Abstract
PURPOSE:
To investigate breast cancer survivors’ inflammatory responses to typhoid vaccine as a window into their innate immune response to novel pathogens.
METHODS:
This double-blind crossover trial randomized 158 breast cancer survivors to either the vaccine/saline placebo or the placebo/vaccine sequence. The relative contributions of age, cardiorespiratory fitness (VO2peak), type of cancer treatment, central obesity, and depression to interleukin (IL)-6, IL-1 receptor antagonist (IL-1Ra), and WBC vaccine responses were assessed pre-injection and 1.5, 3, 4.5, 6, and 7.5 hours post-injection.
RESULTS:
The vaccine produced larger IL-6, IL-1Ra, and WBC responses than placebo, ps<.0001. Prior chemotherapy, higher central obesity, and lower VO2peak were associated with smaller vaccine responses after controlling for baseline inflammation. Vaccine response was summarized by the percent increase in area under the curve (IL-6, WBC) or average post-injection mean (IL-1Ra) for vaccine relative to placebo. Women who received chemotherapy had smaller vaccine responses than women who did not for both IL-6 (44% vs 78%, p<.001) and WBC (26% vs 40%, p<.001); IL-1ra response was not significantly moderated by chemotherapy. Women whose central adiposity was one standard deviation above the mean had smaller vaccine responses than women with average adiposity for IL-6 (33% vs 54%, p<.001), WBC (20% vs 30%, p<.001), and IL-1Ra (2.0% vs 3.2%, p<.001). Women with an average level of VO2peak had smaller vaccine responses than women whose VO2peak was one standard deviation above the mean for IL-6 (54% vs 73%, p<.001), WBC (30% vs 40%, p<.001), and IL-1Ra (3.2% vs. 4.1%, p=0.01). Age and depression did not significantly moderate vaccine responses.
CONCLUSIONS:
This study provided novel data on chemotherapy’s longer-term adverse immune consequences. The data also have an important public health message: even relatively low levels of fitness can benefit the innate immune response to a vaccine.
1. INTRODUCTION
Age-related declines in immune protection heighten the risk for infection and weaken immune responses to vaccines (Crooke et al., 2019). Cancers are predominantly age-related diseases, and treatments including chemotherapy, radiation, and some surgeries are immunosuppressive; survivors’ residual immune deficits may impede the ability of some vaccines to elicit a protective immune response (Denlinger et al., 2014). In addition, chronic diseases/disorders that have been linked with poor vaccine responses include type 2 diabetes, cardiovascular disease, and osteoporosis, all of which are more prevalent among cancer survivors (Pascoe et al., 2014). Poor vaccine responses limit protection. However, more broadly, poor vaccine responses reflect a bigger problem: an increased risk for infectious disease morbidity and mortality.
In this study, we evaluated breast cancer survivors’ inflammatory responses to a typhoid vaccine to provide a window into their innate immune response to novel pathogens. Robust inflammatory responses are an essential immune defense for pathogen control and tissue repair (Pawelec et al., 2014). Inflammation plays a key role in the immune system’s responses to infections and vaccines by stimulating the development of adaptive immune responses. Accordingly, deficits in the initial inflammatory response, a key component of innate immunity, jeopardize the development of both cellular and humoral immune responses (Crooke et al., 2019). We assessed the relative contributions of age, physical fitness, cancer treatment, obesity, and depression because each of these parameters has been associated with poorer vaccine responses (Dhurandhar et al., 2015; Duggal et al., 2019; Goodwin et al., 2006; Kiecolt-Glaser et al., 1996; Madison et al., 2021; Weber and Ljungman, 2018). Importantly, these risk factors share a common element, heightened inflammation.
Although there are contrary data for some vaccines (Moncunill et al., 2020; Neal et al., 2022), higher baseline inflammation predicts poorer vaccine responses (Alter and Sekaly, 2015; Verschoor et al., 2017), For example, older adults with higher interleukin (IL)-6 were significantly less likely to respond to influenza vaccine than those with lower IL-6 (Trzonkowski et al., 2003). Similarly, greater baseline inflammatory gene expression was associated with lower antibody responses, and broad immune activation and excessive inflammation have been identified as transcriptional signatures of weaker protective vaccine responses (Pereira et al., 2020). Chronic inflammation impairs the immune system’s functioning, producing habitual inefficient innate immune responses (Grubeck-Loebenstein et al., 2009), including the acute phase responses that are the focus of this study.
1.1. Aging
“Inflammaging,” the age-related increase in systemic inflammation, inhibits fundamental adaptive immune system vaccine responses by impairing the innate immune system’s antigen presentation and priming tasks. The age-related immune system changes that characterize immunosenescence increase susceptibility to infection and reduce vaccine efficacy (Grubeck-Loebenstein et al., 2009). For example, influenza vaccines have been 70–90% effective in clinical trials with healthy younger adults, but only 17–53% effective in older adults, depending on the viral strain (Goodwin et al., 2006).
1.2. Fitness
Exercise has well-documented anti-inflammatory effects, and regular exercise can dampen the heightened inflammation that accompanies many diseases and disorders, including obesity, coronary artery disease, hypertension, diabetes, stroke, and cancer (Mandsager et al., 2018; Pedersen, 2017). Moreover, regular exercise lowers the risk for respiratory infections, and reduces the risk for both a cancer diagnosis and death from cancer (Gilchrist et al., 2020; Simpson et al., 2020). Higher levels of physical activity promote stronger antibody responses to influenza and pneumococcal vaccines (Duggal et al., 2019).
Unfortunately, the reduced physical activity and associated deconditioning that frequently occur during cancer and its treatment lower physical performance capacity and worsen cardiorespiratory fitness, reflected in lower peak oxygen consumption (VO2peak) (Jones et al., 2009; Mock, 2004; Peel et al., 2014). During chemotherapy many women reduce physical activity and gain weight (Vance et al., 2011), behaviors that fuel inflammation and increase the risk for multiple comorbidities, e.g., type 2 diabetes and cardiovascular disease.
1.3. Cancer treatment
In a longitudinal study that investigated inflammation and comorbidity development over time, breast cancer survivors and non-cancer controls did not differ at the pretreatment baseline, but survivors had greater inflammation 6 and 18 months after their primary cancer treatment compared to controls within a comparable time frame (Alfano et al., 2012). Increases in comorbidities, particularly among later-stage women treated with chemotherapy and radiation, may be one significant pathway (Alfano et al., 2012). However, even early-stage patients often experience persistent fatigue, and over-activation of the inflammatory network appears to be one key mechanism underlying fatigue (Bower and Lamkin, 2013).
Prior cancer treatments may leave survivors at risk for immune suppression (Denlinger et al., 2014). Indeed, cancer survivors have a higher prevalence of influenza and pneumonia than similar adults without a cancer history, and survivors are also more likely to require hospitalization (Abdel-Rahman, 2020; Heo et al., 2017).
1.4. Obesity
Weight gain occurs in 50–96% of women during breast cancer treatment (Vance et al., 2011). Chemotherapy and the onset of menopause are strong clinical predictors of weight gain during and after treatment, and chemotherapy induces menopause in 60% of women over the age of 40 (Goodwin et al., 1999; Vance et al., 2011). Adverse changes in body composition are commonplace after cancer treatment whether or not women gain weight, with increased central obesity and decreased lean mass (Vance et al., 2011), and visceral fat produces inflammatory cytokines (Kyrou et al., 2006).
Obesity has been characterized as a state of chronic inflammation because of the elevated plasma IL-6, TNF-α, and CRP levels (Shelton and Miller, 2010). What is more, the pathways are bidirectional: visceral adipose tissue’s secretion of proinflammatory cytokines can function as a stimulus for HPA axis activation, such that hypercortisolemia enhances adipocyte accumulation, and vice versa (Kyrou et al., 2006). Obesity carries a heightened risk for more severe infections including greater susceptibility to influenza, postsurgical infections, and periodontal disease. Moreover, standardized vaccine doses are not as effective as in the non-obese (Dhurandhar et al., 2015).
