Abstract
Background
Porcine circovirus type 2 (PCV2) and Mycoplasma hyopneumoniae (MHP) are economically significant pathogens in the pig industry. The use of combined vaccines against PCV2 and MHP is one of the most effective ways of protecting pigs from both diseases, and it has become a part of general management.
Objectives
This study evaluated the efficacy of two new bivalent vaccines of PCV2 and MHP (Myco-X and Myco-XD) in SPF pigs. Myco-X and Myco-XD are a combined vaccine of MHP with PCV2b and PCV2d, respectively.
Methods
Sixteen pigs were divided into four groups: Myco-X-vaccinated challenged, Myco-XD-vaccinated challenged, unvaccinated challenged, and unvaccinated unchallenged. Two milliliters of Myco-X were administered intramuscularly, and 0.5 mL of Myco-XD was injected intradermally at 3 wk of age. The pigs were challenged with virulent PCV2d via the intramuscular and intranasal route 4 wk post-vaccination.
Results
All vaccinated pigs showed effective reduction of the clinical signs, the PCV2d load in the blood and nasal swab samples, as well as lung and lymphoid tissue lesions in the challenge test. Compared to unvaccinated challenged animals, the vaccinated challenged animals showed significantly higher (p < 0.05) levels of anti-PCV2 IgG, PCV2d-specific interferon-γ (IFN-γ), and anti-MHP IgG.
Conclusions
Based on clinical, microbiological, serological, and pathological assessments, this study confirmed that both combined vaccines could protect pigs against PCV2 infection caused by PCV2d. On the other hand, further research on the efficacy evaluation of these new vaccines against the MHP challenge and PCV2d/MHP co-challenge is needed.
Keywords: Porcine circovirus, Mycoplasma hyopneumoniae, bivalent vaccine
INTRODUCTION
Porcine circovirus type 2 (PCV2) has been identified as one of the most economically damaging diseases to the global pig industry [1]. PCV2 contributes to post-weaning multisystemic wasting syndrome (PMWS), porcine respiratory disease complex (PRDC), porcine dermatitis and nephritis syndrome (PDNS), reproductive failure, and diarrhea [2,3,4]. PCV2 is a non-enveloped, circular, single-stranded DNA virus belonging to the genus Circovirus, family Circoviridae. Two main proteins are produced from double-stranded DNA intermediates. ORF1 encodes two nonstructural proteins, while the capsid protein is encoded by ORF2 [5]. PCV2 genotypes are classified as PCV2a, PCV2b, PCV2c, PCV2d, and PCV2e based on the differences in ORF2 nucleotide sequences [6]. PCV2 is currently classified into eight genotypes, denoted with lower case letters, “a to h”, in the order of initial identification [7]. PCV2a was the dominant genotype worldwide until 2000 [8,9,10,11,12,13,14]. Currently, PCV genotype 2d, known as mutant PCV2b, is the most common and dominant genotype in the USA [15], Russia [16], China [17], Thailand [18], Vietnam [19], and South Korea [5] because a genotype shift to PCV2d began to occur in 2003 [9,20,21]. The new emergence of the PCV2d genotype has led to vaccine failure, which has rapidly increased the distribution of porcine circovirus associated diseases (PCVAD) outbreaks [22,23]. In challenged pigs, PCV2d tended to have higher virulence, more serious clinical signs, viremia, and pathological lesions than classical PCV2a and 2b [21]. In nationwide PCV2 surveillance in 2016, genotype-specific PCR showed that the incidence of PCV2d infections and PCV2b/d coinfections were 47.8% and 15.9%, respectively, while that of PCV2b infection was only 2.9%. This implies that the PCV2d genotype shift occurred in nationwide and that the coexistence of multiple genotypes is normal in South Korea [5].
Mycoplasma hyopneumoniae (MHP) is common and highly contagious in swine herds [24]. Mycoplasmal pneumonia is caused by MHP and is characterized by a persistent, non-productive cough with high morbidity and low mortality [24]. Enzootic pneumonia, produced by MHP in the presence of opportunistic microorganisms, such as Pasteurella multocida or Actinobacillus pleuropneumoniae, remains a severe chronic respiratory disease in pigs [25]. Enzootic pneumonia reduces the average daily weight gain and delays the marketed age, resulting in severe economic losses in the global pig industry [25].
PCV2 and MHP are economically significant pathogens that are the major agents of PRDC. The vaccination against PCV2 and MHP is one of the most effective ways of controlling both infections [26]. More than half of Korean pig farms presently employ combined vaccines that contain PCV2 and MHP (http://www.kahpha.or.kr; accessed on 29 April 2021). As a result, combination vaccines have become a part of general management [24].
Some bivalent vaccines for PCV2 and MHP manufactured by multinational companies have been introduced into the international markets over the last few years. This study evaluated and compared the efficacy of two newly developing bivalent vaccines, Myco-X (a mixture of PCV2b and MHP) and Myco-XD (a combination of PCV2d and MHP) in specific pathogen-free (SPF) pigs infected with PCV2d based on the clinical, microbiological, serological, and pathological findings.
MATERIALS AND METHODS
Ethical statement
This study was performed at the CAVAC Animal Test Facility under the guidelines established by its Institutional Animal Care and Use Committee (approval numbers: 202124-07 and 202124-08).
Animals
Sixteen SPF piglets were purchased at 3 wk of age from Optipharm (Korea). At 3 wk of age, the pigs were negative for PCV2, PRRSV, and MHP based on serological tests. The sera were negative for PCV2 and PRRSV, and the nasal swabs were negative for MHP through real-time polymerase chain reaction (PCR) [27,28,29].
Experimental design
Myco-X includes inactivated PCV2b and MHP with a nanoparticle adjuvant, while Myco-XD contains inactivated PCV2d and MHP with a water-in-oil type adjuvant.
