Abstract
Objectives
We measure for the first time how commercially available Cannabis flower products affect feelings of fatigue.
Methods
A total of 1,224 people recorded 3,922 Cannabis flower self-administration sessions between June 6, 2016, and August 7, 2019, using the Releaf App. Usage sessions included real-time subjective changes in fatigue intensity levels prior to and following Cannabis consumption, Cannabis flower characteristics (labeled phenotype, cannabinoid potency levels), combustion method, and any potential experienced side effects.
Results
On average, 91.94% of people experienced decreased fatigue following consumption with an average symptom intensity reduction of 3.48 points on a 0–10 visual analog scale (SD = 2.70, d = 1.60, p < 0.001). While labeled plant phenotypes (“C. indica,” “C. sativa,” or “hybrid”) did not differ in symptom relief, people that used joints to combust the flower reported greater symptom relief than pipe or vaporizer users. Across cannabinoid levels, tetrahydrocannabinol, and cannabidiol levels were generally not associated with changes in symptom intensity levels. Cannabis use was associated with several negative side effects that correspond to increased feelings of fatigue (e.g., feeling unmotivated, couch-locked) among a minority of users (<24% of users), with slightly more users (up to 37%) experiencing a positive side effect that corresponds to increased energy (e.g., feeling active, energetic, frisky, or productive).
Conclusions
The findings suggest that the majority of patients experience decreased fatigue from consumption of Cannabis flower consumed in vivo, although the magnitude of the effect and extent of side effects experienced likely vary with individuals' metabolic states and the synergistic chemotypic properties of the plant.
Keywords: Fatigue, Cannabis, Marijuana, Energetics, Cannabidiol, Tetrahydrocannabinol, Stress, Complementary medicine
Federal barriers to conduct clinical investigations on the effects of Cannabis in the USA have resulted in a general lack of understanding of how common and commercially available cannabis-based products may affect even the most basic elements of normative bodily functioning, such as energy and activity levels [1, 2]. Conventional wisdom suggests that frequent Cannabis use results in decreased behavioral activity, goal pursuit, and competitiveness or what has been commonly referred to as “amotivational syndrome” [3, 4, 5]. However, feeling fatigued is also a core feature of many types of illnesses, and several studies have shown that people with chronic pain, cancer, Parkinson's disease, and multiple sclerosis experienced increased energy levels once given the legal ability to purchase and consume medical Cannabis and after substituting Cannabis for other classes of pharmaceutical medications (e.g., opiates, sedatives, antidepressants) [6, 7, 8, 9]. For example, in a recent study with over 1,000 cancer patients, people showed significant reductions across all eight of the symptom categories measured, including fatigue, within 4 months of enrollment in a state-authorized medical cannabis program [10]. However, these results appear to contradict findings from a recent review of adverse events reported by research participants in clinical studies, which found fatigue to be a commonly experienced side effect of administration [11].
Unfortunately, clinical studies in the USA have historically been limited to the investigation of the effects of either: (a) synthetic analog medications and formulates, (b) isolated compounds, or (c) government-issued Cannabis flower that is notorious for having low potency and being of poor quality and hence not generalizable to the breadth of cannabis-based products sold in retail markets throughout the USA [1, 2]. With increasing legal medical and now recreational access and use of Cannabis throughout the USA, particularly among older adults and patients with significant health conditions [12], health providers and consumers still have little formal guidance from the scientific and medical communities on how the cannabis-based products they choose to consume may influence feelings of fatigue and energy levels. No study to date has measured how the breadth of common, commercially available Cannabis flower, the most widely used type of product, affects fatigue levels in actual time, and how labeled product characteristics are correlated with changes in feelings of fatigue and related side effects.
The current study measures for the first time which types of Cannabis flower characteristics among a wide range of common and commercially available products used in vivo are associated with changes in feelings of fatigue in actual time. For measuring session-level Cannabis consumption and its effects, we used the largest database of real-time effects of Cannabis usage in the USA, which was collected by the mobile software application Releaf App [13]. Measurement of the associations between the labeled characteristics and routes of administration of commonly used Cannabis flower products should help guide patients and health providers seeking more individualized treatment options.
Methods
Study Design
This study utilizes anonymized user data collected through the Releaf App, a mobile software application designed to record real-time effects from consuming cannabis for medical use. Data are provided through a confidentiality agreement, with Institutional Review Board approval from the University of New Mexico. The app was designed to help patients monitor the variable effects of cannabis-based products and records the product types, routes of administration, and labeled Cannabis phenotypes and cannabinoid contents of the products consumed. Users indicate the medical conditions for which they are consuming cannabis, real-time symptom intensity levels prior to and following consumption, and any possible side effects experienced, under otherwise naturalistic conditions. Prior to consuming cannabis, users are directed by the app to enter information about the product they intend to consume based on information provided on product labels. Upon starting a treatment session, the user specifies the symptoms to be treated, reports a starting symptom intensity level (on a visual analog scale from 0 to 10), consumes the cannabis product, updates the symptom level, records side effects, and ends the session. The Releaf App includes 47 possible side effects (called “feelings” in the user interface). The user can update the symptom intensity level as frequently as they want and can select multiple side effects.
