Abstract
A rare occurrence of bilateral peripapillary choroidal neovascularization (CNV) in an 18-year-old idiopathic intracranial hypertension (IIH) patient regressed with systemic acetazolamide treatment alone. Multimodal imaging was done, including OCT angiography (OCTA), which showed CNV. No injections were given even though she had macular fluid in her left eye. Nonetheless, the subretinal fluid resolved, and visual acuity improved. This report shows that CNV secondary to IIH can be managed with systemic therapy alone. Moreover, we showed for the first time the ability to diagnose IIH-associated CNV using OCTA.
Keywords: Peripapillary choroidal neovascularization, Idiopathic intracranial hypertension, Acetazolamide, Optical coherence tomography angiography angiography, Multimodal imaging, Vascular endothelial growth factor
Introduction
Idiopathic intracranial hypertension (IIH) is a disorder of unknown cause characterized by elevated intracranial pressure. The typical patient is an obese young woman with headache, tinnitus, transient visual obscurations, and bilateral disc edema. There may or may not be visual loss [1]. In IIH, peripapillary choroidal neovascularization (CNV) is uncommon and a rare cause of visual impairment [2, 3, 4]. The prognosis of peripapillary CNV when it occurs in IIH and other conditions is usually benign unless the CNV and/or associated hemorrhage or exudation extends to involve the fovea [2, 3, 4]. Here, we present a young woman with IIH who developed bilateral peripapillary CNV with polypoidal-like configuration that regressed following systemic treatment with acetazolamide.
Case Report
An 18-year-old Hispanic obese female presented with headache, nausea, and blurry vision for 1 month. Visual acuity was 20/20 in the right eye and 20/150 in the left eye. A trace relative afferent pupillary defect was present in the left eye. On dilated fundus exam, there was bilateral disc elevation with blurry margins and significant intra- and subretinal fluid. In the left eye, the subretinal fluid extended into the fovea. Visual field testing showed an enlarged blind spot in her right eye and a central scotoma on her left eye. MRI of the brain and orbits demonstrated a partially empty sella and no intracranial lesions, consistent with a diagnosis of IIH. The patient was treated with 500-mg oral acetazolamide 2 times a day.
The patient was lost to follow-up but continued to take acetazolamide as prescribed and returned 6 weeks later. Her symptoms had markedly improved. VA improved in her left eye to 20/30, but there was no significant improvement of the disc edema or the intra- and subretinal fluid. However, new grayish peripapillary lesions were noted bilaterally (Fig. 1). OCT imaging of the lesion in the right eye (Fig. 2a) showed an elevated lesion associated with subretinal hyper-reflective material and retinal thickening (Fig. 2a). OCT imaging of the left eye (Fig. 2b) showed a polypoidal-like lesion with intraretinal fluid. Systemic therapy with acetazolamide was increased from 500 mg twice a day to 3 times a day and patient received an urgent referral to a retinal specialist. LP was deferred through shared decision-making with the patient since IIH is most probable per clinical presentation and representative neuroimaging [5, 6].
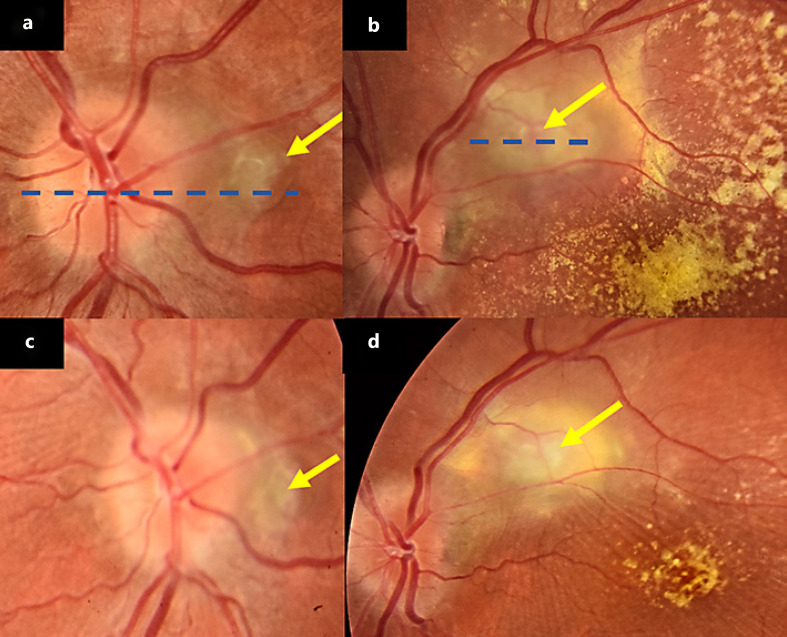
Fig. 1.
