Abstract
Objective
The objective of this subanalysis of data from centres across urban areas in India of the Global Asthma Network (GAN) was to study 1) the prevalence of symptoms of asthma in children and adults, 2) the change in prevalence of asthma and its trigger factors since the International Study of Asthma and Allergies in Childhood (ISAAC), and 3) current asthma treatment practice.
Methods
In this cross-sectional, multicentre, school-based and self-administered questionnaire, responses from children aged 6–7 years and 13–14 years, and their respective parents, were analysed.
Results
The GAN Phase I study included 20 084 children in the 6–7-year age group, 25 887 children in the 13–14-year age group and 81 296 parents. The prevalence of wheeze in the previous 12 months was 3.16%, 3.63% and 3.30% in the three groups, respectively. In comparison to the ISAAC studies, there was a significant reduction in the prevalence of current wheeze (p<0.001). Bivariate analysis revealed a significant reduction in the prevalence of trigger factors. Almost 82% of current wheezers and 70% of subjects with symptoms of severe asthma were not clinically diagnosed as having asthma. The daily use of inhaled corticosteroids (ICS) was less than 2.5% in subjects with current wheeze and those with symptoms of severe asthma but less than 1% used daily ICS when asthma remained undiagnosed.
Conclusion
The prevalence of current wheeze and its causal factors showed a significant reduction compared to previous ISAAC studies. Among subjects with current wheeze and symptoms of severe asthma, the problem of under-diagnosis and under-treatment was widespread.
Short abstract
Data from Indian centres that participated in the multicentre Global Asthma Network showed a significant decline in symptoms of asthma compared to previous studies. The study highlighted under-diagnosis and under-treatment in children and adults with asthma. https://bit.ly/3thevot
Introduction
The prevalence of asthma varies widely among countries/geographical regions and also within countries with different geographies and socioeconomic strata [1, 2]. The Indian Study on Epidemiology of Asthma, Respiratory Symptoms and Chronic Bronchitis in Adults (INSEARCH) estimated the national burden of asthma at 17.23 million with an overall prevalence of 2.05% [3]. The recent Global Burden of Disease (GBD, 1990–2019) estimated the total burden of asthma in India as 34.3 million, accounting for 13.09% of the global burden [4]. It also attributed that there were 13.2 per thousand deaths due to asthma in India [4]. Asthma accounted for 27.9% of disability-adjusted life years (DALYs) in the Indian population [4]. On the whole, India has three times higher mortality and more than two times higher DALYs compared to the global proportion of asthma burden. The disproportionate mortality and morbidity can be explained by global studies with uniform methodology.
The International Study of Asthma and Allergy in Childhood (ISAAC) Phase I (1995) and Phase III (2001–03) are the largest multicentre global studies to be conducted with uniform methodology worldwide [5, 6]. The studies showed around 6% of children in India had current wheezing and identified several environmental factors associated with asthma globally, including environmental tobacco smoke [7], cooking with firewood [8], exposure to heavy truck traffic [9], obesity [10], fast-food consumption [11], dampness in homes [12] and paracetamol/antibiotic use [13].
Over the past two decades there has been a change in the economy, industrialisation, air pollution levels, and environmental and socio-cultural factors in India. Apart from the ISAAC studies, no other worldwide multicentre studies have been conducted to analyse the impact of these changes on the prevalence and severity of asthma. The ISAAC study group was resurrected in 2012 in the form of the Global Asthma Network (GAN), to estimate the current prevalence of symptoms of asthma and allergies [14].
The GAN study included certain aspects that were not covered in the ISAAC studies, including the prevalence of asthma symptoms amongst parents of children and the medications used for control [14]. The objective of this paper was to analyse the GAN Phase I data from Indian centres for 1) the prevalence of asthmatic symptoms in children (aged 6–7 years and 13–14 years) and their adult parents; 2) the change in prevalence of asthmatic symptoms in children compared to previous ISAAC studies and associated environmental factors, and 3) the current use of medicines among children and adults with asthma.
Materials and methods
The GAN Phase I study was a cross-sectional, multi-country, multicentre and questionnaire-based epidemiological research study conducted in schools. The study protocol and methodology were similar to the previous ISAAC Phase III study, details of which are explained in an earlier publication [14]. The GAN Phase I study included seven centres that participated in ISAAC Phase III and two new centres. The new centres (Mysuru and Kolkata, representing south and east India, respectively) were included to better represent all geographical areas. The survey was conducted across nine centres in India (Bikaner, Chandigarh, Delhi, Jaipur, Kolkata, Kottayam, Mysuru, Lucknow and Pune) in the 13–14-year age group and eight centres in the 6–7-year age group of children (Kolkata did not participate in the younger age group). The study was approved by the ethics committee from the respective local centres and was registered in the Clinical Trial Registry, India (CTRI/2018/02/011758).
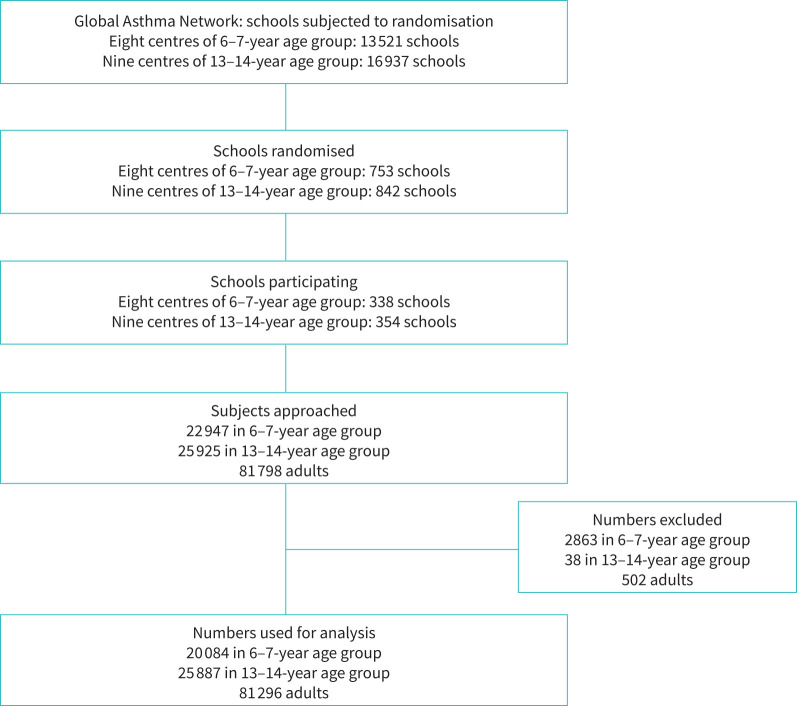
Schools were randomly selected from a pool of total schools in the selected centres (figure 1), with the help of the Indian Institute of Health Management and Research (IIHMR), Jaipur. The geographic boundary was defined for each city and it was divided into four zones. A fraction of schools was randomly selected from each zone. Invitations were sent to the principals of the schools and consenting schools were enrolled in the study. If a school in the randomised list refused to participate, the next school was approached. Passive consent was obtained from the participants. The option of refusal to participate was communicated either by a phone call to the field worker from the parents/guardians or written/verbal refusal by the child to participate.
FIGURE 1.
Flow chart depicting recruitment into the Global Asthma Network study.
