Abstract
Objectives:
This study identified an association between cholesteatoma and progressive sensorineural hearing loss using a large pediatric longitudinal audiologic dataset. Cholesteatoma is a potential sequelae of chronic otitis media with effusion, a commonly observed auditory pathology that can contribute to hearing loss in children. The purpose of this report is to (i) describe the process of identifying the association between cholesteatoma and progressive sensorineural hearing loss in a large pediatric dataset and (ii) describe the audiologic data acquired over time in patients identified with cholesteatoma-associated progressive sensorineural hearing loss.
Design:
Records of patients included in the Audiologic and Genetics Database (n=175,215 patients) were examined using specified criteria defining progressive hearing loss. A linear regression model examined the log frequency of all diagnostic codes in the electronic health record assigned to patients for a progressive hearing loss cohort compared to a stable hearing loss group. Based on findings from the linear regression analysis, longitudinal audiometric air- and bone-conduction thresholds were extracted for groups of subjects with cholesteatoma-associated progressive (n=58 subjects) and stable (n=55 subjects) hearing loss to further analyze changes in hearing over time.
Results:
The linear regression analyses identified that diagnostic codes for cholesteatoma were associated with progressive sensorineural hearing loss in children. The longitudinal audiometric data demonstrated within-subject changes in masked bone-conduction sensitivity consistent with progressive sensorineural hearing loss in children diagnosed with cholesteatoma. Additional analyses showed that mastoidectomy surgeries did not appear to contribute to the observed progressive hearing loss and that a high number of cholesteatoma patients with progressive hearing loss had normal hearing thresholds at their first test.
Conclusions:
The statistical analyses demonstrated an association between cholesteatoma and pediatric progressive sensorineural hearing loss. These findings inform clinical management by suggesting that children with cholesteatoma diagnoses may be at increased risk for progressive sensorineural hearing loss and should receive continued monitoring even after a normal masked bone-conduction baseline has been established.
INTRODUCTION
The question “Will my child’s hearing loss get worse over time?” presents a particular challenge for hearing healthcare professionals as changes in hearing over time are difficult to predict. Progressive sensorineural hearing loss (SNHL) is reported in 4 to 48% of pediatric patients with SNHL (Ruben et al. 1982; Bohme 1985; Parving 1985; Newton & Rowson 1988; Brookhouser et al. 1994; Berrettini et al. 1999; Barreira-Nielsen et al. 2016). Important considerations related to progressive SNHL in pediatric patients are (i) identifying the underlying etiologies, (ii) identifying a sufficient number of patients to understand characteristics, and (iii) defining hearing loss progression.
Etiologies associated with progressive SNHL in pediatric patients can be sub-divided into two main categories: (i) hereditary and (ii) infectious. Hereditary progressive SNHL is associated with non-syndromic and syndromic genotypes and phenotypes and variable inheritance patterns. Non-syndromic hearing loss is reported in 33 to 36% of pediatric patients with progressive SNHL (Brookhouser et al. 1994; Berrettini et al. 1999; Morell et al. 2000). Hereditary syndromes involving progressive hearing loss include, to name a few, Alport, Branchio-oto-renal, Pendred, Usher, and Waardenburg syndromes (e.g. Cremers et al. 1998; Stinckens et al. 2001). Progressive hearing loss, whether non-syndromic or syndromic, is more often associated with autosomal dominant than recessive inheritance patterns (Korver et al. 2017).
Two common underlying etiologies resulting in progressive SNHL due to infectious diseases/disorders are cytomegalovirus (CMV) and chronic otitis media with effusion (OME). Fowler et al. (1997) reported that 50% of children with congenital CMV developed progressive SNHL with a higher occurrence of unilateral progression. On the other hand, while chronic OME is often associated with fluctuating air-conduction thresholds, chronic middle-ear infections also have been associated with potentially progressive extended high-frequency SNHL beyond 8 kHz in children (Hunter et al. 1996). Recurrent OME can lead to the formation of cholesteatoma in the infected middle-ear cavity (e.g. Glasscock et al. 1981). In a cross-sectional study, Rosito et al. (2016) compared bone-conduction (BC) thresholds for ears with a cholesteatoma to normal contralateral ears in the same patients and reported poorer thresholds for the cholesteatoma ears in the frequencies from 2 to 4 kHz.
Large datasets provide an opportunity to gain knowledge about lower incidence conditions affecting auditory function. The Audiologic and Genetics Database (AudGenDB; Pennington et al. 2020) is a large pediatric database that integrates auditory and medical information from the electronic health records of pediatric patients. The availability of a range of audiologic and medical information, including clinical diagnostic coding data, covering the years of patient care facilitates study of changes in hearing and relationships to other conditions over time. Specific to the study reported here, the AudGenDB platform was used to determine whether specific diagnostic codes were more commonly associated with pediatric progressive compared to stable SNHL.
Identifying criteria for progressive SNHL is challenging because published definitions of shifts in hearing typically focus on adult populations and emphasize the higher frequencies as they are most often associated with noise trauma or ototoxicity (ASHA 1994; OSHA 2001). Many pediatric hearing losses, on the other hand, involve lower audiometric frequencies. The recommendations from the Study Group on Genotype Phenotype Correlations (GENDEAF) provide the only published definition of progressive hearing loss that specifically considers younger patients and includes lower frequencies (Mazzoli et al. 2003).
Assessing air- versus bone-conduction thresholds is an additional consideration when examining and defining pediatric progressive hearing loss. Using air-conduction (AC) audiometric thresholds to examine progressive SNHL is challenged by the confounding prevalence of conductive hearing losses related to middle-ear disorders in pediatric populations (e.g., Kaplan et al. 1973; Hogan et al. 1997; Paradise et al. 1997). To avoid the influence of conductive overlays to SNHL, masked BC threshold data are a key consideration when examining pediatric progressive SNHL (Lee & Hood 2014; Lee et al. 2015).
The purpose of this study was two-fold. First, we applied a definition of progressive SNHL to the audiometric data in the AudGenDB dataset to identify diagnostic codes that showed an association with pediatric progressive SNHL. Second, based on the identification of cholesteatoma as having a primary association with pediatric progressive SNHL, we characterized sequential audiometric data for cohorts of patients diagnosed with cholesteatoma with and without progressive hearing loss.
MATERIALS AND METHODS
The AudGenDB Database
The AudGenDB cohort contains pediatric patient data from three institutions (Boston Children’s Hospital, Children’s Hospital of Philadelphia – CHOP, and Vanderbilt University Medical Center – VUMC). AudGenDB contained data from 175,215 pediatric patients at the time of the data query for this study. Only patients from CHOP and VUMC were included in this study as patient information from Boston’s Children’s Hospital had not been integrated into the database at the time of these analyses. The overall inclusion criterion for patients in AudGenDB was at least one audiological evaluation prior to the age of 19 years for CHOP patients and 21 years for VUMC patients; age differences are based on institutional differences in the classification of patients as “pediatric.”
This study was approved by the Vanderbilt University Institutional Review Board. Because data were shared across sites, all demographic and medical information was de-identified by the assignment of arbitrary alias codes with the key to identification maintained only on secure servers at each institution. In accordance with standards set by the Health Insurance Portability and Accountability Act of 1996, no identifying information for VUMC patients was included in any data transfers to the coordinating center at CHOP. This included the change of actual dates (i.e., birth date, test date) to the age of the patient at each encounter, based on comparisons of shifted dates (for further details on the de-identification process, the reader is referred to Pennington et al. 2020).
Audiological data available in AudGenDB included audiograms, speech audiometry, tympanometry, middle-ear muscle reflexes, otoacoustic emissions, and auditory brainstem responses. Medical information available in the database included all diagnostic and procedural codes and limited information related to genetics and radiology.
Inclusion Criteria for Database Query
Pure-tone thresholds tested by AC and BC were extracted for the frequencies of 0.5, 1, 2, and 4 kHz. Inclusion criteria required ear-specific BC thresholds (by use of masking) between −10 and 70 dB hearing level (HL) at all four frequencies for all test dates selected for inclusion. A minimum of three test dates was required to differentiate progression from fluctuation. The focus on BC thresholds is consistent with the analysis of SNHL as AC thresholds can be confounded by conductive hearing loss. This study used International Classification of Diseases Version 9 (ICD-9) diagnostic codes because ICD-10 diagnostic codes had not been entered into the database at the time of these analyses.
