Abstract
The “altered Schaedler flora” (ASF) was developed for colonizing germfree rodents with a standardized microbiota. The purpose of this study was to identify each of the eight ASF strains by 16S rRNA sequence analysis. Three strains were previously identified as Lactobacillus acidophilus (strain ASF 360), Lactobacillus salivarius (strain ASF 361), and Bacteroides distasonis (strain ASF 519) based on phenotypic criteria. 16S rRNA analysis indicated that each of the strains differed from its presumptive identity. The 16S rRNA sequence of strain ASF 361 is essentially identical to the 16S rRNA sequences of the type strains of Lactobacillus murinis and Lactobacillus animalis (both isolated from mice), and all of these strains probably belong to a single species. Strain ASF 360 is a novel lactobacillus that clusters with L. acidophilus and Lactobacillus lactis. Strain ASF 519 falls into an unnamed genus containing [Bacteroides] distasonis, [Bacteroides] merdae, [Bacteroides] forsythus, and CDC group DF-3. This unnamed genus is in the Cytophaga-Flavobacterium-Bacteroides phylum and is most closely related to the genus Porphyromonas. The spiral-shaped strain, strain ASF 457, is in the Flexistipes phylum and exhibits sequence identity with rodent isolates of Robertson. The remaining four ASF strains, which are extremely oxygen-sensitive fusiform bacteria, group phylogenetically with the low-G+C-content gram-positive bacteria (Firmicutes, Bacillus-Clostridium group). ASF 356, ASF 492, and ASF 502 fall into Clostridium cluster XIV of Collins et al. Morphologically, ASF 492 resembles members of this cluster, Roseburia cecicola, and Eubacterium plexicaudatum. The 16S rRNA sequence of ASF 492 is identical to that of E. plexicaudatum. Since the type strain and other viable original isolates of E. plexicaudatum have been lost, strain ASF 492 is a candidate for a neotype strain. Strain ASF 500 branches deeply in the low-G+C-content gram-positive phylogenetic tree but is not closely related to any organisms whose 16S rRNA sequences are currently in the GenBank database. The 16S rRNA sequence information determined in the present study should allow rapid identification of ASF strains and should permit detailed analysis of the interactions of ASF organisms during development of intestinal disease in mice that are coinfected with a variety of pathogenic microorganisms.
The gastrointestinal tracts of mammals, including mice and rats, contain a diverse microecosystem. The ceca of normal mice contain numerous species, and the concentration of bacteria can be as great as 1011 bacteria/g of feces (41, 42). These microorganisms not only provide essential nutrients (e.g., vitamin K) for their hosts but also colonize mucosal niches, which in part helps protect the hosts against microbial pathogens (22, 25, 42, 49, 53, 55). For example, numerous studies have demonstrated the increased susceptibility of germfree mice to a variety of infectious agents compared to that of mice with the normal complement of microorganisms (17).
Gnotobiotic animals colonized with known microbiota have been used to great advantage as models for biomedical research (17). For certain studies, it is particularly desirable to colonize germfree mice with a defined microbiota.
In the mid-1960s, Russell W. Schaedler was the first researcher to colonize germfree mice with selected bacteria isolated from normal mice (40). He subsequently supplied animal breeders with this group of microorganisms (2) for use in colonizing their rodent colonies. These defined bacteria included aerobic bacteria that were easy to grow and some less-oxygen-sensitive anaerobic organisms. The so-called extremely oxygen-sensitive (EOS) fusiform bacteria, which make up the vast majority of the normal microbiota of rodents, were not included due to technical difficulties in isolating and cultivating EOS bacteria (26, 27). Of the defined microbiotas later used for gnotobiotic studies, the one known as the “Schaedler flora” was the most popular. This flora contained eight bacteria, which were designated Escherichia coli var. mutabilis, Streptococcus faecalis, Lactobacillus acidophilus, Lactobacillus salivarius, group N Streptococcus, Bacteroides distasonis, a Clostridium sp., and an EOS fusiform bacterium.
In 1978, the National Cancer Institute (NCI) decided to revise the Schaedler flora or “cocktail” consisting of eight bacteria in order to standardize the microbiota used to colonize axenic (germfree) rodents, including mice, at all NCI contractors, as well as mice used at NCI. Roger Orcutt, therefore, developed the new defined microbiota now known as the “altered Schaedler flora” (ASF), which consisted of four members of the original Schaedler flora (the two lactobacilli, B. distasonis, and the EOS fusiform bacterium), a spiral-shaped bacterium, and three new fusiform EOS bacteria (30).
Although very important, it is very difficult to monitor a gnotobiotic mouse colony with a defined microbiota. Not only is it necessary to demonstrate that the colony is free of any adventitious microorganisms, but it must also be demonstrated that the microorganisms of the specified microbiota are present. In the past, workers monitoring gnotobiotic animals relied on examining the morphology of the microorganisms and performing a limited evaluation of the biochemical traits and growth characteristics of the organisms.