Two age groups of schoolchildren (13–14 years and 6–7 years) and their parents/guardians were given written questionnaires. The heights and weights of the children were recorded on the day the questionnaires were administered. The adolescent group (13–14 years) had to complete a questionnaire about themselves at school and were asked to take the adult questionnaires home for their parents/guardians to complete about their own health. The younger age group (6–7 years) took the questionnaires home for parents/guardians to complete the questionnaire about the health of their wards and themselves. Codes assigned to the children and their respective parents were the same.
The questionnaire used in the study was similar to the previously validated core questionnaire used in ISAAC for both age groups of children. The questionnaire had 70, 50 and 47 questions for the 6–7 years, 13–14 years and the adult groups, respectively (there were an additional two questions for the children and five questions for the adults in the questionnaire used in Indian centres compared to the questionnaire used globally). The items in the questionnaires pertained to demography; symptoms of asthma, rhinoconjunctivitis and atopic eczema; environment and use of medications. The adult questionnaire was derived from the questionnaire used in ISAAC and the European Community Respiratory Health Survey. The questionnaire was available in different languages (English, Hindi, Bengali, Marathi, Punjabi, Kannada and Malayalam). The language translation of the questionnaire was validated by back-translating the questionnaire to the English language according to standard protocols.
A meeting was convened for all principal investigators of their respective centres at the National Data Coordinating Center (NDCC, Asthma Bhawan, Jaipur) to delineate the study pathway. Field investigators and data entry operators received training at IIHMR, Jaipur.
Ten per cent of the data were uploaded online, and the data entry errors were kept below 2% by entering the data twice, first at the site and then the second time by the statistical team at IIHMR, Jaipur. The statistical team consolidated the entire data at IIHMR University, Jaipur, and sent it to the GAN global centre (Auckland, New Zealand) and subsequently to the main data centre (London, UK), where the data were cleaned for consistency and duplicity (figure 1).
Coding of the data was done as per the GAN protocol. Current wheeze was defined as the presence of wheeze in the past 12 months (WHEZ12); this variable was then used to calculate the prevalence of asthma. The other variables were: ever diagnosed as asthma (ASTHMAEV) and wheeze ever (WHEZEV). Dry cough in the night during the past 12 months apart from infection (COUGH12) was termed as nocturnal cough. Severe asthma was defined as a current wheeze with more than four attacks per year or wheeze affecting speech or sleep.
Data management and statistical analysis
The sample size targeted for each age group was 3000 participants, and potentially 6000 adults per centre. This sample size could detect a 5% difference between the two centres with 99% certainty (at a 1% level of significance) [14]. The missing data were not included in the analysis. Statistical tests such as Chi-squared were carried out for bivariate analysis to estimate the distribution and association of current wheeze and severe asthma in different predictors. If the cell frequency was less than 5, the Fisher exact probability test was used. The quantitative data were analysed using STATA version 12 (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP). Prevalence of current wheeze as reported in ISAAC Phase I, III and GAN Phase I studies were compared using Chi-squared test.
Results
In this study, questionnaires were received from 20 084 children in the 6–7-year age group, 25 887 children in the 13–14-year age group and 81 296 adults (table 1). The average response rates were 83.9%, 96.6% and 99.4% for 6–7 years, 13–14 years and adults, respectively. There were 48.0%, 51.1% and 50.2% females in the 6–7 years, 13–14 years and adults, respectively. The mean age of the adults was 37.6±6.4 years.
TABLE 1.
Prevalence of symptoms of asthma, severe asthma, asthma ever and doctor-diagnosed asthma in children and adults across both genders
| Symptoms | 6–7-year age group (n=20 084) | 13–14-year age group (n=25 887) | Adults (n=81 296)# | |||||||||
| Male (n=10 441) | Female (n=9643) | p-value¶ | Total n (%) | Male (n=12 671) | Female (n=13 216) | p-value¶ | Total n (%) | Male (n=40 468) | Female (n=40 815) | p-value¶ | Total n (%) | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||||||
| Current wheeze | 380 (3.64) | 254 (2.63) | <0.0001 | 634 (3.16) | 565 (4.46) | 375 (2.84) | <0.0001 | 940 (3.63) | 1344 (3.32) | 1335 (3.27) | 0.689 | 2680 (3.30) |
| Wheeze ever+ | 757 (7.25) | 498 (5.16) | <0.0001 | 1255 (6.25) | 1139 (8.99) | 778 (5.89) | <0.0001 | 1917 (7.41) | ||||
| Nocturnal cough in past 12 months+ | 1870 (17.91) | 1370 (14.21) | <0.001 | 3240 (16.13) | 3864 (30.49) | 3520 (26.63) | <0.0001 | 7384 (28.52) | ||||
| Asthma severe§ | 192 (1.84) | 128 (1.33) | 0.004 | 320 (1.59) | 258 (2.04) | 157 (1.19) | <0.0001 | 415 (1.60) | 465 (1.15) | 476 (1.17) | 0.790 | 942 (1.16) |
| Asthma ever | 174 (1.67) | 109 (1.13) | 0.0012 | 283 (1.41) | 630 (4.97) | 426 (3.22) | <0.0001 | 1056 (4.08) | 714 (1.76) | 942 (2.31) | <0.0001 | 1656 (2.04) |
| Doctor-diagnosed asthma | 138 (1.32) | 88 (0.91) | 0.0059 | 226 (1.13) | 354 (2.79) | 246 (1.86) | <0.0001 | 600 (2.32) | 517 (1.28) | 659 (1.61) | 0.000 | 1176 (1.45) |
#: sex not defined for 13 adults. ¶: Chi-squared test. +: questions not included in adult questionnaire. §: severe asthma is defined as more than four attacks of wheezing in previous 12 months or wheeze affecting sleep or speech in previous 12 months.
The prevalence of current wheeze was 3.16%, 3.63% and 3.30% among the 6–7 years, 13–14 years and adults, respectively (table 1) with no significant difference between the age groups (p>0.05). A significantly higher prevalence of current wheeze, wheeze ever, asthma ever, doctor-diagnosed asthma and nocturnal cough in the last 12 months was noted in boys in both the age groups of children (p<0.05) (table 1). However, in adults, the prevalence of asthma ever and doctor-diagnosed asthma were significantly higher in women than in men (p<0.001). Severe asthma was prevalent in 1.59%, 1.60% and 1.16% in the 6–7 years, 13–14 years and adults (parents), respectively (table 1). The prevalence of current wheeze, nocturnal cough in the past 12 months and severe asthma varied markedly across centres (supplementary tables 1, 2 and 3).
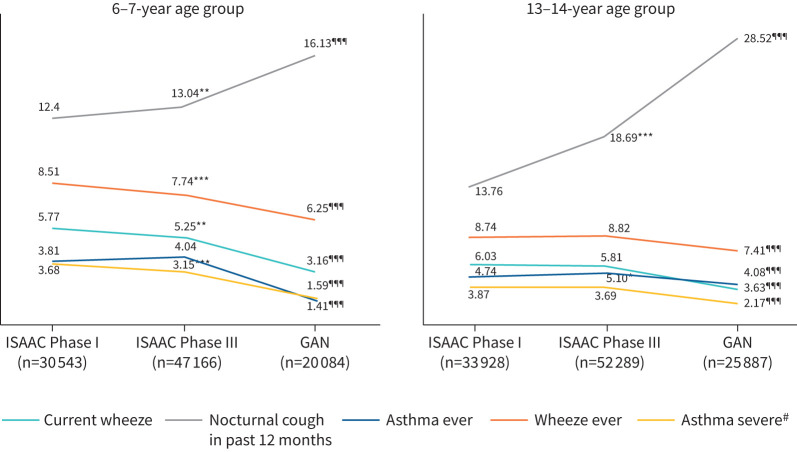
Across ISAAC Phase III and GAN Phase I, in both age groups, there was a significant decrease in the prevalence of current wheeze (p<0.001) and asthma ever (p<0.001), but there was a significant increase in the prevalence of the symptom nocturnal cough (p<0.001, figure 2, supplementary table 4). Bivariate analysis of comparison of various causal factors of current wheeze among children during GAN from ISAAC showed a significant reduction in paracetamol use, maternal smoking, farm exposure, pets in the house and trucks passing outside the house. There was a significant increase in the usage of fresh fruits (table 2).