Sixteen SPF pigs were divided into four groups (four pigs per group) (Table 1). At 3 wk of age, the pigs in the Myco-X-vaccinated group (G1) were injected intramuscularly Myco-X (CAVAC, Korea) with a 2.0 mL dose, while the pigs in the Myco-XD-vaccinated group (G2) were administered Myco-XD (CAVAC) intradermally with a 0.5 mL dose using an intradermal injector (PULSE 250; 30 psi). The unvaccinated groups (2dC and NC) were injected intramuscularly with 2.0 mL of phosphate-buffered saline (PBS, 0.1 M, pH 7.4) at 3 wk of age.
Table 1. Experimental design with vaccination and challenge.
| Group | No. of pigs | Vaccine | Vaccination (3 wk of age) | Challenge (7 wk of age) |
|---|---|---|---|---|
| G1 (PCV2b+MHP) | 4 | Myco-X | 2.0 mL, intramuscularly | PCV2d |
| G2 (PCV2d+MHP) | 4 | Myco-XD | 0.5 mL, intradermally | PCV2d |
| 2dC | 4 | PBS | 2.0 mL, intramuscularly | PCV2d |
| NC | 4 | PBS | 2.0 mL, intramuscularly | ND |
PCV2b, porcine circovirus type 2b; MHP, Mycoplasma hyopneumoniae; PCV2d, porcine circovirus type 2d; G1, Myco-X PCV2d challenge (Myco-X-vaccinated); G2, Myco-XD PCV2d challenge (Myco-XD-vaccinated); 2dC, PCV2d challenge control (unvaccinated challenged); NC, negative control (unvaccinated unchallenged); PBS, phosphate-buffered saline; ND, not done.
At 4 wk post-vaccination, 2.0 mL of a mixture of 2.2 mg/kg xylazine hydrochloride (Rompun; Bayer, Germany) and 2.2 mg/kg zolazepam hydrochloride (Zoletil 50; Virbac, France) was inoculated intramuscularly for cardiac anesthesia, followed by an injection of the virulent PCV2d (strain CBNU 0324, GenBank no. MN545963.1) containing 105.3–5.5 50% fluorescent assay infectious dose (FAID50)/mL into G1, G2 and 2dC pigs, 2.0 mL intramuscularly and 2.0 mL intranasally (1.0 mL in each nostril).
Each group was housed separately under the same air condition-, ventilation-, and temperature-controlled facility, which included access to feed and water ad libitum. The body weight was measured at 0, 1, 2, 3, 4, 5, 6, and 7 wk post-vaccination (WPV). Blood samples were collected every week after vaccination until 7 WPV, and nasal swabs were collected at 4, 5, 6, and 7 WPV. Blood collection was divided into syringes and heparin tubes for the interferon-γ (IFN-γ) measurements. The serum samples were stored without heat inactivation for the viremia measurement. At 7 WPV, all 16 pigs were sedated with an intramuscular injection of a mixture of Rompun and Zoletil and then euthanized by 5.0 mL of 200 mg embutramide, 50 mg mebezonium iodide, and 5 mg tetracaine hydrochloride (T-61; MSD Animal Health, Germany). Tissue samples, such as lung, spleen, tonsil, and lymph nodes, were collected from all pigs at necropsy and divided into formalin-fixed samples and samples for virus antigen detection.
Clinical observation
All the pigs were monitored daily from 4 to 7 WPV for abnormal clinical signs, such as weakness, hair roughness, respiratory signs, and death. The observers were blinded to vaccination and challenge status.
Average daily weight gain
The live body weight of each pig was measured eight times at 0, 1, 2, 3, 4, 5, 6, and 7 wk post-vaccination. The average daily weight gain (ADWG; kg/day) was analyzed over a specific period between 4 and 7 WPV.
Quantification of PCV2d DNA in blood and nasal swabs
DNA was extracted from the serum and nasal swab samples using a commercial kit (MagMax RNA Isolation kit (Thermo Fisher Scientific, USA) to quantify the copy numbers of PCV2d genomic DNA by real-time PCR [30].
Serology
The serum samples were tested for immunoglobulin G (IgG) against PCV2 and MHP using commercial PCV2 ELISA (VDPro PCV2 AB ELISA; Median Diagnostics, Korea) and MHP ELISA (IDEXX, USA) kits, respectively.
Quantification of PCV2-specific IFN-γ in blood
The numbers of PCV2d-specific IFN-γ were measured using a commercial porcine IFN-γ ELISA kit (Quantikine ELISA for Porcine IFN-γ Immunoassay; R&D Systems, USA) after peripheral blood mononuclear cells (PBMC) isolation (SepMate-50; STEMCELL Technologies, Canada) [31,32].
Pathology
The macroscopic lung lesions were scored (Supplementary Fig. 1) [33]. The microscopic lung, tonsil, and inguinal lymph node lesions were analyzed, and the presence of PCV2 in the tissue was identified using a PCV2 antigen immunohistochemistry (IHC) test [2,34].
Statistical analysis
All statistical analyses were conducted by one-way analysis of variance (ANOVA) using SigmaPlot 12.5 (Systat Software Inc., USA). A one-way ANOVA test result with statistical significance was evaluated further using a Tukey’s post hoc test. The p values less than 0.05 were considered significant.
RESULTS
Clinical observation
In the vaccinated challenged (G1 and G2) and unvaccinated unchallenged (NC) groups, each individual showed no significant clinical signs, except that one pig of G2 coughed from 2 wk post-challenge (WPC). On the other hand, the characteristic clinical signs of PCV2, such as weakness, hair roughness, running nose, and cough, were identified in all pigs in the unvaccinated challenged (2dC) group (Table 2).
