Abstract
Chromosome 16 is one of the gene-rich chromosomes; however, approximately 10% of the chromosome 16 sequence is composed of segmental copies, which renders this chromosome instable and predisposes it to rearrangements via frequent nonallelic homologous recombination. Microarray technologies have enabled the analysis of copy number variations (CNV), which may be associated with the risk of developing complex diseases. Through comparative genomic hybridisation in 1,298 patients, we detected 18 cases with chromosome 16 CNV. We identified 2recurrent CNV regions, including 1 at 16p13.11 in 4 patients and another at 16p11.2 in 7 patients. We also detected atypical chromosome 16 rearrangements in 7 patients. Furthermore, we noted an increased frequency of co-occurring genomic changes, supporting the two-hit hypothesis to explain the phenotypic variability in the clinical presentation of CNV syndromes. Our findings can contribute to the creation of a chromosome 16 disease map based on regions that may be associated with disease development.
Keywords: Array CGH, Clinical heterogeneity, Copy number variation, Microdeletion syndrome, Microduplication syndrome, Molecular cytogenetics
Introduction
Chromosome 16, with a total size of 88.7 Mb, harbours 880 protein-coding genes, 19 tRNA genes, and 341 pseudogenes. It is one of the chromosomes with the highest degree of segregation distortion (SD) among human autosomes. As many as 91 genes in chromosome 16 are located in the regions of SD, which are prone to instability. Specifically, SDs clustered along the “p” arm of the chromosome and those within the pericentromeric region (16p11) represent the largest region of chromosomal replication, often mapped to the pericentromeric regions of other chromosomes.
Interestingly, 9.89% of the chromosome 16 sequence is composed of segmental copies (≥90% sequence identity and ≥1 kb length) and a similar portion corresponds to unsecured DNA. According to the “Guide to the Human Genome” (http://www.cshlp.org/ghg5_all/section/dna.shtml), 12.7% of the chromosome 16 region is not sequenced [Walters et al., 2010].
The 16p11.2 locus encompasses several genomic structural variants, including a repetitive interstitial deletion in the ∼600 kb region with 29 genes. At least 17 of these 29 genes present neuronal expression, and a bioinformatic analysis revealed that at least 12 of these genes are involved in single interaction networks [Kumar et al., 2008; Jacquemont et al., 2011; Walsh et al., 2011; Golzio et al., 2012]. The prevalence of this deletion between break points 4 and 5 (breakpoints 4–5, BP4–BP5, 29.6–30.2 Mb, Hg19) in the population is 1/2,000. Studies in model organisms have indicated that specific genes in critical regions are associated with the development of specific phenotypes. For instance, in a previous study using zebrafish, all unique genes in a critical region were systematically inactivated to determine the genetic basis for the development of the macrocephaly phenotype associated with the 16p11.2 deletion. The authors observed that KCTD13 knockout was responsible for the development of the macrocephaly phenotype in zebrafish. Moreover, other recent studies found that the coinheritance of a rare null variant and a hypomorphic TBX6 allele was responsible for the development of congenital scoliosis in 11% of the evaluated cases [Marshall et al., 2008; Wu et al., 2015].
Based on the above reports, deletion or duplication of genes in the critical regions or a combination of noncoding variants in these regions may be responsible for the development of specific phenotypes.
Deletions in the 16p11.2 genomic region are associated with the risk of neurodevelopmental disorders and autism spectrum disorders (ASDs), and 16p11.2 is the most frequently reported single locus. Moreover, this deletion was reported to increase the risk of morbid obesity by 43-fold, predisposing the individuals to a highly penetrating form of obesity [Sebat et al., 2007; Weiss et al., 2008; Jacquemont et al., 2011; Sanders et al., 2011]. In addition, this deletion is also associated with an increased head circumference. The neurological phenotype of the 16p11.2 deletion is characterised by extensive speech articulation abnormalities, hypotonia of the limb and trunk with hyporeflexia, abnormal agility, sacral dimples, seizures or epilepsy, large head size or macrocephaly, and Chiari malformation type I or cerebellar tonsillar ectopia. In those carrying a 16p11.2 duplication, speech articulation abnormalities, hypotonia, abnormal agility, sacral dimples, and seizures or epilepsy with pronounced hyperreflexia have been observed very frequently, whereas hyporeflexia, motion tremors, small head size or microcephaly, cerebral white matter or corpus callosum abnormalities, and ventricular enlargement have been observed relatively less frequently [Bochukova et al., 2010]. Furthermore, carriers of a reciprocal duplication in this region were underweight and were at a high risk of developing microcephaly [Steinman et al., 2016].
Given the diversity of reported clinical features, the effects of deletions in the 16p11.2 regions on neurocognitive development and behaviour must be systematically assessed and asymptomatic, but not comprehensively evaluated, parents must be reported. In the present study, we examined the genotype-phenotype range of chromosome 16 rearrangements. We predicted that the 16p11.2 deletion affects cognitive function, behaviour, growth, and body mass index [Martin et al., 2004; Bijlsma et al., 2009; Bardakjian et al., 2010; Fernandez et al., 2010; Shinawi et al., 2010]. While the link between mutations at 16p11.2 and high risk of neurodevelopmental disorders is well known, the possible involvement of other factors, including the genetic background, which are the key determents of the severity of phenotypic outcome, cannot be ruled out.
