Abstract
Background
Since 2003, the H5 highly pathogenic avian influenza (HPAI) subtype has caused massive economic losses in the poultry industry in South Korea. The role of inland water bodies in avian influenza (AI) outbreaks has not been investigated. Identifying water bodies that facilitate risk pathways leading to the incursion of the HPAI virus (HPAIV) into poultry farms is essential for implementing specific precautionary measures to prevent viral transmission.
Objectives
This matched case-control study (1:4) examined whether inland waters were associated with a higher risk of AI outbreaks in the neighboring poultry farms.
Methods
Rivers, irrigation canals, lakes, and ponds were considered inland water bodies. The cases and controls were chosen based on the matching criteria. The nearest possible farms located within a radius of 3 km of the case farms were chosen as the control farms. The poultry farms were selected randomly, and two HPAI epidemics (H5N8 [2014–2016] and H5N6 [2016–2017]) were studied. Conditional logistic regression analysis was applied.
Results
Statistical analysis revealed that inland waters near poultry farms were significant risk factors for AI outbreaks. The study speculated that freely wandering wild waterfowl and small animals contaminate areas surrounding poultry farms.
Conclusions
Pet birds and animals raised alongside poultry birds on farm premises may wander easily to nearby waters, potentially increasing the risk of AI infection in poultry farms. Mechanical transmission of the AI virus occurs when poultry farm workers or visitors come into contact with infected water bodies or their surroundings. To prevent AI outbreaks in the future, poultry farms should adopt strict precautions to avoid contact with nearby water bodies and their surroundings.
Keywords: Influenza A virus, outbreaks, risk factors, animals, birds
INTRODUCTION
Avian influenza viruses (AIVs) belong to the genus influenza A virus and have caused devastating damage to the poultry industry worldwide [1]. Highly pathogenic avian influenza (HPAI) strains pose a serious economic threat because infected poultry flocks need to be culled [2]. The surface glycoproteins of the virus, such as hemagglutinin and neuraminidase, have been used to categorize AIVs into 18 hemagglutinin and 11 neuraminidase subtypes [3]. Almost all subtype combinations, including 1–9 NA and 1–16 HA, have been discovered in wild birds; thus, they pose the greatest risk when associated with AIV transmission [4].
South Korea has experienced severe consecutive outbreaks of highly pathogenic avian influenza viruses (HPAIVs) in poultry in 2003–2004, 2006–2007, 2008, 2010–2011, and 2014–2015 [5]. A HPAIV (H5N8) outbreak in South Korea in 2014 resulted in millions of culled domestic birds [6]. HPAI strains were found in 38 wild birds and 200 poultry farms, and their nucleotide sequences were similar to the H5N8 strains found in China in 2013 [7]. In November 2016, the HPAI strain, H5N6, was isolated from migratory waterfowl after severe outbreaks in domestic poultry in South Korea [8,9].
Implementing proper biosecurity management practices to control the incidence of HPAI in various poultry production systems is challenging because of the several potential risk factors affecting the transmission of AIVs [10]. Various risk factors have been linked to avian influenza (AI) outbreaks in previous studies. The key risk factors and inflow pathways associated with AI outbreaks were bird housing, sources from where poultry birds were purchased [11], poor sanitation of poultry farms [12], ‘foie gras’ production systems [13], lack of fumigation, no restrictions on visitor entry, no change of boots at the entry point to the farm, and no foot baths at the entry point [14].
Water bodies may function as sources of AIV, and waterfowl (particularly mallards) that act as reservoirs for subtypes H1–H12 are capable of asymptomatic transmission of AIV. On the other hand, water birds can also contaminate the water sources themselves, facilitating the spread of HPAI viruses through various routes [15]. Similarly, domestic ducks and geese also have an influential role in LPAI ecology and allow the asymptomatic transmission of these viruses. Ducks and geese may also play an important role in AIV circulation because they can spread the virus to other birds and animals [16]. When AIV is shed in the aquatic environment by wild birds through feces or other routes, a favorable environment is critical for its long-term survival [17].
Most HPAI (H5N8) cases were reported in the Jeollanam-do, Korea [6], while HPAI (H5N6) cases were mostly reported in the Gyeonggi-do province [18]. Identifying the potential role of water bodies in these HPAI outbreaks would be beneficial for preventing future virus outbreaks. The main purpose of the study was to formulate effective strategies for protection against infections in poultry farms via waterfowl, wild birds, rodents, and other small animals.
MATERIALS AND METHODS
Data collection
The role of different types of inland water bodies in AI epidemics was examined by conducting a 1:4 matched control study for HPAI H5N8 and H5N6 subtype outbreaks that occurred in South Korea in 2014 and 2016, respectively. Comprehensive information about the outbreaks was extracted from the “Epidemiology report for 2016–2017 outbreak of highly pathogenic avian influenza (HPAI) in the Republic of Korea” issued by the Animal and Plant Quarantine Agency (APQA) of South Korea.
Between January 2014 and March 2018, any poultry farm with birds confirmed positive for AI based on rapid antigen detection methods and polymerase chain reaction tests was selected as a “case farm.” Farms at which poultry birds showed no clinical signs, such as lethargy, neurological symptoms, green diarrhea, reduced scattering, and reduction in feed intake during the outbreak period, were selected as “controls.” Since the first HPAIV H5N1 infection at a chicken farm in 2003, the Republic of Korea implemented a very proactive surveillance program on poultry holdings across the country as a prevention and control strategy. For example, all poultry holdings mandate their poultry be tested before slaughter or admission to movements (poultry transportation). Moreover, domestic duck farms, which generally showed vague clinical signs, had bi-weekly surveillance tests during HPAI epidemics. Infected chickens showed marked clinical signs, such as mortality, decreased egg production, and a higher daily fatality rate, which led to the early detection and prevention of future spread.