FIGURE 2.
Time trends in various aspects of asthma including current wheeze, wheeze ever, nocturnal cough, severe asthma and asthma ever through the International Study of Asthma and Allergies in Childhood (ISAAC) Phase I and Phase III, and the Global Asthma Network (GAN) Phase I, in the two age groups of children. #: severe asthma is defined as more than four attacks of wheezing in previous 12 months or wheeze affecting sleep or speech in previous 12 months. *: p<0.05; **: p<0.01; ***: p<0.001 when ISAAC Phase I compared to ISAAC Phase II. ¶¶¶: p<0.001 when ISAAC Phase III compared to GAN Phase I.
TABLE 2.
Time trends in known environmental trigger factors through the International Study of Asthma and Allergies in Childhood (ISAAC) Phase III and Global Asthma Network (GAN) in the two age groups of children
| 6–7-year age group | 13–14-year age group | |||||
| GAN (n=20 084) | ISAAC Phase III (n=47 166) | p-value# | GAN (n=25 887) | ISAAC Phase III (n=52 289) | p-value# | |
| Paracetamol use in last 12 months | ||||||
| Never | 6639 (33.1) | 8945 (19.0) | 0.000 | 9838 (38.0) | 14 689 (28.1) | 0.000 |
| At least once a year | 9496 (47.3) | 21 499 (45.6) | 10 283 (39.7) | 18 749 (35.9) | ||
| At least once per month | 2104 (10.5) | 5940 (12.6) | 4441 (17.2) | 9530 (18.2) | ||
| No response | 1845 (9.2) | 10 782 (22.8) | 1325 (5.1) | 9321 (17.8) | ||
| Farm animals during pregnancy ¶ | ||||||
| Yes | 1307 (6.5) | 3357 (7.1) | 0.000 | |||
| No | 18 051 (89.9) | 34 474 (73.1) | ||||
| No response | 726 (3.6) | 9335 (19.8) | ||||
| Farm animals during first year of life ¶ | ||||||
| Yes | 1059 (5.3) | 4267 (9.0) | 0.000 | |||
| No | 18 297 (91.1) | 33 585 (71.2) | ||||
| No response | 728 (3.6) | 9314 (19.8) | ||||
| Trucks pass through the street | ||||||
| Never | 9999 (49.8) | 9451 (20.0) | 0.374 | 11 063 (42.7) | 10 978 (21.0) | 0.000 |
| Seldom (not often) | 8631 (43.0) | 13 793 (29.2) | 9573 (37.0) | 15 354 (29.4) | ||
| Frequently through the day | 1028 (5.1) | 8276 (17.6) | 2902 (11.2) | 9550 (18.3) | ||
| Almost the whole day | 426 (2.1) | 5808 (12.3) | 1268 (4.9) | 2718 (5.2) | ||
| No response | 0 | 9838 (20.9) | 1081 (4.2) | 13 689 (26.2) | ||
| Child ever breastfed ¶ | ||||||
| Yes | 16 569 (82.5) | 35 215 (74.7) | 0.000 | |||
| No | 2728 (13.6) | 2549 (5.4) | ||||
| No response | 787 (3.9) | 9402 (19.9) | ||||
| Cat in the home during first year of life ¶ | ||||||
| Yes | 991 (4.9) | 3756 (8.0) | 0.000 | |||
| No | 18 360 (91.4) | 34 107 (72.3) | ||||
| No response | 733 (3.7) | 9303 (19.7) | ||||
| Cat in the home in last 12 months | ||||||
| Yes | 985 (4.9) | 3632 (7.7) | 0.000 | 3221 (12.4) | 8491 (16.2) | 0.000 |
| No | 17 875 (89.0) | 34 277 (72.7) | 22 041 (85.1) | 35 480 (67.9) | ||
| No response | 1224 (6.09) | 9257 (19.63) | 625 (2.5) | 8318 (15.9) | ||
| Dog in the home during first year of life ¶ | ||||||
| Yes | 1102 (5.5) | 4102 (8.7) | 0.000 | |||
| No | 18 280 (91.0) | 33 836 (71.7) | ||||
| No response | 702 (3.5) | 9228 (19.6) | ||||
| Dog in the home in last 12 months | ||||||
| Yes | 1200 (6.0) | 3779 (8.0) | 0.000 | 3930 (15.2) | 7934 (15.2) | 0.000 |
| No | 18 150 (90.4) | 34 183 (72.5) | 21 394 (82.6) | 36 000 (68.8) | ||
| No response | 734 (3.6) | 9204 (19.5) | 563 (2.2) | 8355 (16.0) | ||
| Fast food | ||||||
| Never or only occasionally | 13 286 (66.2) | 10 754 (22.8) | 0.000 | 16 535 (63.9) | 12 553 (24.0) | 0.000 |
| Once or twice per week | 3445 (17.2) | 7383 (15.7) | 5200 (20.1) | 12 018 (23.0) | ||
| Most or all days | 462 (2.3) | 9865 (20.9) | 1690 (6.5) | 10 236 (19.6) | ||
| No response | 2891 (13.3) | 19 164 (40.3) | 2462 (9.5) | 17 482 (33.4) | ||
| Antibiotic in first year of life ¶ | ||||||
| Yes | 3350 (16.7) | 17 706 (37.5) | 0.373 | |||
| No | 16 734 (83.3) | 17 711 (37.6) | ||||
| No response | 0 | 11 749 (24.9) | ||||
| Fresh fruit | ||||||
| Never or only occasionally | 4590 (22.9) | 11 179 (23.7) | 0.000 | 7698 (29.7) | 12 031 (23.0) | 0.000 |
| Once or twice per week | 6841 (34.1) | 12 503 (26.5) | 6106 (23.6) | 14 409 (27.6) | ||
| Most or all days | 6341 (31.6) | 11 511 (24.4) | 9255 (35.8) | 15 061 (28.8) | ||
| No response | 2312 (11.4) | 11 973 (25.4) | 2828 (10.9) | 10 788 (20.6) | ||
| Vegetables | ||||||
| Never or only occasionally | 5565 (27.7) | 12 291 (26.1) | 0.000 | 13 150 (50.8) | 12 721 (24.3) | 0.000 |
| Once or twice per week | 5792 (28.8) | 9395 (19.9) | 4666 (18.0) | 11 230 (21.5) | ||
| Most or all days | 5842 (29.1) | 13 726 (29.1) | 5193 (20.1) | 17 681 (33.8) | ||
| No response | 2885 (14.4) | 11 754 (24.9) | 2878 (11.1) | 10 657 (20.4) | ||
| Mother smoking during first year of life | ||||||
| Yes | 209 (1.0) | 385 (0.8) | 0.000 | |||
| No | 19 194 (95.6) | 37 637 (79.8) | ||||
| No response | 681 (3.4) | 9144 (19.4) | ||||
Data are presented as n (%), unless otherwise stated. #: Chi-squared test. ¶: questions included in the 6–7-year age group questionnaire only.