Table 2. Clinical signs of SPF pigs after the challenge with Porcine Circovirus 2d.
| Group | Clinical signs | 0 WPC (4 WPV) | 1 WPC (5 WPV) | 2 WPC (6 WPV) | 3 WPC (7 WPV) |
|---|---|---|---|---|---|
| G1 (PCV2b+MHP) | Weakness | 0/4 | 0/4 | 0/4 | 0/4 |
| Hair roughness | 0/4 | 0/4 | 0/4 | 0/4 | |
| Running nose | 0/4 | 0/4 | 0/4 | 0/4 | |
| Cough | 0/4 | 0/4 | 0/4 | 0/4 | |
| G2 (PCV2d+MHP) | Weakness | 0/4 | 0/4 | 0/4 | 0/4 |
| Hair roughness | 0/4 | 0/4 | 0/4 | 0/4 | |
| Running nose | 0/4 | 0/4 | 0/4 | 0/4 | |
| Cough | 0/4 | 0/4 | 1/4 | 1/4 | |
| 2dC | Weakness | 0/4 | 0/4 | 1/4 | 4/4 |
| Hair roughness | 0/4 | 0/4 | 1/4 | 4/4 | |
| Running nose | 0/4 | 0/4 | 1/4 | 4/4 | |
| Cough | 0/4 | 0/4 | 4/4 | 4/4 | |
| NC | Weakness | 0/4 | 0/4 | 0/4 | 0/4 |
| Hair roughness | 0/4 | 0/4 | 0/4 | 0/4 | |
| Running nose | 0/4 | 0/4 | 0/4 | 0/4 | |
| Cough | 0/4 | 0/4 | 0/4 | 0/4 |
WPC, wk post challenge; WPV, wk post vaccination; PCV2b, porcine circovirus type 2b; MHP, Mycoplasma hyopneumoniae; PCV2d, porcine circovirus type 2d; G1, Myco-X PCV2d challenge (Myco-X-vaccinated); G2, Myco-XD PCV2d challenge (Myco-XD-vaccinated); 2dC, PCV2d challenge control (unvaccinated challenged); NC, negative control (unvaccinated unchallenged).
Average daily weight gain
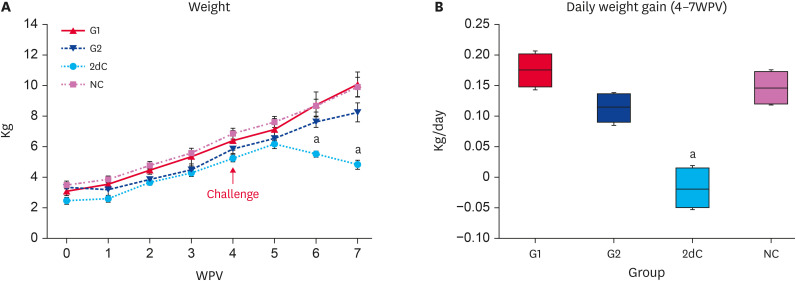
The bodyweight of the G1, G2, and NC pigs was significantly higher (p < 0.05) than that of the 2dC pigs from 6 to 7 WPV. No significant difference was observed between G1, G2, and NC (Fig. 1A and B).
Fig. 1. Bodyweight changes. (A) Mean body weight. (B) Average daily weight gain between 4 to 7 WPV in the different groups.
WPV, wk post-vaccination; PCV2d, porcine circovirus type 2d; G1, Myco-X PCV2d challenge (Myco-X-vaccinated); G2, Myco-XD PCV2d challenge (Myco-XD-vaccinated); 2dC, PCV2d challenge control (unvaccinated challenged); NC, negative control (unvaccinated unchallenged).
aDifferent superscripts indicate a significant difference (p < 0.05) between groups.
Quantification of PCV2d DNA in blood
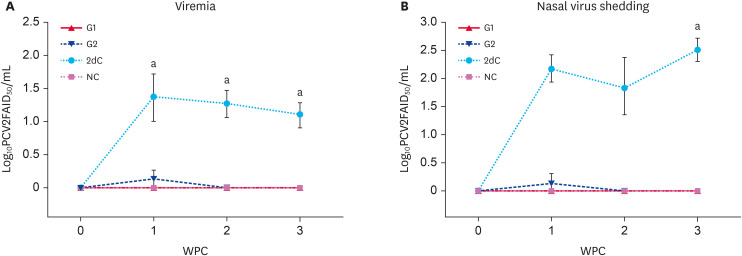
No PCV2 genomic copies were detected in the sera of all four groups at the time of challenge. The PCV2 levels of the G1, G2, and NC animals were significantly lower (p < 0.05) than that of the 2dC animals from 1 to 3 WPC. Genomic copies of PCV2 were not detected in the sera from the G1 and NC animals (Fig. 2A).
Fig. 2. Quantification of PCV2 DNA. (A) Mean values of the genomic copies of PCV2 DNA in the serum samples. (B) Mean values of the genomic copies of PCV2 DNA in the nasal swab samples in the different groups.
PCV2, porcine circovirus type 2; PCV2d, porcine circovirus type 2d; G1, Myco-X PCV2d challenge (Myco-X-vaccinated); G2, Myco-XD PCV2d challenge (Myco-XD-vaccinated); 2dC, PCV2d challenge control (unvaccinated challenged); NC, negative control (unvaccinated unchallenged).
aDifferent superscripts indicate a significant difference (p < 0.05) between groups.
Quantification of PCV2d DNA in nasal swabs
No PCV2 genomic copies were detected in the sera of the four groups before the challenge. The PCV2 levels of the G1, G2, and NC animals were significantly lower (p < 0.05) than that of the 2dC animals at 3 WPC. Genomic copies of PCV2 were not detected in the serum samples from the G1 and NC animals (Fig. 2B).