The goals of this study were, therefore, to identify the bacteria in the ASF by 16S rRNA sequence analysis and to characterize the phylogenetic positions of these organisms relative to those of known bacteria. The long-term goal of our studies is to develop sensitive and specific molecular techniques for monitoring the microbiotas of gnotobiotic animals.
MATERIALS AND METHODS
Bacterial strains and cultivation.
ASF bacteria, including four EOS fusiform anaerobes (Taconic stock culture strains ASF 356, ASF 492, ASF 500, and ASF 502), a spiral-shaped bacterium (Taconic strain ASF 457), two previously identified lactobacilli (Taconic strains ASF 360 and ASF 361), and a Bacteroides sp. (Taconic strain 519), were obtained from Taconic, Germantown, N.Y. The bacteria were cultured anaerobically on Schaedler agar (Difco Laboratories, Detroit, Mich.) supplemented with 5% sterile fetal calf serum (Summit Biotechnology, Ft. Collins, Colo.) in an anaerobic glove chamber containing a 10% CO2–10% H2–80% N2 atmosphere (Coy Laboratory, Grass Lakes, Mich.). Anaerobiosis was monitored with a resazurin indicator. The media were prereduced by placing them inside the chamber 2 days prior to inoculation of bacteria. The temperature in the chamber was maintained at 33 to 35°C.
Extraction of DNA for sequence determination.
Bacteria were harvested, washed twice with 1 ml of sterile phosphate-buffered saline, and then collected by centrifugation at 8,000 × g. The pellets were used for extraction of DNA templates that were used to amplify 16S rRNA by PCR. DNA was extracted from the cell pellets by using a commercial kit (High Pure PCR template preparation kit; Boehringer Mannheim) according to the manufacturer’s instructions.
Amplification of 16S rRNA cistrons by PCR and purification of PCR products.
The 16S rRNA cistrons were amplified with bacterial universal primers F24 and F25 (Table 1). PCR was performed in thin-walled tubes with a Perkin-Elmer model 9700 thermocycler. One microliter of the DNA template was added to a reaction mixture (final volume, 50 μl) containing 20 pmol of each primer, 40 nmol of deoxynucleoside triphosphates, and 1 U of Taq 2000 polymerase (Stratagene, La Jolla, Calif.) in buffer containing Taqstart antibody (Sigma Chemical Co.). In a hot-start protocol, samples were preheated at 95°C for 8 min, and this was followed by amplification in which the following conditions were used: denaturation at 95°C for 45 s, annealing at 60°C for 45 s, and elongation for 1.5 min with an additional 5 s for each cycle. A total of 30 cycles were performed, and then a final elongation step consisting of 72°C for 10 min was performed. The PCR amplification results were examined by electrophoresing preparations in a 1% agarose gel. The DNA was stained with ethidium bromide and visualized under short-wavelength UV light.
TABLE 1.
PCR and sequencing primers
| Primer | Position | Orientation | Specificity | Sequence |
|---|---|---|---|---|
| PCR primers | ||||
| F24 | 9-27 | Forward | Universal | AGTTTGATYMTGGCTCAG |
| F25 | 1525-1541 | Reverse | Universal | AAGGAGGTGWTCCARCC |
| Sequencing primers | ||||
| C75 | 7-27 | Forward | Universal | GAGAGTTTGATYCTGGCTCAG |
| B34 | 344-358 | Forward | Universal | ACGGGAGGCAGCAGY |
| F16 | 789-806 | Forward | Universal | TAGATACCCYGGTAGTCC |
| F18 | 1099-1113 | Forward | Most bacteria | GCAACGAGCGCAACC |
| F19 | 1099-1114 | Forward | Bacteroides | ATAACGAGCGCAACCC |
| E94 | 1522-1541 | Reverse | Universal | GAAGGAGGTGWTCCARCCGCA |
| F20 | 1226-1242 | Reverse | Most bacteria | CCATTGTARCACGTGTG |
| F21 | 1226-1242 | Reverse | β-Proteobacteriaa | CCATTGTATGACGTGTG |
| F17 | 907-926 | Reverse | Universal | CCGTCWATTCMTTTGAGTTT |
| F15 | 519-533 | Reverse | Universal | TTACCGCGGCTGCTG |
| F22 | 344-358 | Reverse | Universal | RCTGCTGCCTCCCGT |
Members of the β subclass of the class Proteobacteria.
16S rRNA sequencing.
Purified DNA obtained from the PCR was sequenced by using an ABI prism cycle sequencing kit (BigDye terminator cycle sequencing kit with AmpliTaq DNA polymerase FS; Perkin-Elmer). The primers in Table 1 were used for sequencing. Quarter dye chemistry was used with primers at a concentration of 80 μM and 1.5 μl of PCR product in a final volume of 20 μl. Cycle sequencing was performed by using a model ABI 9700 apparatus and 25 cycles consisting of denaturation at 96°C for 10 s, annealing, and extension at 60°C for 4 m. Sequencing reactions were performed with a model ABI 377 DNA sequencer.
16S rRNA data analysis.
