Abstract
Aim
Postresuscitation hemodynamics are associated with hospital mortality/functional outcome. We sought to determine whether low-dose steroids started during and continued after cardiopulmonary resuscitation (CPR) affect postresuscitation hemodynamics and other physiological variables in vasopressor-requiring, in-hospital cardiac arrest.
Methods
We conducted a two-center, randomized, double-blind trial of patients with adrenaline (epinephrine)-requiring cardiac arrest. Patients were randomized to receive either methylprednisolone 40 mg (steroids group) or normal saline-placebo (control group) during the first CPR cycle post-enrollment. Postresuscitation shock was treated with hydrocortisone 240 mg daily for 7 days maximum and gradual taper (steroids group), or saline-placebo (control group). Primary outcomes were arterial pressure and central-venous oxygen saturation (ScvO2) within 72 hours post-ROSC.
Results
Eighty nine of 98 controls and 80 of 86 steroids group patients with ROSC were treated as randomized. Primary outcome data were collected from 100 patients with ROSC (control, n = 54; steroids, n = 46). In intention-to-treat mixed-model analyses, there was no significant effect of group on arterial pressure, marginal mean (95% confidence interval) for mean arterial pressure, steroids vs. control: 74 (68–80) vs. 72 (66–79) mmHg] and ScvO2 [71 (68–75)% vs. 69 (65–73)%], cardiac index [2.8 (2.5–3.1) vs. 2.9 (2.5–3.2) L/min/m2], and serum cytokine concentrations [e.g. interleukin-6, 89.1 (42.8–133.9) vs. 75.7 (52.1–152.3) pg/mL] determined within 72 hours post-ROSC (P = 0.12–0.86). There was no between-group difference in body temperature, echocardiographic variables, prefrontal blood flow index/cerebral autoregulation, organ failure-free days, and hazard for poor in-hospital/functional outcome, and adverse events (P = 0.08–>0.99).
Conclusions
Our results do not support the use of low-dose corticosteroids in in-hospital cardiac arrest.
Trial Registration:ClinicalTrials.gov number: NCT02790788 (https://www.clinicaltrials.gov).
Keywords: Heart Arrest, Post-cardiac arrest syndrome, Methylprednisolone, Hydrocortisone, Hemodynamics
Introduction
Post-cardiac arrest syndrome is characterized by myocardial dysfunction and vasodilatory shock.1., 2. Underlying mechanisms include ischemia/reperfusion (I/R) injury, myocardial contracture/interstitial edema, activation of leukocytes/platelets, microvascular plugging, and a “sepsis-like” cytokine storm.1., 2. The associated severe endothelial injury3 and nitric oxide overproduction result in increased microvascular permeability and intravascular volume depletion.1., 2. Brain injury may comprise disruption of the blood–brain barrier, neuronal apoptosis, cytotoxic edema, and impaired cerebral autoregulation.4., 5..
Postresuscitation cortisol synthesis may be compromised by I/R destruction of adrenal tissue.1., 6. Lower postresuscitation levels of corticosteroids and higher levels of interleukin (IL)-6 are associated with increased in-hospital mortality.7., 8., 9., 10. In vasodilatory shock states,11., 12. steroids may attenuate pro-inflammatory [e.g. tumor necrosis factor alpha (TNFα) and IL-6 levels, and endothelial/neutrophil activation] and anti-inflammatory (e.g. IL-10 levels) responses.2., 13., 14. Steroids may also restore vascular responsiveness to catecholamines,15., 16., 17., 18., 19., 20. and attenuate postresuscitation myocardial dysfunction.18., 21. Regarding neuroprotection, steroids may enhance blood–brain barrier integrity18., 22. and thereby mitigate cerebral edema and the associated axonal changes.23
Current evidence supports further study of steroids in cardiac arrest.8 Two prior randomized trials suggested that the vasopressin-steroids-epinephrine (VSE) combination improves short-term and long-term outcomes of patients with in-hospital cardiac arrest.19., 20. However, the combined intervention prevented an assessment of the contribution of steroids to the positive results.19., 20. In this study, we tested the hypothesis that treatment with stress-dose steroids might result in improved early postresuscitation hemodynamics, which are associated with mortality and functional outcome.24 The effect of steroids on systemic inflammation, cerebral blood flow/autoregulation, and longer-term outcomes were also determined.
Methods
Additional methodological details and definitions are provided in the Supplement. We conducted our study in the intensive/coronary care units (ICUs/CCUs), general wards, emergency departments, and operating rooms of 2 Greek tertiary care teaching centers, i.e. Evaggelismos General Hospital, Athens (1200 beds), and Larissa University Hospital, Larissa (700 beds). Eligible patients had vasopressor-requiring in-hospital cardiac arrest according to resuscitation guidelines 2015,25., 26. and return of spontaneous circulation (ROSC) for ≥20 min. Exclusion criteria were age < 18 years; “end-stage” disease with life expectancy < 6 weeks [e.g. metastatic cancer with concurrent organ failure(s); prearrest Sequential Organ Dysfunction Assessment score ≥ 15;27 immunosuppression with new sepsis-associated organ failure(s)]; uncontrollable hemorrhage (e.g. aortic aneurysm rupture); cardiac arrest before hospital admission; prearrest treatment with intravenous corticosteroids; any history of allergic reaction; prearrest evidence of ST segment elevation myocardial infarction; any prior inclusion in or exclusion from the present study; do not-attempt-cardiopulmonary resuscitation (CPR) order; ROSC before adrenaline (epinephrine) administration;20 active peptic ulcer; projected inability for ICU admission within 48 hours of ROSC; and any deviation from the hospital’s standard resuscitation procedure. The last 3 exclusion criteria were added by protocol amendment (see Supplement).
The study was conducted according to the European Union Clinical Trials Regulation No. 536/2014 and the Helsinki Declaration. Details about the obtained institutional review board approvals and the requested and obtained next-of-kin deferred consent19., 20. are provided in the Supplement.
Study design and randomization
We conducted a prospective, randomized, double-blind, placebo-controlled, parallel-group clinical trial. Research Randomizer version 4 (https://www.randomizer.org/) was used for patient group allocation. Two series of 100 unique random numbers were generated before study start. Each number was corresponded to 1 of the consecutively enrolled patients as their study code number. Subgroups with a median number of 7 patients (range: 4–10) and an odd or even first code number were assigned to the control or steroids group, respectively.
CPR and postresuscitation interventions
We enrolled adult in-patients with cardiac arrest due to ventricular fibrillation/pulseless tachycardia (VF/VT) not responsive to 3 shocks,25., 26. or asystole, or pulseless electrical activity (PEA). Study treatments were administered during the first CPR cycle post-enrollment. Patients were randomized to receive either methylprednisolone hydrogen succinate 40 mg (steroids group) or normal saline placebo (control group). Otherwise, resuscitation was performed according to the 2015 Guidelines.25., 26. Following ROSC, patients with postresuscitation shock received either hydrocortisone sodium succinate 240 mg daily for 7 days maximum (steroids group), or saline placebo (control group).19., 20.
Documentation and patient follow-up
CPR attempts were documented according to the Utstein style.19., 20., 28. Hemodynamics, gas-exchange, electrolytes, glucose, lactate, administered fluids, and vasopressor/inotropic support were determined/recorded during CPR, and at 20 min and 4 hours post-ROSC. At these time points, central-venous blood gas analysis was also performed and blood samples were obtained for the determination of cytokines. Postresuscitation cardiac output monitoring (started in the absence of attending physician objection) was to be continued for ≥72 hours post-ROSC. Postresuscitation cardiac function was assessed by transthoracic echocardiography within the first 12 hours and at 72 hours post-ROSC. Blood flow index (BFI) and tissue oxygenation index (TOI) of the prefrontal cortex and vastus lateralis (as lower-extremity BFI/TOI reference) were to be assessed by Near-Infrared Spectroscopy (NIRS) and intravenous Indocyanine Green dye29 at 4 and 72 hours post-ROSC. Core body temperature was recorded hourly for the first 48 hours post-ROSC.
Follow-up at 24, 48, and 72 hours post-ROSC, and then, at 9 a.m. of days 4–10 postrandomization included (1) Determination/recording of hemodynamics/hemodynamic support, gas-exchange, fluid balance (of the preceding 24 hours), arterial blood lactate, and central venous oxygen saturation (ScvO2); (2) Blood sampling for determination of serum cytokines (at 24, 48, 72, and 168 hours post-ROSC); and (3) Recording of laboratory data, and prescribed medication. Results of ≥4 determinations/24 hours of blood glucose were recorded to determine the daily incidence of hyperglycemia (i.e. blood glucose > 200 mg/dL). Follow-up to day 60 post-ROSC included organ failure-free days,19., 20. and ventilator-free days.19., 20. Morbidity/complications throughout ICU/CCU and hospital stay, and times to ICU/CCU and hospital discharge were also recorded.