Following the detection of poultry with the clinical signs of a HPAI infection by poultry owners, farmworkers, or veterinarians, the case must be reported to the APQA, Gimcheon, Korea, in passive surveillance, as required by the Act on Prevention of Contagious Animal Diseases. Government veterinarians visited the reported poultry farms to take samples from sick or dead birds, which were then tested for HPAIV infection [19,20]. If the suspected farm tested positive for HPAIV, it was considered an infected premise (IP). Furthermore, the poultry farms linked epidemiologically to infected premises, such as via sharing vehicles or located at a neighboring distance (e.g., 3 km), underwent HPAIV tests, followed by pre-emptive depopulation. During active surveillance, a depopulated farm that tested positive for HPAIV was designated as a positive premise [19,20]. In all these aspects, without surveillance reports, the control farms are highly unlikely to have infections. The nearest possible control farms located within a 3 km radius of the case farms were chosen to ensure that the study population was exposed to similar ecological risks, such as water bodies, wild birds, and rodents [21]. The study population consisted of ducks and chickens (broilers and layers). All case and control poultry farmhouses measuring > 464.5 m2 were selected randomly according to the matching criteria. According to the literature, increasing the ratio of controls to cases increases the statistical power [22]. Four control farms were chosen for each case farm.
There was no need for an ethical evaluation. Using Google Earth Pro, each case and control poultry farm was located with the help of its geographical coordinates. Visual monitoring was conducted to identify inland water bodies in the surrounding areas for each case and control farm. The measurement of the distance of the water bodies from the nearby poultry farms was performed using the ruler function of Google Earth Pro. With the help of the historical imagery function of Google Earth Pro, visual monitoring of the surrounding areas for each of the farms was conducted at a time point prior to the onset of both outbreaks. For the HPAI (H5N8) epidemic, the images of the surrounding areas of the poultry farms captured between January 2014 and April 2016 were visualized, while the images captured between November 2016 and March 2017 were explored for the HPAI (H5N6) epidemic. Some of the poultry farms were visited to ensure the availability of nearby inland water bodies.
Statistical analysis
By establishing thresholds, the distances of the inland water bodies from the poultry farms were transformed to a dichotomous variable to evaluate the presence of water bodies around the farms. For distances from 10 to 1,200 m, the threshold parameters were considered in 10-m incremental steps. The data were divided into groups for statistical analysis. For each case farm, a maximum of four matched control farms were assigned, and conditional logistic regression was applied using the “clogit” function from the R ‘survival’ package “version 3.1-12” [23]. The presence of inland water bodies around the farm was the predictor variable (independent variable). The occurrence of an HPAI outbreak on the farm was the response variable (dependent variable). All the statistical analysis was performed in statistical software R “i386 4.0.3 version” [24].
RESULTS
Population characteristics
Occurrence status of HPAI (H5N8) in Korea between 2014 and 2016
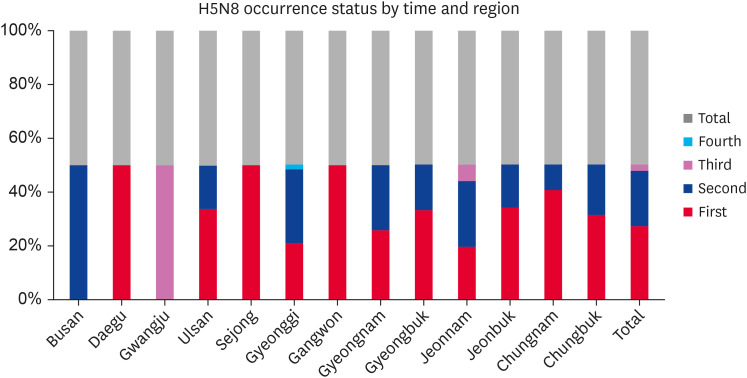
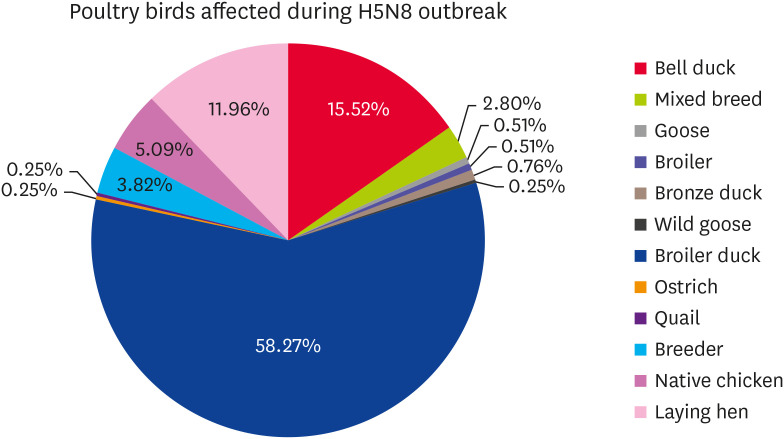
Since the first outbreak of AI subtype H5N8 in Jeollabuk-do province on January 16, 2014, 393 outbreaks have been reported in poultry farms nationwide. The outbreaks spread throughout the country, affecting 13 provinces and 59 cities, counties, and districts, particularly in the west coast provinces of Jeollanam-do and Jeollabuk-do. An analysis of the HPAI (H5N8) occurrence status according to the time and region is shown in Fig. 1, while Fig. 2 presents the types of birds in various poultry farms affected during the H5N8 outbreak season.
Fig. 1. Analysis of the HPAI (H5N8) occurrence status by time and region. Avian influenza outbreak cases recorded at different phases of the outbreak season during 2014–2016.
HPAI, highly pathogenic avian influenza.
Fig. 2. Percentages of different types of birds affected during the H5N8 outbreak season, ducks were the most affected species followed by laying hen.
