Abstract
Background
Associations between height, cancer risk and worse outcome have been reported for several cancers including breast cancer. We hypothesized that in breast cancer clinical trials, tall women should be overrepresented and might have worse prognosis.
Methods
Data of 4,935 women, included from 1990 to 2010 in 5 trials of the Austrian Breast and Colorectal Cancer Study Group (ABCSG), were analyzed retrospectively. The primary objective was to determine differences in height distribution between the ABCSG cohort and the Austrian female population according to a cross-sectional health survey conducted by the Austrian Statistic Center in 2006 and 2007. Secondary endpoints were disease-free survival (DFS) and overall survival (OS) in different height classes and differences of body mass index (BMI) distribution.
Results
Breast cancer patients in the ABCSG cohort were only slightly but statistically significantly smaller compared to unselected Austrian adult females (mean 164.3 vs. 164.8 cm; p < 0.0001) and significantly more patients were seen in the lower body height class (50 vs. 46%; p < 0.0001) when using the median as a cutoff. However, after adjustment for age, the difference in body height between the two cohorts was no longer significant (p = 0.089). DFS and OS in the two upper height groups (≥170 cm) compared to the two lowest height groups (<160 cm) was not significantly different (5-year DFS: 84.7 vs. 83.0%; HR 0.91, 95% CI 0.73–1.13, p = 0.379; 5-year OS: 94.8 vs. 91.7%; HR 0.74, 95% CI 0.55–1.00, p = 0.051). The BMI of ABCSG patients was significantly higher than in the reference population (mean BMI 24.64 vs. 23.96; p < 0.0001).
Conclusions
Our results do not confirm previous findings that greater body height is associated with a higher breast cancer risk and worse outcome.
Keywords: Early breast cancer, Height, Body mass index, Risk, Outcome, Austrian Breast and Colorectal Cancer Study Group, Disease-free survival, Overall survival
Background
Body constitution is thought to influence both incidence and treatment outcome of breast cancer. Obesity is an established risk factor for the development of cancer and is associated with worse prognosis and outcome after antineoplastic therapy [1, 2]. Besides body weight, body height was also shown to have an impact on cancer risk and treatment efficacy, with an increased risk in taller individuals [3, 4, 5, 6, 7]. In the Million Women Study, 1.3 million women without previous history of cancer were followed for cancer incidence for a median of 9.4 years [8]. The relative risk (RR) for developing 1 of 17 documented cancer types increased 1.16 per 10 cm body height. This was significant for 15 of 17 examined tumor types, including breast cancer (RR per 10 cm body height 1.17) [8].
In 2001, Gunnell et al. [9] reviewed 24 cohort and nested case-control studies, which reported a statistical association between height and breast cancer risk. In all but one of the studies, the RR in relation to increasing height was greater than 1 [3]. The reviewed studies were performed in different countries, which is of importance because epidemiologic analyses indicate that geographic patterns of cancer incidence and mortality are associated with variations in population height [9, 10].
Even birth length has been shown to represent a risk factor for breast cancer. In a review of 32 cohort and case-control studies, the association between birth size and breast cancer risk was analyzed. Among the 3 variables birth weight, head circumference, and birth length, length has been shown to be the strongest independent predictor of risk [11]. However, in a prospective study of 453,023 women, birth weight did not add any further information to adult height as a predictor of cancer incidence [12].
The precise nature of the mechanisms underlying these observations remains unclear and speculative. One possible explanation might be that taller people have simply more cells (including stem cells) and, thus, a numerically greater “background” risk of mutations leading to malignant transformation [13]. This is, however, not fully applicable for breast cancer risk, since taller women do not mandatorily have bigger or denser breasts. Another reason could be that people with higher body height have significantly higher plasma levels of insulin-like growth factor 1 (IGF1), which has been linked to an increased risk for several cancer types, breast cancer included [14, 15, 16, 17]. The hormonal environment is another key player in this context. Within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort, the risk for both hormone receptor (HR)-negative and HR-positive breast cancer was positively associated with standing height, leg length, and sitting height and inversely associated with an increasing age at menarche [18, 19]. Women who were tall and had an early menarche (≤13 years) showed an almost 2-fold increased risk for HR-positive breast tumors, but no such increase in risk was observed for HR-negative breast cancer, suggesting a possible hormonal link [19].
Noteworthy, Maehle et al. [18] reported that higher birth length is a negative prognostic factor for breast cancer outcome. This may reflect effects of factors stimulating both longitudinal body growth and metastatic tumor spread. In addition, a relationship between adult body height and breast cancer-related deaths has been described [3, 4].
Body mass index (BMI) is calculated from height and body weight, respectively. Increased BMI has been associated with increased cancer risk in many cancer types, including breast cancer [1, 2, 20]. Increased BMI is largely dominated by body weight mostly caused by an excess of fat tissue. The state of overweight and obesity is now considered a major contributor to cancerogenesis [1]. This is largely attributed to the proinflammatory condition provided by fat tissues and the cytokines released by macrophages therein as well as by significant alterations in the leptin pathway [2].
All these findings led us to hypothesize that in a comprehensive cohort of breast cancer patients, enrolled in Trials of the Austrian Breast and Colon Cancer Study Group (ABCSG), taller women could be overrepresented compared to the general Austrian female population and could have a shorter disease-free survival (DFS) and overall survival (OS) compared to shorter breast cancer patients. Furthermore, we wanted to demonstrate whether BMI had an effect in this regard.
Methods
Participants
Preconditions for eligibility of trials and patients were the following: (1) height and weight were available, (2) data of included patients were available in digital form, and (3) trials were already closed for inclusion. Thus, breast cancer patients from the ABCSG (neo)adjuvant trials numbers 5, 6, 12, 14, and 24 were included, comprising in total 4,935 women. The control group of Austrian adult females was represented by a data set provided by the Austrian Statistic Center, containing the results of a cross-sectional health survey conducted in 2006 and 2007 using a computer-assisted personal interviewing (CAPI) system. In total, 25,130 persons older than 15 years were invited to participate in this survey and 15,474 people were finally interviewed for this survey, including 8,469 women.