Serology for PCV2
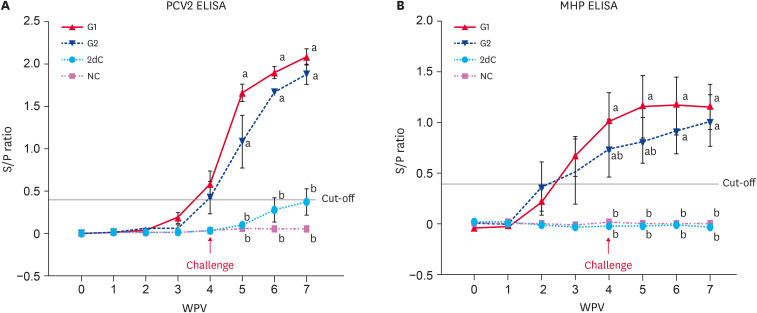
All pigs in the four groups were seronegative at the time of vaccination (3 wk of age; 0 WPV). Anti-PCV2 IgG were detected in the vaccinated challenged (G1 and G2) animals from 2 WPV, and the levels were significantly higher (p < 0.05) than that of the unvaccinated (2dC and NC) animals from 5 to 7 WPV (Fig. 3A).
Fig. 3. Immunological responses against PCV2 and MHP. (A) Mean values of the anti-PCV2 IgG levels. (B) Mean values of the anti-MHP IgG levels in the different groups.
PCV2, porcine circovirus type 2; MHP, Mycoplasma hyopneumoniae; IgG, immunoglobulin G; PCV2d, porcine circovirus type 2d; G1, Myco-X PCV2d challenge (Myco-X-vaccinated); G2, Myco-XD PCV2d challenge (Myco-XD-vaccinated); 2dC, PCV2d challenge control (unvaccinated challenged); NC, negative control (unvaccinated unchallenged).
a,bDifferent letters indicate a significant difference (p < 0.05) between groups.
Serology for MHP
All pigs in the four groups were seronegative at the time of vaccination (3 wk of age; 0 WPV). Anti-MHP IgG were detected in the vaccinated challenged (G1 and G2) animals from 2 WPV, and the levels were significantly higher (p < 0.05) than that of the unvaccinated (2dC and NC) animals from 4 to 7 WPV (Fig. 3B).
PCV2d-specific IFN-γ
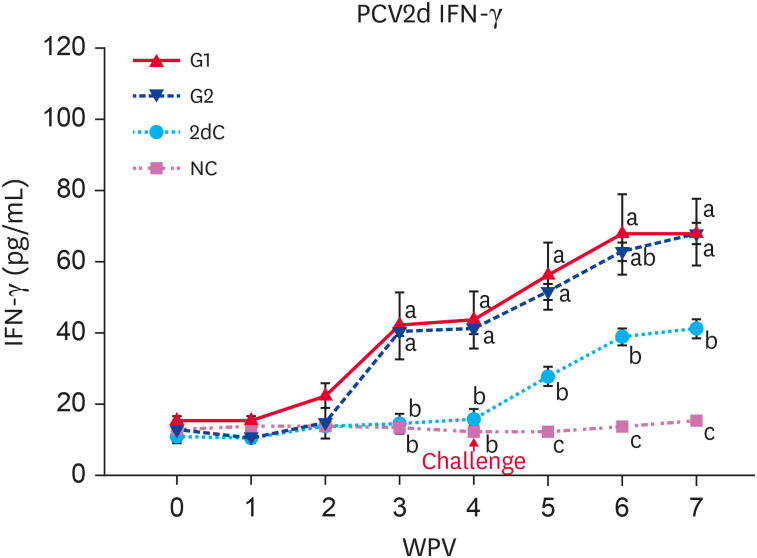
An increase in PCV2d-specific IFN-γ was maintained until the end of the test in the vaccinated challenged (G1 and G2) pigs, starting with a sharp increase from 3 WPV. The PCV2d-specific IFN-γ levels of G1 and G2 pigs were significantly higher (p < 0.05) than that of the unvaccinated (2dC and NC) pigs from 3 to 7 WPV (Fig. 4).
Fig. 4. Mean values of the PCV2d-specific IFN-γ in the different groups.
PCV2d, porcine circovirus type 2d; IFN-γ, interferon-γ; G1, Myco-X PCV2d challenge (Myco-X-vaccinated); G2, Myco-XD PCV2d challenge (Myco-XD-vaccinated); 2dC, PCV2d challenge control (unvaccinated challenged); NC, negative control (unvaccinated unchallenged).
a,b,cDifferent letters indicate a significant difference (p < 0.05) between groups.
Macroscopic lung lesion scores
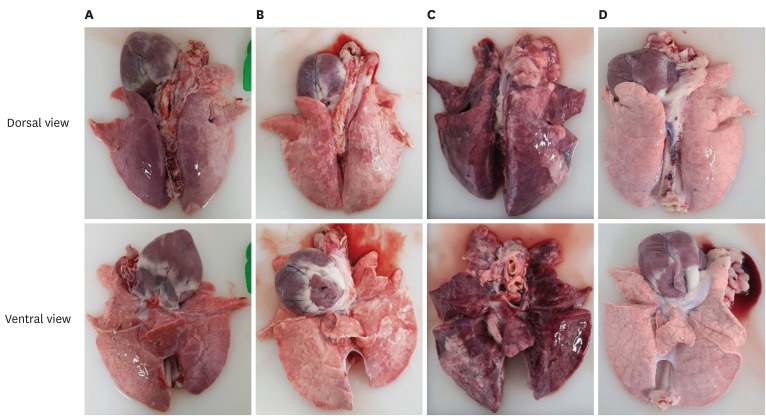
The lung lesions are summarized in Table 3. The lung lesion scores of the G1, G2, and NC pigs were significantly lower (p < 0.05) than that of the 2dC pigs. Severe interstitial pneumonia was observed in the 2dC pigs (Fig. 5).