1.5. Depression
Obesity can promote depression, and, in turn, depression can promote obesity; for example, the risk for developing depression over time is 55% among persons with obesity (Luppino et al., 2010). Furthermore, inflammation can induce depression, and depression primes larger cytokine responses to stressors and pathogens (Kiecolt-Glaser et al., 2015). Individuals with a major depressive disorder (MDD) diagnosis have poorer vaccine responses than those without MDD; heightened subsyndromal depressive symptoms can also impair vaccine responses, as well as eroding vaccine-related protection more rapidly (Ford et al., 2018; Irwin et al., 2011; Madison et al., 2021).
1.6. Current study
Based on this literature, we studied the relative contributions of age, fitness, type of cancer treatment (surgery only, radiation, chemotherapy), obesity, and depression to inflammatory vaccine responses. We chose a typhoid vaccine because its inflammatory sequalae have been well-characterized in multiple studies which have shown that a typhoid vaccination reliably elicits IL-6, IL-1 receptor antagonist (IL-1Ra), and WBC responses without inducing fever or notable discomfort, aside from mild injection site pain (Brydon et al., 2009; Chia et al., 2003; Harrison et al., 2013; Hingorani et al., 2000; Kharbanda et al., 2002; Lacourt et al., 2015; Paine et al., 2013; Wright et al., 2005). The primary outcome was change in IL-6. Secondary outcomes were IL-1Ra and WBC.
2. MATERIALS AND METHODS
2.1. Participants
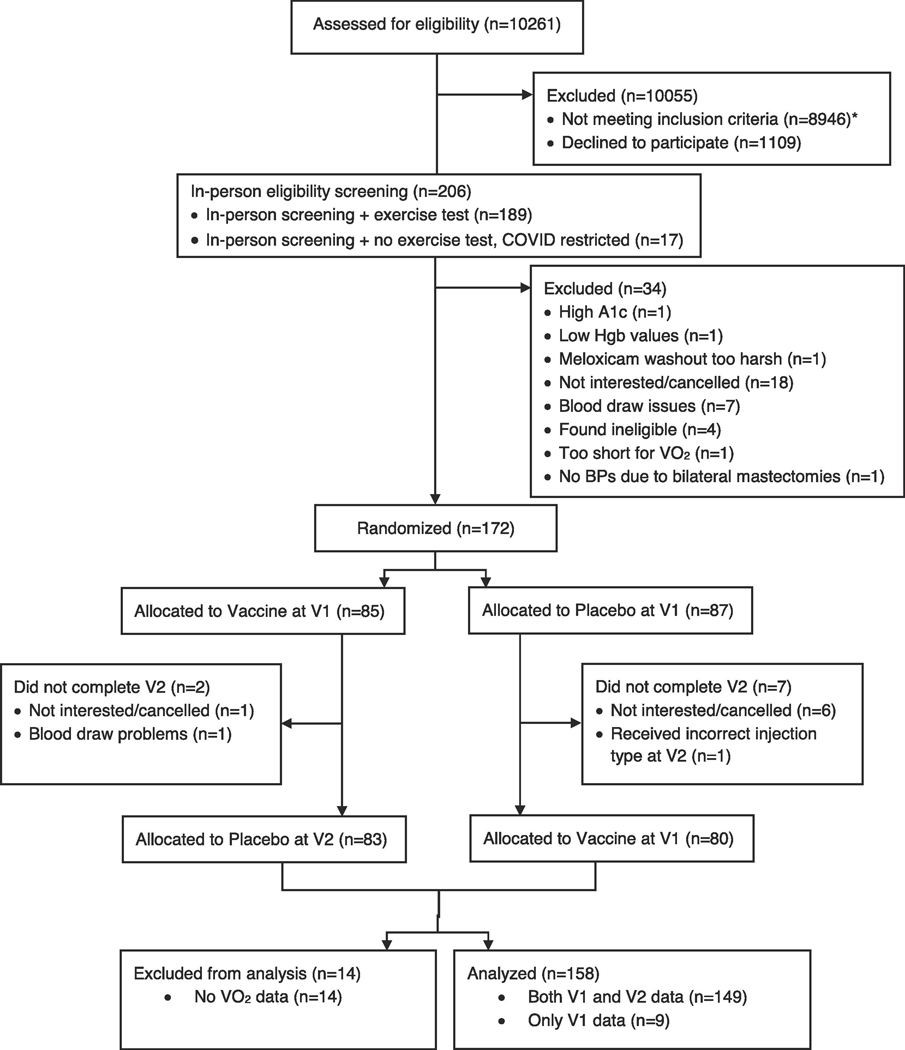
We recruited 172 postmenopausal survivors, ages 36–78, who had been diagnosed with stage I-IIIA breast cancer 1–9 years after the completion of all primary cancer treatment except for longer-term hormonal therapies (tamoxifen, aromatase inhibitors) (Table 1, Figure 1). The primary recruitment sources were the James Cancer Hospital breast cancer clinics, with secondary recruitment through the Army of Women website. Our 172 participants fell short of our 180 recruitment goal because of COVID-19 issues (Figure 1); our final analysis sample was limited to 158 because COVID-19 hospital protocols did not permit VO2 assessments on 14 women (Table 1). The institutional review board approved this study; each participant provided informed consent.
Table 1.
Demographic and clinical characteristics of participants.
| All Participants (n = 172) | Analysis Sample (n = 158) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| N | Mean (SD) or N | Range | N | Mean (SD) or N | Range | |||
| (%) | (%) | |||||||
| Age | 172 | 56.6 (8.4) | 36–78 | 158 | 56.6 (8.4) | 36–78 | ||
| Race | 172 | 158 | ||||||
| White | 159 (92.4%) | 146 (92.4%) | ||||||
| Black | 10 (5.8%) | 10 (6.3%) | ||||||
| Asian | 1 (0.6%) | 0 (0%) | ||||||
| Mixed | 2 (1.2%) | 2 (1.3%) | ||||||
| BMI, kg/m2 | 172 | 158 | ||||||
| <18.5 | 0 (0%) | 0 (0%) | ||||||
| Underweight | ||||||||
| 18.5–24.9 | 61 (35.5%) | 56 (35.4%) | ||||||
| Healthy | ||||||||
| 25–29.9 | 60 (34.9%) | 55 (34.8%) | ||||||
| Overweight | ||||||||
| > 29.9 Obese | 51 (29.7%) | 47 (29.7%) | ||||||
| Trunk fat, kg (DXA) | 172 | 15.7 (6.7) | 3.1–34.3 | 158 | 15.7 (6.7) | 3.1–34.3 | ||
| VO2peak, mL/(kg min) | 158 | 22.0 (5.3) | 10.2–33.6 | 158 | 22.0 (5.3) | 10.2–33.6 | ||
| Years since treatment | 172 | 3.5 (2.3) | 0.8–9.9 | 158 | 3.6 (2.3) | 0.8–9.9 | ||
| Chemotherapy treatment | 172 | 116 (67.4%) | 158 | 108 (68.4%) | ||||
| Radiation treatment | 172 | 105 (61.0%) | 158 | 94 (59.5%) | ||||
| Current Hormone therapy | 172 | 138 (80.2%) | 158 | 128 (81.0%) | ||||
| Cancer stage | 172 | 158 | ||||||
| Stage I | 81 (47.1%) | 75 (47.5%) | ||||||
| Stage II | 82 (47.7%) | 76 (48.1%) | ||||||
| Stage III | 9 (5.2%) | 7 (4.4%) | ||||||
| Any comorbidities | 171 | 22 (12.9%) | 158 | 20 (12.7%) | ||||
| CES-D score | ||||||||
| Visit 1 | 172 | 7.5 (6.8) | 0–37 | 158 | 7.6 (6.9) | 0–37 | ||
| Visit 2 | 163 | 7.7 (6.9) | 0–41 | 149 | 7.8 (7.0) | 0–41 | ||
| Energy/Fatigue* | ||||||||
| Visit 1 | 172 | 60.4 (18.8) | 10–90 | 158 | 60.9 (19.0) | 10–90 | ||
| Visit 2 | 163 | 61.7 (19.7) | 0–100 | 149 | 62.0 (19.8) | 0–100 | ||
| Fasting IL-6, pg/mL | ||||||||
| Visit 1 | 171 | 2.8 (6.1) | 0.4–78.4 | 157 | 2.9 (6.4) | 0.4–78.4 | ||
| Visit 2 | 163 | 2.8 (6.4) | 0.4–80.6 | 149 | 2.8 (6.7) | 0.5–80.6 | ||
| Fasting IL-1Ra, pg/mL | ||||||||
| Visit 1 | 171 | 542 (3 94) | 121 −1991 | 157 | 548 (386) | 121 −1991 | ||
| Visit 2 | 163 | 526 (3 69) | 143–2389 | 149 | 525 (342) | 143–1888 | ||
| Fasting WBC, K/uL | ||||||||
| Visit 1 | 169 | 5.1 (1.4) | 1.9–9.1 | 155 | 5.1 (1.4) | 1.9–9.1 | ||
| Visit 2 | 163 | 5.0 (1.3) | 1.7–10.5 | 149 | 5.0 (1.3) | 1.7–10.5 | ||
From SF-36 RAND Health Survey, higher is less fatigued.