Trials
Three adjuvant (ABCSG 5, 6, and 12) and 2 neoadjuvant (ABCSG 14 and 24) trials, performed between 1990 and 2010, were available for this evaluation. Two of the studies investigated chemotherapeutic regimens only (ABCSG 14 and 24), 1 study both chemotherapy and endocrine treatment (ABCSG 5), and 2 studies investigated endocrine therapy alone (ABCSG 6 and 12). One study (ABCSG 6) included postmenopausal women only, 2 studies (ABCSG 5 and 12) premenopausal women only, and 2 trials (ABCSG 14 and 24) both pre- and postmenopausal breast cancer patients.
In detail, ABCSG 5 compared adjuvant endocrine treatment (tamoxifen and goserelin) in premenopausal patients with estrogen receptor (ER)-positive breast cancer with adjuvant chemotherapy (cyclophosphamide, methotrexate, and fluorouracil [CMF]) [21]. ABCSG 6 compared tamoxifen with tamoxifen plus aminoglutethimide as adjuvant treatment in postmenopausal breast cancer patients with hormone receptor-positive disease [22]. In ABCSG 12, tamoxifen plus goserelin and anastrozole plus goserelin were compared in premenopausal women and the effect of zoledronic acid was investigated [23]. ABCSG 14 investigated the effect of 3 versus 6 cycles of neoadjuvant epirubicin and docetaxel plus granulocyte colony-stimulating factor (G-CSF) on pathologic complete remission rate [24]. ABCSG 24 compared epirubicin, docetaxel, and capecitabine plus G-CSF with epirubicin and docetaxel plus G-CSF as neoadjuvant treatment for early breast cancer [25].
Procedures
The primary aim of this analysis was to determine a difference in height distribution between the ABCSG cohort and the Austrian female population. As secondary endpoints, we defined long-term outcome in different height classes and different classes of BMI of the ABCSG breast cancer cohort. Height of participants was determined in centimeters and weight in kilograms at study entry. Analogous to the Million Women Study [8], patients were divided into 6 height categories: <155 cm, 155–159.9 cm, 160–164.9 cm, 165–169.9 cm, 170–174.9 cm, and ≥175 cm. BMI was classified according to the classification of the World Health Organisation (WHO) into 4 categories: underweight (<18.5), normal weight (18.5–24.99), overweight (≥25), and obese (≥30).
Statistical Methods
The sample of the cross-sectional health survey was spatially stratified in order to achieve an equal size for each supply region to obtain sampling errors and thus results of equal accuracy. The stratification was carried out according to the 32 health care regions, as defined in the Austrian Structural Plan for Health, that is, the same number of people from the population of each care region was included in the sample. To account for a possible bias caused by stratifications, the data from the Austrian health survey were weighted based on the number of citizens of the regions grouped by 5-year age classes and gender. These sampling weights were taken into account for the estimation of frequencies and statistical parameters using dedicated survey procedures. Differences in the body height and BMI class distribution of ABCSG patients and normal Austrian adult females were analyzed using the Rao-Scott χ2 test. In a first step, no adjustment for age was made. In addition, body height was categorized into 2 height classes: above and below the median height of 165 cm. To test for differences between the 2 populations in terms of continuous body height, BMI values, and age (based on mid-values of class intervals), analysis of variance (ANOVA) was used. Since body height data differed slightly but significantly from the normal distribution, results were confirmed by using a nonparametric Kruskal-Wallis test. Because there was no change with respect to inference, only results from ANOVA are given. For BMI data, reciprocal transformation has been used to approximate a normal distribution. An analysis of covariance (ANCOVA) with midpoints of the age classes as covariable was calculated to adjust for the effect of age on the body height and BMI–population relationship.
The risk of recurrence in terms of DFS and OS in the 2 height classes with the tallest patients was compared to the 2 height classes with the shortest patients using the log-rank test stratified by type of therapy (tamoxifen, anastrozole, CMF). Hazard ratios with confidence intervals were computed using univariate, stratified Cox proportional hazards models. These analyses were restricted to the adjuvant trials (5, 6, and 12). Patients from the ABCSG 6 trial were censored at 60 months because they were allowed to switch to another trial. Multivariate Cox proportional hazards regression model stratified by the type of therapy was applied to adjust for tumor stage, nodal stage, tumor grading, hormone receptor status, and BMI. No significant deviation from the proportionality assumption was observed for the univariate and the multivariate models. Analyses were performed using statistical software SAS® v9.3 and v9.4 (SAS Institute, Cary, NC, USA).
Results
In our analysis, 4,935 breast cancer patients from 5 different ABCSG trials were included. Patient characteristics are provided in Table 1 and Table 2. The control group consisted of a data set of 8,469 women registered in the Austrian health survey from 2006/2007 (conducted by Statistik Austria) and was chosen as representative for the Austrian female population (Table 1). The projected population size based on the weights of the survey was 3,624,274 females.
Table 1.
Mean and median age, height, weight, and BMI of patients included in the 5 evaluable ABCSG trials and of women included in the Austrian health survey
| Characteristics | ABCSG trials (N = 4,935) | Austria (N = 3,624,274) | |
|---|---|---|---|
| Age, yearsa | Median (range) | 49 (23, 80) | 43 (17, 87)a |
| Mean (95% CI) | 51 (50, 51) | 47 (47,48)a | |
|
| |||
| Height, cm | Median (range) | 164 (130, 188) | 164.4 (136.0, 190.0) |
| Mean (95% CI) | 164.3 (164.1, 164.4) | 164.8 (164.6, 165.0) | |
|
| |||
| Weight, kgb | Median (range) | 67.0 (35.0, 140.0) | 65.6 (38.0, 164.0) |
| Mean (95% CI) | 68.4 (68.1, 68.8) | 67.1 (66.7, 67.4) | |
|
| |||
| BMIb | Median (range) | 24.5 (14.6, 53.3) | 24.0 (14.4, 67.0) |
| Mean (95% CI)c | 24.6 (24.5, 24.8) | 24.0 (23.9, 24.1) | |
CI, confidence interval; BMI, body mass index.
