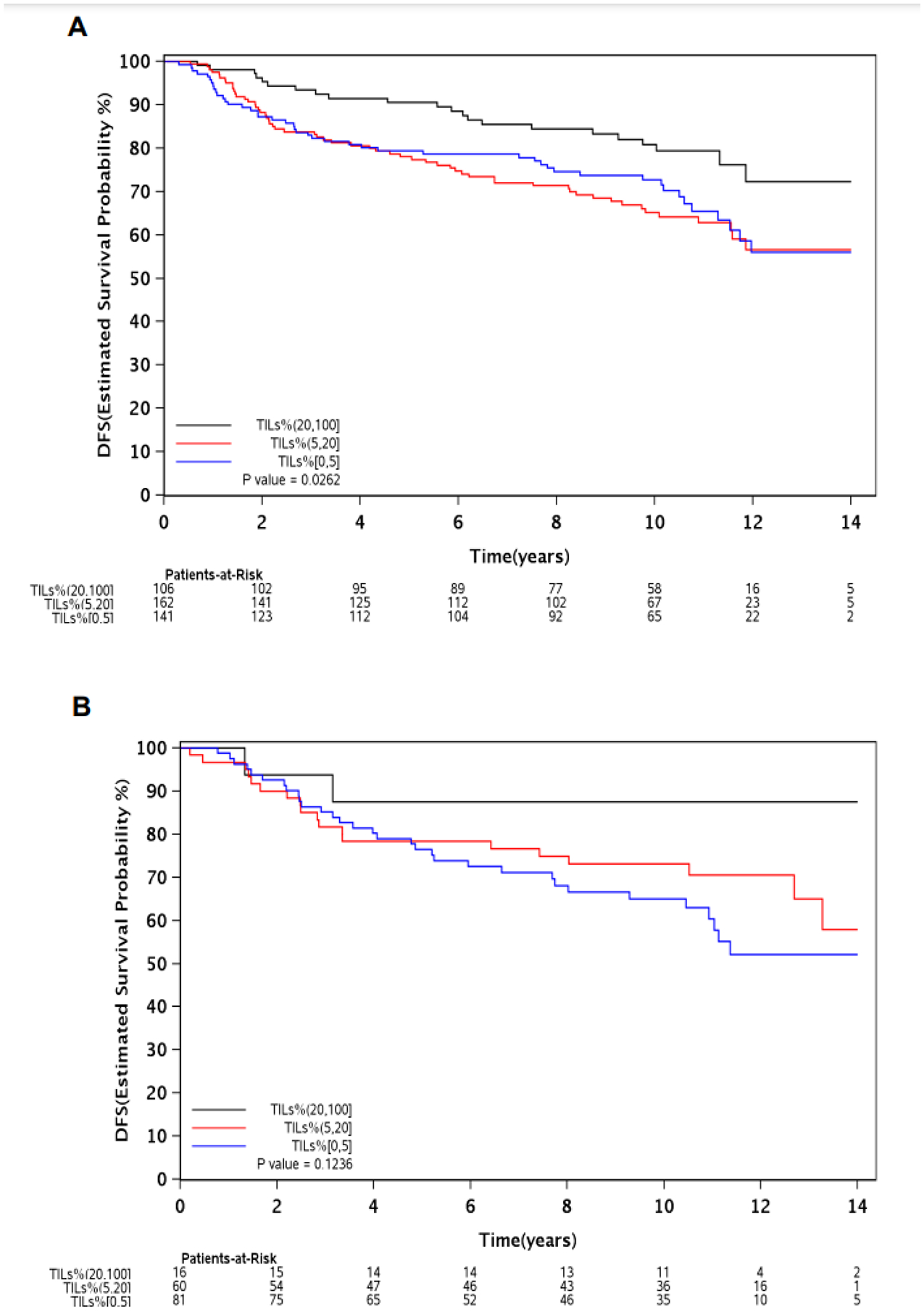
DFS according to TIL levels (>20%, 5–20% and <5%) in patients with ADRB2-high (A), and ADRB2-low (B) tumors.