Definitions of Hearing Loss Categories
Progressive Hearing Loss:
Hearing loss progression (shift) was defined based on the first and last test for each subject. For the database query, progressive hearing loss was defined as a change of ≥10 dB for the BC pure-tone average of 0.5, 1, 2, and 4 kHz (PTA4) from the first to last audiogram. Four published definitions were considered in the decision of using a 10-dB shift to define hearing loss progression. The ASHA (1994) definition provides two criteria: a 20-dB shift at any single frequency (that we will refer to as “DEF1”) or a 10-dB shift at any two adjacent frequencies (“DEF2”). OSHA (2001) defines hearing loss shift using an average of thresholds at 2, 3, and 4 kHz and a value of 10-dB change or greater (“DEF3”). We did not have sufficient data at 3 kHz given the pediatric nature of the population and the general lack of testing at 3 kHz by BC; therefore, we averaged 2 and 4 kHz for our “DEF3” analyses. The GENDEAF (Mazzoli et al., 2003) definition identifies hearing loss shift as 15 dB or greater for the average of thresholds at 0.5, 1, and 2 kHz (“DEF4”). The decision to use a ≥10-dB shift, while less restrictive than definitions such as that recommended by the GENDEAF Group, was adapted from our previous work on progressive hearing loss in subjects included in the AudGenDB dataset (Lee & Hood, 2014; Lee et al. 2015). Following the database query, threshold data at each frequency were reviewed to determine the number (“DEF1” to “DEF4”) of the published definitions of threshold shift that were met for each patient identified with progressive SNHL.
Stable Hearing Loss:
For the linear regression analyses (described below) examining the log frequency of diagnostic codes, a stable hearing loss control group was constituted for comparison to the progressive hearing loss cohort. The stable group was required to meet all of the audiological test inclusion criteria described above with the exception that only two test dates meeting inclusion criteria were required. We required each patient in the stable hearing loss control group to have a change in their masked BC PTA4 of <10 dB HL from the first to last audiogram.
Severity of Hearing Loss:
Hearing loss was categorized by severity based on AC thresholds according to definitions used in AudGenDB (i.e., normal: <15 dB HL; slight: 16–25 dB HL; mild: 26–40 dB HL; moderate: 41–55 dB HL; moderately severe: 56–70 dB HL; severe: 71–90 dB HL; and profound: 91+ dB HL). There was no control for differences in hearing loss configuration between groups.
Statistical Analyses
We generated a linear regression model to examine the log frequency of diagnostic codes for the progressive hearing loss cohort compared to the stable hearing loss control group. Models were created for CHOP and VUMC subjects separately due to differences in diagnostic (ICD-9) coding practices between the two institutions. Residuals from the linear regression were plotted and outliers were identified using the following criteria: (i) high outliers were defined as positive residuals that were larger than the 3rd quartile plus 1.5 times the inter-quartile range and (ii) low outliers were defined as negative residuals that were smaller than the 1st quartile minus 1.5 times the inter-quartile range.
This statistical design identified specific diagnostic codes (i.e., high, positive-residual outliers) that were more prevalent in pediatric progressive hearing loss compared to stable hearing loss. We further calculated chi-square tests to determine significant associations between diagnostic codes and pediatric progressive hearing loss characterized using masked BC thresholds.
Based on the identification of cholesteatoma as a diagnostic code associated with progressive SNHL, we then compared the distributions in the number of mastoidectomy surgeries between our progressive and stable SNHL cohorts and calculated Pearson correlation coefficients to determine whether the number of mastoidectomy surgeries for patients in our cholesteatoma-associated progressive SNHL cohort showed a relationship to the amount of BC shift associated with the progressive SNHL. The number of mastoidectomy surgeries for each subject was identified by review of each subject’s Current Procedural Terminology (CPT) codes. Pearson correlation coefficients examined the relationship between the number of mastoidectomy surgeries and degree of BC PTA4 shift.
RESULTS
Identification of Pediatric Progressive SNHL in AudGenDB
The first analysis identified patients in the AudGenDB cohort who met the study criteria for progressive SNHL. Of the 175,215 patients in AudGenDB, 50,527 patients had BC test results at some but not all 4 frequencies, and 15,581 patients met our strict criteria that required masked BC thresholds at 4 frequencies for at least 3 different test dates. Of those meeting the inclusion criteria, 425 patients were identified with progressive SNHL.
Diagnostic Codes Associated with Pediatric Progressive SNHL
We calculated chi-square tests to examine significant associations between ICD-9 diagnostic codes and pediatric progressive SNHL in the patient data extracted based on meeting BC threshold and test number criteria. Table 1 lists the 29 ICD-9 diagnostic codes that were significantly associated (p < 0.01) with pediatric progressive SNHL sorted based on significance level. With only a few exceptions (e.g., lower joint pain, backache) for which we have no ready explanation, the codes were consistent with hearing loss conditions.
Table 1.
The 29 ICD-9 diagnostic codes that were associated with pediatric progressive SNHL based on chi-square analyses.
| ICD-9 CM | Diagnosis Descriptor | Chi-Square | p-value |
|---|---|---|---|
| 389 | Conductive hearing loss, unspecified | 124.380 | p < 0.001 |
| *385.32 | *Cholesteatoma of middle ear | 123.483 | p < 0.001 |
| 389.03 | Conductive hearing loss, middle ear | 117.894 | p < 0.001 |
| *385.33 | *Cholesteatoma of middle ear and mastoid | 100.925 | p < 0.001 |
| 388.6 | Otorrhea, unspecified | 94.907 | p < 0.001 |
| 384.2 | Perforation of tympanic membrane, unspecified | 92.578 | p < 0.001 |
| 389.05 | Conductive hearing loss, unilateral | 85.451 | p < 0.001 |
| *385.30 | *Cholesteatoma, unspecified | 81.181 | p < 0.001 |
| 389.06 | Conductive hearing loss, bilateral | 66.601 | p < 0.001 |
| 381.81 | Dysfunction of Eustachian tube | 55.528 | p < 0.001 |
| 389.22 | Mixed hearing loss, bilateral | 47.474 | p < 0.001 |
| 380.4 | Impacted cerumen | 47.284 | p < 0.001 |
| 381.3 | Other and unspecified chronic nonsuppurative otitis media | 38.649 | p < 0.001 |
| 382.9 | Unspecified otitis media | 38.528 | p < 0.001 |
| 389.2 | Mixed hearing loss, unspecified | 37.825 | p < 0.001 |
| 381.1 | Chronic serous otitis media, simple or unspecified | 34.196 | p < 0.001 |
| 380.1 | Infective otitis externa, unspecified | 29.715 | p < 0.001 |
| 389.21 | Mixed hearing loss, unilateral | 29.042 | p < 0.001 |
| *389.02 | *Conductive hearing loss, tympanic membrane | 26.598 | p < 0.001 |
| 719.46 | Pain in joint, lower leg | 15.836 | p < 0.001 |
| 388.7 | Otalgia, unspecified | 14.898 | p < 0.001 |
| 381.4 | Nonsuppurative otitis media, not specified as acute or chronic | 11.779 | p < 0.001 |
| 780.79 | Other malaise and fatigue | 11.752 | p < 0.001 |
| 389.18 | Sensorineural hearing loss, bilateral | 11.074 | p < 0.001 |
| 367.1 | Myopia | 9.007 | p < 0.01 |
| 931 | Foreign body in ear | 7.993 | p < 0.01 |
| 389.16 | Sensorineural hearing loss, asymmetrical | 7.711 | p < 0.01 |
| 724.5 | Backache, unspecified | 7.009 | p < 0.01 |
| 781.2 | Abnormality of gait | 6.839 | p < 0.01 |
significant associations with progressive SNHL identified by the linear regression model.
Identification of Association Between Cholesteatoma and Progressive SNHL
Figure 1 shows a representative linear regression model for the CHOP subjects comparing ICD-9 diagnostic code log frequency between progressive and stable hearing loss (panel A) and the resulting outlier diagnostic codes (panel B). Data for the VUMC subjects are not shown as results were similar.
Figure 1.
