Abstract
Vitamin D (VD) is a major regulator of calcium metabolism in many living organisms. In addition, VD plays a key role in regulating innate and adaptive immunity in vertebrates. Neutrophils constitute an important part of the first line of defense against invading microbes; however, the potential effect of VD on neutrophils remains elusive. Thus, in this study zebrafish in different developmental stages were utilized to identify the potential role of VD in the basal homeostasis and functions of neutrophils. Our results showed that addition of exogenous VD<sub>3</sub> promoted granulopoiesis in zebrafish larvae. Reciprocally, neutrophil abundance in the intestine of adult zebrafish with a cyp2r1 mutant, lacking the capacity to 25-hydroxylate VD, was reduced. Moreover, VD-mediated granulopoiesis was still observed in gnotobiotic zebrafish larvae, indicating that VD regulates neutrophil generation independent of the microbiota during early development. In contrast, VD was incapable to influence granulopoiesis in adult zebrafish when the commensal bacteria were depleted by antibiotic treatment, suggesting that VD might modulate neutrophil activity via different mechanisms depending on the developmental stage. In addition, we found that VD<sub>3</sub> augmented the expression of il-8 and neutrophil recruitment to the site of caudal fin amputation. Finally, VD<sub>3</sub> treatment significantly decreased bacterial counts and mortality in zebrafish infected with Edwardsiella tarda (E. tarda) in a neutrophil-dependent manner. Combined, these findings demonstrate that VD regulates granulopoiesis and neutrophil function in zebrafish immunity.
Keywords: Vitamin D3, Granulopoiesis, Microbiota, Neutrophil recruitment, Infection
Introduction
The role of Vitamin D (VD) in the regulation of calcium-phosphate homeostasis and in the control of bone turnover is well known [1]. Vitamin D3 (VD3) is the main form of VD in animals, and it is converted to the hormonal form 1α,25(OH)2D3 by 2 cytochrome P450 (CYP) enzymes, 25-hydroxylase, and 1 alpha-hydroxylase, which are encoded by the cyp2r1 and cyp27b1genes, respectively [2].
Over the past 20 years, numerous studies have shown that VD possesses immunomodulatory functions and plays a critical role in modulating innate and adaptive immunity in mammals [1, 3], and in fish [4]. As one of the first defenders of the innate immunity, neutrophils are rapidly activated upon infection and play an essential role in bacterial clearance [5]. In addition, neutrophils promptly accumulate in large numbers at sites of tissue injury, which limit bacterial translocation, stimulate angiogenesis, and promote tissue restoration [6].
Neutrophil homeostasis is maintained through a careful balance of granulopoiesis, bone marrow (or kidney marrow in fish) storage and release, and migration into vascular compartments and peripheral tissues [7]. Despite extensive evolutionary divergence between teleost fish and mammals, the molecular pathways governing hematopoiesis have been conserved. The process of pluripotent hematopoietic stem cells to myeloid precursors to mature neutrophils is controlled by both extracellular and intracellular factors [8], among which granulocyte colony-stimulating factor (G-CSF), also known as colony-stimulating factor 3 (CSF3), plays a critical role in directing granulopoiesis and maintaining normal neutrophil numbers [5, 9]. Interestingly, it has also been reported that the microbiota was involved in maintaining steady-state granulopoiesis [10].
In addition, myeloperoxidase (mpx) and matrix metalloproteinase9 (mmp9) are stored in neutrophil cytoplasmic granules, and they play an important role in killing pathogens [5]. Mpx is also a specific marker for neutrophils, which can partly reflect the abundance of neutrophils in homeostasis [11]. In addition, lysozyme C (lysc) can also be used as a granulocyte marker [12].
In this study, we used zebrafish as a model and evaluated the effects of VD on neutrophil generation and confirmed if VD could influence granulopoiesis via gut microbiota. We also analyzed the contribution of VD to neutrophil recruitment to the site of tissue injury and for host resistance to bacterial infection.
Materials and Methods
Zebrafish Maintenance
Zebrafish were maintained at 28.5°C in a freshwater circulation system with a light: dark cycle of 14 h:10 h. The fish were fed twice daily with newly hatched brine shrimps (Artemia franciscana). Husbandry and handling of the fish in the present study were approved by the Experimental Animal Ethics Committee of Ocean University of China.
The transgenic Tg (mpx:eGFP) zebrafish used in this study was donated by Dr. Eduardo Villablanca, Karolinska Institutet, Sweden. The generation of cyp2r1−/− zebrafish has been described in a previous report [13].
Feeding Trial
Two experimental diets with 0 or 800 IU VD3/kg diet were designed and formulated in our laboratory. The composition of these experimental diets is shown in Table 1. Wild-type zebrafish at 35 days post fertilization (dpf) around 20–25 mg were randomly assigned into 6 tanks (10 L) with 50 fish/tank, and all fish were fed with 0 IU VD3/kg diet for 1 week. Afterward, each diet was randomly assigned to triplicate tanks, and the fish were fed twice daily.
Table 1.
Dietary formulation of experimental diet (g/kg)
| Ingredients | 0 IU-diet | 800 IU-diet |
|---|---|---|
| Casein (vitamin free) | 388 | 388 |
| Gelatin | 97 | 97 |
| Fish oil | 115 | 115 |
| Starch | 280 | 280 |
| Cellulose | 55 | 55 |
| Monocalcium phosphate | 10 | 10 |
| Choline chloride | 5 | 5 |
| Mineral premix1 | 40 | 40 |
| Vitamin premix (VD3 free)2 | 10 | 10 |
| VD3, IU/kg | 0 | 800 |
VD3, vitamin D3. 1Mineral premix (mg/g diet): calcium lactate, 327; FeSO4, 3.125; MgSO4, 137; NaH2PO4, 87.2; NaCl, 43.5; AlCl3, 0.15; KIO3, 0.125; KCl, 75; CuCl2, 0.1; MnSO4, 0.8; CoCl2, 1; ZnSO4, 3; microcrystalline cellulose, 187.2. 2Vitamin premix (mg/g diet): thiamine HCl, 5; riboflavin, 10; calcium pantothenate, 10; D-biotin, 0.6; pyridoxine HCl, 4; folic acid, 1.5; inositol, 200; L-vitamin C-2-magnesium phosphate, 60; niacin, 6.05; α-vitamin E acetate, 50; vitamin K, 4; retinol acetate, 0.11; microcrystalline cellulose, 648.74.
For antibiotic treatment, zebrafish (2 months, 70–90 mg) were randomly assigned into 4 tanks (10 L) with 50 fish/tank. Half of them were maintained in an aquaculture system with antibiotics (100 μg/mL ampicillin, 10 μg/mL kanamycin, 0.5 μg/mL amphotericin B, and 50 μg/mL gentamycin) for 1 month. The fish were fed twice daily. At the end of the feeding trial, all fish were euthanized in 0.1% tricaine (MS-222), the intestine of each fish was collected, and saved at −80°C for further analysis.
Gene Expression Analysis
Total RNA was extracted from the whole zebrafish larvae treated with control buffer or VD3 by using the RNAeasyTM Animal RNA isolation kit (Beyotime, Shanghai, China) according to the manufacturers' instructions. The quantity of total RNA samples was assessed using NanoDrop® One spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The quality of the extracted RNA was determined by agarose gel electrophoresis. RNA (1 μg) was reversely transcribed to cDNA using the HiScript III RT SuperMix for qPCR with gDNA wiper (Vazyme, Nanjing, China). The actin2 gene was chosen as the reference gene for sample normalization, and all primer sequences of target genes are listed in Table 2. The qRT-PCR reactions were carried out in a quantitative thermal cycler CFX96TM Real Time System (Bio-Rad, Hercules, CA, USA).
Table 2.
