Abstract
Coronavirus disease (COVID-19) was first reported in December 2019, China and later it was found that the causative microorganism is severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). As on 3rd June 2021, SARS-CoV-2 has affected 171049741 people worldwide with 3549710 deaths. Nanomedicine such as nanoparticles, liposomes, lipid nanoparticles, virus-like nanoparticles offer tremendous hopes to treat viral infections including COVID-19. Most importantly target specific ligands can be attached on the surface of them and this makes them more target specific and the loaded drug can be delivered to cellular and molecular level. These properties of nanomedicines can be utilized to deliver drugs or vaccines to treat viral diseases including SARS-CoV-2 infection. This review discusses about SARS-CoV-2 and the potential application of nanomedicines for delivering biological macromolecules like vaccines and drugs for treating COVID-19.
Keywords: Nanomedicine, COVID-19, Nanotechnology, SARS-CoV-2, Nanocarriers, Liposomes
1. Covid-19
Viral diseases are a major cause of death worldwide. The treatment options are vaccination or drugs which inhibit virus multiplication by inhibiting an important step in the virus life cycle. But many viruses, over a period of time, evolve and become drug resistant, which requires better drugs and novel drug delivery approaches for the effective treatment. COVID-19 was first reported in December 2019, China [1]. A group of patients with cough, fever, breathing difficulties and other symptoms were hospitalized and computed tomography (CT) scanning of patients’ lungs showed varied opacities when compared with the images of the lungs of healthy persons [2]. At first, it was thought pneumonia but nucleic acid analysis did not show positive result for known pathogen panels which suggested unknown origin of pneumonia [1]. On 10thJanuary 2020, bronchoalveolar lavage fluid analysis of patients showed a pathogen which has a genetic sequence similar to the betacoronovirus B family. Later, it was found that the virus had ∼50%, ∼80% and ∼96%, similarities to the genome of Middle East respiratory syndrome virus (MERSCoV), severe acute respiratory syndrome virus (SARS-CoV) and bat coronavirus RaTG13 respectively [1]. The novel corona virus causing COVID-19 was named as SARS-CoV-2 (Fig. 1 ). The COVID-19 pandemic has led severe health crisis worldwide. As on 3rd June 2021, SARS-CoV-2 has affected 171049741 people worldwide with 3549710 deaths [3]. The possible mode of transmission is human-to-human through droplets which outspread when an infected person coughs or sneezes and to minimize the possibility of infection by droplets of nose or mouth, the WHO has advised to keep a distance of 1.5 to 2.0 m between people. However, recent studies indicated possibility virus transmission by a distance of 2 m by airborne droplets [4]. This article reviews about SARS-CoV-2 and the potential application of nanomedicines for delivering vaccines and drugs for treating COVID-19.
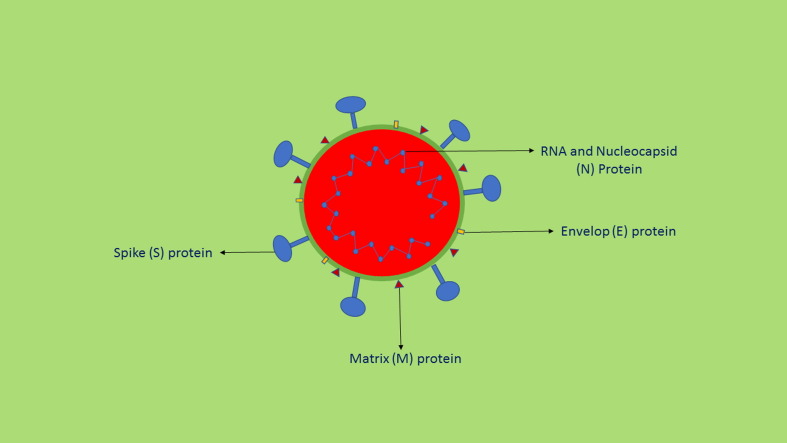
Fig. 1.
Structure of SARS-CoV-2.
2. Mechanism of COVID-19 infection and clinical features
A major determinant for SARS-CoV-2 entry to the host cell is the spike glycoprotein by binding with host cell’s angiotensin converting enzyme 2 (ACE 2), a cellular receptor and the infectivity and fusion are because of significant proteolytic cleavage and by clathrin-dependent and clathrin independent endocytosis. Once internalized into the host’s cell, the virus releases RNA which synthesizes two polyproteins and structural proteins resulting in viral replication. The nucleocapsid is formed by the combination of genomic RNA and nucleocapsid protein and finally the vesicles which contain the viral particles combine with plasma membrane and releases the viruses [5]. The important symptoms are fever, cough, breathlessness and fatigue. The other symptoms which are less common are sore throat, rhinorrhea, headache, dyspnea, loss of appetite, panting, diarrhoea, vomiting and abdominal pain. If the COVID-19 patients have other comorbidities like coronary heart disease, diabetes and hypertension, the severity of the disease increases. The incubation period of the infection has been reported between 5 and 14 days [6]. The symptoms are generally noticed approximately after five days of contracting the infection. Studies have shown that COVID-19 causes severe viral pneumonia. In many patients, the subpleural region of lungs showed presence of glass like opacities and these opacities may produce local and systemic immune reactions resulting in increased inflammatory reactions and interferon administration failed to show effective clinical results [7]. Recent reports reveal that COVID-19, in some patients, damages other organs such as kidney, heart, brain and the eyes. SARS-CoV-2 damages other organs directly or indirectly due to compromise of immune system [8]. A significant number of COVID-19 patients develop acute kidney injury. Acute respiratory distress syndrome (ARDS) is commonly associated with COVID-19 patients with high mortality. Initially COVID-19 patients develop mild symptoms, but a group of patients suddenly develop severe complications like ARDS and sometimes multiorgan failure and death. It is believed that the rapid severe complications are due to cytokine storm. It has recently been reported that coagulopathy as a serious morbidity in COVID-19 patients [9].
In SARS-CoV-2, an early and accurate detection is very important to decrease transmission risk by isolating the infected persons. Clinical diagnosis is mainly based on clinical symptoms and history of contact SARS-CoV-2 infected persons. The common clinical symptoms are cough, fever, sore throat, dyspnea and pneumonia. But serological tests are important for the diagnosis of COVID-19. A rapid detection test for the suspected person is essential to take appropriate steps and decrease transmission. Various tests have been developed based on serological, nanotechnology and molecular methods to detect SARS-CoV-2. Reverse transcription- polymerase chain reaction (RT-PCR) and chest computed tomography have currently been used as screening and diagnostic tests. The other tests performed include quantitative real time RT-PCR and RT-loop mediated isothermal amplification. Immune methods like SARS-CoV-2 specific IgM/IgG detection can be used to current or previous infection [10], [11].
3. Organs involved in COVID-19
An important target of the virus is angiotensin converting enzyme II (ACE2), a surface receptor present in human cells, which is essential for the effective uptake of SARS-CoV-2 in the host cells. During infection, the viral spike glycoprotein binds with the ACE2 of the host cell (peptidase domain of ACE2). Attachment of S protein with ACE2 is an important and the first step of infection, hence disrupting this binding process is a key approach in treatment [12]. Initially, SARS-CoV-2 impairs the respiratory system and thereafter systemically reaches heart, liver and kidneys, but still it is unclear that the disease directly causes organ damage as found in COVID-19 patients [13]. ACE2 is expressed highly in the airways. The other organs where it is expressed highly are the cardiovascular system, central nervous system, gastrointestinal tract and the female reproductive system [14]. The respiratory system serves as the main entry point and binding place for SARS-CoV-2 due to the high expression of ACE2 in the lungs and respiratory tract [15]. Hence, SARSCoV-2 can easily enter into the body by interacting ACE2 in the alveolar cells and this is described as the reason to develop immediate pneumonia and leads to ARDS and failure of multiple organs in severe patients [1]. ACE2 is expressed highly in the cells of cardiovascular system and play a vital role in blood pressure regulation and myocardial contractility [16]. The binding of SARSCoV-2 to ACE2 may result in the formation cardiac inflammation and fibrosis. The CoV-2 can infect the central nervous system by direct infection injury as well as hypoxic injury. The infection of astrocytes, macrophages and microglia can activate brain immune cells which leads to cytokine storm and severe brain damage. The interaction of SARS-CoV-2 with the ACE2 of brain capillary endothelial cells may affect the integrity of the BBB thus helps the entry of virus. Intestinal epithelial cells including pancreas and liver have more ACE2 and TMPRSS2 (transmembrane serine protease 2) and this makes these organs a potential target of CoV-2 [13]. Xiao et al., recently found viral RNA and N protein in the epithelial cells of stomach, duodenum and rectum [17]. ACE2 is expressed more in reproductive system particularly in uterus, placenta and foetal interface of pregnant women. The ACE2 expression in foetal tissue can make it as an important target site for the binding of CoV-2 which further causes morbidity and mortality [18]. High level of inflammatory chemokines and cytokines were observed in COVID-19 patients, which results in cytokine release syndrome (CRS). Advanced forms of CRS particularly combined with ARDS can cause severe multiorgan failure and eventually death. The chemokines and cytokines involved are interleukins 1 or 6 (IL-1, IL-6) or tumour necrosis factor (TNF), hence already available drugs can be tried for treating this syndrome [19]. Further, highly symptomatic COVID-19 patients have a high viral load in their lungs, hence strategies are required not only prevent the infection but also to reduce the viral load to prevent fatal consequences like hyperinflammation of lungs and multiple organ failure [12].
