Abstract
Introduction
Following the easing of COVID-19 restrictions in many countries, a surge in respiratory syncytial virus (RSV) hospitalisations was reported, surpassing yearly trends pre-pandemic. The changes to RSV epidemiology may have unforeseen effects on healthcare systems and populations globally, adding to the burden generated during the pandemic and placing increased demand on resources. Here we aim to identify recent global trends of RSV hospitalisation amongst children aged ≤5 years, to help inform policy makers in the planning of preventative interventions.
Methods
We conducted a scoping review of published literature between January 2009 and May 2021. Using keywords “Hospital admissions, Respiratory syncytial virus, RSV, Bronchiolitis, Children” we located studies using Medline, EMCARE, CINAHL and HMIC. Studies were eligible if they reported on trends/data for RSV hospitalisation amongst children aged ≤5 years. The articles were reviewed by two independent reviewers.
Findings
We assessed 3310 abstracts, reviewed 70 studies and included 56 studies in the final review. Findings were categorised into themes. The review highlighted that, although RSV incidence has been steadily increasing since 2009, the number of reported RSV hospitalisations decreased during lockdown. The highest numbers of hospitalisations were reported in children <1 year of age, particularly 0–2-month-old infants. Globally, RSV hospitalisations tend to peak in the winter months; however, since COVID-19 restrictions have eased, countries are reporting incidence peaks at different times, in contrast to the trends of previous years.
Conclusion
With greater physical interactions due to the relaxation of COVID-19 restriction measures, RSV-related hospitalisations can be seen to increase amongst children aged ≤5 years, possibly surpassing the numbers reported in previous RSV seasons.
Short abstract
With #COVID19 restriction measures being eased globally, #RSV-related hospitalisation among children will increase, possibly surpassing pre-pandemic levels https://bit.ly/35lg4Iv
Introduction
Respiratory syncytial virus (RSV) is an enveloped RNA virus which causes acute lower respiratory infections (ALRI) and bronchiolitis in young children [1] and is responsible for >3 million hospitalisations and over 65 000 deaths globally each year [2]. Although over 80% of infants have experienced at least one RSV infection by the age of 2 years, infants in their first year of life are more likely to experience a severe infection requiring hospitalisation [3]. RSV-related complications other than bronchiolitis include pneumonia, acute otitis media and conjunctivitis [4]. Despite over 50 years of effort, a licensed vaccine is still not available for RSV [5].
RSV is known to create a significant burden on healthcare systems. A systematic review and meta-analysis of 329 studies conducted by Shi et al. in 2017 [6] estimated that there were about 33.1 million (uncertainty range (UR) 21.6–50.3) RSV-ALRI episodes globally, resulting in about 3.2 (UR 2.7–3.8) million hospital admissions and 59 600 (48 000–74 500) in-hospital deaths in (670.5 million) children younger than 5 years. In the UK alone, 450 000 general practitioner (GP) appointments, 29 000 hospitalisations and 83 deaths per year are reported amongst children and are attributed to RSV infection – with the majority of the burden reported in infants [6]. In England, it has been estimated that RSV causes ∼78% (95% CI 75%–83%) of bronchiolitis admissions in children <5 years of age [7]. Reeves et al. (2017) [8] confirm a mean annual admission rate of 35.1 (95% CI 32.9–38.9) per 1000 children aged <1 year and 5.31 (95% CI 4.5–6.6) per 1000 children aged 1–4 years in England when analysing data from 2007 to 2012. Shi et al. (2017) [6] report that direct medical costs associated with hospital care for childhood ALRI have been estimated to range from USD 243 (95% CI 154–341) to USD 559 (269–887) at secondary and tertiary care facilities, respectively, in low- to middle-income countries; and USD 2804 (2001–3683) to USS 7037 (4286–11 311) at secondary and tertiary care facilities, respectively, in high-income countries. These data highlight the importance of managing RSV-related illness in the UK and globally during the pandemic. Furthermore, it is important to consider the direct effects that sociological and economical changes attributable to COVID-19 may have on the epidemiology of RSV in the short, medium and long term. As a result of changes in infection rates and contact patterns, it is likely that levels of immunity in the population are not reflective of recent years. Failure to consider these effects could have an impact on populations and healthcare systems both in the UK and globally.
Throughout much of 2020 and 2021, non-pharmaceutical interventions (NPIs), including physical distancing, reduced social mixing and quarantine measures, were implemented worldwide to limit the spread of SARS-CoV-2. Hygiene measures were also introduced to reduce viral transmission, including the use of face masks, with governments delivering campaigns with the aim of increasing hand hygiene. This was the first time in many years that such dramatic changes to sociological and behavioural dynamics had occurred, leading to changes in the epidemiology of other communicable diseases [9]. Following a nationwide lockdown in 2020, Finland reported a decrease in paediatric hospital admissions caused by respiratory tract infections [10]. The initial trajectory of the RSV season itself was similar to that of previous years; however, 1 week after the start of lockdown, case numbers decreased rapidly, attributed to reduced social mixing and mobility [10].
The changes in RSV epidemiology may have unforeseen effects on healthcare systems and populations globally, adding to the burden generated during the pandemic and placing increased demand on resources. A recent study in Australia highlighted the potential implications of easing COVID-19-related restrictions on numbers of RSV hospitalisations. In Western Australia, RSV case numbers started to increase once physical distancing measures were relaxed, exceeding the seasonal peak reported in the previous eight RSV seasons [11]. A change in the temporal dynamics of RSV was also reported, with the season occurring during spring and summer instead of the winter months [11]. A study in the USA also demonstrated an absence of their usual RSV season during state-wide lockdowns and a delayed resurgence in cases and hospitalisations [12].
The primary objective of this scoping review is to identify and gain insight into the recent trends of RSV-associated hospitalisations globally. Secondary objectives include assessing the impact of the following measures on RSV hospitalisation:
COVID-19 lockdown
Relaxation of COVID-19 restrictions
Methods
A scoping literature review was conducted by two reviewers (M.N. and R.K.). All shortlisted studies were entered into a Microsoft Excel table and shared with the other authors of this article.
Literature search strategy
Key terms including “Hospital admissions, Respiratory syncytial virus, RSV, Bronchiolitis, Children” were searched using Medline, EMCARE, CINAHL and HMIC.
The inclusion criteria were:
Primary and secondary research assessing/reporting RSV illness and hospitalisation globally
Published 2009 onwards
Age group ≤5 years
The exclusion criteria were:
Published 2009 onwards but utilised data from before 2009
Impact assessments of medication/antibiotics/vaccines
Reporting clinical outcomes for RSV patients
Evaluated RSV-related cost of hospitalisation
The shortlisted literature was then categorised into three groups: 1) trends in RSV-associated hospitalisation; 2) seasonality of RSV hospitalisation; and 3) incidence/prevalence of RSV infection. Trends in RSV-associated hospitalisation were further grouped into three themes: i) increase in incidence/rate over time; ii) patient age at time of RSV hospitalisation; and iii) impact of COVID-19 restrictions on number of RSV hospitalisations.
Findings
The literature search located 3310 papers, of which 56 were shortlisted for further review and 56 studies were included in the final review. The studies included in the review are presented in tables 1 and 2. Data, where extractable, have been plotted as figure 1 (RSV incidence) and figure 2 (RSV hospitalisations).
TABLE 1.