Figure 1.
CONSORT diagram, subject flow.
Exclusions included a prior history of any other malignancy except basal or squamous cell skin cancers, strokes, diabetes, anemia, current heart disease or uncontrolled hypertension, liver disease, autoimmune and/or inflammatory diseases, a prior typhoid vaccination, alcohol/drug abuse, smoking, and medical conditions that would have limited participation (e.g., cognitive dysfunction). Medication exclusions included steroids, statins, and other medications with anti-inflammatory actions.
2.2. Study Procedures and Design
Peak oxygen consumption (VO2peak), the gold standard fitness measure (Jones et al., 2009), was evaluated at a screening visit using a graded cycle ergometry exercise test as previously described (Padin et al., 2019). Blood tests confirmed that women were not diabetic or anemic.
In this study’s double-blind crossover design, women were randomized to either the vaccine/placebo or the placebo/vaccine sequence at the first of two full-day study visits. The data manager who prepared the treatment sequence had no study visit involvement. The research assistants did not have the condition assignments. The nurse who inoculated women was not the same nurse who drew blood throughout the day. Blinding was assessed for participants, nurses, and research assistants.
Women were asked to consume their last meal no later than 7:30 pm the night before each of the day-long visits, and also to avoid alcohol and strenuous physical activity two days prior to their appointments. Aspirin and nonsteroidal anti-inflammatory drugs were discontinued one week before each visit.
An intravenous catheter was inserted on admission, and baseline blood samples were drawn following a 20–30 minute adaptation period. Following the blood draw, and before breakfast, women completed baseline questionnaires. A nurse injected saline (the placebo) or Typhoid capsular polysaccharide vaccine (Typhim-Vi, Sanofi Pasteur) into the non-dominant deltoid muscle. Women received standardized meals. Subsequent blood draws occurred every 90 minutes for the next 7.5 hours. Women rated the intensity of physical symptoms (pain, muscle aches, headache, feverishness, focus, memory, and hunger) from 0–9 at each blood draw. The two ~9.5-hour sessions were scheduled 26–420 days apart (M=46.87, SD=47.48); the pandemic delayed some sessions.
Cancer-related fatigue has been well-documented among breast cancer survivors, and inflammatory activity has been associated with its persistence. To address customary or usual fatigue, we administered the SF-36 RAND Health Survey (Ware and Sherbourne, 1992), the most commonly used scale in studies of cancer fatigue and inflammation; its energy/fatigue (vitality) scale assesses fatigue over the last month (Bower et al., 2002).
Depressive symptoms were assessed by the Center for Epidemiological Studies Depression Scale (CES-D) (Basco et al., 1997; Radloff, 1977). The Charlson Index (de Groot et al., 2003; Hall et al., 2004; Katz et al., 1996; Quan et al., 2011) provided data on comorbidities. The Pittsburgh Sleep Quality Index (Buysse et al., 1989) assessed sleep quality.
Central obesity was assessed using dual x-ray absorptiometry (DXA) (model DPX-NT/software version 5.60, GE Lunar, Madison, WI). Table 1 provides BMI data for reference, but we used DXA in analyses because BMI misclassifies adiposity status in roughly one-third of women (Kennedy et al., 2009).
2.3. Blood Samples
We assessed the vaccine’s acute-phase response using IL-6, IL-1Ra and white blood cell count (WBC) following multiple typhoid vaccine studies that have demonstrated reliable changes in these parameters, but not TNF-α (Brydon et al., 2009; Chia et al., 2003; Harrison et al., 2013; Hingorani et al., 2000; Kharbanda et al., 2002; Lacourt et al., 2015; Paine et al., 2013; Wright et al., 2005). Both cytokines, IL-1 and IL-6, have been shown to regulate the liver’s production of acute phase proteins, and IL-1Ra functions as an acute phase protein (Arend et al., 1998; Gabay et al., 1997) Each woman’s stored serum samples were assayed for each inflammatory marker in one run, thus using the same controls for all time points.
We assayed serum IL-6 with the Quantikine HS ELISA kit (R & D Systems, Minneapolis, MN), and serum IL-1Ra using an electrochemilluminescence method with Meso Scale Discovery kits. Sensitivity for IL-6 was 0.03 pg/mL, the intra-assay coefficient of variation was 4.1%, and the inter-assay coefficient of variation was 6.5%; corresponding values for IL-1Ra were 6.3 pg/mL, 4.1% and 8.6%. The hospital lab provided WBC data.
2.4. Statistical Methods
Linear mixed effects models were used to model changes in inflammation across the day for both visits, allowing explicit modeling of the within-subject correlations both within visits and between visits. IL-6 and IL-1Ra values were right-skewed; thus, all analyses for these outcomes used natural log-transformed values (ln) to better approximate normality of residuals. Time since injection was treated as a categorical variable in all models (i.e., trajectories were not assumed to be linear). Random effects included subject-specific visit effects that were allowed to be correlated, thus modeling the different magnitude of within-subject correlations within and across visits. Additionally, random plate effects were included for cytokines (IL-6, IL-1Ra). The Kenward-Roger adjustment to the degrees of freedom was used to control Type 1 error (Kenward and Roger, 1997), and no adjustments were made to p-values to account for multiple testing.
In a first set of models we tested for vaccine effects on outcomes by modeling all time points (including the pre-injection time point) and including fixed effects of injection type, time since injection, and their interaction. The two-way interaction of injection type by time tested for a vaccine effect in these models. In a second set of models testing for moderation of vaccine effects we controlled for baseline outcome levels and modeled the remaining time points as outcomes, thus controlling for pre-injection inflammatory differences across levels of moderators (e.g., differences in baseline inflammation by cardiovascular fitness). These models included the three-way interaction of potential modifier by injection type by time since injection, with all lowerorder interactions. Specifically, we evaluated (in separate models) whether age, cancer treatment, obesity, depression, and physical fitness (VO2peak) moderated the vaccine effect, controlling for baseline inflammation, by testing the three-way interaction term.
In models assessing moderation we used the area under the curve with respect to ground (AUCg) as a summary measure of inflammatory response for outcomes with more than three measurements (IL-6, WBC). AUC with respect to ground was chosen over AUC with respect to increase since we were controlling for baseline inflammation. Since some participants had sporadically missing data points across the day, AUCg for different levels of key predictors (e.g., AUCg for each injection type) was estimated using contrasts within the linear mixed models. This method has been shown to outperform analyses that use AUCg values calculated per individual when there are missing data (Bell et al., 2014), and additionally allowed us to control for the plate effect for models for cytokines. For IL-1Ra, which only had three measurements across the day, we used the estimated average inflammation at 6.5 and 8 hours post-injection as a summary measure of inflammatory response. This was estimated using contrasts in the linear mixed models for differing levels of key predictors.