In the 6–7-year age group absenteeism due to wheeze was noted in 66.1% and 2.5% of children with current wheeze, with and without a concomitant doctor diagnosis of asthma, respectively (table 3). In the 13–14-year age group these numbers were 52.7% and 24.0%, respectively. Hospitalisations at least once in the past year because of breathing problems were noted in 44.3% of children with current wheeze with a concomitant doctor diagnosis of asthma and 1.2% of children with current wheeze without a doctor diagnosis (table 3). In the 13–14-year age group these numbers were 25.5% and 8.7%, respectively. Similarly, exercise-induced wheeze and visits to an emergency department due to breathing trouble were also higher in subjects with doctor-diagnosed asthma.
TABLE 3.
School absenteeism, exercise-induced wheeze and medical assistance required among children and adults with current wheeze with or without doctor-diagnosed asthma, and healthy subjects
| In past 12 months | 6–7-year age group | 13–14-year age group | Adults | |||||||||
| Current wheezers with doctor-diagnosed asthma (n=115) | Current wheezers without doctor-diagnosed asthma (n=519) | Subjects without current wheeze (n=19 450) | p-value # | Current wheezers with doctor-diagnosed asthma (n=239) | Current wheezers without doctor-diagnosed asthma (n=701) | Subjects without current wheeze (n=24 947) | p-value # | Current wheezers with doctor-diagnosed asthma (n=603) | Current wheezers without doctor-diagnosed asthma (n=2077) | Subjects without current wheeze (n=78 616) | p-value # | |
| Missed school due to breathing problem ≥1 day | 76 (66.09) | 13 (2.50) | 53 (0.27) | 0.000 | 126 (52.72) | 168 (23.96) | 1499 (6.01) | 0.000 | ||||
| Wheezy chest with exercise | 39 (33.91) | 117 (22.54) | 399 (2.05) | 0.000 | 103 (43.10) | 237 (33.81) | 1943 (7.79) | 0.000 | ||||
| Urgent visit to doctor due to breathing problem (≥1 time) | 81 (70.43) | 16 (3.08) | 54 (0.28) | 0.000 | 119 (49.79) | 208 (29.67) | 1606 (6.44) | 0.000 | 391 (64.84) | 74 (3.56) | 244 (0.31) | 0.000 |
| Urgent visit to emergency department due to breathing problem (≥1 time) | 42 (36.52) | 5 (0.96) | 18 (0.09) | 0.000 | 53 (22.17) | 73 (10.41) | 598 (2.40) | 0.000 | 180 (29.85) | 32 (1.54) | 106 (0.13) | 0.000 |
| Admitted to hospital due to breathing problem (≥1 time) | 51 (44.35) | 6 (1.16) | 22 (0.11) | 0.000 | 61 (25.52) | 61 (8.70) | 679 (2.72) | 0.000 | 165 (27.36) | 32 (1.54) | 101 (0.13) | 0.000 |
| Limited usual activity (at work or in the home) due to breathing problem | 216 (35.82) | 366 (17.62) | 932 (1.19) | 0.000 | ||||||||
Data are presented as n (%), unless otherwise stated. #: Chi-squared test.
In all groups both inhaled and oral medicines were used in almost equal proportion. However, in subjects in any of the three groups with current wheeze or with symptoms of severe asthma, less than 2.5% of subjects were using daily ICS and less than 2% of subjects used inhaled beta-agonist daily (table 4).
TABLE 4.
Treatment undertaken for current wheeze and severe asthma in children and adults
| Medications used | Current wheeze | Severe asthma# | ||||||||||
| 6–7-year age group (n=634) | 13–14-year age group (n=940) | Adults (n=2680) | 6–7-year age group (n=320) | 13–14-year age group (n=562) | Adults (n=1499) | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Inhaler medicines | 97 | 15.30 | 270 | 28.72 | 487 | 18.17 | 72 | 22.50 | 208 | 37.01 | 389 | 25.95 |
| Nebuliser | 115 | 18.14 | 115 | 12.23 | 309 | 11.53 | 85 | 26.56 | 89 | 15.84 | 231 | 15.41 |
| Oral medications | 76 | 11.99 | 238 | 25.32 | 392 | 14.63 | 61 | 19.06 | 183 | 32.56 | 324 | 21.61 |
| Inhaled β-agonists | ||||||||||||
| Only when needed | 51 | 8.04 | 140 | 14.89 | 239 | 8.92 | 36 | 11.25 | 120 | 21.35 | 188 | 12.54 |
| In short courses | 23 | 3.63 | 27 | 2.87 | 37 | 1.38 | 16 | 5.00 | 26 | 4.63 | 34 | 2.27 |
| Every day | 5 | 0.79 | 9 | 0.96 | 33 | 1.23 | 3 | 0.94 | 9 | 1.60 | 29 | 1.93 |
| ICS | ||||||||||||
| Only when needed | 24 | 3.79 | 43 | 4.57 | 67 | 2.50 | 14 | 4.38 | 34 | 6.05 | 56 | 3.74 |
| In short courses | 13 | 2.05 | 52 | 5.53 | 24 | 0.90 | 11 | 3.44 | 47 | 8.36 | 22 | 1.47 |
| Every day | 5 | 0.79 | 8 | 0.85 | 13 | 0.49 | 3 | 0.94 | 5 | 0.89 | 11 | 0.73 |
| Combined (ICS+LABA) | ||||||||||||
| Only when needed | 18 | 2.84 | 81 | 8.62 | 112 | 4.18 | 10 | 3.13 | 71 | 12.63 | 81 | 5.40 |
| In short courses | 9 | 1.42 | 10 | 1.06 | 22 | 0.82 | 4 | 1.25 | 8 | 1.42 | 18 | 1.20 |
| Every day | 10 | 1.58 | 7 | 0.74 | 37 | 1.38 | 8 | 2.50 | 7 | 1.25 | 32 | 2.13 |
ICS: inhaled corticosteroid; LABA: long-acting β-agonist. #: severe asthma is defined as current wheeze with more than four attacks per year or wheeze affecting speech or activity.
Among subjects with current wheeze, 75–82% remained clinically undiagnosed (table 5). Among subjects with severe asthma, 68–70% of subjects were never clinically diagnosed with asthma (table 5). Among subjects with current wheeze who had undiagnosed asthma, less than 1% took daily ICS (table 5). In subjects with current wheeze who were clinically diagnosed with asthma, use of daily ICS increased to 2–8% in different age groups; this difference was statistically significant (p<0.001). A similar trend was noted in subjects with symptoms of severe asthma (table 5). Almost 40% of doctor-diagnosed patients used beta-agonist inhaled treatment whenever they had symptoms, but less than 7% used it when asthma was not clinically confirmed by a doctor (table 5).
TABLE 5.