Studies on respiratory syncytial virus (RSV)-associated hospital admissions
| Author (year) [ref.]/location | Sample size | Results |
| Hacimustafaoglu et al. (2013) [3] | • The annual incidence of hospitalisation due to RSV+LRTI was 7.8/1000 | |
|
Chung
et al. (2020) [7] Scotland |
43 514 | • Over the 15-year study period, admission rates for children under 2 years old increased 2.20-fold (95% CI 1.4–3.6-fold) from 17.2 (15.9–18.5) to 37.7 (37.4–38.1) admissions per 1000 children per year • Admissions peaked in infants aged 1 month, and in those born in the 3 months preceding the peak bronchiolitis month – September, October and November |
|
Tumba
et al. (2020) [14] Brazil |
263 679 | • The incidence of hospitalisation for bronchiolitis increased by 49% over this period (8.5 to 12.7 per 1000 inhabitants per year), between 2013 and 2014, the incidence rate of hospitalisation for acute bronchiolitis decreased by 8% (12.5 to 11.5 per 1000 inhabitants per year) |
|
Lewis
et al. (2020) [15] England |
3 727 013 | • Bronchiolitis admission rates ranged from 30.9 per 1000 infant-years (95% CI 30.4–31.3) in London to 68.7 per 1000 (95% CI 67.9–69.5) in the North West • Across CCGs: 5.3-fold variation in incidence rates and the epidemic peak ranged from week 49.3 to 52.2 |
|
Lewis
et al. (2020) [16] England |
3 717 329 | • Bronchiolitis admission rate increased from 47.4 (95% CI 46.8–47.9) to 58.9 per 1000 infant-years (95% CI 58.3–59.5) between 2012 and 2016 |
|
Arriola
et al. (2020) [18] USA |
1554 | • Adjusted age-specific RSV hospitalisation rates per 100 000 population were 1970 (95% CI 1787–2177), 897 (95% CI 761–1073), 531 (95% CI 459–624) and 358 (95% CI 317–405) for ages 0–2, 3–5, 6–11 and 12–23 months, respectively |
|
Glatman-Freedman
et al. (2020) [20] Israel |
39 156 | • The hospitalisation load of RSV-related diagnoses was highest in infants <1 year of age (mean yearly rate of 1218.4 per 100 000 infants), rapidly declining in the following years |
|
Hardelid
et al. (2019) [21] Scotland |
169 726 | • There were 5185 RSV admissions among the 169 726 children in the cohort: 48.6% of admissions occurred before the age of 6 months, and 29.6% after the age of 1 year |
|
Reeves
et al. (2020) [22] Europe |
• Average annual RSV-coded admission rates ranged from 20.5 to 22.3 per 1000 children aged <1 year in Scotland, Finland, Norway and Denmark, whereas in children aged 1–4 years rates ranged from 1.25 to 2.24 per 1000 children • Average annual RSV-coded admission rates ranged from 8.6 to 11.7 per 1000 children aged <1 year in England, the Netherlands and Italy, whereas in children aged 1–4 years rates ranged from 0.2 to 0.3 per 1000 children • Annual average RSV-confirmed admission rates were 21.2 per 1000 children <1 year in Scotland and 21.9 per 1000 children <1 year in Finland. For children aged 1–4 years, RSV-confirmed admission rates were 1.6 per 1000 in Finland and 2.1 per 1000 in Scotland |
|
|
Svensson
et al. (2015) [23]
Sweden |
1764 | • The age-specific incidence in infants under 1 year of age was 17.4 per 1000 per year, and the incidence in children aged 1–4 years was 0.6 per 1000 per year • The incidence for all children under 5 years of age was 4.2 per 1000 per year • The risk of being hospitalised for RSV was 17.2 per 1000 live births during the first year of life and 19.6 per 1000 live births before 5 years of age |
|
Britton
et al. (2020) [25]
NSW (Australia) |
• Observed mean±se frequency of RSV detections from April to June, 2020, was 94.3±22.8% lower than predicted on the basis of the underlying trend of 2015–2019 data (mean±se absolute reduced frequency per epidemic month (ARF) 99±24; p=0.026) | |
|
Reeves
et al. (2019) [30]
England |
6758 | • Annual average of 20 359 (95% CI 19 236–22 028) RSV-associated admissions in infants in England from mid-2010 to mid-2012 • RSV-associated admissions peaked in infants aged 6 weeks, and those born September to November |
|
Glick
et al. (2017) [31] USA |
50 157 | • Mean±sd RSV hospitalisation season onset (early November) was 3.3±2.1 weeks before regional activity season onset (early December) • Hospitalisation season offset (early May) was 4.4±2.4 (mean±sd) weeks after activity season offset (mid-April) |
|
Thwaites
et al. (2020) [43] Scotland |
• Between December and January, RSV hospitalisations represented 8.5% of all admissions; this increased to 14.2% between October and March | |
|
Buchwald
et al. (2020) [44] Mali |
1871 | • The incidence of RSV-associated hospitalisations was 45.6 per 1000 person-years |
|
Pelletier
et al. (2021) [24] USA |
5 424 688 | • Decrease in the number of RSV hospital admissions beginning in March 2020 compared to years 2010 to 2019 • Admissions in April 2020 (23 798) were 45.4% lower than previous years, 2010–2019 (median=43 550) |
|
Benitez-Guerra
et al. (2020) [45] Mexico |
294 | • Overall, the hospitalisation rate for RSV-confirmed ARI was 62.6 per 1000 child-years of follow-up |
| Saravanos et al. (2020) [19] Australia | 60 351 | • Under 5s hospitalisation rate was 418 per 100 000 population; under 6 months it was 2224 per 100 000 population; the highest rate was for infants aged 0–2 months (2778 per 100 000 population) |
| Wilder et al. (2021) [26] | 3631 | • Bronchiolitis had fewer median hospitalisations per week in the COVID-19 cohort compared with the pre-COVID-19 cohort: bronchiolitis (1 versus 7; p=0.008) |
|
Mendes-da-Silva
et al. (2019) [46] Portugal |
80 491 | • The mean admission rate was 26.28 and was higher in the northernmost regions of the country • Admission rate rose by an average of 1.6% per year (3.8% in children younger than 3 months) and the average length of stay was 6.1 days and decreased, to a minimum of 5.5 days in 2014 |
|
Rha
et al. (2020) [47] USA |
2969 | • RSV-associated hospitalisation rates were 2.9 per 1000 children <5 years old and 14.7 per 1000 children <6 months old; the highest age-specific rate was observed in 1-month-old infants (25.1 per 1000) |
|
Li
et al. (2021) [48] Global |
1453 | • The median number of RSV-associated ALRI hospitalisations in children younger than 5 years was 8.25 per 1000 (IQR 1.97–48.01), and the median rate of RSV-associated ALRI hospitalisations was 514 (339–866) hospitalisations per 1000 children younger than 5 years |
|
Prasad
et al. (2019) [49] New Zealand |
71 770 | • The seasonal incidence of RSV-associated ARI hospitalisation without accounting for non-tested children was 3.5 (95% CI 3.3–3.7) per 1000 children or 12.2 (95% CI 11.6–12.9) per 1000 child-years at risk |
|
Greenberg
et al. (2020) [50] Israel |
374 late preterm and 2948 term infants | • The mean yearly incidences per 1000 children of RSV bronchiolitis hospitalisations of late preterm and term infants were 35.8±13.0 and 19.6±4.1, respectively (p=0.009) • During RSV seasons the mean incidence rate ratio between groups was 1.82 (95% CI 1.60–2.08) |
|
Fujiogi
et al. (2019) [51] USA |
490 650 | • From 2000 to 2016, the incidence of bronchiolitis hospitalisation decreased from 17.9 to 13.5 per 1000 person-years in US children (25% decrease; p-value trend <0.001) • In contrast, the proportion of bronchiolitis hospitalisations among overall hospitalisations increased from 16% to 18% |
