Abstract
Monoclonal antibody 6F5 (mAb 6F5), which recognizes the mycotoxin deoxynivalenol (DON) (vomitoxin), was used to select for peptides that mimic the mycotoxin by employing a library of filamentous phages that have random 7-mer peptides on their surfaces. Two phage clones selected from the random peptide phage-displayed library coded for the amino acid sequences SWGPFPF and SWGPLPF. These clones were designated DONPEP.2 and DONPEP.12, respectively. The results of a competitive enzyme-linked immunosorbent assay (ELISA) suggested that the two phage displayed peptides bound to mAb 6F5 specifically at the DON binding site. The amino acid sequence of DONPEP.2 plus a structurally flexible linker at the C terminus (SWGPFPFGGGSC) was synthesized and tested to determine its ability to bind to mAb 6F5. This synthetic peptide (designated peptide C430) and DON competed with each other for mAb 6F5 binding. When translationally fused with bacterial alkaline phosphatase, DONPEP.2 bound specifically to mAb 6F5, while the fusion protein retained alkaline phosphatase activity. The potential of using DONPEP.2 as an immunochemical reagent in a DON immunoassay was evaluated with a DON-spiked wheat extract. When peptide C430 was conjugated to bovine serum albumin, it elicited antibody specific to peptide C430 but not to DON in both mice and rabbits. In an in vitro translation system containing rabbit reticulocyte lysate, synthetic peptide C430 did not inhibit protein synthesis but did show antagonism toward DON-induced protein synthesis inhibition. These data suggest that the peptides selected in this study bind to mAb 6F5 and that peptide C430 binds to ribosomes at the same sites as DON.
Deoxynivalenol (DON) (vomitoxin) (Fig. 1A) is one of the sesquiterpene mycotoxins classified as 12,13-epoxy-trichothecenes (28). This compound occurs naturally in infected corn (19, 29), small grains (18, 31), and mixed feeds (29). DON is mainly produced by the fungus Gibberella zeae (Schwein.) Petch (anamorph, Fusarium graminearum Schwabe). At the cellular level, the main toxic effect of DON is inhibition of protein synthesis via binding to ribosomes and interfering with peptidyltransferase (4, 42). In animals, DON can cause anorexia and emesis (vomiting) (37). Other toxic effects of DON include skin irritation, hemorrhaging, hematological changes, human lymphocyte blastogenesis impairment, radiomimetic effects, apoptosis (cytotoxicity), and immunotoxicity (37).
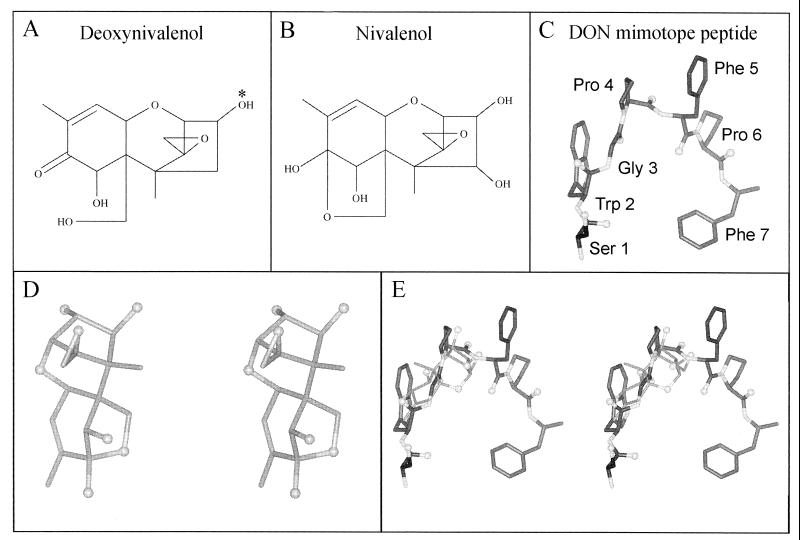
FIG. 1.
Structural comparison of DON and nivalenol with DON mimotope peptide SWGPFPF (DONPEP.2). (A) Two-dimensional representation of DON. The asterisk indicates the site of conjugation of the carrier protein (e.g., BSA) to DON. (B) Two-dimensional structure of nivalenol, an analog of DON whose structure is known. (C) Three-dimensional structural model of the DON mimotope peptide. The white spheres represent oxygen atoms, the white cylinders represent nitrogen atoms, and the grey cylinders represent carbon atoms in panels C through E. (D) Three-dimensional stereo view of the crystallographic structure of nivalenol (CSD entry DUTJOR10 [4a]). (E) Stereo view of the optimal PowerFit superposition of the known nivalenol structure and the DON mimotope peptide structure. Nivalenol aligns with the peptide model main-chain atoms between residues 2 and 5 (TrpGlyProPhe) and partially overlaps the side chains or Trp-2 and Pro-4.
A major way to eliminate DON from human and animal food is to detect contaminated raw materials and divert them from feed and finished food. Compared with other analytic methods, immunoassays have several advantages for rapid field testing, including high specificity, sensitivity, facile sample preparation, and ease of use (33). Following the development of the first monoclonal antibody (mAb) to DON (6), immunological methods, mainly an enzyme-linked immunosorbent assay (ELISA), have been used widely for detection of DON (33). Unfortunately, the efficiency of chemical conjugation of DON to a carrier protein or an enzyme is low because such conjugation involves extensive modification and blocking stages and causes substantial bridge group interference and unwanted cross-reactions (6, 33, 45). Also, when DON is conjugated to a carrier protein, it is weakly immunogenic. Finally, since DON is toxic and is included as a standard and conjugate in immunoassay mixtures, it may pose a toxicity risk to kit users.
One possible alternative to using mycotoxins as immunochemical reagents is to develop protein or peptide mimics that serve the same function. One approach for doing this is via generation of anti-idiotype antibodies (7, 9, 23), whose structures mimic the surface structures of low-molecular-weight biological toxins. Sometimes the sensitivity of the original antibody can be improved by generating anti-anti-idiotype antibodies (8). However, these techniques are time-consuming and costly. A new technique, phage display, allows foreign peptides and proteins to be genetically fused to the N terminus of minor coat protein g3p of the filamentous phage fd, which results in display of the peptides and proteins on the surface of the virion (40). Since random oligonucleotide sequences are inserted into the g3 gene of filamentous phage fd, phage display provides a way to construct extensive peptide libraries that may be screened in order to select peptides with specific affinities or activities (14, 15, 39). Phage-displayed short peptide libraries have been widely used in a number of applications (12, 24), including epitope mapping (14, 39), mapping of protein-protein contacts (22), identification of protease substrates (41), and identification of integrin (32) and other receptors (16), antagonists (35), and ligands (24). Only a few workers have used this technology to select peptide mimics of nonproteinaceous chemicals other than biotin (43) and carbohydrates (21).
Given the feasibility of selecting peptides that mimic nonproteinaceous chemicals, we were interested in determining whether peptide mimics of low-molecular-weight mycotoxins could be identified. Once selected, these peptide mimics might be useful in mycotoxin immunoassays and for developing new antibodies against mycotoxins. In this paper we describe two closely related heptapeptide mimotopes for DON that were selected from a phage-displayed random peptide library and the use of these mimotopes in an analysis of DON by ELISA. One of these mimotope peptides was synthesized and compared with DON for use in immunoassays, in cytotoxicity studies of cell cultures, and in in vitro protein synthesis inhibition studies.