Use of various types of medicines in subjects with current wheeze and severe asthma with or without doctor-diagnosed asthma
| Current wheezers with doctor-diagnosed asthma | Current wheezers without doctor-diagnosed asthma | p-value# | Severe asthma with doctor-diagnosed asthma¶ | Severe asthma without doctor-diagnosed asthma¶ | p-value# | |
| 6–7-year age group | n=115 | n=519 | n=95 | n=225 | ||
| Inhaled β-agonists | ||||||
| Only when needed | 41 (35.65) | 10 (1.93) | 0.000 | 33 (34.74) | 3 (1.33) | 0.000 |
| In short courses | 18 (15.65) | 5 (0.96) | 15 (15.79) | 1 (0.44) | ||
| Every day | 5 (4.35) | 0 (0.0) | 3 (3.16) | 0 (0.0) | ||
| Not using any medicine | 51 (44.35) | 504 (97.11) | 44 (46.31) | 221 (98.23) | ||
| ICS | ||||||
| Only when needed | 16 (13.91) | 8 (1.54) | 0.000 | 11 (11.58) | 3 (1.33) | 0.000 |
| In short courses | 12 (10.43) | 1 (0.19) | 11 (11.58) | 0 (0.0) | ||
| Every day | 4 (3.48) | 1 (0.19) | 3 (3.16) | 0 (0.0) | ||
| Not using any medicine | 83 (72.18) | 509 (98.08) | 70 (73.68) | 222 (98.67) | ||
| Combined (ICS+LABA) | ||||||
| Only when needed | 14 (12.17) | 4 (0.77) | 0.000 | 9 (9.47) | 1 (0.44) | 0.000 |
| In short courses | 6 (5.22) | 3 (0.58) | 4 (4.21) | 0 (0.0) | ||
| Every day | 9 (7.83) | 1 (0.19) | 8 (8.42) | 0 (0.0) | ||
| Not using any medicine | 86 (74.78) | 511 (98.47) | 74 (77.90) | 224 (99.56) | ||
| 13–14-year age group | n=239 | n=701 | n=182 | n=380 | ||
| Inhaled β-agonists | ||||||
| Only when needed | 107 (44.77) | 41 (5.85) | 0.000 | 97 (53.30) | 26 (6.84) | 0.000 |
| In short courses | 18 (7.53) | 22 (3.14) | 17 (9.34) | 7 (1.84) | ||
| Every day | 8 (3.35) | 1 (0.14) | 7 (3.85) | 1 (0.26) | ||
| Not using any medicine | 106 (44.35) | 637 (90.87) | 61 (33.52) | 346 (91.06) | ||
| ICS | ||||||
| Only when needed | 30 (12.55) | 13 (1.85) | 0.000 | 23 (12.64) | 11 (2.89) | 0.000 |
| In short courses | 46 (19.25) | 6 (0.86) | 42 (23.07) | 5 (1.32) | ||
| Every day | 8 (3.35) | 0 (0.0) | 5 (2.75) | 0 (0.0) | ||
| Not using any medicine | 155 (64.85) | 682 (97.29) | 112 (61.54) | 364 (95.79) | ||
| Combined (ICS+LABA) | ||||||
| Only when needed | 65 (27.20) | 16 (2.28) | 0.000 | 58 (31.86) | 13 (3.42) | 0.000 |
| In short courses | 6 (2.51) | 4 (0.57) | 0.012 | 4 (2.20) | 4 (1.05) | |
| Every day | 5 (2.09) | 2 (0.29) | 0.005 | 5 (2.75) | 2 (0.53) | |
| Not using any medicine | 163 (68.20) | 679 (96.86) | 115 (63.19) | 361 (95.0) | ||
| Adults | n=603 | n=2077 | n=467 | n=1032 | ||
| Inhaled β-agonists | ||||||
| Only when needed | 218 (36.15) | 24 (1.16) | 0.000 | 171 (36.62) | 16 (1.55) | 0.000 |
| In short courses | 33 (5.47) | 4 (0.19) | 31 (6.64) | 3 (0.29) | ||
| Every day | 30 (4.98) | 3 (0.14) | 26 (5.57) | 3 (0.29) | ||
| Not using any medicine | 322 (53.40) | 2046 (98.51) | 239 (51.17) | 1010 (97.87) | ||
| ICS | ||||||
| Only when needed | 58 (9.62) | 9 (0.43) | 0.000 | 49 (10.49) | 7 (0.68) | 0.000 |
| In short courses | 21 (3.48) | 3 (0.14) | 19 (4.07) | 3 (0.29) | ||
| Every day | 13 (2.16) | 0 (0.0) | 11 (2.36) | 0 (0.0) | ||
| Not using any medicine | 511 (84.74) | 2065 (99.43) | 388 (83.08) | 1022 (99.03) | ||
| Combined (ICS+LABA) | ||||||
| Only when needed | 101 (16.75) | 11 (0.53) | 0.000 | 73 (15.63) | 8 (0.78) | 0.000 |
| In short courses | 18 (2.99) | 4 (0.19) | 14 (3.00) | 4 (0.39) | ||
| Every day | 37 (6.14) | 0 (0.0) | 32 (6.85) | 0 (0.0) | ||
| Not using any medicine | 447 (74.12) | 2062 (99.28) | 348 (74.52) | 1020 (98.83) | ||
Data are presented as n (%), unless otherwise stated. ICS: inhaled corticosteroid; LABA: long-acting β-agonist. #: the Chi-squared test is used when all expected cell frequencies are ≥5. If expected cell frequency was <5 the Fisher exact probability test was used. ¶: severe asthma is defined as current wheeze with more than four attacks per year or wheeze affecting speech or activity.
Discussion
The GAN Phase I study was the largest multicentre study since the ISAAC Phase III to analyse the prevalence of asthma symptoms in children and adults in India. The prevalence of current wheeze was 3.16%, 3.63% and 3.30% in the 6–7 years, 13–14 years and adults, respectively. The comparison of prevalence of current wheeze across Indian centres in ISAAC Phase I and Phase III, and GAN Phase I, showed a significantly lower prevalence in both the age groups of children in the GAN study (p<0.001), but a higher prevalence for nocturnal cough (p<0.001). Most subjects with symptoms of asthma were not diagnosed clinically with asthma. A significantly higher number of current wheezers/symptoms of severe asthma with a doctor's diagnosis of asthma were taking either inhaled medications or inhaled steroids or oral medications in all three groups (p<0.001) compared to those without a doctor's diagnosis of asthma.
The time trends of prevalence of asthma from our study are contrary to the widely held belief that asthma is increasing in recent decades. However, our study reveals a significant reduction in the prevalence of current wheeze, which has been considered a symptom of asthma. The methodology, recruitment sites and investigators were the same in the ISAAC and GAN studies, thereby allowing for a reasonable comparison of data to assess the time trends. A significant reduction was also noted in the GAN study in the prevalence of most of the causal factors of current wheeze as compared to the previous ISAAC studies, such as paracetamol use, maternal smoking, farm exposure, pets in house, trucks passing outside the house and antibiotic use during the first year of life (table 2). In India strict vehicle emission norms have also been implemented during this period. Conversely, use of factors such as fruit consumption and child ever breastfed were increased significantly in the GAN Phase I study as compared to the ISAAC Phase III study, which are considered as protective factors for asthma [11]. National asthma guidelines were also formulated and initiated, which would have also resulted in better outcomes [15]. The change in aforementioned environmental factors may be a possible explanation for reduction in the prevalence of current wheeze.
Nevertheless, there is a wide variation in the prevalence of wheeze in comparative studies. A questionnaire-based study in adults in India revealed the prevalence of asthma as 2.05% [3]. Another multicentre research study has reported a prevalence of asthma as 2.38% in adults [16]. An ISAAC questionnaire-based study reported the prevalence of bronchial asthma as 13.1% in children aged between 11 and 16 years (n=927). The prevalence of current subjects with asthma (defined as asthma episode(s) in the past 12 months) was 10.2% [17]. Pal et al. [18] profiled 15 epidemiological studies related to asthma in children and reported the prevalence of asthma as 2.74%. Significant time has elapsed since these studies were conducted and published. These studies also had varied methodology, time frames and study population, which could be possible reasons for this difference. The recently published GBD data estimated 34.3 million cases in an Indian population of 1330 million, almost comparable to our study [4].