|
Kramer
et al. (2018) [52] France |
21 930 | • Incidence of RSV-associated hospitalisation in the first year of life per 1000 births was 14.5 (95% CI 13.4–15.6) |
|
Reeves
et al. (2017) [8] England |
• Annual RSV-associated RTI admission rates of 35.1 (95% CI 32.9–38.9) per 1000 children <1 year of age and 5.31 (95% CI 4.5–6.6) per 1000 children 1–4 years of age | |
|
Oakley
et al. (2017) [53] Australia/New Zealand |
3589 | • ICU admission rates ranged from 4.1% to 9.1% with an average of 5.7%. There was evidence of a difference between sites in the rates of ventilatory support use (p<0.001) • Ventilatory support rates ranged from 2.8 to 5.9% across the sites with an average of 4.5% |
|
Cromer
et al. (2017) [54] England |
• Estimated that RSV is responsible for 12 primary care consultations (95% CI 11.9–12.1) and 0.9 admissions to hospital annually per 100 children younger than 5 years (95% CI 0.89–0.90) • In children younger than 6 months, RSV accounted for more than half of all admissions to hospital for acute respiratory conditions and for >70% of those admissions occurring between October and January |
|
|
Sanchez-Luna
et al. (2016) [55] Spain |
1 328 563 discharges | • The total number of yearly hospital discharges for RSV bronchiolitis (ICD-9 code 466.11) in children under 1 year ranged between 5997 (2005) and 8637 (2012) • The hospitalisation rate (discharges per 1000 children under 1 year) for RSV bronchiolitis increased over the period (from 19 to 24.9) |
|
Munoz-Quiles
et al. (2016) [56] Spain |
198 223 | • 5390 were hospitalised with the majority of hospitalisations occurring at <6 months of age (incidence rate of 5.2 per 100 children <6 months per year) and 3106 of the hospitalisations were RSV-positive (incidence rate 3.2 per 100 children <6 months per year) |
|
Saha
et al. (2015) [57] India |
505 | • Annual incidence rates of RSV-associated hospitalisation per 1000 children were highest among infants aged 0–5 months (15.2, 95% CI 8.3–26.8), followed by ages 6–23 months (5.3, 95% CI 3.2–8.7) and lowest among children 24–59 months (0.5, 95% CI 0.1–1.5) |
| Helfrich et al. (2015) [58] | • LPT infants had an absolute hospitalisation rate (AHR) of 2.5%, while term infants had an AHR of 1.3% (p<0.001) • The IDRSV of LPT and term infants was 12.1 and 7.8 per 1000 person-years, respectively |
|
|
Ochoa
et al. (2014) [59] Peru |
335 | • The incidence of RSV respiratory infections that required emergency room management was 103.9 per 1000 child-years, and the incidence of RSV hospitalisations was 116.2 per 1000 child-years (244.9 in infants with a birthweight <1000 g and 88.9 in infants 1000–1500 g; p<0.05) |
|
Nasreen
et al. (2014) [60] Bangladesh |
12 850 | • RSV was associated with 7.9 SARI hospitalisations per 100 000 person-weeks |
| Murray et al. (2014) [61] | • 7189 hospital admissions with a diagnosis of bronchiolitis, 24.2 admissions per 1000 infants under 1 year (95% CI 23.7–24.8), of which 15% (1050/7189) were born preterm (47.3 bronchiolitis admissions per 1000 preterm infants (95% CI 44.4–50.2)) | |
|
Broor
et al. (2014) [29] India |
245 | • RSV accounted for the highest virus-associated hospitalisation incidence (34.6 per 10 000, 95% CI 26.3–44.7) and 20% of hospitalisations |
|
Emukule
et al. (2014) [62] Kenya |
• The average annual incidence of RSV-associated SARI hospitalisation per 1000 persons was 5.2 (95% CI 4.0–6.8) among children <5 years • The incidence of RSV-associated medically attended ILI was 24.6 (95% CI 17.0–35.4) among children <5 years and 0.8 (95% CI 0.3–1.9) among persons ≥5 years |
|
|
Ambrose
et al. (2014) [63] USA |
1646 | • Rates of RSV-related MAARI, outpatient lower respiratory tract illness, emergency department visits and hospitalisation (RSVH) during November to March were 25.4, 13.7, 5.9 and 4.9 per 100 infant-seasons, respectively |
|
Rowlinson
et al. (2013) [64] Egypt |
5342 | • The incidence of RSV-associated hospitalisation and outpatient visits was estimated at 24 and 608 (per 100 000 person-years), respectively • Children aged <1 year experienced the highest incidence of RSV-associated hospitalisations (1745 per 100 000 person-years) |
|
Naorat
et al. (2013) [65] Thailand |
13 982 | • The incidence of RSV-associated ALRI hospitalisation was 85 cases per 100 000 persons per year • The highest rates occurred among children aged <5 years (981 cases per 100 000 persons per year) and <1 year (1543 cases per 100 000 persons per year) |
|
McCracken
et al. (2013) [66] Guatemala |
6626 | • The incidence of RSV-associated hospitalisation for ARI was highest among infants aged <6 months (208 cases/10 000 persons per year) • The incidence of RSV-positive clinic visitation for ARI was highest among infants aged 6–23 months (186 cases/10 000 persons per year) |
|
Eidelman
et al. (2009) [67] Israel |
• On average, 147±17 cases of RSV bronchiolitis were admitted annually in the November–March RSV season, representing 7%–9% of admissions and 10%–14% of hospital days • There was a consistent male preponderance of admissions (55–64%) and 15–23% of admissions were patients <1 month old. • In peak months RSV cases accounted for as many as 40% of the hospitalised infants and was the leading cause of over-occupancy (up to 126%) in the paediatric ward during the winter |
|
|
Hall
et al. (2009) [68] USA |
919 | • Overall, RSV was associated with 20% of hospitalisations, 18% of emergency department visits and 15% of office visits for ARIs from November through April • Average annual hospitalisation rates were 17 per 1000 children under 6 months of age and 3 per 1000 children under 5 years of age |
|
Tong
et al. (2020) [69] USA |
41 610 536 person-years | • The average incidence of all RSV-related healthcare utilisation between 2008 and 2014 was 2.4 per 1000 person-years, with mean incidence for each year of the study ranging from 2.0 to 2.6 per 1000 person-years (RSV-specific rate: 1.5 per 1000 person-years (mean rate for individual years ranging from 1.1 to 1.6 per 1000 person-years)) |
|
Kubale
et al. (2020) [70] Nicaragua |
833 | • The incidence rate of symptomatic RSV was 248.1 cases per 1000 person-years (95% CI 223.2–275.7) • While infants aged 6–11 months had the highest incidence of symptomatic RSV (361.3/1000 person-years, 95% CI 304.4–428.8), infants <3 months had the highest incidence of severe RSV (RSV-associated hospitalisations and/or severe ALRI) |
|
Ueno
et al. (2019) [71] Philippines |
3817 | • Incidence rates for children aged 2–23 months were 124.0 and 51.5 per 1000 child-years for total RSV-LRTI and total severe RSV-LRTI, respectively |
LRTI: lower respiratory tract infection; CCG: clinical commissioning group; ALRI: acute lower respiratory infections; RTI: respiratory tract infection; ICU: intensive care unit; ICD-9: International Classification of Diseases, Ninth Revision; LPT: late preterm; IDRSV: incidence density rate for RSV hospitalisation; SARI: severe acute respiratory infection; ILI: influenza-like illness; MAARI: medically attended acute respiratory infection; ARI: acute respiratory infection.
TABLE 2.