Data from the Austrian health survey did not include the exact age, but age classes of 5-year intervals. Therefore, the mid-values of the class intervals were used to calculate the median and the mean.
Weight and BMI were available for 4,928 patients in the ABCSG population.
Mean calculated after reciprocal transformation of the BMI values.
Table 2.
Characteristics of the patients included in the 5 evaluable ABCSG trials
| Characteristics | ABCSG trials (N = 4,935) |
||||
|---|---|---|---|---|---|
| n | % | ||||
| Menopausal status | Pre- or perimenopausal | 3,053 | 61.9 | ||
| Postmenopausal T1 | 1,882 | 38.1 | |||
|
| |||||
| Tumor stage | 2,862 | 57.0 | |||
| T2 | 1,834 | 37.2 | |||
| T3 | 206 | 4.2 | |||
| T4 | 31 | 0.6 | |||
| Unknown | 2 | <0.1 | |||
|
| |||||
| Nodes | Negative | 2,986 | 60.5 | ||
| Positive | 1,949 | 39.5 | |||
|
| |||||
| Tumor grading | G1 | 611 | 12.4 | ||
| G2 | 2,879 | 58.3 | |||
| G3 | 1,267 | 25.7 | |||
| Unknown | 178 | 3.6 | |||
|
| |||||
| ER status | Negative | 257 | 5.2 | ||
| Positive | 3,761 | 76.2 | |||
| Unknown | 917 | 18.6 | |||
|
| |||||
| PgR status | Negative | 609 | 12.3 | ||
| Positive | 3,404 | 68.0 | |||
| Unknown | 922 | 18.7 | |||
|
| |||||
| HR status | Negative (ER– and PgR–) | 270 | 5.5 | ||
| Positive | 4,263 | 86.4 | |||
| Unknown | 402 | 8.1 | |||
|
| |||||
| HER2a | Negative | 388 | 7.8 | ||
| Positive | 181 | 3.7 | |||
| Unknown | 4,366 | 88.5 | |||
|
| |||||
| Histology | Ductal invasive | 3,012 | 61.0 | ||
| Lobular invasive | 553 | 11.2 | |||
| Other subtypes | 561 | 11.4 | |||
| Unknown | 809 | 16.4 | |||
|
| |||||
| Type of surgery | Breast conserving | 3,327 | 67.4 | ||
| Mastectomy | 1,572 | 31.9 | |||
| Other | 18 | 0.4 | |||
| Unknown | 18 | 0.4 | |||
|
| |||||
| (Neo)adjuvant | Yes | 897 | 18.2 | ||
| chemotherapy | No | 1,514 | 30.7 | ||
| Not applicable | 1,530 | 31.0 | |||
| Unkown | 994 | 20.1 | |||
|
| |||||
| (Neo)adjuvant | Yes | 45 | 24.9 | ||
| trastuzumab if | No | 136 | 75.1 | ||
| HER2-positiveb | |||||
|
| |||||
| Adjuvant endocrine | Yes | 3,333 | 78.2 | ||
| therapy if HR-positivec | No | 930 | 21.8 | ||
|
| |||||
| pCRd in case of neoadjuvant therapye | Yes | 138 | 17.2 | ||
| No | 646 | 80.6 | |||
| Unknown | 18 | 2.2 | |||
ER, estrogen receptor; PgR, progesterone receptor; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; pCR, pathologic complete response.
HER2 was documented in ABCSG 14 and ABCSG 24 only.
Percentages per 181 HER2-positive patients.
Percentages per 4,263 HR-positive patients.
Defined as no invasive tumor in breast irrespective of lymph node involvement (pT0/is).
Percentages per 802 patients who received neoadjuvant therapy.
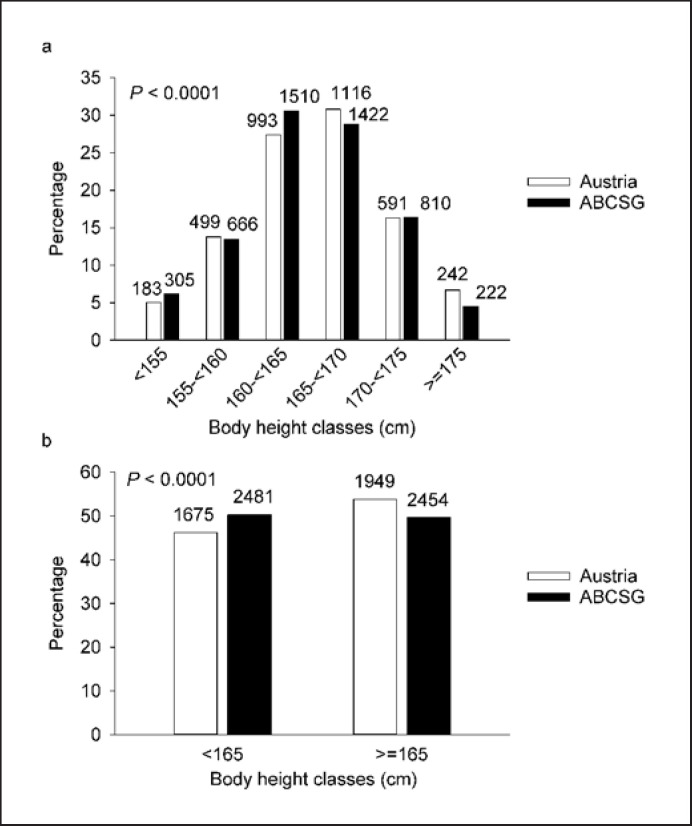
We observed a significant association between body height categories and population; however, the direction of this correlation was opposite to our hypothesis: ABCSG breast cancer patients had a slightly, but statistically significantly, lower mean body height compared to the Austrian adult female population (164.3 vs. 164.8 cm, p < 0.0001; Fig. 1a). When categorized into 2 height classes, according to the median height of the whole population (165 cm), significantly more ABCSG patients were seen in the lower body height class and significantly fewer ABCSG patients in the higher body height class than expected by chance (p < 0.0001; Fig. 1b).