The GAN Phase I global data also show significant changes as per centre, age and income in prevalence of asthma across the world [19]. The world total data showed a significant decline in current wheeze, with both increase and decrease noted as per individual centre. Preliminary data from few other global centres have shown mixed results regarding time trends in the prevalence of current wheeze [20–22]. Data from a single centre in Mexico showed a 7.9% increase in the prevalence of current wheeze in both age groups compared with ISAAC Phase III [20]. On the other hand, the GAN Phase I study conducted in Bangkok suggests a similar prevalence of current wheeze in the younger age group and slightly lower prevalence in the older age group when compared with ISAAC Phase III [21]. Interestingly, lower prevalence of current wheeze was reported from the centres from low-income group countries while it did not change for high-income countries in either age group. However, prevalence of current wheeze was increased in upper middle-income countries [19]. There have been changes in the micro-environment and macro-environment between the phases of studies across the centres, but the exact cause of the trend would be difficult to identify.
The prevalence of current wheeze varied from centre to centre and was consistent with the previous ISAAC studies. Similar heterogeneity was documented in previous ISAAC studies, with intercontinental, intercountry, inter-regional and intra-regional variations [2, 5, 6]. The various cities across India have varying geography, climate, environment and socioeconomic conditions. The lowest prevalence of current wheeze was documented in Bikaner (0.35%) and the highest in Chandigarh (8.54%) in the 6–7-year age group. Bikaner is a desert area located in the western part of India and it experiences dry and hot weather associated with dust storms. Chandigarh, on the other hand, has a cooler and humid climate, situated in the northern part of the country. In the 13–14-year age group, the lowest prevalence was in New Delhi (0.89%) and the highest in Jaipur (6.62%) (supplementary tables 1 and 2, supplementary figure 1). Among adults, New Delhi (0.88%) reported the lowest prevalence and Kottayam (6.02%) the highest prevalence (supplementary table 3). The findings are contradictory as Jaipur is located in western India and has a dry and arid desert climate while New Delhi is situated in the northern part of the country, with poor air quality. Kottayam is situated in southern India with a warm and tropical climate. Such variation is also observed in global data, which is difficult to explain. After ISAAC Phase III, compressed natural gas (CNG) replaced petrol and diesel in nearly all public vehicles in New Delhi in December 2002, including truck traffic [23]. Diesel exhaust particles have been associated with an increase in Th2 and Th17 cytokines, which are responsible for the asthmatic response [24]. Reduction in diesel exhaust particles may in turn have been a contributing factor for a decrease in prevalence in New Delhi. The INSEARCH study also reported variation in the prevalence of bronchial asthma (adults) among the 12 participating centres (from 0.4% in Secunderabad to 4.8% in Kolkata) [3].
The self-reported prevalence of wheeze was significantly more common in boys in both age groups (p<0.001) [25]. It is unclear why there is this gender difference in children; possible explanations are variations in sex hormone levels [26], bronchial lability [27] and allergen sensitivities [28]. Interestingly, among adults this difference was not noted for current wheeze and a significantly higher prevalence of asthma ever and doctor-diagnosed asthma were seen in women, which is consistent with previous reports in adults. Previous health surveys conducted from 2005 to 2006 in adults in India have reported a prevalence of wheeze of 1.8% (95% CI 1.6–2.0) and 1.9% (95% CI 1.8–2.0) in men and women, respectively [29].
Nocturnal cough is a symptom of asthma that has increased in prevalence during GAN Phase I compared to ISAAC Phase I and Phase III. However, nocturnal cough can be attributed to other causes also such as gastro-oesophageal reflux disease, post-nasal drip, bronchiectasis and medication-induced [30]. It is a non-specific symptom and all nocturnal cough would not be due to bronchial asthma. Nonetheless, it is worth noting the significant increase in the prevalence of nocturnal cough; a similar increase was also noted in both the age groups in the worldwide GAN Phase I data, a trend that is difficult to explain [19].
Our study reveals a gap in the diagnosis of asthma. Almost 82% of current wheezers and 70% of subjects with symptoms of severe asthma were not clinically diagnosed as having asthma by a doctor. This gap in diagnosis is high compared to a similar study that reported almost a third of the subjects remained undiagnosed [31]. Under-treatment is another problem; among doctor-diagnosed subjects with wheeze and with symptoms of severe asthma, daily ICS were used in less than 9% among different age groups of subjects (table 5). Under-diagnosis and under-treatment may be attributed to lack of medical facilities, poverty, illiteracy, ignorance on the part of patients, improper techniques of medication intake, non-adherence and poor communication skills of medical personnel [32–34]. Apart from these factors an interesting aspect worth discussing is disease terminology used by asthmatic patients and their treating doctors. When an asthmatic patient consults a doctor, only 71% of doctors refer to “asthma” as the name of their disease and 29% use other terminology. The problem is worse at patient level; only 23% of asthmatic patients call their disease “asthma” while the rest use terminology like swas, dama or cold and cough [35]. Asthma is considered to be a stigma and many parents conceal the disease and therefore avoid medications or give only when a patient is symptomatic or unable to tolerate symptoms. Incorrect notions such as that inhalers are harmful and habit-forming also play a role in treatment non-adherence. Regular use of ICS among undiagnosed current wheezers was thereby very low; under-treatment may account for high school absenteeism, urgent medical consultation to a doctor and emergency department visits (table 3). Even hospitalisation due to breathing problems was significantly high. Similarly, indexes of uncontrolled asthma such as exercise-related wheeze in children and restriction of usual activities were also significantly higher in current wheezers than healthy subjects (table 3). These findings suggest high morbidity and use of hospital resources among current wheezers.
Untreated children with asthma have been found to have higher school absenteeism in similar studies [36]. Exercise-induced asthma, emergency visits, hospitalisation and school absenteeism are markers of uncontrolled asthma [37]. However, all these markers were more significantly affected in subjects having a clinical diagnosis of asthma, probably indicating that more severely affected persons consult a doctor more frequently and thereby have a higher chance of getting clinically diagnosed with asthma. However, earlier studies show that more than half of asthmatic subjects discontinue asthma medications once they tolerate symptoms and this tendency of symptom tolerance persists in more than a third of patients, even after education [32, 33]. This issue needs to be addressed in asthma education programmes as it leads to significant clinical implications in the form of non-compliance.
Our study thus highlights an unmet need in terms of diagnosis and treatment of symptoms of asthma, thereby leading to significant morbidity resulting from the disease. Lack of patient education, concerns about medication side-effects and the stigma associated with inhalation devices might also contribute to this skewed treatment approach [32, 34].
Underdiagnoses, delayed treatment, under-treatment or no treatment may be responsible for increased morbidity due to asthma in terms of DALYs and disproportionately high mortality [1, 4]. These findings emphasise the need for national-level health programmes to address better strategies to diagnose and manage asthma, and also the need to communicate a diagnosis of asthma to the patient.