To this end, we explored the genetic basis of the phenotypic variability among individuals presenting copy number variation (CNV) in chromosome 16p/q. We hypothesised that differences in the genetic background or CNV contribute to phenotypic variability and disease severity.
Materials and Methods
Karyotypes of all patients were determined through the analysis of 20 Giemsa-stained metaphase slides prepared from standard 72 h peripheral blood lymphocyte cultures. Karyotypes were reconstructed following the 2016 edition of the International System for Human Cytogenomic Nomenclature [ISCN, 2016].
Genomic copy number analysis was performed using array CGH with the Agilent Human Genome CGH + SNP Microarray 4 × 180K Kit (Sure Print G3; Agilent Technologies, Santa Clara, CA, USA; International Standards for Cytogenomic Arrays). The array CGH results were validated using FISH.
Molecular karyotypes were expressed according to ISCN 2016. The identified CNVs were classified according to the previously published criteria [Ullmann et al., 2007; Tropeano et al., 2013] and through comparison with the international databases.
A CNV was classified as pathogenic when it was frequently reported as such in the literature and it overlapped genomic imbalance defined in the CNV database for patients with intellectual or developmental disabilities, ASDs, or multiple congenital anomalies. A CNV was classified as likely pathogenic when it was reported in one or more affected individuals with a similar phenotype and when it only partially overlapped a known region with a microdeletion or duplication associated with a CNV syndrome or harboured dose-sensitive genes associated with a specific pathology. A CNV was classified as benign when it was reported in >1% of the normal individuals and identified in the Database of Genomic Variants (DGV).
It is considered benign when a CNV is not listed in databases, is inherited from a phenotypically normal parent, and does not contain genes or overlap with intronic regions. A CNV was classified as a variant of uncertain significance when it had never been reported before, contained a single gene or genes with unknown function, or contained a gene that did not cause a specific pathology in the patient.
Results
Chromosome 16 CNVs in the Cohort
We included 1,298 patients. The most frequent indications among postnatal cases were unexplained intellectual disability (ID) and/or multiple congenital anomalies. Detailed phenotypic findings are included in the online supplementary material (for online suppl. material, see www.karger.com/doi/10.1159/000517762). We detected 18 cases with chromosome 16 CNVs (10 males and 8 females) from the screening of 1,298 participants (1.38%). Table 1 provides patient information and the size and characteristics of each CNV.
Table 1.
List of CNVs and distribution of referral reasons in our cohort
| Case ID | Gender | Cytoband | Coordinates (hg19/Build37) | Size, kb | CNV | Phenotype |
|---|---|---|---|---|---|---|
| 1 | M | p11.2 | 29,656,684–30,190,568 | 534 | Deletion | Seizures, generalized anxiety disorder, manic, retromicrognathia, broad nasal bridge, a history of bronchitis, light-colored skin spotting in the sun, nasolabial sulcus, walking at 18 months |
|
| ||||||
| 2 | M | p11.2 | 29,656,684–30,190,568 | 534 | Deletion | Decrease in sperm motilation, speech articulation, primary infertility |
|
| ||||||
| 3 | F | p11.2 | 29,656,684–30,190,568 | 534 | Deletion | Micrognathia, albinism, and a low hairline |
|
| ||||||
| 4 | M | p11.2 | 29,656,684–30,190,568 | 534 | Deletion | Syndactyly |
|
| ||||||
| 5 | F | p11.2 | 29,656,684–30,190,568 | 534 | Deletion | Growth retardation, atypical autism, psychomotor delay, loss of speech, and epilepsy |
|
| ||||||
| 6 | M | q22.1q22.2 | 68,377,368–72,622,855 | 4,245,488 | Deletion | Micrognathia, large and low-set ears, high-arched palate, straight long nose, overhanging tip of nose, narrow forehead, front fontanel closed, ascending testis, neuromotor growth retardation |