Sequence data were entered into RNA, a program set for data entry, editing, sequence alignment, secondary-structure comparison, similarity matrix generation, and dendrogram construction for 16S rRNA in Microsoft QuickBasic for use with PC computers, and sequences were aligned as previously described (31). Our database contains more than 1,000 sequences obtained in our laboratory and more than 500 sequences obtained from GenBank. Sequences were first checked by BLAST analysis versus all entries in the GenBank database (1). Neighboring sequences for the ASF organisms not already in our database were downloaded and added to our database. Dendrograms were constructed by the neighbor-joining method (37).
Nucleotide sequence accession numbers.
The GenBank accession numbers for the strains examined in this study or used as reference strains are given in Table 2. The 16S rRNA sequences of ASF strains determined in this study have been deposited in the GenBank database under the following accession numbers: ASF 360, AF157050; ASF 361, AF157049; ASF 519, AF157056; ASF 457, AF157055; ASF 356, AF157052; ASF 492, AF157054; ASF 500, AF157051; and ASF 502, AF157053.
TABLE 2.
Strains examined in this study
| Taxon | Strain | Identity | Sequence accession no. |
|---|---|---|---|
| Lactobacillus acidophilus | ASF 360 | Lactobacillus sp. | AF157050 |
| Lactobacillus salivarius | ASF 361 | L. murinus-L. animalis | AF157049 |
| Bacteroides distasonis | ASF 519 | [Bacteroides] sp. | AF157056 |
| Spiral-shaped organism | ASF 457 | Flexistipes phylum | AF157055 |
| Fusiform EOS bacteria | ASF 356 | Clostridium sp. | AF157052 |
| Fusiform EOS bacteria | ASF 492 | E. plexicaudatum | AF157054 |
| Fusiform EOS bacteria | ASF 502 | Clostridium sp. | AF157053 |
| Fusiform EOS bacteria | ASF 500 | Clostridium sp. | AF157051 |
| [Bacteroides] forsythus | ATCC 43037T | L16495 | |
| [Bacteroides] merdae | ATCC 43184T | X83954 | |
| [Bacteroides] distasonis | ATCC 8503T | M86695 | |
| Acetitomaculum ruminis | ATCC 43876T | M59083 | |
| Catonella morbi | ATCC 51271T | X87151 | |
| CDC group DF-3 | CDC F19047 | U41355 | |
| Clostridium neopropionicum | DSM 3847T | X76746 | |
| Clostridium piliforme | Uncultivable | L07416 | |
| Clostridium propionicum | ATCC 25522T | X77841 | |
| Deferribacter thermophilus | MBA1 | U756092 | |
| Eubacterium contortum | ATCC 25540T | L34615 | |
| Eubacterium plexicaudatuma | ATCC 27514T | AF157058 | |
| Flexistipes sinusarabic | DSM 4947 | M59231 | |
| Flexistipes phylum Colobus | Lincoln Park 3 | AF157057 | |
| Flexistipes phylum Rodent-1 | HRI3liv | AF059188 | |
| Flexistipes phylum Rodent-2 | UNSWRSp12 | AF059190 | |
| Flexistipes phylum Rodent-3 | UNSWMCS1 | AF059189 | |
| Geovibrio ferrireducens | PAL-1 | X95744 | |
| Johnsonella ignava | ATCC 51276T | X87152 | |
| Lactobacillus acidophlus | ATCC 1968T (= ATCC 4356T) | M58802 | |
| Lactobacillus animalis | ATCC 35046T | M58807 | |
| Lactobacillus delbrueckii subsp. lactis | ATCC 12315T | M58823 | |
| Lactobacillus mali | ATCC 27053T | M58824 | |
| Lactobacillus murinus | ATCC 35020T | M58826 | |
| Lactobacillus salivarius | ATCC 11741T | AF089108 | |
| Roseburia cecicola | ATCC 33874T | L14676 | |
| Ruminococcus gnavus | ATCC 29149T | X94967 |
E. plexicaudatum type strain ATCC 27514 is not available because it was found to be nonviable.
RESULTS
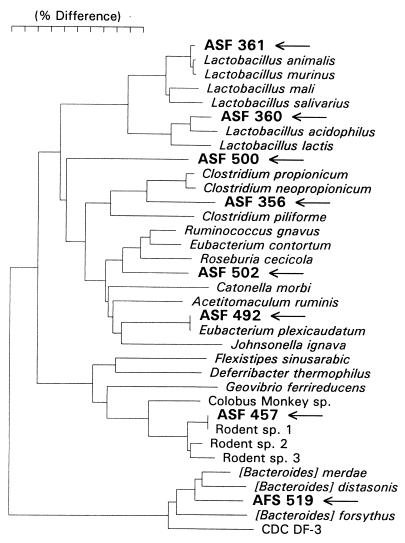
An essentially complete 16S rRNA sequence (length, 1,500 bases) was determined for each of the ASF strains. A neighbor-joining phylogenetic tree that included the closest neighbors of each ASF strain was constructed by using the sequences listed in Table 2 (Fig. 1). The strains previously presumptively identified as L. acidophilus (strain ASF 360), L. salivarius (strain ASF 361), and B. distasonis (strain ASF 519) based on phenotypic criteria were not members of these species but rather were members of neighboring species. The sequence of strain ASF 361 differed from the L. salivarius sequence but was essentially identical to the sequences of Lactobacillus murinis and Lactobacillus animalis (both isolated from mice). Strain ASF 360 is a novel lactobacillus that clusters with L. acidophilus and Lactobacillus lactis. Strain ASF 519 falls into an as yet unnamed genus along with [Bacteroides] distasonis, [Bacteroides] merdae, [Bacteroides] forsythus, and CDC group DF-3. The spiral-shaped strain, strain ASF 457, falls in the Flexistipes phylum and is most closely related to Geovibrio ferrireducens and an organism isolated from the stomach of a Colobus monkey (9). The remaining four ASF strains, which are EOS fusiform bacteria, grouped phylogenetically with the low-G+C-content gram-positive bacteria (Firmicutes, Bacillus-Clostridium group).