a, b Fundus color photo which shows the neovascularization lesions (yellow arrow) with a polyp and significant macular exudation of the left eye. c, d Fundus color photo, 2 months follow-up which shows significant improvement and regression of the lesions.
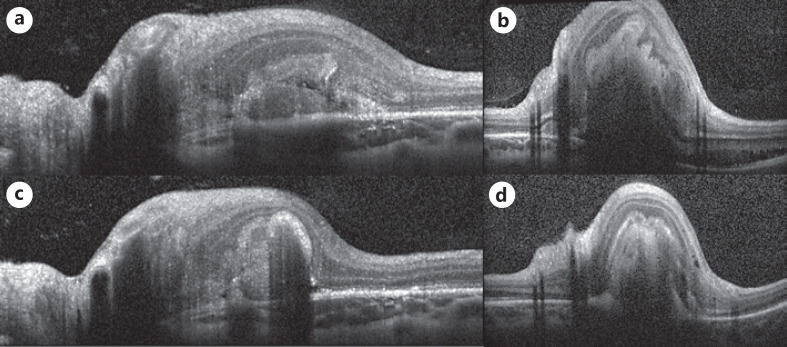
Fig. 2.
SD-OCT cuts over the lesions (dash blue line marked in Fig. 1) at presentation of the (a, b) and 4 months follow-up after initiation of systemic treatment (c, d). SD-OCT, spectral domain OCT.
The patient was again lost to follow-up and returned 2 months later. At this visit, she was evaluated by a retina specialist. Her neurologic symptoms had resolved. VA in her left eye was 20/25. The bilateral peripapillary lesions had decreased in size. Due to the relative improvement of her VA and macular fluid on OCT imaging, no injection of vascular endothelial growth factor (VEGF) inhibitors was given, and the patient was continued on systemic acetazolamide therapy. As the patient was followed over the next 4 months, there was no reactivation of the lesions while only receiving systemic treatment.
On imaging, fluorescein angiography demonstrated late leakage of the peripapillary lesions bilaterally (Fig. 3a, f). Indocyanine green angiography showed bilateral peripapillary polypoidal-like neovascular lesions (Fig. 3b, g). Swept source OCT angiography (OCTA) imaging (PLEX Elite 9000; Carl Zeiss Meditec, Dublin, CA, USA) using a segmentation slab between the retinal pigment epithelium and Bruch's membrane confirmed the presence of bilateral peripapillary CNV as shown by the structural images (Fig. 3c, h) and the flow images (Fig. 3d, e, i, j). On follow-up, spectral domain OCT imaging showed apparent improvement of the polypoidal-like projections and resolution of the subretinal fluid and hyper-reflective material (Fig. 2c, d, 4).
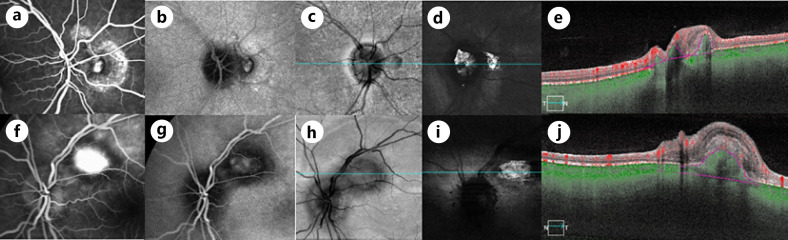
Fig. 3.
Multimodal imaging demonstrating the CNV of the right and left eyes. a, f Late stage FA leakage. b, g Late stage ICG which shows a polyp-like configuration. c, h En face SS-OCT structural image (c, h) and flow images (d, i, e, j) applied to the slab segmented to visualize detectable flow residing between the RPE and Bruch's membrane. FA, fluorescein angiography.