Studies on the seasonality of respiratory syncytial virus (RSV) hospitalisation
| Author (year) [ref.]/location | Sample size | Results |
|
Chung
et al. (2020) [7]
Scotland |
43 514 | • RSV admissions peaked in infants aged 1 month, and in those born in the 3 months preceding the peak bronchiolitis month – September, October and November |
|
Lewis
et al. (2020) [16]
England |
3 727 013 | • Across CCGs, there was a 5.3-fold variation in incidence rates and the epidemic peak ranged from week 49.3 to 52.2 • Admission rates were positively associated with area-level deprivation |
|
Lewis
et al. (2020) [15]
England |
3 717 329 | • Identified some variation in the seasonality of admissions by socioeconomic position: increased deprivation was associated with less seasonal variation and a slightly delayed epidemic peak |
|
Glatman-Freedman
et al. (2020) [20]
Israel |
39 156 | • RSV-related hospitalisations followed a clear seasonal pattern; the peak occurred in January for 14 seasons, in December for two seasons (2014/2015 and 2015/2016) and in February for one season (2004/2005) • A total of 11 RSV hospitalisation seasons started in October, five started in September (2000/2001, 2001/2002, 2002/2003, 2003/2004 and 2014/2015) and one started in November (2009/2010) • A total of 10 RSV hospitalisation seasons ended in April, five in May (2000/2001, 2001/2002, 2002/2003, 2006/2007 and 2011/2012) and two in March (2007/2008 and 2008/2009) |
|
Reeves
et al. (2019) [30]
England |
6758 | • RSV-associated admissions peaked in infants aged 6 weeks, and those born September to November |
|
Glick
et al. (2017) [31]
USA |
50 157 | • Mean±sd RSV hospitalisation season onset (early November) was 3.3±2.1 weeks before regional activity season onset (early December); mean±sd hospitalisation season offset (early May) was 4.4±2.4 weeks after activity season offset (mid-April) • RSV hospitalisation and activity seasons lasted 18 to 32 and 13 to 23 weeks, respectively • Nearly 10% of hospitalisations occurred outside of regional activity season (regional ranges: 5.6–22.4%) |
|
Broor
et al. (2014) [29]
India |
245 | • RSV and influenza virus detection peaked in winter (November to February) and rainy seasons (July), respectively |
| Pangesti et al. (2019) [28] Global (15 WPR countries) | • Generally, temperate countries, both in the Northern and Southern hemispheres, experienced their peak of the epidemic in the winter; in subtropical and tropical countries, the cases peaked mostly in the rainy (wet) season | |
|
Yu
et al. (2019) [72]
China |
4225 | • Identified eight distinctive RSV seasons • On average, the season onset occurred at week 41 (mid-October) and lasted 33 weeks, through week 20 of the next year (mid-May); 97% of all RSV-positive cases occurred during the season • RSV seasons occurred 3–5 weeks earlier and lasted ≈6 weeks longer in RSV subgroup A-dominant years than in RSV subgroup B-dominant years |
|
Leung
et al. (2014) [73]
Hong Kong |
4912 | • Paediatric intensive care unit admissions were higher between October and March |
|
du Prel
et al. (2009) [74]
Germany |
• Influenza A, RSV and adenovirus were correlated with temperature and rhinovirus to relative humidity • In a time series model that included seasonal and climatic conditions, RSV-associated hospitalisations were predictable |
|
|
Chan
et al. (2015) [75]
Hong Kong |
• The activity of RSV lasted longer than influenza, spreading through week 8 to 40 (late-February to late-September) with annual peaks occurring either in week 10 to 15 (early-March to mid-April) or week 29 to 38 (mid-July to mid-September) |
CCG: clinical commissioning group; WPR: World Health Organization Western Pacific Region.
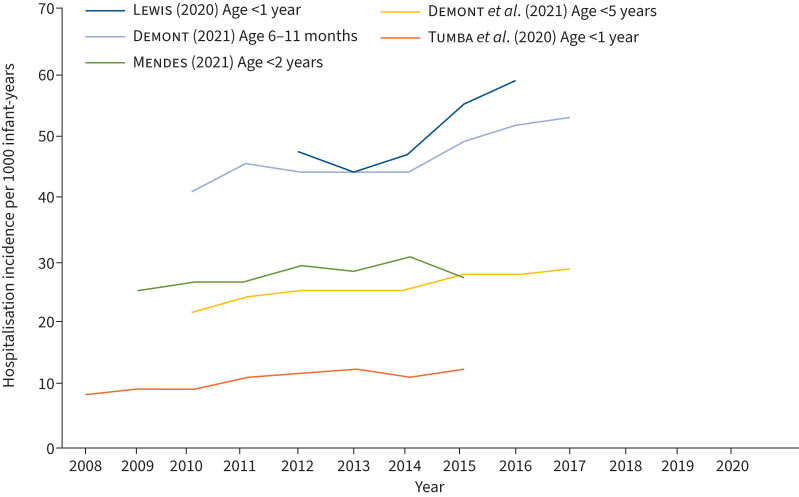
FIGURE 1.
Respiratory syncytial virus (RSV) hospitalisation incidence per 1000 infant-years, from 2008 onwards.
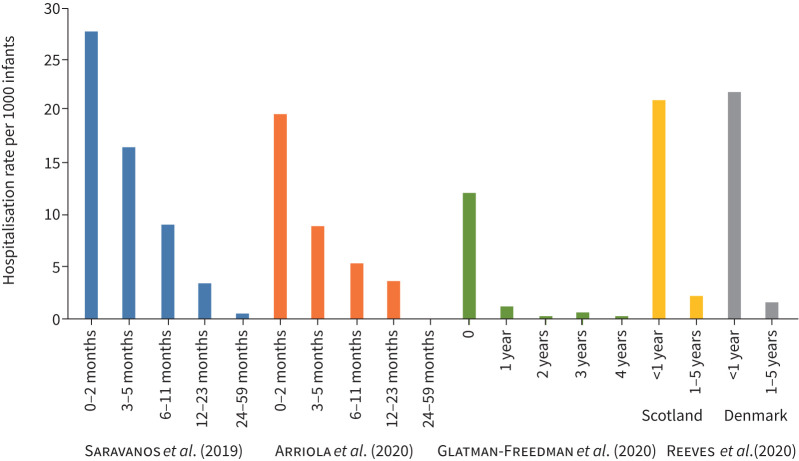
FIGURE 2.
Annual respiratory syncytial virus (RSV)-associated hospitalisation rate per 1000 infants, broken down by age. Please note that the age group brackets of the included studies differ.
RSV-associated hospital admissions (table 1, figure 1)
Increase in incidence/rate over time
We found evidence that the number of infant RSV hospital admissions has been increasing in recent years across the world before the COVID-19 pandemic, with several studies reporting a year-on-year increase in RSV hospitalisations from 2004 [7, 13, 14] (figure 1). Approximately 80% of hospitalised bronchiolitis cases among children under 1 year in England are attributable to RSV [15] (table 1). Lewis et al. (2020) [16] documented the increased bronchiolitis admission rate in England from 47.4 (95% CI 46.8–47.9) to 58.9 per 1000 infant-years (95% CI 58.3–59.5) between 2012 and 2016. A study by Tumba et al. (2020) [14] reported a 49% increase in the incidence of hospitalisation for bronchiolitis in Brazil between January 2008 and December 2015 (8.5 to 12.7 per 1000 infant-years), with RSV attributed as the aetiologic factor. Chung et al. (2020) [7] conducted a 15-year longitudinal study (2001–2016) of RSV admission rates for children under 2 years of age in Scotland and found a 2.20-fold increase (95% CI 1.4–3.6-fold) from 17.2 (15.9–18.5) to 37.7 (37.4–38.1) admissions per 1000 infant-years [7]. A French study by Demont et al. (2021) [17] investigating the economic burden of RSV hospitalisations found that incidence of RSV hospitalisations in under 5s increased from 21.96 to 28.8 per 1000 infant-years between 2010 and 2018. However, the absence of an increased number of RSV cases during the 2020 season is likely attributable to external factors, such as the SARS-CoV-2 pandemic and subsequent behavioural and sociological shifts due to NPIs.