MATERIALS AND METHODS
Reagents.
All inorganic chemicals and organic solvents were reagent grade or better. Crude ovalbumin, bovine serum albumin (BSA) (fraction V), 3,3′,5,5′-tetramethylbenzidine, phenylmethylsulfonyl fluoride, polyoxyethylene sorbitan monolaurate (Tween 20), DON, mouse immunoglobulin G (IgG), and goat anti-mouse IgG peroxidase conjugate were purchased from Sigma Chemical Co. (St. Louis, Mo.). Dimethyl sulfoxide and methanol were purchased from J. T. Baker Inc. (Phillipsburg, N.J.), and nonfat dry milk was obtained from Commander Foods, Inc., (Syracuse, N.Y.). DON-BSA, DON-ovalbumin, and DON-horseradish peroxidase (HRP) conjugates at DON’s 3-hydroxyl group were prepared by using an activated N-hydroxysuccinimide ester as described by Casale et al. (6) and were kindly provided by Frank E. Klein (Neogen Corporation, Lansing, Mich.). Sheep anti-M13 HRP conjugate was purchased from Pharmacia Biotech Inc. (Piscataway, N.J.). m-Maleimidobenzoyl-N-hydroxysulfosuccinimide ester (sulfo-MBS) and p-nitrophenyl phosphate disodium salt tablets were purchased from Pierce Chemical Company (Rockford, Ill.). Anti-DON mAb from hybridoma cell line 6F5 was prepared as described previously (6).
Phage selection by panning-elution.
A phage-displayed heptapeptide library with 2 × 109 sequence complexity (random peptide 7-mers fused to minor coat protein g3p of filamentous coliphage M13) was purchased from New England Biolabs, Inc. (Beverly, Mass.). After amplification in Escherichia coli ER2537 (New England Biolabs, Inc.), the phages in the library were selected by panning-elution as described below.
One hundred microliters of a preparation containing mAb 6F5 (15 μg/ml) in 0.01 M phosphate-buffered saline (PBS) (pH 7.4) was dispensed into each well of disposable Immuno-4 microtiter strips (Dynatech Laboratories, Inc., Chantilly, Va.). The antibody was dried onto the wells in a forced-air oven at 40°C overnight. Blank wells were coated with the same concentration of mouse IgG. The wells were washed four times by filling each well with 300 μl of PBS and aspirating the contents. Nonspecific binding was blocked by incubating 320 μl of 10% nonfat dry milk dissolved in PBS (10% milk–PBS) in each well for 1 h at 37°C and then washing the wells four times with PBS. For panning-elution selection, the recombinant phage-displayed peptide library (diluted with 10% milk–PBS to a concentration of approximately 1010 PFU/ml) was added to the wells (100 μl/well), and the preparations were incubated at 37°C for 1 h. The wells were washed 20 times by filling each well with approximately 300 μl of PBS containing 0.1% (vol/vol) Tween 20 (PBS-T), followed by incubation with 300 μl of PBS per well at 37°C with shaking at 150 rpm for 1 h. The wells were then washed 10 times with PBS-T (300 μl/well). To elute the bound phage in the microtiter wells, 100 μl of DON (100 μg/ml in PBS containing 1% methanol) or PBS containing 1% methanol was added to each well, and the preparations were incubated at 37°C with shaking at 150 rpm for 1 h. The eluted phages were then collected from the microtiter wells and used to reinfect E. coli ER2537 cells in phage titer and amplification experiments. The amplified phage, which contained approximately 8 ng of DON per ml, was used for a subsequent round of panning-elution selection. After four rounds of panning-elution selection, individual plaques were picked from Luria-Bertani plates and used to infect E. coli ER2537 cells in phage production experiments.
Binding to mAb 6F5 was determined for each individual phage clone by ELISA. The amounts of bound phage in mAb 6F5-coated microtiter wells were determined by incubation with 100 μl of sheep anti-M13 HRP conjugate (diluted 1:5,000 in 10% milk–PBS) per well at 37°C for 1 h, followed by incubation with 100 μl of 3,3′,5,5′-tetramethylbenzidine substrate per well at 37°C for 15 min. The absorbance at 450 nm was determined after the reaction was stopped by adding 100 μl of 10% H2SO4 per well.
Competitive ELISA performed with phage-displayed peptide.
To perform a competitive direct ELISA (CD-ELISA) with phage-displayed peptide, Immuno-4 microtiter wells were coated with mAB 6F5 and blocked as described above for panning-elution selection. Various concentrations of DON (0 to 10,000 ng/ml in 1% methanol–PBS) were mixed with equal volumes of phage-displayed peptide (diluted 1:10 in 10% milk–PBS). The mixtures were added to mAB 6F5-coated microtiter wells (100 μl/well), and the preparations were incubated at 37°C for 1 h. After the wells were washed six times with PBS-T (300 μl/well), the amounts of bound recombinant phage were determined by incubating the preparations with 100 μl of sheep anti-M13 HRP conjugate (diluted 1:5,000 in 10% milk–PBS) per well at 37°C for 1 h. Amounts of bound enzyme were determined as described above. For comparison, 50 μl of DON-HRP per well was also mixed with 50 μl of DON at various concentrations (0 to 10,000 ng/ml in 1% methanol–PBS) per well, and the preparations were incubated in mAB 6F5-coated microtiter wells at 37°C for 1 h. Amounts of bound enzyme were determined as described above.
To perform a competitive indirect ELISA with phage-displayed peptide, 100 μl of phage-displayed peptide was dispensed into each well of disposable Immuno-4 microtiter strips, and the peptide was dried onto the wells in a forced-air oven at 40°C overnight. The plates were washed and blocked as described above for the CD-ELISA. Various concentrations of DON (0 to 10,000 ng/ml in 1% methanol–PBS) (50 μl) were added to the wells, and then 50 μl of anti-DON mAB 6F5 (10 μg/ml in 10% milk–PBS) was added to each well. The wells were incubated at 37°C for 1 h. After the wells were washed six times with PBS-T, the amounts of bound anti-DON mAB 6F5 were determined by incubation with goat anti-mouse IgG–HRP conjugate (diluted 1:2,000 with 10% milk–PBS) at 37°C for 1 h. Amounts of bound enzyme were determined as described above.
DNA sequencing.
After specific binding to mAB 6F5 was confirmed by ELISA, 10 ml of recombinant phage particles (1011 PFU/ml in Luria-Bertani medium) from each positive phage clone was used for single-stranded DNA isolation performed with a QIAprep Spin M13 kit (QIAGEN Inc., Chatsworth, Calif.). The single-stranded DNA was sequenced with −28 gIII sequencing primer and −96 gIII sequencing primer (New England Biolabs, Inc.) by using Taq cycle sequencing and dye terminator chemistry at the Michigan State University DNA Sequencing Facility.
Translational fusion with bacterial AP.