4. Current pharmacotherapy approaches for COVID-19
Many drug molecules are under development and testing to treat COVID-19. Drug repurposing is another quick approach to identify potential candidates to prevent or treat COVID-19 and hydroxychloroquine and ivermectin trials are examples of drug repurposing. Current research also focuses on antiviral drugs used to treat viral diseases like SARS-CoV and MERS-CoV and it appears these drugs are promising. Remdesivir, an antiviral drug effective against MERS-CoV, was the first drug tried clinically in the United States for COVID-19 [20]. In an in vitro study, a combination of remdesivir and chloroquine effectively controlled coronavirus infection [21] and it was reported that remdesivir exhibits its activity by inhibiting the nucleotide analog of RNA of RNA-dependent RNA polymerase and further investigation on rhesus macaque infection model of MERS-CoV showed that remdesivir minimized lungs damage and prevents viral replication [22]. Umifenovir, an antiviral drug, is effective against both enveloped and non-enveloped DNA and RNA viruses and are used for treating influenza. In vitro studies showed its efficiency against SARS and now in China its being used in the empirical treatment of COVID-19 [23]. COVID-19 patients who received umifenovir with other antiviral drugs like lopinavir or ritonavir showed significant improvement, better viral clearance and could increase patients discharge rate and decrease mortality rate [24]. Administration of lopinavir/ritonavir in a 54 years old male patient showed null titre values of coronavirus [25]. It was further reported that a combination of interferon-α (INF-α) with lopinavir/ritonavir and INF-α with lopinavir/ritonavir plus ribavirin might be useful to treat COVID-19 [26]. But this combination failed to provide any benefits to severe COVID-19 patients beyond standard care [27]. Hence, more studies are required to confirm their effectiveness to treat COVID-19 patients. Favipiravir, a broad-spectrum antiviral drug inhibits viral replication by inhibiting RNA dependent RNA polymerase of RNA viruses, has shown effectiveness against various viruses such as influenza virus, arenavirus, bunyavirus and filovirus. An early report of a clinical trial revealed that favipiravir has better antiviral activity than lopinavir/ritonavir with milder side effects [28]. Other antiviral drugs like oseltamivir and ribavirin also have been studied to find out their usefulness to treat COVID-19.
Activation of large number of mononuclear macrophages and T lymphocytes happen in COVID-19 infection which results in cytokines such as IL-6 production and this IL-6 binds with IL-6 receptors leading to cytokine storm and severe inflammatory responses in lungs as well as other organs. Hence, monoclonal antibodies which target the IL-6 pathways might be used to prevent cytokine storm. Tocilizumab, a humanized monoclonal antibody and an IL-6 receptor blocker which can bind with the IL-6 receptor with high affinity, reduces inflammatory responses. A retrospective study suggests that it can be used to manage high risk COVID-19 patients with cytokine storm [29]. Further studies showed that tocilizumab improved respiratory functions and immediately reduced the elevated body temperature to normal [30]. Many other studies also support the usefulness of tocilizumab. Coronavirus neutralizing antibodies can be used as they target the spike protein which facilitates virus entry to the host cells. The receptor binding makes irreversible conformational changes in the spike proteins and hence prevents infusion of virus with host cells [31]. CR3022, a SARS-CoV specific human monoclonal antibody, has been suggested along with other neutralizing antibodies against SARS-CoV-2 [32]. Other monoclonal antibodies such as sarilumab, lenzilumab and gimsilumab are in clinical trials. Some promising drug candidates for treating COVID-19 are given in Table 1 .
Table 1.
Some promising drug candidates for treating COVID-19.
| Sl. No. | Drug name | Category |
Reference |
|---|---|---|---|
| 1 | Remdesivir | Nucleotide analogue prodrug which inhibits viral RNA polymerases (antiviral) | [33] |
| 2 | Baricitinib | Janus kinase inhibitor used for treating rheumatoid arthritis | [34] |
| 3 | Favipiravir | Selectively inhibits the RNA dependent RNA- polymerase of RNA viruses (antiviral) | [35] |
| 4 | Dexamethasone | Corticosteroid possess anti-inflammatory and immunosuppressant effects | [36] |
| 5 | Methylprednisolone | Corticosteroid possess anti-inflammatory and immunosuppressant effects | [37] |
| 6 | Tocilizumab | Interlukin-6 receptor inhibitor (humanized monoclonal antibody) | [38] |
| 7 | Bamlanivimab and etesevimab | Bamlanivimab is a neutralizing IgG1K monoclonal antibody. Etesevimab ia s fully human recombinant monoclonal antibody. | [39] |
| 8 | Casirivimab and imdevimab | Monoclonal antibodies | [40] |
| 9 | Ivermectin | Anthelmintic | [41] |
5. Nanomedicine for COVID-19
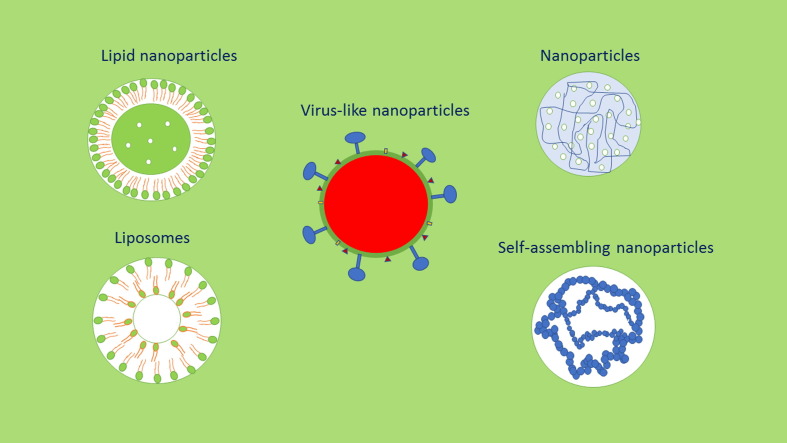
Nanomedicine, application of nanotechnology (nanocarriers) to treat and diagnose diseases, offers tremendous hopes to treat deadly diseases like cancer, neurodegenerative diseases, viral infections and others [42], [43], [44]. Some nanoparticle and liposome-based products such as Doxil®, MyocetTM, Caelyx®, Abraxane®, OnivydeTM and Vyxeos® are available in the market. Many attempts have been made to develop new molecules to eradicate the SARS-CoV-2 virus since the COVID-19 pandemic was declared as a medical emergency. SARS-CoV-2 virus affects the organs of the respiratory system in addition to the other organs. Hence drug targeting especially using nanomedicine such as NPs, liposomes and virus like particles (Fig. 2 ) would be helpful to target the drugs into the affected organs and play a vital role in COVID-19 treatment.
Fig. 2.
Some promising nanomedicines to deliver drugs to treat COVID-19.
Nanoparticles (NPs) are versatile drug delivery carriers and studied extensively to deliver a variety of drug molecules. NPs are further biocompatible, biodegradable and offer the advantage of encapsulating a variety of drugs for site-specific delivery including the brain [45], [46], [47]. NPs can be prepared by many methods using various synthetic and natural polymers [48], [49]. Many vaccines, genes, drugs and antibodies are under development to treat COVID-19 and a high concentration of these substances in non-target organs can cause severe side effects, and these problems might be solved by site specific targeting of these substances by nanomedicine approach. NPs can be surface engineered to reach specific target sites, both extracellular and intracellular and block virus binding to the host cell receptors. This is important to control viral diseases, to minimize side effects, to improve antiviral drug’s safety and overcome drug resistance. NPs also have other advantages in antiviral drug delivery such as improving the drug’s solubility, modifying drug’s pharmacokinetics, hence reduced dose would be required for the antiviral therapy and supresses viral spreading. Drug loaded NPs have been effective in inhibiting viral replication. NPs based on β-cyclodextrin containing acyclovir showed superior activity against clinical isolates of HSV-1 when compared with free acyclovir [50]. Further, acyclovir loaded NPs increased the inhibition of DNA replication in herpes virus infected cells [51]. Hu et al., formulated diphyllin/bafilomycin loaded PEG functionalized PLGA NPs and the drug loaded NPs increased the safety and antiviral activities of the drugs when compared with free diphyllin or bafilomycin. The diphyllin loaded NPs showed activity against H1N1 and H3N2 viral infection. In vivo studies further revealed that diphyllin loaded NPs showed good tolerability in mice, decreased weight loss and viral load in the lungs after H1N1 influenza virus infection and increased mice survival [52]. Methotrexate loaded NPs are currently in Phase I and II clinical trials to study their safety and effectiveness in COVID-19 patients with acute lung injury [53].