The sequences of th primers (5′–3′) used in this study
| Test | Symbol | Forward | Reverse |
|---|---|---|---|
| Genotyping | cyp2r1 | CCCCAAGTTTGCATCTAAGA | GATGCATTACACTGCTATGC [13] |
| csf3r | CTTGTGTGTGTTCAGGAGGA | GGGTGAGTTTCAGAGGATCAG [16] | |
|
| |||
| qRT-PCR | Mpx | TCCAAAGCTATGTGGGATGTGA | GTCGTCCGGCAAAACTGAA [38] |
| lysc | TCGTGTGAAAGCAAGACACTGGGA | ACTCGGTGGGTCTTAAACCTGCTT [12] | |
| csf3a | AACTACATCTGAACCTCCTG | GACTGCTCTTCTGATGTCTG [30] | |
| csf3b | AGAGAACCTACTGAACGACCT | CTTGAACTGGCTGAGTGGAG [16] | |
| il-8 | GTCGCTGCATTGAAACAGAA | GTAACCCATGGAGCAGAGG | |
| mmp9 | CATCCGCAACTACAAGACATTC | GGTCCAGTATTCATCGTCATCA [16] | |
| actin2 | GATGATGAAATTGCCGCACTG | ACCAACCATGACACCCTGATGT [13] | |
csf3b, colony-stimulating factor 3b; mmp9, matrix metalloproteinase 9; mpx, myeloperoxidase; lysc, lysozyme C.
In vivo Imaging
Zebrafish were anesthetized in 0.016% Tricaine (MS-222; Sigma-Aldrich, St. Louis, MO, USA), and then mounted in 2% agarose containing 0.016% Tricaine. Imaging was performed with SMZ25 stereo fluorescence microscope (Nikon, Tokyo, Japan).
Neutrophil Enumeration
Neutrophil numbers were determined by 2 manners. For direct enumeration, manual counting of neutrophil numbers was assisted by the multipoint function in Image J, which records click to avoid duplicate counting. Alternatively, neutrophil units were computed as previously described and validated [14]. Briefly, total fluorescent area of 1 zebrafish larva was measured by using Image J. In order to acquire the average size of neutrophils in this larva, at least 5 neutrophils located in the tail tip were measured to ensure that the neutrophils do not overlap. Finally, neutrophil units were calculated by dividing the “total fluorescent area” value of each embryo by its average value of “neutrophil size.”
Gnotobiotic Zebrafish Husbandry
The generation of gnotobiotic zebrafish in this study followed the established protocols [15]. Briefly, zebrafish embryos were obtained by natural spawning and kept in gnotobiotic zebrafish medium (GZM) at 28.5°C. Next, embryos were incubated in GZM with antibiotics (100 μg/mL ampicillin, 10 μg/mL kanamycin, 0.5 μg/mL amphotericin B, 50 μg/mL gentamycin) for 6–8 h. Under sterile conditions, the embryos were washed with 0.1% PVP-I in GZM for 90 s and rinsed twice with GZM. Thereafter, embryos were washed using 0.003% bleach solution in GZM for 20 min. Tryptic soy agar plate, nutrient broth, brain-heart Infusion broth, and Sab-Dex broth were used for checking sterility.
Caudal Fin Amputation Assay
Larval zebrafish (5 dpf) was anaesthetized with 0.016% Tricaine (MS-222) and mounted on glass slide. Fish were then observed under a stereomicroscope. Tail amputations were performed posterior to the end of the notochord using a surgical scalpel (Surgical Specialties Sharpoint, 72–2,201), and fish were revived into 12-well plates containing GZM. At 0, 4, and 6 h post caudal fin amputation, the fish were euthanized and observed. GFP+ cells at the tail wound margin were enumerated and imaged by using Nikon SMZ25 stereo fluorescence microscope.
Bacterial Challenge
Edwardsiella tarda (E. tarda) was isolated from diseased turbots (Scophthalmus maximus L.), and the identity was confirmed by 16S rRNA gene sequencing. Overnight bacterial cultures were washed with GZM and adjusted to 108 CFU/mL. Zebrafish larva was infected with E. tarda by static immersion at 3 dpf or micro-injections at 5 dpf.
Bacterial Enumeration
Zebrafish larvae in each group were anesthetized at different time points (24, 48, and 72 h post infection [hpi]) after E. tarda immersion. Five larvae were pooled and homogenized in 0.2 mL GZM. Serial dilutions were performed using PBS (10 mM, pH 7.4) of the homogenates. Ten microliters of serial dilutions were plated on deoxycholate hydrogen sulfide lactose agar plates, and black colonies were enumerated after incubation at 28°C for 24 h.
Generation of Neutrophil-Knockdown Zebrafish Larvae
Neutrophil-knockdown zebrafish larvae were generated based on a previous method [16]. Briefly, 2 single-guide (sg) RNAs which specifically targeted the zebrafish csf3r gene were generated (listed in Table 3). The mixture of 2 sgRNAs was microinjected into one-cell stage embryos together with Cas9 protein (M0646T; NEB, San Diego, CA, USA) at a final concentration of 1 μg/μL. Total RNA was extracted at 6 dpf from the whole zebrafish larvae microinjected with control buffer or sgRNAs, and then reversely transcribed to cDNA by using the HiScript III RT SuperMix. Genotyping primers were designed (listed in Table 2), and qRT-PCR verified the validity of the gene editing.
Table 3.
csf3r sgRNA targets used in this study
sg, single-guide.
Calculations and Statistical Methods
All data are expressed as mean ± SEM. The software GraphPad Prism 8.0 was used for all statistical evaluations. Differences between the means were evaluated using t test or 2-way ANOVA with Sidak test, and p value <0.05 was regarded as statistical significance.
Results
Vitamin D Increases the Number of Neutrophils in Zebrafish Larvae
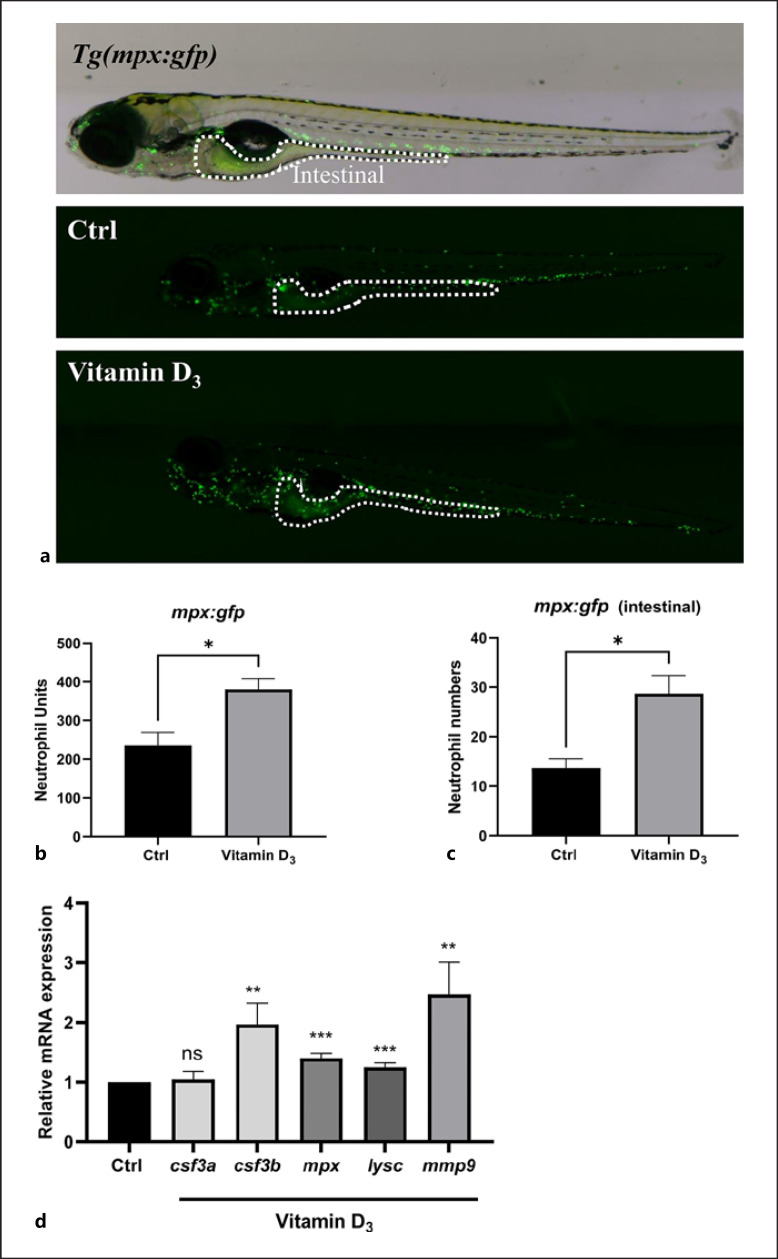
To investigate the effects of VD on neutrophil generation in vivo, Tg(mpx:gfp) zebrafish with GFP-labeled neutrophil-specific mpx were utilized. Tg(mpx:gfp) zebrafish larvae at 2 dpf were exposed to 100 nM VD3 for 4 days, and stereomicroscopic evaluation of GFP+ neutrophils in whole 6 dpf zebrafish showed an increase in neutrophil counts in the VD3-treated group compared with the control group (Fig. 1a, b). Notably, the number of neutrophils located in the zebrafish digestive tracts was significantly increased in the VD3-treated group (Fig. 1c). Gene expression analysis with qRT-PCR revealed that VD3 treatment elevated the expression of the granulopoietic cytokine genes colony-stimulating factor 3b (csf3b), whereas no effect on the production of colony-stimulating factor 3a (csf3a) was detected (Fig. 1d). Likewise, the total neutrophil units in the VD3-treated group was also increased (Fig. 1b). In addition, mpx, the granulocyte marker gene (lys), and the neutrophil cytoplasmic granules gene matrix metalloproteinase9 (mmp9) were also increased in the VD3-treated group compared to the control group (Fig. 1d). These data demonstrated that exogenous addition of VD3 upregulated the number of neutrophils in zebrafish larvae.