A 179-bp DNA fragment encoding the g3p signal peptide, DONPEP.2, and the first 17 amino acids of M13 phage g3p protein was amplified by PCR performed with Pfu DNA polymerase, a sense primer (5′-GCCAAGCTTAGATCTTGGAGCCTTTTTTTTGGAG-3′), and an antisense primer (5′-CCGGTCGACCTGTATGGGATTTTGCTAAACAACT-3′). After gel purification, the amplified DNA was digested with BglII and SalI and was cloned into BamHI-SalI-digested pLIP5 (5), which generated plasmid pQY7. The ligated product was used to transform E. coli DH11S competent cells (Gibco BRL, Gaithersburg, Md.), which generated DH11S/pQY7. DONPEP-alkaline phosphatase (AP) fusion protein was produced from DH11S/pQY7 in SB medium (35 g of Bacto Tryptone/liter, 20 g of Bacto Yeast Extract/liter, 5 g of NaCl/liter) containing ampicillin (100 μg/ml) and was induced by 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). The periplasmic DONPEP-AP fusion protein was extracted by suspending the bacterial cells (1:5, vol/vol) in lysis buffer (50 mM Tris-HCl [pH 8.0], 20% sucrose, 10 mM EDTA, 0.1 mg of lysozyme per ml, 0.5 mM phenylmethylsulfonyl fluoride); the preparation was left on ice for 1 h with agitation before centrifugation for 30 min at 7,000 × g. The supernatant was filtered through a 0.4-μm-pore-size porous filter. The activity of the DONPEP-AP fusion protein was determined by CD-ELISA.
Briefly, Immuno-4 wells were coated with mAB 6F5 and washed as described above. Various concentrations of DON (0 to 10,000 ng/ml in 0.05 M Tris-buffered saline [TBS] [pH 7.4]) were mixed with equal volumes of DONPEP-AP fusion protein (periplasmic extract diluted 1:7 with 2% nonfat dry milk–TBS). The mixtures (100 μl/well) were added to mAB 6F5-coated microtiter wells and incubated at 37°C for 1 h. After the wells were washed six times with 300 μl of TBS containing 0.1% Tween 20, the amounts of bound DONPEP-AP were determined by incubation with a p-nitrophenyl phosphate substrate solution at 37°C for 45 min. The absorbance at 405 nm was determined.
CD-ELISA performed with peptide C430 and its HRP conjugate.
A DON mimotope peptide with a structurally flexible linker and a cysteine residue (NH2-SWGPFPFGGGSC-COOH) was synthesized via [N-(9-fluorenylmethoxycarbonyl)] Fmoc) chemistry at Bio-Synthesis, Inc. (Lewisville, Tex.); this compound was designated C430. Synthetic peptide C430, at various concentrations, was used to compete with DON-HRP for binding to mAB 6F5 in a CD-ELISA. C430 was also chemically conjugated to HRP with a sulfo-MBS cross-linker. The procedure used for the CD-ELISA performed with the C430-HRP conjugate was the same as the procedure used for the CD-ELISA performed with DON-HRP described above, except that C430-HRP (diluted 1:5,000 in blocking buffer) replaced DON-HRP.
Immunization of animals with C430-BSA conjugate.
Synthetic peptide C430 was conjugated to BSA with a sulfo-MBS cross-linker. The conjugate (molar ratio of peptide C430 to the carrier protein BSA, approximately 29:1) was used to inject 7-week-old BALB/c female mice intraperitoneally or 6-month-old New Zealand White rabbits subcutaneously. The initial injections used for mice contained 100 to 200 μg of C430-BSA conjugate in 200 μl of saline-Freund’s complete adjuvant (1:1); these injections were followed at 3-week intervals by boosters consisting of 100 to 200 μg of conjugate in 200 μl of saline-Freund’s incomplete adjuvant (1:1). The initial inoculum used for rabbits consisted of 1 mg of C430-BSA conjugate in 1 ml of saline-Freund’s complete adjuvant (1:1); this was followed at 4-week intervals by boosters consisting of 250 μg of conjugate in 1 ml of saline-Freund’s incomplete adjuvant (1:1). Animals were bled 1 week after each booster injection. Antisera were screened for specific binding in phage-displayed DONPEP.2-coated wells by a competitive indirect ELISA in which C430 or DON was used as the competitive antigen.
Cytotoxicity and effects on protein synthesis in vitro.
Ten-week-old B6C3F1 mice were euthanized by cervical dislocation, and the femurs were removed. Bone marrow cells were flushed from the femurs by using a 1-ml syringe and a 25-gauge needle. Erythrocytes were lysed with 0.83% ammonium chloride. Cell number and viability were determined by trypan blue dye exclusion by using a hemacytometer. The cells were cultured at a concentration of 106 cells/ml in 96-well flat-bottom plates in RPMI-1640 in a humidified incubator containing 5% CO2. RPMI-1640 was supplemented with 100 U of penicillin per ml, 100 μg of streptomycin per ml, 50 μM 2-mercaptoethanol, 1 mM sodium pyruvate, 1 mM nonessential amino acids, 2 mM glutamine, and 10% fetal bovine serum. DON and synthetic peptide C430 were diluted in RPMI-1640. Duplicate cultures were treated. After 18 h of exposure to DON and synthetic peptide C430, cell viability was determined by using an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] conversion assay as described by Marin et al. (26).
To determine the effects on protein synthesis, DON or synthetic peptide C430 (0 or 3.4 μM in a 50-μl [final volume] reaction mixture) was added to 30 μl of a biotin translation mixture (Boehringer Mannheim Corporation, Indianapolis, Ind.) containing reticulocyte lysate, 10 pmol of biotin-lysine-tRNALys, 42 μM amino acids without lysine, spermidine, energy mixture, dithiothreitol, 83 mM potassium acetate, and 0.83 mM magnesium acetate. The mixtures (46 μl) were incubated on ice for 10 min, and then 4 μl of gamma globulin mRNA (0.5 μg/ml) was added. The final reaction mixture (50 μl) was incubated at 30°C for 1 h. The translated protein samples were stored at −80°C before analysis.
For Western blot analysis, 3 μl of protein that was translated in vitro was boiled for 10 min after it was mixed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis loading buffer. The samples then were loaded into a mini sodium dodecyl sulfate–10% polyacrylamide gel and subjected to electrophoresis at 80 V for 2 h. The newly synthesized proteins were detected by electrotransfer to a polyvinylidene difluoride transfer membrane (DuPont NEN Research Products, Boston, Mass.), followed by incubation with streptavidin-peroxidase conjugate (Boehringer Mannheim Corporation). Bound enzyme was visualized by incubation with SuperSignal ULTRA chemiluminescent substrate (Pierce Chemical Company) and by exposing Kodak XAR5 autoradiography film (Kodak, Rochester, N.Y.).
Homologous sequence search and peptide modeling.
The computer program Sequery (10, 13) was used to search for sequences similar to the DONPEP.2 peptide sequence (SWGPFPF) in a database of nonhomologous protein structures derived from the ≤25% identity set of the Protein Data Bank (PDB) Select list (20). Sequery identified all occurrences of protein sequences in the PDB that matched tetrapeptidyl fragments of DONPEP.2. The following conserved residue substitutions were allowed during the sequence search: S for T, W for F, P for ST, and F for ST. The resulting sequence analogs consisted of tetrapeptides that matched a portion of DONPEP.2 peptide and had known three-dimensional structures available in the Brookhaven PDB (1, 3). The secondary structures of these analogs were subsequently analyzed by using the Superpositional Structural Assignment computer program (13). This program superimposed the analogs onto a set of regular secondary structure templates and assigned each analog to the structural category (e.g., α-helix, β-strand, reverse turn) which it matched most closely within a 1.0-Å main-chain root-mean-square positional deviation (RMSD); if no template matched within a 1.0-Å RMSD, the structure of the analog was considered irregular. The analogs that were found to be irregular were analyzed visually by using molecular graphics, so that slightly irregular structures (e.g., a bent α-helical turn) could be assigned to the most appropriate structural category.