Dendrimers are nanosized, radially symmetric, three dimensional, highly branched, monodispersed polymeric scaffolds. Dendrimers have been studied to deliver antiviral drugs [54]. One of the strategies used to control viral infections is to inhibit virus uptake into the host cells and NPs could be used to prevent the interaction between SARS-CoV-2 and ACE2 thus prevent infection. Dendrimers have a tree like branched structure and have the ability to improve the effectiveness of drugs and bioactive compounds. In a study, PAMAM (polyamidoamine) dendrimers were able to bind with angiotensin receptors and acted as an antagonist for viral infection [55]. NPs functionalized with ligands, which are specific to angiotensin converting enzymes readily bind with these enzymes. Hence, these NPs can inhibit viral binding to ACE2 therefore, they can be explored to prevent SARS-CoV-2 infection. Dendrimers have been studied to treat HIV infection and have shown strong interactions with viruses and might increase antiviral activity. They can be engineered with functional ligands to interact with HIV E protein cell receptors of the host and eventually supress viral replication. VivaGel® (SPL7013), a microbicide dendrimer gel and a product of Starpharma, was developed to prevent HIV and HSV infections and available in the market [56]. Starpharma is developing VIRALEZETM COVID-19 nasal spray and it is given that the product has the potential to prevent acquisition and transmission of SARS-CoV-2 and to complement vaccine-based prevention strategies [57]. Polyanionic dendrimers can be used to improve the antiviral activity of drugs. Hence, dendrimers can be studied as a potential carrier to deliver antiviral drugs against COVID-19.
Liposomes, one of the most well-studied nanomedicines used for drug targeting, are vesicles containing one or more lipid bilayers enclosing aqueous spaces. Liposomes are made of mainly natural or synthetic phospholipids and few liposome-based products such as Doxil® and AmBisome® are available in the market. Hence, they have the potential to deliver antiviral drugs for COVID-19 as they have the ability to deliver drugs intracellularly. Liposomes have studied to treat hepatitis B and C viruses and HIV. The lipid composition of liposomes affects cells infectivity and it was reported that cationic and anionic liposomes interacted with equine herpes virus type 1 thus inhibiting infection [58]. Surface functionalization of liposomes with proteins, hydrophilic substances and mAbs improve specificity. Functionalization with PEG reduces unspecific protein adsorption. PEGylated liposomes coated with a HIV directed mAb fragment were specifically taken up by HIV 1 infected cells and produced sustained antiviral activity [59]. Metered dose inhalers, jet nebulizers, dry powder inhalers and soft mist inhalers are used to deliver drugs in the respiratory tract. NPs successfully delivered drugs after intranasal administration to organs such as the brain and lungs. NPs containing corticosteroids showed deep penetration into the lungs when administered as aerosols for treating asthma [60]. Hence, NP-based aerosols appear to be promising to deliver antiviral drugs into the lungs. NPs have been studied to increase the antiviral activity of drugs to treat respiratory infections. In a study after intranasal administration, influenza (H1N1) antigen conjugated chitosan NPs improved the immunogenicity of the antigen [61]. Nanoemulsions are mainly composed of water, oil and surfactant and assembled in nanodroplets and nanoemulsion droplets are stable when diluted and hence can be used for parenteral applications. Nanoemulsions have been studied to deliver vaccines and antiviral drugs. Neutralizing monoclonal antibodies to SARS-CoV-2 can be used for both prophylactic and therapeutic applications. Three monoclonal antibodies have been approved by the FDA for the treatment or prophylaxis of infectious diseases like respiratory syncytial virus, anthrax and Clostridioides difficile [62]. Monoclonal antibody products reduced the mortality by Ebola virus infection [63]. This supports the usefulness of monoclonal antibodies to treat COVID-19. But they have limitations such as issues related to bioavailability and viral diversity. Hence, it is important to observe the emergence of resistant viral mutations [62].
Nanovesicles derived cell membranes like exosomes play a vital role in drug targeting because they are biocompatible, surface engineered and cross the biological barriers [Xia et al., 2020]. Spike CAR-NK cells and Spike CAR macrophage cells showed good targetability and neutralizing capacity of SARS-CoV-2 pseudotyped virus [63]. But CAR-immune cell therapy may cause cytokine release syndrome, therefore an alternative may be use of nanovesicles. Nanovesicles have the capacity to load drugs including antiviral drugs. In a study Zhu et al. developed a nanovesicle derived from bispecific CAR-T cells which express CR3022 and B38 (single chain fragment variables) to specifically target SARS-CoV-2. Nanovesicles which express CR3022 and B38 showed stronger capacity for neutralizing spike pseudovirus infection when compared with the nanovesicles which express CR3022 and B38 alone. Further, the nanovesicles which express CR3022 and B38 could decrease the occurrence of viral resistance and their binding capacity to the spike protein of SARS-CoV-2 as well as the drug loading capacity help site-specific delivery of remdesivir to 293 T cells which overexoress spike protein [64]. This study shows the ability of nanovesicles to target antiviral drugs to treat COVID-19. In another study, Li et al. developed human lung spheroid cells (LSCs) derived ACE2 nanodecoys. The ACE2 nanodecoys can attach with SARS-CoV-2 and neutralize them for protecting the lung cells of the host from infection. The LSC ACE2 nanodecoys stayed in the lungs of mice after inhalation for a period of 72 h and increased the removal of SARS-CoV-2 mimics from the lungs. Further, four doses of inhalation of nanodecoys in Cynomolgus macaques, which were administered with live SARS-CoV-2, increased viral clearance with decreased lung injury [65]. This study suggests the potential usefulness of LSC nanodecoys in treating COVID-19.
The main target site of SARS-CoV-2 is nasal mucosa hence targeting ACE2 and DPP4 (dipeptidyl peptidase 4) (virus specific receptors) is a promising strategy and this can be achieved by conjugating receptor specific ligands like peptide or mAb onto the nanoparticles [19]. The mAbs or peptides was used as ACE2 or DPP4 antagonist to block receptors functions which resulted in preventing cell entry of CoVs [66]. Another strategy is to design the peptides or mAbs in such that to target the AE2 or DPP4 receptors by conjugating the peptides onto the nanomedicines. In this way, these nanomedicines could be used to deliver the drugs into the infected cells while preventing virus entry into the normal cells [67]. But no study described the usefulness of these peptides or mAbs to target drugs to SARS-CoV-2 [19]. It was reported that synthetic S proteins showed antiviral activity, by blocking ACE2, in SARS associated CoV [68] and may be used as a targeting ligand [19]. Reactive oxygen species (ROS) play a role in immunological responses and removing virus, but excess level of ROS oxidize cellular proteins and membrane lipids and quickly kill the cells infected with virus. This can damage the normal cells of lungs and heart which results in organ failures. An antioxidant therapy can be useful to decrease COVID-19 related cardiac problems. Nanoparticles containing antioxidants (nanoantioxidants) could be useful to reduce the oxidative stress in preventing and treating viral diseases including COVID-19 [69].
The safe and effective entry of NPs into the cells is important to achieve targetability and exhibit therapeutic activity. Further, the intracellular fate of NPs is crucial if the nanocarrier is intended for delivering specific drug or gene to the nucleus or the cytosol or any other specific intracellular site. More knowledge, about the different mechanisms involved in the uptake of NPs by the cells, is essential to develop safe and effective nanomedicines by modifying physicochemical properties of NPs to improve their cellular uptake followed by their targeting to the specific cellular organelle [70]. When NPs come and contact with plasma membrane of a cell, they interact with plasma membrane components or extracellular matrix and internalized into the cell mainly by the process of endocytosis. Endocytosis results in engulfing of NPs by invagination of cell membrane and pinching off to produce endocytic vesicles, followed by their transport to the specific intracellular organelle. The NPs fate, after administration, is related to NP-protein association and dissociation. This association and dissociation play a vital role to interact with biological surfaces in general and receptors in particular. NPs which entered into the cell via endocytosis may remain in the endocytic vesicles and therefore limit their usefulness to target intracellular level [70]. One of the effective strategies to overcome this is surface modification of NPs. Cell penetrating peptides and peptides with nuclear localization sequences can be used to guide the NPs into the cell interior including the cell nuclei [71], [72].