Fig. 1.
VD3 promotes the granulopoiesis in zebrafish larvae. Zebrafish larvae at 2 dpf were exposed to 100 nM VD3 for 2 days, GZM was replaced at 4 dpf, and 100 nM VD3 were added simultaneous. a The merged images of Tg(mpx:gfp) zebrafish (6 dpf) showed the abundance and localization of GFP+ neutrophils. White dashed line indicates the intestine. b The size of neutrophil population was represented by neutrophil unit as described in Materials and Methods. Graph shows quantification of neutrophil units in whole zebrafish larva at 6 dpf. c Enumeration of intestinal-associated GFP+ cells in 6 dpf larvae. d The gene expression by qRT-PCR of csf3a, csf3b, mpx, lysc, and mmp9 from 6 dpf zebrafish larvae treated with 0 or 100 nM VD3 for 4 days (results are combined from 2 independent experiments, n = 4 replicates/group/experiment, 10–20 larvae/replicate). Data are represented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. VD3, vitamin D3; dpf, days post fertilization; mpx, myeloperoxidase; csf3a, colony-stimulating factor 3a; csf3b, colony-stimulating factor 3b; lysc, lysozyme C; mmp9, matrix metalloproteinase9; GZM, gnotobiotic zebrafish medium.
Vitamin D Regulates Granulopoiesis in Zebrafish Intestines
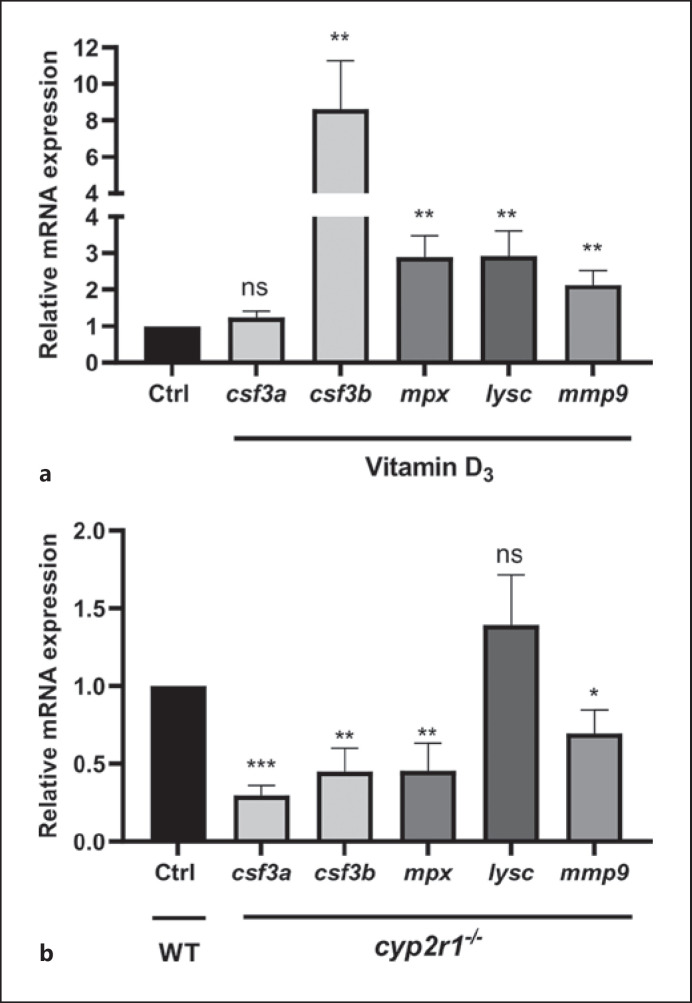
Next, the effects of VD3 on the neutrophil population in zebrafish intestines at different development stages were examined. Consistent with the results in zebrafish larvae, the genes of csf3b, mpx, lysc, and mmp9, were expressed at higher levels in juvenile zebrafish (35 dpf) fed for 2 weeks with a diet containing 800 IU VD3 compared to the 0 IU group (Fig. 2a). Moreover, the gene expression of csf3a, csf3b, mpx, mmp9 in cyp2r1−/− zebrafish was significantly decreased compared to wild-type (WT) control zebrafish at adult stages (Fig. 2b). These transcriptional differences suggested that VD3 contributed to the number and distribution of the neutrophil population in zebrafish intestines.
Fig. 2.
VD3 contributes to the granulopoiesis in zebrafish intestine. a The gene expression of csf3a, csf3b, mpx, lysc, and mmp9 in the gut of zebrafish (35 dpf) fed 0 or 800 IU VD3/kg diets for 2 weeks (n = 12 replicates/group, 2–3 zebrafish/replicate). b Transcript levels of csf3a, csf3b, mpx, lysc, and mmp9 in the gut of WT and cyp2r1 mutant zebrafish in 3 months (n = 8 replicates/genotype). Data are represented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. csf3a, colony-stimulating factor 3a; csf3b, colony-stimulating factor 3b; lysc, lysozyme C; mmp9, matrix metalloproteinase9; dpf, days post fertilization; VD3, vitamin D3; WT, wild-type; mpx, myeloperoxidase.