To facilitate comparison of effect sizes across the set of potential effect modifiers, we standardized each continuous predictor using z-scores, and report the effects of each for a one unit increase in the z-score (e.g., a one standard deviation increase in the predictor). Binary modifiers (e.g., chemotherapy) were used without modification. Additional covariates were included in all models to guard against confounding. All models controlled for the main effects of visit order (first versus second visit), cancer stage, time since cancer treatment, receipt of hormone therapy (yes/no), and presence of comorbidities. We initially also included sleep quality (Pittsburgh Sleep Quality Index) in models, but it was not significant, and results were unchanged when it was removed. Additionally, all potential effect modifiers (age, cancer treatment, obesity, depression, and physical fitness) were included as main effects in all models even when they were not the target predictor of interest in interaction terms. Analyses were conducted in SAS version 9.4 (Cary, North Carolina).
Bang et al. blinding indices (Bang et al., 2004) were used to assess blinding of participants and experimenters, separately for each injection type. This index ranges from −1 to 1 with a blinding index of zero indicating perfect blinding, i.e., random guessing, and a value of one indicating complete unblinding, i.e., all guesses correct. the blinding index, which was calculated using R (Team, 2020).
2.5. Sample Size
Sample size was determined a priori based on ensuring adequate power for the three-way interaction of depression by injection type by time using estimates from pilot data to inform the calculation. A sample size of 180 subjects was estimated to provide 94% power for this interaction. However, we were unable to fully recruit and assess cardiovascular fitness (VO2peak) on a subset of the sample whose visits occurred when COVID-19-related restrictions were in place, resulting in 158 subjects with enough data such that they could be included in analysis models (Table 1). This sample size was estimated to provide 88% power for the three-way interaction.
3. Results
3.1. Vaccine Effects on Inflammatory Responses
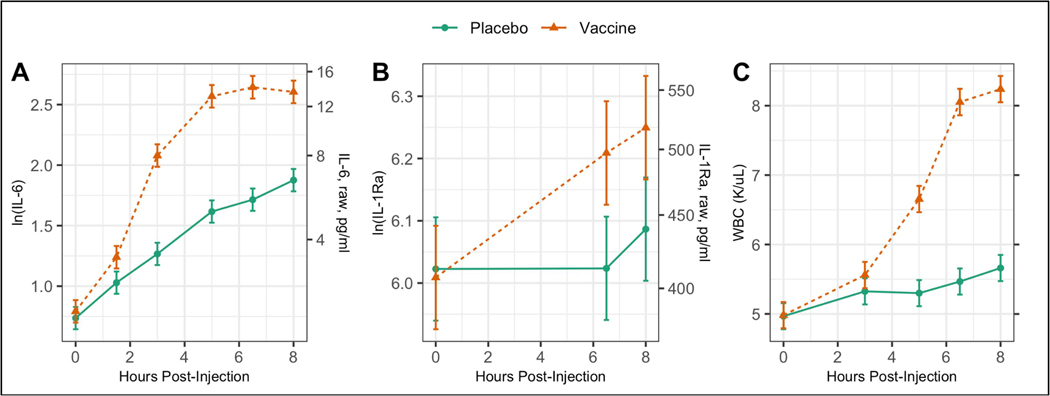
All inflammatory measures (IL-6, IL-1Ra, WBC) demonstrated significantly larger responses to the vaccine as compared to the placebo as evidenced by significant injection type by time interactions (p<.0001 for all). Figure 2 shows the estimated trajectories. IL-6 and WBC produced the most pronounced vaccine responses; the IL-1Ra response was less pronounced. Of note, for all three outcomes the mean level at the end of the day was significantly higher than at baseline for the placebo visit (p<.001 for all), underscoring the importance of having this condition as a control. In addition to these significant inflammatory responses, there were also significant vaccine effects on self-rated measures of pain (p<.0001), aches (p<.0001), and headache (p=0.02), as shown in Supplemental Figure 1.
Figure 2:
Estimated trajectories of (A) serum IL-6, (B) serum IL-1Ra, and (C) WBC after injection with either placebo or typhoid vaccine. Results are from linear mixed models controlling for age, cancer treatment, hormone treatment, obesity, depression, physical fitness, visit order, cancer stage, time since cancer treatment, and presence of comorbidities.
3.2. Effects of Age, Cancer Treatment, Obesity, Depression, and Physical Fitness on Baseline Inflammation
Higher central obesity and lower cardiorespiratory fitness (VO2peak) were associated with higher pre-injection levels of IL-6, IL-1Ra, and WBC (p<.001 for all; Supplemental Table 1). Pre-injection IL-6 was also associated with chemotherapy treatment (higher IL-6 for survivors who received chemotherapy, p=.008) and current hormone therapy (higher IL-6 for women not undergoing hormone therapy, p=.03). Neither age nor depression were significantly associated with any of the inflammatory markers at baseline (p>.05 for all).
3.3. Effects of Age, Cancer Treatment, Obesity, Depression, and Physical Fitness on Vaccine Responses
The IL-6 and WBC vaccine responses were significantly moderated by central obesity, VO2peak, and prior chemotherapy (p<.001 for all, Table 2), but not age, depression, prior radiation therapy, or hormone therapy (p>.08 for all). After controlling for baseline differences in inflammation, prior chemotherapy, higher central obesity, and lower cardiorespiratory fitness were associated with smaller vaccine responses. Relative to placebo, the vaccine increased the IL-6 AUCg by 78% for women who did not receive chemotherapy versus only 44% among those who did. For a woman at the average levels of central obesity and cardiorespiratory fitness, the vaccine produced a 54% increase in IL-6 AUCg relative to placebo. If central obesity was one standard deviation above the mean, the vaccine produced only a 33% increase; if VO2peak was one standard deviation above the mean, the vaccine produced a 73% increase. Relative to placebo, the vaccine increased WBC AUCg by 40% for women who did not receive chemotherapy but only 26% for women who did receive chemotherapy. For a woman at the average levels of central obesity and cardiorespiratory fitness, the vaccine produced a 30% increase in WBC AUCg relative to placebo. If central obesity was one standard deviation above the mean the vaccine produced only a 20% increase, and if VO2peak was one standard deviation above the mean the vaccine produced a 40% increase. Among these effects, for both IL-6 and WBC the largest effect sizes were for the effect of prior chemotherapy, followed by central obesity and VO2peak.
Table 2.
Estimated area under the curve with respect to ground (AUCg) for serum IL-6 and WBC, for each injection type (placebo, vaccine) by levels of potential effect modifiers. Results from separate linear mixed effects models for each moderator, controlling for baseline (pre-injection) outcome level, age, cancer treatment, hormone treat-ment, obesity, depression, physical fitness, visit order, cancer stage, time since cancer treatment, and presence of comorbidities.