Outcome Measures Primary:
[1] Arterial blood pressure; and [2] ScvO2 at 20 min and at 4, 24, 48, and 72 hours post-ROSC.
Secondary: [1] Cardiac output at 4, 24, 48 and 72 hours post-ROSC (changed by protocol amendment from primary to secondary outcome - see Supplement); [2] Left and right ventricular end-diastolic areas (LVEDA and RVEDA, respectively), left and right ventricular ejection fraction, and eccentricity index within the first 12 hours, and at 72 hours post-ROSC; [3] prefrontal cortex BFI at 4 and 72 hours post-ROSC; [4] Body temperature over the first 48 hours post-ROSC;30., 31., 32. [5] Serum concentrations of TNFα, IL-1 beta, IL-6, IL-8, IL-10, at 4, 24, 48, and 72 hours post-ROSC; [6] Number of organ failure-free days19., 20. within days 1–60 post-ROSC; [7] Survival to hospital discharge with good neurological outcome, defined as Cerebral Performance Category Score of 1 or 2;19., 20. and [8] Potentially corticosteroid-associated complications such as hyperglycemia (during days 1–10 post-ROSC), infections, bleeding peptic ulcers, and paresis throughout hospital stay.
Statistical analysis
Based on hemodynamic data collected within 24 hours postresuscitation,19., 20. we predicted that the day-1 mean (SD) of mean arterial pressure (MAP) would be 71.0 (19.1) and 88.0 (25.1) mmHg in the control and steroids group, respectively. These predictions were consistent with day-1 estimates of mixed model analyses of pooled VSE study data.19., 20. These VSE hemodynamic data probably reflected only the effect of steroids,15., 33. because plasma half-life of vasopressin is 21 min.34 For the current study, a total of 78 patients (39 in each group) with available day-1 MAP data was required to detect an effect size d of 0.761 with α = 0.015 and power = 0.80. Target enrollment was set at 100 patients with ROSC for ≥20 min to compensate for possible dropouts or missing data due to patient death within the first 24 hours post-ROSC.19., 20. For an expected ROSC rate of ≥50%,19., 20. a maximum of 200 patients would need to be randomized to the study treatments.
A primary intention-to-treat (ITT) and a secondary per-protocol analysis were performed. Distribution normality was tested by the Kolmogorov-Smirnov test. Data are reported as mean (95% confidence interval (CI)), mean (SD), median (IQR), and number (percentage), unless otherwise specified. Continuous variables were compared by a two-tailed, independent samples t-test or the Mann-Whitney exact U test. Dichotomous and categorical variables were compared by two-sided Fisher's exact test. Linear mixed-model analyses (fixed factors: group, time, group * time, study center, and insulin infusion rate;20., 35. random factor: “patients”20., 35.) were used to analyze variable data obtained at multiple, consecutive time points of follow-up; dependent variables with skewed distributions (e.g. cytokines) were log-transformed. P-values of ≥2 consecutive comparisons were subjected to the Bonferroni correction (i.e. multiplied by the number of the consecutive comparisons).
Multivariable Cox regression was used to analyze survival data and determine hazard ratios (HRs) and their 95% CIs for potential predictors of poor outcome (i.e. death or survival with CPC score of 3 or 420); tested predictors were study center (Evaggelismos vs. Larissa); group (steroids vs. control); cardiac arrest cause (cardiac vs. noncardiac), area (monitored vs. non-monitored), and rhythm (VF/VT vs. asystole/PEA); bicarbonate and adrenaline dose during CPR; and cardiac arrest occurrence on working day vs. holiday, and during the morning-to-late-evening (07:00–23:00) shift or the night (23:00–07:00) shift.20
To avert potential bias due to post-randomization exclusion,36 data from patients with no ROSC were included in the analyses of survival/neurological outcome and of non-outcome cardiac arrest variables (for details on analysis protocol modification see Supplement).
Significance was set at (two-sided) P < 0.05. Sample size was calculated using G*Power version 3.1 (Heinrich Heine University, Germany). Analyses were conducted using SPSS version 26 (SPSS).
Results
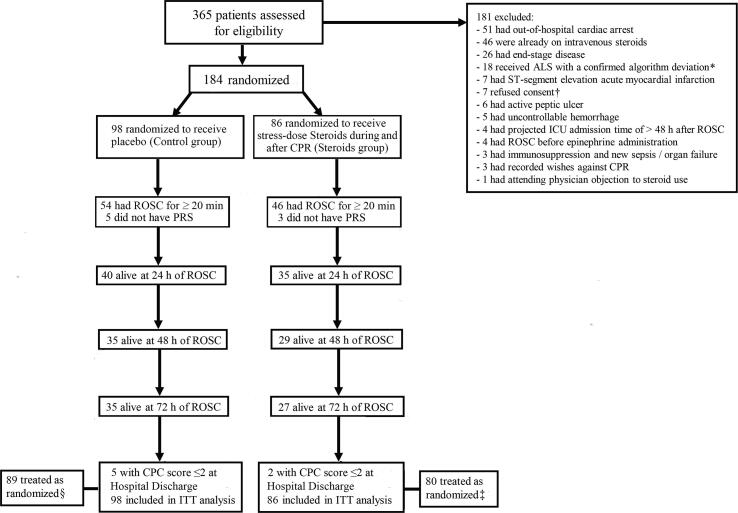
From November 4, 2016 to May 22, 2018, 184 patients were randomized to the control group (n = 98), or the steroids group (n = 86); respective ROSC rates were 54/98 (55.1%) and 46/86 (53.5%) (P = 0.88). Patient follow-up was completed on August 11, 2018. Baseline characteristics were generally similar; between-group differences of ≥10% were present in the frequency of preexisting coronary artery disease, acute cardiovascular disease as hospital admission cause, and 2 cardiac arrest causes (Table 1). Fig. 1 displays the study flow chart. Eighty nine controls (90.8%) and 80 steroids group patients (93.0%) were treated as randomized; additional details, including the post-ROSC use of steroids are reported in the Supplement. Results of per-protocol analyses are not presented, because they were similar to those of the ITT analyses.
Table 1.
Patient characteristics before cardiac arrest and causes of cardiac arrest.
| Characteristic | Control group (n = 98) | Steroids group (n = 86) |
|---|---|---|
| Age – yra | 76.0 (67.0–83.0) | 76.0 (63.8–83.3) |
| Male sex – no. (%) | 54 (55.1) | 49 (57.0) |
| Body-mass index – kg/m2a | 26.0 (23.5–28.1) | 25.8 (22.8–29.5) |
| Pre-arrest hospital stay – daysa | 1.0 (0.5–1.0) | 1.0 (0.0–3.0) |
| Comorbidity– no. (%) | ||
| Cardiovascular– no. (%) | ||
| Hypertension | 64 (65.3) | 62 (72.1) |
| Diabetes | 31 (31.6) | 28 (32.6) |
| Coronary artery disease | 39 (39.8) | 25 (29.1) |
| Cardiac arrhythmia | 26 (26.5) | 18 (20.9) |
| Peripheral vascular disease | 26 (26.5) | 17 (19.8) |
| Valvular heart disease | 12(12.2) | 9 (10.5) |
| Cardiac conduction disturbances | 12 (12.2) | 11(12.8) |
| Non-cardiovascular-no. (%)b | 77 (78.6) | 71 (82.6) |
| Hospital Admission Cause – no. (%)c | ||
| Acute digestive disease | 17 (17.3) | 20 (23.3) |
| Acute respiratory disease | 22 (22.4) | 16 (18.6) |
| Acute cardiovascular disease | 36 (36.7) | 23 (26.7) |
| Acute neurologic disease | 12 (12.2) | 12 (14.0) |
| Trauma | 7 (7.1) | 2 (2.3) |
| Malignancy | 2 (2.0) | 7 (8.1) |
| Acute renal disease | 8 (8.2) | 12 (14.0) |
| Other | 4 (4.1) | 8 (9.3) |
| Cause(s) of cardiac arrest-no.(%)d | ||
| Hypotension | 38 (38.8) | 43 (50.0) |
| Respiratory depressione or failuref | 46 (46.9) | 40 (46.5) |
| Myocardial ischemia/infarction | 26 (26.5) | 13 (15.1) |
| Metabolic | 26 (26.5) | 29 (33.7) |
| Arrhythmia | 14 (14.3) | 12 (14.0) |
| Otherg | 8 (8.2) | 5 (5.8) |
Data presented as median (interquartile range).