FIG. 1.
16S rRNA-based phylogenetic tree for ASF organisms and related species. The scale bar represents a 10% difference in nucleotide sequences, as determined by measuring the lengths of the horizontal lines connecting any two species. The Colobus monkey species isolate was obtained from Lincoln Park Zoo, Chicago, Ill., courtesy of Shelia Davis. The 16S rRNA sequence data for rodent species 1, 2, and 3 were obtained from reference 35.
DISCUSSION
Germfree mice and rats that are monoassociated with a bacterium or a particular microbiota are commonly used in biomedical research. The ASF has been widely used since the 1980s as a group of defined bacteria for colonizing the gastrointestinal tracts of commercially available mice and rats used for biomedical research. In this report we provide an initial taxonomic description of these bacteria based on a 16S rRNA analysis.
Lactobacilli are common colonizers of the gastrointestinal mucosal and squamous epithelia of mice (20, 34, 38, 39, 41). Historically, most of the indigenous lactobacilli either have not been identified to the species level or have been identified as minor variants of human species by using a limited number of biochemical tests (20, 34, 39). Unfortunately, different vertebrate species often contain unique bacterial species that are distinct from phenotypically similar human-associated species. Thus, ASF 360 and ASF 361 were identified as minor phenotypic variants of L. acidophilus and L. salivarius, respectively. With the emerging use of molecular techniques, such as restriction endonuclease fingerprinting, DNA-DNA hybridization, plasmid content analysis, and 16S rRNA sequencing, classification and identification of lactobacilli have been greatly improved (6, 7, 16, 33, 36, 43, 47, 52, 58). 16S rRNA sequence analysis has clearly demonstrated that ASF 360 and ASF 361 are distinct from each other and distinct from L. acidophilus or L. salivarius. However, as shown in Fig. 1, the 16S rRNA sequence of ASF 361 appears to be essentially identical to the 16S rRNA sequences of the previously described species L. murinus and L. animalis. L. murinus strains have been isolated from the intestinal tracts of mice and rats (21). L. animalis strains have been isolated from the dental plaque and alimentary tracts of animals (8). The type strains of L. murinus and L. animalis appear to belong to a single species. While L. animalis strains isolated from mice may belong to the same species as L. murinus strains, strains isolated from other mammalian sources may belong to different species. Therefore, a thorough examination of L. murinus and L. animalis strains is necessary to resolve these taxonomic issues. The possibility that these species are identical was suggested previously (24). L. murinus was named 2 years before L. animalis was named, and therefore the name L. murinus has priority according to the rules of nomenclature.
Bacteroides spp. are microbes that are commonly found in the intestinal tracts of mammals. Many Bacteroides species, including B. distasonis, have been isolated from the ceca of conventional mice and characterized (39, 50, 51). These bacteria were included in the genus Bacteroides because they are nonmotile, gram-negative, strictly anaerobic, non-spore-forming rods which do not produce butyric acid (50, 51). However, after many of the early studies were performed, it was recognized that the genus Bacteroides contained species representing several genera. A majority of the species previously included in the genus Bacteroides have been placed in the genera Porphyromonas, Prevotella, and Bacteroides sensu stricto (44–46). [B.] distasonis is not a true member of the genus Bacteroides but rather falls in a novel genus closely related to the genus Porphyromonas (32). Strain ASF 519 is related to [B.] distasonis but is clearly a distinct species. [B.] distasonis, [B.] merdae, [B.] forsythus, CDC group DF-3 (54), and strain ASF 519 comprise a novel unnamed genus in the Cytophaga-Flavobacterium-Bacteroides phylum.
Strain ASF 457, a spiral-shaped obligately anaerobic bacterium, was described as a spirochete by Orcutt et al. (30). Bacteria with spiral-shaped morphology are commonly found in large numbers mixed with tapered rods in the mucus layers of the ceca and colons of mice (18, 39). As determined by 16S rRNA analysis, this bacterium is related to G. ferrireducens, a dissimilatory, Fe(III)-reducing bacterium (3), Deferribacter thermophilus (19), and Flexistipes sinusarabici (10, 28) in the Flexistipes phylum (23). Within the level of sequencing error, the sequence of strain ASF 457 is identical to sequences of rodent isolates described by B. R. Robertson (35) and deposited in the GenBank database (accession no. AF059186 to AF05988). ASF 457 and the Robertson strains probably are isolates of the same species. Two other Robertson strains (accession no. AF059189 and AF05990) and a strain isolated from the stomach of a Colobus monkey (9) belong to related species. It appears that the Flexistipes phylum contains species that inhabit mammalian gastrointestinal tracts, as well as iron-reducing environmental isolates.