Abbreviations: ADRB2, beta-2 adrenergic receptor gene; DFS, disease-free survival; HR, hazard ratio; TILs, tumor-infiltrating lymphocytes.
ADRB2 expression and 8-gene trastuzumab-benefit signature subgroups
When applying the 8-gene signature to our population, a total of 140 patients were categorized as no-benefit, 522 as moderate-benefit and 570 as large-benefit from trastuzumab. No significant association between ADRB2 expression and the 8-gene trastuzumab-benefit signature subgroups was observed in our study (p=0.32). No significant interaction occurred between ADRB2-high expression and moderate-benefit (p=0.59) or large-benefit (p=0.68) trastuzumab subgroups for the outcome DFS.
Discussion
In the present post-hoc analysis of the NCCTG-N9831 trial, a high expression of ADRB2 was associated with a favorable prognosis in patients with HER2-positive early-stage breast cancer, with this effect being mainly observed in those who received trastuzumab plus chemotherapy (arms B&C). Interestingly, the ADRB2-high expression profile also identified a subgroup of patients who appeared to benefit from adjuvant trastuzumab in the trial. The observed correlation between ADRB2 expression and TIL levels suggests that ADRB2 may exert its effects by participating in lymphocyte recruitment and immune activation to potentialize trastuzumab anti-tumor effect.
Previous studies assessed the impact of ADBR2 expression on the outcomes of patients with breast cancer. When evaluating the expression of adrenergic receptor genes in 1,924 breast cancer patients (out of whom only 50 were HER2-positive), Rivero et al. found that a high expression of ADRB2 was associated with a longer distant-metastasis-free survival.14 Our group has previously assessed ADRB2 expression in 175 patients with HER2-positive early-stage breast cancer and observed a longer DFS in those whose tumors presented high levels of ADRB2 expression. In this study, ADRB2 expression was correlated with the expression of genes involved in immune activation, whereas it was negatively correlated with genes involved in angiogenesis and cell proliferation, suggesting potential biological mechanisms through which ADRB2 may exert its effects.15 By assessing ADRB2 expression in patients enrolled on a phase III trial, the present study reinforces the role of ADRB2 high expression as a potential prognostic factor in patients with HER2-positive early-stage breast cancer.
Chronic stress and adrenergic activation induce angiogenesis, stimulate cell motility and facilitate metastases development in preclinical models of breast cancer.30,31 In response to high catecholamine levels observed in states of chronic stress, a negative feedback mechanism occurs, consisting in the downregulation of adrenergic receptors mediated by an increase in their degradation and suppression of their expression.32 In this context, a high ADRB2 expression might be an indirect marker of low stress levels, which would potentially justify the prognostic effect of ADRB2 expression observed in our study. Moreover, as ADRB2 expression can be modulated by factors such as physical exercise (increases expression) and chronic stress (reduces its expression), interventions to minimize chronic stress or stimulate exercise are worthy of evaluation in future studies.33–35 Notably, the practice of physical exercise has been shown to reduce the risk of recurrence in patients with early-stage breast cancer.36
In patients with HER2-positive early-stage breast cancer, high TIL levels are usually associated with a favorable prognosis as well as predictors of pathologic complete response in several studies.37,38 Preclinical data suggest that ß2AR participates in several stages of immune activation by inducing the differentiation of T cells, increasing interferon gamma secretion and enhancing the activity of T cytotoxic lymphocytes.8 On this basis, the correlation between TIL levels and ADRB2 expression observed in our study highlights the role of the ß2AR as a potential mediator of immune activation in breast cancer patients. Furthermore, high TIL levels were prognostic mainly in patients whose tumors presented a high ADRB2 expression, suggesting that ß2AR may not only participate in TIL recruitment but also stimulate their anti-cancer activity.