The study sample includes sessions using flower products with the fatigue symptom reported and with a starting fatigue intensity level greater than 0. We exclude from the analyses symptom levels updated 4 h or more after the start of the session, i.e., we use the last symptom level recorded within 4 h and include robustness checks using 1 to 3 h as the cutoff. The resulting analyzed sample includes 1,224 users who completed 3,922 sessions between June 6, 2016, and August 7, 2019.
Study Outcomes
The main study outcome is the change in the user-reported symptom intensity level following the use of Cannabis. We measure the change in the symptom intensity level (symptom relief) as ending symptom level minus starting symptom level. The resulting symptom intensity measure ranges between −10 (maximum relief) and 9 (minimum relief/maximum exacerbation of symptom intensity). For side effects, we characterize the 47 possible side effects as negative side effects (17), positive side effects (19), and context-specific side effects (11). Context-specific side effects are those side effects, which cannot easily be categorized as positive or negative, e.g., feeling “high” or “tingly.” For regression analysis, we construct measures of side effects as dummy variables indicating if the user reported any of the side effects in the category and continuous variables measuring the proportion of total side effects in each category that the user reported.
Statistical Analysis
The effect of Cannabis use on the fatigue symptom level is first examined using means comparisons. Fixed-effects panel data regression models are then used to analyze the effects of flower characteristics on symptom relief and the onset of side effects. User-specific fixed effects are included in all regressions to account for user characteristics, which do not vary over the period of observation for that user. The average cannabis patient in our study tracks their symptom relief in the app for 26.6 days (90th percentile is 76 days), meaning that important factors, such as years of cannabis use, diagnosis of mental health disorders, and education, are unlikely to change during the sample period and are thus accounted for by the individual fixed effects. Through the inclusion of user-specific fixed effects, the effects of flower characteristics are estimated by comparing symptom relief in different sessions reported by the same user, rather than comparing across users. As trade-offs exist between potency and dosing, we also include the natural log of the number of doses as a covariate in all our regressions. We use the natural log to account for outliers in our sample. The raw, unlogged mean of the dose variable is 8.49 (standard deviation = 9.65) and the 99th percentile is 47. Standard errors are clustered at the user level to account for heteroscedasticity and arbitrary correlation at the user level. Analyses were conducted using Stata 15.1.
Results
Table 1 presents descriptive statistics for the product characteristics (labeled plant phenotype, combustion method, and tetrahydrocannabinol [THC] and cannabidiol [CBD] potency levels), the starting and ending symptom intensity levels, and the prevalence of side effects. On average, users reported a starting symptom intensity level of 6.08 and an ending symptom intensity level of 2.60, suggesting an average symptom relief of 3.48 (SD = 2.70, d = 1.60, p <0.001). Among users who recorded any side effects, 68% reported at least one negative side effect, 96% reported at least one positive effect, and 81% reported at least one context-specific side effect. The most common negative side effects are dry mouth (32%) and feeling foggy (24%), the most common positive side effects are feeling relaxed (62%) and peaceful (55%), and the most common context-specific side effects are feeling high (46%) and tingly (32%) (online suppl. Table S1 [see www.karger.com/doi/10.1159/000524057 for all online suppl. material] reports the prevalence and categorization of the side effects and the average symptom relief in sessions in which that side effect was reported).
Table 1.
Descriptive statistics
| Mean | SD | Min | Max | |
|---|---|---|---|---|
| Panel A: labeled plant phenotype (3,511 symptom sessions, 1,118 users) | ||||
| Hybrid | 0.48 | 0.50 | 0 | 1 |
| C. indica | 0.18 | 0.38 | 0 | 1 |
| C. sativa | 0.35 | 0.48 | 0 | 1 |
| Panel B: combustion method (3,667 symptom sessions, 1,147 users) | ||||
| Joint | 0.17 | 0.38 | 0 | 1 |
| Pipe | 0.49 | 0.50 | 0 | 1 |
| Vape | 0.34 | 0.47 | 0 | 1 |
| Panel C: THC (1,469 symptom sessions, 517 users) | ||||
| % THC | 19.09 | 7.33 | 0 | 35 |
| THC <10% | 0.14 | 0.35 | 0 | 1 |
| THC 10–19% | 0.39 | 0.49 | 0 | 1 |
| THC 20–35% | 0.47 | 0.50 | 0 | 1 |
| Panel D: CBD (901 symptom sessions, 369 users) | ||||
| % CBD | 5.95 | 7.65 | 0 | 35 |
| CBD <1% | 0.34 | 0.47 | 0 | 1 |
| CBD 1–9% | 0.37 | 0.48 | 0 | 1 |
| CBD 10–35% | 0.29 | 0.45 | 0 | 1 |
| Panel E: outcome and control variables (3,922 symptom sessions, 1,224 users) | ||||
| Symptom change | –3.48 | 2.70 | –10 | 9 |
| Starting symptom level | 6.08 | 2.10 | 1 | 10 |
| Ending symptom level | 2.60 | 2.24 | 0 | 10 |
| Total dose | 8.49 | 9.65 | 1 | 149 |
| Panel F: side effects (3,247 sessions, 1,020 users) | ||||
| Any negative side effect | 0.68 | 0.47 | 0 | 1 |
| Negative side effects, % | 0.13 | 0.16 | 0 | 1 |
| Any positive side effect | 0.96 | 0.20 | 0 | 1 |
| Positive side effects, % | 0.31 | 0.21 | 0 | 1 |
| Any context-specific side effect | 0.81 | 0.39 | 0 | 1 |
| Context-specific side effects, % | 0.23 | 0.23 | 0 | 1 |
Sample includes sessions with fatigue as the symptom reported. Nineteen positive, seventeen negative, and eleven context-specific side effects were available for selection.