Occurrence status of the H5N6 outbreak
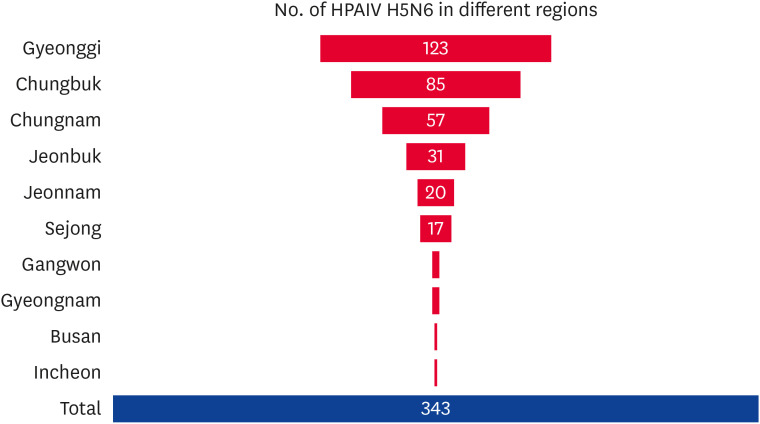
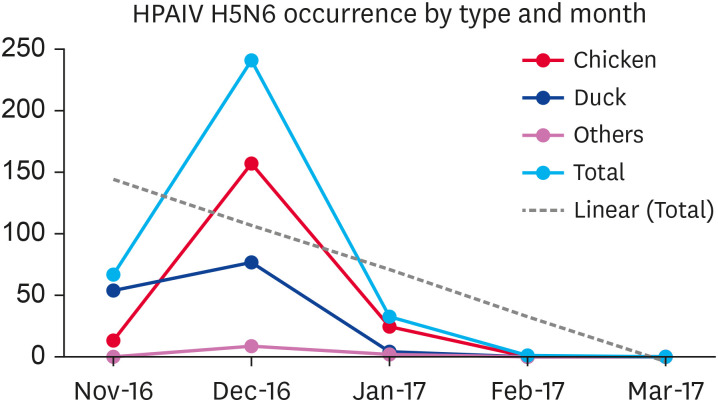
Between November 16, 2016, and March 3, 2017, 343 AI cases were reported in 107 days. Fig. 3 presents the H5N6 cases in different regions, while Fig. 4 shows the various poultry birds affected by type and month during the outbreak season.
Fig. 3. Number of cases of H5N6 outbreak in different regions. Gyeonggi region was the most affected followed by Chungbuk and Chungnam.
HPAIV, highly pathogenic avian influenza virus.
Fig. 4. Number of cases of different types of birds affected during each month of the H5N6 outbreak period. From mid-November to December end the cases were on the peak, then at the start of January a clear decline of cases can be seen.
HPAIV, highly pathogenic avian influenza virus.
During the HPAI (H5N8) epidemic from 2014 to 2016, 393 outbreaks were reported. Of these, 71 cases and 259 controls were studied. Three hundred and forty-three outbreaks were reported during the 2016–2017 HPAI (H5N6) epidemic. Of these, 80 cases and 286 controls were studied. During the AI outbreaks, the minimum and maximum distances of inland water bodies from case and control farms were as follows: HPAI (H5N8) outbreak: case farms, 4–1,358 m, and control farms, 8–2,086 m; HPAI (H5N6) outbreak: case farms, 4–1,330 m, and control farms, 10–3,001 m. Supplementary File 1 presents the distances of all the case and control farms from their neighboring water bodies during both outbreaks.
All HPAI (H5N8) outbreak cases occurred during the four phases of the outbreak season. Phase one of the outbreak lasted for 194 days from January 16, 2014, to July 29, 2014, and resulted in 212 outbreak cases. Phase two occurred from September 24, 2014, to June 10, 2015, lasting for 261 days and resulting in 162 cases. Phase three occurred from September 14, 2015, to November 11, 2015, lasting for 62 days and resulting in 17 cases. Phase four occurred from March 23, 2016, to April 5, 2016, lasting for 13 days and resulting in two cases. Supplementary File 2 shows the distances of the inland water bodies from case farms during phases 1 and 2.
The H5N6 avian influenza outbreak season started in November 2016 and continued until March 2017. During this season, 67 outbreak cases were recorded in November 2016, 243 in December 2016, 30 in January 2017, two in February 2017, and only one in March 2017. Supplementary File 3 shows the distances of the inland water bodies from the case farms during November and December 2016.
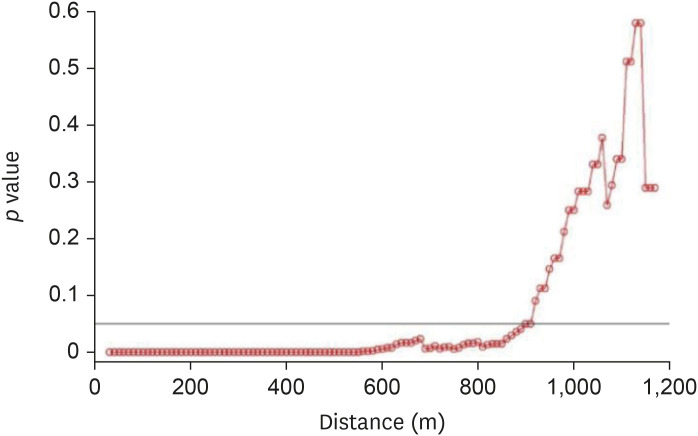
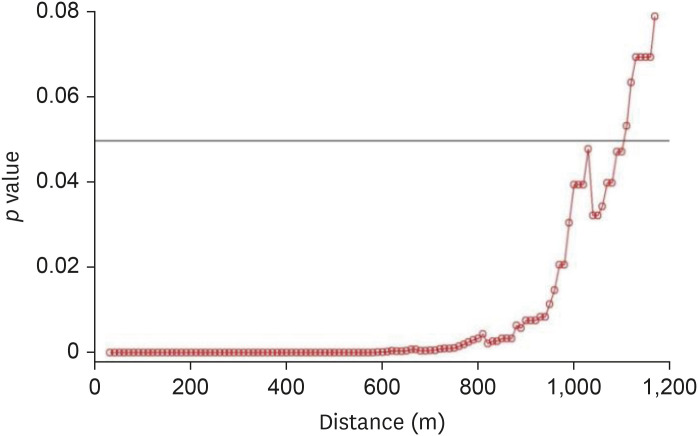
When p values < 0.05 were used to define significance [25], the results from both outbreak periods showed strong significance when neighboring inland water bodies were present within a 10–890-m distance from HPAI (H5N8) outbreak and within a 10–1,100-m distance from HPAI (H5N6) outbreak.