Fig. 1.
Differences in body height distribution between the ABCSG cohort and the Austrian female population in 6 (a) and 2 (b) height classes. The numbers of females on top of the bars of the Austrian population have to be mutiplied by 1,000.
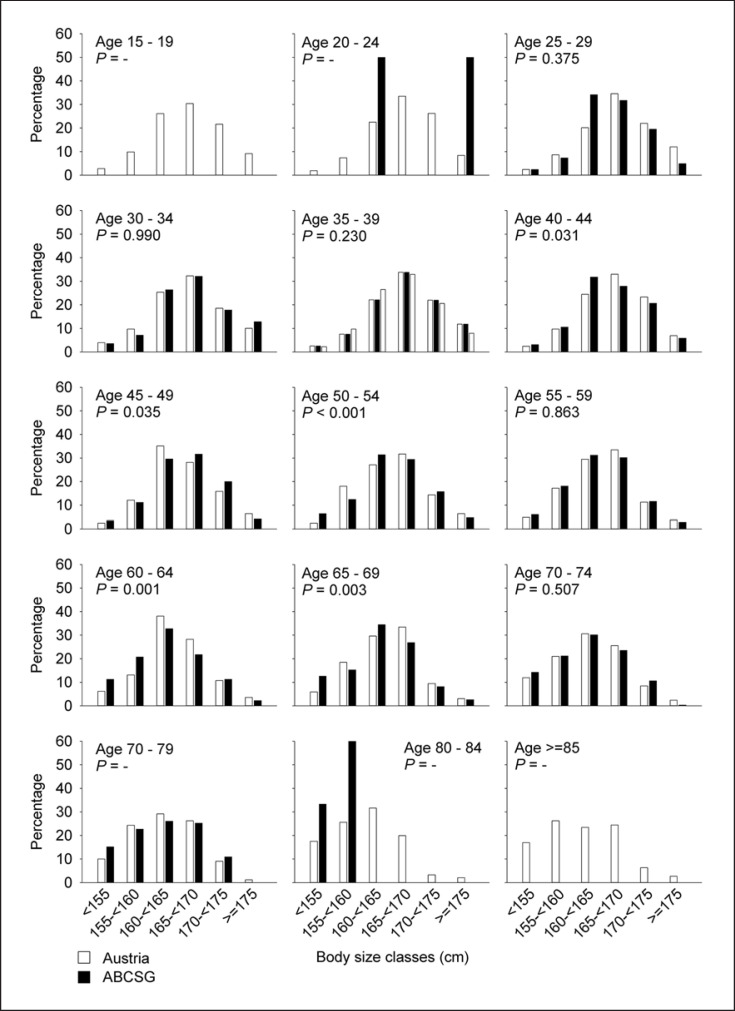
Age had a weak but significant effect (R2 = 0.086; p < 0.0001) on body height. After adjusting for age, the difference between Austrian adult females and ABCSG patients with respect to body height was not significant anymore (ANCOVA: F = 2.90, p = 0.089). Significant differences in terms of frequency distributions across height classes occurred in the age classes 40–44, 45–49, 50–54, 60–64, and 65–69 years (Fig. 2). Significant differences in terms of height comparisons were found in the age classes 35–39, 40–44, 60–64, and 65–69 years. In these height classes, body height was lower for ABCSG patients compared to Austrian adult females. The mean difference was 0.53 cm.
Fig. 2.
Differences in body height distribution between the ABCSG cohort and the Austrian female population per age classes.
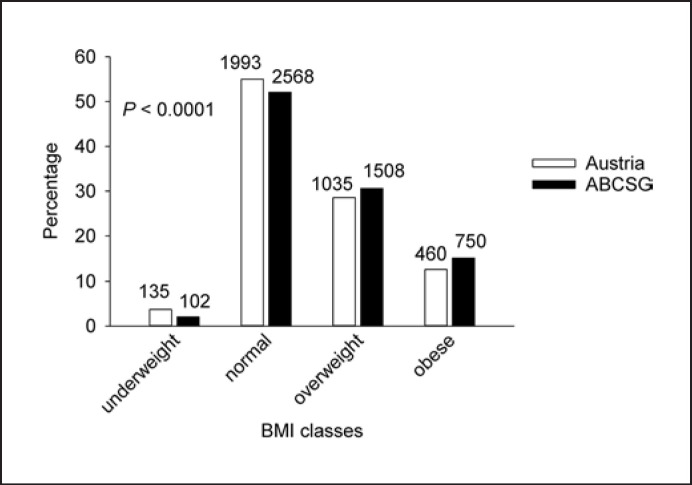
We observed a significant difference between the breast cancer population and the control group with respect to BMI as well. Compared to Austrian adult females, the mean BMI of ABCSG patients was significantly higher (24.64 vs. 23.96, p < 0.0001). These results were independent of the effect of age (Fig. 3).
Fig. 3.
Differences in BMI distribution between the ABCSG cohort and the Austrian female population in 4 BMI classes. The numbers of females on top of the bars of the Austrian population have to be mutiplied by 1,000.