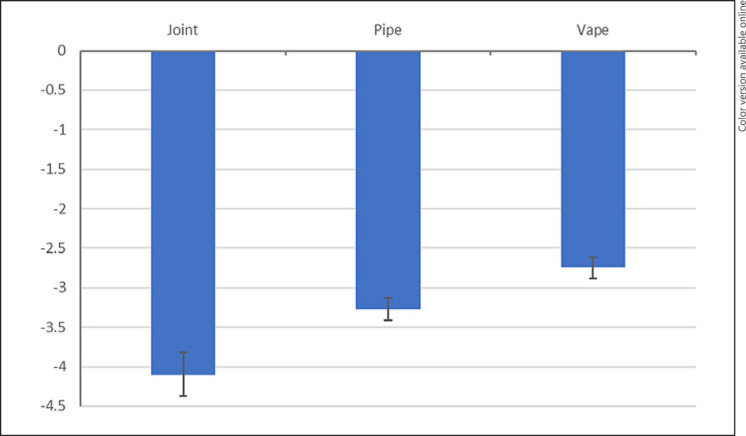
Table 2 shows the results for the effects of product characteristics on fatigue symptom relief. Each column reports results from a separate regression. Column 1 shows the effect of the labeled plant phenotype. The “hybrid” plant phenotype is omitted as the base category. Findings suggest that symptom relief does not vary by the labeled plant phenotype. Column 2 suggests that vaping Cannabis flower is associated with less symptom relief than using a joint. Column 3 suggests that THC and CBD levels cannot explain any difference in fatigue symptom relief. In column 4, all product characteristics are included in the regression. The effect of combustion method becomes stronger. Overall, people that used joints reported the largest symptom relief, relative to users of pipes and vaporizers. Vaping is associated with the least symptom relief. Figure 1 plots average symptom relief from individual fixed-effects models controlling for product type, combustion method, THC and CBD levels, starting symptom level, and individual fixed effects. The coefficients for the other product characteristics remain generally insignificant except for mid-level CBD potency, which is weakly associated with greater symptom relief. Online supplementary Table S2 shows various alternative modeling specifications: THC and CBD levels coded as continuous variables, maximum symptom relief used as the outcome variable (lowest recorded symptom level within 4 h, rather than last, minus starting symptom level), and samples restricted by using the last symptom level recorded within 1–3 h, instead of 4 h. Sample sizes are smaller because some individuals who update their symptom level within 4 h do not update their symptom level within 1–3 h. Findings confirm that the combustion method is the only significant independent predictor of fatigue symptom relief. Vaporizers and pipes are similarly inferior to joints for one-hour symptom relief, but vaporizers appear to become less effective relative to pipes over a more extended period, i.e., vaporizers may offer the least sustained effect on relief from fatigue symptoms.
Table 2.