Table 3. Gross scoring of lung lesions and histopathological findings in the lungs, tonsils, and lymph nodes of SPF pigs challenged with porcine circovirus 2d.
| Group | Gross lung lesions* | Histopathology | PCV2 antigen∥ | ||||
|---|---|---|---|---|---|---|---|
| Lung† | Tonsil‡ | Lymph node§ | Lung | Tonsil | Lymph node | ||
| G1 (PCV2b+MHP) | 12.00 ± 4.97a,b | miPP (4/4) | None | None | 0/4 | 0/4 | 0/4 |
| G2 (PCV2d+MHP) | 32.75 ± 27.83a | miPP (4/4) | None | None | 0/4 | 1/4 | 0/4 |
| 2dC | 98.75 ± 2.50c | miPP (2/4) | LD (4/4) | LD (4/4) | 3/4 | 3/4 | 4/4 |
| moPP (2/4) | IB (3/4) | IB (4/4) | |||||
| NC | 0.75 ± 0.50b | miPP (1/4) | None | None | 0/4 | 0/4 | 0/4 |
PCV2d, porcine circovirus type 2d; PCV2, porcine circovirus type 2; PCV2b, porcine circovirus type 2b; MHP, Mycoplasma hyopneumoniae; G1, Myco-X PCV2d challenge (Myco-X-vaccinated); G2, Myco-XD PCV2d challenge (Myco-XD-vaccinated group); 2dC, PCV2d challenge control (unvaccinated challenged); NC, negative control (unvaccinated unchallenged); LD, lymphoid depletion and histiocytic replacement in the lymphoid follicles; IB, basophilic intracytoplasmic viral inclusion bodies in lymph nodes.
a,b,cSignificantly differences (p < 0.05) between groups are indicated by different superscripted lower-case letters.
*Lung lesion score ranged from 0 to 12.5 depending on the lobes.
†The presence of mild or moderate peribronchiolar and perivascular inflammatory cell infiltration in the lungs.
‡The presence of lymphoid depletion and histiocytic replacement in the lymphoid follicles, and basophilic intracytoplasmic viral inclusion bodies in the tonsils.
§The presence of lymphoid depletion and histiocytic replacement in the lymphoid follicles, and basophilic intracytoplasmic viral inclusion bodies in the lymph nodes.
∥The presence of PCV2 antigen in different tissue.
Fig. 5. Gross lung lesions in lungs in different groups. (A) G1, (B) G2, (C) 2dC, and (D) NC groups.
PCV2d, porcine circovirus type 2d; G1, Myco-X PCV2d challenge (Myco-X-vaccinated); G2, Myco-XD PCV2d challenge (Myco-XD-vaccinated); 2dC, PCV2d challenge control (unvaccinated challenged); NC, negative control (unvaccinated unchallenged).
Microscopic lesions
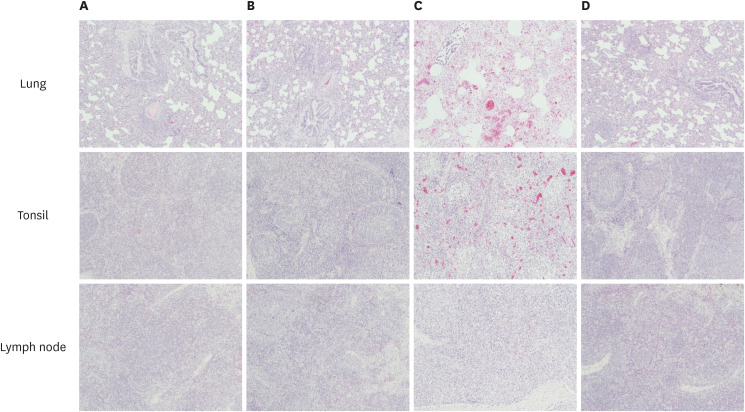
The lesions in the lungs, tonsils, and inguinal lymph nodes are presented (Table 3). Microscopic lesions were not found in the lungs of the NC animals except that one animal showed mild peribronchiolar and perivascular inflammatory cell infiltration. Peribronchiolar and perivascular inflammatory cell infiltration and alveolar wall thickening were observed mainly in the lungs of the vaccinated challenged (G1 and G2) animals at minimal to mild levels. In contrast, the lung lesions in the 2dC animals were accompanied by severe damaging reactions. Histopathological changes indicative of PCV2 infection were not identified in the tonsils and inguinal lymph nodes of the G1, G2, and NC pigs. On the other hand, in the 2dC pigs, lymphoid depletion and histiocytic replacement in the lymphoid follicles were observed in the tonsils and inguinal lymph nodes at mild to severe levels. In addition, basophilic intracytoplasmic viral inclusion antibodies were observed in all the tonsils and lymph nodes of 2dC pigs except for the tonsil of one animal (Fig. 6).
Fig. 6. Histopathological findings in the lungs, tonsils, and lymph nodes in different groups (H&E ×100). (A) G1, (B) G2, (C) 2dC, and (D) NC groups.
PCV2d, porcine circovirus type 2d; G1, Myco-X PCV2d challenge (Myco-X-vaccinated); G2, Myco-XD PCV2d challenge (Myco-XD-vaccinated); 2dC, PCV2d challenge control (unvaccinated challenged); NC, negative control (unvaccinated unchallenged).