Microbiota Is Involved in VD-Mediated Regulation of Granulopoiesis
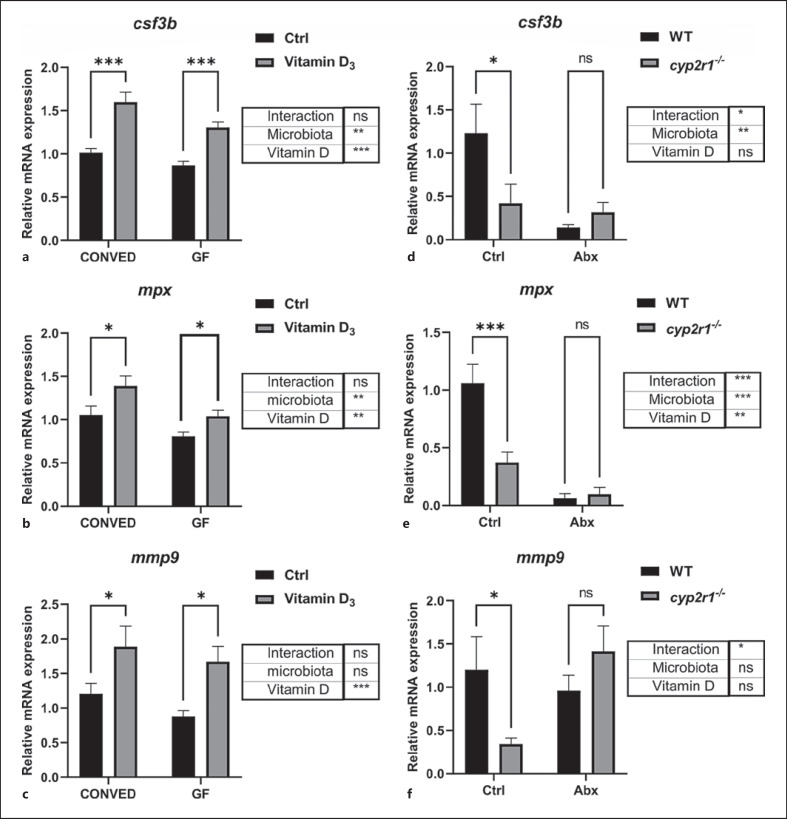
To test whether the microbiota had any role in VD-mediated regulation of granulopoesis, the expression of csf3b, mpx, and mmp9 was assessed in both conventionalized (CONVED) and germ-free GF zebrafish. Interestingly, VD3 treatment resulted in upregulation of the 3 genes in both CONVED and GF zebrafish (Fig. 3a-c). These findings indicated that VD3-enhanced neutrophil activity was independent of the microbiota in the larvae stage.
Fig. 3.
Microbiota impact on granulopoiesis in zebrafish. a–c The gene expression of csf3b (a), mpx (b), and mmp9 (c) in CONVED or GF zebrafish larvae (6 dpf) treated 0 or 100 nM VD3 for 4 days (results are combined from 2 independent experiments, n = 12 replicates/group, 10–20 larvae/replicate). d–f WT and cyp2r1 mutant zebrafish in 3 months were treated with antibiotics for 1 week, and the gene expression of csf3b (d) mpx (e), and mmp9 (f) transcripts in the gut was analyzed (n = 8 replicates/genotype). Two-way ANOVA with Sidak test was used to test significance. Data are represented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. csf3b, colony-stimulating factor 3; mmp9, matrix metalloproteinase9; dpf, days post fertilization; VD3, vitamin D3; WT, wild-type; CONVED, conventionalized; GF, germ-free; mpx, myeloperoxidase.
Next, the intestines in adult cyp2r1−/− and WT zebrafish were dissected. Consistent with the results of Figure 2b, cyp2r1−/− zebrafish had reduced gene expression of csf3b, mpx, mmp9 compared to WT controls under normal conditions. Notably, when the microbiota was depleted with antibiotics, no significant reduction of gene expression was detected in cyp2r1−/− zebrafish compared to WT zebrafish. Thus, there is a significant interaction between VD-mediated effects on intestinal gene expression and the microbiota in adult zebrafish (Fig. 3d-f).
Vitamin D Promotes Neutrophil Recruitment to the Wound Margin
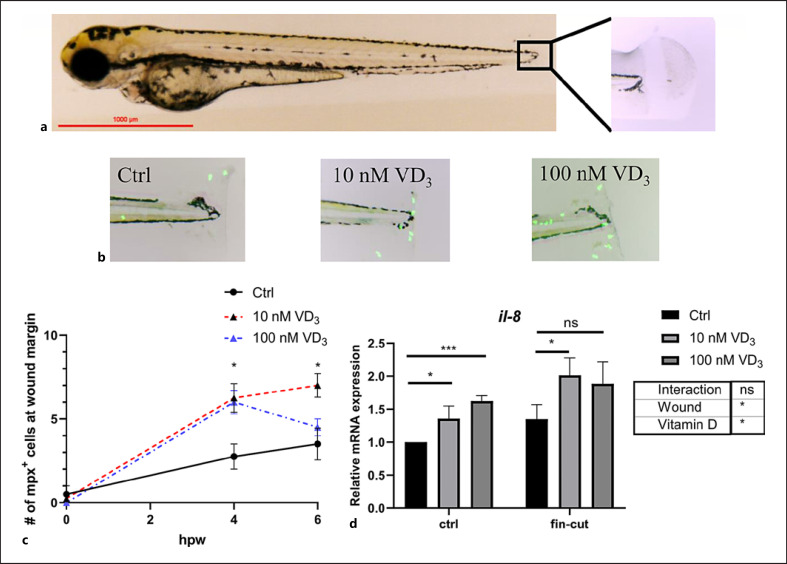
To assess the effects of VD on neutrophil migration, we performed caudal fin amputations on Tg(mpx:gfp) zebrafish larvae and quantified the recruitment of neutrophils to the wound margin. We observed that the count of GFP+ neutrophils in the wound margin was higher in the VD3-treated group than the control group at 4 h post wounding (hpw). At 6 hpw, only the group treated with 10 nM VD3 showed increased neutrophil recruitment, while the recruitment of neutrophils to the wound margin in the group treated with a higher VD3 dose (100 nM) was similar to controls (Fig. 4c). The gene expression of neutrophil chemoattractant factor il-8 in whole zebrafish larvae was quantified in homeostasis and after caudal fin amputation. A 2-way ANOVA analysis revealed that both VD3 incubation and caudal fin amputation treatment increased the expression of il-8, while there were no significant interactions between VD3 treatment and caudal fin amputation. In addition, the group treated with 100 nM VD3 exhibited a moderate increase compared with the group treated with 10 nM, at 6 hpw (Fig. 4d). These results indicate that VD3 enhanced the recruitment of neutrophils to the wound, and it corresponded with increased expression of the il-8 gene.
Fig. 4.
VD3 enhances neutrophil recruitment to the wound site of amputated fish. a–c The caudal fin of 6 dpf Tg(mpx:gfp) zebrafish. Imaging and quantification of GFP+ neutrophils recruited to tail wound at 4 hpw (n = 4/group) (b). d qRT-PCR analysis of IL-8 transcripts in 6 dpf zebrafish larvae treated 0 nM, 10 nM, or 100 nM VD3 for 4 days, with and without caudal fin amputation (n = 6 replicates/group, 6–15 larvae/replicate). Two-way ANOVA with Sidak test was used to test significance. For c, statistical comparisons were performed within each time point. Data are represented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. dpf, days post fertilization; VD3, vitamin D3; WT, wild-type; hpw, hours post wounding; mpx, myeloperoxidase.