The GAN Phase I study was a self-reported, questionnaire-based study; objective measures of assessment of asthma and its severity, such as spirometry, were not used for diagnosis of the disease. Conducting spirometry for every child across all centres would not have been logistically feasible and would not have allowed for comparison with earlier studies. Because it was a questionnaire-based study, answers were subject to the understanding of the children and adults. Adolescents may have a poor perception of their symptoms; thus questionnaire-based assessment tools may underestimate the actual prevalence of the illness. Cough is a common symptom in subjects with asthma, especially children. Although wheeze is more specific for a diagnosis of asthma, its sensitivity is low and may therefore underestimate the prevalence of asthma. However, the previous ISAAC studies have used this particular question to calculate the prevalence of asthma [2, 5, 6]. The validated ISAAC questionnaire is the most authentic means of collecting childhood asthma prevalence globally [14, 38]. Furthermore, because the study aimed to compare the current prevalence of wheeze with the previous ISAAC data, a similar questionnaire was used to maintain consistency. The study was limited by the fact that the centres participated voluntarily, and were not randomly selected. Although the participating centres were voluntary they represented the various geographies including north, south, east, west and central. Further, each centre was divided into four zones, and a fraction of schools were randomly selected from each zone to ensure appropriate representation. This same methodology was adopted by the GAN Phase I study worldwide and the previous ISAAC studies as well. The study population was representative of the population of school-going children of the respective nine centres. Despite these steps to strive for equal representation from across centres, the prevalence of asthma varies across rural and urban areas, and within cities itself due to differences in socioeconomic conditions, thus to generalise the results to the entire country or the world would not be appropriate. Potential bias could exist as some children may have refused to participate and all children may not be attending school. Furthermore, not all schools across the country were covered. Recall bias could also exist, as exposures in the first year of life could have been forgotten or incorrectly remembered. However, the same protocol was used in the previous ISAAC studies, thereby allowing for valid comparison of time trends. Prevalence of current wheeze in adults could be obtained for only those adults (age group 31–44 years) whose children were in a particular age group and were attending the participating schools; thus, a selection bias may exist.
Conclusions
The prevalence of current wheeze in the 6–7 years, 13–14 years and adults was 3.16%, 3.63% and 3.30%, respectively. There was a significant reduction in current wheeze in GAN Phase I compared with the previous ISAAC Phase III study. It was also associated with a significant reduction in the frequency of exposure to causal factors such as paracetamol use, maternal smoking, farm exposure, pets in the house and trucks passing outside the house. The problem of under-diagnosis and under-treatment of asthma was noted. Up to 82% of subjects with current wheeze and up to 70% of subjects with symptoms of severe asthma remain undiagnosed and less than 1% of undiagnosed subjects take the recommended daily ICS. Even among current wheezers with a clinical diagnosis of asthma, use of daily ICS was relatively low. The study provides valuable insights into changing trends in the prevalence of current wheeze in India and highlights under-diagnosis and under-treatment. The findings could help in planning management strategies for this non-communicable disease.
Acknowledgements
We are thankful to the children, parents and school staff who contributed to the study. We would like to thank the team at the Chest Research Foundation for their relentless efforts as partners in the coordination and execution of this study across centres. We would like to thank Dr K.R. Jat, Dr Pankaj Jorwal, Dr Rakesh Lodha, Dr Rashmi Yadav and Dr Tuhina Verma for valuable data collection at various sites. Ms Deepika Chaudhary, Ms Divya Sharma, Ms Gazala Parveen, Ms P. Lingambika, Ms Nek Parveen, Ms Pallavi, Ms Pooja, Ms Preeti Bala, Ms Monika, Ms Priya Chugh, Ms Puja, Ms Rashmi Mundada, Ms Shivani Sharma, Ms N. Vidyashree, Mr Aadi Kumar, Mr Ajil, Mr A. Akhil, Mr Anil Kumar, Mr Ankit, Mr Balaji, Mr Dushyant Acharya, Mr Hasib Rehman, Mr Jitendra Kumar, Mr Kamlesh Kumar, Mr Mohd. Javed, Mr Pankaj Rathore, Mr Prafful, Mr Rahul Kumar, Mr M. Raju, Mr Rakesh, Mr P. Ravi Kumar, Mr Ravinder Duggal, Mr Ravindra, Mr Saizan, Mr Samir, Mr Sandeep, Mr Sanjeev, Mr Sathish Chandran, Mr Sumanta Ghata, Mr Suvajit Das, Mr Treedeep, Mr Vedprakash, Mr Vishnu and Mr B. Vyshakh have been instrumental in collecting data and its entry across various sites in India. We would also like to thank Ms Sushmeeta Chhowala for valuable coordination between centres and supervision in manuscript preparation.
Provenance: Submitted article, peer reviewed.
This study was registered in the Clinical Trial Registry, India (CTRI/2018/02/011758).
Conflict of interest: There is no conflict of interest to report by any of the authors.
Support statement: This study was supported by the Cipla Foundation, Mumbai, India. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.India State-Level Disease Burden Initiative CRD Collaborators . The burden of chronic respiratory diseases and their heterogeneity across the states of India: the Global Burden of Disease Study 1990–2016. Lancet Glob Health 2018; 6: e1363–e1374. doi: 10.1016/S2214-109X(18)30409-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh S, Sharma BB, Sharma SK, et al. Prevalence and severity of asthma among Indian school children aged between 6 and 14 years: associations with parental smoking and traffic pollution. J Asthma 2016; 53: 238–244. doi: 10.3109/02770903.2015.1087558 [DOI] [PubMed] [Google Scholar]
- 3.Jindal SK, Aggarwal AN, Gupta D, et al. Indian study on epidemiology of asthma, respiratory symptoms and chronic bronchitis in adults (INSEARCH). Int J Tuberc Lung Dis 2012; 16: 1270–1277. doi: 10.5588/ijtld.12.0005 [DOI] [PubMed] [Google Scholar]
- 4.GBD Compare. Viz Hub . (2021, June 30). https://vizhub.healthdata.org/gbd-compare/
- 5.Beasley R, Ellwood P, Asher I. International patterns of the prevalence of pediatric asthma the ISAAC program. Pediatr Clin North Am 2003; 50: 539–553. doi: 10.1016/S0031-3955(03)00050-6 [DOI] [PubMed] [Google Scholar]
- 6.Asher MI, Montefort S, Björkstén B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006; 368: 733–743. doi: 10.1016/S0140-6736(06)69283-0 [DOI] [PubMed] [Google Scholar]
- 7.Mitchell EA, Beasley R, Keil U, et al. The association between tobacco and the risk of asthma, rhinoconjunctivitis and eczema in children and adolescents: analyses from Phase Three of the ISAAC programme. Thorax 2012; 67: 941–949. doi: 10.1136/thoraxjnl-2011-200901 [DOI] [PubMed] [Google Scholar]
- 8.Wong G, Brunekreef B, Ellwood P, et al. Cooking fuels and prevalence of asthma: a global analysis of phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Lancet Respir Med 2013; 1: 386–394. doi: 10.1016/S2213-2600(13)70073-0 [DOI] [PubMed] [Google Scholar]