| AUCg, Mean (SE) | Difference in Difference (High-Low) | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Moderator | Level* | Placebo | Vaccine | Difference | Mean (SE) | 95% CI | P-value |
|
| ||||||||
| ln(IL-6) | Age | Z-score = 0 | 9.4 (0.52) | 14.6 (0.53) | 5.2 (0.32) | |||
| Z-score = 1 | 10.1 (0.59) | 14.9 (0.60) | 4.8 (0.45) | −0.37 (0.32) | (−1.01, 0.26) | 0.25 | ||
| CESD | Z-score = 0 | 9.4 (0.52) | 14.6 (0.53) | 5.2 (0.32) | ||||
| Z-score = 1 | 9.5 (0.57) | 14.7 (0.60) | 5.1 (0.46) | −0.03 (0.33) | (−0.69, 0.62) | 0.92 | ||
| Trunk Fat | Z-score = 0 | 9.5 (0.52) | 14.6 (0.53) | 5.1 (0.29) | ||||
| Z-score = 1 | 10.4 (0.63) | 13.8 (0.63) | 3.5 (0.42) | −1.67 (0.29) | (−2.25, −1.09) | <0.0001 | ||
| VO2peak | Z-score = 0 | 9.5 (0.52) | 14.6 (0.53) | 5.1 (0.30) | ||||
| Z-score = 1 | 9.1 (0.61) | 15.7 (0.62) | 6.6 (0.42) | 1.48 (0.30) | (0.89, 2.07) | <0.0001 | ||
| Chemotherapy | No | 8.6 (0.70) | 15.3 (0.71) | 6.7 (0.54) | ||||
| Yes | 10.0 (0.54) | 14.4 (0.55) | 4.4 (0.38) | − 2.28 (0.66) | (−3.57, −0.98) | 0.0007 | ||
| Radiation | No | 8.9 (0.62) | 14.0 (0.62) | 5.0 (0.50) | ||||
| Yes | 10.0 (0.58) | 15.2 (0.60) | 5.2 (0.42) | 0.22 (0.66) | (−1.08, 1.52) | 0.74 | ||
| Hormone Tx | No | 9.8 (0.76) | 13.8 (0.78) | 4.0 (0.73) | ||||
| Yes | 9.4 (0.51) | 14.9 (0.52) | 5.4 (0.35) | 1.42 (0.81) | (−0.18, 3.02) | 0.08 | ||
| WBC | Age | Z-score = 0 | 27.5 (0.54) | 35.9 (0.55) | 8.4 (0.46) | |||
| Z-score = 1 | 27.1 (0.66) | 35.9 (0.67) | 8.8 (0.65) | 0.41 (0.46) | (−0.50, 1.32) | 0.38 | ||
| CESD | Z-score = 0 | 27.5 (0.54) | 35.9 (0.55) | 8.4 (0.46) | ||||
| Z-score = 1 | 27.6 (0.63) | 35.7 (0.66) | 8.1 (0.65) | − 0.29 (0.47) | (−1.21, 0.64) | 0.54 | ||
| Trunk Fat | Z-score = 0 | 27.6 (0.53) | 35.9 (0.54) | 8.3 (0.40) | ||||
| Z-score = 1 | 28.1 (0.69) | 33.5 (0.69) | 5.5 (0.57) | − 2.82 (0.40) | (−3.61, −2.02) | <0.0001 | ||
| VO2peak | Z-score = 0 | 27.6 (0.54) | 35.9 (0.54) | 8.4 (0.42) | ||||
| Z-score = 1 | 26.6 (0.70) | 37.2 (0.72) | 10.6 (0.59) | 2.28 (0.42) | (1.45, 3.12) | <0.0001 | ||
| Chemotherapy | No | 27.4 (0.81) | 38.3 (0.83) | 10.9 (0.76) | ||||
| Yes | 27.1 (0.55) | 34.2 (0.55) | 7.1 (0.53) | −3.77 (0.92) | (−5.59, −1.94) | <0.0001 | ||
| Radiation | No | 27.7 (0.69) | 36.1 (0.69) | 8.4 (0.72) | ||||
| Yes | 27.4 (0.63) | 35.7 (0.66) | 8.3 (0.60) | − 0.13 (0.95) | (−2.00, 1.74) | 0.89 | ||
| Hormone Tx | No | 28.1 (0.90) | 34.9 (0.91) | 6.9 (1.0) | ||||
| Yes | 27.5 (0.52) | 36.3 (0.53) | 8.7 (0.51) | 1.83 (1.16) | (−0.47, 4.12) | 0.12 | ||
Z-score = 0 is the AUCg at the average level of the moderator in the sample (e.g., at average age), and Z-score = 1 is the AUCg at one standard deviation above the average level of the moderator (e.g., age that is one SD above the mean age).
The IL-1Ra vaccine response was significantly moderated by both central obesity (p<.001) and cardiorespiratory fitness (p=.01), with higher central obesity and lower VO2peak associated with smaller vaccine responses (Table 3). For a woman at the average levels of central obesity and cardiorespiratory fitness, average post-injection IL-1Ra was 3.2% higher after the vaccine compared to placebo. If central obesity was one standard deviation above the mean the vaccine produced only a 2.0% increase, and if VO2peak was one standard deviation above the mean the vaccine produced a 4.1% increase. Additionally, the IL-1Ra vaccine response was moderated by hormone therapy (p=.048), with higher inflammatory response for cancer survivors using hormone therapy (Table 3). For women using hormone therapy, the vaccine produced a 3.7% increase in IL-1Ra, while IL-1Ra increased only 1.9% among women not using hormone therapy. Among these effects, the largest effect size was for hormone therapy, followed by central obesity and VO2peak. Age, depression, prior chemotherapy, and prior radiation therapy did not significantly moderate the IL-1Ra vaccine response (p>.08).
Table 3.
Estimated average serum IL-1ra mean post-injection, for each injection type (placebo, vaccine) by levels of potential effect modifiers. Results from separate linear mixed effects models for each moderator, controlling for baseline (pre-injection) IL-1ra, age, cancer treatment, hormone treatment, obesity, depression, physical fitness, visit order, cancer stage, time since cancer treatment, and presence of comorbidities.
| Post-Injection Mean (SE) | Difference in Difference (High-Low) | |||||||
| Outcome | Moderator | Level* | Placebo | Vaccine | Difference | Mean (SE) | 95% CI | P-value |
|
| ||||||||
| ln(IL-1Ra) | Age | Z-score = 0 | 6.11 (0.03) | 6.32 (0.03) | 0.21 (0.02) | |||
| Z-score = 1 | 6.10 (0.03) | 6.31 (0.03) | 0.21 (0.03) | 0.004 (0.02) | (−0.041, 0.050) | 0.85 | ||
| CESD | Z-score =0 | 6.11 (0.03) | 6.32 (0.03) | 0.21 (0.02) | ||||
| Z-score =1 | 6.14 (0.03) | 6.33 (0.03) | 0.19 (0.03) | −0.013 (0.02) | (−0.058, 0.032) | 0.57 | ||
| Trunk Fat | Z-score =0 | 6.14 (0.05) | 6.34 (0.05) | 0.20 (0.02) | ||||
| Z-score =1 | 6.17 (0.05) | 6.29 (0.05) | 0.12 (0.03) | −0.073 (0.02) | (−0.113, −0.034) | 0.0004 | ||
| VO2peak | Z-score = 0 | 6.14 (0.05) | 6.34 (0.05) | 0.20 (0.02) | ||||
| Z-score = 1 | 6.12 (0.05) | 6.37 (0.05) | 0.25 (0.03) | 0.053 (0.02) | (0.012, 0.094) | 0.01 | ||
| Chemotherapy | No | 6.09 (0.04) | 6.35 (0.04) | 0.26 (0.04) | ||||
| Yes | 6.12 (0.03) | 6.30 (0.03) | 0.18 (0.03) | −0.077 (0.05) | (−0.171, 0.017) | 0.11 | ||
| Radiation | No | 6.11 (0.04) | 6.27 (0.04) | 0.16 (0.04) | ||||
| Yes | 6.12 (0.03) | 6.36 (0.03) | 0.24 (0.03) | 0.082 (0.05) | (−0.009, 0.174) | 0.08 | ||
| Hormone Tx | No | 6.13 (0.04) | 6.24 (0.04) | 0.11 (0.05) | ||||
| Yes | 6.13 (0.03) | 6.36 (0.03) | 0.23 (0.02) | 0.114 (0.06) | (0.001, 0.226) | 0.048 | ||
Z-score = 0 is the post-injection mean at the average level of the moderator in the sample (e.g., at average age), and Z-score = 1 is the post-injection mean at one standard deviation above the average level of the moderator (e.g., age that is one SD above the mean age).
Though prior radiation treatment did not moderate vaccine responses, it was significantly associated with overall levels of inflammation after both injections, even after controlling for baseline inflammation. Cancer survivors who had prior radiation treatment had significantly higher post-injection IL-6 (main effect of radiation: p=.02) and higher post-injection IL-1Ra (main effect of radiation: p=.02) across the day. Supplemental Figure 2 shows estimated post-injection trajectories for all outcomes.