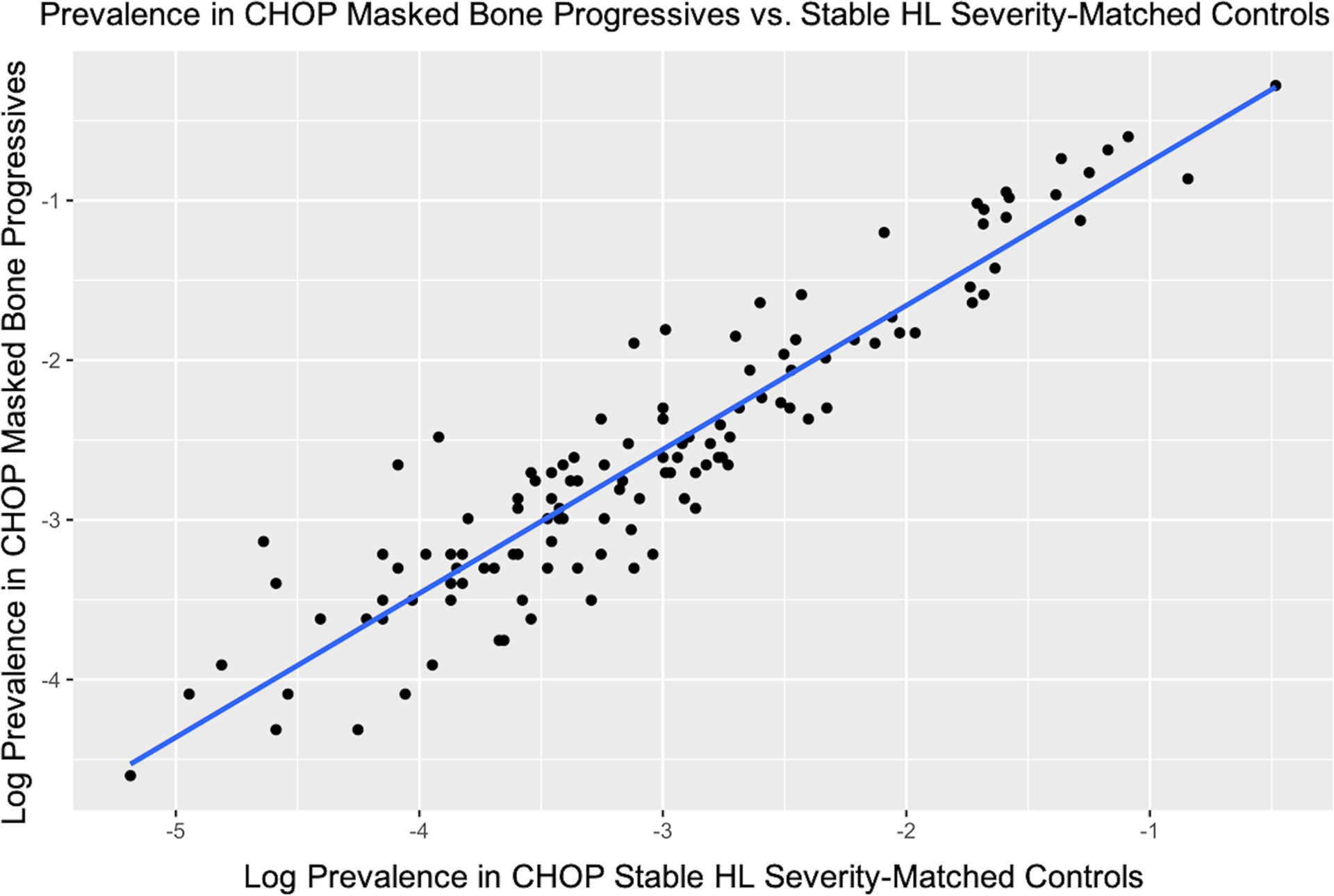
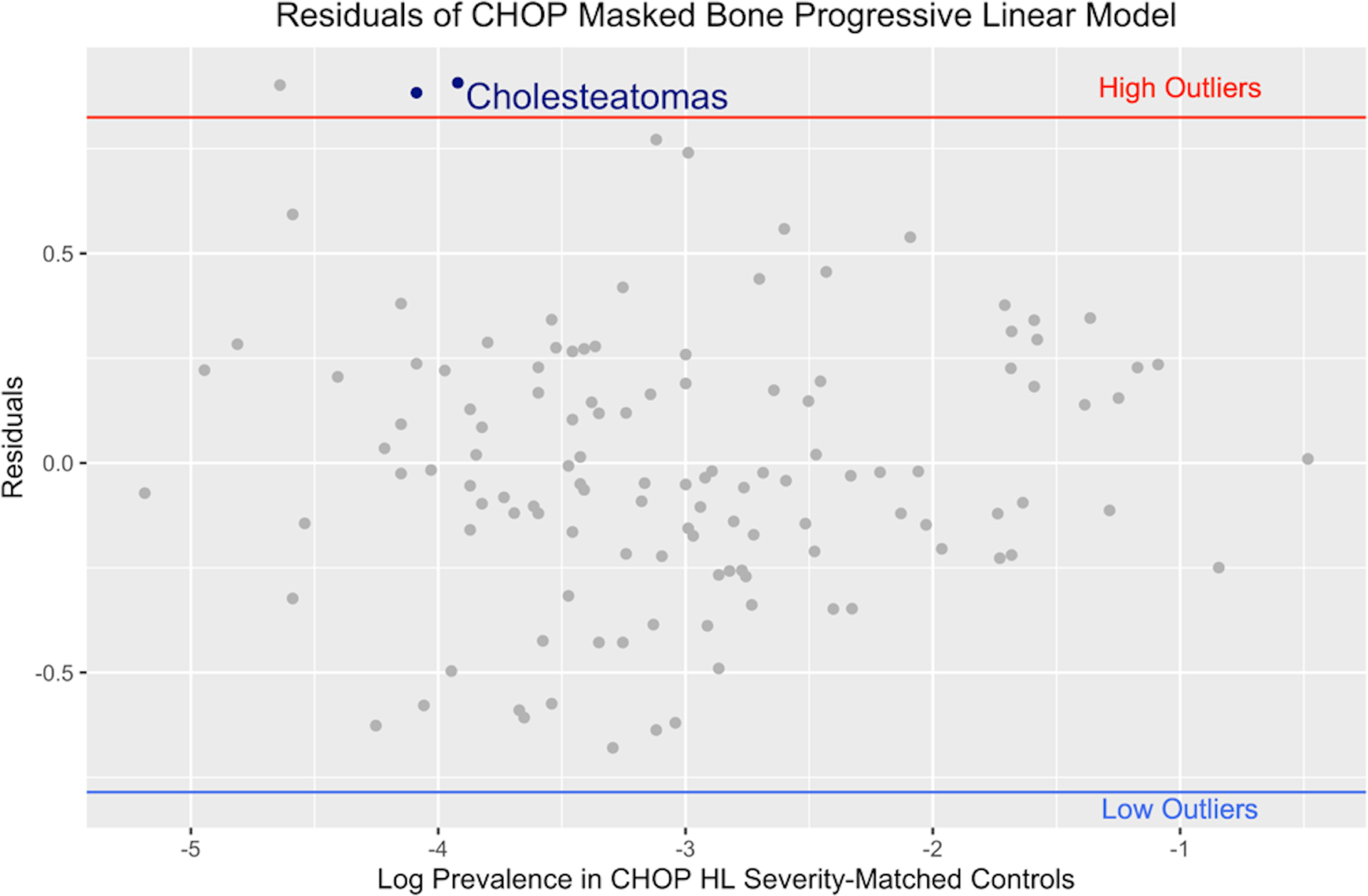
Linear regression analyses (panel A) comparing the log frequency of diagnostic codes between a progressive hearing loss cohort and stable hearing loss control group. The residuals from the linear regression (panel B) were used to identify outlier diagnostic codes that occurred more frequently in the progressive hearing loss cohort (high outliers). Various cholesteatoma diagnostic codes were identified as high, positive-residual outliers (highlighted in blue in Panel B). Analyses shown are for CHOP subjects. Comparable analyses were completed separately for VUMC patients (data not shown).
ICD-9 diagnostic codes identified as high, positive-residual outliers included 385.32 “Cholesteatoma of middle ear”, 385.33 “Cholesteatoma of middle ear and mastoid”, 385.30 “Cholesteatoma, unspecified”, and 389.02 “Conductive hearing loss, tympanic membrane”. All of the cholesteatoma diagnostic codes identified as high, positive-residual outliers from the linear regression analyses demonstrated significant associations with progressive hearing loss for the chi-square tests (indicated with an * in Table 1).
Cohort with Progressive SNHL and Cholesteatoma Diagnosis
Of those patients identified with a cholesteatoma diagnosis code, the final cohort with progressive SNHL comprised 68 ears from 58 subjects. Demographic and other subject information are shown in Table 2. This final subject group was derived after cross-checking all test results with inclusion criteria. The majority of subjects in this group had unilateral cholesteatoma with approximately 15% diagnosed with bilateral cholesteatoma. The mean age difference from first to last test covered 7.8 years with an average of 7.5 tests per subject that met our inclusion criteria. Given the clinical nature of the data and the consideration only of data meeting our inclusion criteria, there was no specific test number in the sequence of tests or time from first test that was associated with the progression.
Table 2.