Effects of flower characteristics on symptom relief
| Outcome = symptom change (ending – starting symptom) | (1) | (2) | (3) | (4) |
|---|---|---|---|---|
| Panel A: labeled plant phenotype, omitted category = hybrid | ||||
| C. indica | 0.073 | – | – | 0.425 |
| (0.097) | – | – | (0.309) | |
| C. sativa | 0.034 | – | – | 0.001 |
| (0.076) | – | – | (0.229) | |
|
| ||||
| Panel B: combustion method, omitted category = joint | ||||
| Pipe | – | –0.040 | – | 0.812** |
| (0.129) | – | (0.401) | ||
| Vape | – | 0.292** | – | 1.288** |
| – | (0.118) | – | (0.519) | |
|
| ||||
| Panel C: THC and CBD, omitted categories = THC <10% and CBD <1% | ||||
| THC 10–19% | – | – | 0.125 | 0.072 |
| – | – | (0.254) | (0.244) | |
| THC 20–35% | – | – | –0.049 | 0.022 |
| – | – | (0.268) | (0.269) | |
| CBD 1–9% | – | – | –0.278 | –0.401* |
| – | – | (0.204) | (0.241) | |
| CBD 10–35% | – | – | 0.226 | 0.132 |
| – | – | (0.188) | (0.191) | |
| Starting symptom level | –0.668*** | –0.666*** | –0.660*** | –0.606*** |
| (0.026) | (0.026) | (0.063) | (0.068) | |
| Log dose | –0.206*** | –0.227*** | –0.199 | –0.171 |
| (0.067) | (0.067) | (0.157) | (0.171) | |
| Constant | 0.902*** | 0.853*** | 1.030** | –0.257 |
| (0.211) | (0.203) | (0.522) | (0.726) | |
| Sessions, n | 3,493 | 3,647 | 724 | 632 |
| R 2 | 0.286 | 0.289 | 0.270 | 0.257 |
| Users, n | 1,113 | 1,141 | 311 | 270 |
All regressions are estimated using a fixed-effects model. C. indica and C. sativa are relative to hybrid, THC categories are relative to THC between 0 and 9%, CBD categories are relative to 0% CBD, and pipe and vape are relative to joint. Standard errors, clustered at the individual user level, are shown in parentheses.
p < 0.01.
p < 0.05.
p < 0.10.
Fig. 1.
Adjusted fatigue relief refers to the covariate-adjusted change in symptom severity, obtained from a fixed-effects model controlling for subspecies, combustion method, THC and CBD categories, log of dose, and starting symptom level.
Table 3 presents regressions of side effects on product characteristics. Use of products labeled as “C. indica” is associated with more reporting of negative side effects and may increase context-specific side effects. C. sativa is weakly associated with increased reporting of positive side effects. Higher THC levels are weakly associated with increased reporting of context-specific side effects, but do not affect the reporting of negative or positive side effects. Higher CBD levels are associated with decreased reporting of negative and possibly context-specific side effects.
Table 3.
Effects of flower characteristics on side effects
| (1) negative | (2) negative, % | (3) positive | (4) positive, % | (5) context-specific | (6) context-specific, % | |
|---|---|---|---|---|---|---|
| C. indica | 0.117 | 0.043** | –0.005 | –0.043 | 0.077 | 0.058** |
| (0.089) | (0.018) | (0.020) | (0.028) | (0.067) | (0.026) | |
|
| ||||||
| C. sativa | –0.046 | –0.006 | 0.001 | 0.032* | 0.048 | 0.025 |
| (0.063) | (0.015) | (0.023) | (0.019) | (0.061) | (0.022) | |
|
| ||||||
| Pipe | –0.166 | –0.024 | 0.023 | –0.037 | 0.141 | –0.007 |
| (0.162) | (0.033) | (0.020) | (0.024) | (0.136) | (0.050) | |
|
| ||||||
| Vape | –0.169 | –0.057 | –0.023 | –0.068 | 0.083 | –0.066 |
| (0.169) | (0.040) | (0.020) | (0.044) | (0.135) | (0.063) | |
|
| ||||||
| THC 10–19% | 0.019 | –0.007 | 0.006 | 0.027 | 0.112 | 0.060** |
| (0.095) | (0.015) | (0.023) | (0.034) | (0.080) | (0.027) | |
|
| ||||||
| THC 20–35% | 0.012 | 0.004 | –0.005 | 0.033 | 0.085 | 0.046* |
| (0.119) | (0.017) | (0.023) | (0.029) | (0.064) | (0.025) | |
|
| ||||||
| CBD 1–9% | –0.082 | 0.012 | –0.022 | 0.005 | –0.083* | 0.004 |
| (0.084) | (0.018) | (0.029) | (0.031) | (0.045) | (0.023) | |
|
| ||||||
| CBD 10–35% | –0.225*** | –0.007 | –0.023 | –0.014 | –0.096 | –0.026 |
| (0.079) | (0.013) | (0.016) | (0.023) | (0.066) | (0.021) | |
|
| ||||||
| Starting symptom level | 0.018 | 0.002 | –0.002 | 0.002 | –0.010 | 0.000 |
| (0.021) | (0.005) | (0.006) | (0.006) | (0.012) | (0.005) | |
|
| ||||||
| Log dose | 0.086 | 0.008 | 0.035 | 0.025** | 0.051* | 0.037*** |
| (0.053) | (0.011) | (0.025) | (0.013) | (0.030) | (0.013) | |
|
| ||||||
| Constant | 0.632*** | 0.096** | 0.927*** | 0.238*** | 0.635*** | 0.103* |
| (0.181) | (0.044) 543 | (0.068) 543 | (0.049) 543 | (0.140) 543 | (0.061) 543 | |
|
| ||||||
| Observations | 543 | 543 | 543 | 543 | 543 | 543 |
|
| ||||||
| R 2 | 0.055 | 0.049 | 0.016 | 0.065 | 0.030 | 0.079 |
|
| ||||||
| Users, N | 231 | 231 | 231 | 231 | 231 | 231 |
All regressions are estimated using a fixed-effects model. C. indica and C. sativa are relative to hybrid, THC categories are relative to THC between 0 and 9%, CBD categories are relative to 0% CBD, and pipe and vape are relative to joint. Standard errors, clustered at the individual user level, are shown in parentheses.
p < 0.01.
p < 0.05.
p < 0.10.