|
| ||||||
| 7 | F | p11.2 | 29,656,684–30,190,568 | 534 | Duplication | Wide and depressed nasal bridge, epicanthus, high palate, facial asymmetry, frontal bossing and low-set protruding ears, retrognathia, frontal fontanel open and wide, bilateral internal epicantal folds, low posterior hairline, short neck, bilateral palmar crease in both hands, toes arise from a single root, ear cartilage anomaly, toenails hyperconvex and cleft lip |
|
| ||||||
| 8 | M | p11.2 | 29,824,794–29,031,059 | 206 | Duplication | Micrognathia, retromicrognathia, dysplastic left ear, V-shaped facial structure, atypical autism, ADHD |
|
| ||||||
| 9 | M | q24.1 | 84,473,191–84,567,551 | 94,361 | Duplication | Cleft palate lip, corpus callosum agenesis, asymmetrically small for gestational age |
|
| ||||||
| 10 | F | p13.11p12.3 | 15,404,452–18,631,981 | 3,228 | Duplication | Craniosynostosis |
|
| ||||||
| 11 | F | p13.11 | 14,910,205–16,276,115 | 1,366 | Duplication | Recurrent pregnancy loss |
|
| ||||||
| 12 | M | p13.11 | 15,125,829–16,229,700 | ˜1.103 | Duplication | Autism spectrum disorder |
|
| ||||||
| 13 | M | p13.11 | 14,910,205–16,229,700 | ˜1.319 | Duplication | Recurrent pregnancy loss |
|
| ||||||
| 14 | M | q24.3 | 89,824,688–89,874,708 | ˜50 | Deletion | Epilepsy and growth retardation |
|
| ||||||
| 15 | F | q24.3 | 89,824,688–89,874,708 | ˜50 | Deletion | Normal (patient #14's mother) |
|
| ||||||
| 16 | M | q24.3 | 89,520,504–89,732,173 | ˜211 | Duplication | High risk of double screening test and an increase in nuchal tranlucency |
|
| ||||||
| 17 | M | q24.3 | 89,520,504–89,732,173 | ˜211 | Duplication | Normal |
|
| ||||||
| 18 | F | q23.2q24.3 | 81,491,655–90,148,393 | 8,657 | Duplication | Intellectual disability |
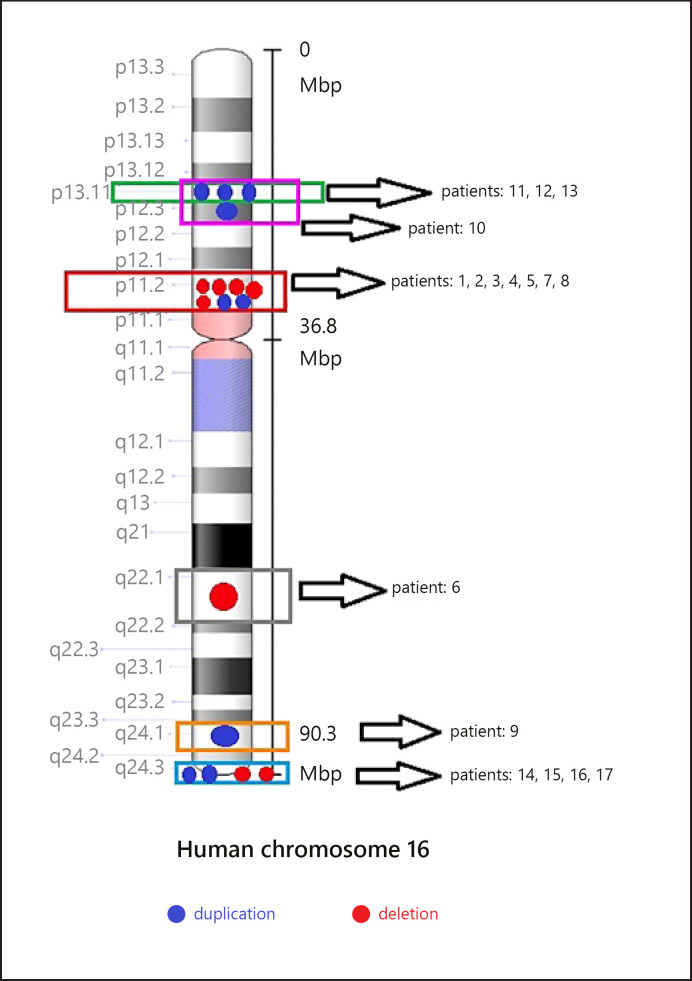
We identified 2 common regions with CNVs, namely 16p13.11 in 4 patients and 16p11.2 in 7 patients. We also detected atypical rearrangements of chromosome 16 in 7 patients (Fig. 1).
Fig. 1.
Distribution of CNVs among chromosome 16.
16p13.11 duplication is a common aberration. In the present cohort, the clinical features of the four patients carrying this aberration varied in general, with phenotypes ranging from ASD to craniosynostosis. The overall size of the 16p13.11 duplications was 1,800 kb on average. However, the phenotypic effect of the 16p13.11 duplication could not be inferred with clear clinical findings.
Recurrent Microdeletion/Duplication of 16p11.2
We detected 2 duplications and 5 microdeletions in the distal chromosomal region of 534 kb at 16p11.2 in 7 patients (4 males and 3 females). The region included 9 OMIM genes (ATXN2L, TUFM, SH2B1, ATP2A1, RABEP2, CD19, NFATC2IP, SPNS1, and LAT). All microdeletions and duplications were of de novo origin. All boundaries of break points overlapped blocks of segmental duplications, indicating mechanisms involving nonallelic homologous recombination.
Atypical Rearrangements in Chromosome 16
We identified atypical rearrangements of chromosome 16 in 7 patients (4 males and 3 females). In patient #18, a de novo duplication of 8,657 kb was detected at 16q23.2q24.3; this patient presented a very complex phenotype with growth delay, microcephaly, and severe ID. We detected a familial deletion at 16q24.3 in patient #14 and the mother (#15). We detected a paternally inherited familial duplication at 16q21.1q24.3 in patient #16 and his father. Finally, in patients #9 and #6, we detected a de novo microduplication at 16q24.1 and a de novo deletion of 4.2 Mb at 16q22.1q22.2, respectively (Table 1).
Discussion
In the present study, we confirmed the importance of pathogenic CNVs in chromosome 16 in determining the phenotype of patients. We identified 18 patients carrying 8 microdeletions and 10 microduplications during diagnostic investigation of 23 postnatal cases.