- 9.Brunekreef B, Stewart AW, Anderson HR, et al. Self-reported truck traffic on the street of residence and symptoms of asthma and allergic disease: a global relationship in ISAAC phase 3. Environ Health Perspect 2009; 117: 1791–1798. doi: 10.1289/ehp.0800467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell EA, Beasley R, Björkstén B, et al. The association between BMI, vigorous physical activity and television viewing and the risk of symptoms of asthma, rhinoconjunctivitis and eczema in children and adolescents: ISAAC Phase Three. Clin Exp Allergy 2013; 43: 73–84. doi: 10.1111/cea.12024 [DOI] [PubMed] [Google Scholar]
- 11.Ellwood P, Asher MI, García-Marcos L, et al. Do fast foods cause asthma, rhinoconjunctivitis and eczema? Global findings from the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three. Thorax 2013; 68: 351–360. doi: 10.1136/thoraxjnl-2012-202285 [DOI] [PubMed] [Google Scholar]
- 12.Weinmayr G, Gehring U, Genuneit J, et al. Dampness and moulds in relation to respiratory and allergic symptoms in children: results from Phase Two of the International Study of Asthma and Allergies in Childhood (ISAAC Phase Two). Clin Exp Allergy 2013; 43: 762–774. doi: 10.1111/cea.12107 [DOI] [PubMed] [Google Scholar]
- 13.Beasley R, Clayton T, Crane J, et al. Association between paracetamol use in infancy and childhood, and risk of asthma, rhinoconjunctivitis, and eczema in children aged 6–7 years: analysis from Phase Three of the ISAAC programme. Lancet 2008; 372: 1039–1048. doi: 10.1016/S0140-6736(08)61445-2 [DOI] [PubMed] [Google Scholar]
- 14.Ellwood P, Asher MI, Billo NE, et al. The Global Asthma Network rationale and methods for phase I global surveillance: prevalence, severity, management and risk factors. Eur Respir J 2017; 49: 1601605. doi: 10.1183/13993003.01605-2016 [DOI] [PubMed] [Google Scholar]
- 15.Agarwal R, Dhooria S, Aggarwal AN, et al. Guidelines for diagnosis and management of bronchial asthma: joint recommendations of National College of Chest Physicians (India) and Indian Chest Society. Ind J Chest Dis Allied Sci 2015; 57: 5–52. [PubMed] [Google Scholar]
- 16.Aggarwal AN, Chaudhry K, Chhabra SK, et al. Prevalence and risk factors for bronchial asthma in Indian adults: a multicentre study. Indian J Chest Dis Allied Sci 2006; 48: 13–22. [PubMed] [Google Scholar]
- 17.Bhalla K, Nehra D, Nanda S, et al. Prevalence of bronchial asthma and its associated risk factors in school-going adolescents in Tier-III North Indian City. J Family Med Prim Care 2018; 7: 1452–1457. doi: 10.4103/jfmpc.jfmpc_117_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pal R, Dahal S, Pal S. Prevalence of bronchial asthma in Indian children. Indian J Community Med 2009; 34: 310–316. doi: 10.4103/0970-0218.58389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asher MI, Rutter CE, Bissell K, et al. Worldwide trends in the burden of asthma symptoms in school-aged children: Global Asthma Network Phase I cross-sectional study. Lancet 2021; 398: 1569–1580. doi: 10.1016/S0140-6736(21)01450-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Del-Rio-Navarro BE, Navarrete-Rodríguez EM, Berber A, et al. The burden of asthma in an inner-city area: a historical review 10 years after ISAAC. World Allergy Organ J 2020; 13: 100092. doi: 10.1016/j.waojou.2019.100092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chinratanapisit S, Suratannon N, Pacharn P, et al. Prevalence and severity of asthma, rhinoconjunctivitis and eczema in children from the Bangkok area: The Global Asthma Network (GAN) phase I. Asian Pac J Allergy Immunol 2019; 37: 226–231. [DOI] [PubMed] [Google Scholar]
- 22.Gashi V, Ahmetaj L. The prevalence of self-reported respiratory symptoms, asthma and use of asthma medication among young adolescents from Southeast Kosovo. Med Arc 2020; 74: 19–23. doi: 10.5455/medarh.2020.74.19-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar N, Foster A. Air quality interventions and spatial dynamics of air pollution in Delhi and its surroundings. Int J Environ Waste Manag 2009; 4: 85–111. doi: 10.1504/IJEWM.2009.026886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muñoz X, Barreiro E, Bustamante V, et al. Diesel exhausts particles: their role in increasing the incidence of asthma. Reviewing the evidence of a causal link. Sci Total Environ 2019; 652: 1129–1138. doi: 10.1016/j.scitotenv.2018.10.188 [DOI] [PubMed] [Google Scholar]
- 25.Kynyk JA, Mastronarde JG, McCallister JW. Asthma, the sex difference. Curr Opin Pulm Med 2011; 17: 6–11. doi: 10.1097/MCP.0b013e3283410038 [DOI] [PubMed] [Google Scholar]
- 26.Lauzon-Joset JF, Mincham KT, Abad AP, et al. Oestrogen amplifies pre-existing atopy-associated Th2 bias in an experimental asthma model. Clin Exp Allergy 2020; 50: 391–400. doi: 10.1111/cea.13544 [DOI] [PubMed] [Google Scholar]
- 27.Verity CM, Vanheule B, Carswell F, et al. Bronchial lability and skin reactivity in siblings of asthmatic children. Arch Dis Child 1984; 59: 871–876. doi: 10.1136/adc.59.9.871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sears MR, Burrows B, Flannery EM, et al. Atopy in childhood gender and allergen related risks for development of hay fever and asthma. Clin Exper Allergy 1993; 23: 941–948. doi: 10.1111/j.1365-2222.1993.tb00279.x [DOI] [PubMed] [Google Scholar]
- 29.Agrawal S, Pearce N, Ebrahim S. Prevalence and risk factors for self-reported asthma in an adult Indian population: a cross-sectional survey. Int J Tuberc Lung Dis 2013; 17: 275–282. doi: 10.5588/ijtld.12.0438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marchesani F, Cecarini L, Pela R, et al. Causes of chronic persistent cough in adult patients: the results of a systematic management protocol. Monaldi Arch Chest Dis 1998; 53: 510–514. [PubMed] [Google Scholar]
- 31.Kaur B, Anderson HR, Austin J, et al. Prevalence of asthma symptoms, diagnosis, and treatment in 12–14 year old children across Great Britain (International Study of Asthma and Allergies in Childhood, ISAAC UK). BMJ 1998; 316: 118–124. doi: 10.1136/bmj.316.7125.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh V, Sinha HV, Gupta R. Barriers in the management of asthma and attitudes towards complementary medicine. Respir Med 2002; 96: 835–840. doi: 10.1053/rmed.2002.1368 [DOI] [PubMed] [Google Scholar]
- 33.Singh V, Gupta R. Influence of a clinic-based group intervention on asthma perception and management in India. J Asthma Allergy Educ 2013; 4: 217–225. doi: 10.1177/2150129713478635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lycett H, Wildman E, Raebel EM, et al. Treatment perceptions in patients with asthma: synthesis of factors influencing adherence. Respir Med 2018; 141: 180–189. doi: 10.1016/j.rmed.2018.06.032 [DOI] [PubMed] [Google Scholar]
- 35.Singh N, Singh S, Jain NK, et al. Respiratory disease terminology: discordance between pulmonologists and patients. Lung India 2017; 34: 9–12. doi: 10.4103/0970-2113.197092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeatts K, Shy C, Sotir M, et al. Health consequences for children with undiagnosed asthma-like symptoms. Arch Pediatr Adolesc Med 2003; 157: 540–544. doi: 10.1001/archpedi.157.6.540 [DOI] [PubMed] [Google Scholar]
- 37.Ostrom NK, Eid NS, Craig TJ, et al. Exercise-induced bronchospasm in children with asthma in the United States: results from the exercise-induced bronchospasm landmark survey. Allergy Asthma Proc 2011; 32: 425–430. doi: 10.2500/aap.2011.32.3502 [DOI] [PubMed] [Google Scholar]
- 38.ISAAC Steering Committee . International Study of Asthma and Allergies in Childhood. 2nd Edn. Auckland: /Münster, ISAAC Phase One Manual, 1993. [Google Scholar]