Vitamin D Restrains Infection with a Gram-Negative Bacterium in the Zebrafish
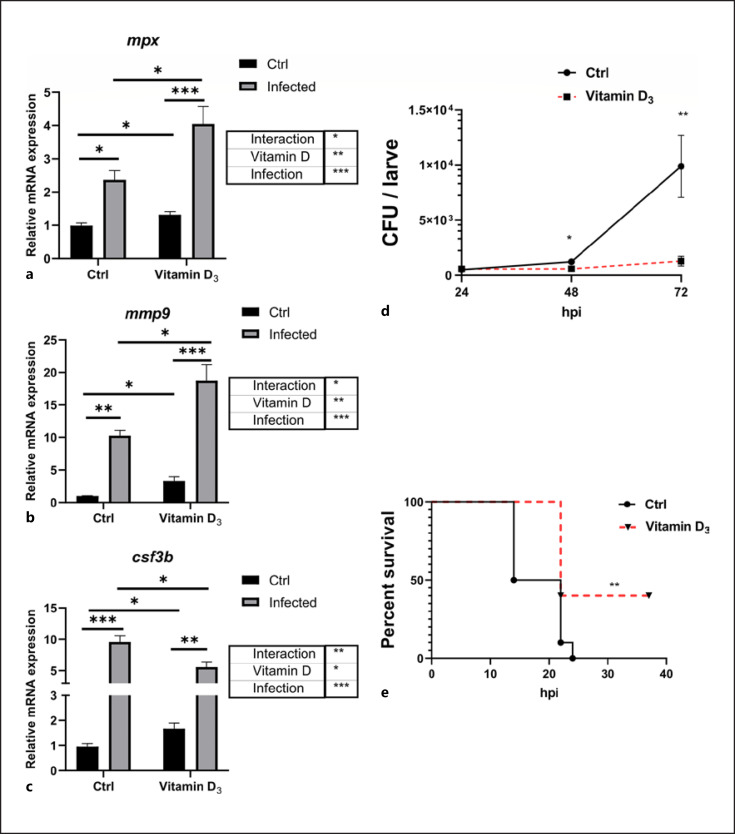
Neutrophils are conditioned by host factors and also respond to microbially derived signals, such as LPS and other pathogen-associated molecular patterns, allowing for proper responses to inflammatory stimuli [17]. To define the effects of VD3 in the process against infection, E. tarda, a Gram-negative bacterium known to cause major damage in aquaculture, was used to challenge 6 dpf zebrafish larvae. The mRNA expression of mpx and mmp9 was increased significantly at the transcriptional level after E. tarda infection (immersion model), and the VD3-treated group had higher mpx and mmp9 expression than the control group. There was a significant interaction between VD3 and E. tarda challenge on mpx and mmp9 expressions (Fig. 5a, b). Additionally, during E. tarda infection, bacterial counts showed a profound increase after 72 h post infection (hpi) in the control group, while the growth of E. tarda was inhibited in the group supplemented with VD3 (Fig. 5d). We also challenged zebrafish larvae by microinjection of E. tarda. Compared with the control group, the VD3 group exhibited significantly greater survival after 36 hpi (Fig. 5e). Collectively, these data demonstrated that supplementation with VD3 enhanced the activity of neutrophils and improved the ability of zebrafish to cope with infection caused by E. tarda in both mmersion and injection models.
Fig. 5.
VD3 restrains pathogen infection in zebrafish. a–c Transcript levels of mpx (a), mmp9 (b), and csf3b (c) in unstimulated and E. tarda-exposed zebrafish larvae, pretreated with 0 or 100 nM VD3 (n = 4 replicates/group, 10–20 larvae/replicate). Two-way ANOVA with Sidak test was used to test significance. d Zebrafish larvae at 3 dpf were immersed with 1 × 108 CFU/mL E. tarda. After 24, 48, and 72 h, the bacteria burden in the larvae was counted (n ≥ 24 larvae/group/experiment, 3 independent experiments). e Zebrafish larvae at 3 dpf were pretreated with control buffer or VD3 (50 nM) for 48 h. Afterward, the larvae were microinjected with E. tarda (approximately 200 bacteria/larva). The survival of the larvae in each group was recorded up to 36 hpi (n = 10 larvae/group/experiment, 3 independent experiments). Statistical analysis was conducted by Log-rank test. Data are represented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. csf3b, colony-stimulating factor 3b; mmp9, matrix metalloproteinase9; dpf, days post fertilization; VD3, vitamin D3; E. tarda, Edwardsiella tarda; hpi, hours post infection; mpx, myeloperoxidase.
Neutrophils Are Required for Vitamin D3-Mediated Control of E. tarda Infection
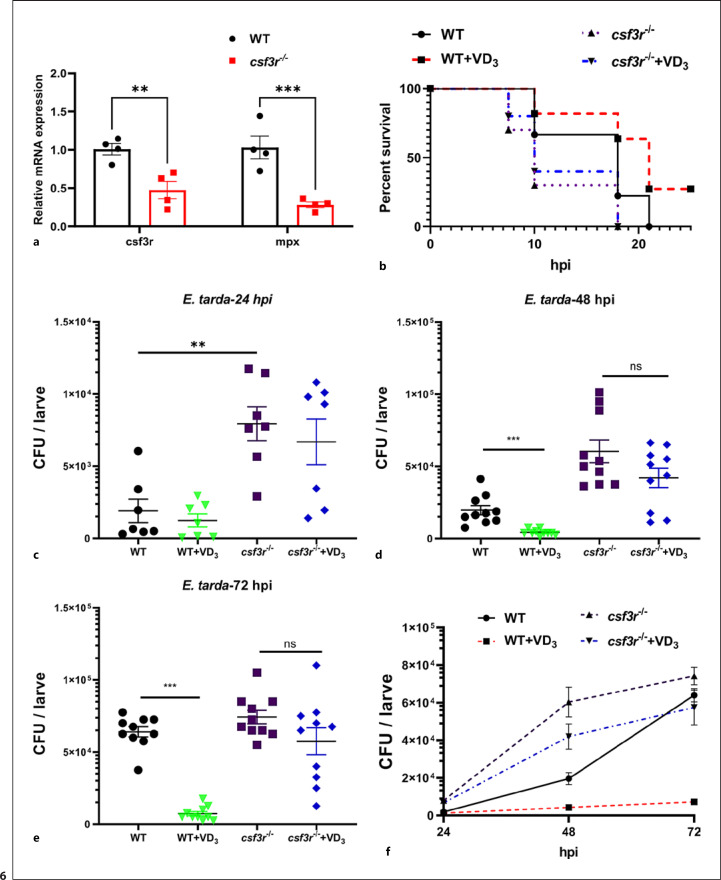
Finally, the role of neutrophils in VD3-mediated control of E. tarda infection in zebrafish larvae was investigated. Neutrophil-knockdown zebrafish larvae were generated through the CRISPR/Cas9 system, and F0 mosaic mutant larvae (crispants) were acquired. The efficiency of this gene editing was confirmed by qRT-PCR analysis for csf3r transcription expression (Fig. 6a). When zebrafish larvae were challenged by the microinjection of E. tarda at 5 dpf, csf3r−/− crispants showed lower survival rate than the WT counterparts (Fig. 6b). Notably, all csf3r−/− crispants died at 18 hpi, which was independent of pretreatment with VD3 (Fig. 6b). Consistently, VD3 pretreatment significantly suppressed the bacterial load in WT zebrafish larvae at 48 and 72 hpi (Fig. 6c-e), whereas VD3 did not exhibit any significant effects the bacterial load in csf3r−/− crispants (Fig. 6c-f). Thus, neutrophils are essential for VD3 to control bacterial growth in zebrafish larvae.
Fig. 6.
Neutrophils are required for VD3-mediated control of bacterial growth in zebrafish. a Embryos microinjected with control buffer or sgRNAs targeting csf3r were collected at 6 dpf. Genotyping of csf3r amplicons was analyzed by qRT-PCR, and transcript levels of mpx were also tested. b Zebrafish larvae at 5 dpf were microinjected with E. tarda (approximately 200 bacteria/larva). The survival of the larvae in each group was recorded up to 25 hpi (n = 10 larvae/group/experiment, 2 independent experiments). Statistical analysis was conducted by Log-rank test. c–f Zebrafish larvae were immersed with 1 × 108 CFU/mL E. tarda at 3 dpf. After 24, 48, and 72 h, the bacteria burden in the larvae was counted (n ≥ 14 larvae/group/experiment, 2 independent experiments). Data are represented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. dpf, days post fertilization; VD3, vitamin D3; E. tarda, Edward-siella tarda; hpi, hours post infection; mpx, myeloperoxidase; WT, wild-type.
Discussion
In this study, we have shown that VD is an important regulator of granulopoiesis and neutrophil activities in zebrafish in vivo. Moreover, we provided evidence that the microbiota is involved in VD-regulated neutrophil function depending on the developmental stage of the fish. Finally, VD3 supplementation increased neutrophil recruitment to the wound site in zebrafish and protected zebrafish against bacterial infection.