The association of the 2 recurrent CNVs (16p13.11 and 16p11.2) detected in our cohort with increased risk of neurodevelopmental disorders is well known [Nuttle et al., 2016]. The common denominator was that neurodevelopmental disorders were present to varying degrees in all postnatal cases. Moreover, the reported CNVs were similar to those detected in the 11 affected males and 7 affected females in the present study. Additionally, our findings support the speculations regarding the male-biased autosomal effects of chromosome 16 CNVs [Tropeano et al., 2013], and this trend remained true even when considering the de novo events alone (8 males and 6 females).
In our cohort, 7 of the detected CNVs were deletions and 7 were duplications. Although the numbers presented here are very low, these data prove that duplications are generally associated with benign features, with low penetrance in phenotypes. Among the 14 cases with de novo CNVs, the frequency of deletions and duplications was the same (1:1, seven each).
Four of the 10 microduplications were clustered at 16p13.11, and all were inherited from apparently normal parents. Deletions in this region are associated with ID, microcephaly, and epilepsy. Patients with reciprocal duplications present with distinct behavioural problems, in addition to ID and/or childhood apraxia of speech. As reported in previous studies, the frequency of 16p13.11 duplications is comparable between the normal and affected populations, indicating that the variant phenotype is similar to the normal phenotype [Kirchhoff et al., 2005; Rosenberg et al., 2006; Ullmann et al., 2007; Hannes et al., 2009].
Despite the phenotypic variability, deletions inherited from clinically normal parents may lead to the development of a pathogenic phenotype. However, the role of duplications (either de novo or hereditary) in the development of clinical phenotypes remains unknown, and this aberration may be a rare benign variant. Finally, according to Kuang et al. [2011], recurrent 16p13.11 duplications are a risk factor for thoracic aortic aneurysm and dissection.
The phenotypic variability of genomic changes in chromosome 16 may be explained by the recent “two-hit” hypothesis. According to this hypothesis, co-occurrence of few rare variants with great effect contributes to the phenotypic heterogeneity of genomic disorders [Girirajan et al., 2012]. In our cohort, 16p13.11 duplications produced a mild phenotypic effect (Table 1). Indeed, although the classic recurrent 16p13.11 duplication is a benign variant, it is noteworthy that the carriers of this duplication in our cohort presented with severe language delay and behavioural problems, in addition to ID.
Many recurrent de novo variations, including 16p11.2 deletions, are closely associated with autism and other psychiatric and developmental disorders [Sanders et al., 2011; Hanson et al., 2015]. In the present study, we confirmed these associations, as all de novo variations in our cohort were 16p11.2 microdeletions.
Through unbiased whole-brain analysis, a previous study revealed that the genomic copy number at the 16p11.2 BP4–BP5 locus was associated with changes in brain anatomy in a dose-dependent manner, and these structural changes were frequently reported in patients diagnosed with ASDs or seizure disorders. In addition, changes in brain anatomy were closely correlated with the phenotypic traits of individuals carrying 16p11.2 deletions or duplications [Maillard-Wermelinger, 2015]. Specifically, eating behaviour-associated reciprocal volume changes at the key nodes of the reward circuit − the striatum, medio-dorsal thalamus, orbito-frontal cortex, and insula − may be important for explaining the mirror body mass index phenotype in 16p11.2 CNV carriers. Consistently, reciprocal changes in language areas − the middle and upper temporal gyrus and caudate − may be responsible for language deficiencies reported in the carriers of deletion but not in the carriers of duplication [Kaminsky et al., 2011]. Analyses of cortex anatomy revealed reduced cortical thickness in both 16p11.2 deletion and duplication carriers.
Given the significant overlap in the transcript levels of genes at the 16p11.2 BP4–BP5 locus, we avoid drawing conclusions regarding the potential differential contribution of a single gene to brain anatomy.
In a study based on the ClinGen database, 16p11.2 deletions were the second most commonly identified microdeletions, occurring at a frequency of 1 in 235 tested cases with intellectual and developmental disabilities [Miller et al., 2015]. In the present cohort, 5 patients carried 16p11.2 deletions and 2 patients carried 16p11.2 duplications, which is consistent with the reported trend.
In most cases, these microdeletions are not inherited, as evidenced by the de novo origin of deletions in approximately 75% paediatric cases in a previous study [Stoppel et al., 2018]. In our cohort, all 16p11.2 deletions (n = 7) were de novo. The clinical presentation of 16p11.2 microdeletions and duplications may vary across patients, depending on the size of the chromosome fragment lost or duplicated.