Descriptive characteristics of users are shown in online supplementary Table S3. Approximately 72% of sessions in the analyzed sample have user characteristics information reported. Male users experienced greater symptom relief compared to female users. Symptom relief does not seem to differ by user age and user experience. Table 4 compares the effect of flower characteristics on symptom relief by user characteristics. Similar to the full sample analysis, subsample analyses by gender, age, and self-reported experience with Cannabis show that using joints is associated with greater symptom relief in all subgroups except for male and experienced users, although the coefficient on vaporizer is generally larger and more statistically significant than the coefficient for pipe. Use of products labeled as “C. indica” is weakly associated with less symptom relief among users aged 40 years and younger. THC levels are not significant predictors of fatigue symptom relief for any subgroups except for users who are not experienced with Cannabis. For these users, higher levels of THC are associated with less symptom relief. Individuals under 40 years experienced increased symptom relief with CBD levels between 1 and 9%, while the results suggest a similar, less statistically significant relationship for females. CBD levels between 10 and 35% are associated with less symptom relief among users aged 40 years and above and users who are not experienced.
Table 4.
Effects of flower characteristics on symptom relief: by user characteristics
| Male | Female | Age < = 40 years | Age >40 years | Experienced | Not experienced | |
|---|---|---|---|---|---|---|
| C. indica | 0.217 | 0.779 | 1.234* | –0.433 | –0.006 | 1.016 |
| (0.503) | (0.646) | (0.629) | (0.305) | (0.263) | (0.617) | |
|
| ||||||
| C. sativa | –0.119 | 0.113 | –0.177 | 0.283 | 0.334 | 0.147 |
| (0.424) | (0.380) | (0.428) | (0.340) | (0.335) | (0.392) | |
|
| ||||||
| Pipe | 0.709 | 1.132** | 1.582*** | 1.065** | 0.930 | 0.726 |
| (0.720) | (0.457) | (0.415) | (0.433) | (0.728) | (0.536) | |
|
| ||||||
| Vape | 1.449 | 1.837*** | 2.346*** | 1.427** | 1.079 | 1.715** |
| (0.870) | (0.570) | (0.605) | (0.544) | (0.773) | (0.672) | |
|
| ||||||
| THC 10–19% | 0.650 | 0.060 | –0.038 | 0.340 | 0.148 | 0.545* |
| (0.664) | (0.314) | (0.520) | (0.216) | (0.576) | (0.305) | |
|
| ||||||
| THC 20–35% | 0.116 | 0.292 | –0.236 | 0.581 | –0.447 | 1.056** |
| (0.766) | (0.353) | (0.391) | (0.427) | (0.605) | (0.407) | |
|
| ||||||
| CBD 1–9% | –0.260 | –0.522* | –0.847** | –0.308 | –0.319 | –0.307 |
| (0.427) | (0.313) | (0.420) | (0.207) | (0.483) | (0.284) | |
|
| ||||||
| CBD 10–35% | –0.748 | 0.111 | 0.049 | 0.286* | –0.236 | 0.707*** |
| (0.574) | (0.228) | (0.353) | (0.145) | (0.506) | (0.182) | |
|
| ||||||
| Starting symptom level | –0.758*** | –0.465*** | –0.634*** | –0.470*** | –0.660*** | –0.510*** |
| (0.091) | (0.102) | (0.086) | (0.105) | (0.064) | (0.130) | |
|
| ||||||
| Log dose | –0.562* | 0.062 | –0.361* | 0.273 | –0.220 | 0.167 |
| (0.290) | (0.211) | (0.205) | (0.189) | (0.197) | (0.268) | |
|
| ||||||
| Constant | 1.006 | –1.894* | –0.283 | –2.430** | 0.311 | –2.644* |
| (1.156) | (1.069) | (0.765) | (1.084) | (0.901) | (1.399) | |
|
| ||||||
| Observations | 175 | 278 | 263 | 173 | 251 | 207 |
|
| ||||||
| R2 | 0.376 | 0.248 | 0.387 | 0.195 | 0.391 | 0.247 |
|
| ||||||
| Users, N | 77 | 91 | 111 | 52 | 95 | 76 |
All regressions are estimated using a fixed-effects model. C. indica and C. sativa are relative to hybrid, THC categories are relative to THC between 0 and 9%, CBD categories are relative to 0% CBD, and pipe and vape are relative to joint. Standard errors, clustered at the individual user level, are shown in parentheses.
p < 0.01.
p < 0.05.
p < 0.1.