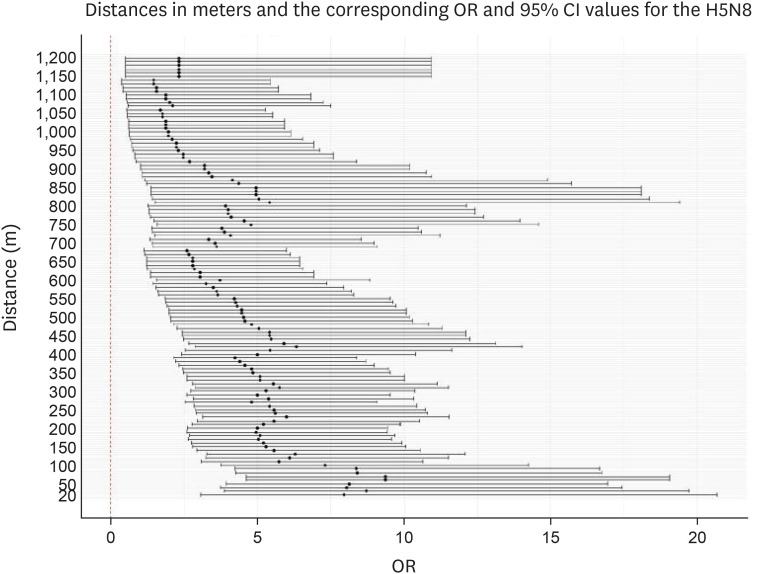
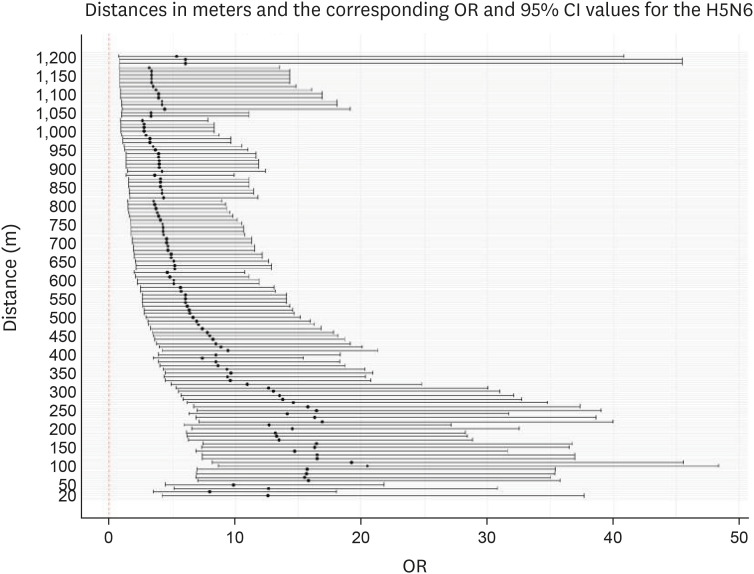
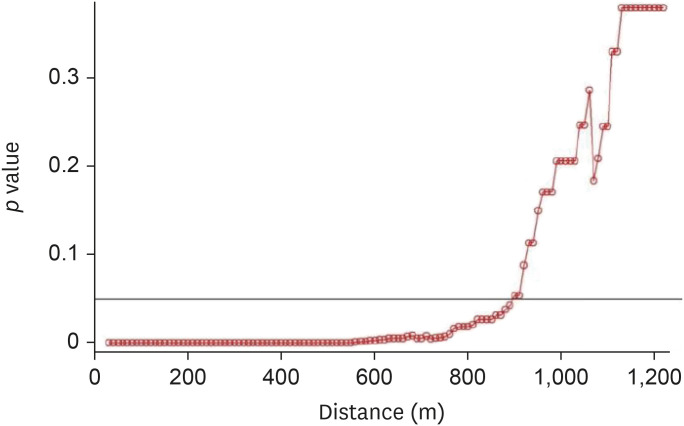
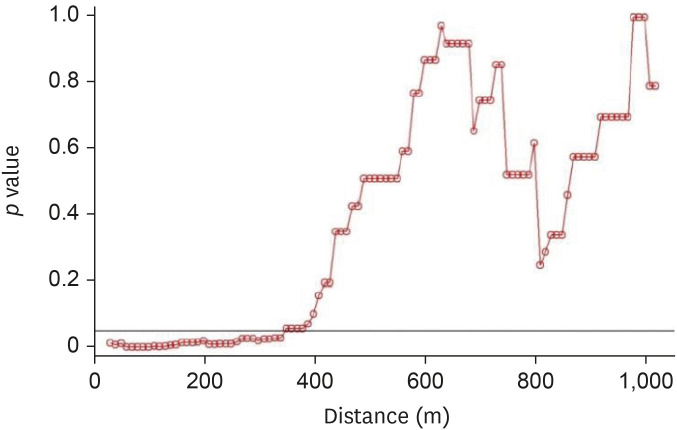
For the 2014–2016 seasonal outbreak, p-values were determined using the Wald test with respect to the distance from the poultry farms as per conditional logistic regression analysis, as shown in Fig. 5. Similarly, Fig. 6 presents the results for the 2016–2017 seasonal outbreak. The odds ratio (OR) and 95% confidence interval (CI) results for the 2014–2016 outbreak period are shown using a forest plot in Fig. 7, and those for the 2016–2017 outbreak period are depicted in Fig. 8.
Fig. 5. Results of Wald tests showing significance levels (p values) as per conditional logistic regression analysis for the 2014–2016 avian influenza outbreak. Neighboring inland water bodies at each distance from the poultry farm were evaluated using R software.