Furthermore, 16p11.2 microdeletions are mostly characterised by neurocognitive developmental delay, ID, and ASD. In addition, the phenotypic spectrum associated with this deletion is much wider and includes delays in speech or motor development, low muscle tone, hypo- or hyperreflexia, language impairment (apraxia or dysarthria), obese tendency, short stature, and hyperinsulinemic hypoglycaemia. Consistent with previous reports, speech abnormalities were detected in patients #1 and #5 carrying this deletion in our cohort. In addition, various craniofacial abnormalities have been reported in patients carrying this chromosomal abnormality, such as macro- or microcephaly, hypertelorism, full cheeks, posteriorly rotated ears, downward-sloping palpebral fissures, deep-set eyes, ptosis, and a small nose with a wide nasal bridge. Abnormalities of fingers and toes, such as clinodactyly and syndactyly, have also been observed. Further, 16p11.2 microdeletions are associated with dermatological changes, such as café au lait spots and sacral dimples [Bachmann-Gagescu et al., 2010; Steinman et al., 2016; Demopoulo et al., 2018; Shriberg et al., 2019; Stingl et al., 2020]. Genomic aberrations are clustered in regions corresponding to the major processes of craniofacial development (frontonasal, medial nasal, maxillary, and mandibular development). Specifically, 16p11.2 deletions are associated with significantly larger frontal and maxillary dimensions and a shorter and narrower nose. In contrast, 16p11.2 duplications are associated with smaller frontal dimensions and a significantly wider nose and longer nasal bridge. Additionally, 16p11.2 duplications are associated with a narrower labiomental angle (LMA), consistent with a more protrusive chin. Deletion carriers exhibited a wider LMA, although the effect did not reach statistical significance [Qiu et al., 2019]. The molecular mechanisms underlying the clinical presentation of patients with 16p11.2 microdeletions remain unclear and warrant further research. Overall, the phenotypic variability of clinical presentation among the carriers of chromosome 16 aberrations and the relatively low penetration of 16p11.2 deletions in our cohort may, in fact, be explained by the two-hit hypothesis.
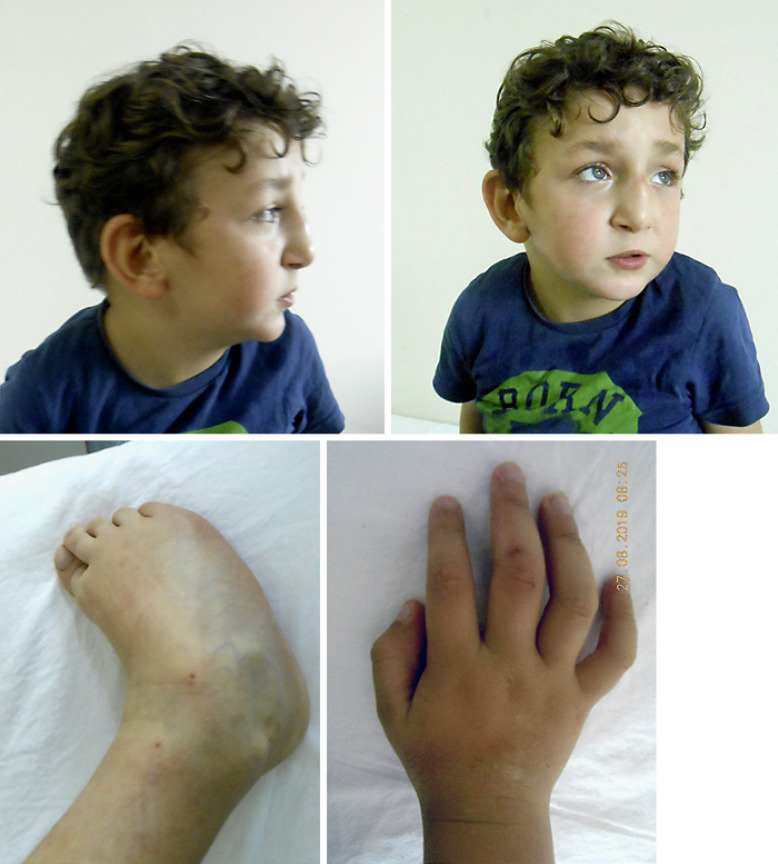
In a previous study, a male paediatric patient carried an interstitial deletion at 16q22.1q22.2, instead of the 16p11.2 and 16p13.11 chromosomal regions. As such, over 26 patients with different interstitial long-arm deletions in chromosome 16 have been reported to date. Patients with interstitial long-arm deletions in chromosome 16 share clinical features such as growth retardation, microcephaly, high and prominent forehead, prominent metopic suture, wide anterior fontanel, hypertelorism, wide nasal bridge, low-set and dysplastic ears, cleft palate micrognathia, short neck, narrow thorax, wide toes, ID, muscle hypotonia, congenital heart defects, and gastrointestinal and renal anomalies [Knoblauch et al., 2000]. Most of these clinical findings were observed in patient #6 in our cohort (Fig. 2), who carried a deletion of approximately 4,000 kb (Fig. 3).
Fig. 2.
Phenotypic images of a patient with deletion 16q22.1q22.2 (patient #6).
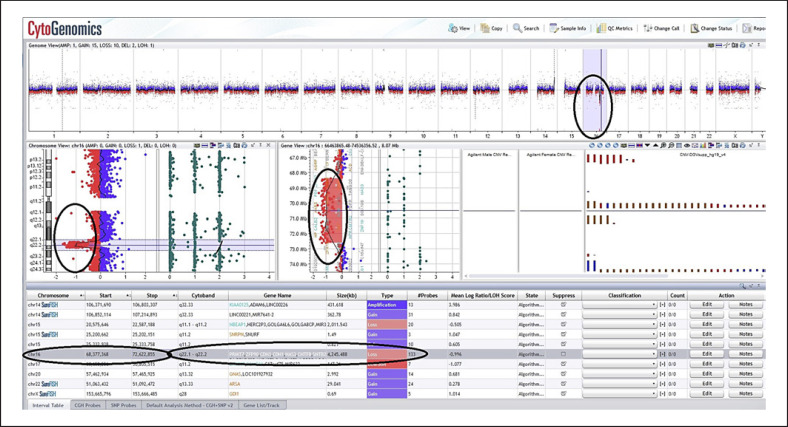
Fig. 3.