Age at time of RSV hospitalisation (figure 2)
Several studies have confirmed an increase in RSV hospitalisations with a decrease in patient age, with infants <1 year old having the highest burden of RSV hospitalisations, with admissions peaking for those below 2 months of age [18–23] (figure 2). Reeves et al. (2020) [22] found the mean annual RSV-coded admission rates ranged from 20.5 to 22.3 per 1000 children aged <1 year in Scotland, Finland, Norway and Denmark, as compared with a range of 1.25 to 2.24 per 1000 children in children aged 1–4 years. Arriola et al. (2020) [18] reported similar findings with the adjusted age-specific RSV hospitalisation rates per 1000 population being 19.7 (95% CI 17.87–21.77), 8.97 (95% CI 7.61–10.73), 5.31 (95% CI 4.59–6.24) and 3.58 (95% CI 3.17–4.05) for ages 0–2, 3–5, 6–11 and 12–23 months, respectively. Saravanos et al. (2019) [19] also found that the highest RSV hospitalisation rate was for infants aged <2 months (27.78 per 1000 population). Glatman-Freedman et al.’s (2020) [20] study in Israel concurred with the other studies, with hospitalisation load of RSV-related diagnoses reported as highest in infants <1 year of age (mean yearly rate of 12.18 per 1000 infants), rapidly declining in the following years. According to Hardelid et al. (2019) [21], the overall RSV admission rate in the first, second and third year of life was 21.9, 7.0 and 2.0 per 1000 infant-years, respectively, indicating a clear reduction in hospitalisation with age. Demont et al. (2021) [17] found that the most burdened age group in terms of RSV hospitalisation was aged <1 year, representing 69% of hospitalisations. Incidence of RSV hospitalisations in this population increased from 0.52 to 0.74 per 1000 infant-years between 2010 and 2018 and was significantly higher than in other age groups.
Impact of COVID-19 restrictions on RSV
Three of the studies conducted during the COVID-19 pandemic confirmed a change in RSV hospitalisation dynamics during periods of NPI, as compared with previous years [24–26]. Pelletier et al. (2020) [24] demonstrated a decrease in the number of RSV hospital admissions in the USA beginning in March 2020 compared with the period from 2010 to 2019. In April 2020, hospital admissions were reduced by 45.4% compared with previous years (23 798 in April 2020 compared with a median (interquartile range) of 43 550 (42 110–43 946) in April 2010–2019). An Australian study conducted by Britton et al. (2020) [25] observed a reduced mean frequency of RSV detections between April and June 2020, which was 94.3% lower than predicted based on 2015–2019 data (mean±se 99±24; p=0.026). Furthermore, Wilder et al. (2020) [26] found that the mean number of bronchiolitis admissions was lower in the COVID-19 cohort than the pre-COVID-19 cohort (1 versus 7; p=0.008). A retrospective analysis of the sentinel surveillance system for viruses in Austria by Redlberger-Fritz et al. (2021) [27] also confirmed a rapid reduction in RSV prevalence, a week after national lockdown measures were imposed in Austria in March 2020. The study confirmed 19 RSV detections after lockdown compared to 82 (95% CI 65−104) (p<0.001) detections observed in the equivalent period during the previous seasons [27]. Together, these studies suggest an association between the introduction of COVID-19 social distancing and lockdown measures and a reduction in burden of RSV hospitalisations amongst infants.
Seasonality of RSV infections (table 2)
A global study by Pangesti et al. (2019) [28] established that generally in temperate countries located in the Northern and Southern hemispheres, a peak of RSV admissions occurs in winter months, while in subtropical and tropical countries, admissions peak mostly during the rainy season. The results of a 17-year Israeli study by Glatman-Freedman et al. (2020) [20] reported that RSV-related hospitalisations followed a clear seasonal pattern, in which the peak occurred in January for 14 seasons, in December for two seasons (2014/2015 and 2015/2016) and in February for one season (2004/2005). In India, Broor et al. [29] observed RSV detection to peak in winter (November–February) and in rainy seasons.
Three studies in the UK produced similar findings, with a peak in admissions seen in infants born during the winter months of September to December [7, 15, 16]. Reeves et al. (2019) [30] found that RSV-associated admissions peaked in infants born in September to November. Similarly, Chung et al. (2020) [7] found that RSV admissions peaked in infants born in the 3 months preceding December, the month when cases of bronchiolitis are highest. Across the Clinical Commissioning Groups in England, Lewis et al. (2020) [16] found there was a 5.3-fold variation in incidence with area-level deprivation and the epidemic peak ranged from week 49.3 to 52.2. A study by Glick et al. (2020) [31], comparing paediatric RSV hospitalisations with regional RSV activity in the USA, produced similar findings to the British studies and confirmed that the mean RSV hospitalisation season fell in early November.
Furthermore, in the study by Reeves et al. (2020) [22], biennial peaks were seen in RSV-coded admission rates for Finland, Norway and Denmark, with a higher admission rate one year followed by a lower rate in the next. This shows a clear pattern of seasonality within RSV that should enable a country to predict the approximate timeframe in which an increased number of infections and hospitalisations might occur.
Discussion
This scoping review provides an overview of the global trends in RSV-associated detections and hospitalisations reported in the literature since 2009 to present. RSV hospitalisations have been widely studied, given the impact on infant mortality and financial burden on healthcare systems. We located studies from Mali, Nicaragua, Philippines, Spain, England, Israel, Hong Kong, Austria, USA and beyond to inform trends amongst individuals from diverse and varied socioeconomic backgrounds.
The results of our review indicate that the incidence of RSV hospitalisations has been increasing longitudinally and that the highest number of hospitalisations is reported in 0–2-month-old infants. Green et al. (2016) [13] suggest that the general increase in bronchiolitis admissions may not be due to changes in the severity or transmissibility of RSV but to changes to healthcare; changes in healthcare policies such as admission thresholds, as well as an increase in hospital bed availability, have been suggested as drivers of the increase, rather than a true increase in incidence. Tumba et al. (2020) [14] suggest that the increase is down to external factors, speculating that the increase in bronchiolitis hospitalisations seen in Brazilian children could be due to an increase in caesarean sections being performed, prematurity rates and increased urbanisation. Premature babies are prone to developing incomplete lung function and are more susceptible to respiratory problems [14]. Nenna et al. (2017) [32] found both indoor and outdoor pollution to be risk factors for acute bronchiolitis in infants aged <3 years old. In addition, a study by Robledo-Aceves et al. (2018) [33] showed that neonatal infection with other respiratory viruses, including rhinovirus, were risk factors for bronchiolitis. Hardelid et al. (2019) [21] reported premature birth, having older siblings, maternal age <30, low socioeconomic status and delayed infant vaccination as risk factors significantly associated with increased RSV hospitalisation. Additionally, Carbonell et al. (2012) [34] found that male sex, tobacco smoking while pregnant, month of birth, duration of breastfeeding, number of siblings at school and number of smokers in a household all contributed to the risk of RSV hospitalisation amongst infants. Similar risk factors were associated with the need for mechanical ventilation and supplemental oxygen in a prospective cohort study of 299 RSV-positive infants admitted to the Alder Hey Children's Hospital, Liverpool [35].
We also found some evidence for an association between socioeconomic status, deprivation and RSV admissions; early RSV epidemic peaks have been associated with areas of higher population densities, such as London and Manchester in England [15] and in Scotland [7].
The reduction in RSV-associated hospitalisations during the COVID-19 pandemic can possibly be attributed to the implementation of public health interventions to prevent or reduce the transmission of SARS-CoV-2 [25–27, 36]. Handwashing and isolation are known effective measures against nosocomial RSV transmission. A very high uptake (>84%) of enhanced hygiene and physical distancing measures could explain the large reduction in RSV incidence and hospitalisation reported in Australia in March 2020 [25]. Significant reductions in other diseases associated with social distancing measures were also reported, e.g. in overall hospitalisations for bronchiolitis, pneumonia and asthma during the initial SARS-CoV-2 outbreak, compared with the same calendar period in the 4 previous years [26]. By contrast, there was no change in hospitalisations for those conditions, such as cellulitis, gastroesophageal reflux disease and urinary tract infection, not known to be associated with viral infections and social distancing measures [26].
Chung et al.'s (2020) [7] study of bronchiolitis amongst children under 2 years of age in Scotland concluded that the increase in bronchiolitis admissions may be due to similar factors which have contributed to an increase in all paediatric hospital admissions in Scotland: infants attending day-care at younger age (resulting in an earlier exposure to pathogens); a decrease in out-of-hours care availability due to changes of GP practice contracts; and changes in parental expectations of treatment. Given the varying healthcare and social systems across the globe, these reasons might not be generalisable elsewhere but do provide potential insights.