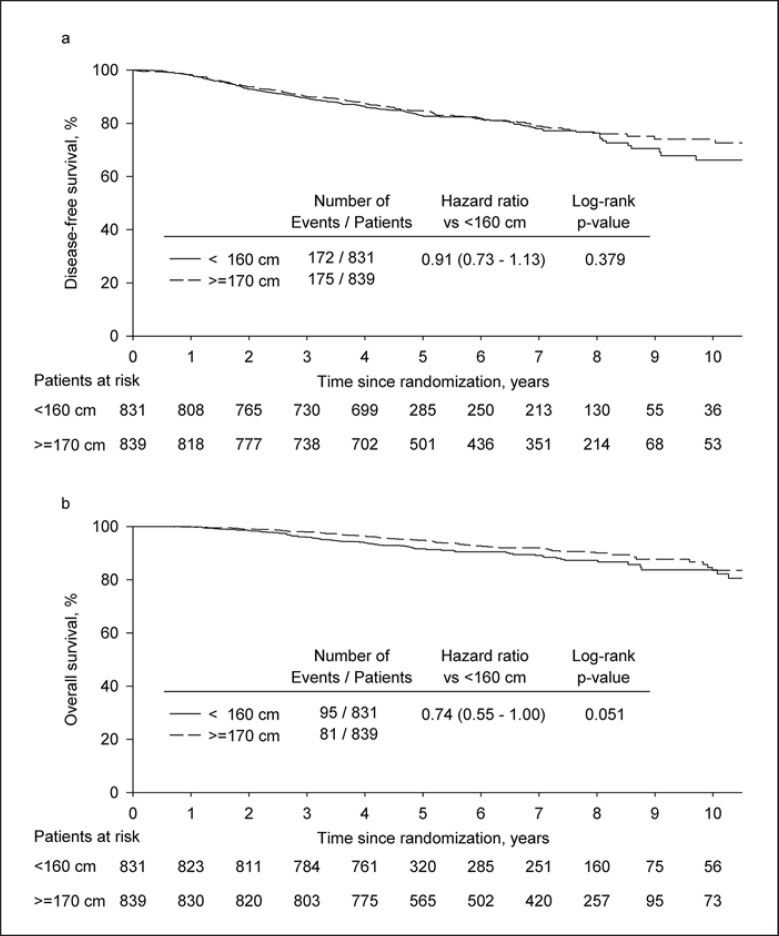
The median follow-up in the combined analysis of the trials ABCSG 5, 6, and 12 was 73.1 months (95% CI 71.7–82.3). The risk of an event in the 2 height classes with the tallest patients (≥170 cm) compared to the 2 classes with the shortest patients (<160 cm) was not significantly different for DFS (log-rank, 5-year DFS: 84.7 vs. 83.0%; HR 0.91, 95% CI 0.73–1.13, p = 0.379; multivariate Cox model: HR 0.88, 95% CI 0.69–1.12, p = 0.281). For OS, a borderline significance was reached with an absolute difference in 5-year OS of 3.1% (log-rank, 5-year OS: 94.8 vs. 91.7%; HR 0.74, 95% CI 0.55–1.00, p = 0.051; multivariate Cox model: HR 0.73, 95% CI 0.51–1.04, p = 0.080; Fig. 4).
Fig. 4.
Kaplan-Meier plots for disease-free survival (a) and overall survival (b) of patients ≥170 cm (N = 839) versus patients <160 cm (N = 831) from the trials ABCSG 5, 6, and 12.
Discusion
In this retrospective data analysis of 4,935 breast cancer patients included in clinical trials of the ABCSG, we found a significant difference in height distribution between the ABCSG population and the Austrian adult female population. In these studies, premenopausal as well as postmenopausal women were included and treated in neoadjuvant or adjuvant intention with endocrine therapy and/or chemotherapy according to their risk profile. Since up to 33% of all Austrian breast cancer patients are included in ABCSG trials, the ABCSG trial collective is a representative population sample enabling the planned comparison.
In contrast to our hypothesis, the ABCSG population was significantly shorter than the normal Austrian adult female population (p < 0.0001). According to the literature, taller women should be overrepresented in cancer cohorts because of the widely described positive correlation between cancer risk and height. This association brought up in large epidemiologic comparisons was not confirmed by our data. After adjusting for age, however, the difference in height between the ABCSG population and the general population was not statistically significant anymore (p = 0.089). Age is one potential confounding factor as it may be related to both height (the general adult population gets taller over the years in many countries [26]) and incidence of cancer (higher age is associated with higher incidence of cancer [27]). Interestingly, the Million Women Study stratified all analyses by age and adjusted − among others − for socioeconomic status and BMI. Nevertheless, a significant association between height and cancer risk was found [8].
It should be considered that our patient population was exclusively built up by patients enrolled into clinical trials and presents, therefore, a very homogenous group with regard to the inclusion criteria and the risk factor distribution. Probably, much stronger risk factors for breast cancer carcinogenesis, such as the hormonal environment, mask the influence of increased cell turnover mediated by growth factors or the larger cell amount in taller individuals. In the EPIC cohort, the highest risk for ER/progesterone receptor (PR)+ breast cancer was found in tall women who had an early menarche (≤13 years). Their risk was almost 2-fold increased in comparison to the group with a late menarche and short stature. For ER–/PR– breast cancer, this association appeared to be less strong. The menopause, in turn, seems to influence the “height effect” on the risk for ER+/PR+ breast cancer as well, since a stronger correlation in postmenopausal women was observed in the EPIC cohort [19]. Unfortunately, other known risk factors for breast cancer development, such as socioeconomic status, region of residence, age at menarche, parity, age at first delivery, use of hormone replacement therapy, physical activity, smoking status, and alcohol consumption, were not recorded in the included trials. This is certainly, besides the retrospective design, one of the major limitations of this analysis.