Demographic data for children with cholesteatoma diagnosis included in the progressive and stable SNHL cohorts.
| Progressive | Stable | ||||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Number of Subjects | 58 | - | 55 | - | |
| Female | 32 | 55.2% | 21 | 38.2% | |
| Male | 26 | 44.8% | 34 | 61.8% | |
| n | % | n | % | ||
| Number of Ears | 68 | - | 66 | - | |
| Bilateral | 10 | 14.7% | 11 | 16.7% | |
| Unilateral LE | 20 | 29.4% | 19 | 28.8% | |
| Unilateral RE | 28 | 41.2% | 25 | 37.9% | |
| Mean | sd | Mean | sd | ||
| Age at first test (yrs) | 10.9 | 3.8 | 10.8 | 4.0 | |
| Age at last test (yrs) | 18.7 | 7.1 | 14.8 | 4.3 | |
| Age from first to last (yrs) | 7.8 | 6.1 | 4.0 | 2.8 | |
| Mean | sd | Mean | sd | ||
| Number of tests meeting inclusion criteria | 7.5 | 4.8 | 5.2 | 3.5 | |
As noted in the methods section, 4 published definitions were considered in the decision of using a 10-dB shift to define hearing loss progression. For the 68 ears included in the cholesteatoma-associated progressive SNHL group, 53 ears met “DEF1”, 65 ears met “DEF2”, 54 ears met “DEF3”, and 26 ears met “DEF4”. Individual ears met from 1 to 4 of the definitions with 23 ears meeting criteria for all 4 definitions, 20 ears meeting 3 definitions, 21 ears meeting 2 definitions, and 4 ears meeting only 1 definition. In total, 35 of the 68 ears demonstrated PTA shifts >15 dB, with 26 of these for the frequencies 0.5, 1, and 2 kHz (“DEF4”) and 9 for higher frequencies (2 and 4 kHz; “DEF3”).
PTA4 shift for BC thresholds ranged from 10 to 57.5 dB (Table 3 and Figure 2c). Shift data were also reviewed for each frequency individually for children with cholesteatoma and progressive SNHL. The average shifts for each frequency were >15 dB and slightly greater PTA4 shifts were observed for 2 and 4 kHz than the lower frequencies (Table 3). Though some ears showed negative BC shifts for some individual frequencies, these negative shifts were offset by larger shifts at other frequencies so that the masked BC PTA4 shift was ≥10 dB for all ears in this cohort. The individual frequency showing the greatest shift was distributed across frequencies; fewer shifts were observed at 0.5 kHz, though 19 ears showed greatest shift at multiple frequencies.
Table 3.
Mean, standard deviation (sd), and range (minimum to maximum) of BC shift from the first to last test for each individual frequency and for masked BC PTA4 for ears meeting inclusion criteria for cholesteatoma-associated progressive and stable SNHL.
| Progressive | Stable | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 kHz | 1 kHz | 2 kHz | 4 kHz | PTA4 | 0.5 kHz | 1 kHz | 2 kHz | 4 kHz | PTA4 | ||
| Mean BC Shift (dB) | 15.81 | 16.47 | 19.26 | 19.93 | 17.87 | −2.20 | 0.08 | −0.98 | 0.91 | −0.55 | |
| sd | 9.80 | 11.91 | 14.10 | 17.99 | 10.05 | 7.50 | 6.04 | 7.35 | 8.18 | 4.49 | |
| BC Shift Range (dB) | −5 to 50 | −5 to 60 | 0 to 65 | −10 to 70 | 10 to 57.5 | −15 to 15 | −15 to 15 | −20 to 15 | −15 to 15 | −8.75 to 8.75 | |