In recent years, there has been a dramatic resurgence in research interest in VD due to its various biological functions, including the immunomodulation of innate immune cells and inflammatory responses [3, 18]. For example, it was reported that VD can regulate macrophage differentiation [19], the activation of T lymphocytes [20], and B-cell function [21]. Moreover, it has been demonstrated that VD signaling plays an important role in the expansion of hematopoietic stem cells and progenitors [22, 23]. However, little is known about the effects of VD on neutrophil development or functions. As one of the main myeloid cell types, neutrophil development is highly conserved in vertebrates [24], and a high percentage of hematopoiesis is committed to the production of neutrophils [25]. During vertebrate development, successive waves of primitive and definitive hematopoiesis can be found in distinct anatomical sites, and they contribute to both embryonic and adult hematopoiesis [26]. The generation of neutrophils occurs in zebrafish from 2 dpf [27, 28], thus we used this time point and treated zebrafish larvae with exogenous VD3 for 4 days and enumerated neutrophil numbers. Interestingly, our results showed that VD3 promoted the abundance of neutrophils in the early development of zebrafish larvae (Fig. 1a, b). The relative gene expression of the neutrophilic genes mpx, lysc, and mmp9 was increased (Fig. 1d).
It has previously been shown that granulopoiesis is regulated by transcription factors and cytokine signals [29]. In particular, G-CSF/CSF3 is an important cytokine involved in granulopoiesis, and it stimulates the survival, proliferation, differentiation, and function of neutrophil precursors and mature neutrophils [9]. In fact, there are 2 csf3 subtypes encoded by the genes csf3a and csf3b in zebrafish [30], and our results showed that only csf3b, but not csf3a, was upregulated in zebrafish larvae treated with VD3. On account of the different temporal and spatial expressions of csf3a and csf3b during embryogenesis in zebrafish [30], these 2 proteins may have different functions in development. To further confirm the effects of VD on neutrophils, RNA was extracted from the gut of cyp2r1+/+ and cyp2r1−/− zebrafish at the age of 3 mpf. In accordance with the results from zebrafish fed without dietary VD3, the gene expression of csf3b and mpx was decreased in cyp2r1−/− zebrafish (Fig. 2b). Therefore, we believe that VD influences neutrophil granulopoiesis and abundance in the intestine via the regulation of G-CSF/CSF3.
It is noteworthy that zebrafish larvae were treated with VD3 instead of the active metabolite 1α,25(OH)2D3 in our study. So far, there is no evidence to prove that VD3 itself directly stimulates the host immune system. A previous study has demonstrated that the metabolite 25(OH)D3 induces the antimicrobial peptide expression in macrophages when TLRs in macrophages are activated [31]. Further results have shown that TLRs activation upregulates the expression of cyp27b1 in macrophages to metabolize 25(OH)D3 into the active 1α,25(OH)2D3 [31], which is highlighting the importance of the active metabolite 1α,25(OH)2D3 in host immunity. As we analyzed in this study, the gene expression of cyp2r1 and cyp27b1, which encode 2 critical enzymes converting VD3 into the active 1α,25(OH)2D3, was enhanced in exogenous VD3-treated fish larvae (data not shown). Interestingly, it has also been reported that the exogenous estrogen can be absorbed by zebrafish larvae (0–5 dpf) [32]. Accordingly, it is speculated that zebrafish larvae uptake exogenous VD3, which is further metabolized into the active 1α,25(OH)2D3 exerting the immune functions.
The gut can be considered as the largest compartment of the immune system, and it is constantly exposed to multiple foreign antigens [33, 34]. The microbiota plays a fundamental role in the induction, education, and function of the immune system [35]. It has been reported that commensal microbiota may contribute to IL-17 and G-CSF production, thus regulating neutrophil homeostasis [36]. In addition, circulating and bone marrow-derived neutrophils are reduced in antibiotic-exposed neonatal mice [37], and germ-free zebrafish exhibit a severe neutropenia [38]. Thus, there seems to be a close link between microbiota and neutrophil physiology. Interestingly, it has been shown that VD/VDR signaling has a significant impact on the intestinal microbiota [39, 40], and it has been suggested that the effect of VD on health is partially mediated through the microbiome [41]. Therefore, we hypothesized that the communication between VD and microbiota in zebrafish might influence neutrophil behavior. To test our hypothesis, the microbiota in the intestine of juvenile zebrafish was depleted by antibiotic treatment. Notably, we found that the decrease in the gene expression of mpx, csf3b, and mmp9 diminished in the intestine of cyp2r1−/− zebrafish compared to WT fish when the microbiota was depleted (Fig. 3d-f), which confirmed the contribution of microbiota to VD-mediated neutrophil functions. However, the supplementation with VD3 still upregulated the gene expression of csf3b, mpx, and mmp9 in gnotobiotic zebrafish at 6 dpf (Fig. 3a-c), which suggested that VD3-induced granulopoiesis was independent on the microbiota in the early developmental stage of zebrafish. Interestingly, a recent report demonstrated that VD promoted G-CSF production in placental explants [42] Considering the fact that the whole gastrointestinal tube opens in the zebrafish at 6 dpf, and commensal bacteria colonize the intestine of zebrafish over the course of several weeks [43, 44], we suggest that VD/VDR signaling might directly regulate neutrophil granulopoiesis and the gene expression of csf3 in the early development as a transcription factor.
Neutrophils promptly accumulate in large numbers at sites of tissue injury or infection against the potential microbial invasion. Caudal fin amputation of larval zebrafish is a valuable in vivo model of inflammation and wound repair [45]. Interestingly, the previous studies articulated that neutrophils were the primary cells scavenging apoptotic bodies and small cell debris within 6 h post amputation [46], and IL-8 (also known as CXCL8) is known to be one of the most potent chemoattractant molecules for guiding neutrophils to reach the injured sites [47]. A previous study revealed that 1α,25(OH)2D3, the active form of VD3, enhanced IL-8 production by neutrophils in vitro [48]. Interestingly, our results confirmed that 1α,25(OH)2D3 augmented gene expression of IL-8 in zebrafish larva, and further improved neutrophil recruitment to the wound site (Fig. 4). These results demonstrated that VD could regulate neutrophil behaviors and promote neutrophil recruitment to the injury sites in an IL-8 dependent manner, which potentially contributes to injury protection and subsequent wound healing.
VD deficiency has been associated with a range of bacterial infections [49], and our previous study has identified the importance of VD for pneumococcal killing by human neutrophils [50]. It is well established that neutrophils play a critical role during bacterial infection in zebrafish larvae [16, 51]. In addition, csf3r knockdown zebrafish, in which neutrophil counts are substantially reduced, exhibit a higher mortality and bacterial colonization than WT controls [16]. Interestingly, our results confirmed that VD3 treatment reduced the mortality and bacterial colonization in E. tarda-infected zebrafish larvae (Fig. 5). Consistent with previous reports [16, 51], E. tarda infection resulted in significant upregulation of the neutrophil-specific genes mpx, csf3b, and mmp9. Our results showed that VD3 pretreatment further augmented the expression of mpx and mmp9 in zebrafish larvae following E. tarda infection. In fact, mpx is one of the key components in primary granules of neutrophils, and it is an important part of the innate immune system for host defense against invading microorganisms and neutrophil functions [52]. Besides, mmp9 is also present in the tertiary granules of neutrophils and implicated in neutrophil extravasation, the degradation of extracellular matrix, and activation of IL-1β [5, 53]. Furthermore, we confirmed that neutrophils are essential for VD3-mediated effects on E. tarda bacterial growth in zebrafish, since VD3 was inactive in csf3r−/− crispants (Fig. 6). Combined, our results showed that VD enhanced neutrophil functions during bacterial infection, which is beneficial for bacterial clearance in the host.