Microarray results of proband #6 sample. The results show genomic imbalance of deletions in the 16q22.1q22.2 chromosome region.
In a study by Rivera et al. [1985], a 2-month-old male patient presenting with delayed growth and development, brachycephaly, large anterior fontanelle, low-set folded ears, micrognathia, aortic coarctation, floppy abdominal muscles, and pes varus exhibited a 46,XY,del(16)(q21;q23) de novo karyotype. This observation corroborates both the distinctness of the 16q monosomy syndrome and the pathogenic role of the 16q21 locus. In a study of Knoblauch et al. [2000], the phenotype of the 16q deletion syndrome was attributed to the deletion of critical bands at 16q11.2q13, 16q21, and 16q22.1.
Although the phenotypic expression of full trisomy 16 is well defined, reports of partial trisomy of distal 16q are rare. It is challenging to determine the genotype-phenotype correlation in this trisomy because all previous cases carried unbalanced translocations, and the clinical manifestations were attributed to partial monosomy rather than trisomy. We believe that our patient carrying a 16q24.1 duplication with no simultaneous deletion in another chromosome is the best candidate for genotype-phenotype analysis thus far. In this case, however, it is difficult to establish the correlation between a phenotype and a trisomy-specific cytogenetic band. All reported cases of trisomy harboured break points or duplications. In contrast, in our case, additional chromosome imbalances associated with rearrangements, all of which significantly affected the phenotype, were noted. Therefore, the phenotype resulting from the 16q24 duplication is likely the result of the interactions between duplicated and non-duplicated genes involved in pathogenesis and more likely of gene overdose. To the best of our knowledge, there are no previous reports of phenotypes associated with genes located within the duplicated 16q24 region. The gene map of chromosome 16q24.1 may offer insights into this duplication. The microduplication detected in the present study was 94 kb in size and only contained 2 genes (ATP2C2 and TLDC1 [mEAK-7]).
Unlike lower eukaryotes, mammals possess a second gene, ATP2C2, which encodes a member of the Ca2+/Mn(2+)-ATPases–SPCA2 secretory pathway family. Human SPCA2 shares 64% amino acid identity with hSPCA1 − the defective protein in Hailey-Hailey disease. Mn neurotoxicity and Parkinsonism are closely linked, and SPCA2 plays an important physiological role in the brain. Variations in ATP2C2 and word repetition ability in certain language disorders may be related [Xiang et al., 2005].
mEAK-7 interacts with the mammalian target of rapamycin (mTOR) in the lysosomes and activates an alternative mechanistic/mTOR signalling pathway in human cells. mEAK-7 knockout markedly decreased the lysosomal localisation of mTOR, while mEAK-7 overexpression increased the lysosomal activation of mTOR. Furthermore, deletion of the C-terminus of mEAK-7 significantly reduced mTOR activation. While mEAK-7 knockdown reduced cell proliferation and migration, mEAK-7 overexpression enhanced the potency of cellular effects [Nguyen et al., 2018].
Conclusions
The present study underscores the importance of extensive molecular cytogenetic investigations in dysmorphic patients with ID and neurodevelopmental disorders. With advances in molecular cytogenetic tools, additional cases of chromosome 16 aberrations will definitely be unveiled. In the present cohort, patients with chromosome 16p rearrangements presented with diverse neuropsychiatric phenotypes with a broad spectrum of severity. Molecular refinement of these new cases will allow us to create a precise phenotypic map of chromosome 16 aberrations.
Statement of Ethics
The protocols used in this study were in compliance with the Declaration of Helsinki and were approved by the Ethics Committee of the Trakya University Faculty of Medicine (2021/43). The study was conducted following the routine diagnostic procedures of the Department of Medical Genetics, Faculty of Medicine, Trakya University. Written informed consent was obtained from the parent/legal guardian of participants prior to the study.
Conflict of Interest Statement
The authors declare that they have no conflicts of interest.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Contributions
All authors contributed to conception, genetic examinations and counseling, data analysis and interpretation. Emine Ikbal Atli prepared the manuscript. All authors read and approved the manuscript.
Data Availability Statement
All data generated or analysed during this study are included in this article [and/or] its supplementary material files. Further enquiries can be directed to the corresponding author.
Supplementary Material
Supplementary data
Acknowledgement
We express our gratitude towards the patients and their families as well as the referring physicians.