Fig. 6. Results of Wald tests showing significance levels (p values) as per conditional logistic regression analysis for the 2016–2017 avian influenza outbreak. Neighboring inland water bodies at each distance from the poultry farm were evaluated using R software.
Fig. 7. Forest plot showing ORs as black dots with corresponding 95% CI values as lines, as per conditional logistic regression analysis for the 2014–2016 avian influenza outbreak. Neighboring inland water bodies at each distance from the poultry farm were evaluated using R software.
OR, odds ratio; CI, confidence interval.
Fig. 8. Forest plot showing ORs as black dots with corresponding 95% CI values as lines, as per conditional logistic regression analysis for the 2016–2017 avian influenza outbreak. Neighboring inland water bodies at each distance from the poultry farm were evaluated using R software.
OR, odds ratio; CI, confidence interval.
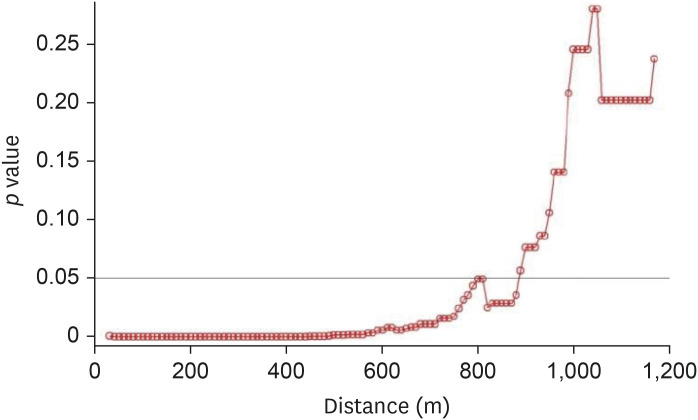
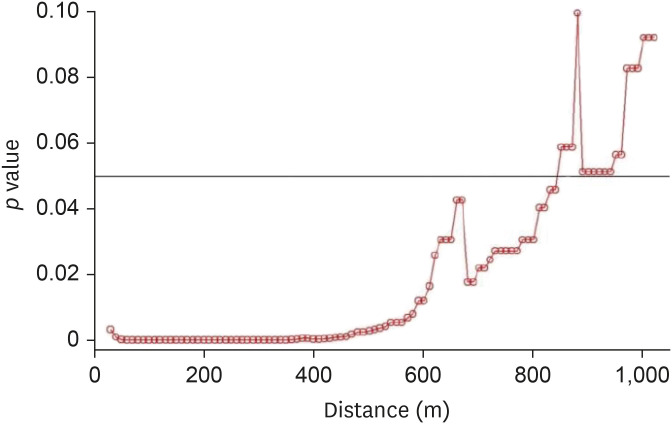
The inland water bodies had different degrees of impact on AI outbreak cases during the different phases of AI outbreaks. During phase 1 of the H5N8 epidemic, high significance was observed when the inland water bodies were present within a 10–890-m distance from the poultry farms (Fig. 9). During phase 2, a significant effect was observed when the water bodies were located within a 10–340-m distance (Fig. 10). During phases 3 and 4 of the epidemic, control farms were found in the vicinity of only two case farms, while no control farms could be found in the vicinity of the remaining case farms.
Fig. 9. Results of Wald tests showing significance levels (p values) as per conditional logistic regression analysis phase 1 of the 2014–2016 avian influenza outbreak. Neighboring inland water bodies at each distance from the poultry farm were evaluated using R software.
Fig. 10. Results of Wald tests showing significance levels (p values) as per conditional logistic regression analysis for phase 2 of the 2014–2016 avian influenza outbreak. Neighboring inland water bodies at each distance from the poultry farm were evaluated using R software.
During the H5N6 outbreak period, inland water bodies had significant effects on the HPAI epidemic during November and December 2016. The inland water bodies had a significant influence on HPAI outbreaks according to the distance from poultry farms. In November 2016, those present within a 10–880-m distance showed significant influence (Fig. 11), while in December 2016, those within a 10–840-m distance had significant influence (Fig. 12). Only a few HPAI outbreaks were observed during January, February, and March 2017.
Fig. 11. Results of Wald tests showing significance levels (p values) as per conditional logistic regression analysis for the month of November 2016, during the avian influenza outbreak. Neighboring inland water bodies at each distance from the poultry farm were evaluated using R software.
Fig. 12. Results of Wald tests showing significance levels (p values) as per conditional logistic regression analysis for the month of December 2016, during the avian influenza outbreak. Neighboring inland water bodies at each distance from the poultry farm were evaluated using R Software.
DISCUSSION
The study populations located near water bodies in Jeollanam-do and Gyeonggi-do provinces were significantly impacted during both outbreaks. Jeollanam-do province has densely populated agricultural lands with large water bodies [26]. According to a report issued by the APQA of South Korea, HPAI (H5N6) infections were reported primarily near destinations where migratory birds resided, such as rivers, wetlands, and agricultural lands in Gyeonggi-do province of the Republic of Korea. Rich, fertile agricultural land with abundant water resources provides the most conducive environment for the spread of AIV to wild birds and domestic poultry farms [27]. Wild waterfowl, rodents, and humans can mechanically transmit AIV infections to nearby poultry farms.