Throughout the regressions, higher starting symptom levels are consistently associated with greater symptom relief, but have no effect on side-effect reporting. The natural log of dose is associated with greater symptom relief only in regressions that do not control for THC and CBD. Once we control for potency, the effect of dose becomes much weaker and generally statistically insignificant. Higher doses are associated with greater reporting of positive and context-specific side effects, but are not associated with differential negative side-effect reporting.
Discussion
By measuring how common and commercially available Cannabis flower products affect momentary fatigue levels, we provide a significant, initial contribution to the scientific literature on normative ethological effects of Cannabis consumption. Using the largest database of real-time effects of Cannabis usage in the USA, we found that combusting whole, dried Cannabis flower has a generally fast-acting and energetic effect for the majority of people that have symptoms of fatigue. While some user sessions resulted in increased fatigue or fatigue-related side-effect experiences, the vast majority of people (about 92%) reported an overall decrease in their perceived fatigue intensity levels, averaging roughly 3.5 points on a standard 0–10 visual analog scale. Overall, higher CBD levels were inversely associated with the reporting of negative side effects, and males, older patients, and more experienced users reported the greatest symptom relief. These findings highlight the need for more fine-grained analyses of how phytochemical consumption may interact with the user's endocrine, metabolic, cardiovascular, and other (e.g., musculoskeletal) bodily systems to produce variable visceral and physiological effects.
The consistent finding that vaporization of Cannabis flower resulted in worse outcomes than joints, particularly for more sustained symptom relief over a longer period, suggests that the temperature at which the phytochemicals combust may affect changes in fatigue. Both pipes and vaporizers generally provide less relief from fatigue, perhaps also related to dosage and the relatively easier task of inhaling repeatedly on a burning joint versus relighting a pipe or using a vaporizer. In the overall sample and for all subgroups except inexperienced users, THC, CBD, and labeled plant phenotypes (e.g., “C. sativa” vs. “C. sativa”) were not independent predictors of symptom relief, suggesting that other cannabinoids and noncannabinoid chemical constituents, such as terpenes and terpenoids, may affect the perception of mental and physical fatigue or the relationship between THC, CBD, and fatigue. Terpenes and terpenoids that are present in concentrations above 0.05% are of pharmacological interest [14], and terpenes such as alpha-pinene and D-limonene are highly bioavailable; the bioavailability of alpha-pinene is about 60% and that of D-limonene is about 70% [15, 16, 17]. Because of their lipophilicity and small molecular size, many terpenes and terpenoids are thought to cross the blood-brain barrier with relative ease [18].
The bicyclic monoterpene, alpha-pinene, and the cyclic monoterpene, D-limonene, are the most and the second most widely abundant terpenes, respectively, found in nature [16, 19, 20]. For example, in Salvia lavandulifolia, the concentration of alpha-pinene has recently been reported to be approximately 4.8–5.5%, and D-limonene was reported to be approximately 2.8–4.4% [21]. Researchers measuring the effects of essential oil derived from S. lavandulifolia have observed attenuation of mental fatigue during task performance as well as higher subjective ratings of alertness during a 4-h Cognitive Demand Battery assessment, likely in part due to the ability of alpha-pinene and D-limonene to inhibit acetylcholinesterase (AChE) [22]. Similar cognitive and attentional improvements have been observed in other studies using extracts of sage with less potent AChE-inhibiting properties [23, 24, 25].
Additional terpenes and terpenoids that can be found at therapeutic levels in the Cannabis plant, including pulegone, 1,8-cineole (eucalyptol), alpha-terpineol, terpinen-4-ol, and p-cymene, also demonstrate AChE inhibition [23, 26]. Among these, eucalyptol has been observed to have the strongest AChE inhibitory effects, followed by alpha-pinene and terpinen-4-ol [21]. Other research measuring AChE and butyrylcholinesterase activity in essential oils from three different species of pine (P. heldreichii subsp.) found that leucodermis is also a potent inhibitor of AChE and butyrylcholinesterase [27]. Synergistic therapeutic effects have been observed, suggesting that essential oils derived from whole plants such as sage demonstrate stronger effects than their individual constituents alone [18]. This synergy or molecular symphony is often referred to as the “entourage effect” that is commonly reported in full-spectrum cannabis products (i.e., whole dried flower), and which is absent when individual constituents (e.g., cannabinoids, terpenoids, flavonoids, and other phytochemicals) are evaluated in isolation.