References
- Bachmann-Gagescu R, Mefford HC, Cowan C, Glew GM, Hing AV, Wallace S, et al. Recurrent 200-kb deletions of 16p11.2 that include the SH2B1 gene are associated with developmental delay and obesity. Genet Med. 2010;12((10)):641–7. doi: 10.1097/GIM.0b013e3181ef4286. [DOI] [PubMed] [Google Scholar]
- Bardakjian TM, Kwok S, Slavotinek AM, Schneider AS. Clinical report of microphthalmia and optic nerve coloboma associated with a de novo microdeletion of chromosome 16p11.2. Am J Med Genet A. 2010;152A((12)):3120–3. doi: 10.1002/ajmg.a.33492. [DOI] [PubMed] [Google Scholar]
- Bijlsma EK, Gijsbers AC, Schuurs-Hoeijmakers JH, van Haeringen A, Fransen van de Putte DE, Anderlid BM, et al. Extending the phenotype of recurrent rearrangements of 16p11.2: deletions in mentally retarded patients without autism and in normal individuals. Eur J Med Genet. 2009;52((2-3)):77–87. doi: 10.1016/j.ejmg.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Bochukova EG, Huang N, Keogh J, Henning E, Purmann C, Blaszczyk K, et al. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature. 2010;463((7281)):666–70. doi: 10.1038/nature08689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demopoulos C, Kothare H, Mizuiri D, Henderson-Sabes J, Fregeau B, Tjernagel J, et al. Abnormal speech motor control in individuals with 16p11.2 deletions. Sci Rep. 2018;8((1)):1274. doi: 10.1038/s41598-018-19751-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez BA, Roberts W, Chung B, Weksberg R, Meyn S, Szatmari P, et al. Phenotypic spectrum associated with de novo and inherited deletions and duplications at 16p11.2 in individuals ascertained for diagnosis of autism spectrum disorder. J Med Genet. 2010;47((3)):195–203. doi: 10.1136/jmg.2009.069369. [DOI] [PubMed] [Google Scholar]
- Girirajan S, Rosenfeld JA, Coe BP, Parikh S, Friedman N, Goldstein A, et al. Phenotypic heterogeneity of genomic disorders and rare copy-number variants. N Engl J Med. 2012;367((14)):1321–31. doi: 10.1056/NEJMoa1200395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golzio C, Willer J, Talkowski ME, Oh EC, Taniguchi Y, Jacquemont S, et al. KCTD13 is a major driver of mirrored neuroanatomical phenotypes of the 16p11.2 copy number variant. Nature. 2012;485((7398)):363–7. doi: 10.1038/nature11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannes FD, Sharp AJ, Mefford HC, de Ravel T, Ruivenkamp CA, Breuning MH, et al. Recurrent reciprocal deletions and duplications of 16p13.11: The deletion is a risk factor for MR/MCA while the duplication may be a rare benign variant. J Med Genet. 2009;46((4)):223–32. doi: 10.1136/jmg.2007.055202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson E, Bernier R, Porche K, Jackson FI, Goin-Kochel RP, Snyder LG, et al. The cognitive and behavioral phenotype of the 16p11.2 deletion in a clinically ascertained population. Biol Psychiatry. 2015;77((9)):785–93. doi: 10.1016/j.biopsych.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISCN 2016 ISCN 2016: An International System for Human Cytogenomic Nomenclature (2016) Cytogenet Genome Res. 2016;149((1–2)):1–140. [Google Scholar]
- Jacquemont S, Reymond A, Zufferey F, Harewood L, Walters RG, Kutalik Z, et al. Mirror extreme BMI phenotypes associated with gene dosage at the chromosome 16p11.2 locus. Nature. 2011;478((7367)):97–102. doi: 10.1038/nature10406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky EB, Kaul V, Paschall J, Church DM, Bunke B, Kunig D, et al. An evidence-based approach to establish the functional and clinical significance of copy number variants in intellectual and developmental disabilities. Genet Med. 2011;13((9)):777–84. doi: 10.1097/GIM.0b013e31822c79f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff M, Gerdes T, Brunebjerg S, Bryndorf T. Investigation of patients with mental retardation and dysmorphic features using comparative genomic hybridization and subtelomeric multiplex ligation dependent probe amplification. Am J Med Genet A. 2005;139((3)):231–3. doi: 10.1002/ajmg.a.31019. [DOI] [PubMed] [Google Scholar]
- Knoblauch H, Thiel G, Tinschert S, Körner H, Tennstedt C, Chaoui R, et al. Clinical and molecular cytogenetic studies of a large de novo interstitial deletion 16q11.2-16q21 including the putative transcription factor gene SALL1. J Med Genet. 2000;37((5)):389–92. doi: 10.1136/jmg.37.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang SQ, Guo DC, Prakash SK, McDonald ML, Johnson RJ, Wang M, et al. Recurrent chromosome 16p13.1 duplications are a risk factor for aortic dissections. PLoS Genet. 2011;7((6)):e1002118. doi: 10.1371/journal.pgen.1002118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar RA, KaraMohamed S, Sudi J, Conrad DF, Brune C, Badner JA, et al. Recurrent 16p11.2 microdeletions in autism. Hum Mol Genet. 2008;17((4)):628–38. doi: 10.1093/hmg/ddm376. [DOI] [PubMed] [Google Scholar]
- Maillard-Wermelinger A. The 16p11.2 rearrangements: genetic paradigms for obesity and neurodevelopmental disorders. Thesis. 2015 [Google Scholar]
- Marshall CR, Noor A, Vincent JB, Lionel AC, Feuk L, Skaug J, et al. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82((2)):477–88. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Han C, Gordon LA, Terry A, Prabhakar S, She X, et al. The sequence and analysis of duplication-rich human chromosome 16. Nature. 2004;432((7020)):988–94. doi: 10.1038/nature03187. [DOI] [PubMed] [Google Scholar]