6. Nanomedicine for vaccine delivery
Vaccines intervene with the virus before the virus infects the host. Vaccines provide long-lasting effectiveness against the virus. Vaccines provide specific viral antigens into the body and often these antigens are presented on the cell surface of antigen presenting cells (APCs) and this is important for the adaptive immune system. The adaptive immune system recognizes such antigens and releases antibodies or activate the T cells to destroy them. Further, memory B cells produce antibodies specific to the virus on the cell membrane, which recognize the virus, activate immediate immune response to kill the virus [19]. The identification of genetic sequence of SARS-CoV-2 has initiated the development of vaccines against COVID-19. The vaccines are in different stages of clinical trials and many are in preclinical development (Table 2 ). Vaccines can be developed from viral antigens such as vectored vaccines, recombinant proteins, whole attenuated or inactivated virus and also can be developed from RNA- or DNA-encoding of viral antigens. The COVID-19 vaccines such as Pfizer/BioNTech Comirnaty vaccine, Covishield and AstraZeneca vaccines (developed by AstraZeneca/Oxford), Janssen/Ad26.COV 2.S (developed by Johnson & Johnson), Moderna COVID-19 vaccine and Sinopharm COVID-19 vaccine are listed in the WHO Emergency Use Listing (EUL) [73]. The US-FDA on 11th December 2020 issued emergency use authorization (EUA) to the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) to prevent COVID-19 disease caused by SARS-CoV-2 in individuals of 16 years age or older [74] and Moderna COVID-19 vaccine (mRNA-1273) in individuals of 18 years age and older [75]. The AstraZeneca’s COVID-19 has been approved for emergency supply in the UK by MHRA [76]. The BNT162b2 is a lipid nanoparticle formulated, mRNA vaccine that encodes the spike glycoprotein of SARS-CoV-2 [77]. The mRNA-1273 is a lipid NP-encapsulated mRNA- based vaccine that encodes for a full length, S protein of SARS-CoV-2 [78]. The mRNA vaccines deliver antigen encoding mRNA to the ribosomes to produce antigens. Various types of cells uptake the mRNA vaccine after vaccination and then activates the immune system via the MHC-I and MHC-II pathways. When APCs uptake the mRNA vaccine, the APCs express the target protein as an endogenous antigen and thereafter stimulates the CD8+ T cells via the MHC-I pathway. If non-APCs uptake the mRNA vaccine, then the non-APCs translate and secrete the target protein which is thereafter internalized by the APCs. Thereafter, the APCs stimulate the CD8+ T cells via the MHC-II pathway [79]. Both DNA and RNA vaccines have some advantages over conventional vaccines like low cost and simple purification. But there are some drug delivery issues such as degradation, less bioavailability and easy clearance from the body. These problems can be overcome by nanomedicine-based approaches of drug targeting.
Table 2.
Clinical trial status of some promising vaccines for the prophylaxis of SARS-CoV-2 infection (www.clinicaltrials.gov) Accessed on May 5, 2021.
| Candidate and clinical trial identifier number | Sponsor | Study title and Clinical trial status |
Characteristics/ Description |
Actual enrolment | Actual study start date and estimated study completion date |
|---|---|---|---|---|---|
| mRNA-1273 NCT04283461 |
National Institute of Allergy and Infectious Diseases (NIAID) | Safety and immunogenicity study of 2019-nCoV vaccine (mRNA-1273) for prophylaxis of SARS-CoV-2 infection (COVID-19) Phase 1 Open-label, dose-ranging study to determine the safety and immunogenicity of mRNA-1273 |
mRNA-based vaccine is encapsulated in a lipid nanoparticle that encodes for a full length, S protein of SARS-CoV-2. | 120 participants | March 16, 2020 November 22, 2022 |
| LV-SMENP NCT04276896 |
Shenzhen Geno-Immune Medical Institute | Immunity and safety of Covid-19 synthetic minigene vaccine Phase 1/2Multicentre study to determine the safety and efficacy of Lentiviral Minigene Vaccine (LV-SMENP) |
LV-SMENP vaccine is prepared by modifying dendritic cells with lentivirus vectors expressing COVID-19 minigene SMENP and immune modulatory genes | 100 participants (estimated) |
March 24, 2020 December 31, 2024 |
| INO-4800 NCT04336410 |
Inovio Pharmaceuticals | Safety, tolerability and immunogenicity of INO-4800 for COVID-19 in healthy volunteers Phase 1 Open-label study to determine the safety, tolerability and immunogenicity of INO-4800 |
Contains the plasmid PGX9501 which encodes full length S protein (Vaccine is administered intradermally followed by electroporation using CELLECTRA® 2000 | 120 participants | April 3, 2020 January 2022 |
| COV001 NCT04324606 |
University of Oxford | A study of a candidate COVID-19 vaccine (COV001) Phase 1/2 Single-blinded, randomized multicentre study to determine the efficacy, safety and immunogenicity of ChAdOx1 nCoV-19 |
ChAdOx1 nCoV-19 is a Chimpanzee adenovirus vectored vaccine expressing the SARS-CoV-2 spike protein | 1090 participants | April 23, 2020 October 2021 |
| BNT162b1 and BNT162b2 NCT04368728 |
BioNTech SE | Study to describe the safety, tolerability, immunogenicity, and efficacy of RNA vaccine candidates against COVID-19 in healthy individuals Phase 2/3 A placebo- controlled, randomized, observer-blind, dose finding study to determine the safety, tolerability, immunogenicity and efficacy |
BNT162b1 is a lipid nanoparticle formulated, nucleoside modified mRNA vaccine that encodes the trimerized receptor binding domain of spike glycoprotein of SARS-CoV-2 | 46,663 participants (estimated) | April 29. 2020 April 6, 2023 |
| Recombinant novel coronavirus vaccine (adenovirus type 5 vector) NCT04313127 |
CanSino Biologicals Inc. | Phase I clinical trial of a COVID-19 vaccine in 18–60 healthy adults (CTCOVID-19) Phase 1Single centre opel-label, dose-escalating study to determine the safety, reactogenicity and immunogenicity of recombinant novel coronavirus vaccine (adenovirus type 5 vector) |
Recombinant novel coronavirus vaccine (adenovirus type 5 vector) | 108 participants | March 16, 2020 December 20, 2022 |
| Pathogen-specific artificial antigen presenting cell (aAPC) NCT04299724 |
Shenzhen Geno-Immune Medical Institute | Safety and immunity of Covid-19 aAPC vaccine Phase 1 Open-label study to determine the safety and immunity of artificial antigen presenting cell vaccine |
aAPC vaccine is prepared by modifying lentivirus including immune modulatory genes and the viral minigenes to the artificial antigen presenting cells | 100 participants (estimated) |
February 15, 2020 December 31, 2024 |
| GX-19 NCT04445389 |
Genexine, Inc. | Safety and immunogenicity study of GX-19, a COVID-19 preventive DNA vaccine in healthy adults Phase 1/2 Multicenter, randomized, double blind, placebo controlled study to study the safety, tolerability and immunogenicity of GX-19 |
GX-19 is a SARS-CoV-2 spike (S) DNA based vaccine | 210 participants (estimated) | June 17, 2020 June 17, 2022 |
| Ad26.COV2.S NCT04505722 |
Janssen Vaccines & Prevention B.V. | A study of Ad26.COV2.S for the prevention of SARS-CoV-2-mediated COVID-19 in adult participants (ENSEMBLE) Phase 3 Randomized, double-blind, placebo controlled study to investigate the efficacy and safety of Ad26.CoV2.S. |
Ad26.COV2.S is a recombinant, replication-incompetent adenovirus serotype 26 vector encoding a SARS-CoV-2 spike protein. |
44,325 participants | September 7, 2020 January 2, 2023 |
| Gam-COVID-Vac NCT04436471 |
Gamaleya Research Institute of Epidemiology and Microbiology, Health Ministry of the Russian Federation | An open study of the safety, tolerability and immunogenicity of the drug “Gam-COVID-Vac” vaccine against COVID-19 Phase 1/2 An open two stage non-randomized study to assess the safety, tolerability and immunogenicity of Gam-COVID-Vac |
Gam-COVID-Vac is a recombinant adenovirus vector based on human adenovirus type 26 containing the SARS-CoV-2 S protein | 38 participants | June 17, 2020 August 10, 2020 |
| SARS-CoV-2rS/Matrix-M1 Adjuvant NCT04611802 |
Novavax | A study to evaluate the efficacy, immune response, and safety of COVID-19 vaccine in adults ≥ 18 years with a pediatric expansion in adolescents (12–17 years) at risk for SARS-CoV-2 Phase 3 Randomized, observer blinded, placebo controlled study to determine the efficacy, safety and immunogenicity of SARS-CoV-2 rS/Matrix-M1 adjuvant |
SARS-CoV-2 rS/Matrix-M1 is a SARS-CoV-2 recombinant spike protein nanoparticle vaccine with Matrix-M1 adjuvant | 33,000 participants (estimated) |
December 27, 2020 June 30, 2023 |
| BBV152 NCT04641481 |
Bharat Biotech International Limited | An efficacy and safety clinical trial of an investigational COVID-19 vaccine (BBV152) in adult volunteers Phase 3 Event-driven, randomized, double blind, placebo controlled, multicentre study to evaluate the efficacy, safety and immunogenicity of BBV152. |