| Count | 68 | 68 | 68 | 68 | 68 | 66 | 66 | 66 | 66 | 66 | |
Figure 2.
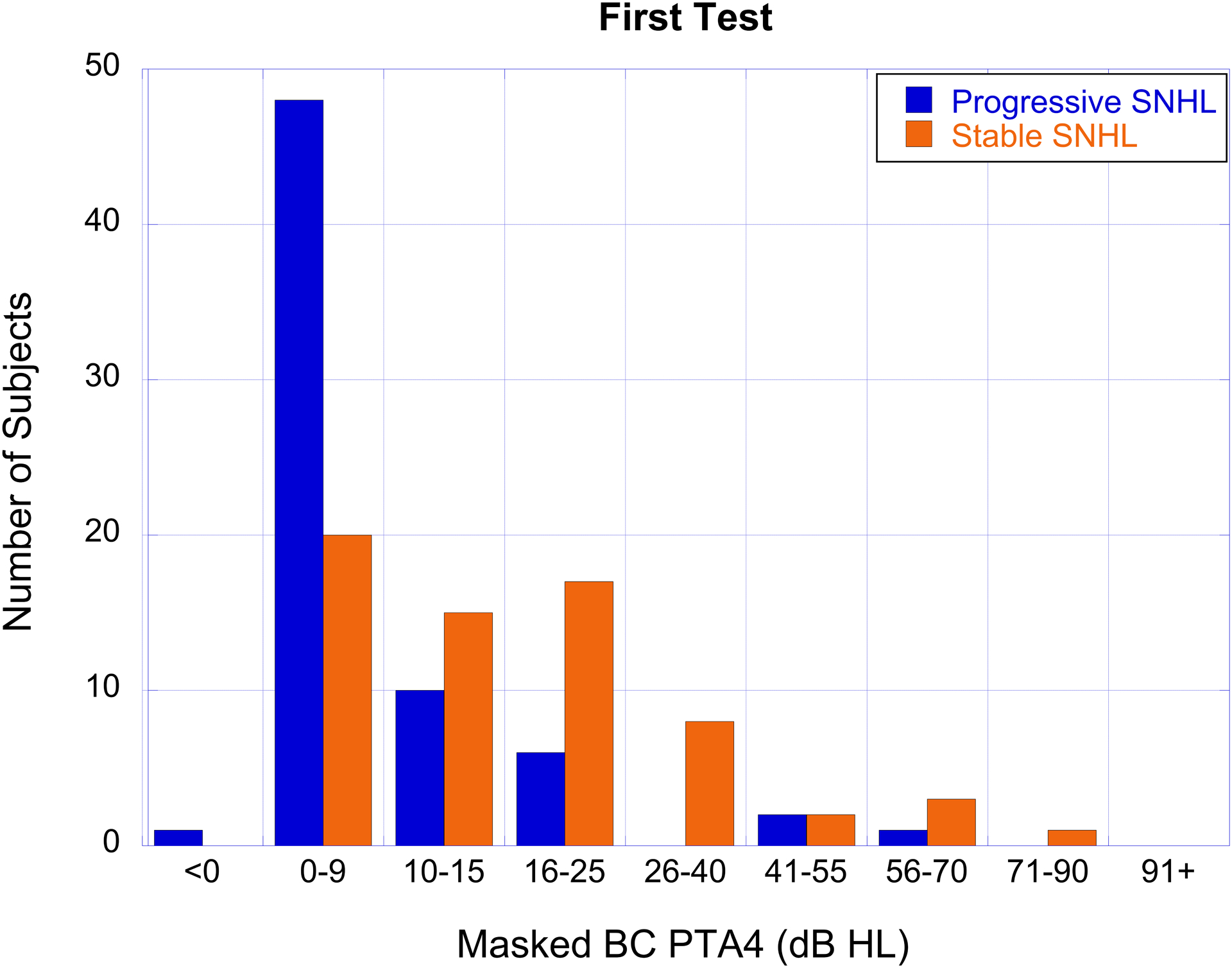
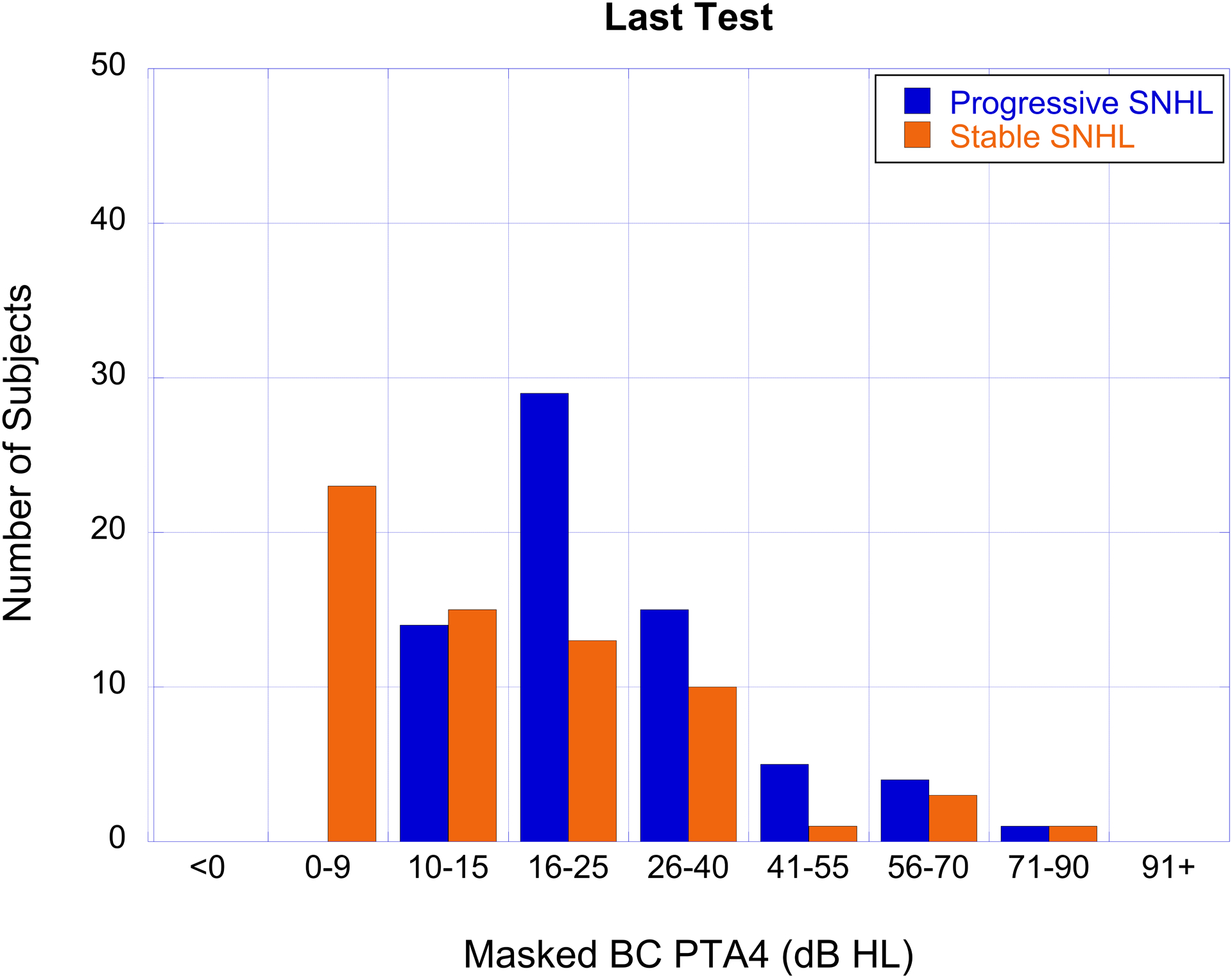
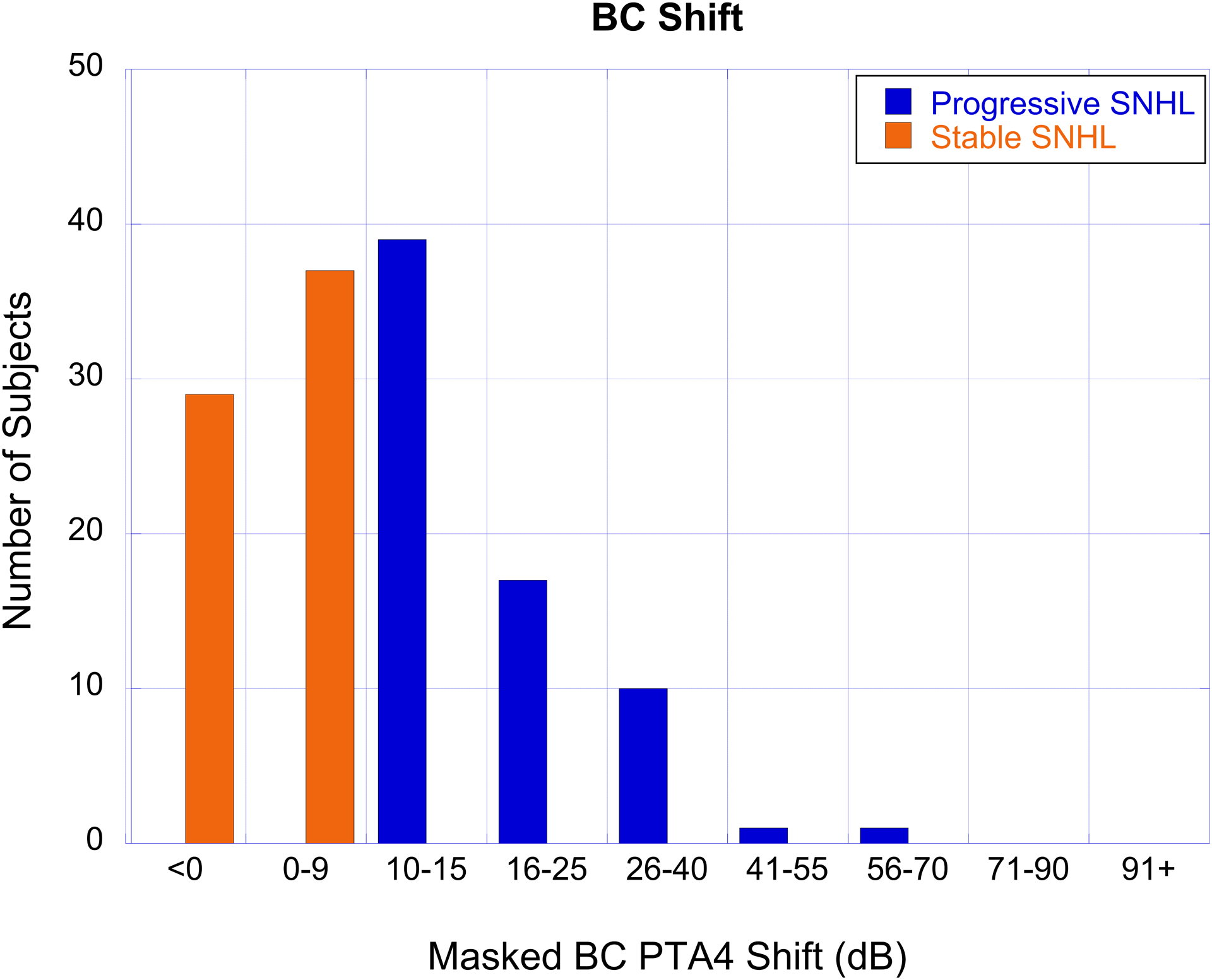
The number of subjects with cholesteatoma-associated progressive SNHL (blue bars) and stable SNHL (orange bars) as a function of hearing loss severity based on masked BC PTA4 for the first test (panel A), last test (panel B), and the degree of BC PTA4 shift (panel C).
The degree of hearing loss and shift, organized by categories described in the methods section, are shown for air- and bone-conduction in Table 4. AC thresholds were most often in the 26–55 dB HL range while BC thresholds were most often <15 dB HL on the first test and in the 10–40 dB range on the last test (Figure 2a, b). Air-bone gaps averaged 28.13 dB on the first test and 24.98 dB on the last test. A large percentage of ears in this cohort showed progression of BC thresholds from sensitivity that was initially in the normal hearing range (Figure 2a, b).
Table 4.
The number of ears with progressive and stable SNHL for each hearing loss severity category shown for the first and last audiometric tests.
| Air Conduction (PTA4) | Bone Conduction (PTA4) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Test (Number of Ears) |
Last Test (Number of Ears) |
Shift (Number of Ears) |
First Test (Number of Ears) |
Last Test (Number of Ears) |
Shift (Number of Ears) |
||||||||
| dB HL | P | S | P | S | P | S | P | S | P | S | P | S | |
| < 0 | 0 | 0 | 0 | 0 | 15 | 38 | 1 | 0 | 0 | 0 | 0 | 29 | |
| 0 – 9 | 1 | 0 | 0 | 0 | 14 | 29 | 48 | 20 | 0 | 23 | 0 | 37 | |
| 10 – 15 | 4 | 0 | 1 | 2 | 9 | 2 | 10 | 15 | 14 | 15 | 39 | 0 | |
| 16 – 25 | 9 | 9 | 5 | 8 | 9 | 3 | 6 | 17 | 29 | 13 | 17 | 0 | |
| 26 – 40 | 31 | 21 | 18 | 28 | 14 | 2 | 0 | 8 | 15 | 10 | 10 | 0 | |
| 41 – 55 | 17 | 14 | 19 | 13 | 4 | 0 | 2 | 2 | 5 | 1 | 1 | 0 | |
| 56 – 70 | 4 | 16 | 11 | 9 | 2 | 0 | 1 | 3 | 4 | 3 | 1 | 0 | |
| 71 – 90 | 1 | 2 | 9 | 3 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | |
| 91+ | 1 | 4 | 5 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
“P” = progressive SNHL cohort; “S” = stable SNHL cohort.
Stable SNHL and Cholesteatoma Diagnosis
The stable SNHL cohort was comprised of 66 ears from 55 subjects with a diagnosis of cholesteatoma. Demographic and other subject information are shown in Table 2. The majority of subjects in this group had unilateral cholesteatoma with approximately 17% diagnosed with bilateral cholesteatoma. The mean age difference from first to last test covered over 4.0 years with an average of 5.2 tests per subject meeting the inclusion criteria. Degree of hearing loss, based on BC PTA4 for the first audiogram, was compared between the progressive and stable cohorts. Hearing loss severity was distributed across similar ranges for both the progressive and stable SNHL groups.