Includes chronic respiratory, neurologic, digestive, renal, endocrine, psychiatric, ocular, musculoskeletal, and autoimmune disease, malignancy, morbid obesity, substance abuse, and immunosuppression; 1 control patient (1.0%) had prior treatment with oral prednisone (15 mg/day), and 1 control patient (1.0%) and 1 Steroids group patient (1.2%) had prior treatment with inhaled corticosteroids.
Some patients had more than one cause of hospital admission; “other” causes included 2 cases of hyponatremia, 2 cases of drug-related QT prolongation, and 1 case of erysipelothrix infection, substance abuse-related respiratory depression, acute hypothyroidism, food refusal, lower extremity gangrene, elective coronary artery bypass grafting, immunosuppression-associated sepsis, and septic shock caused by Panton-Valentine-positive methicillin-resistant Staphylococcus aureus.
In some patients, there were more than 1 major disturbances precipitating the cardiac arrest.
Occurring during spontaneous breathing.
Occurring after endotracheal intubation and initiation of mechanical ventilation.
Includes 2 cases of drug-related polymorphic ventricular tachycardia (torsades des pointes), 2 cases of central venous catheterization-related hemothorax, 2 cases of bradycardia/asystole due to an acute rise in intracranial pressure, and 1 case of abdominal compartment syndrome, massive unilateral pleural effusion, upper airway obstruction during feeding, drug-related hyperkalemia, acute myocarditis-associated arrhythmia, drug-associated hypomagnesemia, and vagotonic arrest.
Fig. 1.
The study flow chart. ROSC, return of spontaneous circulation; ALS, advance life support; ICU, intensive care unit; CPR, cardiopulmonary resuscitation; PRS, postresuscitation shock. *, Pertains to any confirmed deviation from the ALS algorithms presented in references 25 and 26; two patients (control, n = 1) were excluded after randomization. †, Consent was refused within 48 hours of ROSC in all cases; four of these patients were initially randomized to the control group. ‡, Four patients were started on open label, stress-dose hydrocortisone within 24 hours post-ROSC; in 3 of these patients, the prescribed hydrocortisone dose differed from the dose specified by the study protocol; study treatment was withheld in another 3 patients within 24 hours post-ROSC; consequently, a total of 6 patients were not treated as randomized. §, Nine patients were started on open label stress-dose hydrocortisone within 24 hours post-ROSC; consequently, these patients were not treated as randomized.
Peri-arrest data
There was no significant difference in recorded cardiac arrest characteristics, and peri-arrest physiological variables and therapeutic interventions (supplemental Tables S1 and S2).
Study outcomes and vasopressor infusion rate
Table 2 displays summary results on study outcomes, besides functional in-hospital outcome. Additional details are reported in the Supplement (as indicated in Table 2).
Table 2.
Summary results on study outcomes (besides functional in-hospital outcome, which is presented in Fig. 3).
| ITT analyses, first 72 hours post-ROSC |
Exploratory ITT analyses, 10-day follow-up |
Additional presentation a | |||||
|---|---|---|---|---|---|---|---|
| Primary outcomes b | Control group | Steroids group | P-value | Control group | Steroids group | P-value | |
| Systolic arterial pressure, EMM (95% CI), mmHg | 109 (100–120) | 111 (102–120) | 0.64 | 119 (110–128) | 118 (110–127) | 0.97 | Figure S1 |
| Diastolic arterial pressure, EMM (95% CI), mmHg | 54 (49–60) | 55 (50–60) | 0.71 | 55 (51–61) | 54 (50–59) | 0.44 | Figure S2 |
| Mean arterial pressure, EMM (95% CI), mmHg | 72 (66–79) | 74 (68–80) | 0.61 | 77 (71–83) | 75 (70–81) | 0.78 | Figure 2A |
| Central venous oxygen saturation, EMM (95% CI), (%) | 69 (65–73) | 71 (68–75) | 0.17 | 70 (65–74) | 72 (68–77) | 0.18 | Figure S3 |
| Secondary outcomes | Control group | Steroids group | P-value | Control group | Steroids group | P-value | |
| Cardiac index, EMM (95% CI), L/min/m2 BSA c | 2.9 (2.5–3.2) | 2.8 (2.5–3.1) | 0.79 | 2.9 (2.5–3.2) | 3.1 (2.7–3.5) | 0.33 | Figure S4 |
| ITT analyses, at 12 hours post-ROSC | ITT analyses, at 72 hours post-ROSC | ||||||
| Echocardiographic variables d | Control group | Steroids group | P-value | Control group | Steroids group | P-value | |
| LVEDA, median (IQR) at 12 hours, mean (SD) at 72 hours, cm2 | 19.4 (15.9–29.2) | 24.0 (17.8–30.6) | >0.99 | 18.5 (2.9) | 23.6 (7.9) | 0.051 e | Table S3 |
| RVEDA, mean (SD), cm2 | 13.0 (4.0) | 12,1 (4.2) | 0.98 | 12.6 (4.1) | 14.3 (3.7) | 0.48 | Table S3 |
| LVEF, median (IQR), (%) | 50.0 (37.5–50.0) | 45.0 (30.0–55.0) | 0.62 | 55.0 (45.0–55.0) | 45.0 (32.5–55.0) | 0.44 | Table S3 |
| RVEF, mean (SD), (%) | 42.7 (9.3) | 41.3 (8.7) | >0.99 | 44.7 (7.1) | 42.2 (8.4) | 0.94 | Table S3 |
| Secondary outcomes |
ITT analyses, within 4-12 hours of ROSC f |
ITT analyses, at 72 hours post- ROSC |
Additional presentationa | ||||
| NIRS-derived variablesg | Control group | Steroids group | P-value | Control group | Steroids group | P-value | |
| Prefrontal cortex BFI, median (IQR), nM/s, 1st Measurement | 2.8 (1.7–4.1) | 3.3 (2.4–4.5) | 0.60 | 2.4 (1.8–5.4) | 3.7 (1.5–6.4) | >0.99 | Table S4 |
| Prefrontal cortex BFI, median (IQR), nM/s, 2nd Measurement | 3.9 (1.6–4.9) | 3.3 (2.8–4.9) | >0.99 | 3.9 (1.8–4.9) | 3.3 (1.6–7.2) | >0.99 | Table S4 |
| ITT analyses, over 48 hours of ROSC |
|||||||
| Control group | Steroids group | P-value | |||||
| Core body temperature, mean (SD), degrees Celsius h | 36.4 (1.1) | 36.2 (1.1) | 0.03 | Table S5 | |||
| ITT analyses, first 72 hours post-ROSC |
Exploratory ITT analyses, 7-day follow-up |
||||||
| Serum cytokine concentrations i | Control group | Steroids group | P-value | Control group | Steroids group | P-value | |
| Tumor necrosis factor alpha, EMM (95% CI), pg/mL | 83.1 (46.1–149.9) | 91.4 (53.0–157.8) | 0.70 | 82.3 (45.5–148.9) | 90.4 (52.2–156.5) | 0.70 | Figure S5 |
| Interleukin 1-beta, EMM (95% CI), pg/mL | 99.5 (84.9–116.7) | 109.5 (94.0–127.4) | 0.12 | 99.8 (45.5–148.9) | 110.5 (52.2–156.3) | 0.12 | Figure S6 |
| Interleukin 6, EMM (95% CI), pg/mL | 75.7 (42.8–133.9) | 89.1 (52.1–152.3) | 0.48 | 78.3 (44.7–137.2) | 81.6 (48.1–138.4) | 0.86 | Figure 2C |
| Interleukin 8, EMM (95% CI), pg/mL | 146.7 (90.6–237.3) | 138.3 (87.5–218.5) | 0.75 | 143.9 (89.0–232.6) | 129.5 (82.0–204.6) | 0.58 | Figure S7 |
| Interleukin 10, EMM (95% CI), pg/mL | 22.8 (11.9–43.6) | 31.5 (17.2–57.8) | 0.22 | 21.6 (11.3–41.0) | 28.8 (15.8–52.7) | 0.27 | Figure S8 |
| Secondary outcomes | ITT analyses, 60-day follow-up |
Additional presentation a | |||||
| Organ failure free days j | Control group | Steroids group | P-value | NA | |||
| Circulatory failure free days, median (IQR), max. | 0.0 (0.0–12.8), 59.0 | 0.0 (0.0–26.8), 60.0 | 0.84 | ||||
| Neurologic failure free days, median (IQR), max. | 0.0 (0.0–0.0), 59.0 | 0.0 (0.0–1.0), 56.0 | 0.33 | ||||
| Renal failure free days, median (IQR), max. | 1.0 (0.0–13.0), 60.0 | 1.0 (0.0–44.0), 60.0 | 0.97 | ||||
| Respiratory failure free days, median (IQR), max. | 3.0 (0.0–31.8), 60.0 | 3.0 (0.0–46.8), 60.0 | 0.83 | ||||
| Coagulation failure free days, median (IQR), max. | 7.0 (0.0–29.8), 60.0 | 3.5 (0.0–48.3), 60.0 | 0.79 | ||||
| Hepatic failure free days, median (IQR), max. | 7.0 (0.0–31.0), 60.0 | 5.0 (0.5–52.5), 60.0 | 0.92 | ||||
| ITT analyses, follow-up until hospital discharge |
|||||||
| Adverse events potentially associated with steroids k | Control group | Steroids group | P-value | Table S7 | |||
| Sepsis /septic shock, no./total no. (%) | 17/54 (31.5) | 16/46 (34.8) | 0.83 | ||||
| Hospital-acquired pneumonia, no./total no. (%) | 7/54 (13.0) | 11/46 (23.9) | 0.20 | ||||
| Urinary tract infection, no/total no. (%) | 3/54 (5.6) | 0/46 (0.0) | 0.25 | ||||
| Paresis, no./total no. (%) | 4/54 (7.4) | 4/46 (8.7) | >0.99 | ||||
| Upper gastrointestinal bleeding– no./total no. (%) | 1/54 (1.9%) | 0/46 (0.0) | >0.99 | ||||
| Episodes of hyperglycemia/patient-day, median (IQR), max. | 0 (0–2), 9 | 0 (0–2), 7 | 0.63 | ||||
| Episodes of hypernatremia/patient-day, median (IQR), max. | 0 (0–0), 2 | 0 (0–0), 3 | 0.68 | ||||
ITT, intention to treat; ROSC, return of spontaneous circulation; EMM, estimated marginal mean (by linear mixed-model analyses; see also Statistical Analysis); CI, confidence interval; BSA, body surface area; LVEDA and RVEDA, left and right ventricular end-diastolic area, respectively; LVEF and RVEF, left and right ventricular ejection fraction, respectively; NIRS, near-infrared spectroscopy; NA, not applicable. P-values corresponding to ≥2 consecutive comparisons were subjected to the Bonferroni correction.