BBV152 is a whole virion inactivated SARS-CoV-2 vaccine. | 25,800 participants | November 16, 2020 December 2022 |
Many preclinical studies support the usefulness of delivering antiviral drugs, recombinant virus subunit vaccines, attenuated viral antigens delivery using nanocarriers to treat viral infections. NPs have shown effectiveness to deliver small interfering RNA (siRNA) to treat diseases such as infections, malignancies, neurological diseases and autoimmune disease. Various antigens like HBsAg, tetanus toxoid, malaria antigens, Listeria monocytogenes and Bacillus anthracis were successfully loaded into PLGA NPs and produced sustained cellular and humoral immune response. Antigenic substances can be encapsulated into the nanocarriers or attached on their surface. The encapsulation strategy is used to protect the antigens from proteolytic degradation and target them to APCs. In the case of direct attachment of antigens on the surface, the NPs mimic the virus itself. Purified immunoglobulins, from COVID-19 patient plasma, attached on a NP may be a promising NP vaccine against coronaviruses [19]. SARS-CoV-2 gains entry into the body through the nasal cavity, especially through mucosal epithelial cells which include mucous producing goblet cells and ciliated cells [80]. NPs are suitable for administration by various route such as oral, intravenous, subcutaneous, intramuscular and intranasal, therefore can be used to target vaccines to lymph nodes and penetrate across mucosal and epithelial barriers which includes the airways, gastrointestinal and nasal barriers. Vaccines administration through intravenous or intramuscular routes induced systemic immunity, but the mucosal response was weak [81]. Administration of vaccines through nasal route, to activate mucosal surface immunity and systemic immunity seems encouraging [19]. Administration of vaccine by nasal route enhanced the propagation of antigen-specific lymphocytes, improved cytokine production and induction of antigen-specific antibodies in comparison with systemic or subcutaneous administration [82]. Hence nasal sprays especially nano nasal sprays are promising to deliver vaccines. Self-assembling protein NPs were also studied for vaccine delivery. In a study, a combination of coronavirus S protein loaded NPs and Matrix M1 increased the immunity against coronaviruses in mice. Further, the vaccination increased the presence of neutralizing antibodies [83].
Virus like particles (VLPs) are viral protein-based particles, have the capacity to self-assemble into spherical NPs with size ranging between 20 and 200 nm. Chimeric spherical VLPs based on MERS-CoV viral proteins increased both cellular and humoral immunity in mice [84]. In another study Kato et al., prepared MERS-CoV VLPs including the full S protein of MERS-CoV using insect cells [85]. VLNs prepared from HCoV-NL63′s structural proteins M, E, S efficiently transfected nasal mucosal ciliary cells [86]. The VLPs can be used as a strategy to deliver drugs to treat COVID-19 as they are similar to virus, have the ability to escape from immune clearance, penetrate through the mucous to reach lungs as well as other affected sites [19]. Medicago, a Canada based biopharmaceutical company, started phase I clinical trials in July 2020 for its VLP-based vaccine against SARS-Cov-2 and planning a phase 2/3 trial in October 2020 [87]. Lipid based NPs like solid lipid NPs (SLNs) or liposomes have high similarities with virus particles. Further, they have similar surface structures as viruses. The efficiency of liposomes to deliver genes have been studied by many authors. The mRNA based COVID-19 vaccines are in clinical development [16]. For example, Moderna’s mRNA-1273 (lipid NP capsule) is in clinical trial phase 3 [88]. LUNAR®COV19 (ARCT-021), a self-replicating mRNA vaccine formulated in a lipid NP, was developed by Arcturus Therapeutics is in clinical trial phase 1/2 [89]. Self-replicating mRNA construct encodes an RNA-dependent RNA polymerase complex which is required for self-amplification and also the components which are present in the nonreplicating constructs. Self-replication enhances the magnitude and prolong construct expression and therefore encoded immunogen production. Further, it is possible to include multiple gene sequences into the same replicon. An Alphavirus derived replicon RNA vaccine stabilized in lipid NPs induced strong antibody responses in mice and these antibodies neutralized SARS-CoV-2 [90]. These studies show the feasibilities of using lipid NPs to deliver RNA vaccines.
The other nanomedicines which can be used for delivering mRNA are cationic nanoliposomes, PEG-lipid functionalized dendrimers, nanoemulsion, polyethylenimine NPs and polymeric NPs. Naked DNA also faces systemic stability problems by nucleases. Polymeric NPs, lipid-based cationic NPs and inorganic NPs have been suggested for DNA-based vaccines. PLGA NPs have been widely studied to deliver DNA vaccines and the results showed increased systemic antigen specific antibody responses [91]. NPs functionalized with PEG can avoid RES uptake hence increase systemic circulation time and enhance target specificity. NPs were able to target both innate immune systems such as macrophages, monocytes and neutrophils and the adaptive systems like T cells and B cells. NPs can modulate APCs and which is important for COVID-19 vaccine approach [92]. Antigen loaded NPs delivery to dendritic cells can enhance T cell immunity. A major challenge in COVID-19 vaccine research is to find out various approaches which are able to stimulate T cells and B cells immunity against SARS-CoV-2. Further, it is also required to develop next generation vaccine strategies which are useful for specific subgroups or individual with impaired immunity [93]. Another important strategy is to introduce nanomedicine-based COVID-19 vaccines which have the capacity on the cellular presentation of the selected antigen [13]. Nanomedicines can help to improve the immune response against specific antigens. Immune targeted nanomedicines are expected to intensify hosts’ immune responses. Further, nanomedicines functionalized with virus specific target molecules can enhance the vaccines’ efficiency as well as their effectiveness to prevent viral infections [12]. APCs present in the lymphoid organs are very near to T cells which provides an ideal microenvironment to effectively amplify T cell responses. In a study, dendritic cells were effectively and precisely targeted in in vivo using RNA-lipoplexes after intravenous administration by adjusting the net charge of the lipid carrier without functionalizing the particles with specific molecular ligands for cancer immunotherapy [94]. In the case of COVID-19, mRNA-1273 is in the first phase of clinical trial [95]. The mRNA vaccine encodes the S-2P antigen containing the SARS-CoV-2 glycoprotein. The lipid nanoparticle capsule consisting of four lipids and it was prepared in a fixed ratio of mRNA and lipid [96]. The RNA vaccine development involves the utilization of a proper delivery system to increase the stability and thus the translatability intracellularly. But encapsulating mRNA vaccines encoding SARS-CoV-2 proteins into nanomedicines for site specific targeting and clinical translation is a great challenge. The efficiency of protein-based vaccines such as viral vector, recombinant protein, attenuated or inactivated vaccines have well documented against many viral infections with licenced products. All these approaches can be studied for SARS-CoV-2.
7. Nanomedicine for vaccine adjuvant delivery
Inactivated and recombinant protein vaccines sometimes require adjuvants to enhance their immunogenicity. NPs have the capacity to deliver molecular adjuvants and many a time NP alone has adjuvant property for the incorporated antigen. Vaccine adjuvant NPs can overcome the disadvantages of traditional way of delivering molecular vaccine adjuvants. Adjuvants which are licenced to use in vaccines include alum (aluminium salts), MF59 (a squalene-based emulsion adjuvant), AS01 (a liposome-based adjuvant), AS03 (a squalene-based emulsion adjuvant), AF03 (a squalene-based emulsion adjuvant), AS04 (consist of TLR4 agonist MPL (3-O-desacyl-4′-monophosphoryl lipid A) and aum) and virosomes. MF59 is a nanoadjuvant and showed good adjuvant activity includes humoral and T helper type 1 immune responses [97]. Alum is used in licenced vaccines such as DTap, Hib, hepatitis A and hepatitis B. Virosomes is used in Inflexal® V and Invivac® influenza vaccine and hepatitis A vaccine (Epaxal®). AS04 is licenced to use in human papilloma virus vaccine (CervarixTM) and hepatitis B virus vaccine (Fendrix®) [98]. MF59 and AS03 has licenced for influenza vaccines used for the geriatric populations [99]. AS01 has licenced for herpes zoster subunit vaccine for the geriatric population of 70 years or above [100].
