Abstract
We combined the use of low inoculation titers (300 ± 100 CFU/ml) and enumeration of culturable cells to measure the osmoprotective potentialities of dimethylsulfoniopropionate (DMSP), dimethylsulfonioacetate (DMSA), and glycine betaine (GB) for salt-stressed cultures of Escherichia coli. Dilute bacterial cultures were grown with osmoprotectant concentrations that encompassed the nanomolar levels of GB and DMSP found in nature and the millimolar levels of osmoprotectants used in standard laboratory osmoprotection bioassays. Nanomolar concentrations of DMSA, DMSP, and GB were sufficient to enhance the salinity tolerance of E. coli cells expressing only the ProU high-affinity general osmoporter. In contrast, nanomolar levels of osmoprotectants were ineffective with a mutant strain (GM50) that expressed only the low-affinity ProP osmoporter. Transport studies showed that DMSA and DMSP, like GB, were taken up via both ProU and ProP. Moreover, ProU displayed higher affinities for the three osmoprotectants than ProP displayed, and ProP, like ProU, displayed much higher affinities for GB and DMSA than for DMSP. Interestingly, ProP did not operate at substrate concentrations of 200 nM or less, whereas ProU operated at concentrations ranging from 1 nM to millimolar levels. Consequently, proU+ strains of E. coli, but not the proP+ strain GM50, could also scavenge nanomolar levels of GB, DMSA, and DMSP from oligotrophic seawater. The physiological and ecological implications of these observations are discussed.
Glycine betaine (GB) [(CH3)3N+CH2-COO−; 2-trimethylammonioacetate] and 3-dimethylsulfoniopropionate (DMSP) [(CH3)2S+CH2-CH2-COO−] are produced by a wide variety of halophilic photosynthetic organisms, including marine algae, phytoplankton, cyanobacteria, and/or nearshore halophilic higher plants, in which they apparently accumulate as cytosolic osmolytes (3, 35, 39, 48, 50). In contrast, 2-dimethylsulfonioacetate (DMSA) [(CH3)2S+CH2-COO−], the closest sulfonium analog of GB, is found only as a secondary solute in two species of marine algae, and our knowledge of the biological function(s) of DMSA is rudimentary (6, 42, 46). However, it has been established that many bacterial species, including Escherichia coli, respond to hyperosmotic stress by accumulating high levels of GB and DMSP from their environments (8, 11, 40, 42). These compounds primarily serve as cytosolic osmolytes that allow for rehydration of stressed cells exposed to hyperosmotic environments (7, 8). GB and DMSP also act as “compatible solutes” because high cytosolic concentrations of these compounds do not disturb the functioning of cellular proteins and counteract the destabilizing effects of salts (13, 20, 38). Moreover, GB and its analogs are also called “osmoprotectants” because they stimulate bacterial growth in media with inhibitory osmolarities and extend the range of salinities at which bacteria can grow (6, 8, 18). In this respect, GB and DMSP are among the most effective osmoprotectants for E. coli and other enteric bacteria, such as Salmonella typhimurium and Klebsiella pneumoniae (35, 40, 46).