The amplification and/or hyperexpression of HER2 is the standard method adopted in clinical practice to select patients who will receive HER2 blockade.1 Nonetheless, the fact that a significant proportion of patients with HER2-positive early-stage breast cancer present recurrences despite receiving the standard treatment whereas some patients enrolled on the NCCTG-N9831 trial are alive and recurrence-free after being treated with chemotherapy alone highlights the importance of identifying those who may not benefit from HER2 blockade, to whom alternatives to trastuzumab are needed.4 Moreover, trastuzumab administration is associated with substantial financial costs; with a 1-year length; and with a risk (albeit modest) of clinically relevant toxicities such as cardiac events.2,39–42 The identification of patients who do not benefit from trastuzumab would thus spare these individuals from unnecessary toxicities, offer them an opportunity to receive alternative treatments or to be enrolled on clinical trials, as well as to optimize financial resource application.4 In our study, only patients whose tumors had a high ADRB2 expression appeared to benefit from trastuzumab. If these findings are further validated, ADRB2 expression has the potential to be explored as a predictive biomarker to help clinicians in identifying patients who need alternatives to trastuzumab treatment. Furthermore, by providing prognostic information, ADRB2 expression may also identify a subset of patients with a favorable prognosis who may be candidates to de-escalation strategies.43
To further understand the mechanisms by which ADRB2 exerts its effects in HER2-positive early breast cancer, in light of the preclinical data that shows a significant cross-talk between HER2 and ß2AR pathways, we investigated the association between ADRB2 expression and a 8-gene trastuzumab-benefit signature that relies on the expression of genes related to the estrogen receptor and HER2 pathways.17 In the studies that described this signature, patients with HER2-positive early breast cancer who did not benefit from trastuzumab presented high levels of estrogen receptor and intermediate/low levels of HER2 expression, being most frequently categorized as luminal A and B per PAM50 subtypes, whereas those who benefitted from trastuzumab presented more frequently high levels of HER2 expression.44 ADRB2 expression was not associated with the 8-gene signature profiles that predict trastuzumab benefit in the present study, suggesting that ADRB2 effects may occur independently of its interaction with HER2 signalling. Notably, in previous studies with the 8-gene signature, patients who benefited from trastuzumab had higher TIL levels than those who identified as having no trastuzumab benefit.28,45 As in our study, in which ADRB2 expression was associated with TIL levels, these results suggest that the 8-gene signature might also be a marker of T-cell recruitment and immune activation in these patients. Noteworthy, these findings have to be interpreted cautiously as TIL levels alone were not predictive of trastuzumab benefit in the NCCTG-N9831 population.45
A high expression of ß2AR in immunohistochemistry has been previously associated with a worse prognosis in patients with HER2-positive breast cancer, whereas our study assessing the same pathway - but at the gene expression level - shows different results.46 Previous studies have demonstrated a very poor correlation between gene expression and protein synthesis.47,48 The lack of immunohistochemistry for ß2AR is a limitation of the present study. Whether ADRB2 expression correlates with ß2AR or if ß2AR expression in immunohistochemistry is prognostic/predictive in patients with HER2-positive breast cancer is a question that remains open and needs to be addressed in future studies. Hypothetically, ADRB2 may exert its effects through intracellular signalling at the RNA level rather than at the protein level (ß2AR).