Virus like particles, PLGA NPs, cationic liposomes, nanoemulsion and cholesterol bearing nanogel are also studied for their adjuvant properties. Some nanomaterials alone perform adjuvant activity by increasing antigen presentation and through their inherent immunoactivation properties. Cyclic dinucleotides (CDNs) have vaccine adjuvants property. Cyclic di-GMP (cdGMP) loaded PEGylated lipid NPs (NP-cgGMP) delivered CDNs to the draining lymph nodes and increased the adjuvant activity of CDNs [101]. Hamdy et al., used PLGA NPs to co-deliver antigen ovalbumin along with monophosphoryl lipid A as adjuvant to induct CD4+ and CD8+ T cell responses [102]. In another study, Toll-like receptors ligands and antigen hemagglutinin loaded calcium phosphate NPs induced both innate and adaptive immunities by activating dendritic cells [103]. In the case of SARs-CoV-2, adjuvants could be useful for patients with impaired immunological functions and patients with other comorbidities which results in immune dysfunctions [13]. Vaccine adjuvants are expected to reduce the antigen dose required for COVID-19. A SARS-CoV-2 recombinant spike protein NPs vaccine with Matrix-M adjuvant (a saponin-based adjuvant) is in phase I clinical trial to evaluate its safety and immunogenicity [104]. Hence, it is believed that a combination of vaccine and adjuvants play an important role especially in the case of old age people and immune compromised patients.
8. Future perspectives
Nanomedicines such as NPs, lipid NPs and VLPs have been attracted the researches as delivery carriers for drug molecules/vaccines as they increase the antigen stability, antigen processing and immunogenicity, and also the sustained and targeted delivery of antigens. But the reproducibility and large-scale industrial production of drug/vaccine loaded target-specific ligand conjugated nanomedicines are a major challenge [105]. Introducing new antiviral drugs and their availability for the general public requires years as many regulatory steps are required to prove the efficacy and safety of the drugs and vaccines. The search for new drugs, repurposing of existing drugs as well as developing of new drug molecules for COVID-19 either from synthetic or natural origin, necessitates a thorough understanding of molecular mechanisms of SARS-CoV-2 infection and further consequences in cellular and molecular level. Most importantly antiviral drugs and vaccines generally target a particular viral species/strains, hence may not be effective on other species/strains as well as mutated genes [106]. Therefore, it is imperative to come up with new drug molecules and vaccines which are effective against various strains including the mutant ones of SARS-CoV-2. Nanomedicines such as NPs, liposomes, monoclonal antibodies and virus like particles can be utilized to target drugs, which are effective against COVID-19, in cellular and molecular levels as the nanomedicine-based novel drug delivery systems have shown their effectiveness to target anticancer drugs into the cancerous cells. Hence, nanomedicine is expected as a powerful platform for repurposing of existing antiviral drugs to improve COVID-19 treatment. But the safety aspects of the nanomedicines should be studied properly in order to produce a biocompatible and safe product. It has been reported that artificial intelligence enabled nanomedicines can be used to treat advanced cancer [107]. Hence, it is believed that a combination of nanomedicines and artificial intelligence would be very effective for the early detection of SARS-CoV-2 infection, repurposing of existing antiviral drugs and introducing new antiviral drug moieties for treating COVID-19.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology 2020;200642. [DOI] [PMC free article] [PubMed]
- 3.COVID-19 - Situation Update Worldwide, European Centre for Disease Prevention and Control 2020, https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases. Accessed 4th June 2021.
- 4.Setti L, Passarini F, De Gennaro, et al. Airborne transmission route of COVID-19: why 2 meters/6 feet of inter-personal distance could not be enough. Int J Environ Res Public Health 2020;17:2932. [DOI] [PMC free article] [PubMed]
- 5.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10(2):102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R., et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172(9):577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lei J., Li J., Li X., Qi X. CT imaging of the 2019 novel coronavirus (2019-NCoV) pneumonia. Radiology. 2020;295(1):18. doi: 10.1148/radiol.2020200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eftekhari A., Alipour M., Chodari L., Maleki Dizaj S., Ardalan M., Samiei M., et al. A comprehensive review of detection methods for SARS-CoV-2. Microorganisms. 2021;9(2):232. doi: 10.3390/microorganisms9020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rashidzadeh H., Danafar H., Rahimi H., et al. Nanotechnology against the novel coronavirus (severe acute respiratory syndrome coronavirus 2): diagnosis, treatment, therapy and future perspectives. Nanomedicine (Lond) 2021;16:497–516. doi: 10.2217/nnm-2020-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiss C., Carriere M., Fusco L., Capua I., Regla-Nava J.A., Pasquali M., et al. Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano. 2020;14(6):6383–6406. doi: 10.1021/acsnano.0c03697. [DOI] [PubMed] [Google Scholar]
- 13.Chauhan G., Madou M.J., Kalra S., Chopra V., Ghosh D., Martinez-Chapa S.O. Nanotechnology for COVID-19: therapeutics and vaccine research. ACS Nano. 2020;14(7):7760–7782. doi: 10.1021/acsnano.0c04006. [DOI] [PubMed] [Google Scholar]
- 14.Hamming I., Timens W., Bulthuis MLC, Lely A.T., Navis G.J., van Goor H. Tissue Distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner A.J., Hiscox J.A., Hooper N.M. ACE2: From vasopeptidase to SARS virus receptor. Trends Pharmacol Sci. 2004;25(6):291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oudit G.Y., Crackower M.A., Backx P.H., Penninger J.M. The role of ACE2 in cardiovascular physiology. Trends Cardiovasc Med. 2003;13(3):93–101. doi: 10.1016/s1050-1738(02)00233-5. [DOI] [PubMed] [Google Scholar]
- 17.Xiao F., Tang M., Zheng X., Liu Y.e., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M., Chen L., Zhang J., Xiong C., Li X., Chan R.W.Y. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS ONE. 2020;15(4):e0230295. doi: 10.1371/journal.pone.0230295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinrich M.A., Martina B., Prakash J. Nanomedicine strategies to target coronavirus. Nano Today. 2020;35:100961. doi: 10.1016/j.nantod.2020.100961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., et al. novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Wit E., Feldmann F., Cronin J., Jordan R., Okumura A., Thomas T., et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci USA. 2020;117(12):6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J., Zhou L., Yang Y., Peng W., Wang W., Chen X. Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics. Lancet Respir Med. 2020;8(3):e11–e12. doi: 10.1016/S2213-2600(20)30071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng L., Li C., Zeng Q.i., Liu X.i., Li X., Zhang H., et al. Arbidol combined with LPV/r versus LPV/r alone against corona virus disease 2019: a retrospective cohort study. J Infect. 2020;81(1):e1–e5. doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim J., Jeon S., Shin H.Y., et al. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Korean Med Sci. 2020;35 doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan J., Zou R., Zeng L., Kou S., Lan J., Li X., et al. The correlation between viral clearance and biochemical outcomes of 94 COVID-19 infected discharged patients. Inflamm Res. 2020;69(6):599–606. doi: 10.1007/s00011-020-01342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chavez S., Long B., Koyfman A., Liang S.Y. Coronavirus Disease (COVID-19): a primer for emergency physicians. Am J Emerg Med. 2021;44:220–229. doi: 10.1016/j.ajem.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo P., Liu Y.i., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020;92(7):814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu B., Xu X., Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med. 2020;18:164. doi: 10.1186/s12967-020-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C., Li W., Drabek D., Okba N.M.A., van Haperen R., Osterhaus A.D.M.E., et al. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun. 2020;11(1) doi: 10.1038/s41467-020-16256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian X., Li C., Huang A., Xia S., Lu S., Shi Z., et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microbes Infect. 2020;9(1):382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goletti D., Cantini F. Baricitinib therapy in Covid-19 pneumonia — an unmet need fulfilled. N Engl J Med. 2021;384(9):867–869. doi: 10.1056/NEJMe2034982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dabbous H.M., Abd-Elsalam S., El-Sayed M.H., et al. Efficacy of favipiravir in COVID-19 treatment: a multi-center randomized study. Arch Virol. 2021;166:949–954. doi: 10.1007/s00705-021-04956-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Horby P., Lim W.S., Emberson J.R., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edalatifard M., Akhtari M., Salehi M., Naderi Z., Jamshidi A., Mostafaei S., et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: Results from a randomised controlled clinical trial. Eur Respir J. 2020;56(6):2002808. doi: 10.1183/13993003.02808-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao H., Zhu Q.i., Zhang C., Li J., Wei M., Qin Y., et al. Tocilizumab combined with favipiravir in the treatment of COVID-19: A multicenter trial in a small sample size. Biomed Pharmacother. 2021;133:110825. doi: 10.1016/j.biopha.2020.110825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.US Food and Drug Administration. Fact sheet for health care providers emergency use authorization (EUA) of bamlanivimab and etesevimab. FDAhttps://www.fda.gov/media/145802/download. Accessed 9th May 2021.