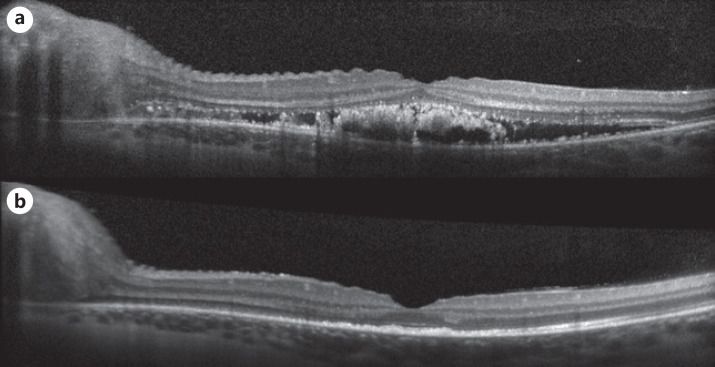
Fig. 4.
Foveal SD-OCT cut of the left eye shows improvement of foveal intra- and subretinal fluid at presentation (a) and 4 months follow-up (b). SD-OCT, spectral domain OCT.
Discussion
Peripapillary CNV is a rare complication in patients with IIH [3, 4]. Data on the prevalence of this complication are limited with only a few cases and case series reported [3, 7, 8]. The majority of cases with IIH-associated peripapillary CNV are unilateral and do not affect visual acuity [3]. Our patient developed bilateral peripapillary CNV with foveal involvement and decreased vision in her left eye.
CNV is considered a rare [1, 3] and late complication of IIH. In a small case series, the mean elapsed time to development of IIH-associated CNV was 41 months [4]. However, our patient developed these lesions relatively early, only 3 months after the onset of symptoms, and 2 months after her initial evaluation and treatment. The pathogenesis behind the development of peripapillary CNV in IIH patients is unknown. One possible etiology is persistent hypoperfusion of the peripapillary neurosensory retina and RPE due to the chronic swollen state of the optic disc. This might explain why CNV develops late in the disease course [9, 10]. An alternative explanation is that the physical deformity of peripapillary tissues causes breaks in Bruch's membrane and a pathway for the ingrowth of CNV similar to the mechanism of CNV formation in a choroidal rupture [2].
The gold standard modality for the diagnosis of CNV is fluorescein angiography/indocyanine green angiography imaging. This is the first case, to our knowledge, where CNV secondary to IIH was confirmed using OCTA.
Treatment options include photodynamic therapy, focal laser, observation, and anti-VEGF injections [1]. Several case reports have demonstrated improvement in IIH-associated peripapillary CNV with subretinal and intraretinal fluid in patients treated with anti-VEGF agents [3, 4, 7, 8, 11, 12]. Lesions without macular fluid are usually observed. Our patient was not treated with injections even though she had macular fluid in her left eye. Nonetheless, the subretinal fluid resolved, which may be explained by systemic acetazolamide treatment or may be the natural history of IIH-associated peripapillary CNV.
In conclusion, peripapillary CNV is a rare complication of IIH. To our knowledge, this is the first case to document IIH-associated peripapillary CNV using OCTA.
Statement of Ethics
This study protocol was reviewed and approved by the Institutional Review Board of the University of Miami Miller School of Medicine, IRB numbers 20043451 and 20120997. The study was performed in accordance with the tenets of the Declaration of Helsinki and complied with the Health Insurance Portability and Accountability Act of 1996. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Conflict of Interest Statement
Philip Rosenfeld receives research support from Carl Zeiss Meditec, Inc., and the University of Miami co-owns a patent that is licensed to Carl Zeiss Meditec, Inc. Philip Rosenfeld also receives grant support from Boehringer-Ingelheim and Stealth BioTherapeutics. He is also a consultant for Apellis, Boehringer-Ingelheim, Carl Zeiss Meditec, Chengdu Kanghong Biotech, Ocunexus Therapeutics, Ocudyne, and Unity Biotechnology. Philip Rosenfeld has equity interest in Apellis, Verana Health, and Ocudyne. The remaining authors have no disclosures.