PCV2 antigen Immunohistochemistry
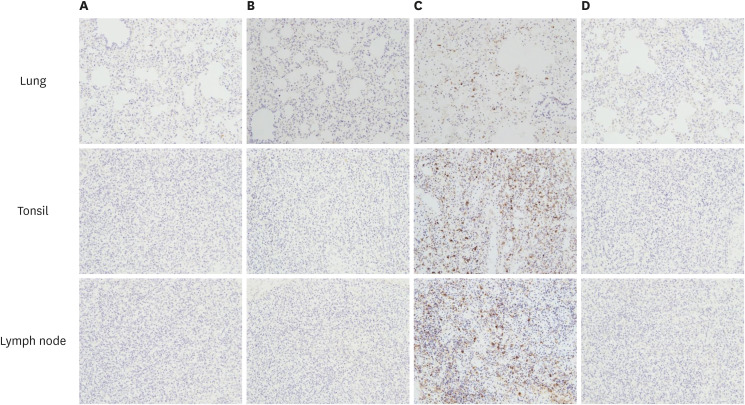
The presence of PCV2 in lungs, tonsils, and inguinal lymph nodes are shown (Table 3). The PCV2 antigen was not detected in the lungs, tonsils, and inguinal lymph nodes of the NC animals. The PCV2 antigen was not detected in the lungs of the vaccinated challenged (G1 and G2) animals. In contrast, the PCV2 antigen was detected in the lungs of three of the 2dC animals. The PCV2 antigen was not detected in the tonsils and inguinal lymph nodes of the G1 and G2 animals except for the tonsil of one animal in the G2 group. On the other hand, the PCV2 antigen was detected in the tonsils, and inguinal lymph nodes of 2dC animals except for the tonsil of one animal (Fig. 7).
Fig. 7. Immunohistochemistry of PCV2 antigen in the lungs, tonsils, and lymph nodes in different groups (×200). (A) G1, (B) G2, (C) 2dC, and (D) NC groups.
PCV2, porcine circovirus type 2; PCV2d, porcine circovirus type 2d; G1, Myco-X PCV2d challenge (Myco-X-vaccinated); G2, Myco-XD PCV2d challenge (Myco-XD-vaccinated); 2dC, PCV2d challenge control (unvaccinated challenged); NC, negative control (unvaccinated unchallenged).
DISCUSSION
An experimental challenge study is essential for evaluating and comparing the efficacy of new vaccines. This study confirmed that two newly developing bivalent vaccines of PCV2 and MHP could effectively protect pigs from PCV2d challenge. In particular, Myco-X containing PCV2b demonstrates itself to cross-protect against PCV2d challenge, which is the most prevalent genotype worldwide.
Both humoral and cell-mediated responses are important for assessing the efficacy of new bivalent vaccines. Pigs vaccinated with Myco-X or Myco-XD produced anti-PCV IgG and PCV2d-specific IFN-γ through successful immune responses. Both immunological responses reduced the clinical signs, PCV2 viremia, PCV2 nasal shedding, lung, tonsil, and lymphoid lesions, and PCV2 antigen in those lesions.
When a vaccine contains multiple antigens, there is a possibility of interference between them. On the other hand, the two bivalent vaccines induced anti-PCV2 IgG, PCV2d-specific IFN-γ, and anti-MHP IgG and reduced the clinical signs, viremia, nasal shedding, and lung, tonsil, and lymphoid lesions. The results showed no interference between the antigens in those two bivalent vaccines.
There is no reliable correlation between humoral immune response and protection against MHP [35,36]. In this study, however, vaccinated animals seroconverted after vaccination while immunized animals with another company’s bivalent vaccine seroconverted only after the MHP challenge in a previous study [37].
The microscopic lesions caused by PCV2d challenge showed the following: a moderate degree of peribronchiolar and perivascular inflammatory cell infiltration; moderate to severe degree of congestion and edema in the interlobular septa; minimal to mild hemorrhage in the lungs; moderate to some degree of lymphoid depletion; histiocytic replacement in the lymphoid follicles; the presence of basophilic intracytoplasmic viral inclusion bodies in tonsil and lymph nodes in the 2dC pigs. An injection of bivalent vaccines of PCV2 and MHP reduced the severity of lung, tonsil, and lymphoid lesions, and PCV2 antigen in those lesions compared to 2dC pigs.
Both bivalent vaccines of PCV2 and MHP could protect pigs against PCV2d despite only four animals per group being used. Field trials with a large number of pigs per group will be needed to confirm the vaccine efficacy. In addition, when MHP infection precedes a PCV2 infection, it contributes to the severity of the PCV2-associated lesions [34]. Further studies on the efficacy of these new vaccines against MHP challenge and PCV2d/MHP co-challenge will be needed.
Most commercially available PCV2 vaccines in the market are made of the PCV genotype 2a, and they often result in vaccine failure because of PCV2d infection. It is speculated that it is caused by the genotype difference between the vaccine strain and virulent field strain. On the other hand, this study differed from other studies [22,23], which might be related to the formula of the vaccines.
Overall, these two newly developing bivalent vaccines of PCV2 and MHP can protect pigs from PCV2d, assist pig practitioners and producers in reducing the number of vaccinations in pigs, and are expected to control two economically significant diseases, PCV2d infection and enzootic pneumonia.
Footnotes
Funding: This study was supported by BK21 PLUS, the Research Institute for Veterinary Science, Seoul National University, and Rural Development Administration (Project No. PJ01398501) and the Research Institute for Veterinary Science, Seoul, South Korea.
Conflict of Interest: This experiment was carried out independently without any directions from the vaccine company. The authors declared no conflicts of interest.
- Conceptualization: Lim J, Yoo HS.
- Data curation: Lim J, Jin M.
- Formal analysis: Lim J.
- Funding acquisition: Yoo HS.
- Investigation: Lim J, Jin M.
- Methodology: Jin M, Yoon I.
- Project administration: Yoo HS.
- Resources: Jin M, Yoon I.
- Software: Lim J, Jin M.
- Supervision: Yoon I, Yoo HS.
- Validation: Lim J.
- Visualization: Jin M.
- Writing - original draft: Lim J.
- Writing - review & editing: Lim J, Yoo HS.
SUPPLEMENTARY MATERIAL
Gross pig lung lesion scoring system (100 points) and area (bars) where sections were taken for microscopic examination.