GB and DMSP are released into marine sediments, seawater, and estuarine waters as a result of the natural decay of halophytes, as well as the exposure of halophytes to fluctuating salinity levels that are caused by inflowing freshwater and twice-daily tides (24, 25, 49). Furthermore, GB and DMSP might be important to human health because environmental osmoprotectants favor survival and could promote proliferation of pathogenic bacteria, such as enterotoxigenic strains of E. coli in sediments, recreational waters, and shellfish production zones that may be contaminated by sewage effluents from upstream urban and rural communities (14–18). However, the concentrations of GB and DMSP in natural environments are at least 3 to 4 orders of magnitude lower (1 to 10 nM [27, 30, 51]) than the concentrations of these compounds that provide maximal osmoprotection to pure bacterial cultures grown under controlled laboratory conditions (10 to 500 μM [11, 12, 31, 41, 42]). Moreover, most studies performed with such cultures have measured bacterial growth by using spectrophotometric methods which require high cell densities that are not likely to be found in natural environments (31). Furthermore, it is notable that the GB and DMSP concentrations in seawater are also considerably lower (ca. 3 logs lower) than the calculated affinities (Km values) of well-characterized osmoporters, such as the ProP and ProU GB-proline transporters of S. typhimurium (4, 5), as well as the GB or DMSP porters of many other bacteria (1, 23, 32, 45). These differences are particularly intriguing because natural populations of free-living bacteria can salvage nanomolar levels of GB, choline (a precursor of GB), and DMSP from seawater and can accumulate osmotically significant levels of GB and DMSP from their natural habitats (28, 29, 52). However, it is not yet known which species of free-living bacteria can salvage very low concentrations of environmental osmoprotectants. Surprisingly, it is also not known if nanomolar levels of GB and other osmoprotectants can effectively confer enhanced salinity tolerance to bacteria (i.e., stimulate bacterial growth at inhibitory osmolarities). Also, the transporters and the genes involved in DMSP and DMSA uptake have not been identified in any bacterium, although competition studies have suggested that GB porters of E. coli and marine bacteria also recognize DMSP as a substrate (11, 19, 29, 52).
The objectives of this study were (i) to determine the lowest concentration(s) of GB, DMSA, and DMSP that could still alleviate osmotic inhibition of growth when E. coli was cultured at very low cell densities, (ii) to evaluate the osmoprotective activities and uptake kinetics of the three methylated onium compounds in E. coli strains expressing either the ProP osmoporter or the ProU osmoporter (19, 34), and (iii) to determine whether E. coli cells maintained in oligotrophic seawater could take up very low levels of environmental osmoprotectants.
MATERIALS AND METHODS
Bacterial strains.
A set of E. coli strains expressing either both, one, or none of the two GB-proline transport systems (i.e., ProP and ProU) that operate in wild-type E. coli K-12 (7, 34) were used in this study. Strain MC4100 [F− araD139 Δ(argF-lac)U169 rpsL150 relA1 deoC1 ptsF25 flbB5301 rbsR] was used as the parental strain (37). Strain GM50 [MC4100 Φ(proU-lacZ+)3 (λplacMu55)] (37) and strain BK32 [MC4100 Δ(putPA)101 Δ(proP)2] (21) are defective in ProU- and ProP-mediated GB uptake, respectively. Strain MKH13 [MC4100 Δ(putPA)101 Δ(proP)2 Δ(proU)608] is deficient in both proline transport and GB uptake activities (21).
Media and growth conditions.
Bacteria were pregrown aerobically at 37°C in M63 minimal medium containing 10 mM glucose and 15 mM ammonium sulfate as the carbon and nitrogen sources, respectively (18). Cultures (10 ml) were grown in 50-ml test tubes (inclined at an angle of 30°) with rotary shaking at 200 rpm. The osmotic strength of M63 medium was raised by adding NaCl to final concentrations ranging from 0.3 to 0.8 M. Natural surface seawater was collected at high tide at Paimpol (anse du Guilben, northern Brittany, France), filtered with Whatman no. 1 paper, autoclaved (30 min, 121°C), and stored at 4°C. The osmolalities of M63 medium and seawater were determined by using a freezing point depression microosmometer. At the collection site, the osmolality of seawater was similar to the osmolality of M63 medium supplemented with 0.6 M NaCl (1,360 mosmol/kg of water).
Osmoprotectants were added to M63 medium or seawater from filter-sterilized stock solutions. GB was purchased from Sigma Chimie, St. Quentin Fallavier, France. Unlabeled DMSA and DMSP, as well as [methyl-14C]GB (2.04 GBq · mmol−1), [methyl-14C]DMSA (2.04 GBq · mmol−1), [1-14C]DMSA (0.28 GBq · mmol−1), and [1-14C]DMSP (37 MBq · mmol−1), were synthesized as described previously (42).