- 40.US Food and Drug Administration. Fact sheet for health care providers emergency use authorization (eua) of casirivimab and imdevimab. https://www.fda.gov/media/143892/download. Accessed 9th May 2021.
- 41.Ahmed S., Karim M.M., Ross A.G., Hossain M.S., Clemens J.D., Sumiya M.K., et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis. 2021;103:214–216. doi: 10.1016/j.ijid.2020.11.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson B., Geetha K.M. Neurotherapeutic applications of nanomedicine for treating Alzheimer’s disease. J Control Release. 2020;325:25–37. doi: 10.1016/j.jconrel.2020.05.044. [DOI] [PubMed] [Google Scholar]
- 43.Wilson B., Ambika T.V., Dharmesh Kumar Patel R., Jenita J.L., Priyadarshini S.R.B. Nanoparticles based on albumin: preparation, characterization and the use for 5-flurouracil delivery. Int J Biol Macromol. 2012;51(5):874–878. doi: 10.1016/j.ijbiomac.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 44.Wilson B., Paladugu L., Priyadarshini S.R.B., Jenita J.J.L. Development of albumin-based nanoparticles for the delivery of abacavir. Int J Biol Macromol. 2015;81:763–767. doi: 10.1016/j.ijbiomac.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 45.Wilson B., Samanta M.K., Santhi K., Kumar K.P.S., Paramakrishnan N., Suresh B. Poly(n-butylcyanoacrylate) nanoparticles coated with polysorbate 80 for the targeted delivery of rivastigmine into the brain to treat Alzheimer’s disease. Brain Res. 2008;1200:159–168. doi: 10.1016/j.brainres.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 46.Wilson B., Lavanya Y., Priyadarshini S.R.B., Ramasamy M., Jenita J.L. Albumin nanoparticles for the delivery of gabapentin: preparation, characterization and pharmacodynamic studies. Int J Pharm. 2014;473(1-2):73–79. doi: 10.1016/j.ijpharm.2014.05.056. [DOI] [PubMed] [Google Scholar]
- 47.Wilson B., Selvam J., Mukundan G.K., Premakumari K.B., Jenita J.L. Albumin nanoparticles coated with polysorbate 80 for the targeted delivery of antiepileptic drug levetiracetam into the brain. Drug Deliv Transl Res. 2020;10(6):1853–1861. doi: 10.1007/s13346-020-00831-3. [DOI] [PubMed] [Google Scholar]
- 48.Wilson B., Samanta M.K., Santhi K., Sampathkumar K.P., Ramasamy M., Suresh B. Chitosan nanoparticles as a novel delivery system for anti-Alzheimer’s drug tacrine. Nanomed: Nanotech Biol Med. 2010;6:144–152. doi: 10.1016/j.nano.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Wilson B., Mohamed Alobaid B.N., Geetha K.M., Jenita J.L. Chitosan nanoparticles to enhance nasal absorption and brain targeting of sitagliptin to treat Alzheimer's disease. J Drug Delivery Sci Technol. 2021;61:102176. doi: 10.1016/j.jddst.2020.102176. [DOI] [Google Scholar]
- 50.Cavalli R., Donalisio M., Civra A., et al. Enhanced antiviral activity of acyclovir loaded into b-cyclodextrinpoly(4-acryloylmorpholine) conjugate nanoparticles. J Control Release. 2009;137:116–122. doi: 10.1016/j.jconrel.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Lee E.C., Nguyen C.T.H., Strounina E., Davis-Poynter N., Ross B.P. Structure-activity relationships of GAG mimetic-functionalized mesoporous silica nanoparticles and evaluation of acyclovir-loaded antiviral nanoparticles with dual mechanisms of action. ACS Omega. 2018;3(2):1689–1699. doi: 10.1021/acsomega.7b01662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu C.M.J., Chen Y.T., Fang Z.S., Chang W.S., Chen H.W. Antiviral efficacy of nanoparticulate vacuolar ATPase inhibitors against influenza virus infection. Int J Nanomedicine. 2018;14:8579–8593. doi: 10.2147/IJN.S185806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.https://clinicaltrials.gov/ct2/show/NCT04352465. Accessed 7th January 2021.
- 54.Gajbhiye V., Kumar P.V., Jain N.K. A systematic review on therapeutic dendrimers. J Pharm Pharmacol. 2009;61:989–1003. doi: 10.1211/jpp/61.08.0002. [DOI] [PubMed] [Google Scholar]
- 55.Hennig R., Veser A., Kirchhof S., Goepferich A. Branched polymer–drug conjugates for multivalent blockade of angiotensin II receptors. Mol Pharm. 2015;12(9):3292–3302. doi: 10.1021/acs.molpharmaceut.5b00301. [DOI] [PubMed] [Google Scholar]
- 56.Cardoso V.M.d.O., Moreira B.J., Comparetti E.J., Sampaio I., Ferreira L.M.B., Lins P.M.P., et al. Is nanotechnology helping in the fight against COVID-19? Front Nanotechnol. 2020;2 doi: 10.3389/fnano.2020.588915. [DOI] [Google Scholar]
- 57.https://www.starpharma.com/vivagel/spl7013-covid-19-nasal-spray. Accessed 7th January 2021.
- 58.Kolyvushko O., Latzke J., Dahmani I., Osterrieder N., Chiantia S., Azab W. Differentially-charged liposomes interact with alphaherpesviruses and interfere with virus entry. Pathogens. 2020;9:1–9. doi: 10.3390/pathogens9050359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clayton R., Ohagen A., Nicol F., Del Vecchio A.M., Jonckers T.H.M., Goethals O., et al. Sustained and specific in vitro inhibition of HIV-1 replication by a protease inhibitor encapsulated in gp120-targeted liposomes. Antiviral Res. 2009;84(2):142–149. doi: 10.1016/j.antiviral.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Singh A.P., Biswas A., Shukla A., Maiti P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct Target Ther. 2019;4:1–21. doi: 10.1038/s41392-019-0068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Q., Zheng X., Zhang C., Shao X., Zhang X.i., Zhang Q., et al. Conjugating influenza a (H1N1) antigen to ntrimethylaminoethylmethacrylate chitosan nanoparticles improves the immunogenicity of the antigen after nasal administration. J Med Virol. 2015;87(11):1807–1815. doi: 10.1002/jmv.24253. [DOI] [PubMed] [Google Scholar]
- 62.Marovich M., Mascola J.R., Cohen M.S. Monoclonal antibodies for prevention and treatment of COVID-19. JAMA. 2020;324:131–132. doi: 10.1001/jama.2020.10245. [DOI] [PubMed] [Google Scholar]
- 63.Ma M., Badeti S., Geng K., Liu D. Efficacy of targeting SARS-CoV-2 by CARNK cells. BioRxiv. 2020;38:337. [Google Scholar]
- 64.Zhu T., Xiao Y., Meng X., Tang L., Li B., Zhao Z., et al. Nanovesicles derived from bispecific CAR-T cells targeting the spike protein of SARS-CoV-2 for treating COVID-19. J Nanobiotechnology. 2021;19(1) doi: 10.1186/s12951-021-01148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Z., Wang Z., Dinh P.U.C. Cell-mimicking nanodecoys neutralize SARS-CoV-2 and mitigate lung injury in a non-human primate model of COVID-19. Nat Nanotechnol. 2021;16:942–951. doi: 10.1038/s41565-021-00923-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ohnuma K., Haagmans B.L., Hatano R., Raj V.S., Mou H., Iwata S., et al. Inhibition of Middle East respiratory syndrome coronavirus infection by anti-CD26 monoclonal antibody. J Virol. 2013;87(24):13892–13899. doi: 10.1128/JVI.02448-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng B.-J., Guan Y.i., He M.-L., Sun H., Du L., Zheng Y., et al. Synthetic peptides outside the spike protein heptad repeat regions as potent inhibitors of SARS-associated coronavirus. Antivir Ther. 2005;10(3):393–403. [PubMed] [Google Scholar]
- 69.Ali J.F., Rezvan H., Maryam H., et al. Nanoantioxidant/antioxidant therapy in 2019-nCoV: a new approach to reactive oxygen species mechanisms. Curr Drug Ther. 2021;16:291–298. [Google Scholar]
- 70.Behzadi S., Serpooshan V., Tao W., Hamaly M.A., Alkawareek M.Y., Dreaden E.C., et al. Cellular uptake of nanoparticles: journey inside the cell. Chem Soc Rev. 2017;46(14):4218–4244. doi: 10.1039/c6cs00636a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krpeti Z, Anguissola S, Garry D, et al. In Nanomaterials: Impact on Cells and Cell Organelles D.G. Capco, Y. Chen (eds.), Nanomaterial, Advances in Experimental Medicine and Biology 811, 2014. Springer Science+Business Media Dordrecht. [DOI] [PubMed]
- 72.Nativo P., Prior I.A., Brust M. Uptake and intracellular fate of surface-modifi ed gold nanoparticles. ACS Nano. 2008;2(8):1639–1644. doi: 10.1021/nn800330a. [DOI] [PubMed] [Google Scholar]
- 73.https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19. Accessed on 5th June 2021.