BC threshold shifts for PTA4 in the stable group ranged from −8.75 to +8.75 dB (Table 3 and Figure 2c). Evaluation of individual frequency BC thresholds indicated mean shifts at each frequency ranging from −2.2 dB at 0.5 kHz to 0.91 dB at 4 kHz (Table 3). The range of individual frequency BC threshold shifts did not exceed 15 dB for any frequency and occurrences of larger shifts at a single frequency were offset by smaller shifts at other frequencies so that none of the ears in the stable SNHL cohort showed a PTA4 shift ≥10 dB or met any of the 4 published definitions for progressive hearing loss. AC thresholds were most often in the 26–70 dB HL ranges while BC thresholds were most often in the 0–40 dB HL range on the first and last tests (Table 4). Air-bone gaps averaged 28.88 dB on the first test and 25.06 dB on the last test.
Impact of Mastoidectomy Surgery
An important consideration regarding cholesteatoma-associated progressive hearing loss is the potential effect of mastoidectomy surgical procedures on hearing sensitivity. We began by comparing the number of mastoidectomy surgeries between the cholesteatoma-associated progressive SNHL cohort (n=58 subjects) and the cholesteatoma with stable SNHL cohort (n=55 subjects). We identified 39 subjects with cholesteatoma-associated progressive SNHL and 31 subjects with stable SNHL and a cholesteatoma diagnosis who received at least 1 mastoidectomy surgical procedure. The number of subjects as a function of the number of mastoidectomy surgeries for both the cholesteatoma-associated progressive SNHL and stable SNHL cohorts are shown in Figure 3. The majority of subjects with cholesteatoma-associated progressive SNHL received 2 mastoidectomy surgeries while the majority of subjects with stable SNHL received 1 mastoidectomy surgery (Figure 3).
Figure 3.
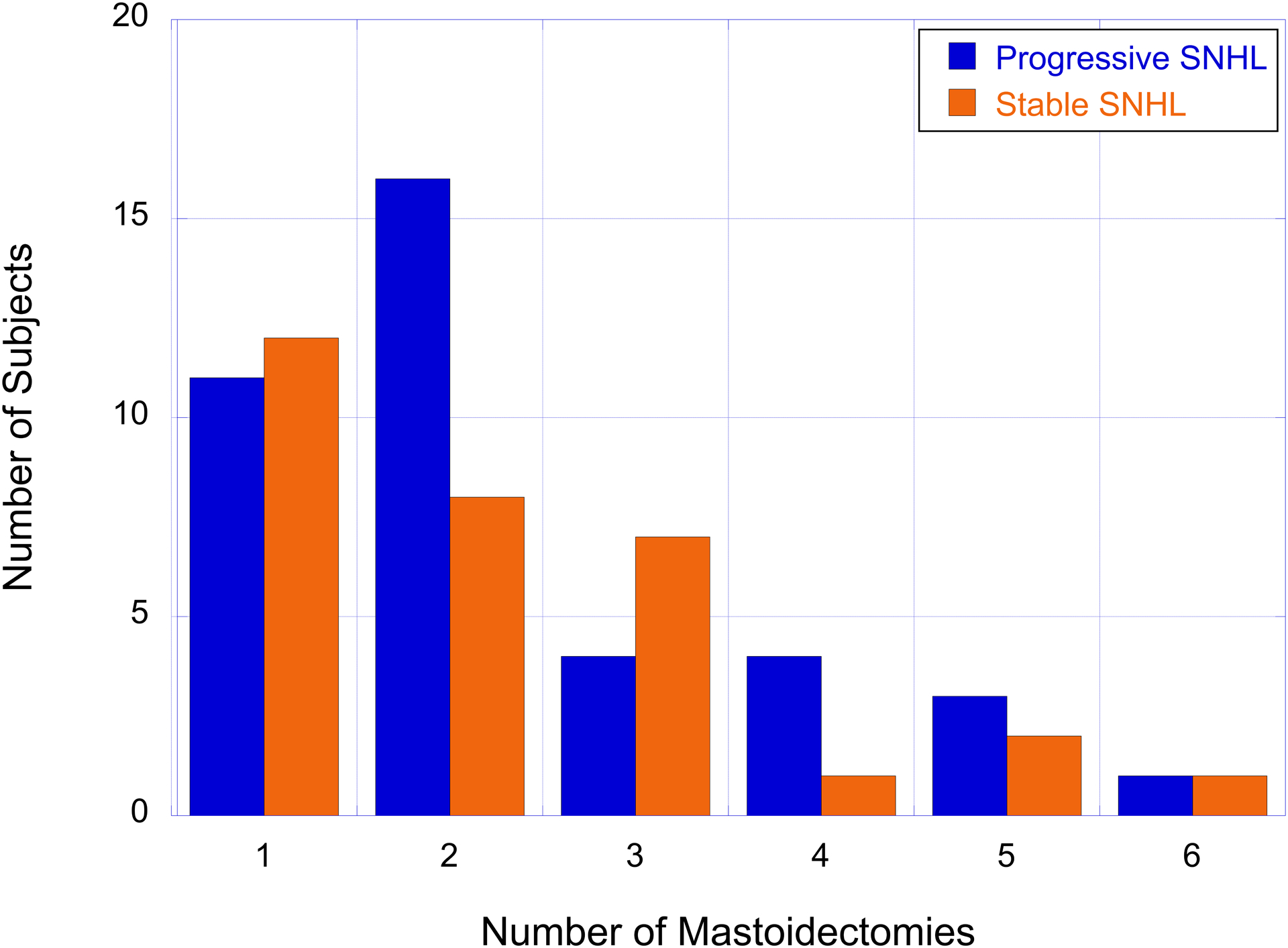
The number of subjects with cholesteatoma-associated progressive SNHL (blue bars) and stable SNHL (orange bars) as a function of the number of mastoidectomy surgeries.
Pearson correlation coefficients then examined the relationship between the number of mastoidectomy surgeries and degree of BC PTA4 shift for patients with cholesteatoma-associated progressive SNHL. A positive correlation between the number of mastoidectomy surgeries and degree of BC PTA4 shift would be suggestive of a larger number of mastoidectomy surgeries possibly contributing to cholesteatoma-associated progressive SNHL. The number of mastoidectomy surgeries was not correlated with BC PTA4 shift in the left ear (r=0.03, p=0.89). We observed a trend towards a negative correlation between the number of mastoidectomy surgeries and BC PTA4 shift in the right ear, though this trend was not significant (r=−0.21, p=0.29).
Bone-conduction versus air-conduction thresholds
The data in this study focus on BC thresholds, based upon our earlier studies (Lee & Hood 2014; Lee et al. 2015). Clinically, AC threshold testing is completed on a more regular basis than BC threshold testing, particularly when air-bone gaps are less than 10 dB. Based on this current clinical practice, we were interested in whether AC thresholds might indicate comparable hearing loss progression to that observed for masked BC thresholds. All ears with a cholesteatoma diagnosis meeting the inclusion criteria and demonstrating progressive SNHL also had AC thresholds for all four frequencies, allowing for calculation of AC and BC PTA4. Shifts in the PTA4 from the first to last test were compared for AC and BC. While all 68 ears met the criteria for cholesteatoma-associated progressive SNHL using the masked BC data, only 39 ears (57%) met the criteria for progression using AC thresholds. Figure 4 shows AC and BC threshold shifts for each of the 68 ears. AC threshold shifts (red bars) are displayed from lowest (left) to highest (right) values and BC threshold shifts (blue bars) are matched for each ear.
Figure 4.