This column cites mainly supplemental Figures and Tables with detailed reporting of the results of the ITT analyses.
Ranges of % missing follow-up datapoints through day 10 post-ROSC for the presented variables: control group, 0.5–25.6%; steroids group, 1.8–24.7%.
% missing follow-up datapoints through day 10 post-ROSC: control group, 20.0%; steroids group, 28.6%.
Ranges of number of patients with available data for the presented variables: at 12 hours post-ROSC, control group, 16–29; steroids group, 20–28; at 72 hours post-ROSC, control group, 9–15; steroids group, 13–17.
The uncorrected P value of 0.025 was statistically significant.
Following ICU admission, day-1 data on prefrontal cortex BFI were actually collected at a median (IQR) of 5.5 (3.8–12.0) and 8.8 (4.0–12.0) hours post-ROSC in steroids and control group, respectively (P = 0.35).
Number of patients with available data: at 12 hours post-ROSC, control group, 14; steroids group, 15; at 72 hours post-ROSC, control group, 14; steroids group, 11; at 72 hours post-ROSC, mean PaCO2 was approximately 5 mmHg lower in steroids group vs. control (P = 0.005).
Number of patients with available data: ITT analysis, control group, 42; steroids group, 38.
Ranges of % missing follow-up datapoints through day 10 post-ROSC for the presented variables: control group, 14.1–14.1%; steroids group, 20.2–20.7%.
Number of patients with available data: ITT analyses, all variables (besides hepatic failure free days), control group, 54; steroids group, 46; ITT analysis, hepatic failure free days, control group, 51; steroids group, 45.
Number of patients with available data: ITT analyses (all variables), control group, 54; steroids group, 46.
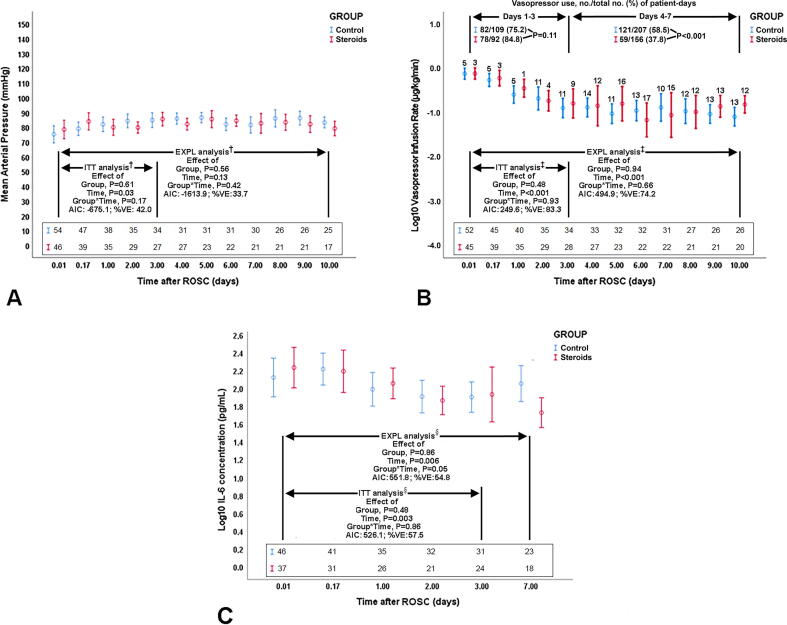
In mixed-model analyses, there was no significant effect of group, group * time, and center on primary outcomes [i.e. arterial pressure (Fig. 2A) and ScvO2], or on cardiac index. Regarding patients receiving vasopressors, estimates for vasopressor infusion rates (non-outcome variable) were similar in the 2 groups (P ≥ 0.48) (Fig. 2B). There was no difference in patient-days of vasopressor use (non-outcome variable) over days 1–3 post-ROSC (P = 0.11); control vs. steroids group had more patient-days of vasopressor use over days 4–10 post-ROSC (P < 0.001) (Fig. 2B).
Fig. 2.
Time course of mean arterial pressure (A), log-transformed vasopressor infusion rate in patients who received vasopressors and no./total no. (%) of patient-days of vasopressor use (B), and log-transformed interleukin (IL)-6 (C) in the Steroids and Control group. Data are presented as mean (95% confidence interval). ITT analysis: intention-to-treat mixed-model analysis corresponding to the first 72 hours postresuscitation; EXPL: exploratory mixed-model analyses corresponding to days 1–10 (A, B) or to days 1–7 (C) postresuscitation. In B, the nos./total nos. (%) of patient days of vasopressor use were compared by Fisher’s exact test. ROSC, return of spontaneous circulation. AIC: Akaike information criterion; %VE: percent variance explained, which reflects the squared Pearson correlation coefficient determined by linear regression with observed variable values as dependent variable and mixed model-estimated variable values as independent variable. A, B, and C: The sequences of numbers just above the horizontal axes represent nos. of patients participating in the analysis at the respective follow-up time points. B: The numbers on the top of the bars reflect numbers of patients not receiving any vasopressor support at the corresponding time-points of follow-up. †, Additional model information: A, ITT and EXPL analysis: effect of center and insulin infusion rate, P = 0.17 to 0.52. ‡, Additional model information: B, ITT and EXPL analysis: effect of center, insulin infusion rate, and blood glucose, P = 0.22 to 0.71. §, Additional model information: C, ITT and EXPL analysis: effect of center P = 0.10 to 0.11. †,‡,§, Log-transformed values were actually used in all analyses, because data exhibited skewed distributions; in A, we present the actually observed/recorded values of mean arterial pressure in mmHg, solely for the purpose of a simplified and clear presentation.
There was no significant, between-group difference in day-1 and day-3 echocardiographic variables and prefrontal cortex BFI. Mean body temperature over the first 48 hours post-ROSC was slightly lower (i.e. by 0.2–0.3 °C) in the steroids group vs. control (P ≤ 0.03).
There was no significant effect of group, group * time, and center on serum cytokine concentrations (Fig. 2C). There was no significant between-group difference in organ failure free days and adverse events.
Survival and neurological outcome
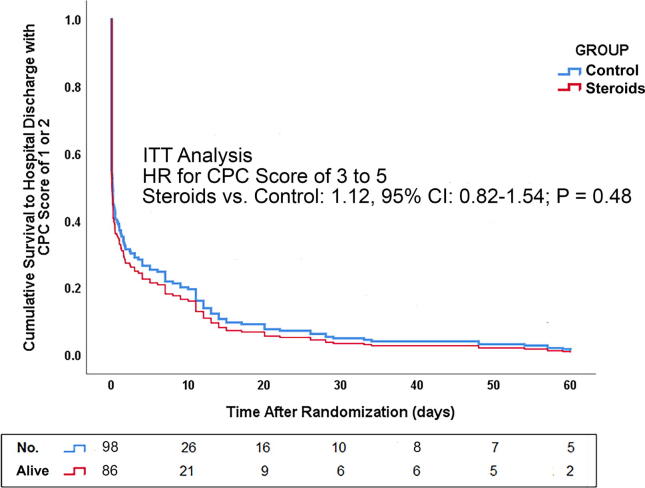
The steroids vs. control HR for poor functional outcome was nonsignificant (P = 0.28–0.48; Fig. 3). For Cox model details, see Table S6.