The majority of the members of the gastrointestinal microbiota of mice and rats are fusiform bacteria or tapered rods and are referred to in broad terms as EOS bacteria. These bacteria outnumber facultatively anaerobic bacteria by as much as 100 to 1 and aerobic bacteria by thousands to one (39). Although large numbers of the EOS fusiform bacteria or tapered rods are present (18, 20), only a few of these organisms have been cultivated, and fewer still have been named and extensively studied (48, 50, 57). Because it is difficult to identify these organisms at the species and genus levels, older taxonomic studies often grouped these bacteria on the basis of morphological criteria and growth characteristics (18) and in many cases considered them members of the genera Eubacterium, Fusobacterium, and Clostridium (20, 50, 57). The four EOS fusiform ASF strains belong in the low-G+C-content gram-positive bacterial group (Firmicutes, Bacillus-Clostridium group). Strain ASF 356 is most closely related to Clostridium propionicum. Strain ASF 492 possesses a subpolar tuft of flagella that is inserted subterminally, an unusual morphological characteristic shared by Roseburia cecicola (48) and Eubacterium plexicaudatum (57). The ASF 492 sequence clearly differentiates this organism from R. cecicola, but unfortunately, the type strain and other viable strains of E. plexicaudatum have been lost (56). The American Type Culture Collection still had vials of ATCC 27514T that were never released because they were found to be nonviable. The complete 16S rRNA sequence of ATCC 27514T (a nonviable strain kindly provided by the American Type Culture Collection) was determined, and this sequence was identical to the 16S rRNA sequence of strain ASF 492. Elsewhere, we will propose that ASF 492 should become the neotype strain for E. plexicaudatum. Our results demonstrate that 16S rRNA sequence analysis is an ideal tool for determining the molecular identities of archival or reference organisms which are no longer viable. Strain ASF 502 is most closely related to Ruminococcus gnavus. Strains ASF 356, ASF 492, and ASF 502 fall into Clostridium cluster XIV of Collins et al. (5). Strain ASF 500 branches deeply in the low-G+C-content gram-positive phylogenetic tree but is not closely related to any organism currently in the GenBank database.
Our findings again highlight the pitfalls of placing human and animal isolates with similar phenotypic characteristics in a single species. Taxonomic analysis of the family Pasteurellaceae (32), as well as many other organisms, has indicated that individual mammalian organisms have their own unique associated species. Molecular techniques, such as 16S rRNA sequencing, easily detect the existence of polyphyletic groups and can be used to prevent misclassification based phenotypic similarity.
Our findings also illustrate the taxonomic complexities of the normal flora of the mouse. Clearly, most mouse floras are much more diverse than the ASF in mice maintained under strict germfree conditions to prevent introduction of other bacterial species adept at colonizing the murine lower bowel. It is common for investigators to stipulate that mice have been maintained under specific-pathogen-free conditions. Unfortunately, this term is misinterpreted in most scientific publications and is commonly used to mask a lack of detailed information regarding the microbial pathogen status of the animals being studied. Because mice are housed in a “pathogen-free” environment and are periodically screened by viral serology and/or intestinal culture methods for known pathogenic bacteria and parasites, it is frequently assumed that infectious agents are not present and do not contribute to the pathogenesis of the disease being studied. Invariably, in these studies the intestinal flora is considered the normal flora. Indeed, a number of newly recognized murine enterohepatic helicobacters, which are fastidious microaerobes, were placed in this category; they were ignored because they are difficult to culture and because there were no previous data attributing any importance to the presence of large numbers of these spiral organisms in the crypts of the lower intestines of mice. It is now known that several of these helicobacters, most notably Helicobacter hepaticus, can cause serious gastrointestinal disease in a number of inbred and mutant mice (4, 11–13, 15). Also, it is important to recognize that certain members of the microflora of the intestine may be protective. This was clearly illustrated in young neonatal IL-10−/− mice susceptible to inflammatory bowel disease at a young age. These mice were protected against the development of colitis by oral administration of Lactobacillus sp. (29). The authors hypothesized that the Lactobacillus sp. prevented bacterial adherence to gut mucosa and subsequent bacterial translocation.
This research provided an unambiguous molecular approach to identify the AFS organisms. Our information should allow workers who utilize ASF-colonized mice to more precisely monitor the microbiota of these gnotobiotic animals by using 16S rRNA-based probe or PCR techniques. The availability of the PCR probes should also result in more accurate quality control of the defined murine microbiota and prevent infections of mice with microbial pathogens (11, 14).