Phase 1 of the HPAI (H5N8) epidemic began at a large wintering location for wild migratory ducks and spread to neighboring duck farms [7]. Ducks and other waterfowl allowed to graze freely may contaminate the water in the surrounding areas [28]. Thus, these migratory waterfowl could have contaminated the surrounding areas and caused HPAI outbreaks in the adjacent duck farms. Lee et al. [29] reported that during the HPAI (H5N8) epidemic from 2014 to 2016, an extension of the habitat range of migratory and non-migratory sub-populations of wild migratory waterfowl was related to the highest risk of disease outbreaks. The second phase of the HPAI (H5N8) epidemic began during the autumn migration period of wild migrating waterfowl and resulted in HPAI outbreaks in adjacent duck farms. According to the Korean APQA, during this phase, fewer duck farms were impacted than that in the first phase of the HPAI epidemic. This may be because, after the phase 1 epidemic, the biosecurity precautions were implemented at a higher level during the autumn migration period. A comparison of phase 1 with phase 2 showed that the inland water bodies were more associated with HPAI outbreaks during phase 1 than during phase 2 of the outbreak season. Because the Korean APQA reported that duck farms were the first to be affected during both phases of the outbreak season, it is reasonable to conclude that water bodies hosting wild migrating waterfowl pose a greater risk to neighboring duck farms than to chickens or other bird farms. Chicken farms may be the most affected regarding inter-farm transmission. Duck farms may also be affected because confirmation of HPAI (H5N8) infections in ducks is delayed because of the mild clinical symptoms in these birds. On the other hand, the significance of migrating waterfowl in the poultry farm transmission chain remains debatable [30].
According to the Korean APQA, the HPAI (H5N6) outbreak season began in the last month of the autumn migration period of migratory waterfowl, and most outbreaks occurred during November and December 2016. Strong significant associations were observed between the distance to inland water bodies and the occurrence of HPAI outbreaks during November and December. Duck and chicken farms were affected during both months of the outbreak season. During the HPAI (H5N6) epidemic, inland water bodies hosting wild migratory waterfowl affected the nearby duck farms and chicken farms, whereas during the HPAI (H5N8) outbreak, duck farms were primarily affected. Compared to other HPAI strains, the HPAI H5N8 strain evolving in the Republic of Korea is believed to be more adapted to the “Anseriformes” order, causing reduced mortality but higher transmissibility in ducks [31]. Water has a diverse range of physicochemical properties [32] and a significant impact on the survival of AIV [33].
Consistent H5 HPAI outbreaks have swept the country and been detected in various wild birds. The western part of South Korea is home to an abundance of poultry farms and migratory bird habitats, including mallards. Through active and passive surveillance, 398 HPAI (H5N8) viruses were isolated after detection in poultry, while 58 were isolated from wild birds during the four consecutive HPAI (H5N8) outbreaks in 2014 and 2016 [34]. The isolation of viruses from wild birds indicates the presence of AI circulation among water bodies and wild birds, as surface water act as an important source of AIV infections in domestic poultry [35]. Waterfowl relocating to surrounding areas of inland waters may contaminate the neighboring farms or cause environmental contamination near water bodies. In addition, humans and small animals, such as rodents, may transmit the viruses mechanically after coming into contact with contaminated environments [36]. Furthermore, domestic cats and dogs can become infected if they consume infected chickens. Humans who come into direct contact with cats and dogs may also be infected because cats and dogs are at risk of contracting HPAI (H5N1) virus infections [37].
The study findings are in accordance with those of a study conducted in Japan. Shimizu et al. [38] reported that inland bodies of water could increase the risk of water contamination in nearby poultry farms. Small animals, such as rodents, can be found both inside and in areas surrounding poultry houses and may share the habitat with aquatic birds that freely move in and out of the poultry environment. Velkers et al. [39] indicated that rodents might play a role in AIV transmission from one poultry house to another and from wild birds to poultry. Inland water bodies near poultry farms have been associated with an increased risk of HPAIV epidemics because water bodies provide an ideal environment for the spread of infection among various wild animals, including waterfowl [40].
This study had some limitations. The physicochemical properties of water bodies, such as streams, rivers, ponds, lakes, and rivers, were not investigated. Furthermore, a consistent evaluation of the inland water bodies that were exposed to wild birds and animals was not performed. Similarly, no investigation was conducted regarding the presence of rodents within or in areas surrounding poultry houses. As per the control strategy of ring culling implemented by the Republic of Korea, the HPAI infected poultry farms and geographically neighboring farms within a 3 km radius were immediately depopulated [19,20]. An in-depth analysis of different water bodies, including the physical and chemical properties, is recommended. Such analysis should be conducted based on water samples collected from various water bodies located around the affected areas during AIV outbreaks or bird migration periods. These practices will assist in identifying the types of inland water bodies that provide an appropriate aquatic environment for the spread of HPAI.
In conclusion, the presence of water bodies might play a vital role in HPAI outbreaks at poultry farms, and HPAIV transmission is associated with direct contact with nearby water bodies or areas around water bodies that are visited by waterfowl and free-grazing ducks. Workers and pet animals at poultry farms may be considered high-risk pathways for HPAIV transmission to poultry. If the physicochemical properties of the water bodies were suitable for HPAIV survival for a longer period, there would undoubtedly be a higher risk. These findings suggest that birds in poultry farms near water bodies may be infected by HPAIV more frequently because these water bodies and surrounding areas are often contaminated with HPAIV owing to contact with free-roaming wild birds and small animals. Therefore, poultry farms should strictly adhere to all applicable biosecurity measures to avoid direct or indirect contact with the surrounding inland water bodies. Such measures include using nets on farms, barricading all entries to farm premises, properly fumigating and disinfecting areas inside and outside farms (particularly areas surrounding water bodies), covering stored water, properly disinfecting nearby stagnant waters, and restricting the entry of visitors and vehicles. Most importantly, personal sanitation should be practiced regularly when entering a poultry farm. During the migration of wild birds, the government should spray inland waters surrounding areas with proper disinfection to inactivate the AIV on a large scale.
ACKNOWLEDGEMENTS
The authors wish to thank all the members of the Animal and Plant Quarantine Agency, Korea for providing the data to perform the work. The cooperation of poultry farms workers in collecting additional information is also appreciated.
Footnotes
Funding: This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) grant funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (No. 1545026102).
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Lee J, Yoo D, Suh S , Lee CM.
- Data curation: Ahmad S, Koh K, Yoo D.
- Formal analysis: Ahmad S, Koh K.
- Investigation: Ahmad S, Koh K, Yoo D, Lee J.
- Methodology: Ahmad S, Koh K.