A three-dimensional structural model of DONPEP.2 peptide was created based on the structural analogs by using the molecular modeling software Insight II (Molecular Simulations Inc., San Diego, Calif.) and a Silicon Graphics Indigo2 Extreme computer. The overlapping residues of the sequence analogs were superimposed, and a consensus structure was created, which consisted of the peptide main-chain atoms and proline side chains. Side chains for the nonproline residues were added to the model by using the rotamer library of Insight II. Final side chain positions were selected based on consensus between the analogs and the absence of steric overlap. Interactions within the model were then optimized by using 100 steps of backbone-restrained steepest-descent energy minimization with the cff91 force field of the Discover 3 module of Insight II. The stereochemical quality of the model was validated with Procheck (25).
The structural and chemical similarities between DON and the DONPEP.2 peptide model were analyzed by using the program PowerFit (MicroSimulations Inc., Mahwah, N.J.), which tests a large number of random three-dimensional alignments of two structures by using a Monte Carlo search algorithm. The quality of the superpositions was scored by using a complementarity function measuring van der Waals and electrostatic overlap between the two structures. The three-dimensional structure of nivalenol (Cambridge Structural Database [CSD] code, DUTJOR10), which was obtained from the CSD (4a), was used in place of DON for the superposition, as a three-dimensional structure for DON was not available. Nivalenol binds to mAB 6F5 with slightly less affinity than DON binds to mAB 6F5 (6). The side chain angles of the Ser, Trp, and Phe residues in the peptide were allowed to rotate during PowerFit superposition, and only superpositions with the stereochemically acceptable side chain conformation were accepted. No bonds in the rigid nivalenol structure were allowed to rotate. The results of the alignment were analyzed visually with Insight II.
RESULTS
Panning-elution selection of specific phages.
After each round of panning-elution selection, the number of eluted phage was determined. Enrichment for specific phages with affinity to mAb 6F5 was observed after two rounds of panning-elution selection. The phages with affinity to mAb 6F5 were enriched approximately 6 orders of magnitude after the fourth round of panning-elution. Fifteen individual phage plaques from the fourth-round-selected phages were randomly isolated and used to infect E. coli ER2537 for phage reamplification. Each of the 15 phage isolates was tested to determine its binding to mAb 6F5 by ELISA. Five of these phage isolates, designated DONPEP.1, DONPEP.2, DONPEP.3, DONPEP.4, and DONPEP.12, exhibited binding to mAb 6F5.
Phage single-stranded DNA were isolated, and the nucleotide sequence of each of the five positive phage isolates was determined. Four of the five isolates sequenced had the same DNA sequence (5′-AGTTGGGGTCCTTTTCCGTTT-3′), which encoded the consent peptide sequence SWGPFPF, while isolate DONPEP.12 differed by four nucleotides (5′-TCTTGGGGTCCGCTTCCTTTT-3′), which resulted in a change in the fifth amino acid from Phe to Leu (the different nucleotides are underlined).
Use of DON mimotope peptide in competitive ELISA.
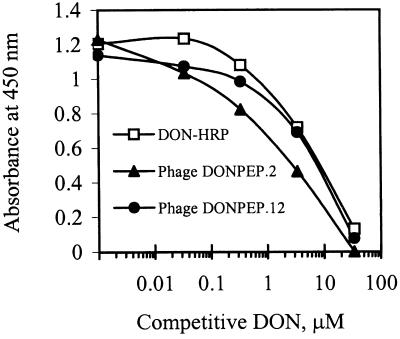
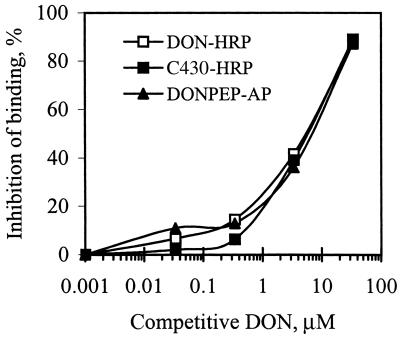
To determine whether the positive recombinant phage peptides actually mimicked the epitope recognized by mAB 6F5 or just bound nonspecifically to the surface of the antibody molecule outside the antigen binding site, two different positive phage clones were tested for binding to mAB 6F5 by performing a CD-ELISA (Fig. 2). Binding of phage clones DONPEP.2 and DONPEP.12 to immobilized mAB 6F5 was competitively inhibited by free DON. This strongly suggested that these two phage clones bound to the antigen binding site of the mAb, mimicking, in part, the structural epitope of DON.
FIG. 2.
Competition between phage-displayed mimotope peptides and DON for binding to immobilized mAB 6F5 in CD-ELISA. Various concentrations of free DON competed with equal volumes of phage-displayed peptides (at a constant concentration) for binding to immobilized mAB 6F5. Bound phage peptide was detected with HRP-conjugated sheep anti-M13 IgG and then measured by absorbance. DON-HRP was included as a positive control.
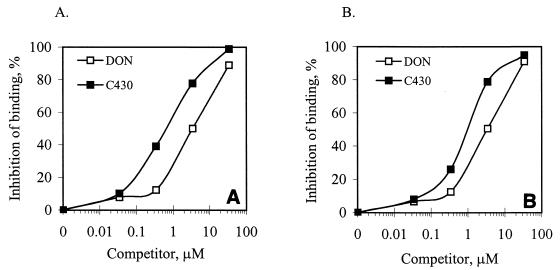
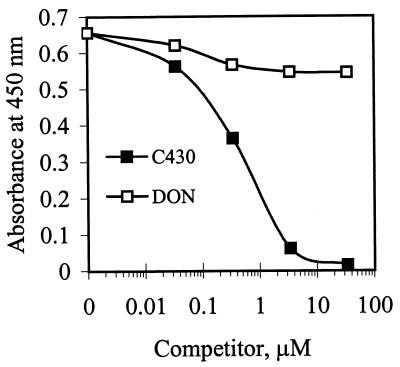
To confirm that the sequence obtained from the phage library bound specifically to mAB 6F5 independent of the phage structural context, synthetic peptide C430 was tested for binding by performing a CD-ELISA. Binding of DON-HRP to immobilized mAB 6F5 was inhibited by free C430 (Fig. 3A), while binding of the C430-HRP conjugate to immobilized mAB 6F5 was inhibited by free DON (Fig. 3B). This indicated that the peptide alone was sufficient for binding to the antibody, independent of the phage structural context. When C430 was used to compete with DON-HRP or C430-HRP for binding to mAB 6F5, the 50% inhibitory concentrations were 0.64 to 0.8 μM, whereas 3.4 μM free DON was required to obtain 50% inhibition of DON-HRP or C430-HRP binding to the same antibody. This indicated that mAb 6F5 had a higher affinity for C430 than for DON. In a similar CD-ELISA, none of the individual amino acids in C430 (at concentrations up to 34 μM) significantly inhibited binding to DON-HRP. This suggested that the sequence in C430 was important for specific binding to mAB 6F5, since individual amino acids did not bind to mAB 6F5 specifically.
FIG. 3.
Competition between synthetic peptide C430 and DON for binding to mAB 6F5. (A) DON and synthetic peptide C430 competing with DON-HRP for binding to immobilized mAB 6F5. (B) DON and synthetic peptide C430 competing with C430-HRP for binding to immobilized anti-DON mAB 6F5.