Funding Sources
Research supported by grants from the Salah Foundation, an unrestricted grant from the Research to Prevent Blindness, Inc. (New York, NY, USA), and the National Eye Institute Center Core Grant (P30EY014801) to the Department of Ophthalmology, University of Miami Miller School of Medicine. The funding organizations had no role in the design or conduct of the present research.
Author Contributions
O.T., P.J.R., and J.T. contributed to the design and implementation of this report, to the analysis of the results, and to the writing of the manuscript. L.W., P.I., D.R., and Y.S. contributed to the implementation of this report, to the analysis of the results, and to the writing of the manuscript. All authors attest that they meet the current ICMJE criteria for Authorship.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1.Wall M. Update on idiopathic intracranial hypertension. Neurol Clin. 2017;35((1)):45–57. doi: 10.1016/j.ncl.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morse PH, Leveille AS, Antel JP, Burch JV. Bilateral juxtapapillary subretinal neovascularization associated with pseudotumor cerebri. Am J Ophthalmol. 1981;91((3)):312–7. doi: 10.1016/0002-9394(81)90282-8. [DOI] [PubMed] [Google Scholar]
- 3.Wendel L, Lee AG, Boldt HC, Kardon RH, Wall M. Subretinal neovascular membrane in idiopathic intracranial hypertension. Am J Ophthalmol. 2006;141((3)):573–4. doi: 10.1016/j.ajo.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 4.Ozgonul C, Moinuddin O, Munie M, Lee MS, Bhatti MT, Landau K, et al. Management of peripapillary choroidal neovascular membrane in patients with idiopathic intracranial hypertension. J Neuroophthalmol. 2019;39((4)):451–7. doi: 10.1097/WNO.0000000000000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vosoughi AR, Margolin EA, Micieli JA. Can lumbar puncture be safely deferred in patients with mild presumed idiopathic intracranial hypertension? J Neuroophthalmol. 2021 doi: 10.1097/WNO.0000000000001411. [DOI] [PubMed] [Google Scholar]
- 6.Margolis MS, DeBusk AA, Moster ML, Falardeau JM, Eggenberger ER, Sergott RC, et al. Lumbar puncture for diagnosis of idiopathic intracranial hypertension in typical patients. J Neuroophthalmol. 2021;41((3)):375–8. doi: 10.1097/WNO.0000000000001319. [DOI] [PubMed] [Google Scholar]
- 7.Kaeser PF, Borruat FX. Peripapillary neovascular membrane: a rare cause of acute vision loss in pediatric idiopathic intracranial hypertension. J AAPOS. 2011;15((1)):83–6. doi: 10.1016/j.jaapos.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Belliveau MJ, Xing L, Almeida DR, Gale JS, ten Hove MW. Peripapillary choroidal neovascular membrane in a teenage boy: presenting feature of idiopathic intracranial hypertension and resolution with intravitreal bevacizumab. J Neuroophthalmol. 2013;33((1)):48–50. doi: 10.1097/WNO.0b013e318281b7b9. [DOI] [PubMed] [Google Scholar]
- 9.Hayreh MS, Hayreh SS. Optic disc edema in raised intracranial pressure. I. Evolution and resolution. Arch Ophthalmol. 1977;95((7)):1237–44. doi: 10.1001/archopht.1977.04450070135013. [DOI] [PubMed] [Google Scholar]
- 10.Castellarin AA, Sugino IK, Nasir M, Zarbin MA. Clinicopathological correlation of an excised choroidal neovascular membrane in pseudotumour cerebri. Br J Ophthalmol. 1997;81((11)):994–1000. doi: 10.1136/bjo.81.11.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee IJ, Maccheron LJ, Kwan AS. Intravitreal bevacizumab in the treatment of peripapillary choroidal neovascular membrane secondary to idiopathic intracranial hypertension. J Neuroophthalmol. 2013;33((2)):155–7. doi: 10.1097/WNO.0b013e31827c6b49. [DOI] [PubMed] [Google Scholar]
- 12.Altan-Yaycioglu R, Canan H, Saygi S. Intravitreal ranibizumab injection in peripapillary CNVM related to idiopathic intracranial hypertension. J Pediatr Ophthalmol Strabismus. 2017;54:e27–30. doi: 10.3928/01913913-20170201-04. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.