- 74.https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine. Accessed on 7th January 2021.
- 75.https://www.modernatx.com/covid19vaccine-eua. Accessed 5th June 2021.
- 76.https://www.astrazeneca.com/media-centre/press-releases/2020/astrazenecas-covid-19-vaccine-authorised-in-uk.html. Accessed on 7th January 2021.
- 77.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.https://clinicaltrials.gov/ct2/show/NCT04283461. Accessed on 5th June 2021.
- 79.Wadhwa A., Aljabbari A., Lokras A., Foged C., Thakur A. Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics. 2020;12(2):102. doi: 10.3390/pharmaceutics12020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sungnak W., Huang N.i., Bécavin C., Berg M., Queen R., Litvinukova M., et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Al-Halifa S., Gauthier L., Arpin D., Bourgault S., Archambault D. Nanoparticle-based vaccines against respiratory viruses. Front Immunol. 2019;10:22. doi: 10.3389/fimmu.2019.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mapletoft J.W., Latimer L., Babiuk L.A., van Drunen Littel-van den Hurk S. Intranasal immunization of mice with a bovine respiratory syncytial virus vaccine induces superior immunity and protection compared to those by subcutaneous delivery or combinations of intranasal and subcutaneous prime-boost strategies. Clin Vaccine Immunol. 2010;17(1):23–35. doi: 10.1128/CVI.00250-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coleman C.M., Liu Y.V., Mu H., Taylor J.K., Massare M., Flyer D.C., et al. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine. 2014;32(26):3169–3174. doi: 10.1016/j.vaccine.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang C., Zheng X., Gai W., Wong G., Wang H., Jin H., et al. Novel chimeric virus-like particles vaccine displaying MERS-CoV receptor-binding domain induce specific humoral and cellular immune response in mice. Antiviral Res. 2017;140:55–61. doi: 10.1016/j.antiviral.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kato T., Takami Y., Kumar Deo V., Park E.Y. Preparation of virus-like particle mimetic nanovesicles displaying the S protein of Middle East respiratory syndrome coronavirus using insect cells. J Biotechnol. 2019;306:177–184. doi: 10.1016/j.jbiotec.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Naskalska A., Dabrowska A., Nowak P., Szczepanski A., Jasik K., Milewska A., et al. Novel coronavirus-like particles targeting cells lining the respiratory tract. PLoS ONE. 2018;13(9):e0203489. doi: 10.1371/journal.pone.0203489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Medicago Begins Phase I Clinical Trials for Its COVID-19 Vaccine Candidate, Medicago, 2020 www.medicago.com. Accessed 5 October 2020.
- 88.https://clinicaltrials.gov/ct2/show/NCT04470427. Accessed 6th October 2020.
- 89.https://clinicaltrials.gov/ct2/show/NCT04480957. Accessed 6th October 2020.
- 90.Erasmus JH, Khandhar AP, O'Connor MA, et al. An Alphavirus-derived replicon RNA vaccine induces SARS-CoV-2 neutralizing antibody and T cell responses in mice and nonhuman primates. Sci Transl Med 2020;12:eabc9396. [DOI] [PMC free article] [PubMed]
- 91.Wang G., Pan L.i., Zhang Y., Wang Y., Zhang Z., Lü J., et al. Intranasal delivery of cationic plga nano/microparticles-loaded FMDV DNA vaccine encoding IL-6 elicited protective immunity against FMDV challenge. PLoS ONE. 2011;6(11):e27605. doi: 10.1371/journal.pone.0027605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Steinman R.M., Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 93.Loutfy M.R., Blatt L.M., Siminovitch K.A., Ward S., Wolff B., Lho H., et al. Interferon Alfacon-1 plus corticosteroids in severe acute respiratory syndrome: A preliminary study. JAMA. 2003;290(24):3222. doi: 10.1001/jama.290.24.3222. [DOI] [PubMed] [Google Scholar]
- 94.Kranz L.M., Diken M., Haas H., Kreiter S., Loquai C., Reuter K.C., et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534(7607):396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 95.https://clinicaltrials.gov/ct2/show/NCT04283461?term=NCT04283461&draw=2&rank=1. Accessed 13 September 2020.
- 96.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., et al. An mRNA vaccine against SARS-CoV-2 — Preliminary report. N Engl J Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu M., Wang R., Nie G. Applications of nanomaterials as vaccine adjuvants. Hum Vaccines Immunother. 2014;10(9):2761–2774. doi: 10.4161/hv.29589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Awate S., Babiuk L.A., Mutwiri G. Mechanisms of action of adjuvants. Front Immunol. 2013;4:114. doi: 10.3389/fimmu.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nicholson K.G., Abrams K.R., Batham S., Clark T.W., Hoschler K., Lim W.S., et al. Immunogenicity and safety of a two-dose schedule of whole-virion and AS03A-adjuvanted 2009 Influenza A (H1N1) vaccines: A randomised, multicentre, age-stratified, head-to-head trial. Lancet Infect Dis. 2011;11(2):91–101. doi: 10.1016/S1473-3099(10)70296-6. [DOI] [PubMed] [Google Scholar]
- 100.Cunningham A.L., Lal H., Kovac M., Chlibek R., Hwang S.-J., Díez-Domingo J., et al. Efficacy of the Herpes Zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–1032. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- 101.Hanson M.C., Crespo M.P., Abraham W., Moynihan K.D., Szeto G.L., Chen S.H., et al. Nanoparticulate sting agonists are potent lymph node-targeted vaccine adjuvants. J Clin Invest. 2015;125(6):2532–2546. doi: 10.1172/JCI79915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hamdy S., Elamanchili P., Alshamsan A., Molavi O., Satou T., Samuel J. Enhanced antigen-specific primary CD4+ and CD8+ responses by codelivery of ovalbumin and toll-like receptor ligand monophosphoryl lipid A in Poly (D, L-lactic-co-glycolic acid) nanoparticles. J Biomed Mater Res, Part A. 2007;81A(3):652–662. doi: 10.1002/jbm.a.31019. [DOI] [PubMed] [Google Scholar]
- 103.Sokolova V., Knuschke T., Kovtun A., Buer J., Epple M., Westendorf W.AM. The use of calcium phosphate nanoparticles encapsulating toll-like receptor ligands and the antigen hemagglutinin to induce dendritic cell maturation and T cell activation. Biomaterials. 2010;31:5627–5633. doi: 10.1016/j.biomaterials.2010.03.067. [DOI] [PubMed] [Google Scholar]
- 104.https://clinicaltrials.gov/ct2/show/NCT04368988. Accessed 13th September 2020.
- 105.Muthu M.S., Wilson B. The challenges posed by the scale-up of nanomedicines. Nanomedicine (London) 2010;5:169–171. doi: 10.2217/nnm.12.3. [DOI] [PubMed] [Google Scholar]
- 106.Zumla A., Chan J.F.W., Azhar E.I., Hui D.S.C., Yuen K.-Y. Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discovery. 2016;15(5):327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wilson B., Km G. Artificial intelligence and related technologies enabled nanomedicine for advanced cancer treatment. Nanomedicine (London) 2020;15(5):433–435. doi: 10.2217/nnm-2019-0366. [DOI] [PubMed] [Google Scholar]