While the mobile software technology used for collecting real-time, individual-level recordings of Cannabis' effects provides a partial solution to the challenge of measuring the pharmacodynamics and other experienced effects of consuming the breadth of commercially available cannabis products sold at medical and recreational dispensaries and black market dealers, the current study does have important limitations. The primary drawback is the lack of a comparison group, such as people that did not use Cannabis to treat fatigue or Cannabis consumed independent of using the app. People who treat symptoms of fatigue with Cannabis or have failed to adequately benefit from alternative treatment options may be particularly more likely to report benefits from Cannabis. The potential bias from app use is less clear and may be affected by knowledge and feelings about the Releaf App or technological app use in general, and/or due to attrition from either dissatisfaction with cannabis or the app or to satisfaction with existing cannabis use and no further need to explore product options. Although our study used the largest sample of real-time assessments of Cannabis' effects on fatigue to date, we did not include the many phytochemicals in the Cannabis plant, nonflower cannabis products, or individual factors that vary over time, such as concomitant use of other substances (e.g., nicotine, alcohol, caffeine, and prescription drugs), which likely influences cannabis users' experiences. Our results that high levels of THC and CBD decrease symptom relief among less experienced users suggest that tolerance may play a role and that the mantra “start low, go slow” may be good advice for users new to medical Cannabis.
Despite these limitations, the current study shows that whole natural Cannabis flower has fast-acting and energetic effects for the majority of users who use it for treating fatigue. However, novice users generally should avoid high levels of THC, and perhaps high levels of CBD, although the latter should be weighed against the apparent ability of higher levels of CBD to reduce the probability of reporting a negative side effect. Future research would benefit from investigating real-time effects of Cannabis usage on behavioral and mental fatigue under altered bodily states and how different phytochemicals in the Cannabis plant aggregate and/or interact in its mental and physical effects in healthy people and clinical populations.
Statement of Ethics
By using the Releaf App users consent to the clearly described Privacy Policy stating their anonymized data may be available to outside researchers. The study followed all appropriate guidelines and was deemed exempt from further review by the University of New Mexico Institutional Review Board.
Conflict of Interest Statement
F.B., K.K., and B.H. are employed by MoreBetter, Ltd. The authors report no other conflicts of interests.
Funding Sources
This research was supported in part by the University of New Mexico Medical Cannabis Research Fund.
Author Contributions
J.M.V. and S.S.S. conceived the study. F.B., K.K., and B.H. independently designed and developed the Releaf AppTM and server infrastructure as part of their effort to help create an education tool for medical cannabis patients. X.L. conducted the analyses. J.M.V., S.S.S., J.P.D., and X.L. drafted the manuscript. All the authors contributed substantially to its intellectual content and revision.
Data Availability Statement
The data are available upon request and approval from MoreBetter, Ltd.
Supplementary Material
Supplementary data
Acknowledgments
We thank all the donors to the University of New Mexico, Medical Cannabis Research Fund for making this research possible.
References
- 1.Stith SS, Vigil JM. Federal barriers to cannabis research. Science. 2016;352:1182. doi: 10.1126/science.aaf7450. [DOI] [PubMed] [Google Scholar]
- 2.National Academies of Sciences Engineering and Medicine . The Health Effects of cannabis and cannabinoids: the current state of evidence and recommendations for research (2017) Washington, DC: The National Academies Press; 2017. [PubMed] [Google Scholar]
- 3.Lac A, Luk JW. Testing the amotivational syndrome: marijuana use longitudinally predicts lower self-efficacy even after controlling for demographics, personality, and alcohol and cigarette use. Prev Sci. 2018;19((2)):117–26. doi: 10.1007/s11121-017-0811-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DavidSmith EMD. Acute and chronic toxicity of marijuana. J Psychedelic Drugs. 1968;2((1)):37–48. [Google Scholar]
- 5.Lynskey M, Hall W. The effects of adolescent cannabis use on educational attainment: a review. Addiction. 2000 Nov;95((11)):1621–30. doi: 10.1046/j.1360-0443.2000.951116213.x. [DOI] [PubMed] [Google Scholar]
- 6.Vigil JM, Stith SS, Adams IM, Reeve AP. Associations between medical cannabis and prescription opioid use in chronic pain patients: a preliminary cohort study. PLoS One. 2017;12((11)):e0187795–13. doi: 10.1371/journal.pone.0187795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stith SS, Vigil JM, Adams IM, Reeve AP. Effects of legal access to cannabis on scheduled II–V drug prescriptions. J Am Med Dir Assoc. 2018 Jan;19((1)):59–64.e1. doi: 10.1016/j.jamda.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Piper BJ, Dekeuster RM, Beals ML, Cobb CM, Burchman CA, Perkinson L, et al. Substitution of medical cannabis for pharmaceutical agents for pain, anxiety, and sleep. J Psychopharmacol. 2017 May;31((5)):569–75. doi: 10.1177/0269881117699616. [DOI] [PubMed] [Google Scholar]