Bacterial growth was monitored by counting colonies on Luria-Bertani agar plates and/or by measuring the optical densities at 570 nm (OD570) of cell suspensions with a Turner spectrophotometer. All of the data below are means based on at least three independent experiments, and the standard deviations for the spectrophotometric and culturable count determinations were less than 5% and less than 15%, respectively.
Uptake and fate of radiolabeled osmoprotectants.
E. coli cells grown to the mid-exponential phase in M63 medium were harvested by centrifugation (5,000 × g, 10 min) and were resuspended at an OD570 of 1 in M63 medium containing 0.3 M NaCl and 10 mM glucose. The cells were incubated in this medium for 45 to 60 min in order to obtain full induction of the ProU and ProP osmoporters (8, 34). Uptake of [methyl-14C]GB, [methyl-14C]DMSA, and [1-14C]DMSP was then monitored at room temperature (20 to 22°C) in the same medium. Transport was terminated by filtering cell suspensions with Whatman GF/F filters. Each filter was then rinsed with 5 ml of transport medium without osmoprotectant. The radioactivity captured by the filtered cells was measured by liquid scintillation counting (19, 41).
For kinetic studies, time-dependent measurements of 14C-labeled osmoprotectant uptake were obtained for each concentration of radioactive substrate. Kinetic experiments were performed with dense and dilute cell suspensions of strains BK32 and GM50 in order to evaluate the capacities of ProU and ProP to take up osmoprotectants in the presence of a wide range of substrate concentrations. In the kinetic experiments performed with dense suspensions (OD570, 1; ca. 2 × 108 CFU/ml), the concentrations of [14C]GB, [14C]DMSA, and [14C]DMSP ranged from 0.1 μM to 4 mM. For each concentration of 14C-labeled osmoprotectant, four 40-μl aliquots of cell suspension were filtered at 30-s intervals over a 2-min period. In the kinetic experiments performed with dilute cell suspensions, dense suspensions (OD570, 1) were diluted 1:1,000 in transport medium before the radiolabeled osmoprotectant was added (final cell density, ca. 2 × 105 CFU/ml). In these experiments, [14C]GB and [methyl14C]DMSA were supplied without isotopic dilution at final concentrations ranging from 1 nM to 40 μM in a final volume of 2 to 100 ml. Time-dependent uptake assays (at four times over a 5- to 12-min period) were performed by filtering between 0.5 and 20 ml of cell suspension for each time point, depending on the concentration of [14C]GB or [14C]DMSA. The amounts of internalized osmoprotectants were always linear with time (data not shown). Unless indicated otherwise, uptake rates were expressed in nanomoles per minute per milligram of cell protein, which were determined by the method of Lowry et al. (33).
To investigate the fate of [methyl-14C]DMSA and [1-14C]DMSP, cultures of E. coli MC4100 were grown to the mid-log phase in M63 medium with or without 0.5 M NaCl. Then, 5 ml of a cell suspension was supplemented with 200,000 dpm of radiolabeled DMSA or DMSP and transferred into a Warburg vial whose center well contained a piece of filter paper soaked with 20 μl of 5 M KOH, which was used to trap the 14CO2 that might evolve from catabolism of [14C]DMSA or [14C]DMSP. The vial was sealed with a rubber stopper and incubated overnight at 37°C with shaking at 100 rpm. At the end of the experiment, the cells were centrifuged and extracted in 80% ethanol. The radioactivities of the ethanol-soluble and insoluble extracts, as well as 14CO2, were then measured by liquid scintillation counting (17, 42).
Uptake of nanomolar concentrations of osmoprotectants by confined cultures of E. coli exposed to seawater.