Fig. 3.
Probability of survival with a Cerebral Performance Category (CPC) score of 1 or 2 until day 60 after the return of spontaneous circulation (ROSC), which was identical to survival to hospital discharge with a CPC score of 1 or 2; intention-to-treat (ITT) analysis. “No. Alive:” reflects total number of participants minus (1) those who died before the corresponding time point; and (2) those in whom neurologic failure (i.e. Glasgow Coma Scale score of ≤9) was confirmed (before the corresponding time point) and was followed either by death before hospital discharge (without any intervening neurologic failure free day) or by determination of a CPC score of 3 or 4 at hospital discharge (again, without any intervening neurologic failure free day). Therefore, “No. Alive” reflects patients who could still achieve hospital discharge with a CPC score of 1 or 2.
Non-outcome variables
Table 3 displays main results on non-outcome variables. Additional details are reported in the Supplement (as indicated in Table 3).
Table 3.
Main results on non-outcome variables.
| ITT analyses, first 72 hours post-ROSC |
Exploratory ITT analyses, 10-day follow-up |
Additional presentation a | |||||
|---|---|---|---|---|---|---|---|
| Hemodynamic support and related variables b | Control group | Steroids group | P-value | Control group | Steroids group | P-value | Figures S11–S18 |
| Vasopressor infusion rate EMM (95% CI), μg/kg/min c | 0.30 (0.16–0.55) | 0.31 (0.18–0.52) | 0.89 | 0.18 (0.11–0.29) | 0.16 (0.10–0.27) | 0.78 | Figure 2B |
| Fluid balance, EMM (95% CI), mL | 927 (–93 to 1948) | 2125 (1306–2944) | 0.01 | 1071 (408–1735) | 1652 (1048–2257) | 0.07 | Figure S11 |
| Arterial blood lactate, EMM (95% CI), mmol/L | 4.5 (1.7–7.3) | 6.2 (3.8–8.6) | 0.20 | 2.9 (0.4–5.4) | 5.7 (3.5–7.9) | 0.03 | Figure S12 |
| Arterial blood pH, EMM (95% CI) | 7.36 (7.32–7.41) | 7.31 (7.27–7.35) | 0.004 | 7.35 (7.30–7.40) | 7.34 (7.28–7.39) | 0.69 | Figure S18 |
| ITT analysis, at 12 hours post-ROSC |
ITT analysis, at 72 hours post- ROSC |
||||||
| Control group | Steroids group | P-value | Control group | Steroids group | P-value | ||
| Cerebral autoregulation (by NIRS) adequate, d no./total no. (%) e | 5/14 (35.7) | 6/15 (40.0) | >0.99 | 6/14 (42.9) | 4/11 (36.4) | >0.99 | Table S4 |
| ITT analysis, 60-day follow-up |
|||||||
| Control group | Steroids group | P-value | |||||
| Shock reversal in patients with PRS, no./total no. (%) f | 16/49 (32.7) | 16/43 (37.2) | 0.68 | NA | |||
| Days to first cessation of vasopressors, median (IQR); max g | 2.0 (1.3-4.8); 9.0 | 3.5 (3.0-6.0); 8.0 | 0.18 | NA | |||
| Exploratory analysis, first 72 hours post-ROSC |
Additional presentation a | ||||||
| Control group | Steroids group | P-value | Supplemental Results and Figure S19 | ||||
| Mean systemic filling pressure, EMM (95% CI),mmHg h | 20.2 (18.9–21.6) | 20.0 (18.1–22.0) | 0.86 | ||||
| Cardiac pump performance, EMM (95% CI), fraction of unity h | 0.51 (0.47–0.55) | 0.46 (0.42–0.50) | 0.10 | ||||
| Systemic vascular resistance, EMM (95% CI), dynes * s/cm5h | 1131 (1012–1248) | 1113 (993–1232) | 0.83 | ||||
| Control group | Steroids group | P-value | |||||
| Ventilator free days, median (IQR), max. i | 0.0 (0.0-2.0), 58.0 | 0.0 (0.0-5.0), 53.0 | 0.77 | NA | |||
| Length of hospital stay, days – median (IQR); max. | 7.5 (0.9-32.5); 218.0 | 4.5 (0.7-41.3); 147.0 | 0.25 | Table S8j | |||
ITT, intention to treat; ROSC, return of spontaneous circulation, EMM, estimated marginal mean (by linear mixed-model analyses; see also.
Statistical Analysis); CI, confidence interval; NIRS, near-infrared spectroscopy; BS, broad spectrum; PRS, postresuscitation shock. NA, not applicable. P-values corresponding to ≥2 consecutive comparisons were subjected to the Bonferroni correction.
This column cites mainly supplemental Figures and Tables with detailed reporting of the results of the ITT analyses.
Ranges of % missing follow-up datapoints through day 10 post-ROSC for the presented variables: control group, 2.2–13.4%; steroids group, 3.0–13.8%; see Supplement for further details.
Calculated as the sum of the infusion rates of norepinephrine (in μg/kg/min), dopamine/2 (in μg/kg/min), and epinephrine (in μg/kg/min).40
Cerebral autoregulation was defined as adequate according to the following criterion: Pearson correlation coefficient between mean arterial pressure and prefrontal cortex tissue oxygenation index of <0.3;34 see Methods and Supplemental Methods for further details.
No. of patients with available data, ITT analysis at 12 hours post-ROSC, control group, 14; steroids group, 15; ITT analysis at 72 hours post-ROSC, control group, 14; steroids group, 11.
Defined as maintenance of a mean arterial pressure of >70 mmHg without any vasopressor support for a minimum of 24 hours.
Variable pertains solely to patients who had PRS followed by shock reversal.
Formulas of determination are presented in the Supplement; data originate from all patients who received cardiac output monitoring. (control group, 32; steroids group, 27); a cardiac pump performance of 1.00 is considered to reflect normal heart function.39
No. of patients with available data, ITT analysis, control group, 54; steroids group, 46.
Supplemental Table S8 displays additional details on the length of intensive/coronary care unit stay.
Mixed-model estimates for steroids group vs. control included more positive fluid balance (first 72 hours post-ROSC), higher arterial lactate (days 1–10 post-ROSC), and lower arterial pH (first 72 hours post-ROSC) (all P ≤ 0.03).
In exploratory analyses of day-1 and day-3 NIRS measurements, cerebral autoregulation was adequate37 (see also footnote of Table 3) over an MAP range of 70–110 mmHg38 in similar proportions of steroids and control NIRS-subgroup patients (P > 0.99).
There were significant between-group differences in the number of patient-days with use of various drug classes through day 10. Drug classes included vasopressors (see also Fig. 2B), antibiotics, antiarrhythmics, antiepileptics and antihypertensives; see Supplement for details.
In exploratory analyses, there was no significant, between-group difference in the proportions of patients with postresuscitation shock and subsequent shock reversal (see footnote of Table 3 and Supplement), or the times (in days) to first post-ROSC cessation of vasopressors. Also, determinants of vasopressor responsiveness (i.e. mean systemic filling pressure, cardiac pump performance and systemic vascular resistance)39., 40. did not differ between steroids group and control patients with available data (Table 3; see also Supplement).
There was no significant between-group difference in ventilator free days, or postresuscitation ICU/CCU and hospital stay.
Discussion
We evaluated the effect of intra-arrest methylprednisolone and post-ROSC hydrocortisone on early postresuscitation circulatory and systemic inflammation endpoints, reportedly associated with long-term outcomes.24., 7., 8., 9., 10., 41., 42., 43., 44. Our results suggest lack of physiological benefit of corticosteroid supplementation in in-hospital cardiac arrest. Steroids did not affect postresuscitation MAP/ScvO2, cardiac function, prefrontal cortex BFI, and systemic inflammation. Accordingly, steroids did not improve postresuscitation organ dysfunction or survival/neurological outcome.