- 9.Kindred JH, Li K, Ketelhut NB, Proessl F, Fling BW, Honce JM, et al. Cannabis use in people with parkinson's disease and multiple sclerosis: a web-based investigation. Complement Ther Med. 2017;33:99–104. doi: 10.1016/j.ctim.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Anderson SP, Zylla DM, McGriff DM, Arneson TJ. Impact of Medical Cannabis on patient-reported symptoms for patients with cancer enrolled in minnesota's medical cannabis program. J Oncol Pract. 2019 Apr;15((4)):e338–45. doi: 10.1200/JOP.18.00562. [DOI] [PubMed] [Google Scholar]
- 11.Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313((24)):2456–73. doi: 10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- 12.Han BH, Palamar JJ. Trends in cannabis use among older adults in the United States, 2015–2018. JAMA Intern Med. 2020;180((4)):609–11. doi: 10.1001/jamainternmed.2019.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Releaf App. Cannabis treatment tracking & research [Internet] [cited 2019 Jun 10]. Available from: https://releafapp.com/
- 14.Adams TB, Taylor SV. Handbook of essential oils science, technology, and applications. 1st ed. CRC Press; 2010. Safety evaluation of essential oils: a constituent-based approach. [Google Scholar]
- 15.Filipsson AF, Löf A, Hagberg M, Hjelm EW, Wang Z. D-limonene exposure to humans by inhalation: uptake, distribution, elimination, and effects on the pulmonary function. J Toxicol Environ Health. 1993 Jan;38((1)):77–88. doi: 10.1080/15287399309531702. [DOI] [PubMed] [Google Scholar]
- 16.Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011 Aug;163((7)):1344–64. doi: 10.1111/j.1476-5381.2011.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falk AA, Hagberg MT, Löf AE, Wigaeus-Hjelm EM, Wang ZP. Uptake, distribution and elimination of alpha-pinene in man after exposure by inhalation. Scand J Work Environ Health. 1990 Oct;16((5)):372–8. doi: 10.5271/sjweh.1771. [DOI] [PubMed] [Google Scholar]
- 18.Savelev SU, Okello EJ, Perry EK. Butyryl- and acetyl-cholinesterase inhibitory activities in essential oils of salvia species and their constituents. Phytother Res. 2004 Apr;18((4)):315–24. doi: 10.1002/ptr.1451. [DOI] [PubMed] [Google Scholar]
- 19.Noma Y, Asakawa Y. Handbook of essential oils: science, technology, and applications. 2nd ed. CRC Press; 2015. Biotransformation of monoterpenoids by microorganisms, insects, and mammals. [Google Scholar]
- 20.Andre CM, Hausman JF, Guerriero G. Cannabis sativa: the plant of the thousand and one molecules. Front Plant Sci. 2016 Feb 4;7:19. doi: 10.3389/fpls.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cutillas AB, Carrasco A, Martinez-Gutierrez R, Tomas V, Tudela J. Composition and antioxidant, antienzymatic and antimicrobial activities of volatile molecules from Spanish salvia lavandulifolia (Vahl) essential oils. Molecules. 2017 Aug 21;22((8)):1382. doi: 10.3390/molecules22081382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tildesley NTJ, Kennedy DO, Perry EK, Ballard CG, Savelev S, Wesnes KA, et al. Salvia lavandulaefolia (Spanish Sage) enhances memory in healthy young volunteers. Pharmacol Biochem Behav. 2003 Jun;75((3)):669–74. doi: 10.1016/s0091-3057(03)00122-9. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy DO, Dodd FL, Robertson BC, Okello EJ, Reay JL, Scholey AB, et al. Monoterpenoid extract of sage (Salvia lavandulaefolia) with cholinesterase inhibiting properties improves cognitive performance and mood in healthy adults. J Psychopharmacol. 2011 Aug;25((8)):1088–100. doi: 10.1177/0269881110385594. [DOI] [PubMed] [Google Scholar]
- 24.Scholey AB, Tildesley NTJ, Ballard CG, Wesnes KA, Tasker A, Perry EK, et al. An extract of Salvia (sage) with anticholinesterase properties improves memory and attention in healthy older volunteers. Psychopharmacology. 2008 May;198((1)):127–39. doi: 10.1007/s00213-008-1101-3. [DOI] [PubMed] [Google Scholar]
- 25.Tildesley NT, Kennedy DO, Perry EK, Ballard CG, Wesnes KA, Scholey AB. Positive modulation of mood and cognitive performance following administration of acute doses of Salvia lavandulaefolia essential oil to healthy young volunteers. Physiol Behav. 2005 Jan 17;83((5)):699–709. doi: 10.1016/j.physbeh.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 26.McPartland JM, Russo EB. Cannabis therapeutics in HIV/AIDS. Routledge; 2012. Cannabis and Cannabis extracts: greater than the sum of their parts? [Google Scholar]
- 27.Bonesi M, Menichini F, Tundis R, Loizzo MR, Conforti F, Passalacqua NG, et al. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of Pinus species essential oils and their constituents. J Enzyme Inhib Med Chem. 2010 Oct;25((5)):622–8. doi: 10.3109/14756360903389856. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
The data are available upon request and approval from MoreBetter, Ltd.