DONPEP-AP fusion protein collected from the culture supernatant or periplasmic extract exhibited AP activity, and its specific binding to mAB 6F5 was similar to that of DON-HRP (Fig. 4). This suggested that the DON mimotope peptide sequence was structurally stable in a different protein structural context.
FIG. 4.
CD-ELISA performed with DONPEP-AP fusion protein. Binding of the DONPEP-AP fusion protein to immobilized mAB 6F5 was inhibited by free DON. Competition of free DON with DON-HRP was used as a control.
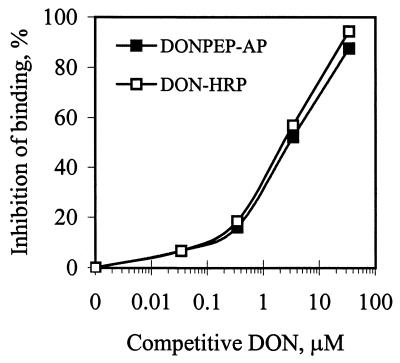
CD-ELISA for wheat extract spiked with DON.
To test the feasibility of using DON mimotope peptide sequences as immunochemical reagents for DON immunoassays in food and feed, a CD-ELISA was performed with wheat extracts spiked with DON. Both C430-HRP conjugate and DONPEP-AP fusion protein exhibited binding to immobilized mAB 6F5 in the wheat extract (Fig. 5). All three conjugates produced similar linear inhibition curves at DON concentrations ranging from 0.1 to 10 μg/ml in wheat extract. However, a slightly lower level of absorbance was observed in the CD-ELISA performed with C430-HRP and DONPEP-AP in wheat extract than in PBS buffer, indicating that the wheat extract interfered with binding of the mimotope peptide to anti-DON mAB 6F5 to some degree.
FIG. 5.
Use of C430-HRP and DONPEP-AP in a DON immunoassay (CD-ELISA) performed with wheat extract spiked with DON. Immulon-4 microtiter wells were coated with mAB 6F5 antibody, and DON-HRP was used as a positive control.
Immunization with C430-BSA conjugate.
If the selected peptides represent a true image of the DON surface structure, they might elicit an immunoresponse similar to that of the original DON epitope and, therefore, serve as alternative antigens for DON. When rabbits and mice were immunized with C430-BSA conjugate, both types of animals produced antibody specific to the DON mimotope peptide sequence after the second injection of C430-BSA. After four injections, the antiserum exhibited a strong antibody response against the DON mimotope peptide sequence (Fig. 6). A synthetic peptide C430 concentration of 0.39 μM resulted in 50% inhibition of antiserum binding to the immobilized phage-displayed peptide. However, binding of antisera to immobilized phage-displayed peptide was not inhibited by free DON in solution, indicating that antibodies in the immunized animals were not specific for DON.
FIG. 6.
Specificity of antibody from C430-BSA-immunized mice. DON and C430 at various concentrations were used to inhibit the binding of antisera to immobilized DONPEP.2 phage. Bound mouse antibodies were detected with HRP-conjugated goat anti-mouse IgG, and the amounts of these antibodies were measured by absorbance.
Cytotoxicity and effects on protein synthesis in vitro.
One of the effects of DON is to cause cytotoxicity through cell apoptosis (34). To determine if the DON mimotope peptide was cytotoxic, synthetic peptide C430 (0, 0.34, 3.4, and 34 μM) was compared with DON (0 and 3.4 μM) in order to determine the cytotoxic effects of these compounds to bone marrow cells. As expected, DON at a concentration of 3.4 μM caused 40 to 60% cell death after 18 h of incubation. Synthetic peptide C430, however, did not have any adverse effect on the viability of bone marrow cells in culture at C430 concentrations up to 34 μM. When combined with DON, C430 at any of the concentrations tested did not significantly increase or decrease the cell viability resulting from the presence of DON, suggesting that there was no significant synergism or antagonism between the DON mimotope peptide and DON with respect to bone marrow cell death.
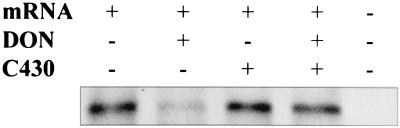
In a cell-free translation system, DON at a concentration 3.4 μM significantly inhibited new protein synthesis, while C430 caused no inhibition (Fig. 7). However, protein synthesis was not inhibited by 3.4 μM DON when it was mixed with 3.4 μM C430. This suggested that C430 was antagonistic to the inhibitory effects of DON on in vitro protein synthesis.
FIG. 7.
Effects of DON (3.4 μM) and synthetic peptide C430 (3.4 μM) on protein synthesis in vitro with rabbit reticulocyte lysate. The translation template was gamma globulin mRNA. The plus and minus signs indicate whether reagents were added.
Structural model of the DON mimotope peptide and comparison with the structure of DON.
Within the set of nonhomologous protein structures, 31 analogs of DONPEP.2 tetrapeptides were found. The sequence search was limited to four residues, because close sequence matches to more than four residues are rarely found in the Brookhaven PDB. The secondary structures of these tetrapeptides, which were assigned based on close backbone superposition with ideal secondary structures (α-helices, β-strands, reverse turns), were predominately in the β-strand conformation (Table 1).
TABLE 1.
Secondary structures characteristics of DONPEP.2 sequence analogsa
| Analog | Conformation | Backbone RMSD (Å) | Peptide | PDB code | Chain | Residue range |
|---|---|---|---|---|---|---|
| SWGP | DON mimotope peptide | 1–4 | ||||
| SWGS | Strand | 0.580 | Penicillin amidohydrolase | 1pnk | B | 64–67 |
| SWGT | Strand | 1.264 | Sulfhydryl proteinase | 9pap | 176–179 | |
| SWGT | Strand | 0.946 | Xylanase | 1xnb | 84–87 | |
| TWGP | Strand | 0.794 | Bluetongue virus coat protein | 1bvp | 1 | 118–121 |
| SFGT | Strand | 0.734 | Creatinase | 1chm | A | 325–328 |
| SFGT | Strand | 0.453 | Phosphotransferase | 3pmg | A | 377–380 |
| WGPF | DON mimotope peptide | 2–5 | ||||
| FGSF | Turn | 0.897 | Fe(III) superoxide dismutase | 1isc | A | 100–103 |
| FGSY | Strand | 0.465 | Beta-lactamase | 2blt | A | 322–325 |
| FGSY | Strand | 0.730 | Flavodoxin | 4fxn | 85–88 | |
| FGTY | Strand | 0.530 | Simian virus 40 coat protein | 1sva | 1 | 221–224 |
| WGTY | Turn | 0.412 | Xylanase | 1xnb | 85–88 | |
| FGPW | Strand | 0.956 | Vitelline membrane protein 1 | 1vmo | A | 126–129 |
| WGTW | Turn | 0.820 | Glycosyl transferase | 1bpl | B | 215–218 |
| WGTW | Strand | 0.660 | Vitelline membrane protein 1 | 1vmo | A | 70–73 |
| GPFP | DON mimotope peptide | 3–6 | ||||
| GPFP | Strand | 1.080 | Adenylosuccinate synthetase | 1ade | A | 276–279 |
| GPFP | Turn | 0.821 | N-Cadherin | 1nch | A | 15–18 |
| GPFP | Turn | 0.518 | Photosynthetic reaction center | 1pcr | H | 54–57 |
| GPFT | Turn | 0.966 | Aconitase | 8acn | 324–327 | |
| GPFT | Turn | 0.702 | Monellin | 1mol | A | 9–12 |
| GPYP | Strand | 0.909 | Dioxygenase | 2pcd | M | 445–448 |
| GTFP | Strand | 1.110 | Black beetle virus coat protein | 2bbv | C | 131–134 |
| GTFP | Turn | 0.732 | Glycosyl transferase | 1xyz | A | 806–809 |
| PFPF | DON mimotope peptide | 4–7 | ||||
| PFSF | Strand | 1.119 | N-Acetylneuraminate lyase | 1nal | 1 | 112–115 |
| PFSY | Turn | 0.888 | Acid phosphatase | 1kbp | A | 218–221 |
| PFTY | Strand | 1.127 | Dialkyglycine decarboxylase | 2dkb | 170–173 | |
| PYSY | Turn | 0.528 | Transthyretin | 1ttb | A | 113–116 |
| PYTF | Strand | 0.599 | Dethiobiotin synthase | 1dts | 73–76 | |
| PYTF | Strand | 0.498 | Zinc endopeptidase | 1iae | 16–19 | |
| PYTF | Strand | 0.554 | Acid phosphatase | 1kbp | A | 127–130 |
| TYPY | Turn | 0.952 | Adenylosuccinate synthetase | 1ade | A | 234–237 |
| TYPY | Strand | 1.086 | Sulfhydryl proteinase | 9pap | 85–88 |
The backbone superpositional RMSD values were based on a least-squares fit of analogs of the DON mimetic peptide, SWGPFPF, with regular secondary structure templates. Each analog was assigned the conformation of the template with which it had the lowest backbone superpositional RMSD. The analogs for SWGP start in strand conformation and then show a preference for forming a turn starting at the glycine residue. The final sections of the peptide analogs favor strand conformations.