Current results on hemodynamic variables differ from those reported by the VSE studies.19., 20. Indeed, mixed-model analyses of pooled VSE study data19., 20. revealed a significantly higher MAP (by approximately 9–10 mmHg) over the first 72 hours post-ROSC in the intervention group. Group*time estimates suggested that the VSE-associated MAP increase could peak at 17 mmHg through the first 24 hours post-ROSC and then decline to 4–6 mmHg over the subsequent 48 hours. This MAP benefit was determined at a similar level of support with vasopressors and fluids in the VSE and control groups. In contrast, current mixed-model estimates of MAP values were similar in the steroids and control group. Group*time estimates suggested a slightly and nonsignificantly higher MAP (by 5–6 mmHg) within the first 4 hours post-ROSC in the steroids group; however, this was followed by very similar MAP values at the subsequent follow-up time points (Fig. 2A). Vasopressor infusion rates, vasopressor use and determinants of vasopressor responsiveness39., 40. did not differ between the 2 groups during the first 72 hours post-ROSC; however, steroids group patients received more fluids and had a higher arterial lactate during follow-up.
The neutral results on ScvO2, cardiac index, echocardiographic variables, and prefrontal cortex BFI were consistent with the results on arterial pressure, indicating a similar systemic and regional-cerebral hemodynamic profile in the 2 groups. Our neutral results on shock reversal and vasopressor requirements were consistent with the main findings of a prior, small (n = 50), randomized study with different major characteristics.45 In the latter study: (1) 76% of the participants had out-of-hospital cardiac arrest; (2) median downtime to ALS was 21 min - as opposed to 2 min in the current study (Table S1); and (3) median time to administration of stress-dose hydrocortisone was 9.7 hours after ROSC45., 46.- as opposed to the current study’s intra-arrest methylprednisolone.
Our results on early postresuscitation IL-6 levels (Table 2 and Fig. 2C) are consistent with those reported by 3 prior studies.9., 19., 45. However, in contrast with 2 of these studies,19., 45. we found no between-group difference in post-ROSC cytokine concentrations. Collectively, our results imply a potential, early resistance to previously documented circulatory and immunomodulatory mechanisms of steroid action.47., 14., 15., 16., 17., 18., 19., 20. Notably, compared to the current steroids group, our combined, prior VSE groups19., 20. had a markedly shorter median ALS duration (i.e. 14 min vs. 27 min in the steroids group; Table S1), and substantially higher rates of ROSC after just 2–3 vasopressor doses (i.e. 32.0–48.9% vs. 5.8–15.1% in the steroids group; Table S1). However, a long low-flow/ischemia time may increase the I/R-induced oxidation of the glucocorticoid receptor (GR).49., 50., 51. This may enhance the proteasome degradation of the GR,49., 50. with consequent decrease in GR expression51 and resistance to steroids. Such resistance might wane later-on, as implied by our day 4–10 results on vasopressor use (Fig. 2B). These speculations/interpretations should be evaluated in future studies.
Our results on organ failure-free days and functional in-hospital outcome are consistent with neutral findings on survival and/or functional outcome reported by 2 retrospective observational studies (n = 262–458),52., 53. and the randomized study of Donnino et al.45 However, 2 large, retrospective observational studies (n = 2333–10890)54., 55. suggested associations between postresuscitation steroid use and survival to hospital discharge. Accordingly, 2 recent systematic reviews of the conflicting published evidence were inconclusive about steroid use in cardiac arrest.56., 57.
Study limitations
ROSC was not an outcome in this study, because methylprednisolone was not combined with vasopressin during CPR.19., 20., 58. However, the early, nongenomic vasoconstrictor effect of methylprednisolone15., 30., 48., 59., 60., 61., 62. can be expected within 30–60 min post-administration,15., 19., 59., 60. and should therefore mainly concern patients with ROSC. Also, vasopressor dose was not part of the primary outcome, despite its pre-specified recording along with MAP. However, there was no between-group difference in vasopressor dose or use over the time-frame of MAP determination as primary outcome.
Our prediction for a possible, steroid-related increase in MAP of 17 mmHg could be regarded as overoptimistic. The current study could detect only a large effect size. However, prior control subgroup data (n = 13)20 suggested a hydrocortisone-associated median rise in MAP of 24 mmHg within 4–24 hours post-ROSC (unpublished observations; see Supplement for details). Furthermore, postresuscitation disease may be considered as a sepsis-like syndrome,11., 12. and prior data from patients with septic shock suggested hydrocortisone-associated MAP increases of 15–30 mmHg.15.
Additional limitations included (1) limited sample size precluding reliable evaluation of long-term outcomes19., 20. and contributing to “baseline imbalances”36 (Table 1); such imbalances in conjunction with treatment individualization in small study groups might partly explain the observed differences in the prescribed medication (Tables 3 and S9); (2) group cross-contamination by steroids’ use in 9 controls with vasopressor-refractory hemodynamic instability;20 (3) lack of determinations of cortisol levels,19., 45. and GR expression;51 (4) inability to assess factors such as steroids’ accessibility to the GR,62., 63. GR functionality,63., 64. GR interaction with other proteins,59., 63., 65. and GR accessibility to the genome;63 and (5) missing echocardiographic and NIRS data from >50% of the patients of both groups.
Study strength
This study comprised the first randomized controlled evaluation of the effect of steroids on multiple post-ROSC physiological mechanisms potentially affecting functional outcome. Furthermore, the results were consistently neutral across all of the conducted analyses.
Implications for practice and research
Our results do not support the use of stress-dose steroids in in-hospital cardiac arrest. Future research should further elucidate the possible role of interventions targeted at improving post-ROSC hemodynamics and attenuating inflammation.
Author contributions
Study concept: Mentzelopoulos. Study Design: Mentzelopoulos, Ischaki, Malachias. Acquisition of Data: Pappa, Vrettou, Giannopoulos, Karlis, Adamos, Pantazopoulos, Megalou, Agaliotis, Papadaki, Baladima, Aggelopoulos, Lasithiotaki, Lagiou, Temperikidis, Louka, Asimakos, Kougias, Xintara, Papadonta, Koutsothymiou, Zakynthinos E. Drafting of the manuscript: Mentzelopoulos. Critical revision of the manuscript for important intellectual content: all authors. Statistical expertise: Mentzelopoulos, Kougias, Aggelopoulos. Near-infrared spectroscopy expertise: Louvaris, Mentzelopoulos, Malachias. Determination of serum cytokine concentrations: Karavana. Obtained funding: Mentzelopoulos, Zakynthinos S. Administrative, technical, or material support: Mentzelopoulos, Zakynthinos S, Zakynthinos E, Makris. Study supervision: Mentzelopoulos, Ischaki, Malachias, Vrettou, Makris. Full access to all of the data in the study and responsibility for the integrity of the data and the accuracy of the data and analysis: Mentzelopoulos. The contributions of the second, third, fourth, fifth, and forelast author were equally important.
Funding/support
This work has been funded in part by (1) the Thorax Research Foundation, Athens, Greece; (2) the Special Account for Research Grants of the National and Kapodistrian University of Athens, Greece; and (3) by Astellas Pharmaceuticals ABEE, Athens, Greece (GRE-STL-2875).
Role of the sponsor
The funding bodies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosure
There is no disclosure to be made by anyone of the authors regarding any conflict of interest.
Study organization and personnel
Study Chairpersons: Spyros D. Mentzelopoulos, MD, PhD (principal investigator); Spyros G. Zakynthinos, MD, PhD (study director and chair). Statistical Analyses: Epameinondas Aggelopoulos, MD, PhD, Marios Kougias, MD, PhD, Spyros D. Mentzelopoulos. Study Pharmacists team: Sotirios Malachias, MD, PhD, Eleni Ischaki, MD, PhD (Evaggelismos General Hospital), Demosthenes Makris, MD, PhD (Larissa University hospital). Independent Main End Point and Safety Monitoring Committee: Zafeiria Mastora, MD, PhD and Eleni Magira, MD, PhD (Evaggelismos General Hospital); Zoi Daniil, MD, PhD (Larissa University Hospital); Quality and data security assurance: Zafeiria Mastora, Eleni Magira, Zoi Daniil.