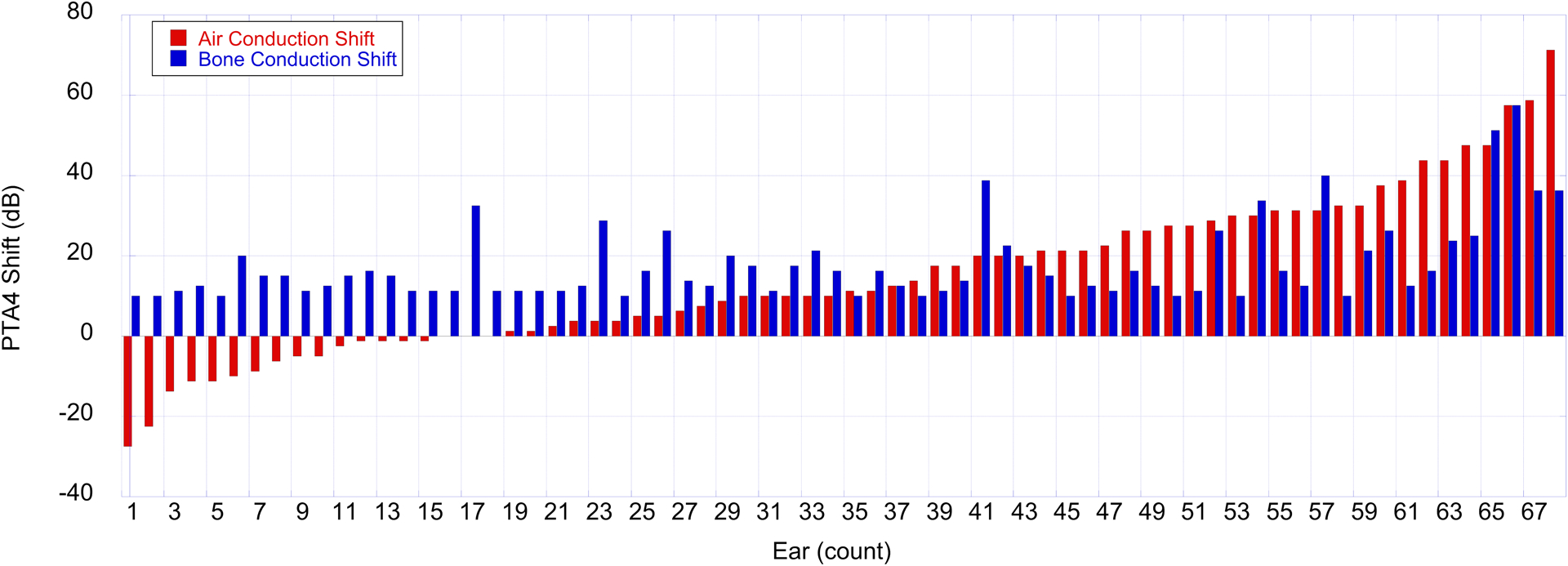
PTA4 BC and AC threshold shifts between the first and last tests for 68 ears with cholesteatoma that meet criteria for progressive hearing loss. BC threshold shifts (blue bars) indicate that all ears have shifts to poorer hearing of at least 10 dB. AC thresholds shifts (red bars) indicate ears with improved hearing for the last test (left), more stable hearing (middle), and poorer hearing for the last test (right). Note the differences in identifying progressive hearing loss between BC and AC thresholds.
DISCUSSION
While SNHL related to cholesteatoma has been reported in a cross-sectional study (Rosito et al. 2016), the present study provides within-subject, sequential audiometric data that demonstrate progression in cholesteatoma-associated SNHL in children. This observation extends the findings of Rosito et al. (2016) who identified SNHL based on data obtained at a single test date for each subject. Consistent with the cross-sectional findings of Rosito et al. (2016), we identified slightly greater BC threshold shifts for 2 and 4 kHz in children with a cholesteatoma.
Several previous studies have reported associations between OME and SNHL (e.g. Feinmesser et al. 1980; Lashin et al. 1988; Hunter et al. 1996). The exact pathophysiology underlying the association between OME/cholesteatoma and SNHL is unknown; however, inflammation of cochlear and/or auditory neural structures following viral effusion from the middle-ear cavity to the cochlea via the round window membrane may contribute to sensorineural changes in hearing following middle-ear pathology. Furthermore, cholesteatoma may erode the ossicular chain, primarily affecting the incus and articulation between the incus and stapes (Dornelles et al. 2007; Albera et al. 2012). Albera et al. (2012) showed that erosion of the ossicular chain secondary to cholesteatoma resulted in poorer AC and BC thresholds when compared to cases of cholesteatoma without ossicular chain erosion, suggesting that erosion of the ossicular chain by cholesteatoma can affect sensorineural aspects of hearing presumably with minimal effect on cochlear and auditory neural structures. Further research that elucidates the specific mechanisms by which middle-ear infectious diseases and processes affect sensorineural aspects of hearing over time is warranted.
The definition of pediatric progressive hearing loss that we employed in this study required ≥ 10-dB shift in the masked BC PTA for frequencies from 0.5 to 4 kHz. We used a 4-frequency PTA rather than the 3-frequency PTA used by Mazzoli et al. (2003) in order to include cases of pediatric progressive SNHL that occur in the higher frequency range (i.e., 4 kHz). This provided a more inclusive sample of the pediatric progressive SNHL population for our initial inquiry related to identifying diagnostic codes associated with pediatric progressive SNHL. The work of Rosito et al. (2016) showed greater BC threshold shifts at 2 and 4 kHz in children with cholesteatoma further supporting our decision to include 4 kHz thresholds into our 4-frequency BC PTA. A change of 10 dB is often considered within the expected test-retest repeatability range for pediatric audiologic testing. All patients identified with progressive hearing loss according to our definition also met at least 1 of the 4 published definitions of progressive hearing loss with many patients meeting multiple published definitions of progressive hearing loss.
Our strict inclusion criteria requiring masked BC data for 4 audiometric frequencies for at least 3 test dates limited the percentage of the full dataset available for analyses and precluded accurate estimations of incidence. In pediatric clinical practice, there are many instances when masked BC audiometric thresholds may not be able to be obtained, especially for 4 frequencies in each ear with masking. Our inclusion criteria, though designed to accurately assess ear-specific sensorineural aspects of hearing loss, may have introduced a potential bias in our sample. It is possible that the children included in our analyses were only those with sufficient developmental and cognitive capabilities to complete masked BC testing for 4 frequencies. Future studies that employ less strict BC inclusion criteria may better represent clinical practices and a greater range of developmental and cognitive abilities.
Current clinical protocols related to monitoring hearing loss in children are less likely to require the continued monitoring of masked BC thresholds of children after a normal masked BC baseline has been obtained at initial testing. Rather, it is more likely for AC thresholds to be monitored over time. Not monitoring BC threshold sensitivity over time is often related to time constraints associated with pediatric testing in fast-paced clinics. Further, threshold testing with masking requires the ability to attend to a tone while ignoring a simultaneous noise, which may be beyond the developmental capabilities of some children. However, the data from this study suggest the need for continued monitoring of BC threshold sensitivity, even after a normal baseline has been obtained. This appears particularly true for clinical monitoring protocols for children with a cholesteatoma as they may be at risk for developing progressive SNHL.
Figure 4 highlights the discrepancy between hearing loss progression identified based on AC versus BC threshold criteria. While all subjects included in this study demonstrated progressive hearing loss for masked BC thresholds, only 57% of these ears demonstrated progressive hearing loss using AC test results. Also, 6 subjects demonstrated improvements in thresholds of 10 dB or more when using AC despite having progressive hearing loss based on BC thresholds. These data support re-evaluating and re-structuring current clinical guidelines to include continued monitoring of BC thresholds for assessment and management of children with a history of cholesteatoma.
Regarding the potential for mastoidectomy surgeries to contribute to the observed cholesteatoma-associated progressive SNHL, our analyses did not identify a statistically significant association between the number of mastoidectomy surgeries and amount of BC PTA4 shifts for either ear. While we did not identify an association, future investigation would be appropriate with larger numbers of subjects, less restrictive inclusion criteria, and examination of relationships at specific frequencies.
The use of large datasets has an important role in research. However, the limitations associated with the retrospective, multi-institutional nature of the current study should also be carefully considered. While test protocols varied across clinic sites, each institution contributing data to AudGenDB used standard within-institution protocols that followed best-practice procedures. In addition, diagnostic coding practices among practitioners within and across institutions can introduce variability. This study analyzed the relationship between pediatric progressive hearing loss and diagnostic codes for each institution separately in order to control for differences in coding practices between the two institutions; however, we could not account for differences in coding practices among clinicians within an institution. Nevertheless, 3 out of 5 ICD-9 cholesteatoma of the middle ear codes were significantly associated with progressive hearing loss based on the chi-square tests reported by this study. This provided evidence that cholesteatoma may contribute to childhood progressive SNHL regardless of how the cholesteatoma diagnosis was coded.
A significant percentage of the cholesteatoma-associated progressive SNHL cohort had normal AC and BC thresholds for their first test. Later onset of progressive hearing loss raises a particular clinical dilemma in identification of hearing loss as early as possible. These children may pass regularly scheduled school hearing screening tests, depending on the timing of the onset of cholesteatoma and hearing loss progression. We also found that later-onset pediatric progressive SNHL progressed to mainly slight or mild hearing losses, which could also be missed depending on the environment of a screening test and the intensity level screened. Thus, these children may spend a longer period of time with undiagnosed and untreated hearing loss that could result in speech and language delays (e.g. Yoshinaga-Itano et al. 2008).
Longitudinal analyses that characterize pediatric progressive hearing loss over time have the potential to provide valuable information; however, these studies are currently lacking in the literature. While this study is an important first step in the longitudinal analysis of pediatric progressive hearing loss, the present analyses focused only on 4-frequency, BC PTA differences between the first and last audiograms from records with at least 3 tests. Future studies that effectively quantify the timeline and frequency-specific characteristics of pediatric progressive hearing loss and, specifically, cholesteatoma-associated progressive hearing loss are warranted. AudGenDB is uniquely designed and suited for such analyses.