ACKNOWLEDGMENTS
This work was supported in part by NIH grants R01DE-10374 (to F.E.D.), R01DE-11443 (to B.J.P.) R01DK52413 (to D.B.S. and J.G.F.), R01CA-67529 (to J.G.F. and D.B.S.), P01CA 26731 (to J.G.F.), and RR01046 (to J.G.F.).
We thank the American Type Culture Collection for providing a vial of the nonviable type strain of E. plexicaudatum.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. GappedBLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker D E. The commercial production of mice with a specified flora. Natl Cancer Inst Mongr. 1966;20:161–166. [PubMed] [Google Scholar]
- 3.Caccavo F, Jr, Coates J D, Rosselo-Mora R A, Ludwig W, Schleifer K H, Lovely D R, McInerney M J. Geovibrio ferrireducens, a phylogenetically distinct dissimilatory Fe(III)-reducing bacterium. Arch Microbiol. 1996;165:370–379. doi: 10.1007/s002030050340. [DOI] [PubMed] [Google Scholar]
- 4.Cahill R J, Foltz C J, Fox J G, Dangler C A, Powrie F, Schauer D B. Inflammatory bowel disease: an immunity-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infect Immun. 1997;65:3126–3131. doi: 10.1128/iai.65.8.3126-3131.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins M D, Lawson P A, Willems A, Cordoba J J, Fernandez-Garayzabal J, Garcia P, Hippe J, Farrow J A E. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int J Syst Bacteriol. 1994;44:812–826. doi: 10.1099/00207713-44-4-812. [DOI] [PubMed] [Google Scholar]
- 6.Collins M D, Phillips B A, Zanoni P. Deoxyribonucleic acid homology studies of Lactobacillus casei, Lactobacillus paracasi sp. nov., subsp. paracasei and subsp. tolerans, and Lactobacillus rhamnosus sp. nov., comb. nov. Int J Syst Bacteriol. 1989;39:105–108. [Google Scholar]
- 7.Collins M D, Rodrigues U, Ash C, Aguirre M, Farrow J A E, Martinez Murcia A, Phillips B A, Williams A M, Wallbanks S. Phylogenetic analysis of the genus Lactobacillus and related lactic acid bacteria as determined by reverse transcriptase sequencing of 16S rRNA. FEMS Microbiol Lett. 1991;77:5–12. [Google Scholar]
- 8.Dent V E, Williams R A D. Lactobacillus animalis sp. nov., a new species of Lactobacillus from the alimentary canal of animals. Zentbl Bakteriol Parasitenkd Infektionskr Hyg Abt 1 Orig. 1982;3:377–387. [Google Scholar]
- 9.Dewhirst, F. E., J. Zdziarski, S. Davis, and J. G. Fox. Unpublished data.
- 10.Fiala G, Woese C R, Langworthy T A, Stetter K O. Flexistipes sinusarabici, a novel genus and species of eubacteria occurring in the Atlantis II Deep brines of the Red Sea. Arch Microbiol. 1990;154:120–126. [Google Scholar]
- 11.Fox J G, Dewhirst F E, Tully J G, Paster B J, Yan L, Taylor N S, Collins M J, Gorelick P L, Ward J M. Helicobacter hepaticus sp. nov, a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J Clin Microbiol. 1994;32:1238–1245. doi: 10.1128/jcm.32.5.1238-1245.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox J G, Lee A. The role of Helicobacter species in newly recognized gastrointestinal tract diseases of animals. Lab Anim Sci. 1997;47:222–255. [PubMed] [Google Scholar]
- 13.Fox J G, Li X, Yan L, Cahill R J, Hurley R, Lewis R, Murphy J C. Chronic proliferative hepatitis in A/JCr mice associated with persistent Helicobacter hepaticus infection: a model of helicobacter-induced carcinogenesis. Infect Immun. 1996;64:1548–1558. doi: 10.1128/iai.64.5.1548-1558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox J G, MacGregor J, Shen Z, Li X, Lewis R, Dangler C A. Comparison of methods to identify Helicobacter hepaticus in B6C3F1 used in a carcinogenesis bioassay. J Clin Microbiol. 1998;36:1382–1387. doi: 10.1128/jcm.36.5.1382-1387.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox J G, Yan L, Dewhirst F E, Paster B J, Shames B, Murphy J C, Hayward A, Belcher J C, Mendes E N. Helicobacter bilis sp. nov., a novel Helicobacter isolated from bile, livers, and intestines of aged, inbred mouse strains. J Clin Microbiol. 1995;33:445–454. doi: 10.1128/jcm.33.2.445-454.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujisawa T, Benno Y, Yaeshima T, Mitsuoka T. Taxonomic study of the Lactobacillus acidophilus group, with recognition of Lactobacillus gallinarum sp. nov. and Lactobacillus johnsonii sp. nov. and synonymy of Lactobacillus acidophilus group A (Johnson et al. 1980) with the type strain of Lactobacillus amylovorus (Nakamura 1981) Int J Syst Bacteriol. 1992;42:487–491. doi: 10.1099/00207713-42-3-487. [DOI] [PubMed] [Google Scholar]