Redlberger-Fritz et al. (2021) [27] state that the Austrian national lockdown led to 70% reduction of mobility and had a significant impact on the prevalence of respiratory viruses in Austria, including RSV. The study also cites Belingheri et al. (2020) [36] confirming that lockdown measures led to a reduction in epidemic diseases of childhood in Italy, including chickenpox, rubella, pertussis and measles. The reductions are attributed to the forced lockdown, closure of schools and public meeting places and the intensive use of masks in the general population leading to a reduction in inhalation of airborne respiratory droplets [36]. The study also highlights that a reduction in viral diseases during the pandemic could be a result of a decrease in individuals visiting emergency departments due to the fear of being infected at the hospital [36]. However, the global reduction in RSV hospitalisations of such a magnitude cannot be solely attributed to individuals avoiding emergency care. Hence, one can conclude that the preventative measures in place for COVID-19 also curbed the transmission of other viral diseases, including RSV.
Our findings suggest that there is a distinct seasonal pattern to RSV incidence and RSV hospitalisations, with a peak in RSV infections observed during the winter in temperate regions; however, there is no general agreement as to why this occurs. It has been suggested that this may be due to a link between temperature and RSV infection [37], or the inhalation of the air in cold temperatures causing nasal passages to cool thus inhibiting their respiratory defence [38]. However, it has also been argued that the seasonal peaks of RSV are associated with higher environmental temperature [39] or the crowding of susceptible individuals indoors during cold winter weather leading to greater transmission of respiratory viruses [40]. Relative humidity and rainfall have also been suggested to affect RSV activity [37, 39, 41].
Having older siblings at nursery or school has also been identified to increase the risk of RSV hospitalisation. Attending closed settings outside of the home increases infection risks through greater contact with other children [21, 42].
This review has a number of limitations. Since this is a scoping review, the literature search may have missed some relevant publications within grey literature and articles from the Cochrane Library that might encompass further eligible studies. We also could not provide cumulative means for the trends in hospitalisations as we were unable to extract data from all the studies. None of the eligible studies were critically appraised and might include biases owing to respective study designs, definitions and sources of data. Definitions for hospitalisation may vary from study to study and among countries. We also have not grouped the studies/findings based on low-, middle- and high-income countries.
Conclusion
Evidence strongly suggests that RSV hospitalisation amongst children aged ≤5 years has increased in the past decade and follows a distinct seasonal pattern. The highest number of hospitalisations was reported in the 0–2-month-old age group. When comparing the <1-year-old age group with the 1–5-year-old group, hospitalisations were considerably higher in the infants <1 year old. RSV hospitalisation rates decreased with the introduction of social distancing measures; in some cases a reduction of up to 94.3% as compared with previous years has been reported. As countries have begun to lift restrictions, emerging reports of spikes in RSV hospitalisations from Australia, Austria and the UK are concerning. Countries have also reported out of season spikes, with the southern states of the USA reporting a spike in the summer months of 2021, at variance with the predictable seasonality of RSV in the Northern Hemisphere. Furthermore, with older siblings returning to school, infants born during lockdowns, particularly those born prematurely, will be highly prone to contracting RSV from their siblings. Further research is required to understand the changes in the epidemiology of RSV due to waves of COVID-19 restrictions and subsequent easements to support planning for increased demands on often already overburdened paediatric intensive care and medical services.
Acknowledgements
We would like to extend our gratitude to our colleagues at the UK Health Security Agency: Hannah Williams and Joseph Shingleton for providing valuable advice on the search strategy, and Thomas Finnie and Andre Charlett for reviewing the final draft of the paper.
Provenance: Submitted article, peer reviewed.
Conflict of interest: The authors have nothing to disclose.
References
- 1.Yassine HM, Sohail MU, Younes N, et al. Systematic review of the respiratory syncytial virus (RSV) prevalence, genotype distribution, and seasonality in children from the Middle East and North Africa (MENA) Region. Microorganisms 2020; 8: 713. doi: 10.3390/microorganisms8050713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambert L, Sagfors AM, Openshaw PJ, et al. Immunity to RSV in early-life. Front Immunol 2014; 5: 466. doi: 10.3389/fimmu.2014.00466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hacimustafaoglu M, Celebi S, Bozdemir SE, et al. RSV frequency in children below 2 years hospitalized for lower respiratory tract infections. Turk J Pediatr 2013; 55: 130–139. [PubMed] [Google Scholar]
- 4.Wrotek A, Kobiałka M, Grochowski B, et al. Respiratory complications in children hospitalized with respiratory syncytial virus infection. Adv Exp Med Biol 2020; 1279: 113–120. doi: 10.1007/5584_2020_530 [DOI] [PubMed] [Google Scholar]
- 5.Graham BS. Vaccine development for respiratory syncytial virus. Curr Opin Virol 2017; 23: 107–112. doi: 10.1016/j.coviro.2017.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi T, McAllister DA, O'Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390: 946–958. doi: 10.1016/S0140-6736(17)30938-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung A, Reeves RM, Nair H, et al. Hospital admission trends for bronchiolitis in Scotland, 2001–2016: a national retrospective observational study. J Infect Dis 2020; 222: Suppl 7, S592–S598. doi: 10.1093/infdis/jiaa323 [DOI] [PubMed] [Google Scholar]
- 8.Reeves RM, Hardelid P, Gilbert R, et al. Estimating the burden of respiratory syncytial virus (RSV) on respiratory hospital admissions in children less than five years of age in England, 2007–2012. Influenza Other Respir Viruses 2017; 11: 122–129. doi: 10.1111/irv.12443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park KY, Seo S, Han J, et al. Respiratory virus surveillance in Canada during the COVID-19 pandemic: an epidemiological analysis of the effectiveness of pandemic-related public health measures in reducing seasonal respiratory viruses test positivity. PLoS One 2021; 16: e0253451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuitunen I, Artama M, Makela L, et al. Effect of social distancing due to the COVID-19 pandemic on the incidence of viral respiratory tract infections in children in Finland during early 2020. Pediatr Infect Dis J 2020; 39: e423–e427. doi: 10.1097/INF.0000000000002845 [DOI] [PubMed] [Google Scholar]
- 11.Foley DA, Yeoh DK, Minney-Smith CA, et al. The interseasonal resurgence of respiratory syncytial virus in Australian children following the reduction of coronavirus disease 2019-related public health measures. Clin Infect Dis 2021; 73: e2829–e2830. doi: 10.1093/cid/ciaa1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agha R, Avner JR. Delayed seasonal RSV surge observed during the COVID-19 pandemic. Pediatrics 2021; 148: e2021052089. doi: 10.1542/peds.2021-052089 [DOI] [PubMed] [Google Scholar]