Potential limitations have to be considered when interpreting our findings. Not all patients included in the NCCTG-N9831 trial had evaluable samples for gene expression analyses and TIL assessment, although patients enrolled in this study were representative of the trial’s population.21 An interaction between ADRB2 expression and treatment arm for the outcome DFS – which would strengthen our findings - could not be demonstrated. As this is to our knowledge the largest cohort of breast cancer patients in which ADRB2 expression has been evaluated as a biomarker, we defined the ideal cut-point to categorize ADRB2-high versus low expression groups based on our own population, increasing the risk of “overfitting”. However, the validation of the cut-point in a different cohort of patients with HER2-positive early-stage breast cancer yielded similar results, suggesting this cut-point may be applicable for future studies exploring the role of ADRB2 expression in patients with breast cancer.23
After the publication of the NCCTG-N9831 trial, pertuzumab, neratinib and trastuzumab-emtansine (T-DM1) have been incorporated into the treatment of HER2-positive early-stage breast cancer.41,42,49–51 It is unknown if the results of this study can be extrapolated to patients who received these contemporary therapies. Patients whose tumors were classified as ADRB2-high may represent those who exercise regularly, and thus physical exercise habits may have impacted their prognosis.35 Data regarding patient exercise habits and concomitant medications such as beta-blockers/beta-agonists that could have influenced our results were not evaluable, precluding analyses adjusted for these factors. Gene expression analyses were performed in samples obtained before enrolment, therefore any potential effect of trastuzumab in ADRB2 expression could not be evaluated dynamically. Although preclinical data supports a cross-talk between HER2 and ß2AR pathways, we did not observe an association between ADRB2 expression and the 8-gene signature containing genes related to HER2 and estrogen receptor pathways, reinforcing that further studies are needed to understand the potential mechanism through which ß2AR exerts its effects in HER2-positive breast cancer. Although the correlation between ADRB2 expression and TIL levels suggests that ADRB2 may act as an immune-modulator, TIL levels alone were not prognostic in patients who received trastuzumab in the NCCTG-N9831 trial.52
Despite the above-mentioned limitations, having the analyses performed in a subset of patients enrolled in a randomized controlled trial strengthens our findings. Moreover, the fact that a single gene (ADRB2) was used instead of complex multigene signatures renders the study’s methods more feasible to be reproduced in future studies.
Conclusion
This post-hoc analysis of the NCCTG-N9831 trial suggests that a high ADRB2 expression is a favorable prognostic factor and may identify a subset of patients with HER2-positive early-stage breast cancer who benefit from adjuvant trastuzumab. The ß2AR may exert its effects on HER2-positive breast cancer by rendering the tumoral stroma more immunogenic and stimulating TIL infiltration.
Supplementary Material
Highlights.
Beta-2 adrenergic receptor (ß2AR) mediates inflammation and T cell activation.
Previous data suggest ß2AR gene (ADRB2) expression is prognostic in HER2+ breast cancer.
We assessed if ADRB2 expression impacted outcomes of patients enrolled in the NCCTG-N9831 trial.
High ADRB2 expression was associated with a favorable prognosis and predicted trastuzumab benefit.
ADRB2 may exert its effects via immune activation and lymphocyte recruitment.
Clinical practice points.
Although the development of HER2 blockade has dramatically improved the outcomes of patients with HER2-positive breast cancer, additional prognostic and predictive biomarkers are needed to identify patients who do not benefit from HER2 blockade and to spare these individuals from unnecessary toxicities, offering them an opportunity to receive alternative treatments or to be enrolled on clinical trials, as well as to optimize financial resource application. Previous studies suggest that a high expression of the beta-2 adrenergic receptor gene (ADRB2) – involved in inflammation and lymphocyte activation - is associated with a favorable prognosis in patients with HER2-positive breast cancer. In this post-hoc analysis of the NCCTG-N9831 trial, a high ADRB2 expression was associated with a longer disease-free survival and also identified a subset of patients who benefited from the addition of trastuzumab to adjuvant chemotherapy. The expression of ADRB2 correlated with stromal tumor infiltrating lymphocyte (TIL) levels, suggesting that the beta-2 adrenergic receptor may interact with the tumoral immune microenvironment in HER2-positive breast cancer. ADRB2 expression arises as a promising biomarker to be further explored in patients with HER2-positive breast cancer.