To construct a three-dimensional structural model of SWGPFPF from the tetramer analogs in Table 1, we began by superimposing the analogs for residues 1 to 4 (SWGP), which all formed a β-strand. The superpositions for residues 2 to 5 (WGPF) and 3 to 6 (GPFP) indicated that the central region of the peptide could form either a β-strand or a reverse turn. However, the pair of Pro residues in the sequence resulted in a significant kink even in the β-strand-like analogs (all of which had a superpositional RMSD of ≥0.9 Å in the GPFP region). Similarly, in the turn-forming analogs, the two Pro residues made it impossible to form an ideal reverse turn. Thus, it seems likely that the central region forms a loose, inverted U-shaped turn flanked by residues in the β-strand conformation, which is prevalent in the analogs for residues 4 to 7 (Table 1). The conformation of the turn shown in Fig. 1C is based on superposition of the turn-forming analogs for residues 2 to 5.
The similarity between DON and the SWGPFPF model was determined by using PowerFit to evaluate favorable three-dimensional superpositions of nivalenol (Fig. 1B and D), a close analog of DON (Fig. 1A), onto the peptide model. Ten independent superpositions, beginning with random conformations, were performed by using the PowerFit program. Three of the ten structures with low superpositional energies showed a preference for the DON analog to align in a specific position along the peptide backbone of the model in the region spanning from the second-residue (Trp) main-chain nitrogen to the fifth-residue (Phe) carbonyl carbon (Fig. 1E). The remaining superpositions showed a preference for nivalenol to align along the peptide backbone as well, although it aligned with shorter sections. Also, side chain atoms of the second residue (Trp) in SWGPFPF overlapped with atoms of nivalenol in several of the superposition results.
DISCUSSION
In a search for DON mimotope peptides, we screened a phage-displayed random heptapeptide library and identified two closely related peptide sequences, DONPEP.2 (SWGPFPF) and DONPEP.12 (SWGPLPF), that specifically bind with high affinity to anti-DON mAb 6F5. To our knowledge, this is the first time that mimotope peptides for a nonproteinaceous, low-molecular-weight mycotoxin have been found.
In a competitive ELISA, DON could compete with selected peptides for binding to mAb 6F5, while the synthetic peptide also competed with DON for binding to the same antibody, which strongly suggested that these clones bind to the same antigen binding site of the mAb. The fact that these peptides can be used in ELISA has several implications. From a safety standpoint, it is advantageous to use a nontoxic peptide as an immunochemical reagent which replaces the toxic compound DON in an immunoassay. Second, conjugation of a peptide to peroxidase or other proteins is much easier than conjugation of DON to the same enzyme. Finally, if the peptide is genetically fused with a reporter enzyme, such as AP, chemical conjugation can be avoided. In practice, commercial ELISA for DON detect a lower range of concentrations (0.5 to 1 μg/ml; 1.7 to 3.4 μM).
Although many mimotope peptides for protein antigens have been isolated from phage-displayed peptide libraries, there have been only a few cases in which peptides have been selected as mimics of nonproteinaceous chemicals (21, 38). It is interesting to compare the peptide sequences selected with mAb 6F5 to peptide sequences that are known to interact with carbohydrate binding sites in order to identify whether there are general features common to the sequences. Our sequences were similar to the sequences of the carbohydrate mimics in that there was a clear preference for aromatic groups and proline residues (21, 38). This is not unreasonable since these amino acids resemble sugar moieties in size and cyclic shape. Furthermore, the proline residues may serve an important structural role by orienting the aromatic side chains spatially in a manner similar to the orientation of the branched carbohydrates which they mimic.
Although the peptide may have alternative conformations, the prevalent structure in the peptide analogs is an inverted-U-shaped turn. The absence of any superpositions in which nivalenol aligned predominately with one of the aromatic side chains suggests that the side chains of the peptide may not be the major epitopes recognized by mAb 6F5. Although in several of the superposition results Trp side chain atoms of SWGPFPF corresponded to nivalenol atoms, the peptide backbone, especially in the region of the first proline residue, forms the most DON-like structure in the peptide.
If a small peptide completely mimics the epitope of a certain antigen, when the peptide is conjugated on a suitable carrier protein or displayed on a phage, it can elicit antibodies directed against the native antigen whose epitope the peptide represents (2, 27, 30). Although our selected peptides and DON competed with each other for binding to mAB 6F5 and C430 can antagonize the inhibitory effects of DON in an in vitro protein synthesis system, no specific antibodies for DON were produced from C430-BSA conjugate-immunized animals when high-affinity antibodies to the peptide were detected in these animals. It is not clear whether the selected peptides mimic the surface structure of DON in some respects or bind to the cleft by an entirely different mechanism. The latter possibility was described in the case of streptavidin-binding peptides discovered with a phage-displayed library (43), in which the peptides associated with the binding site in a fashion that was quite different than the fashion used by the natural ligand, biotin. Another possibility is that there may be more than one epitope in the peptide and that the moiety which mimics DON has poor immunogenicity. This proposed mechanism is supported by the observation that a specific antibody for DON was rarely detected in large numbers of animals immunized with DON-carrier protein conjugates (6).