3.4. Baseline Inflammation and Baseline Fatigue as Moderators of Vaccine Responses
We also assessed the question of whether baseline inflammation and/or baseline fatigue moderated vaccine responses. The IL-6 vaccine response was significantly moderated by pre-injection inflammation (p<.001), such that cancer survivors with higher baseline inflammation had smaller responses. For a woman whose pre-injection IL-6 (log-transformed) was one standard deviation above the mean, the vaccine produced a 27% increase compared to placebo, significantly smaller than the 53% increase for a woman at the average pre-injection IL-6 level. Neither the WBC (p=0.10) nor IL-1Ra (p=0.07) vaccine responses were moderated by pre-injection levels of these measures. Baseline fatigue did not moderate the IL-6 (p=0.77), WBC (p=0.32), or IL-1Ra (p=0.81) responses.
3.5. Blinding
Participants were not completely blinded to the injection type, which was expected due to the difference in arm pain between the two injection types. The Bang et al. blinding index (Bang et al., 2004) for participants during the placebo visit was 0.35 (95% CI: 0.26, 0.45) and for the vaccine visit it was 0.44 (95% CI: 0.26, 0.45). There was evidence that blinding was much more effective for both the experimenters and the nurse. For the morning experimenter, these indices were −0.03 (95% CI: −0.08, 0.02) and 0.05 (95% CI: 0.01, 0.10) for the placebo and vaccine visits, respectively. For the afternoon experimenter, these indices were 0.08 (95% CI: 0.03, 0.13) and 0.13 (95% CI: 0.07, 0.20) for the placebo and vaccine visits, respectively. For the nurse, these indices were −0.02 (95% CI: −0.08, 0.03) and 0.04 (95% CI: −0.003, 0.09) for the placebo and vaccine visits, respectively.
4. Discussion
A robust inflammatory response plays a crucial role in the acute response to infection and wounding (Jenny, 2012). Age, cancer treatment, obesity, depression, and physical fitness have all been associated with heightened low-grade chronic inflammation, and higher inflammation has predicted poorer responses to diverse vaccines including influenza, varicella zoster virus, yellow fever, and hepatitis B (Alter and Sekaly, 2015; Pereira et al., 2020; Verschoor et al., 2017). After accounting for baseline differences in inflammation, prior chemotherapy, lower fitness, and greater central obesity were all associated with lower inflammatory vaccine responses. Comparisons of the relative effect sizes showed that chemotherapy had the largest impact on IL-6 and WBC levels, but was not significant for IL-1Ra. Central obesity had the second largest effect size for all three inflammatory markers, while cardiorespiratory fitness had modestly smaller but significant effects across all three indices. Women receiving hormone therapy had larger IL-1Ra responses than those who did not, but it did not have significant IL-6 or WBC effects.
Neither age nor depressive symptoms showed significant relationships with inflammatory responses. However, our participants’ average age was only 57, and age-related vaccine impairments are observed in the elderly (Goodwin et al., 2006). Similarly, major depression has been a primary focus in vaccine studies (Ford et al., 2018; Irwin et al., 2011), and only 14% of our participants’ CES-D scores reached the major depression threshold at either visit, limiting our assessment of depression’s impact.
Importantly, time since primary treatment completion, which ranged from 1–9 years, was not related to vaccine responses. The women in our study who received chemotherapy were 3.8 years younger than those who did not, and the groups did not differ on fitness, central obesity, depression, or comorbidities. In this light, the chemotherapy-treated women’s lower inflammatory responses provide novel evidence of persistent adverse chemotherapy-related immune alterations.
A review of 27 clinical trials and observational studies concluded that breast cancer survivors’ cardiorespiratory fitness was 25% lower than that of healthy sedentary women (Peel et al., 2014). Viewed another way, the average 50-year-old survivor’s VO2max was most similar to that of a sedentary 60-year-old woman, and declines in fitness persisted even seven years after treatment completion. Consistent with these studies, our participants’ average V02peak, 22.0 mL/(kg min), paralleled data from this review, 22.2 mL/(kg min) (Peel et al., 2014), and our participants’ modal fitness classification was very poor. However, even among our low fitness sample, women who were relatively more fit had larger inflammatory vaccine responses. In concert with antibody studies that have looked at fitness (Duggal et al., 2019), these data show how physical activity promotes vaccine responsiveness.
Exercise need not be vigorous to be beneficial. A large prospective study of older women showed that accelerometer-measured standing, with or without ambulation, was associated with lower all-cause mortality, particularly among those with high sedentary time (Jain et al., 2020). Similarly, other researchers found that low leisure-time physical activity was associated with a 10% lower risk of bacterial infection compared to sedentary behavior (Pape et al., 2016). Our study shows how such minimal differences in activity may confer health benefits.
Our study was not designed to address the particular innate immune pathways, but other typhoid vaccine studies have provided helpful information in this regard. For example, Paine and colleagues reported that the typhoid vaccine produced a significant increase in granulocytes, as well as nonsignificant increases in monocytes and lymphocytes (Paine et al., 2013). Additionally, there may be de-margination of neutrophils that have been ”sticking” to vascular and/or lymphoid tissues shortly after vaccination, and this de-margination may reflect a vaccine response.
Our study focused on the initial, rapid inflammatory response, and we did not assess antibody responses to the vaccine, one limitation. This vaccine produces an antibody response in 90% of recipients (Plotkin and Bouveret-Le Cam, 1995), and this efficacy is such that a weaker initial reaction may not diminish its ultimate efficacy and protective immunity.
Although there is a clear theoretical basis for expecting that a robust initial inflammatory vaccine response should correspond to more efficient recruitment of the adaptive immune system, some studies suggest that a less robust inflammatory vaccine response may predict better outcomes. For example, protection following malaria immunization was heightened by a “moderately elevated inflammatory state” before vaccination (Neal et al., 2022), and another study also suggested that a more activated immune system prior to immunization responded better to the malaria vaccine (Moncunill et al., 2020).
However, in other studies pre-vaccination inflammation-related gene signatures predicted influenza antibody titers one month post-vaccination across multiple seasons (Nakaya et al.). Similarly, greater baseline inflammatory gene expression was associated with lower hepatitis B antibody responses, and broad immune activation and excessive inflammation were transcriptional signatures of weak hepatitis B vaccine responses (Bartholomeus et al., 2018; Fourati et al., 2016; Pereira et al., 2020). In our study, baseline IL-6 had a strong moderating effect on the IL-6 vaccine response; as expected, higher baseline IL-6 predicted a lower IL-6 vaccine response. Accordingly, blocking inflammation has been suggested as a potential strategy to enhance vaccine efficacy (Alter and Sekaly, 2015; Pereira et al., 2020), and this could be a particularly relevant strategy for groups such as older adults and cancer survivors.
Vaccination provides a useful model to assess the immune system’s response to novel pathogens because it affords uniform antigen exposure across individuals as well as a dynamic assessment of the inflammatory response, a central component of the innate immune system’s first line of defense. Our study provides a new window on chemotherapy’s persistent adverse immune consequences, a finding that deserves further attention. The data also have an important public health message for cancer survivors: even relatively low levels of fitness can benefit immune system functioning and health.
Supplementary Material
Highlights.
Innate immunity plays a key role in immune responses to infections and vaccines. A typhoid vaccine provided a window into the inflammatory immune response. Chemotherapy was associated with poorer vaccine responses in cancer survivors Even relatively low fitness appeared to benefit inflammatory vaccine responses.
Funding:
The study was supported in part by NIH grants CA186251, CA172296, UL1TR001070, and CA016058. The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Competing Interests: The authors have no conflicts of interest to declare.