- 13.Green CA, Yeates D, Goldacre A, et al. Admission to hospital for bronchiolitis in England: trends over five decades, geographical variation and association with perinatal characteristics and subsequent asthma. Arch Dis Child 2016; 101: 140–146. doi: 10.1136/archdischild-2015-308723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tumba K, Comaru T, Machado C, et al. Temporal trend of hospitalizations for acute bronchiolitis in infants under one year of age in BRAZIL between 2008 and 2015. Rev Paul Pediatr 2020; 38: e2018120. doi: 10.1590/1984-0462/2020/38/2018120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis KM, De Stavola B, Hardelid P. Geospatial and seasonal variation of bronchiolitis in England: a cohort study using hospital episode statistics. Thorax 2020; 75: 262–268. doi: 10.1136/thoraxjnl-2019-213764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis K, De Stavola B, Hardelid P. Is socioeconomic position associated with bronchiolitis seasonality? A cohort study. J Epidemiol Community Health 2021; 75: 76–83. [DOI] [PubMed] [Google Scholar]
- 17.Demont C, Petrica N, Bardoulat I, et al. Economic and disease burden of RSV-associated hospitalizations in young children in France, from 2010 through 2018. BMC Infect Dis 2021; 21: 730. doi: 10.1186/s12879-021-06399-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arriola CS, Kim L, Langley G, et al. Estimated burden of community-onset respiratory syncytial virus-associated hospitalizations among children aged <2 years in the United States, 2014–15. J Pediatric Infect Dis Soc 2020; 9: 587–595. doi: 10.1093/jpids/piz087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saravanos GL, Sheel M, Homaira N, et al. Respiratory syncytial virus-associated hospitalisations in Australia, 2006–2015. Med J Aust 2019; 210: 447–453. doi: 10.5694/mja2.50159 [DOI] [PubMed] [Google Scholar]
- 20.Glatman-Freedman A, Kaufman Z, Applbaum Y, et al. Respiratory syncytial virus hospitalization burden: a nation-wide population-based analysis, 2000–2017. J Infect 2020; 81: 297–303. doi: 10.1016/j.jinf.2020.05.078 [DOI] [PubMed] [Google Scholar]
- 21.Hardelid P, Verfuerden M, McMenamin J, et al. The contribution of child, family and health service factors to respiratory syncytial virus (RSV) hospital admissions in the first 3 years of life: birth cohort study in Scotland, 2009 to 2015. Euro Surveill 2019; 24: 1800046. doi: 10.2807/1560-7917.ES.2019.24.1.1800046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reeves RM, van Wijhe M, Tong S, et al. Respiratory syncytial virus-associated hospital admissions in children younger than 5 years in 7 European countries using routinely collected datasets. J Infect Dis 2020; 222: Suppl 7, S599–S605. doi: 10.1093/infdis/jiaa360 [DOI] [PubMed] [Google Scholar]
- 23.Svensson C, Berg K, Sigurs N, et al. Incidence, risk factors and hospital burden in children under five years of age hospitalised with respiratory syncytial virus infections. Acta Paediatr 2015; 104: 922–926. doi: 10.1111/apa.13061 [DOI] [PubMed] [Google Scholar]
- 24.Pelletier JH, Rakkar J, Au AK, et al. Trends in US pediatric hospital admissions in 2020 compared with the decade before the COVID-19 pandemic. JAMA Netw Open 2021; 4: e2037227. doi: 10.1001/jamanetworkopen.2020.37227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Britton PN, Hu N, Saravanos G, et al. COVID-19 public health measures and respiratory syncytial virus. Lancet Child Adolesc Health 2020; 4: e42–e43. doi: 10.1016/S2352-4642(20)30307-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilder JL, Parsons CR, Growdon AS, et al. Pediatric hospitalizations during the COVID-19 pandemic. Pediatrics 2020; 146: e2020005983. doi: 10.1542/peds.2020-005983 [DOI] [PubMed] [Google Scholar]
- 27.Redlberger-Fritz M, Kundi M, Aberle SW, et al. Significant impact of nationwide SARS-CoV-2 lockdown measures on the circulation of other respiratory virus infections in Austria. J Clin Virol 2021; 137: 104795. doi: 10.1016/j.jcv.2021.104795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pangesti KNA, El Ghany MA, Kesson AM, et al. Respiratory syncytial virus in the western Pacific region: a systematic review and meta-analysis. J Glob Health 2019; 9: 020431. doi: 10.7189/jogh.09.020431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broor S, Dawood FS, Pandey BG, et al. Rates of respiratory virus-associated hospitalization in children aged <5 years in rural northern India. J Infect 2014; 68: 281–289. doi: 10.1016/j.jinf.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reeves RM, Hardelid P, Panagiotopoulos N, et al. Burden of hospital admissions caused by respiratory syncytial virus (RSV) in infants in England: a data linkage modelling study. J Infect 2019; 78: 468–475. doi: 10.1016/j.jinf.2019.02.012 [DOI] [PubMed] [Google Scholar]
- 31.Glick AF, Kjelleren S, Hofstetter AM, et al. RSV hospitalizations in comparison with regional RSV activity and inpatient palivizumab administration, 2010–2013. Hosp Pediatr 2017; 7: 271–278. doi: 10.1542/hpeds.2016-0124 [DOI] [PubMed] [Google Scholar]
- 32.Nenna R, Cutrera R, Frassanito A, et al. Modifiable risk factors associated with bronchiolitis. Ther Adv Respir Dis 2017; 11: 393–401. doi: 10.1177/1753465817725722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robledo-Aceves M, Moreno-Peregrina MJ, Velarde-Rivera F, et al. Risk factors for severe bronchiolitis caused by respiratory virus infections among Mexican children in an emergency department. Medicine (Baltimore) 2018; 97: e0057. doi: 10.1097/MD.0000000000010057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carbonell X, Fullarton JR, Gooch KL, et al. The evolution of risk factors for respiratory syncytial virus-related hospitalisation in infants born at 32–35 weeks’ gestational age: time-based analysis using data from the FLIP-2 study. J Perinat Med 2012; 40: 685–691. doi: 10.1515/jpm-2011-0248 [DOI] [PubMed] [Google Scholar]
- 35.Semple MG, Taylor-Robinson DC, Lane S, et al. Household tobacco smoke and admission weight predict severe bronchiolitis in infants independent of deprivation: prospective cohort study. PLoS One 2011; 6: e22425. doi: 10.1371/journal.pone.0022425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belingheri M, Paladino ME, Piacenti S, et al. Effects of COVID-19 lockdown on epidemic diseases of childhood. J Med Virol 2021; 93: 153–154. doi: 10.1002/jmv.26253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan PW, Chew FT, Tan TN, et al. Seasonal variation in respiratory syncytial virus chest infection in the tropics. Pediatr Pulmonol 2002; 34: 47–51. doi: 10.1002/ppul.10095 [DOI] [PubMed] [Google Scholar]
- 38.Eccles R. An explanation for the seasonality of acute upper respiratory tract viral infections. Acta Otolaryngol 2002; 122: 183–191. doi: 10.1080/00016480252814207 [DOI] [PubMed] [Google Scholar]
- 39.Shek LP-C, Lee B-W. Epidemiology and seasonality of respiratory tract virus infections in the tropics. Paediatr Respir Rev 2003; 4: 105–111. doi: 10.1016/S1526-0542(03)00024-1 [DOI] [PubMed] [Google Scholar]
- 40.Monto AS. Epidemiology of viral respiratory infections. Am J Med 2002; 112: 6, Suppl 1, 4–12. doi: 10.1016/S0002-9343(01)01058-0 [DOI] [PubMed] [Google Scholar]
- 41.Thongpan I, Vongpunsawad S, Poovorawan Y. Respiratory syncytial virus infection trend is associated with meteorological factors. Sci Rep 2020; 10: 10931. doi: 10.1038/s41598-020-67969-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munywoki PK, Koech DC, Agoti CN, et al. The source of respiratory syncytial virus infection in infants: a household cohort study in rural Kenya. J Infect Dis 2014; 209: 1685–1692. doi: 10.1093/infdis/jit828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thwaites R, Buchan S, Fullarton J, et al. Clinical burden of severe respiratory syncytial virus infection during the first 2 years of life in children born between 2000 and 2011 in Scotland. Eur J Pediatr 2020; 179: 791–799. doi: 10.1007/s00431-019-03564-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buchwald AG, Tamboura B, Tennant SM, et al. Epidemiology, risk factors, and outcomes of respiratory syncytial virus infections in newborns in Bamako, Mali. Clin Infect Dis 2020; 70: 59–66. doi: 10.1093/cid/ciz157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benitez-Guerra D, Pina-Flores C, Zamora-Lopez M, et al. Respiratory syncytial virus acute respiratory infection-associated hospitalizations in preterm Mexican infants: a cohort study. Influenza Other Respir Viruses 2020; 14: 182–188. doi: 10.1111/irv.12708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mendes-da-Silva A, Goncalves-Pinho M, Freitas A, et al. Trends in hospitalization for acute bronchiolitis in Portugal: 2000–2015. Pulmonology 2019; 25: 154–161. doi: 10.1016/j.pulmoe.2018.05.002 [DOI] [PubMed] [Google Scholar]