The toxic effects of trichothecenes are presumably related to the inhibition of protein synthesis by these compounds. The inhibitory activities of trichothecenes are due to their ability to bind to the 60S subunits of eukaryotic ribosomes and to interfere with peptidyltransferase activity (4, 37, 42). Presumably, peptides are agonists only if they undergo the same specific contacts and interactions with the ribosomes as DON. The mimotope peptides selected from the phage-displayed library in this study, however, exhibited antagonism to DON-induced protein synthesis inhibition. This suggested that the synthetic peptide might bind to the same site on a ribosome as DON but in an orientation that does not mimic the function of DON. It is notable that C430 did not potentiate or attenuate DON in causing cell death in a cell culture model. This may have been due to the fact that it was difficult for the peptide to cross the cell membrane (17). DON, which has a four-fold-lower molecule weight and a specific structure, may be more readily absorbed into cells (44).
In addition to the toxic effects of trichothecenes on animals and humans, there is a growing body of evidence which indicates that these compounds, including DON, can enhance the virulence of plant-pathogenic Fusarium species on plant hosts (36, 46). The trichothecene-producing Fusarium fungi cause a broad range of plant diseases, including head and seedling blight of small grains, such as wheat and rye, ear and stalk rot of maize, stem rot of carnation, and seedling blight and root rot of a number of other plant species, including beans, clover, and tomato (11). If the fungal virulence associated with DON is mediated through protein synthesis inhibition and the mimotope peptides described here are antagonistic to DON activity in plants, transgenic plants expressing the peptides (possibly fused to another protein) might exhibit reduced levels of wheat head scab and other plant diseases caused by the DON-producing pathogenic Fusarium species.
In summary, we identified two closely related mimotope peptides for DON and demonstrated the potential for using these peptides in DON immunoassays. We also demonstrated that the mimotopes are antagonistic to DON-induced protein synthesis inhibition. The structure of DON is mimicked only indirectly by the DON mimotope peptides, and further understanding of the mode of binding of these peptides to the same antibody or ribosomes should help elucidate the mechanisms of DON toxicity and may lead to prevention of plant and animal diseases associated with this mycotoxin.
ACKNOWLEDGMENTS
This work was supported by USDA-NRI grant 9702545, by Public Health Service grant E5-03358, by the Michigan State University Agricultural Experiment Station, by the MSU Crop and Food Bioprocessing Center, and by the MSU National Food Safety and Toxicology Center.
We are grateful to Frederic Ducancel (Protein Engineering and Research Department, C.E.A. Saclay, Gif-sur-Yvette, France) for a generous gift of plasmid pLIP5. We also thank Frank Klein (Neogen Company) for help in making several conjugates. We also acknowledge Rebecca Uzarski for help with the cytotoxicity assay.
REFERENCES
- 1.Abola E E, Sussman J L, Prilusky J, Manning N O. Protein data bank: archives of three-dimensional macromolecular structures. Methods Enzymol. 1997;277:556–571. doi: 10.1016/s0076-6879(97)77031-9. [DOI] [PubMed] [Google Scholar]
- 2.Arnon R. Synthetic peptides as the basis for vaccine design. Mol Immunol. 1991;28:209–215. doi: 10.1016/0161-5890(91)90063-p. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein F C, Koetzel T F, Williams J B, Meyer E F, Jr, Brice M D, Rodger J R, Kennard O, Shimanouchi T, Tasumi M. The protein data bank: a computer-based archival file for macromolecule structures. J Mol Biol. 1977;112:535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- 4.Betina V. Structure-activity relationships among mycotoxins. Chem Biol Interact. 1989;71:105–146. doi: 10.1016/0009-2797(89)90030-6. [DOI] [PubMed] [Google Scholar]
- 4a.Cambridge Crystallographic Data Centre. 12 February 1991. [12 February 1991, posting date. [Online.] Cambridge Structural Database. http://www.ccdc.cam.ac.uk. [March 1998, last date accessed.]
- 5.Carrier A, Ducancel F, Settiawan N B, Cattolico L, Maillere B, Leonetti M, Drevet P, Menez A, Boulain J C. Recombinant antibody-alkaline phosphatase conjugates for diagnosis of human IgGs: application to anti-HBsAg detection. J Immunol Methods. 1995;181:177–186. doi: 10.1016/0022-1759(94)00344-v. [DOI] [PubMed] [Google Scholar]
- 6.Casale W L, Pestka J J, Hart L P. Enzyme-linked immunosorbent assay employing monoclonal antibody specific for deoxynivalenol (vomitoxin) and several analogues. J Agric Food Chem. 1988;36:663–668. [Google Scholar]
- 7.Chanh T C, Huot R I, Schick M R, Hewetson J F. Anti-idiotypic antibodies against a monoclonal antibody specific for the trichothecene mycotoxin T-2. Toxicol Appl Pharmacol. 1989;100:201–207. doi: 10.1016/0041-008x(89)90306-2. [DOI] [PubMed] [Google Scholar]
- 8.Chanh T C, Rappocciolo G, Hewetson J F. Monoclonal anti-idiotype induces protection against the cytotoxicity of the trichothecene mycotoxin T-2. J Immunol. 1990;144:4721–4728. [PubMed] [Google Scholar]
- 9.Chu F S, Huang X, Maragos C M. Production and characterization of anti-idiotype and anti-anti-idiotype antibodies against fumonisin B-1. J Agric Food Chem. 1995;43:261–267. [Google Scholar]
- 10.Collawn J F, Kuhn L A, Liu L-F S, Tainer J A, Trowbridge I S. Transplanted LDL and mannose-6-phosphate receptor internalization signals promote high-efficiency endocytosis of the transferrin receptor. EMBO J. 1991;10:3247–3252. doi: 10.1002/j.1460-2075.1991.tb04888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook R J. Fusarium diseases of wheat and other small grains in North America. In: Nelson P E, Toussoun T A, Cook R J, editors. Fusarium: diseases, biology, and taxonomy. University Park: The Pennsylvania State University Press; 1981. pp. 39–52. [Google Scholar]
- 12.Cortese R, Monaci P, Nicosia A, Luzzago A, Felici F, Galfre G, Pessi A, Tramontano A, Sollazzo M. Identification of biologically active peptides using random libraries displayed on phage. Curr Opin Biotechnol. 1995;6:73–80. doi: 10.1016/0958-1669(95)80012-3. [DOI] [PubMed] [Google Scholar]
- 13.Craig L, Sanschagrin P C, Rozak A, Lackie S, Kuhn L A, Scott J K. The role of structure in antibody cross-reactivity between peptides and folded proteins. J Mol Biol. 1998;281:183–201. doi: 10.1006/jmbi.1998.1907. [DOI] [PubMed] [Google Scholar]
- 14.Cwirla S E, Peters E A, Barrett R W, Dower W J. Peptides on phage: a vast library of peptides for identifying ligands. Proc Natl Acad Sci USA. 1990;87:6378–6382. doi: 10.1073/pnas.87.16.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devlin J J, Panganiban L C, Devlin P E. Random peptide libraries: a source of specific protein binding molecules. Science. 1990;249:404–406. doi: 10.1126/science.2143033. [DOI] [PubMed] [Google Scholar]