Funding and support
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821, U10CA180882, and U24CA196171 (to the Alliance for Clinical Trials in Oncology). Also supported in part by funds from Genentech, Genomic Health, Breast Cancer Research Foundation; and Fondation Cancer Luxembourg (to F.R. and C.D.). Roche/Genentech provided trastuzumab for this study. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. https://acknowledgments.alliancefound.org
Competing interests
RC is currently employed by Novartis Pharma – Switzerland (but was not at the time this study was conceived, developed and finalized); received speaker honoraria from Boehringer Ingelheim, AstraZeneca and Janssen, and travel grants from AstraZeneca and Pfizer, outside of the submitted work. EP and AMA received research grants from Genentech and NIH (to the institution) for the NCCTG-N9831 study. AA has acted as advisor, received fees for lectures and research grants from Roche, Lilly, Amgen, ESAI, BMS, Pfizer, Novartis and MSD. MP is a board member of Radius; has received consultant honoraria from: AstraZeneca, Lilly, MSD, Novartis, Odonate, Pfizer, Roche-Genentech, Crescendo Biologics, Periphagen, Huya, Debiopharm, PharmaMar, G1 Therapeutics, Menarini, Seattle Genetics and Immunomedics. Institut Jules Bordet has received research grants from Roche/GNE, Radius, AstraZeneca, Lilly, MSD, GSJ/Novartis, Synthon, Servier, and Pfizer. SB received speaker honoraria from Targos, MSD and Natera, and research funds for his institution from Dako/Agilent and Roche/Ventana. EdA received honoraria from Roche-Genentech, research grant from Roche-Genentech (to the institution), and travel grants from Roche-Genentech and GlaxoSmithKline, outside the submitted work. FR, CD, EAT, YM and CDA declare no disclosures.
Glossary
- ß2AR
Beta-2 adrenergic receptor
- ADRB2
Beta-2 adrenergic receptor gene
- CI
confidence interval
- Cm
centimetres
- DFS
disease-free survival
- HER2
human epidermal growth factor receptor 2
- HR
hazard ratio
- NCCTG
North Central Cancer Treatment Group
- REMARK
Reporting Recommendations for Tumor Marker Prognostic Studies
- TILs
tumor infiltrating lymphocytes
- T-DM1
trastuzumab-emtansine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Prior presentations: The present study was presented as a poster at the European Society for Medical Oncology (ESMO) virtual conference that occurred between 18–22nd of September, 2020.
Ethics approval and consent to participate: The Mayo Clinic institutional review board and the Correlative Science Committee of the North American Breast Cancer Group approved this study.
References
- 1.Loibl S, Gianni L. HER2-positive breast cancer. The Lancet. 2017;389(10087):2415–2429. doi: 10.1016/S0140-6736(16)32417-5 [DOI] [PubMed] [Google Scholar]
- 2.Balduzzi S, Mantarro S, Guarneri V, et al. Trastuzumab-containing regimens for metastatic breast cancer. Cochrane Database Syst Rev. 2014;(6):CD006242. doi: 10.1002/14651858.CD006242.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gingras I, Gebhart G, de Azambuja E, Piccart-Gebhart M. HER2-positive breast cancer is lost in translation: time for patient-centered research. Nat Rev Clin Oncol. 2017;14(11):669–681. doi: 10.1038/nrclinonc.2017.96 [DOI] [PubMed] [Google Scholar]
- 4.Dieci MV, Miglietta F, Griguolo G, Guarneri V. Biomarkers for HER2-positive metastatic breast cancer: Beyond hormone receptors. Cancer Treat Rev. 2020;88:102064. doi: 10.1016/j.ctrv.2020.102064 [DOI] [PubMed] [Google Scholar]