- Miller DT, Chung W, Nasir R, Shen Y, Steinman KJ, Wu BL, et al. 16p11.2 Recurrent Microdeletion. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. Gene Reviews® [Internet] Seattle (WA): University of Washington, Seattle; 2009. Sep 22, 1993-2021 [Updated 2015 Dec 10] [Google Scholar]
- Nguyen JT, Ray C, Fox AL, Mendonça DB, Kim JK, Krebsbach PH. Mammalian EAK-7 activates alternative mTOR signaling to regulate cell proliferation and migration. Sci Adv. 2018;4((5)):eaao5838. doi: 10.1126/sciadv.aao5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttle X, Giannuzzi G, Duyzend MH, Schraiber JG, Narvaiza I, Sudmant PH, et al. Emergence of a Homo sapiens-specific gene family and chromosome 16p11.2 CNV susceptibility. Nature. 2016;536((7615)):205–9. doi: 10.1038/nature19075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Arbogast T, Lorenzo SM, Li H, Tang SC, Richardson E, et al. Oligogenic Effects of 16p11.2 Copy-Number Variation on Craniofacial Development. Cell Rep. 2019;28((13)):3320–3328e4. doi: 10.1016/j.celrep.2019.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera H, Vargas‐Moyeda E, Möller M, Torres‐Lamas A, Cantú JM. Monosomy 16q: a distinct syndrome Apropos of a de novo del(16) (q2100q2300) Clin Genet. 1985;28:84–6. doi: 10.1111/j.1399-0004.1985.tb01223.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg C, Knijnenburg J, Bakker E, Vianna-Morgante AM, Sloos W, Otto PA, et al. Array-CGH detection of micro rearrangements in mentally retarded individuals: Clinical significance of imbalances present both in affected children and normal parents. J Med Genet. 2006;43((2)):180–6. doi: 10.1136/jmg.2005.032268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70((5)):863–85. doi: 10.1016/j.neuron.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316((5823)):445–9. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinawi M, Liu P, Kang SH, Shen J, Belmont JW, Scott DA, et al. Recurrent reciprocal 16p11.2 rearrangements associated with global developmental delay, behavioural problems, dysmorphism, epilepsy, and abnormal head size. J Med Genet. 2010;47((5)):332–41. doi: 10.1136/jmg.2009.073015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriberg LD, Strand EA, Jakielski KJ, Mabie HL. Estimates of the prevalence of speech and motor speech disorders in persons with complex neurodevelopmental disorders. Clin Linguist Phon. 2019;33((8)):707–36. doi: 10.1080/02699206.2019.1595732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman KJ, Spence SJ, Ramocki MB, Proud MB, Kessler SK, Marco EJ, et al. 16p11.2 deletion and duplication: Characterizing neurologic phenotypes in a large clinically ascertained cohort. Am J Med Genet A. 2016 Nov;170((11)):2943–55. doi: 10.1002/ajmg.a.37820. [DOI] [PubMed] [Google Scholar]
- Stingl CS, Jackson-Cook C, Couser NL. Ocular findings in the 16p11.2 microdeletion syndrome: a case report and literature review. Case Rep Pediatr. 2020;2020:2031701. doi: 10.1155/2020/2031701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppel LJ, Kazdoba TM, Schaffler MD, Preza AR, Heynen A, Crawley JN, et al. R-baclofen reverses cognitive deficits and improves social interactions in two lines of 16p11.2 deletion mice. Neuropsychopharmacology. 2018;43((3)):513–24. doi: 10.1038/npp.2017.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropeano M, Ahn JW, Dobson RJ, Breen G, Rucker J, Dixit A, et al. Male-biased autosomal effect of 16p13.11 copy number variation in neurodevelopmental disorders. PLoS ONE. 2013;8((4)):e61365. doi: 10.1371/journal.pone.0061365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmann R, Turner G, Kirchhoff M, Chen W, Tonge B, Rosenberg C, et al. Array CGH identifies reciprocal 16p13.1 duplications and deletions that predispose to autism and/or mental retardation. Hum Mutat. 2007;28((7)):674–82. doi: 10.1002/humu.20546. [DOI] [PubMed] [Google Scholar]
- Walsh KM, Bracken MB. Copy number variation in the dosage-sensitive 16p11.2 interval accounts for only a small proportion of autism incidence: a systematic review and meta-analysis. Genet Med. 2011;13((5)):377–84. doi: 10.1097/GIM.0b013e3182076c0c. [DOI] [PubMed] [Google Scholar]
- Walters RG, Jacquemont S, Valsesia A, de Smith AJ, Martinet D, Andersson J, et al. A new highly penetrant form of obesity due to deletions on chromosome 16p11.2. Nature. 2010;463((7281)):671–5. doi: 10.1038/nature08727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, Fossdal R, et al. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 2008;358((7)):667–75. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- Wu N, Ming X, Xiao J, Wu Z, Chen X, Shinawi M, et al. TBX6 null variants and a common hypomorphic allele in congenital scoliosis. N Engl J Med. 2015;372((4)):341–50. doi: 10.1056/NEJMoa1406829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M, Mohamalawari D, Rao R. A novel isoform of the secretory pathway Ca2+,Mn(2+)-ATPase, hSPCA2, has unusual properties and is expressed in the brain. J Biol Chem. 2005;280((12)):11608–14. doi: 10.1074/jbc.M413116200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
All data generated or analysed during this study are included in this article [and/or] its supplementary material files. Further enquiries can be directed to the corresponding author.