- 16.Doorbar J, Winter G. Isolation of a peptide antagonist to the thrombin receptor using phage display. J Mol Biol. 1994;244:361–369. doi: 10.1006/jmbi.1994.1736. [DOI] [PubMed] [Google Scholar]
- 17.Firth K L, Brownel H L, Raptis L. Improved procedure for electroporation of peptides into adherent cells in situ. BioTechniques. 1997;23:644–646. doi: 10.2144/97234bm21. [DOI] [PubMed] [Google Scholar]
- 18.Hart L P, Braselton W E. Distribution of vomitoxin in dry milled fractions of wheat infected with Gibberella zeae. J Agric Food Chem. 1983;31:657–659. doi: 10.1021/jf00117a046. [DOI] [PubMed] [Google Scholar]
- 19.Hart L P, Braselton W E, Stebbins T C. Production of zearalenone and deoxynivalenol in commercial sweet corn. Plant Dis. 1982;66:1133–1135. [Google Scholar]
- 20.Hobohm U, Scharf M, Schneider R, Sander C. Selection of representative protein data sets. Protein Sci. 1992;1:409–417. doi: 10.1002/pro.5560010313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoess R, Brinkmann U, Handel T, Pastan I. Identification of a peptide which binds to the carbohydrate-specific monoclonal antibody B3. Gene. 1993;128:43–49. doi: 10.1016/0378-1119(93)90151-r. [DOI] [PubMed] [Google Scholar]
- 22.Hong S S, Boulanger P. Protein ligands of the human adenovirus type 2 outer capsid identified by biopanning of a phage-displayed peptide library on separate domains of wild-type and mutant penton capsomers. EMBO J. 1995;14:4714–4727. doi: 10.1002/j.1460-2075.1995.tb00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu K H, Chu F S. Production and characterization of anti-idiotype and anti-anti-idiotype antibodies from a monoclonal antibody against aflatoxin. J Agric Food Chem. 1994;42:2353–2359. [Google Scholar]
- 24.Katz B A. Structural and mechanistic determinants of affinity and specificity of ligands discovered or engineered by phage display. Annu Rev Biophys Biomol Struct. 1997;26:27–45. doi: 10.1146/annurev.biophys.26.1.27. [DOI] [PubMed] [Google Scholar]
- 25.Laskowski R A, MacArthur M W, Moss D S, Thornton J M. Procheck: a program to check stereochemical quality of protein structures. J Appl Crystallogr. 1993;26:283–291. [Google Scholar]
- 26.Marin M L, Wong S-S, Pestka J J. Increased IL-1, IL-6 and TNFα secretion and mRNA levels in WEHI-3 cells exposed to cyclopiazonic acid. Toxicology. 1996;114:67–79. doi: 10.1016/s0300-483x(96)03416-6. [DOI] [PubMed] [Google Scholar]
- 27.Minenkova O O, Ilyichev A A, Kishchenko G P, Petrenko V A. Design of specific immunogens using filamentous phage as the carrier. Gene. 1993;128:85–88. doi: 10.1016/0378-1119(93)90157-x. [DOI] [PubMed] [Google Scholar]
- 28.Mirocha C J, Pathre S V, Christensen C M. Chemistry of Fusarium and Stachybotrys mycotoxins. In: Wyllie T D, Morehouse L G, editors. Mycotoxic fungi, mycotoxins, mycotoxicoses: an encylopedic handbook. Mycotoxic fungi and chemistry of mycotoxins. New York, N.Y: Marcel Dekker; 1977. pp. 346–349. [Google Scholar]
- 29.Mirocha C J, Pathre S V, Schauerhamer B, Christensen C M. Natural occurrence of Fusarium toxins in feedstuff. Appl Environ Microbiol. 1976;32:553–556. doi: 10.1128/aem.32.4.553-556.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motti C, Nuzzo M, Meola A, Galfre G, Felici F, Cortese R, Nicosia A, Monaci P. Recognition by human sera and immunogenicity of HBsAg mimotopes selected from an M13 phage display library. Gene. 1994;146:191–198. doi: 10.1016/0378-1119(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 31.Neish G A, Cohen H. Vomitoxin and zearalenoen production by Fusarium graminearum from winter wheat and barley in Ontario. Can J Plant Sci. 1981;61:811–815. [Google Scholar]
- 32.O’Neil K T, Hoess R H, Jackson S A, Ramachandran N S, Mousa S A, DeGrado W F. Identification of novel peptide antagonists for GPIIb/IIIa from a conformationally constrained phage peptide library. Proteins. 1992;14:509–515. doi: 10.1002/prot.340140411. [DOI] [PubMed] [Google Scholar]
- 33.Pestka J J, Abouzied M N, Sutikno Immunological assays for mycotoxin detection. Food Technol. 1995;49:120–128. [Google Scholar]
- 34.Pestka J J, Yan D, King L E. Flow cytometric analysis of the effects of in vitro exposure to vomitoxin (deoxynivalenol) on apoptosis in murine T, B and IgA+ cells. Food Chem Toxicol. 1994;32:1125–1136. doi: 10.1016/0278-6915(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 35.Pierce H H, Adey N, Kay B K. Identification of cyclized calmodulin antagonists from a phage display random peptide library. Mol Divers. 1996;1:259–265. doi: 10.1007/BF01715530. [DOI] [PubMed] [Google Scholar]
- 36.Proctor R H, Hohn T M, McCormick S P. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol Plant Microbe Interact. 1995;8:593–601. doi: 10.1094/mpmi-8-0593. [DOI] [PubMed] [Google Scholar]
- 37.Rotter B A, Prelusky D B, Pestka J J. Toxicology of deoxynivalenol (vomitoxin) J Toxicol Environ Health. 1996;48:101–134. doi: 10.1080/009841096161447. [DOI] [PubMed] [Google Scholar]
- 38.Scott J K, Loganathan D, Easley R B, Gong X, Goldstein I J. A family of concanavalin A-binding peptides from a hexapeptide epitope library. Proc Natl Acad Sci USA. 1992;89:5398–5402. doi: 10.1073/pnas.89.12.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott J K, Smith G P. Searching for peptide ligands with an epitope library. Science. 1990;249:386–390. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]
- 40.Smith G P. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315–1317. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 41.Smith M M, Shi L, Navre M. Rapid identification of highly active and selective substrates for stromelysin and matrilysin using bacteriophage peptide display libraries. J Biol Chem. 1995;270:6440–6449. doi: 10.1074/jbc.270.12.6440. [DOI] [PubMed] [Google Scholar]
- 42.Thompson W L, Wannemacher J. Structure-function relationships of 12,13-epoxytrichothecene mycotoxins in cell culture: comparison to whole animal lethality. Toxicon. 1986;24:985–994. doi: 10.1016/0041-0101(86)90004-8. [DOI] [PubMed] [Google Scholar]
- 43.Weber P C, Pantoliano M W, Thompson L D. Crystal structure and ligand-binding studies of a screened peptide complexed with streptavidin. Biochemistry. 1992;31:9350–9354. doi: 10.1021/bi00154a004. [DOI] [PubMed] [Google Scholar]
- 44.Witt M F, Pestka J J. Uptake of the naturally occurring 3-alpha-hydroxy isomer of T-2 toxin by a murine B cell hybridoma. Food Chem Toxicol. 1990;28:21–28. doi: 10.1016/0278-6915(90)90131-6. [DOI] [PubMed] [Google Scholar]
- 45.Xiao H, Clarke J R, Marquardt R R, Frohlich A A. Improved methods for conjugating selected mycotoxins to carrier proteins and dextran for immunoassays. J Agric Food Chem. 1995;43:2092–2097. [Google Scholar]
- 46.Yuan Q, Clarke J R, Zhou H R, Linz J E, Pestka J J, Hart L P. Molecular cloning, expression, and characterization of a functional single-chain Fv antibody to the mycotoxin zearalenone. Appl Environ Microbiol. 1997;63:263–269. doi: 10.1128/aem.63.1.263-269.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]