- 5.Brandão M, Caparica R, Malorni L, Prat A, Carey LA, Piccart M. What Is the Real Impact of Estrogen Receptor Status on the Prognosis and Treatment of HER2-Positive Early Breast Cancer? Clin Cancer Res. 2020;26(12):2783–2788. doi: 10.1158/1078-0432.CCR-19-2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warne T, Moukhametzianov R, Baker JG, et al. The structural basis for agonist and partial agonist action on a β(1)-adrenergic receptor. Nature. 2011;469(7329):241–244. doi: 10.1038/nature09746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armaiz-Pena GN, Allen JK, Cruz A, et al. Src activation by β-adrenoreceptors is a key switch for tumour metastasis. Nat Commun. 2013;4(1):1403. doi: 10.1038/ncomms2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swanson MA, Lee WT, Sanders VM. IFN-gamma production by Th1 cells generated from naive CD4+ T cells exposed to norepinephrine. J Immunol Baltim Md 1950. 2001;166(1):232–240. [DOI] [PubMed] [Google Scholar]
- 9.Slota C, Shi A, Chen G, Bevans M, Weng N. Norepinephrine preferentially modulates memory CD8 T cell function inducing inflammatory cytokine production and reducing proliferation in response to activation. Brain Behav Immun. 2015;46:168–179. doi: 10.1016/j.bbi.2015.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashrafi S, Shapouri R, Shirkhani A, Mahdavi M. Anti-tumor effects of propranolol: Adjuvant activity on a transplanted murine breast cancer model. Biomed Pharmacother. 2018;104:45–51. doi: 10.1016/j.biopha.2018.05.002 [DOI] [PubMed] [Google Scholar]
- 11.Triulzi T, Regondi V, De Cecco L, et al. Early immune modulation by single-agent trastuzumab as a marker of trastuzumab benefit. Br J Cancer. 2018;119(12):1487–1494. doi: 10.1038/s41416-018-0318-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varadan V, Gilmore H, Miskimen KLS, et al. Immune Signatures Following Single Dose Trastuzumab Predict Pathologic Response to PreoperativeTrastuzumab and Chemotherapy in HER2-Positive Early Breast Cancer. Clin Cancer Res. 2016;22(13):3249–3259. doi: 10.1158/1078-0432.CCR-15-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griguolo G, Pascual T, Dieci MV, Guarneri V, Prat A. Interaction of host immunity with HER2-targeted treatment and tumor heterogeneity in HER2-positive breast cancer. J Immunother Cancer. 2019;7(1):90. doi: 10.1186/s40425-019-0548-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivero EM, Martinez LM, Bruque CD, Gargiulo L, Bruzzone A, Lüthy IA. Prognostic significance of α‐ and β2‐adrenoceptor gene expression in breast cancer patients. Br J Clin Pharmacol. Published online July 31, 2019:bcp.14030. doi: 10.1111/bcp.14030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caparica R, Richard F, Brandão M, Awada A, Sotiriou C, de Azambuja E. Prognostic and Predictive Impact of Beta-2 Adrenergic Receptor Expression in HER2-Positive Breast Cancer. Clin Breast Cancer. Published online March 2020:S1526820920300239. doi: 10.1016/j.clbc.2020.01.007 [DOI] [PubMed] [Google Scholar]
- 16.Luttrell LM, Rocca GJD, van Biesen T, Luttrell DK, Lefkowitz RJ. Gβγ Subunits Mediate Src-dependent Phosphorylation of the Epidermal Growth Factor Receptor: A SCAFFOLD FOR G PROTEIN-COUPLED RECEPTOR-MEDIATED Ras ACTIVATION. J Biol Chem. 1997;272(7):4637–4644. doi: 10.1074/jbc.272.7.4637 [DOI] [PubMed] [Google Scholar]
- 17.Shi M, Liu D, Duan H, et al. The β2-adrenergic receptor and Her2 comprise a positive feedback loop in human breast cancer cells. Breast Cancer Res Treat. 2011;125(2):351–362. doi: 10.1007/s10549-010-0822-2 [DOI] [PubMed] [Google Scholar]
- 18.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus Adjuvant Chemotherapy for Operable HER2-Positive Breast Cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122 [DOI] [PubMed] [Google Scholar]