References
- 1.Chae C. A review of porcine circovirus 2-associated syndromes and diseases. Vet J. 2005;169(3):326–336. doi: 10.1016/j.tvjl.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Opriessnig T, Meng XJ, Halbur PG. Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J Vet Diagn Invest. 2007;19(6):591–615. doi: 10.1177/104063870701900601. [DOI] [PubMed] [Google Scholar]
- 3.An DJ, Roh IS, Song DS, Park CK, Park BK. Phylogenetic characterization of porcine circovirus type 2 in PMWS and PDNS Korean pigs between 1999 and 2006. Virus Res. 2007;129(2):115–122. doi: 10.1016/j.virusres.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 4.Zheng G, Lu Q, Wang F, Xing G, Feng H, Jin Q, et al. Phylogenetic analysis of porcine circovirus type 2 (PCV2) between 2015 and 2018 in Henan Province, China. BMC Vet Res. 2020;16(1):6. doi: 10.1186/s12917-019-2193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon T, Lee DU, Yoo SJ, Je SH, Shin JY, Lyoo YS. Genotypic diversity of porcine circovirus type 2 (PCV2) and genotype shift to PCV2d in Korean pig population. Virus Res. 2017;228:24–29. doi: 10.1016/j.virusres.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Olvera A, Cortey M, Segalés J. Molecular evolution of porcine circovirus type 2 genomes: phylogeny and clonality. Virology. 2007;357(2):175–185. doi: 10.1016/j.virol.2006.07.047. [DOI] [PubMed] [Google Scholar]
- 7.Franzo G, Segalés J. Porcine circovirus 2 (PCV-2) genotype update and proposal of a new genotyping methodology. PLoS One. 2018;13(12):e0208585. doi: 10.1371/journal.pone.0208585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung AK, Lager KM, Kohutyuk OI, Vincent AL, Henry SC, Baker RB, et al. Detection of two porcine circovirus type 2 genotypic groups in United States swine herds. Arch Virol. 2007;152(5):1035–1044. doi: 10.1007/s00705-006-0909-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dupont K, Nielsen EO, Baekbo P, Larsen LE. Genomic analysis of PCV2 isolates from Danish archives and a current PMWS case-control study supports a shift in genotypes with time. Vet Microbiol. 2008;128(1-2):56–64. doi: 10.1016/j.vetmic.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Grau-Roma L, Crisci E, Sibila M, López-Soria S, Nofrarias M, Cortey M, et al. A proposal on porcine circovirus type 2 (PCV2) genotype definition and their relation with postweaning multisystemic wasting syndrome (PMWS) occurrence. Vet Microbiol. 2008;128(1-2):23–35. doi: 10.1016/j.vetmic.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Carman S, Cai HY, DeLay J, Youssef SA, McEwen BJ, Gagnon CA, et al. The emergence of a new strain of porcine circovirus-2 in Ontario and Quebec swine and its association with severe porcine circovirus associated disease--2004-2006. Can J Vet Res. 2008;72(3):259–268. [PMC free article] [PubMed] [Google Scholar]
- 12.Wang F, Guo X, Ge X, Wang Z, Chen Y, Cha Z, et al. Genetic variation analysis of Chinese strains of porcine circovirus type 2. Virus Res. 2009;145(1):151–156. doi: 10.1016/j.virusres.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Cortey M, Pileri E, Sibila M, Pujols J, Balasch M, Plana J, et al. Genotypic shift of porcine circovirus type 2 from PCV-2a to PCV-2b in Spain from 1985 to 2008. Vet J. 2011;187(3):363–368. doi: 10.1016/j.tvjl.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Franzo G, Cortey M, Segalés J, Hughes J, Drigo M. Phylodynamic analysis of porcine circovirus type 2 reveals global waves of emerging genotypes and the circulation of recombinant forms. Mol Phylogenet Evol. 2016;100:269–280. doi: 10.1016/j.ympev.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Noll L, Lu N, Porter E, Stoy C, Zheng W, et al. Genetic diversity and prevalence of porcine circovirus type 3 (PCV3) and type 2 (PCV2) in the Midwest of the USA during 2016-2018. Transbound Emerg Dis. 2020;67(3):1284–1294. doi: 10.1111/tbed.13467. [DOI] [PubMed] [Google Scholar]
- 16.Raev SA, Yuzhakov AG, Alekseev KP, Kostina LV, Gulyukin MI, Stepanova TV, et al. Transmission of porcine circovirus genotype type 2 (PCV2) in Russia and genotype association (PCV2d) with porcine dermatitis and nephropathy syndrome (PDNS) IOP Conf Ser Earth Environ Sci. 2019;315(4):042026 [Google Scholar]
- 17.Hou Z, Wang H, Feng Y, Song M, Li Q, Li J. Genetic variation and phylogenetic analysis of Porcine circovirus type 2 in China from 2016 to 2018. Acta Virol. 2019;63(4):459–468. doi: 10.4149/av_2019_413. [DOI] [PubMed] [Google Scholar]
- 18.Thangthamniyom N, Sangthong P, Poolperm P, Thanantong N, Boonsoongnern A, Hansoongnern P, et al. Genetic diversity of porcine circovirus type 2 (PCV2) in Thailand during 2009-2015. Vet Microbiol. 2017;208:239–246. doi: 10.1016/j.vetmic.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Dinh PX, Nguyen MN, Nguyen HT, Tran VH, Tran QD, Dang KH, et al. Porcine circovirus genotypes and their copathogens in pigs with respiratory disease in southern provinces of Vietnam. Arch Virol. 2021;166(2):403–411. doi: 10.1007/s00705-020-04878-y. [DOI] [PubMed] [Google Scholar]