Cultures which were used to evaluate the ability of E. coli cells to scavenge nanomolar levels of radiolabeled osmoprotectants from seawater were pregrown to the mid-exponential phase (OD570, 1; ca. 2 × 108 CFU/ml) in M63 medium supplemented with 0.3 M NaCl in order to obtain osmoadapted cells. Then, 5 ml of a bacterial cell suspension was transferred directly into a diffusion chamber which was immersed in 1 liter of autoclaved seawater containing a nanomolar concentration of a radiolabeled osmoprotectant (ca. 100,000 dpm). The diffusion chamber was composed of a screw-cap bottomless Eppendorf tube prolonged by a dialysis bag whose bottom was sealed with a tight press-on clamp. The lower part of the bag was installed in a glass beaker below the surface of the seawater, which was stirred at 50 rpm. The upper end of the dialysis bag was sealed to the Eppendorf tube with several layers of self-adhesive tape and a rubber band; it was kept above the seawater. Prior to the uptake experiment, the beaker was washed with a 0.1 mM solution of unlabeled osmoprotectant in order to avoid nonspecific adsorption of the radiolabeled compound to the glassware. The beaker was then rinsed thoroughly with sterile distilled water in order to remove unbound traces of the unlabeled osmoprotectant. Uptake assays were performed at room temperature (20 to 22°C). Time-dependent measurements of [14C]GB, [14C]DMSA, and [14C]DMSP uptake rates were obtained by filtering subsamples of the confined bacterial cells onto Whatman GF/F filters (as described above) and rinsing the filtered cells twice with 1 ml of autoclaved seawater.
Stability of radiolabeled osmoprotectants.
GB and DMSA were chemically stable under all of our experimental conditions. However, DMSP underwent limited spontaneous degradation to dimethylsulfide and acrylic acid (2 to 5% in 10 to 15 h). Therefore, control experiments without bacteria were performed along with the experiments that required long-term incubation of DMSP with E. coli cultures, particularly metabolism experiments and experiments in which we measured DMSP uptake for long periods of time.
RESULTS
E. coli MC4100 is responsive to nanomolar concentrations of osmoprotectants.
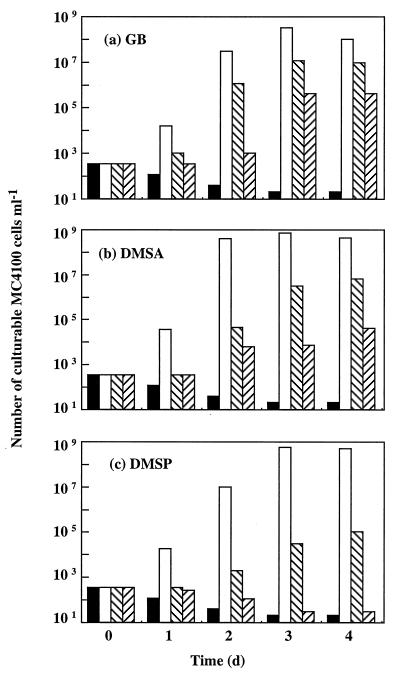
E. coli MC4100 was inoculated at a very low cell density (300 ± 100 CFU/ml) into M63 medium containing 0.8 M NaCl, which prevented growth of this bacterium (18, 19). GB, DMSA, and DMSP were supplied as putative osmoprotectants at initial concentrations ranging from 10−12 to 10−3 M. Bacterial growth was monitored by counting colonies for 4 days. Growth of MC4100 was not detected after 4 days of incubation in hyperosmotic M63 medium without an osmoprotectant (Fig. 1) or in stressed suspensions that contained only 10−12 M GB, 10−12 M DMSA, or 10−12 M DMSP (data not shown). However, substantial growth occurred during the first day in stressed cultures that contained one of the three osmoprotectants at a concentration of 1 mM. At this stage, the cell densities of the three cultures had already increased ca. 2 orders of magnitude (Fig. 1). This indicates that six to seven generations of MC4100 cells grew during the first day of incubation with 1 mM GB, 1 mM DMSA, or 1 mM DMSP. Ultimately, an osmoprotectant concentration of 1 mM resulted in an approximately 7-log increase in the final number of culturable E. coli MC4100 cells after 2 days of growth in hyperosmotic M63 medium (Fig. 1).