Conclusion
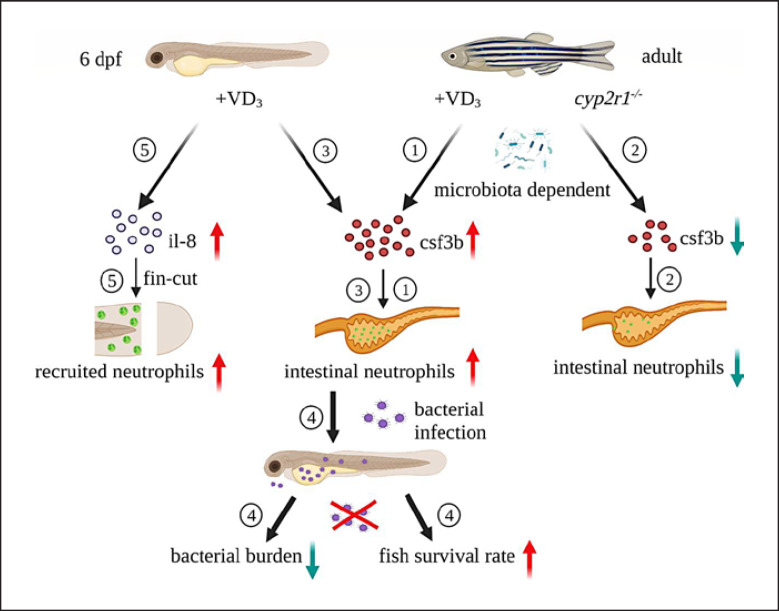
Our findings have outlined a role for VD3 as an important regulator of neutrophil generation and function. Moreover, VD3 enhances neutrophil recruitment to the wound site and restrains the proliferation of pathogenic bacteria in infected zebrafish. Importantly, neutrophils are required for VD3-mediated control of bacterial infection (Fig. 7).
Fig. 7.
Schematic model of the regulation of granulopoiesis and neutrophil functions by VD3 in zebrafish. ① In adult zebrafish, the gene expression of csf3b and the neutrophil markers were enhanced in the intestine of the fish fed with VD3-containing diet, compared to those fed with non-VD3 diet. ② The gene expression of csf3b and neutrophil abundance in the intestine of adult zebrafish with a cyp2r1 mutant were reduced. Interestingly, microbiota is involved in VD-mediated granulopoiesis in adult zebrafish. ③ Addition of exogenous VD3 promoted granulopoiesis in zebrafish larvae. However, VD-regulated neutrophil generation independent of the microbiota during early development. ④ VD3 treatment significantly decreased bacterial counts and mortality in zebrafish larvae infected with E. tarda in a neutrophil-dependent manner. ⑤ VD3 augmented the gene expression of IL-8 and neutrophil recruitment to the site of caudal fin amputation. csf3b, colony-stimulating factor 3b; VD3, vitamin D3; E. tarda, Edwardsiella tarda.
Statement of Ethics
Husbandry and handling of the fish in the present study were approved by the Experimental Animal Ethics Committee of Ocean University of China, and the procedures were performed strictly according to the Management Rule of Laboratory Animals (Chinese order No. 676 of the State Council, revised on 1 March 2017).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was supported by the National Natural Science Foundation of China (Grant No. 31972802); Natural Science Foundation of Shandong Province (Grant No. ZR2019MC041); National Key R&D Program of China (Grant No. 2018YFD0900400); Youth Talent Program supported by Laboratory for Marine Fisheries Science and Food Production Processes, Pilot National Laboratory for Marine Science Technology (Qingdao) (Grant No. 2018-MFS-T11); and the Fundamental Research Funds for the Central Universities (Grant No. 201961025).
Author Contributions
X.L. designed and performed the experiments, analyzed the data, and wrote the manuscript; Y.L., R.S., and S.L. performed the experiments; Z.Y. generated cyp2r1−/− zebrafish and revised the manuscript, G.H.G. and P.B. supervised the project and revised the manuscript; M.W. acquired the financial supports, supervised the project, designed the experiments, analyzed the data, and wrote the manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1.Sassi F, Tamone C, D'Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. 2018 Nov;10((11)):1656. doi: 10.3390/nu10111656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones G, Prosser DE, Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res. 2014 Jan;55((1)):13–31. doi: 10.1194/jlr.R031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantorna MT, Rogers CJ, Arora J. Aligning the paradoxical role of vitamin D in gastrointestinal immunity. Trends Endocrinol Metab. 2019 Jul;30((7)):459–66. doi: 10.1016/j.tem.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lock E-J, Waagbø R, Wendelaar Bonga S, Flik G. The significance of vitamin D for fish: a review. Aquac Nutr. 2010 Sep;16((1)):100–16. [Google Scholar]
- 5.Liew PX, Kubes P. The neutrophil's role during health and disease. Physiol Rev. 2019 Apr;99((2)):1223–48. doi: 10.1152/physrev.00012.2018. [DOI] [PubMed] [Google Scholar]
- 6.Phillipson M, Kubes P. The healing power of neutrophils. Trends Immunol. 2019 Jul;40((7)):635–47. doi: 10.1016/j.it.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence SM, Corriden R, Nizet V. The ontogeny of a neutrophil: mechanisms of granulopoiesis and homeostasis. Microbiol Mol Biol Rev. 2018 Feb;82((1)):e00057–17. doi: 10.1128/MMBR.00057-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Havixbeck JJ, Barreda DR. Neutrophil development, migration, and function in teleost fish. Biology. 2015 Nov;4((4)):715–34. doi: 10.3390/biology4040715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pazhakh V, Clark S, Keightley MC, Lieschke GJ. A GCSFR/CSF3R zebrafish mutant models the persistent basal neutrophil deficiency of severe congenital neutropenia. Sci Rep. 2017 Mar;7:44455. doi: 10.1038/srep44455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salva S, Alvarez S. The role of microbiota and immunobiotics in granulopoiesis of immunocompromised hosts. Front Immunol. 2017 May;8:507. doi: 10.3389/fimmu.2017.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renshaw SA, Loynes CA, Trushell DM, Elworthy S, Ingham PW, Whyte MK. A transgenic zebrafish model of neutrophilic inflammation. Blood. 2006 Dec;108((13)):3976–8. doi: 10.1182/blood-2006-05-024075. [DOI] [PubMed] [Google Scholar]
- 12.Murdoch CC, Espenschied ST, Matty MA, Mueller O, Tobin DM, Rawls JF. Intestinal Serum amyloid A suppresses systemic neutrophil activation and bactericidal activity in response to microbiota colonization. PLoS Pathog. 2019 Mar;15((3)):e1007381. doi: 10.1371/journal.ppat.1007381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng X, Shang G, Wang W, Chen X, Lou Q, Zhai G, et al. Fatty acid oxidation in zebrafish adipose tissue is promoted by 1alpha,25(OH)2D3. Cell Rep. 2017 May;19((7)):1444–55. doi: 10.1016/j.celrep.2017.04.066. [DOI] [PubMed] [Google Scholar]
- 14.Ellett F, Lieschke GJ. Computational quantification of fluorescent leukocyte numbers in zebrafish embryos. Methods Enzymol. 2012 Feb;506:425–35. doi: 10.1016/B978-0-12-391856-7.00046-9. [DOI] [PubMed] [Google Scholar]
- 15.Pham LN, Kanther M, Semova I, Rawls JF. Methods for generating and colonizing gnotobiotic zebrafish. Nat Protoc. 2008 Dec;3((12)):1862–75. doi: 10.1038/nprot.2008.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Lin L, Chen W, Zheng X, Zhang Y, Liu Q, et al. Neutrophil plays critical role during Edwardsiella piscicida immersion infection in zebrafish larvae. Fish Shellfish Immunol. 2019 Apr;87:565–72. doi: 10.1016/j.fsi.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 17.El-Benna J, Hurtado-Nedelec M, Marzaioli V, Marie JC, Gougerot-Pocidalo MA, Dang PM. Priming of the neutrophil respiratory burst: role in host defense and inflammation. Immunol Rev. 2016 Sep;273((1)):180–93. doi: 10.1111/imr.12447. [DOI] [PubMed] [Google Scholar]
- 18.Martens PJ, Gysemans C, Verstuyf A, Mathieu AC. Vitamin D's effect on immune function. Nutrients. 2020 Apr;12((5)):1248. doi: 10.3390/nu12051248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wasnik S, Rundle CH, Baylink DJ, Yazdi MS, Carreon EE, Xu Y, et al. 1,25-Dihydroxyvitamin D suppresses M1 macrophages and promotes M2 differentiation at bone injury sites. JCI Insight. 2018 Sep;3((17)):e98773. doi: 10.1172/jci.insight.98773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Ødum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol. 2010 Apr;11((4)):344–9. doi: 10.1038/ni.1851. [DOI] [PubMed] [Google Scholar]
- 21.Rolf L, Muris AH, Hupperts R, Damoiseaux J. Vitamin D effects on B cell function in autoimmunity. Ann N Y Acad Sci. 2014 May;1317((1)):84–91. doi: 10.1111/nyas.12440. [DOI] [PubMed] [Google Scholar]