- 19.Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32(33):3744–3752. doi: 10.1200/JCO.2014.55.5730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29(25):3366–3373. doi: 10.1200/JCO.2011.35.0868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez EA, Thompson EA, Ballman KV, et al. Genomic Analysis Reveals That Immune Function Genes Are Strongly Linked to Clinical Outcome in the North Central Cancer Treatment Group N9831 Adjuvant Trastuzumab Trial. J Clin Oncol. 2015;33(7):701–708. doi: 10.1200/JCO.2014.57.6298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan J-B, Yeakley JM, Bibikova M, et al. A versatile assay for high-throughput gene expression profiling on universal array matrices. Genome Res. 2004;14(5):878–885. doi: 10.1101/gr.2167504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haibe-Kains B, Desmedt C, Loi S, et al. A Three-Gene Model to Robustly Identify Breast Cancer Molecular Subtypes. JNCI J Natl Cancer Inst. 2012;104(4):311–325. doi: 10.1093/jnci/djr545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28(1):105–113. doi: 10.1200/JCO.2009.23.7370 [DOI] [PubMed] [Google Scholar]
- 25.Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31(7):860–867. doi: 10.1200/JCO.2011.41.0902 [DOI] [PubMed] [Google Scholar]
- 26.Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32(27):2959–2966. doi: 10.1200/JCO.2013.55.0491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–271. doi: 10.1093/annonc/mdu450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pogue-Geile KL, Song N, Serie DJ, et al. Validation of the NSABP/NRG Oncology 8-Gene Trastuzumab-benefit Signature in Alliance/NCCTG N9831. JNCI Cancer Spectr. 2020;4(5):pkaa058. doi: 10.1093/jncics/pkaa058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pogue-Geile KL, Kim C, Jeong J-H, et al. Predicting Degree of Benefit From Adjuvant Trastuzumab in NSABP Trial B-31. JNCI J Natl Cancer Inst. 2013;105(23):1782–1788. doi: 10.1093/jnci/djt321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sloan EK, Priceman SJ, Cox BF, et al. The Sympathetic Nervous System Induces a Metastatic Switch in Primary Breast Cancer. Cancer Res. 2010;70(18):7042–7052. doi: 10.1158/0008-5472.CAN-10-0522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antonova L, Aronson K, Mueller CR. Stress and breast cancer: from epidemiology to molecular biology. Breast Cancer Res. 2011;13(2):208. doi: 10.1186/bcr2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lohse MJ, Engelhardt S, Danner S, Böhm M. Mechanisms of beta-adrenergic receptor desensitization: from molecular biology to heart failure. Basic Res Cardiol. 1996;91 Suppl 2:29–34. doi: 10.1007/bf00795359 [DOI] [PubMed] [Google Scholar]
- 33.Hadcock JR, Malbon CC. Down-regulation of beta-adrenergic receptors: agonist-induced reduction in receptor mRNA levels. Proc Natl Acad Sci. 1988;85(14):5021–5025. doi: 10.1073/pnas.85.14.5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jockers R, Angers S, Da Silva A, et al. β 2 -Adrenergic Receptor Down-regulation: EVIDENCE FOR A PATHWAY THAT DOES NOT REQUIRE ENDOCYTOSIS. J Biol Chem. 1999;274(41):28900–28908. doi: 10.1074/jbc.274.41.28900 [DOI] [PubMed] [Google Scholar]
- 35.Azadpour N, Tartibian B, Koşar ŞN. Effects of aerobic exercise training on ACE and ADRB2 gene expression, plasma angiotensin II level, and flow-mediated dilation: a study on obese postmenopausal women with prehypertension. Menopause N Y N. 2017;24(3):269–277. doi: 10.1097/GME.0000000000000762 [DOI] [PubMed] [Google Scholar]
- 36.Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: A systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015;54(5):635–654. doi: 10.3109/0284186X.2014.998275 [DOI] [PubMed] [Google Scholar]