Our second hypothesis was that taller patients with early breast cancer should have a shorter DFS and OS compared to shorter patients. Besides evaluating the effect of body height alone, our results were adjusted for age, tumor stage, nodal stage, tumor grading, hormone receptor status of estrogen and progesterone, and BMI. Again, our hypothesis was not confirmed. There was no statistical difference in univariate and multivariate analysis for DFS and OS, respectively. The univariate log-rank test for OS showed a borderline significance (HR 0.74, 95% CI 0.55–1.00, p = 0.051), however, with a trend toward better prognosis for taller breast cancer patients, opposite to our initial hypothesis.
High BMI is an established and widely described risk factor for the development of many different cancer sites [2]. This applies in particular to breast cancer. In accordance with these findings, we could detect an overrepresentation of women with higher BMI in the ABCSG cohort compared to the Austrian female population. This risk factor is of special interest since overweight or obesity at the time of diagnosis seems to be a poor prognostic factor associated with nodal involvement as well as increased disease-specific and overall mortality [28, 29, 30]. Furthermore, a reduced efficacy of adjuvant aromatase inhibitor therapy was reported for obese postmenopausal women, suggesting a certain under-dosage in these women [31, 32]. Unfortunately, adjustment for socioeconomic status, which is strongly related to BMI [33], was not possible in our analysis.
Conclusions
Our results did not confirm previous findings that greater body height significantly increases breast cancer risk or deteriorates prognosis of this disease. Further studies are warranted to confirm the results of this retrospective data analysis. The hypothesis of a predominance of women with higher BMI in the early breast cancer cohort compared to the general female population was clearly supported in our study cohort.
Statement of Ethics
All procedures performed in the 5 included studies were in accordance with the ethical standards of the national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All 5 studies were approved by ethics committees and institutional review boards in all participating institutions. Written informed consent was obtained from all study participants. For this retrospective analysis, no additional informed consent was obtained.
Conflict of Interest Statement
Verena Sagaster declares the following relations: Employment: none; Leadership: none; Stock or other Ownership: none; Honoraria: none; Consulting or Advisory Role: Bayer, Novartis, Eli Lilly; Speakers Bureau: none; Research Funding: none; Patents, Royalties, Other Intellectual Property: none; Expert Testimony: none; Travel, Accommodation, Expenses: none; Other Relationships: none.
Florian Fitzal declares the following relations: Employment: none; Leadership: none; Stock or other Ownership: none; Honoraria: none; Consulting or Advisory Role: Pfizer, Roche; Speakers Bureau: none; Research Funding: Novartis, Pfizer, Roche, AstraZeneca, Comesa, Bondimed, Nanostring; Patents, Royalties, Other Intellectual Property: none; Expert Testimony: none; Travel, Accommodation, Expenses: Novartis, Pfizer, Roche, AstraZeneca, Comesa, Bondimed, Nanostring; Other Relationships: none.
Yelena Devyatko declares the following relations: Employment: none; Leadership: none; Stock or other Ownership: none; Honoraria: none; Consulting or Advisory Role: none; Speakers Bureau: none; Research Funding: none; Patents, Royalties, Other Intellectual Property: none; Expert Testimony: none; Travel, Accommodation, Expenses: Roche; Other Relationships: none.
Georg Pfeiler declares the following relations: Employment: none; Leadership: none; Stock or other Ownership: none; Honoraria: Amgen, Lilly, Roche, AstraZeneca, Novartis, Accord, Pfizer; Consulting or Advisory Role: Amgen, Lilly, Roche, AstraZeneca, Novartis, Accord, Pfizer; Speakers Bureau: none; Research Funding: none; Patents, Royalties, Other Intellectual Property: none; Expert Testimony: none; Travel, Accommodation, Expenses: none; Other Relationships: none.
Michael Gnant declares the following relations: Employment: Snadoz; Leadership: none; Stock or other Ownership: none; Honoraria: Amgen, AstraZeneca, Celgene, Eli Lilly, Invectys, Pfizer, Nansotring, Novartis, Roche, Medison; Consulting or Advisory Role: AstraZeneca, Eli Lilly; Speakers Bureau: none; Research Funding: none; Patents, Royalties, Other Intellectual Property: none; Expert Testimony: none; Travel, Accommodation, Expenses: Amgen, AstraZeneca, Eli Lilly, Ipsen, Pfizer, Roche, Medison; Other Relationships: none.
All other authors declare that they do not have any conflicts of interest in direct or indirect relationship with this research.
Funding Sources
This retrospective analysis was funded by the ABCSG. The design of the study, the collection, interpretation, and analysis of the data, the preparation of this report, or the decision to publish were not influenced by the funding source of the 5 clinical trials.
The corresponding author had full access to all data. C.F. had access to the raw data of all studies. The corresponding author assumes the final responsibility for the decision to submit for publication.
Author Contributions
Conceptualization: R.G.: ideas; formulation or evolution of overarching research goals and aims. Data curation: C.F.: management activities to annotate (produce dataset), data preparation, and maintain research data (including software code, where it is necessary to interpret the data itself) for initial use and later reuse. Formal analysis: C.F.: application of statistical, mathematical, computational, or other formal techniques to analyze or synthesize study data. Funding acquisition: M.G., G.S., R.G.: acquisition of the financial support for the project leading to this publication. Investigation: S.P.G., M.P.: conducting a research and investigation process, specifically performing the experiments, or data/evidence collection. Methodology: S.P.G., M.P., G.R., R.G., C.F.: development or design of methodology; creation of models. Project administration: S.P.G., M.P., G.R., M.G., R.G.: management and coordination responsibility for the research activity planning and execution. Resources: S.P.G., M.P., G.R., B.M., S.G.-R., G.S., V.S., F.F., R.E., Y.D., M.B., H.S., C.S., T.B., C.S., G.P., M.S., R.H., D.H., H.R., W.K., U.W., M.G.: provision of study materials, reagents, materials, patients, laboratory samples, animals, instrumentation, computing resources, or other analysis tools. Supervision: R.G., S.P.G., M.P.: oversight and leadership responsibility for the research activity planning and execution, including mentorship external to the core team. Validation: S.P.G., M.P., C.F., G.R., B.M., S.G.-R., G.S., V.S., F.F., R.E., Y.D., M.B., H.S., C.S., T.B., C.S., G.P., M.S., R.H., D.H., H.R., W.K., U.W., M.G.: verification, whether as a part of the activity or separate, of the overall replication/reproducibility of results/experiments and other research outputs. Visualization: C.F.: preparation, creation, and/or presentation of the published work, specifically visualization/data presentation. Writing − original draft preparation: S.P.G., M.P., G.R.: creation and/or presentation of the published work, specifically writing the initial draft (including substantial translation). Writing − review and editing: C.F., B.M., S.G.-R., G.S., V.S., F.F., R.E., Y.D., M.B., H.S., C.S., T.B., C.S., G.P., M.S., R.H., D.H., H.R., W.K., U.W., M.G.: preparation, creation, and/or presentation of the published work by those from the original research group, specifically critical review, commentary or revision − including pre- or post-publication stages.