- 47.Rha B, Curns AT, Lively JY, et al. Respiratory syncytial virus-associated hospitalizations among young children: 2015–2016. Pediatrics 2020; 146: e20193611. doi: 10.1542/peds.2019-3611 [DOI] [PubMed] [Google Scholar]
- 48.Li Y, Johnson EK, Shi T, et al. National burden estimates of hospitalisations for acute lower respiratory infections due to respiratory syncytial virus in young children in 2019 among 58 countries: a modelling study. Lancet Respir Med 2021; 9: 175–185. doi: 10.1016/S2213-2600(20)30322-2 [DOI] [PubMed] [Google Scholar]
- 49.Prasad N, Newbern EC, Trenholme AA, et al. Respiratory syncytial virus hospitalisations among young children: a data linkage study. Epidemiol Infect 2019; 147: e246. doi: 10.1017/S0950268819001377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greenberg D, Dagan R, Shany E, et al. Incidence of respiratory syncytial virus bronchiolitis in hospitalized infants born at 33–36 weeks of gestational age compared with those born at term: a retrospective cohort study. Clin Microbiol Infect 2020; 26: 256.e1–256.e5. doi: 10.1016/j.cmi.2019.05.025 [DOI] [PubMed] [Google Scholar]
- 51.Fujiogi M, Goto T, Yasunaga H, et al. Trends in bronchiolitis hospitalizations in the United States: 2000–2016. Pediatrics 2019; 144: e20192614. doi: 10.1542/peds.2019-2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kramer R, Duclos A, VRS study group in Lyon, et al. Cost and burden of RSV related hospitalisation from 2012 to 2017 in the first year of life in Lyon, France. Vaccine 2018; 36: 6591–6593. doi: 10.1016/j.vaccine.2018.09.029 [DOI] [PubMed] [Google Scholar]
- 53.Oakley E, Chong V, Borland M, et al. Intensive care unit admissions and ventilation support in infants with bronchiolitis. Emerg Med Australas 2017; 29: 421–428. doi: 10.1111/1742-6723.12778 [DOI] [PubMed] [Google Scholar]
- 54.Cromer D, van Hoek AJ, Newall AT, et al. Burden of paediatric respiratory syncytial virus disease and potential effect of different immunisation strategies: a modelling and cost-effectiveness analysis for England. Lancet Public Health 2017; 2: e367–e374. doi: 10.1016/S2468-2667(17)30103-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanchez-Luna M, Elola FJ, Fernandez-Perez C, et al. Trends in respiratory syncytial virus bronchiolitis hospitalizations in children less than 1 year: 2004–2012. Curr Med Res Opin 2016; 32: 693–698. doi: 10.1185/03007995.2015.1136606 [DOI] [PubMed] [Google Scholar]
- 56.Munoz-Quiles C, Lopez-Lacort M, Ubeda-Sansano I, et al. Population-based analysis of bronchiolitis epidemiology in Valencia, Spain. Pediatr Infect Dis J 2016; 35: 275–280. doi: 10.1097/INF.0000000000000993 [DOI] [PubMed] [Google Scholar]
- 57.Saha S, Pandey BG, Choudekar A, et al. Evaluation of case definitions for estimation of respiratory syncytial virus associated hospitalizations among children in a rural community of northern India. J Glob Health 2015; 5: 010419. doi: 10.7189/jogh.05.020419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Helfrich AM, Nylund CM, Eberly MD, et al. Healthy late-preterm infants born 33-36+6 weeks gestational age have higher risk for respiratory syncytial virus hospitalization. Early Hum Dev 2015; 91: 541–546. doi: 10.1016/j.earlhumdev.2015.06.009 [DOI] [PubMed] [Google Scholar]
- 59.Ochoa TJ, Bautista R, Davila C, et al. Respiratory syncytial virus-associated hospitalizations in pre-mature infants in Lima, Peru. Am J Trop Med Hyg 2014; 91: 1029–1034. doi: 10.4269/ajtmh.13-0648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nasreen S, Luby SP, Brooks WA, et al. Population-based incidence of severe acute respiratory virus infections among children aged <5 years in rural Bangladesh, June-October 2010. PloS One 2014; 9: e89978. doi: 10.1371/journal.pone.0089978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murray J, Saxena S, Sharland M. Preventing severe respiratory syncytial virus disease: passive, active immunisation and new antivirals. Arch Dis Child 2014; 99: 469–473. doi: 10.1136/archdischild-2013-303764 [DOI] [PubMed] [Google Scholar]
- 62.Emukule GO, Khagayi S, McMorrow ML, et al. The burden of influenza and RSV among inpatients and outpatients in rural western Kenya, 2009–2012. PLoS One 2014; 9: e105543. doi: 10.1371/journal.pone.0105543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ambrose CS, Anderson EJ, Simoes EAF, et al. Respiratory syncytial virus disease in preterm infants in the U.S. born at 32–35 weeks gestation not receiving immunoprophylaxis. Pediatr Infect Dis J 2014; 33: 576–582. doi: 10.1097/INF.0000000000000219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rowlinson E, Dueger E, Taylor T, et al. Incidence and clinical features of respiratory syncytial virus infections in a population-based surveillance site in the Nile Delta Region. J Infect Dis 2013; 208: Suppl 3, S189–S196. doi: 10.1093/infdis/jit457 [DOI] [PubMed] [Google Scholar]
- 65.Naorat S, Chittaganpitch M, Thamthitiwat S, et al. Hospitalizations for acute lower respiratory tract infection due to respiratory syncytial virus in Thailand, 2008–2011. J Infect Dis 2013; 208: S238–S245. doi: 10.1093/infdis/jit456 [DOI] [PubMed] [Google Scholar]
- 66.McCracken JP, Prill MM, Arvelo W, et al. Respiratory syncytial virus infection in Guatemala, 2007–2012. J Infect Dis 2013; 208: S197–S206. doi: 10.1093/infdis/jit517 [DOI] [PubMed] [Google Scholar]
- 67.Eidelman AI, Megged O, Feldman R, et al. The burden of respiratory syncytial virus bronchiolitis on a pediatric inpatient service. Isr Med Assoc J 2009; 11: 533–536. [PubMed] [Google Scholar]
- 68.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009; 360: 588–598. doi: 10.1056/NEJMoa0804877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tong S, Amand C, Kieffer A, et al. Incidence of respiratory syncytial virus related health care utilization in the United States. J Glob Health 2020; 10: 020422. doi: 10.7189/jogh.10.020422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kubale J, Kuan G, Gresh L, et al. Assessing the incidence of symptomatic respiratory syncytial virus illness within a prospective birth cohort in Managua, Nicaragua. Clin Infect Dis 2020; 70: 2029–2035. doi: 10.1093/cid/ciz585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ueno F, Tamaki R, Saito M, et al. Age-specific incidence rates and risk factors for respiratory syncytial virus-associated lower respiratory tract illness in cohort children under 5 years old in the Philippines. Influenza Other Respir Viruses 2019; 13: 339–353. doi: 10.1111/irv.12639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu J, Liu C, Xiao Y, et al. Respiratory syncytial virus seasonality, Beijing, China, 2007–2015. Emerg Infect Dis 2019; 25: 1127–1135. doi: 10.3201/eid2506.180532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leung T, Lam D, Miu T, et al. Epidemiology and risk factors for severe respiratory syncytial virus infections requiring pediatric intensive care admission in Hong Kong children. Infection 2014; 42: 343–350. doi: 10.1007/s15010-013-0557-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.du Prel J-B, Puppe W, Grondahl B, et al. Are meteorological parameters associated with acute respiratory tract infections? Clin Infect Dis 2009; 49: 861–868. doi: 10.1086/605435 [DOI] [PubMed] [Google Scholar]
- 75.Chan PKS, Tam WWS, Lee TC, et al. Hospitalization incidence, mortality, and seasonality of common respiratory viruses over a period of 15 years in a developed subtropical city. Medicine (Baltimore) 2015; 94: e2024. doi: 10.1097/MD.0000000000002024 [DOI] [PMC free article] [PubMed] [Google Scholar]