Previous presentations of the current work or related material
This work has been presented in part at the International Symposium on Intensive Care and Emergency Medicine Virtual Meeting (e-ISICEM), September 15-20, 2020, Brussels, Belgium, and the 29th Congress of the Greek Society of Pulmonology, December 17-20, 2020, Athens, Greece. Medical University of Vienna, Monash, University of Washington Joint Resuscitation Rounds [Zoom Meeting], December 8, 2021.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resplu.2022.100252.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Mentzelopoulos S.D., Zakynthinos S.G. Post-cardiac arrest syndrome: pathological processes, biomarkers and vasopressor support, and potential therapeutic targets. Resuscitation. 2017;121:A12–A14. doi: 10.1016/j.resuscitation.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Jozwiak M., Bougouin W., Geri G., Grimaldi D., Cariou A. Post-resuscitation shock: recent advances in pathophysiology and treatment. Ann Intensive Care. 2020;10:170. doi: 10.1186/s13613-020-00788-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fink K., Schwarz M., Feldbrügge L., et al. Severe endothelial injury and subsequent repair in patients after successful cardiopulmonary resuscitation. Crit Care. 2010;14:R104. doi: 10.1186/cc9050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reis C., Akyol O., Araujo C., et al. Pathophysiology and the Monitoring Methods for Cardiac Arrest Associated Brain Injury. Int J Mol Sci. 2017;18:129. doi: 10.3390/ijms18010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.González-Ibarra F.P., Varon J., López-Meza E.G. Therapeutic hypothermia: critical review of the molecular mechanisms of action. Front Neurol. 2011;2:4. doi: 10.3389/fneur.2011.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper M.S., Stewart P.M. Corticosteroid insufficiency in acutely ill patients. N Engl J Med. 2003;348:727–734. doi: 10.1056/NEJMra020529. [DOI] [PubMed] [Google Scholar]
- 7.Hékimian G., Baugnon T., Thuong M., et al. Cortisol levels and adrenal reserve after successful cardiac arrest resuscitation. Shock. 2004;22:116–119. doi: 10.1097/01.shk.0000132489.79498.c7. [DOI] [PubMed] [Google Scholar]
- 8.Pantazopoulos I.N., Zakynthinos S.G., Mentzelopoulos S.D. Corticosteroids and inflammation after cardiac arrest. Resuscitation. 2016;99:e7–e8. doi: 10.1016/j.resuscitation.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 9.Vaahersalo J., Skrifvars M.B., Pulkki K., et al. FINNRESUSCI Laboratory Study Group. Admission interleukin-6 is associated with post resuscitation organ dysfunction and predicts long-term neurological outcome after out-of-hospital ventricular fibrillation. Resuscitation. 2014;85:1573–1579. doi: 10.1016/j.resuscitation.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 10.Kim J.J., Hyun S.Y., Hwang S.Y., et al. Hormonal responses upon return of spontaneous circulation after cardiac arrest: a retrospective cohort study. Crit Care. 2011;15:R53. doi: 10.1186/cc10019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adrie C., Adib-Conquy M., Laurent I., et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106:562–568. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
- 12.Adrie C., Laurent I., Monvhi M., Cariou A., Dhainou J.F., Spaulding C. Postresuscitation disease after cardiac arrest: a sepsis-like syndrome? Curr Opin Crit Care. 2004;10:208–212. doi: 10.1097/01.ccx.0000126090.06275.fe. [DOI] [PubMed] [Google Scholar]
- 13.Keh D., Boehnke T., Weber-Cartens S., et al. Immunologic and hemodynamic effects of “low-dose” hydrocortisone in septic shock: a double-blind, randomized, placebo-controlled, crossover study. Am J Respir Crit Care Med. 2003;167:512–520. doi: 10.1164/rccm.200205-446OC. [DOI] [PubMed] [Google Scholar]
- 14.Barnes P.J., Adcock I. Anti-inflammatory actions of steroids: molecular mechanisms. Trends Pharmacol Sci. 1993;14:436–441. doi: 10.1016/0165-6147(93)90184-l. [DOI] [PubMed] [Google Scholar]
- 15.Annane D., Bellissant E., Sebille V., et al. Impaired pressor sensitivity to noradrenaline in septic shock patients with and without impaired adrenal function reserve. Br J Clin Pharmacol. 1998;46:589–597. doi: 10.1046/j.1365-2125.1998.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Annane D., Renault A., Brun-Buisson C., et al. Hydrocortisone plus Fludrocortisone for Adults with Septic Shock. N Engl J Med. 2018;378:809–818. doi: 10.1056/NEJMoa1705716. [DOI] [PubMed] [Google Scholar]
- 17.Venkatesh B., Finfer S., Cohen J., et al. ADRENAL Trial Investigators and the Australian-New Zealand Intensive Care Society Clinical Trials Group. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. N Engl J Med. 2018;378:797–808. doi: 10.1056/NEJMoa1705835. [DOI] [PubMed] [Google Scholar]
- 18.Koliantzaki I., Zakynthinos S.G., Mentzelopoulos S.D. In: Resuscitation. Gullo A., Ristagno G., editors. Milano; Springer: 2014. The Potential Contribution of Corticosteroids to Positive Cardiac Arrest Outcomes; pp. 143–155. [Google Scholar]
- 19.Mentzelopoulos S.D., Zakynthinos S.G., Tzoufi M., et al. Vasopressin, epinephrine, and corticosteroids for in-hospital cardiac arrest. Arch Intern Med. 2009;169:15–24. doi: 10.1001/archinternmed.2008.509. [DOI] [PubMed] [Google Scholar]
- 20.Mentzelopoulos S.D., Malachias S., Chamos C., et al. Vasopressin, steroids, and epinephrine and neurologically favorable survival after in-hospital cardiac arrest: a randomized clinical trial. JAMA. 2013;310:270–279. doi: 10.1001/jama.2013.7832. [DOI] [PubMed] [Google Scholar]
- 21.Finkel M.S., Oddis C.V., Jacob T.D., Watkins S.C., Hattler B.G., Simmons R.L. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992;257:387–389. doi: 10.1126/science.1631560. [DOI] [PubMed] [Google Scholar]
- 22.Kröll S., El-Gindi J., Thanabalasundaram G., Panpumthong P., Schrot S., Hartmann C., Galla H.J. Control of the blood-brain barrier by glucocorticoids and the cells of the neurovascular unit. Ann N Y Acad Sci. 2009;1165:228–239. doi: 10.1111/j.1749-6632.2009.04040.x. [DOI] [PubMed] [Google Scholar]
- 23.Kozler P., Riljak V., Pokorný J. Methylprednisolone reduces axonal impairment in the experimental model of brain oedema. Neuro Endocrinol Lett. 2011;32:831–835. [PubMed] [Google Scholar]
- 24.Trzeciak S., Jones A.E., Kilgannon J.H., et al. Significance of arterial hypotension after resuscitation from cardiac arrest. Crit Care Med. 2009;37:2895–2903. doi: 10.1097/ccm.0b013e3181b01d8c. [DOI] [PubMed] [Google Scholar]
- 25.Link M.S., Berkow L.C., Kudenchuk P.J., et al. Part 7: Adult Advanced Cardiovascular Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S444–S464. doi: 10.1161/CIR.0000000000000261. Erratum in: Circulation. 2015;132:e385. [DOI] [PubMed] [Google Scholar]
- 26.Soar J., Nolan J.P., Böttiger B.W., et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 3. Adult advanced life support. Resuscitation. 2015;95:100–147. doi: 10.1016/j.resuscitation.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Vincent J.L., Moreno R., Takala J., et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 28.Cummins R.O., Chamberlain D., Hazinski M.F., et al. Recommended guidelines for reviewing, reporting, and conducting research on in-hospital resuscitation: the in-hospital “Utstein style.”. Circulation. 1997;95:2213–2239. doi: 10.1161/01.cir.95.8.2213. [DOI] [PubMed] [Google Scholar]
- 29.Boushel R., Langberg H., Olesen J., et al. Regional blood flow during exercise in humans measured by near – infrared spectroscopy and indocyanine green. J Appl Physiol. 2000;89:1868–1878. doi: 10.1152/jappl.2000.89.5.1868. [DOI] [PubMed] [Google Scholar]
- 30.Tatro J.B. Endogenous antipyretics. Clin Infect Dis. 2000;31(Suppl 5):S190–S201. doi: 10.1086/317519. [DOI] [PubMed] [Google Scholar]
- 31.Tuure L., Hämäläinen M., Nummenmaa E., Moilanen T., Moilanen E. Downregulation of microsomal prostaglandin E synthase-1 (mPGES-1) expression in chondrocytes is regulated by MAP kinase phosphatase-1 (MKP-1) Int Immunopharmacol. 2019;71:139–143. doi: 10.1016/j.intimp.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 32.Bro-Jeppesen J., Hassager C., Wanscher M., et al. Targeted temperature management at 33°C versus 36°C and impact on systemic vascular resistance and myocardial function after out-of-hospital cardiac arrest: a sub-study of the Target Temperature Management Trial. Circ Cardiovasc Interv. 2014;7:663–672. doi: 10.1161/CIRCINTERVENTIONS.114.001556. [DOI] [PubMed] [Google Scholar]