Availability of Data and Material
The datasets analyzed during the current study are not publicly available due to confidentiality obligation but are available from the corresponding author on reasonable request.
References
- 1.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010 Oct;123((3)):627–35. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 2.Vucenik I, Stains JP. Obesity and cancer risk: evidence, mechanisms, and recommendations. Ann N Y Acad Sci. 2012 Oct;1271:37–43. doi: 10.1111/j.1749-6632.2012.06750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davey Smith G, Hart C, Upton M, Hole D, Gillis C, Watt G, et al. Height and risk of death among men and women: aetiological implications of associations with cardiorespiratory disease and cancer mortality. J Epidemiol Community Health. 2000 Feb;54((2)):97–103. doi: 10.1136/jech.54.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiren S, Häggström C, Ulmer H, Manjer J, Bjorge T, Nagel G, et al. Pooled cohort study on height and risk of cancer and cancer death. Cancer Causes Control. 2014 Feb;25((2)):151–9. doi: 10.1007/s10552-013-0317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang S, Lv G, Chen W, Jiang J, Wang J. Height and kidney cancer risk: a meta-analysis of prospective studies. J Cancer Res Clin Oncol. 2015 Oct;141((10)):1799–807. doi: 10.1007/s00432-014-1870-5. [DOI] [PubMed] [Google Scholar]
- 6.Markozannes G, Tzoulaki I, Karli D, Evangelou E, Ntzani E, Gunter MJ, et al. Diet, body size, physical activity and risk of prostate cancer: An umbrella review of the evidence. Eur J Cancer. 2016 Dec;69:61–9. doi: 10.1016/j.ejca.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 7.Campbell PT, Newton CC, Kitahara CM, Patel AV, Hartge P, Koshiol J, et al. Body Size Indicators and Risk of Gallbladder Cancer: Pooled Analysis of Individual-Level Data from 19 Prospective Cohort Studies. Cancer Epidemiol Biomarkers Prev. 2017 Apr;26((4)):597–606. doi: 10.1158/1055-9965.EPI-16-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green J, Cairns BJ, Casabonne D, Wright FL, Reeves G, Beral V. Height and cancer incidence in the Million Women Study: prospective cohort, and meta-analysis of prospective studies of height and total cancer risk. Lancet Oncol. 2011 Aug;12((8)):785–94. doi: 10.1016/S1470-2045(11)70154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunnell D, Okasha M, Smith GD, Oliver SE, Sandhu J, Holly JM. Height, leg length, and cancer risk: a systematic review. Epidemiol Rev. 2001;23((2)):313–42. doi: 10.1093/oxfordjournals.epirev.a000809. [DOI] [PubMed] [Google Scholar]
- 10.Albanes D, Taylor PR. International differences in body height and weight and their relationship to cancer incidence. Nutr Cancer. 1990;14((1)):69–77. doi: 10.1080/01635589009514078. [DOI] [PubMed] [Google Scholar]
- 11.Silva Idos S, De Stavola B, McCormack V. Birth size and breast cancer risk: re-analysis of individual participant data from 32 studies. PLoS Med. 2008 Sep 30;5((9)):e193. doi: 10.1371/journal.pmed.0050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang TO, Reeves GK, Green J, Beral V, Cairns BJ. Birth weight and adult cancer incidence: large prospective study and meta-analysis. Ann Oncol. 2014 Sep;25((9)):1836–43. doi: 10.1093/annonc/mdu214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albanes D, Winick M. Are cell number and cell proliferation risk factors for cancer? J Natl Cancer Inst. 1988 Jul 20;80((10)):772–4. doi: 10.1093/jnci/80.10.772. [DOI] [PubMed] [Google Scholar]
- 14.Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998 May 9;351((9113)):1393–6. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 15.Canzian F, McKay JD, Cleveland RJ, Dossus L, Biessy C, Boillot C, et al. Genetic variation in the growth hormone synthesis pathway in relation to circulating insulin-like growth factor-I, insulin-like growth factor binding protein-3, and breast cancer risk: results from the European prospective investigation into cancer and nutrition study. Cancer Epidemiol Biomarkers Prev. 2005 Oct;14((10)):2316–25. doi: 10.1158/1055-9965.EPI-04-0874. [DOI] [PubMed] [Google Scholar]
- 16.Lagiou P, Hsieh CC, Lipworth L, Samoli E, Okulicz W, Troisi R, et al. Insulin-like growth factor levels in cord blood, birth weight and breast cancer risk. Br J Cancer. 2009 Jun 2;100((11)):1794–8. doi: 10.1038/sj.bjc.6605074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zatko T, Matejovicova B, Boledovicova M, Vondrakova M, Bezakova A, Sirotkin AV. Growth and obesity and its association with plasma level of steroid hormones and insulin-like growth factor-I (IGF-I) in Slovak female students. Bratisl Lek Listy. 2013;114((10)):573–80. doi: 10.4149/bll_2013_123. [DOI] [PubMed] [Google Scholar]