ClinicalTrials.gov identifier: NCT02415387
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Rahman O, 2020. Prevalence and healthcare utilization of acute respiratory infections among cancer survivors in the United States: a population-based study. Expert Reviews in Respiratory Medicine. [Google Scholar]
- Alfano CM, Imayama I, Neuhouser ML, Kiecolt-Glaser JK, Smith AW, Meeske K, McTiernan A, Bernstein L, Baumgartner KB, Ulrich CM, Ballard-Barbash R, 2012. Fatigue, inflammation, and omega-3 and -6 fatty acid intake among breast cancer survivors. J. Clin. Oncol. 30, 1280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter G, Sekaly RP, 2015. Beyond adjuvants: Antagonizing inflammation to enhance vaccine immunity. Vaccine 33, B55–B59. [DOI] [PubMed] [Google Scholar]
- Arend WP, Malyak M, Guthridge CJ, Gabay C, 1998. Interleukin-1 receptor antagonist: role in biology. Annu. Rev. Immunol. 16, 27–55. [DOI] [PubMed] [Google Scholar]
- Bang H, Ni L, Davis CE, 2004. Assessment of blinding in clinical trials. Control. Clin. Trials 25, 143–156. [DOI] [PubMed] [Google Scholar]
- Bartholomeus E, De Neuter N, Meysman P, Suls A, Keersmaekers N, Elias G, Jansens H, Hens N, Smits E, Van Tendeloo V, Beutels P, Van Damme P, Ogunjimi B, Laukens K, Mortier G, 2018. Transcriptome profiling in blood before and after hepatitis B vaccination shows significant differences in gene expression between responders and non-responders. Vaccine 36, 6282–6289. [DOI] [PubMed] [Google Scholar]
- Basco MR, Krebaum SR, Rush AJ, 1997. Outcome measures of depression. In: Strupp HH, Horowitz LM, Lambert MJ (Eds.), Measuring patient changes in mood, anxiety, and personality disorders. American Psychological Association, Washington D. C., pp. 207–245. [Google Scholar]
- Bell ML, King MT, Fairclough DL, 2014. Bias in Area Under the Curve for Longitudinal Clinical Trials With Missing Patient Reported Outcome Data: Summary Measures Versus Summary Statistics. Sage Open 4. [Google Scholar]
- Bower JE, Ganz PA, Aziz N, Fahey JL, 2002. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom. Med. 64, 604–611. [DOI] [PubMed] [Google Scholar]
- Bower JE, Lamkin DM, 2013. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav. Immun. 30 Suppl, S48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L, Walker C, Wawrzyniak A, Whitehead D, Okamura H, Yajima J, Tsuda A, Steptoe A, 2009. Synergistic effects of psychological and immune stressors on inflammatory cytokine and sickness responses in humans. Brain, Behavior, and Immunity 23, 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ, 1989. Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Chia S, Ludlam CA, Fox KAA, Newby DE, 2003. Acute systemic inflammation enhances endothelium-dependent tissue plasminogen activator release in men. J. Am. Coll. Cardiol. 41, 333–339. [DOI] [PubMed] [Google Scholar]
- Crooke SN, Ovsyannikova IG, Poland GA, Kennedy RB, 2019. Immunosenescence: A systems-level overview of immune cell biology and strategies for improving vaccine responses. Exp. Gerontol. 124, 110632. [Google Scholar]
- de Groot V, Beckerman H, Lankhorst GJ, Bouter LM, 2003. How to measure comorbidity. a critical review of available methods. J. Clin. Epidemiol. 56, 221–229. [DOI] [PubMed] [Google Scholar]
- Denlinger CS, Ligibel JA, Are M, Baker KS, Demark-Wahnefried W, Dizon D, Friedman DL, Goldman M, Jones L, King A, Ku GH, Kvale E, Langbaum TS, Leonardi-Warren K, McCabe MS, Melisko M, Montoya JG, Mooney K, Morgan MA, Moslehi JJ, O’Connor T, Overholser L, Paskett ED, Peppercorn J, Raza M, Rodriguez MA, Syrjala KL, Urba SG, Wakabayashi MT, Zee P, McMillian NR, Freedman-Cass DA, 2014. Survivorship: Immunizations and Prevention of Infections, Version 2.2014. Journal of the National Comprehensive Cancer Network 12, 1098–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhurandhar NV, Bailey D, Thomas D, 2015. Interaction of obesity and infections. Obesity Reviews 16, 1017–1029. [DOI] [PubMed] [Google Scholar]
- Duggal NA, Niemiro G, Harridge SDR, Simpson RJ, Lord JM, 2019. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nature Reviews Immunology 19, 563–572. [Google Scholar]
- Ford BN, Yolken RH, Dickerson FB, Teague TK, Irwin MR, Paulus MP, Savitz J, 2018. Reduced immunity to measles in adults with major depressive disorder. Psychol. Med, 1–7. [Google Scholar]
- Fourati S, Cristescu R, Loboda A, Talla A, Filali A, Railkar R, Schaeffer AK, Favre D, Gagnon D, Peretz Y, Wang IM, Beals CR, Casimiro DR, Carayannopoulos LN, Sekaly RP, 2016. Pre-vaccination inflammation and B-cell signalling predict age-related hyporesponse to hepatitis B vaccination. Nature communications 7. [Google Scholar]
- Gabay C, Smith MF, Eidlen D, Arend WP, 1997. Interleukin 1 receptor antagonist (IL-1Ra) is an acute-phase protein. J. Clin. Invest. 99, 2930–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist SC, Howard VJ, Akinyemiju T, Judd SE, Cushman M, Hooker SP, Diaz KM, 2020. Association of Sedentary Behavior With Cancer Mortality in Middle-aged and Older US Adults. JAMA Oncology. [Google Scholar]
- Goodwin K, Viboud C, Simonsen L, 2006. Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine 24, 1159–1169. [DOI] [PubMed] [Google Scholar]
- Goodwin PJ, Ennis M, Pritchard KI, McCready D, Koo J, Sidlofsky S, Trudeau M, Hood N, Redwood S, 1999. Adjuvant treatment and onset of menopause predict weight gain after breast cancer diagnosis. J. Clin. Oncol. 17, 120–129. [DOI] [PubMed] [Google Scholar]
- Grubeck-Loebenstein B, Della Bella S, Iorio AM, Michel JP, Pawelec G, Solana R, 2009. Immunosenescence and vaccine failure in the elderly. Aging Clinical and Experimental Research 21, 201–209. [DOI] [PubMed] [Google Scholar]
- Hall WH, Ramachandran R, Narayan S, Jani AB, Vijayakumar S, 2004. An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer 4, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Cooper E, Voon V, Miles K, Critchley HD, 2013. Central autonomic network mediates cardiovascular responses to acute inflammation: Relevance to increased cardiovascular risk in depression? Brain Behavior and Immunity 31, 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J, Chun M, Oh YT, Noh OK, Kim L, 2017. Influenza Among Breast Cancer Survivors in South Korea: A Nationwide Population-Based Study. In Vivo 31, 967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani AD, Cross J, Kharbanda RK, Mullen MJ, Bhagat K, Taylor M, Donald AE, Palacios M, Griffin GE, Deanfield JE, MacAllister RJ, Vallance P, 2000. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation 102, 994–999. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Levin MJ, Carrillo C, Olmstead R, Lucko A, Lang N, Caulfield MJ, Weinberg A, Chan ISF, Clair J, Smith JG, Marchese RD, Williams HM, Beck DJ, McCook PT, Johnson G, Oxman MN, 2011. Major depressive disorder and immunity to varicella-zoster virus in the elderly. Brain Behavior and Immunity 25, 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain P, Bellettiere J, Glass N, LaMonte MJ, Di C, Wild RA, Evenson KR, LaCroix AZ, 2020. The Relationship of Accelerometer-Assessed Standing Time With and Without Ambulation and Mortality: The WHI OPACH Study. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. [Google Scholar]
- Jenny NS, 2012. Inflammation in Aging: Cause, Effect, or Both? Discov. Med. 13, 451–460. [PubMed] [Google Scholar]
- Jones LW, Eves ND, Haykowsky M, Freedland SJ, Mackey JR, 2009. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncology 10, 598–605. [DOI] [PubMed] [Google Scholar]
- Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW, 1996. Can comorbidity be measured by questionnaire rather than medical record review? Med. Care 34, 73–84. [DOI] [PubMed] [Google Scholar]
- Kennedy AP, Shea JL, Sun G, 2009. Comparison of the Classification of Obesity by BMI vs. dual-energy X-ray Absorptiometry in the Newfoundland Population. Obesity 17, 2094–2099. [DOI] [PubMed] [Google Scholar]
- Kenward MG, Roger JH, 1997. Small sample inference for fixed effects from restricted maximum likelihoodsample inference for fixed effects from restricted maximum likelihood. Biometrics 53, 983–997. [PubMed] [Google Scholar]
- Kharbanda RK, Walton B, Allen M, Klein N, Hingorani AD, MacAllister RJ, Vallance P, 2002. Prevention of inflammation-induced endothelial dysfunction - A novel vasculo-protective action of aspirin. Circulation 105, 2600–2604. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Derry HM, Fagundes CP, 2015. Inflammation: Depression Fans the Flames and Feasts on the Heat. Am. J. Psychiatry 172, 1075–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Gravenstein S, Malarkey WB, Sheridan J, 1996. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc. Natl. Acad. Sci. U. S. A. 93, 3043–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrou I, Chrousos GP, Tsigos C, 2006. Stress, visceral obesity, and metabolic complications. Stress, Obesity, and Metabolic Syndrome, pp. 77–110. [Google Scholar]
- Lacourt TE, Houtveen JH, Veldhuijzen van Zanten JJ, Bosch JA, Drayson MT, Van Doornen LJ, 2015. Negative affectivity predicts decreased pain tolerance during low-grade inflammation in healthy women. Brain Behavior and Immunity 44, 32–36. [DOI] [PubMed] [Google Scholar]
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, Zitman FG, 2010. Overweight, Obesity, and Depression: A Systematic Review and Meta-analysis of Longitudinal Studies. Arch. Gen. Psychiatry 67, 220–229. [DOI] [PubMed] [Google Scholar]
- Madison AA, Shrout MR, Renna ME, Kiecolt-Glaser JK, 2021. Psychological and Behavioral Predictors of Vaccine Efficacy: Considerations for COVID-19. Perspectives on Psychological Science 16, 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandsager K, Harb S, Cremer P, Phelan D, Nissen SE, Jaber W, 2018. Association of Cardiorespiratory Fitness With Long-term Mortality Among Adults Undergoing Exercise Treadmill Testing. JAMA Network Open 1, e183605-e183605. [Google Scholar]
- Mock V, 2004. Evidence-based treatment for cancer-related fatigue. Journal of the National Cancer Institute Monographs 32, 112–118. [Google Scholar]
- Moncunill G, Scholzen A, Mpina M, Nhabomba A, Hounkpatin AB, Osaba L, Valls R, Campo JJ, Sanz H, Jairoce C, Williams NA, Pasini EM, Arteta D, Maynou J, Palacios L, Duran-Frigola M, Aponte JJ, Kocken CHM, Agnandji ST, Mas JM, Mordmuller B, Daubenberger C, Sauerwein R, Dobano C, 2020. Antigen-stimulated PBMC transcriptional protective signatures for malaria immunization. Sci. Transl. Med. 12. [Google Scholar]
- Nakaya Helder I., Hagan T, Duraisingham Sai S., Lee Eva K., Kwissa M, Rouphael N, Frasca D, Gersten M, Mehta Aneesh K., Gaujoux R, Li G-M, Gupta S, Ahmed R, Mulligan Mark J., Shen-Orr S, Blomberg Bonnie B., Subramaniam S, Pulendran B, Systems Analysis of Immunity to Influenza Vaccination across Multiple Years and in Diverse Populations Reveals Shared Molecular Signatures. Immunity 43, 1186–1198. [Google Scholar]
- Neal ML, Duffy FJ, Du Y, Aitchison JD, Stuart KD, 2022. Preimmunization correlates of protection shared across malaria vaccine trials in adults. Npj Vaccines 7. [Google Scholar]
- Padin AC, Wilson SJ, Bailey BE, Malarkey WB, Lustberg MB, Farrar WB, Povoski SP, Agnese DM, Reinbolt RE, Wesolowski R, Williams N, Sardesai S, Ramaswamy B, Noonan AM, Vandeusen JB, Haas GJ, Kiecolt-Glaser JK, 2019. Physical Activity After Breast Cancer Surgery: Does Depression Make Exercise Feel More Effortful than It Actually Is? Int. J. Behav. Med. 26, 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine NJ, Ring C, Bosch JA, Drayson MT, Veldhuijzen van Zanten JJ, 2013. The time course of the inflammatory response to the Salmonella typhi vaccination. Brain Behav. Immun. 30, 73–79. [DOI] [PubMed] [Google Scholar]
- Pape K, Ryttergaard L, Rotevatn TA, Nielsen BJ, Torp-Pedersen C, Overgaard C, BØGgild H, 2016. Leisure-Time Physical Activity and the Risk of Suspected Bacterial Infections. Medicine and Science in Sports and Exercise 48. [Google Scholar]
- Pascoe AR, Fiatarone Singh MA, Edwards KM, 2014. The effects of exercise on vaccination responses: A review of chronic and acute exercise interventions in humans. Brain Behavior and Immunity 39C, 33–41. [Google Scholar]
- Pawelec G, Goldeck D, Derhovanessian E, 2014. Inflammation, ageing and chronic disease. Curr. Opin. Immunol. 29, 23–28. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, 2017. Anti-inflammatory effects of exercise: role in diabetes and cardiovascular disease. Eur. J. Clin. Invest. 47, 600–611. [DOI] [PubMed] [Google Scholar]
- Peel AB, Thomas SM, Dittus K, Jones LW, Lakoski SG, 2014. Cardiorespiratory Fitness in Breast Cancer Patients: A Call for Normative Values. Journal of the American Heart Association 3. [Google Scholar]
- Pereira B, Xu X-N, Akbar AN, 2020. Targeting Inflammation and Immunosenescence to Improve Vaccine Responses in the Elderly. Frontiers in Immunology 11, 583019–583019. [Google Scholar]
- Plotkin SA, Bouveret-Le Cam N, 1995. A new typhoid vaccine composed of the Vi capsular polysaccharide. Arch. Intern. Med. 155, 2293–2299. [PubMed] [Google Scholar]
- Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel JM, Sundararajan V, 2011. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 173, 676–682. [DOI] [PubMed] [Google Scholar]
- Radloff LS, 1977. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1, 385–401. [Google Scholar]
- Shelton RC, Miller AH, 2010. Eating ourselves to death (and despair): the contribution of adiposity and inflammation to depression. Prog. Neurobiol. 91, 275–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson RJ, Campbell JP, Gleeson M, Kruger K, Nieman DC, Pyne DB, Turner JE, Walsh NP, 2020. Can exercise affect immune function to increase susceptibility to infection? Exerc. Immunol. Rev. 26, 8–22. [PubMed] [Google Scholar]
- Team, R.C., 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Trzonkowski P, Mysliwska J, Szmit E, Wieckiewicz J, Lukaszuk K, Brydak LB, Machala M, Mysliwski A, 2003. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination--an impact of immunosenescence. Vaccine 21, 3826–3836. [DOI] [PubMed] [Google Scholar]
- Vance V, Mourtzakis M, McCargar L, Hanning R, 2011. Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obesity Reviews 12, 282–294. [DOI] [PubMed] [Google Scholar]
- Verschoor CP, Lelic A, Parsons R, Evelegh C, Bramson JL, Johnstone J, Loeb MB, Bowdish DME, 2017. Serum C-Reactive Protein and Congestive Heart Failure as Significant Predictors of Herpes Zoster Vaccine Response in Elderly Nursing Home Residents. J. Infect. Dis. 216, 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE, Sherbourne CD, 1992. The MOS 36-Item-Short-Form Health Survey (SF-36): I. Conceptual framework and item selection. Med. Care 30, 473–483. [PubMed] [Google Scholar]
- Weber T, Ljungman P, 2018. Stringent vaccination of cancer patients: is it that important. Ann. Oncol. 29, 1348–1349. [DOI] [PubMed] [Google Scholar]
- Wright CE, Strike PC, Brydon L, Steptoe A, 2005. Acute inflammation and negative mood: Mediation by cytokine activation. Brain Behavior and Immunity 19, 345–350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.