- 22.Cortes M, Chen MJ, Stachura DL, Liu SY, Kwan W, Wright F, et al. Developmental vitamin D availability impacts hematopoietic stem cell production. Cell Rep. 2016 Oct;17((2)):458–68. doi: 10.1016/j.celrep.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medrano M, Carrillo-Cruz E, Montero I, Perez-Simon JA. Vitamin D: effect on haematopoiesis and immune system and clinical applications. Int J Mol Sci. 2018 Sep;19((9)):2663. doi: 10.3390/ijms19092663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cowland JB, Borregaard N. Granulopoiesis and granules of human neutrophils. Immunol Rev. 2016 Sep;273((1)):11–28. doi: 10.1111/imr.12440. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy AD, DeLeo FR. Neutrophil apoptosis and the resolution of infection. Immunol Res. 2009 Dec;43((1–3)):25–61. doi: 10.1007/s12026-008-8049-6. [DOI] [PubMed] [Google Scholar]
- 26.Ottersbach K, Smith A, Wood A, Göttgens B. Ontogeny of haematopoiesis: recent advances and open questions. Br J Haematol. 2010 Feb;148((3)):343–55. doi: 10.1111/j.1365-2141.2009.07953.x. [DOI] [PubMed] [Google Scholar]
- 27.Le Guyader D, Redd MJ, Colucci-Guyon E, Murayama E, Kissa K, Briolat V, et al. Origins and unconventional behavior of neutrophils in developing zebrafish. Blood. 2008 Jan 1;111((1)):132–41. doi: 10.1182/blood-2007-06-095398. [DOI] [PubMed] [Google Scholar]
- 28.Murayama E, Kissa K, Zapata A, Mordelet E, Briolat V, Lin HF, et al. Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity. 2006 Dec;25((6)):963–75. doi: 10.1016/j.immuni.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 29.Ward AC, Loeb DM, Soede-Bobok AA, Touw IP, Friedman AD. Regulation of granulopoiesis by transcription factors and cytokine signals. Leukemia. 2000 Jun;14((6)):973–90. doi: 10.1038/sj.leu.2401808. [DOI] [PubMed] [Google Scholar]
- 30.Stachura DL, Svoboda O, Campbell CA, Espín-Palazón R, Lau RP, Zon LI, et al. The zebrafish granulocyte colony-stimulating factors (Gcsfs): 2 paralogous cytokines and their roles in hematopoietic development and maintenance. Blood. 2013 Dec;122((24)):3918–28. doi: 10.1182/blood-2012-12-475392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006 Mar;311((5768)):1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 32.Souder JP, Gorelick DA. Quantification of estradiol uptake in zebrafish embryos and larvae. Toxicol Sci. 2017 Aug;158((2)):465–74. doi: 10.1093/toxsci/kfx107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mowat AM. To respond or not to respond: a personal perspective of intestinal tolerance. Nat Rev Immunol. 2018 Jun;18((6)):405–15. doi: 10.1038/s41577-018-0002-x. [DOI] [PubMed] [Google Scholar]
- 34.Martins RR, Ellis PS, MacDonald RB, Richardson RJ, Henriques CM. Resident immunity in tissue repair and maintenance: the zebrafish model coming of age. Front Cell Dev Biol. 2019 Feb;7:12. doi: 10.3389/fcell.2019.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belkaid Y, Harrison OJ. Homeostatic immunity and the microbiota. Immunity. 2017 Apr;46((4)):562–76. doi: 10.1016/j.immuni.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mei J, Liu Y, Dai N, Hoffmann C, Hudock KM, Zhang P, et al. Cxcr2 and Cxcl5 regulate the IL-17/G-CSF axis and neutrophil homeostasis in mice. J Clin Invest. 2012 Mar;122((3)):974–86. doi: 10.1172/JCI60588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deshmukh HS, Liu Y, Menkiti OR, Mei J, Dai N, O'Leary CE, et al. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat Med. 2014 May;20((5)):524–30. doi: 10.1038/nm.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanther M, Tomkovich S, Xiaolun S, Grosser MR, Koo J, Flynn EJ, et al. Commensal microbiota stimulate systemic neutrophil migration through induction of serum amyloid A. Cell Microbiol. 2014 Jul;16((7)):1053–67. doi: 10.1111/cmi.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Thingholm LB, Skiecevičienė J, Rausch P, Kummen M, Hov JR, et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat Genet. 2016 Nov;48((11)):1396–406. doi: 10.1038/ng.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas RL, Jiang L, Adams JS, Xu ZZ, Shen J, Janssen S, et al. Vitamin D metabolites and the gut microbiome in older men. Nat Commun. 2020 Nov;11((1)):5997. doi: 10.1038/s41467-020-19793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waterhouse M, Hope B, Krause L, Morrison M, Protani MM, Zakrzewski M, et al. Vitamin D and the gut microbiome: a systematic review of in vivo studies. Eur J Nutr. 2019 Oct;58((7)):2895–910. doi: 10.1007/s00394-018-1842-7. [DOI] [PubMed] [Google Scholar]
- 42.Zhang JY, Wu P, Chen D, Ning F, Lu Q, Qiu X, et al. Vitamin D promotes trophoblast cell induced separation of vascular smooth muscle cells in vascular remodeling via induction of G-CSF. Front Cell Dev Biol. 2020 Dec;8:601043. doi: 10.3389/fcell.2020.601043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brugman S. The zebrafish as a model to study intestinal inflammation. Dev Comp Immunol. 2016 Nov;64:82–92. doi: 10.1016/j.dci.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 44.Wallace KN, Pack M. Unique and conserved aspects of gut development in zebrafish. Dev Biol. 2003 Mar;255((1)):12–29. doi: 10.1016/s0012-1606(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 45.Miskolci V, Squirrell J, Rindy J, Vincent W, Sauer JD, Gibson A, et al. Distinct inflammatory and wound healing responses to complex caudal fin injuries of larval zebrafish. Elife. 2019 Jul;8:e45976. doi: 10.7554/eLife.45976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li L, Yan B, Shi YQ, Zhang WQ, Wen ZL. Live imaging reveals differing roles of macrophages and neutrophils during zebrafish tail fin regeneration. J Biol Chem. 2012 Jul;287((30)):25353–60. doi: 10.1074/jbc.M112.349126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Oliveira S, Reyes-Aldasoro CC, Candel S, Renshaw SA, Mulero V, Calado A. Cxcl8 (IL-8) mediates neutrophil recruitment and behavior in the zebrafish inflammatory response. J Immunol. 2013 Apr;190((8)):4349–59. doi: 10.4049/jimmunol.1203266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen L, Eapen MS, Zosky GR. Vitamin D both facilitates and attenuates the cellular response to lipopolysaccharide. Sci Rep. 2017 Mar;7:45172. doi: 10.1038/srep45172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Derbyshire EJ, Calder PC. Respiratory tract infections and antibiotic resistance: a protective role for vitamin D? Front Nutr. 2021 Mar;8:652469. doi: 10.3389/fnut.2021.652469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Subramanian K, Bergman P, Henriques-Normark B. Vitamin D promotes pneumococcal killing and modulates inflammatory responses in primary human neutrophils. J Innate Immun. 2017 Feb;9((4)):375–86. doi: 10.1159/000455969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu X, Chang X, Wu H, Xiao J, Gao Y, Zhang Y. Role of intestinal inflammation in predisposition of Edwardsiella tarda infection in zebrafish (Danio rerio) Fish Shellfish Immunol. 2014 Dec;41((2)):271–8. doi: 10.1016/j.fsi.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 52.Aratani Y. Myeloperoxidase: its role for host defense, inflammation, and neutrophil function. Arch Biochem Biophys. 2018 Feb;640:47–52. doi: 10.1016/j.abb.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood. 2000 Oct;96((8)):2673–81. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.