- 18.Maehle BO, Vatten LJ, Tretli S. Birth length and weight as predictors of breast cancer prognosis. BMC Cancer. 2010;10:115. doi: 10.1186/1471-2407-10-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritte R, Lukanova A, Tjonneland A, Olsen A, Overvad K, Mesrine S, et al. Height, age at menarche and risk of hormone receptor-positive and -negative breast cancer: a cohort study. Int J Cancer. 2013 Jun 1;132((11)):2619–29. doi: 10.1002/ijc.27913. [DOI] [PubMed] [Google Scholar]
- 20.Parr CL, Batty GD, Lam TH, Barzi F, Fang X, Ho SC, et al. Body-mass index and cancer mortality in the Asia-Pacific Cohort Studies Collaboration: pooled analyses of 424,519 participants. Lancet Oncol. 2010 Aug;11((8)):741–52. doi: 10.1016/S1470-2045(10)70141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jakesz R, Hausmaninger H, Kubista E, Gnant M, Menzel C, Bauernhofer T, et al. Randomized adjuvant trial of tamoxifen and goserelin versus cyclophosphamide, methotrexate, and fluorouracil: evidence for the superiority of treatment with endocrine blockade in premenopausal patients with hormone-responsive breast cancer--Austrian Breast and Colorectal Cancer Study Group Trial 5. J Clin Oncol. 2002 Dec 15;20((24)):4621–7. doi: 10.1200/JCO.2002.09.112. [DOI] [PubMed] [Google Scholar]
- 22.Schmid M, Jakesz R, Samonigg H, Kubista E, Gnant M, Menzel C, et al. Randomized trial of tamoxifen versus tamoxifen plus aminoglutethimide as adjuvant treatment in postmenopausal breast cancer patients with hormone receptor-positive disease: Austrian breast and colorectal cancer study group trial 6. J Clin Oncol. 2003 Mar 15;21((6)):984–90. doi: 10.1200/JCO.2003.01.138. [DOI] [PubMed] [Google Scholar]
- 23.Gnant M, Mlineritsch B, Stoeger H, Luschin-Ebengreuth G, Knauer M, Moik M, et al. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann Oncol. 2015 Feb;26((2)):313–20. doi: 10.1093/annonc/mdu544. [DOI] [PubMed] [Google Scholar]
- 24.Steger GG, Galid A, Gnant M, Mlineritsch B, Lang A, Tausch C, et al. Pathologic complete response with six compared with three cycles of neoadjuvant epirubicin plus docetaxel and granulocyte colony-stimulating factor in operable breast cancer: results of ABCSG-14. J Clin Oncol. 2007 May 20;25((15)):2012–8. doi: 10.1200/JCO.2006.09.1777. [DOI] [PubMed] [Google Scholar]
- 25.Steger G, Greil R, Jakesz R, Lang A, Mlineritsch B, Melbinger-Zeinitzer E, et al. Final Results of ABCSG-24, a Randomized Phase III Study Comparing Epirubicin, Docetaxel, and Capecitabine (EDC) to Epirubicin and Docetaxel (ED) as Neoadjuvant Treatment for Early Breast Cancer and Comparing ED/EDC + Trastuzumab (T) to ED/EDC as Neoadjuvant Treatment for Early HER-2 Positive Breast Cancer. Cancer Res. 2010 Feb;69((24 suppl)):1081–1081. [Google Scholar]
- 26.NCD Risk Factor Collaboration (NCD-RisC) A century of trends in adult human height. eLife. 2016 Jul;5:e13410. doi: 10.7554/eLife.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White MC, Holman DM, Boehm JE, Peipins LA, Grossman M, Henley SJ. Age and cancer risk: a potentially modifiable relationship. Am J Prev Med. 2014 Mar;46((3 Suppl 1)):S7–15. doi: 10.1016/j.amepre.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carmichael AR, Bates T. Obesity and breast cancer: a review of the literature. Breast. 2004 Apr;13((2)):85–92. doi: 10.1016/j.breast.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Doyle C, Kushi LH, Byers T, Courneya KS, Demark-Wahnefried W, Grant B, et al. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J Clin. 2006 Nov-Dec;56((6)):323–53. doi: 10.3322/canjclin.56.6.323. [DOI] [PubMed] [Google Scholar]
- 30.Niraula S, Ocana A, Ennis M, Goodwin PJ. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: a meta-analysis. Breast Cancer Res Treat. 2012 Jul;134((2)):769–81. doi: 10.1007/s10549-012-2073-x. [DOI] [PubMed] [Google Scholar]
- 31.de Azambuja E, McCaskill-Stevens W, Francis P, Quinaux E, Crown JP, Vicente M, et al. The effect of body mass index on overall and disease-free survival in node-positive breast cancer patients treated with docetaxel and doxorubicin-containing adjuvant chemotherapy: the experience of the BIG 02-98 trial. Breast Cancer Res Treat. 2010 Jan;119((1)):145–53. doi: 10.1007/s10549-009-0512-0. [DOI] [PubMed] [Google Scholar]
- 32.Pfeiler G, Konigsberg R, Fesl C, Mlineritsch B, Stoeger H, Singer CF, et al. Impact of body mass index on the efficacy of endocrine therapy in premenopausal patients with breast cancer: an analysis of the prospective ABCSG-12 trial. J Clin Oncol. 2011 Jul 1;29((19)):2653–9. doi: 10.1200/JCO.2010.33.2585. [DOI] [PubMed] [Google Scholar]
- 33.Vieira LS, Bierhals IO, Vaz JDS, Meller FO, Wehrmeister FC, Assuncao MCF. Socioeconomic status throughout life and body mass index: a systematic review and meta-analysis. Cad Saude Publica. 2019;35((10)):e00125518. doi: 10.1590/0102-311X00125518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are not publicly available due to confidentiality obligation but are available from the corresponding author on reasonable request.