- 33.Annane D. Glucocorticoids in the treatment of severe sepsis and septic shock. Curr Opin Crit Care. 2005;11:449–453. doi: 10.1097/01.ccx.0000176691.95562.43. [DOI] [PubMed] [Google Scholar]
- 34.Baumann G., Dingman J.F. Distribution, blood transport, and degradation of antidiuretic hormone in man. J Clin Invest. 1976;57:1109–1116. doi: 10.1172/JCI108377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown H., Prescott R. In: Applied Mixed Models in Medicine. 2nd ed. Brown H., Prescott R., editors. John Wiley and Sons Ltd; Chichester, West Sussex, England: 2006. Normal Mixed Models; pp. 33–106. [Google Scholar]
- 36.Fergusson D., Aaron S.D., Guyatt G., Hébert P. Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ. 2002;325:652–654. doi: 10.1136/bmj.325.7365.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ono M., Brady K., Easley R.B., et al. Duration and magnitude of blood pressure below cerebral autoregulation threshold during cardiopulmonary bypass is associated with major morbidity and operative mortality. J Thorac Cardiovasc Surg. 2014;147:483–489. doi: 10.1016/j.jtcvs.2013.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ameloot K., Genbrugge C., Meex I., et al. An observational near-infrared spectroscopy study on cerebral autoregulation in post-cardiac arrest patients: time to drop 'one-size-fits-all' hemodynamic targets? Resuscitation. 2015;90:121–126. doi: 10.1016/j.resuscitation.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Sondergaard S., Parkin G., Aneman A. Central venous pressure: soon an outcome-associated matter. Curr Opin Anaesthesiol. 2016;29:179–185. doi: 10.1097/ACO.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 40.Åneman A., Wilander P., Zoerner F., Lipcsey M., Chew M.S. Vasopressor Responsiveness Beyond Arterial Pressure: A Conceptual Systematic Review Using Venous Return Physiology. Shock. 2021;56:352–359. doi: 10.1097/SHK.0000000000001762. [DOI] [PubMed] [Google Scholar]
- 41.Bro-Jeppesen J., Kjaergaard J., Stammet P., et al. TTM-Trial Investigators. Predictive value of interleukin-6 in post-cardiac arrest patients treated with targeted temperature management at 33 °C or 36 °C. Resuscitation. 2016;98:1–8. doi: 10.1016/j.resuscitation.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 42.Kilgannon J.H., Roberts B.W., Jones A.E., et al. Arterial blood pressure and neurologic outcome after resuscitation from cardiac arrest. Crit Care Med. 2014;42:2083–2091. doi: 10.1097/CCM.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 43.Laurikkala J., Wilkman E., Pettilä V., et al. Mean arterial pressure and vasopressor load after out-of-hospital cardiac arrest: Associations with one-year neurologic outcome. Resuscitation. 2016;105:116–122. doi: 10.1016/j.resuscitation.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 44.Roberts B.W., Kilgannon J.H., Hunter B.R., et al. Association Between Elevated Mean Arterial Blood Pressure and Neurologic Outcome After Resuscitation From Cardiac Arrest: Results From a Multicenter Prospective Cohort Study. Crit Care Med. 2019;47:93–100. doi: 10.1097/CCM.0000000000003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donnino M.W., Andersen L.W., Berg K.M., et al. Collaborating Authors from the Beth Israel Deaconess Medical Center’s Center for Resuscitation Science Research Group. Corticosteroid therapy in refractory shock following cardiac arrest: a randomized, double-blind, placebo-controlled, trial. Crit Care. 2016;20:82. doi: 10.1186/s13054-016-1257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mentzelopoulos S.D., Mongardon N., Xanthos T., Zakynthinos S.G. Possible significance of hemodynamic and immunomodulatory effects of early stress-dose steroids in cardiac arrest. Crit Care. 2016;20:211. doi: 10.1186/s13054-016-1384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skyschally A., Haude M., Dörge H., et al. Glucocorticoid treatment prevents progressive myocardial dysfunction resulting from experimental coronary microembolization. Circulation. 2004;109:2337–2342. doi: 10.1161/01.CIR.0000127961.66744.F4. [DOI] [PubMed] [Google Scholar]
- 48.Ullian M.E. The role of corticosteriods in the regulation of vascular tone. Cardiovasc Res. 1999;41:55–64. doi: 10.1016/s0008-6363(98)00230-2. [DOI] [PubMed] [Google Scholar]
- 49.Divald A., Powell S.R. Proteasome mediates removal of proteins oxidized during myocardial ischemia. Free Radic Biol Med. 2006;40:156–164. doi: 10.1016/j.freeradbiomed.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 50.Calise J., Powell S.R. The ubiquitin proteasome system and myocardial ischemia. Am J Physiol Heart Circ Physiol. 2013;304:H337–H349. doi: 10.1152/ajpheart.00604.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vassiliou A.G., Floros G., Jahaj E., et al. Decreased glucocorticoid receptor expression during critical illness. Eur J Clin Invest. 2019;49:e13073. doi: 10.1111/eci.13073. [DOI] [PubMed] [Google Scholar]
- 52.Grafton S.T., Longstreth W.T., Jr. Steroids after cardiac arrest: a retrospective study with concurrent, nonrandomized controls. Neurology. 1988;38:1315–1316. doi: 10.1212/wnl.38.8.1315. [DOI] [PubMed] [Google Scholar]
- 53.Jastremski M., Sutton-Tyrrell K., Vaagenes P., Abramson N., Heiselman D., Safar P. Glucocorticoid treatment does not improve neurological recovery following cardiac arrest. Brain Resuscitation Clinical Trial I Study Group. JAMA. 1989;262:3427–3430. [PubMed] [Google Scholar]
- 54.Niimura T., Zamami Y., Koyama T., et al. Hydrocortisone administration was associated with improved survival in Japanese patients with cardiac arrest. Sci Rep. 2017;7:17919. doi: 10.1038/s41598-017-17686-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsai M.S., Chuang P.Y., Huang C.H., et al. Postarrest Steroid Use May Improve Outcomes of Cardiac Arrest Survivors. Crit Care Med. 2019;47:167–175. doi: 10.1097/CCM.0000000000003468. [DOI] [PubMed] [Google Scholar]
- 56.Li Y., Zhang J., Cai N., He F. Efficacy and safety of corticosteroid therapy in patients with cardiac arrest: a systematic review of randomised controlled trials. Eur J Clin Pharmacol. 2020;76:1631–1638. doi: 10.1007/s00228-020-02964-3. [DOI] [PubMed] [Google Scholar]
- 57.Sahebnasagh A., Najmeddin F., Najafi A., et al. Efficacy of Glucocorticoid Administration in Patients with Cardiac Arrest: A Systematic Review of Clinical Studies. Curr Med Chem. 2021 doi: 10.2174/0929867328666210531145617. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 58.Andersen L.W., Isbye D., Kjærgaard J., et al. Effect of Vasopressin and Methylprednisolone vs Placebo on Return of Spontaneous Circulation in Patients With In-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA. 2021;326:1586–1594. doi: 10.1001/jama.2021.16628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Limbourg F.P., Liao J.K. Nontranscriptional actions of the glucocorticoid receptor. J Mol Med (Berl) 2003;81:168–174. doi: 10.1007/s00109-003-0418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Czock D., Keller F., Rasche F.M., Häussler U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet. 2005;44:61–98. doi: 10.2165/00003088-200544010-00003. [DOI] [PubMed] [Google Scholar]
- 61.Christ M., Meyer C., Sippel K., Wehling M. Rapid aldosterone signaling in vascular smooth muscle cells: involvement of phospholipase C, diacylglycerol and protein kinase C alpha. Biochem Biophys Res Commun. 1995;213:123–129. doi: 10.1006/bbrc.1995.2106. [DOI] [PubMed] [Google Scholar]
- 62.Yang S., Zhang L. Glucocorticoids and vascular reactivity. Curr Vasc Pharmacol. 2004;2:1–12. doi: 10.2174/1570161043476483. [DOI] [PubMed] [Google Scholar]
- 63.Williams S., Ghosh C. Neurovascular glucocorticoid receptors and glucocorticoids: implications in health, neurological disorders and drug therapy. Drug Discov Today. 2020;25:89–106. doi: 10.1016/j.drudis.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fruchter O., Kino T., Zoumakis E., Alesci S., De Martino M., Chrousos G., Hochberg Z. The human glucocorticoid receptor (GR) isoform beta differentially suppresses GR{alpha}-induced transactivation stimulated by synthetic glucocorticoids. J Clin Endocrinol Metab. 2005;90:3505–3509. doi: 10.1210/jc.2004-1646. [DOI] [PubMed] [Google Scholar]
- 65.Rajapandi T., Greene L.E., Eisenberg E. The molecular chaperones Hsp90 and Hsc70 are both necessary and sufficient to activate hormone binding by glucocorticoid receptor. J Biol Chem. 2000;275:22597–22604. doi: 10.1074/jbc.M002035200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.