- Resources: Ahmad S, Koh K, Yoo D, Suh S , Lee CM.
- Software: Ahmad S, Koh K.
- Supervision: Lee J, Suh S , Lee CM.
- Validation: Ahmad S, Koh K, Yoo D, Lee J, Suh S, Lee CM.
- Visualization: Ahmad S, Koh K.
- Writing - original draft: Ahmad S, Koh K.
- Writing - review & editing: Ahmad S, Koh K, Yoo D, Lee J, Suh S, Lee CM.
SUPPLEMENTARY MATERIALS
Distances of H5N8 and H5N6.
Distances of H5N8: phases 1 and 2.
Distances of H5N6: phases 1 and 2.
References
- 1.Gharieb R, Mohamed M, Khalil A, Ali A. Influenza A viruses in birds and humans: Prevalence, molecular characterization, zoonotic significance and risk factors’ assessment in poultry farms. Comp Immunol Microbiol Infect Dis. 2019;63:51–57. doi: 10.1016/j.cimid.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Chatziprodromidou IP, Arvanitidou M, Guitian J, Apostolou T, Vantarakis G, Vantarakis A. Global avian influenza outbreaks 2010-2016: a systematic review of their distribution, avian species and virus subtype. Syst Rev. 2018;7(1):17. doi: 10.1186/s13643-018-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tahir MF, Abbas MA, Ghafoor T, Dil S, Shahid MA, Bullo MM, et al. Seroprevalence and risk factors of avian influenza H9 virus among poultry professionals in Rawalpindi, Pakistan. J Infect Public Health. 2019;12(4):482–485. doi: 10.1016/j.jiph.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Bouwstra R, Gonzales JL, de Wit S, Stahl J, Fouchier RA, Elbers AR. Risk for low pathogenicity avian influenza virus on poultry farms, the Netherlands, 2007–2013. Emerg Infect Dis. 2017;23(9):1510–1516. doi: 10.3201/eid2309.170276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mo IP, Bae YJ, Lee SB, Mo JS, Oh KH, Shin JH, et al. Review of avian influenza outbreaks in South Korea from 1996 to 2014. Avian Dis. 2016;60(1) Suppl:172–177. doi: 10.1637/11095-041715-Review. [DOI] [PubMed] [Google Scholar]
- 6.Yoon H, Moon OK, Jeong W, Choi J, Kang YM, Ahn HY, et al. H5N8 highly pathogenic avian influenza in the Republic of Korea: epidemiology during the first wave, from January through July 2014. Osong Public Health Res Perspect. 2015;6(2):106–111. doi: 10.1016/j.phrp.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong J, Kang HM, Lee EK, Song BM, Kwon YK, Kim HR, et al. Highly pathogenic avian influenza virus (H5N8) in domestic poultry and its relationship with migratory birds in South Korea during 2014. Vet Microbiol. 2014;173(3-4):249–257. doi: 10.1016/j.vetmic.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Kwon JH, Lee DH, Jeong JH, Yuk SS, Erdene-Ochir TO, Noh JY, et al. Isolation of an H5N8 highly pathogenic avian influenza virus strain from wild birds in Seoul, a highly urbanized area in South Korea. J Wildl Dis. 2017;53(3):630–635. doi: 10.7589/2016-07-161. [DOI] [PubMed] [Google Scholar]
- 9.Lee EK, Lee YN, Kye SJ, Lewis NS, Brown IH, Sagong M, et al. Characterization of a novel reassortant H5N6 highly pathogenic avian influenza virus clade 2.3.4.4 in Korea, 2017. Emerg Microbes Infect. 2018;7(1):103. doi: 10.1038/s41426-018-0104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aditama TY, Samaan G, Kusriastuti R, Sampurno OD, Purba W, Misriyah, et al. Avian influenza H5N1 transmission in households, Indonesia. PLoS One. 2012;7(1):e29971. doi: 10.1371/journal.pone.0029971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudhry M, Rashid HB, Angot A, Thrusfield M, Bronsvoort BM, Capua I, et al. Risk factors for avian influenza H9 Infection of chickens in live bird retail stalls of Lahore district, Pakistan 2009–2010. Sci Rep. 2018;8(1):5634. doi: 10.1038/s41598-018-23895-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang XX, Cheng W, Yu Z, Liu SL, Mao HY, Chen EF. Risk factors for avian influenza virus in backyard poultry flocks and environments in Zhejiang Province, China: a cross-sectional study. Infect Dis Poverty. 2018;7(1):65. doi: 10.1186/s40249-018-0445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guinat C, Artois J, Bronner A, Guérin JL, Gilbert M, Paul MC. Duck production systems and highly pathogenic avian influenza H5N8 in France, 2016-2017. Sci Rep. 2019;9(1):6177. doi: 10.1038/s41598-019-42607-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gompo TR, Shah BR, Karki S, Koirala P, Maharjan M, Bhatt DD. Risk factors associated with avian influenza subtype H9 outbreaks in poultry farms in Kathmandu valley, Nepal. PLoS One. 2020;15(4):e0223550. doi: 10.1371/journal.pone.0223550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Achenbach JE, Bowen RA. Transmission of avian influenza A viruses among species in an artificial barnyard. PLoS One. 2011;6(3):e17643. doi: 10.1371/journal.pone.0017643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Cui P, Zeng X, Jiang Y, Li Y, Yang J, et al. Characterization of avian influenza H5N3 reassortants isolated from migratory waterfowl and domestic ducks in China from 2015 to 2018. Transbound Emerg Dis. 2019;66(6):2605–2610. doi: 10.1111/tbed.13324. [DOI] [PubMed] [Google Scholar]