- 20.Opriessnig T, Gerber PF, Xiao CT, Mogler M, Halbur PG. A commercial vaccine based on PCV2a and an experimental vaccine based on a variant mPCV2b are both effective in protecting pigs against challenge with a 2013 U.S. variant mPCV2b strain. Vaccine. 2014;32(2):230–237. doi: 10.1016/j.vaccine.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Kim K, Hahn TW. Evaluation of novel recombinant porcine circovirus type 2d (PCV2d) vaccine in pigs naturally infected with PCV2d. Vaccine. 2021;39(3):529–535. doi: 10.1016/j.vaccine.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Xiao CT, Halbur PG, Opriessnig T. Global molecular genetic analysis of porcine circovirus type 2 (PCV2) sequences confirms the presence of four main PCV2 genotypes and reveals a rapid increase of PCV2d. J Gen Virol. 2015;96(Pt 7):1830–1841. doi: 10.1099/vir.0.000100. [DOI] [PubMed] [Google Scholar]
- 23.Xiao CT, Harmon KM, Halbur PG, Opriessnig T. PCV2d-2 is the predominant type of PCV2 DNA in pig samples collected in the U.S. during 2014-2016. Vet Microbiol. 2016;197:72–77. doi: 10.1016/j.vetmic.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Um H, Yang S, Oh T, Park K, Cho H, Suh J, et al. Comparative evaluation of growth performance between bivalent and trivalent vaccines containing porcine circovirus type 2 (PCV2) and Mycoplasma hyopneumoniae in a herd with subclinical PCV2d infection and enzootic pneumonia. Vaccines (Basel) 2021;9(5):450. doi: 10.3390/vaccines9050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maes D, Sibila M, Kuhnert P, Segalés J, Haesebrouck F, Pieters M. Update on Mycoplasma hyopneumoniae infections in pigs: knowledge gaps for improved disease control. Transbound Emerg Dis. 2018;65(Suppl 1):110–124. doi: 10.1111/tbed.12677. [DOI] [PubMed] [Google Scholar]
- 26.Chae C. Porcine respiratory disease complex: Interaction of vaccination and porcine circovirus type 2, porcine reproductive and respiratory syndrome virus, and Mycoplasma hyopneumoniae . Vet J. 2016;212:1–6. doi: 10.1016/j.tvjl.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 27.Wasilk A, Callahan JD, Christopher-Hennings J, Gay TA, Fang Y, Dammen M, et al. Detection of U.S., Lelystad, and European-like porcine reproductive and respiratory syndrome viruses and relative quantitation in boar semen and serum samples by real-time PCR. J Clin Microbiol. 2004;42(10):4453–4461. doi: 10.1128/JCM.42.10.4453-4461.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubosson CR, Conzelmann C, Miserez R, Boerlin P, Frey J, Zimmermann W, et al. Development of two real-time PCR assays for the detection of Mycoplasma hyopneumoniae in clinical samples. Vet Microbiol. 2004;102(1-2):55–65. doi: 10.1016/j.vetmic.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Gagnon CA, del Castillo JR, Music N, Fontaine G, Harel J, Tremblay D. Development and use of a multiplex real-time quantitative polymerase chain reaction assay for detection and differentiation of Porcine circovirus-2 genotypes 2a and 2b in an epidemiological survey. J Vet Diagn Invest. 2008;20(5):545–558. doi: 10.1177/104063870802000503. [DOI] [PubMed] [Google Scholar]
- 30.Opriessnig T, Xiao CT, Halbur PG, Gerber PF, Matzinger SR, Meng XJ. A commercial porcine circovirus (PCV) type 2a-based vaccine reduces PCV2d viremia and shedding and prevents PCV2d transmission to naïve pigs under experimental conditions. Vaccine. 2017;35(2):248–254. doi: 10.1016/j.vaccine.2016.11.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerace E, Mandanici F, Pasquali P, Falduto M, Vitale M, Di Marco Lo Presti V, et al. M. bovis infection in pigs: improvement of the γ-IFN assay efficiency in this species using a maintenance medium. Tuberculosis (Edinb) 2018;108:151–154. doi: 10.1016/j.tube.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Bhatt KH, Neller MA, Srihari S, Crooks P, Lekieffre L, Aftab BT, et al. Profiling HPV-16-specific T cell responses reveals broad antigen reactivities in oropharyngeal cancer patients. J Exp Med. 2020;217(10):e20200389. doi: 10.1084/jem.20200389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halbur PG, Paul PS, Frey ML, Landgraf J, Eernisse K, Meng XJ, et al. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol. 1995;32(6):648–660. doi: 10.1177/030098589503200606. [DOI] [PubMed] [Google Scholar]
- 34.Opriessnig T, Thacker EL, Yu S, Fenaux M, Meng XJ, Halbur PG. Experimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual infection with Mycoplasma hyopneumoniae and porcine circovirus type 2. Vet Pathol. 2004;41(6):624–640. doi: 10.1354/vp.41-6-624. [DOI] [PubMed] [Google Scholar]
- 35.Djordjevic SP, Eamens GJ, Romalis LF, Nicholls PJ, Taylor V, Chin J. Serum and mucosal antibody responses and protection in pigs vaccinated against Mycoplasma hyopneumoniae with vaccines containing a denatured membrane antigen pool and adjuvant. Aust Vet J. 1997;75(7):504–511. doi: 10.1111/j.1751-0813.1997.tb14383.x. [DOI] [PubMed] [Google Scholar]
- 36.Thacker EL, Thacker BJ, Boettcher TB, Jayappa H. Comparison of antibody production, lymphocyte stimulation, and protection induced by four commercial Mycoplasma hyopneumoniae bacterins. J Swine Health Prod. 1998;6:107–112. [Google Scholar]
- 37.Park C, Jeong J, Choi K, Chae C. Efficacy of a new bivalent vaccine of porcine circovirus type 2 and Mycoplasma hyopneumoniae (Fostera™ PCV MH) under experimental conditions. Vaccine. 2016;34(2):270–275. doi: 10.1016/j.vaccine.2015.11.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gross pig lung lesion scoring system (100 points) and area (bars) where sections were taken for microscopic examination.