- 37.Nuciforo P, Prat A, Llombart A, et al. Tumor-infiltrating lymphocytes (TILs) in HER2-positive (HER2+) early breast cancer treated with neoadjuvant lapatinib and trastuzumab without chemotherapy in the PAMELA Trial. Ann Oncol. 2017;28:v46. doi: 10.1093/annonc/mdx362.006 [DOI] [Google Scholar]
- 38.Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. doi: 10.1016/S1470-2045(17)30904-X [DOI] [PubMed] [Google Scholar]
- 39.Dahabreh IJ, Linardou H, Siannis F, Fountzilas G, Murray S. Trastuzumab in the adjuvant treatment of early-stage breast cancer: a systematic review and meta-analysis of randomized controlled trials. The Oncologist. 2008;13(6):620–630. doi: 10.1634/theoncologist.2008-0001 [DOI] [PubMed] [Google Scholar]
- 40.Neyt M, Albrecht J, Cocquyt V. An economic evaluation of Herceptin in adjuvant setting: the Breast Cancer International Research Group 006 trial. Ann Oncol Off J Eur Soc Med Oncol. 2006;17(3):381–390. doi: 10.1093/annonc/mdj101 [DOI] [PubMed] [Google Scholar]
- 41.Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194–1220. doi: 10.1093/annonc/mdz173 [DOI] [PubMed] [Google Scholar]
- 42.Denduluri N, Somerfield MR, Eisen A, et al. Selection of Optimal Adjuvant Chemotherapy Regimens for Human Epidermal Growth Factor Receptor 2 (HER2) -Negative and Adjuvant Targeted Therapy for HER2-Positive Breast Cancers: An American Society of Clinical Oncology Guideline Adaptation of the Cancer Care Ontario Clinical Practice Guideline. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34(20):2416–2427. doi: 10.1200/JCO.2016.67.0182 [DOI] [PubMed] [Google Scholar]
- 43.Dieci MV, Vernaci G, Guarneri V. Escalation and de-escalation in HER2 positive early breast cancer. Curr Opin Oncol. 2019;31(1):35–42. doi: 10.1097/CCO.0000000000000492 [DOI] [PubMed] [Google Scholar]
- 44.Pogue-Geile KL, Song N, Jeong J-H, et al. Intrinsic Subtypes, PIK3CA Mutation, and the Degree of Benefit From Adjuvant Trastuzumab in the NSABP B-31 Trial. J Clin Oncol. 2015;33(12):1340–1347. doi: 10.1200/JCO.2014.56.2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim RS, Song N, Gavin PG, et al. Stromal Tumor-infiltrating Lymphocytes in NRG Oncology/NSABP B-31 Adjuvant Trial for Early-Stage HER2-Positive Breast Cancer. JNCI J Natl Cancer Inst. 2019;111(8):867–871. doi: 10.1093/jnci/djz032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu D, Deng Q, Sun L, et al. A Her2-let-7-β2-AR circuit affects prognosis in patients with Her2-positive breast cancer. BMC Cancer. 2015;15(1):832. doi: 10.1186/s12885-015-1869-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2012;13(4):227–232. doi: 10.1038/nrg3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maier T, Güell M, Serrano L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009;583(24):3966–3973. doi: 10.1016/j.febslet.2009.10.036 [DOI] [PubMed] [Google Scholar]
- 49.von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med. 2017;377(2):122–131. doi: 10.1056/NEJMoa1703643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin M, Holmes FA, Ejlertsen B, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(12):1688–1700. doi: 10.1016/S1470-2045(17)30717-9 [DOI] [PubMed] [Google Scholar]
- 51.von Minckwitz G, Huang C-S, Mano MS, et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med. 2019;380(7):617–628. doi: 10.1056/NEJMoa1814017 [DOI] [PubMed] [Google Scholar]
- 52.Perez EA, Ballman KV, Tenner KS, et al. Association of Stromal Tumor-Infiltrating Lymphocytes With Recurrence-Free Survival in the N9831 Adjuvant Trial in Patients With Early-Stage HER2-Positive Breast Cancer. JAMA Oncol. 2016;2(1):56. doi: 10.1001/jamaoncol.2015.3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