- 17.Germeraad EA, Elbers AR, de Bruijn ND, Heutink R, van Voorst W, Hakze-van der Honing R, et al. Detection of low pathogenic avian influenza virus subtype H10N7 in poultry and environmental water samples during a clinical outbreak in commercial free-range layers, Netherlands 2017. Front Vet Sci. 2020;7:237. doi: 10.3389/fvets.2020.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryu S, Lim JS, Cowling BJ, Chun BC. Low risk of avian influenza A (H5N6) transmission to depopulation workers in Korea. Influenza Other Respi Viruses. 2018;12(3):412–415. doi: 10.1111/irv.12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim WH, Bae SH, Cho S. Spatiotemporal dynamics of highly pathogenic avian influenza subtype H5N8 in poultry farms, South Korea. Viruses. 2021;13(2):274. doi: 10.3390/v13020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoo DS, Chun B, Min KD, Lim JS, Moon OK, Lee KN. Elucidating the local transmission dynamics of highly pathogenic avian influenza H5N6 in the Republic of Korea by integrating phylogenetic information. Pathogens. 2021;10(6):691. doi: 10.3390/pathogens10060691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim WH, An JU, Kim J, Moon OK, Bae SH, Bender JB, et al. Risk factors associated with highly pathogenic avian influenza subtype H5N8 outbreaks on broiler duck farms in South Korea. Transbound Emerg Dis. 2018;65(5):1329–1338. doi: 10.1111/tbed.12882. [DOI] [PubMed] [Google Scholar]
- 22.Kang MS, Choi SH, Koh IS. The effect of increasing control-to-case ratio on statistical power in a simulated case-control SNP association study. Genomics Inform. 2009;7(3):148–151. [Google Scholar]
- 23.Therneau T. A Package for Survival Analysis in R. R Package Version 3.1-12. New York: Springer; 2020. [Google Scholar]
- 24.R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 25.Andrade C. The P value and statistical significance: misunderstandings, explanations, challenges, and alternatives. Indian J Psychol Med. 2019;41(3):210–215. doi: 10.4103/IJPSYM.IJPSYM_193_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang HS, Yang JC, Oh SH, Lee YM, Chang KS. A study on the flora of 15 islands in the western sea of Jeollanamdo province, Korea. J Asia Pac Biodivers. 2013;6(2):281–310. [Google Scholar]
- 27.Papp Z, Clark RG, Parmley EJ, Leighton FA, Waldner C, Soos C. The ecology of avian influenza viruses in wild dabbling ducks (Anas spp.) in Canada. PLoS One. 2017;12(5):e0176297. doi: 10.1371/journal.pone.0176297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prosser DJ, Palm EC, Takekawa JY, Zhao D, Xiao X, Li P, et al. Movement analysis of free-grazing domestic ducks in Poyang Lake, China: a disease connection. Int J Geogr Inf Sci. 2016;30(5):869–880. doi: 10.1080/13658816.2015.1065496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee K, Yu D, Martínez-López B, Yoon H, Kang SI, Hong SK, et al. Fine-scale tracking of wild waterfowl and their impact on highly pathogenic avian influenza outbreaks in the Republic of Korea, 2014-2015. Sci Rep. 2020;10(1):18631. doi: 10.1038/s41598-020-75698-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Dijk JGB, Kleyheeg E, Soons MB, Nolet BA, Fouchier RAM, Klaassen M. Weak negative associations between avian influenza virus infection and movement behaviour in a key host species, the mallard Anas platyrhynchos . Oikos. 2015;124(10):1293–1303. [Google Scholar]
- 31.Kang HM, Lee EK, Song BM, Jeong J, Choi JG, Jeong J, et al. Novel reassortant influenza A(H5N8) viruses among inoculated domestic and wild ducks, South Korea, 2014. Emerg Infect Dis. 2015;21(2):298–304. doi: 10.3201/eid2102.141268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mihai ME, Tecu C, Ivanciuc AE, Necula G, Lupulescu E, Onu A. Survival of H5N1 influenza virus in water and its inactivation by chemical methods. Roum Arch Microbiol Immunol. 2011;70(2):78–84. [PubMed] [Google Scholar]
- 33.Pinon A, Vialette M. Survival of viruses in water. Intervirology. 2018;61(5):214–222. doi: 10.1159/000484899. [DOI] [PubMed] [Google Scholar]
- 34.Song BM, Lee EK, Lee YN, Heo GB, Lee HS, Lee YJ. Phylogeographical characterization of H5N8 viruses isolated from poultry and wild birds during 2014-2016 in South Korea. J Vet Sci. 2017;18(1):89–94. doi: 10.4142/jvs.2017.18.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinshaw VS, Webster RG, Turner B. Water-bone transmission of influenza A viruses? Intervirology. 1979;11(1):66–68. doi: 10.1159/000149014. [DOI] [PubMed] [Google Scholar]
- 36.Fujimoto Y, Usui T, Ito H, Ono E, Ito T. Susceptibility of wild passerines to subtype H5N1 highly pathogenic avian influenza viruses. Avian Pathol. 2015;44(4):243–247. doi: 10.1080/03079457.2015.1043235. [DOI] [PubMed] [Google Scholar]
- 37.Butler D. Can cats spread avian flu? Nature. 2006;440(7081):135–136. doi: 10.1038/440135a. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu Y, Hayama Y, Yamamoto T, Murai K, Tsutsui T. Matched case-control study of the influence of inland waters surrounding poultry farms on avian influenza outbreaks in Japan. Sci Rep. 2018;8(1):3306. doi: 10.1038/s41598-018-21695-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Velkers FC, Blokhuis SJ, Veldhuis Kroeze EJ, Burt SA. The role of rodents in avian influenza outbreaks in poultry farms: a review. Vet Q. 2017;37(1):182–194. doi: 10.1080/01652176.2017.1325537. [DOI] [PubMed] [Google Scholar]
- 40.Fang LQ, de Vlas SJ, Liang S, Looman CW, Gong P, Xu B, et al. Environmental factors contributing to the spread of H5N1 avian influenza in mainland China. PLoS One. 2008;3(5):e2268. doi: 10.1371/journal.pone.0002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distances of H5N8 and H5N6.
Distances of H5N8: phases 1 and 2.
Distances of H5N6: phases 1 and 2.