Though we focused on behavioral BC audiometric threshold data in this study, the role of a battery of audiologic measures that include physiologic assessment in diagnosing conductive hearing loss secondary to cholesteatoma in children is important. Physiologic measures such as standard single-frequency tympanometry, wideband acoustic immittance, otoacoustic emissions, and auditory brainstem responses provide valuable information related to middle-ear, cochlear, and auditory neural function. These objective measures can be key components in the evaluation of auditory function in children who are not able to provide reliable behavioral responses. Future studies investigating progressive changes in auditory function in children with cholesteatoma would benefit from employing auditory physiologic measures.
Conclusion:
Several cholesteatoma diagnostic codes are associated with progressive SNHL in children. Cholesteatoma may impact sensorineural aspects of hearing via erosion of the ossicular chain in the middle-ear space or by viral infection of cochlear and/or auditory neural structures. Of note, a significant percentage of children with cholesteatoma-associated progressive SNHL have normal BC threshold sensitivity at their first hearing test. The combination of these findings highlighted an important need for continued assessment of masked BC thresholds in children who have cholesteatoma diagnostic codes towards monitoring their risk for progressive SNHL.
ACKNOWLEDGEMENTS
The authors wish to thank Janice Creel for her work in coordinating data retrieval, entry, and transfer from Vanderbilt University Medical Center to Children’s Hospital of Philadelphia for inclusion into AudGenDB.
Funded, in part, by the National Institute on Deafness and Other Communication Disorders Grant Number 1R24DC012207 (PI: EBC, Co-I: LJH).
Footnotes
The authors have no conflicts of interest to disclose.
Portions of this work were presented at the 2018 Annual Scientific and Technology Conference of the American Auditory Society, Scottsdale, Arizona, and at the 2019 Frontiers in Hearing Conference, Estes Park, Colorado.
REFERENCES
- Albera R, Canale A, Piumetto E, Lacilla M, & Dagna F (2012). Ossicular chain lesions in cholesteatoma. Acta Otorhinolaryngologica Italica, 32(5), 309. [PMC free article] [PubMed] [Google Scholar]
- American Speech-Language-Hearing Association. (1994). Audiologic Management of Individuals Receiving Cochleotoxic Drug Therapy [Guidelines].
- Barreira-Nielsen C, Fitzpatrick E, Hashem S, Whittingham J, Barrowman N, & Aglipay M (2016). Progressive hearing loss in early childhood. Ear and hearing, 37(5), e311–e321. [DOI] [PubMed] [Google Scholar]
- Berrettini S, Ravecca F, Sellari-Franceschini S, Matteucci F, Siciliano G, Ursino F (1999). Progressive sensorineural hearing loss in childhood. Pediatr Neurol, 20(2), 130–6. [DOI] [PubMed] [Google Scholar]
- Bohme G (1985). Progression of early childhood sensorineural hearing damage]. Laryngol Rhinol Otol (Stuttg), 64(9), 470–2. [PubMed] [Google Scholar]
- Brookhouser PE, Worthington DW, & Kelly WJ (1994). Fluctuating and/or progressive sensorineural hearing loss in children. Laryngoscope, 104(8 Pt 1), 958–64. [DOI] [PubMed] [Google Scholar]
- Cremers CW, Admiraal RJ, Huygen PL, Bolder C, Everett LA, Joosten FB, Green ED, van Camp G, & Otten BJ (1998). Progressive hearing loss, hypoplasia of the cochlea and widened vestibular aqueducts are very common features in Pendred’s syndrome. International journal of pediatric otorhinolaryngology, 45(2), 113–123. [DOI] [PubMed] [Google Scholar]
- Dornelles C, Rosito LPS, Meurer L, Costa SSD, Argenta A, & Alves SL (2007). Hystology findings’ correlation between the ossicular chain in the transoperative and cholesteatomas. Revista Brasileira de Otorrinolaringologia, 73, 738–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinmesser R, Weissel MJ, Levi H, & Weiss S (1980). Bullous myringitis: its relation to sensorineural hearing loss. The Journal of Laryngology & Otology, 94(6), 643–647. [DOI] [PubMed] [Google Scholar]
- Fowler KB, McCollister FP, Dahle AJ, Boppana S, Britt WJ & Pass RF (1997). Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. The Journal of pediatrics, 130(4), 624–630. [DOI] [PubMed] [Google Scholar]
- Glasscock III ME, Dickins JR, & Wiet R (1981). Cholesteatoma in children. The Laryngoscope, 91(10), 1743–1753. [DOI] [PubMed] [Google Scholar]
- Hogan SC, Stratford KJ, & Moore D (1997). Duration and recurrence of otitis media with effusion in children from birth to 3 years: Prospective study using monthly otoscopy and tympanometry. BMJ, 314, 350–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter LL, Margolis RH, Rykken JR, Le CT, Daly KA, & Giebink GS (1996). High frequency hearing loss associated with otitis media. Ear and hearing, 17(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Kaplan GJ, Fleshman JK, Bender TR, Baum C, & Clark PS (1973). Long-term effects of otitis media: A ten-ear cohort study of Alaskan Eskimo children. Pediatrics, 52, 577–585. [PubMed] [Google Scholar]
- Korver AMH, Smith RJH, Van Camp G, Schleiss MR, Bitner-Glindzicz MAK, Lustig LR, Shin-ichi U, & Boudewyns AN (2017). Congenital hearing loss. Nat Rev Dis Primers, 3, 16094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashin N, Zaher S, Ragab A, & ElGabri TH (1988). Hearing loss in bullous myringitis. Ear, nose, & throat journal, 67(4), 206–210. [PubMed] [Google Scholar]
- Lee J, and Hood LJ (2014). Defining and describing progressive hearing loss in pediatric populations. Poster presented at the Annual Scientific and Technology Meeting of the American Auditory Society. [Google Scholar]
- Lee J, Crenshaw EB, & Hood LJ (2015). Tracking changes in bone conduction thresholds using definitions of progression. Poster presented at the Annual Scientific and Technology Meeting of the American Auditory Society. [Google Scholar]
- Mazzoli M, Van Camp G, Newton V, Giarbini N, Declau F, & Parving A (2003). Recommendations for the description of genetic and audiological data for families with nonsyndromic hereditary hearing impairment. Audiol Med, 1, 148–50. [Google Scholar]
- Morell RJ, Friderici KH, Wei S, Elfenbein JL, Friedman TB, & Fisher RA (2000). A new locus for late-onset, progressive, hereditary hearing loss DFNA20 maps to 17q25. Genomics, 63(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Newton VE and Rowson VJ (1988). Progressive sensorineural hearing loss in childhood. Br J Audiol, 22(4), 287–95. [DOI] [PubMed] [Google Scholar]
- Occupational Safety and Health Administration. (2001). Occupational Injury and Illness Recording and Reporting Requirements, Vol. 66:§ 1904.10. [PubMed] [Google Scholar]
- Paradise JL, Rockette HE, Colborn KD, Bernard BS, Smith CG, Kurs-Lasky M, & Janosky JE (1997). Otitis mediia in 2253 Pittsburgh-area infants: Prevalence and risk factors during the first two years of life. Pediatrics, 99, 318–333. [DOI] [PubMed] [Google Scholar]
- Parving A (1985). Hearing disorders in childhood, some procedures for detection, identification and diagnostic evaluation. Int J Pediatr Otorhinolaryngol, 9(1), 31–57. [DOI] [PubMed] [Google Scholar]
- Pennington JW, Ruth B, Miller JM, Peterson J, Xu B, Masino AJ, Krantz I, Manganella J, Gomes T, Stiles D, Kenna M, Hood LJ, Germiller J, & Crenshaw EB (2020). Perspective on the Development of a Large-Scale Clinical Data Repository for Pediatric Hearing Research. Ear and Hearing, 41(2), 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosito LS, Netto LS, Teixeira AR, & Costa SSD (2016). Sensorineural hearing loss in cholesteatoma. Otology & Neurotology, 37(3), 214–217. [DOI] [PubMed] [Google Scholar]
- Ruben RJ, Levine R, Baldinger E, Silver M, Umano H, Fishman G, Feldman W, Stein M, & Kruger B (1982). Moderate to severe sensorineural hearing impaired child: analysis of etiology, intervention, and outcome. Laryngoscope, 92(1), 38–46. [DOI] [PubMed] [Google Scholar]
- Stinckens C, Standaert L, Casselman JW, Huygen PL, Kumar S, Van de Wallen J, & Cremers CW (2001). The presence of a widened vestibular aqueduct and progressive sensorineural hearing loss in the branchio-oto-renal syndrome. A family study. Int J Pediatr Otorhinolaryngol, 59(3), 163–72. [DOI] [PubMed] [Google Scholar]
- Yoshinaga-Itano C, Johnson CDC, Carpenter K, & Brown AS (2008). Outcomes of children with mild bilateral hearing loss and unilateral hearing loss. Seminars in Hearing, 29(2), 196–211. [Google Scholar]