- 17.Gordon H A, Pesti L. The gnotobiotic animals as a tool in the study of host microbial relationships. Bacteriol Rev. 1971;35:390–429. doi: 10.1128/br.35.4.390-429.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon J H, Dubos R. The anaerobic bacterial flora of the mouse cecum. J Exp Med. 1970;132:251–260. doi: 10.1084/jem.132.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene A C, Patel B K C, Sheehy A J. Deferribacter thermophilus gen. nov., a novel thermophilic manganese- and iron-reducing bacterium isolated from the petroleum reservoir. Int J Syst Bacteriol. 1997;47:505–509. doi: 10.1099/00207713-47-2-505. [DOI] [PubMed] [Google Scholar]
- 20.Harris M A, Reddy C A, Carter G R. Anaerobic bacteria from the large intestine of mice. Appl Environ Microbiol. 1976;31:907–912. doi: 10.1128/aem.31.6.907-912.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemme D, Raibaud P, Ducluzeau R, Galpin J V, Sicard P, Van Heljenoort J. Lactobacillus murinus sp. nov., a new species of the autochtoneous dominant flora of the digestive tract of rat and mouse. Ann Microbiol (Paris) 1980;131:297–308. [PubMed] [Google Scholar]
- 22.Hentges D J, Stein A J, Casey S W, Que J U. Protective role of intestinal flora against infection with Pseudomonas aeruginosa in mice: influence of antibiotics on colonization resistance. Infect Immun. 1985;47:118–122. doi: 10.1128/iai.47.1.118-122.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hugenholtz P, Pitulle C, Hershberger K, Pace N R. Novel division level bacterial diversity in a Yellowstone hot spring. J Bacteriol. 1998;180:366–376. doi: 10.1128/jb.180.2.366-376.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kandler O, Weiss N. Genus Lactobacillus Beijerinck 1901, 212AL. In: Sneath P H A, Mair N S, Sharpe N E, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 2. Baltimore, Md: Williams and Wilkins; 1986. pp. 1209–1234. [Google Scholar]
- 25.Kennedy M J, Volz P A. Ecology of Candida albicans gut colonization: inhibition of Candida adhesion, colonization, and dissemination from the gastrointestinal tract by bacterial antagonism. Infect Immun. 1985;49:654–663. doi: 10.1128/iai.49.3.654-663.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee A, Gordon J, Dubos R. Enumeration of the oxygen sensitive bacteria usually present in the intestine of healthy mice. Nature (London) 1968;220:1137–1139. doi: 10.1038/2201137a0. [DOI] [PubMed] [Google Scholar]
- 27.Lee A, Gordon J, Dubos R. The mouse intestinal flora with emphasis on the strict anaerobes. J Exp Med. 1971;133:339–352. doi: 10.1084/jem.133.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ludwig W, Wallner G, Tesch A, Klink F. A novel eubacterial phylum: comparative nucleotide sequence analysis of a tuf-gene of Flexistipes sinusarabici. FEMS Microbiol Lett. 1991;78:139–144. doi: 10.1016/0378-1097(91)90147-3. [DOI] [PubMed] [Google Scholar]
- 29.Madsen K L, Doyle J S, Jewell L D, Tavernini M M, Fedorak R N. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116:1107–1114. doi: 10.1016/s0016-5085(99)70013-2. [DOI] [PubMed] [Google Scholar]
- 30.Orcutt R P, Gianni F J, Judge R J. Development of an “Altered Schaedler Flora” for NCI gnotobiotic rodents. Microecol Ther. 1987;17:59. [Google Scholar]
- 31.Paster B J, Dewhirst F E. Phylogeny of Campylobacter, wolinellas, Bacteroides gracilis, and Bacteroides ureolyticus by 16S ribosomal ribonucleic acid sequencing. Int J Syst Bacteriol. 1988;38:56–62. [Google Scholar]
- 32.Paster B J, Dewhirst F E, Olsen I, Fraser G. Phylogeny of Bacteroides, Prevotella, Porphyromonas spp., and related bacteria. J Bacteriol. 1994;176:725–732. doi: 10.1128/jb.176.3.725-732.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pot B, Hertel C, Ludwig W, Descheemaeker P, Kersters K, Schleifer K H. Identification and classification of Lactobacillus acidophilus, L. gasseri and L. johnsonii strains by SDS-PAGE and rRNA-targeted oligonucleotide probe hybridization. J Gen Microbiol. 1993;139:513–517. doi: 10.1099/00221287-139-3-513. [DOI] [PubMed] [Google Scholar]
- 34.Roach S, Savage D C, Tannock G W. Lactobacilli isolated from the stomach of conventional mice. Appl Environ Microbiol. 1977;33:1197–1203. doi: 10.1128/aem.33.5.1197-1203.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robertson B R. The molecular phylogeny and ecology of spiral bacteria from the mouse gastrointestinal tract. Ph.D. thesis. Sydney, Australia: The University of New South Wales; 1998. [Google Scholar]
- 36.Rodtong S, Tannock G W. Differentiation of lactobacillus strains by ribotyping. Appl Environ Microbiol. 1993;59:3480–3484. doi: 10.1128/aem.59.10.3480-3484.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 38.Savage D C. Microbial interference between indigenous yeast and lactobacilli in the rodent stomach. J Bacteriol. 1969;98:1278–1283. doi: 10.1128/jb.98.3.1278-1283.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savage D C. Associations of indigenous microorganisms with gastrointestinal mucosal epithelia. Am J Clin Nutr. 1970;23:1495–1501. doi: 10.1093/ajcn/23.11.1495. [DOI] [PubMed] [Google Scholar]
- 40.Schaedler R W, Dubos R, Costello R. Association of germfree mice with bacteria isolated from normal mice. J Exp Med. 1965;122:77–82. doi: 10.1084/jem.122.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaedler R W, Dubos R, Costello R. The development of the bacterial flora in the gastrointestinal tract of mice. J Exp Med. 1965;122:59–66. doi: 10.1084/jem.122.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaedler R W, Orcutt R P. Gastrointestinal microflora. In: Foster H L, Small J D, Fox J G, editors. The mouse in biomedical research. III. New York, N.Y: Academic Press; 1983. pp. 327–345. [Google Scholar]
- 43.Schleifer K H. Recent changes in the taxonomy of lactic acid bacteria. FEMS Microbiol Rev. 1987;46:201–203. [Google Scholar]
- 44.Shah H, Collins M D. Prevotella, a new genus to include Bacteroides melaninogenicus and related species formerly classified in the genus Bacteroides. Int J Syst Bacteriol. 1990;40:205–208. doi: 10.1099/00207713-40-2-205. [DOI] [PubMed] [Google Scholar]
- 45.Shah H N, Collins M D. Proposal for reclassification of Bacteroides asaccharolyticus, Bacteroides gingivalis, and Bacteroides endodontalis in a new genus, Porphyromonas. Int J Syst Bacteriol. 1988;38:128–131. [Google Scholar]
- 46.Shah H N, Collins M D. Proposal to restrict the genus Bacteroides (Castellami and Chalmers) to Bacteroides fragillis and closely related species. Int J Syst Bacteriol. 1989;39:85–87. [Google Scholar]
- 47.Stahl M, Molin G, Persson A, Ahrne S, Stahl S. Restriction endonuclease pattern and multivariate analysis as a classification tool for Lactobacillus spp. Int J Syst Bacteriol. 1990;40:189–193. [Google Scholar]
- 48.Stanton T B, Savage D C. Roseburia cecicola gen. nov., a motile obligately anaerobic bacterium from a mouse cecum. Int J Syst Bacteriol. 1983;33:618–627. [Google Scholar]
- 49.Steffen E K, Berg R D. Relationship between cecal population levels of indigenous bacterial and translocation to the mesenteric lymph nodes. Infect Immun. 1983;39:1252–1259. doi: 10.1128/iai.39.3.1252-1259.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Syed S A. Biochemical characteristics of Fusobacterium and Bacteroides species from mouse cecum. Can J Microbiol. 1972;18:169–174. doi: 10.1139/m72-027. [DOI] [PubMed] [Google Scholar]
- 51.Tannock G W. Characteristics of Bacteroides isolated from the cecum of conventional mice. Appl Environ Microbiol. 1977;33:745–750. doi: 10.1128/aem.33.4.745-750.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tannock G W, Fuller R, Pedersen K. Lactobacillus succession in the piglet digestive tract demonstrated by plasmid profiling. Appl Environ Microbiol. 1990;56:1310–1316. doi: 10.1128/aem.56.5.1310-1316.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson G E. Control of intestinal flora in animals and humans: implications for toxicology and health. J Environ Pathol Toxicol. 1978;1:113–123. [PubMed] [Google Scholar]
- 54.Vandamme P, Vancanneyt M, Van Belkum A, Segers P, Quint W G V, Kersters K, Paster M B J, Dewhirst F E. Polyphasic analysis of strains of the genus Capnocytophaga and Centers for Disease Control. Int J Syst Bacteriol. 1996;46:782–791. doi: 10.1099/00207713-46-3-782. [DOI] [PubMed] [Google Scholar]
- 55.Wells C L, Maddaus M A, Reynolds C M, Jechorek R P, Simmons R L. Role of anaerobic flora in the translocation of aerobic and facultative anaerobic intestinal bacteria. Infect Immun. 1987;55:2689–2694. doi: 10.1128/iai.55.11.2689-2694.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilkins, T. D. 1999. Personal communication.
- 57.Wilkins T D, Fulghum R S, Wilkins J H. Eubacterium plexicaudatum sp. nov., an anaerobic bacterium with a subpolar tuft of flagella, isolated from a mouse cecum. Int J Syst Bacteriol. 1974;24:408–411. [Google Scholar]
- 58.Zhong W, Millsap K, Bialkowska-Hobrzanska H, Reid G. Differentiation of Lactobacillus species by molecular typing. Appl Environ Microbiol. 1998;64:2418–2423. doi: 10.1128/aem.64.7.2418-2423.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]