FIG. 1.
Effects of a wide range of GB (a), DMSA (b), and DMSP (c) concentrations on the growth of E. coli wild-type strain MC4100 in hyperosmotic M63 medium. The bacterium was inoculated at a low cell density (300 ± 100 CFU ml−1) into M63 medium containing 0.8 M NaCl without osmoprotectant (solid bars) or into M63 medium containing osmoprotectant at a concentration of 1 mM (open bars), 1 μM (left-to-right cross-hatched bars), or 1 nM (right-to-left cross-hatched bars). Growth was measured by counting colonies. The unstressed control culture grown in M63 medium without NaCl and with or without one of the three osmoprotectants reached a maximal cell density of ca. 109 CFU ml−1 after 1 day of incubation. d, day.
Very strong stimulation of bacterial growth at high salinity also occurred after 2 days of culture of MC4100 with either 1 μM GB or 1 μM DMSA (Fig. 1a and b) and after 3 days of culture with either 1 μM DMSP (Fig. 1c) or as little as 1 nM GB or 1 nM DMSA (Fig. 1a and b). Ultimately, the total number of culturable MC4100 cells increased ca. 5 orders of magnitude after 3 to 4 days of growth in hyperosmotic M63 medium containing either 1 μM GB or 1 μM DMSA (Fig. 1a and b). Meanwhile, the final cell densities of the stressed cultures grown with either 1 μM DMSP (Fig. 1c) or 1 nM GB (Fig. 1a) increased ca. 3 log factors. Finally, the increase in the number of culturable MC4100 cells observed with 1 nM DMSA was about 2 log factors after 4 days of growth (Fig. 1b), but no growth stimulation was detected in the presence of 1 nM DMSP (Fig. 1c). Thus, DMSP was significantly less osmoprotective than DMSA for parental strain MC4100, and DMSA was less osmoprotective than GB.
Growth stimulation by nanomolar concentrations of osmoprotectants requires the presence of a functional ProU transport system.
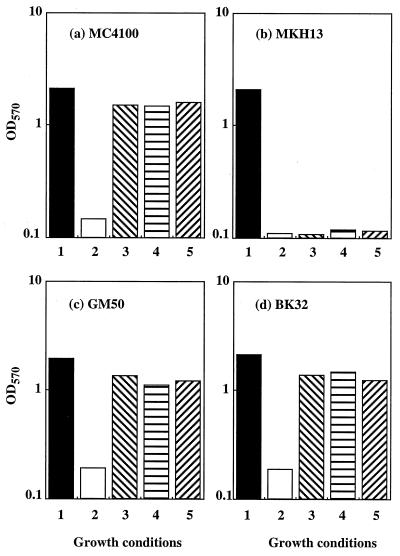
The following two GB-proline transporters operate in wild-type strains of E. coli and S. typhimurium: (i) ProP, a low-affinity transport system; and (ii) ProU, a high-affinity, binding protein-dependent transport system (2, 4, 5, 10, 34). In E. coli, ProP and ProU also mediate the uptake of several other osmoprotectants, including betaines, pipecolic acid, and ectoine (19, 21, 22, 47), and are thought to be involved in DMSA and DMSP uptake. The fact that DMSP was less osmoprotective than GB and DMSA (Fig. 1) suggested that DMSP was transported less efficiently or that it exhibited less functionality than its two analogs. Alternatively, DMSP might not be transported via the same routes as GB and DMSA. The individual contributions of ProP and ProU to osmoprotection of E. coli by GB, DMSA, and DMSP were evaluated by using a set of strains that differ from each other by mutations that affect either ProU or ProP or both ProU and ProP. The bacteria were inoculated at a high cell density (ca. 107 CFU/ml) into M63 medium with or without 0.8 M NaCl. The osmoprotectants were supplied at a concentration of 1 mM to ensure that all possible uptake routes for these compounds would operate at saturation capacity. Maximal growth yields were measured spectrophotometrically after 24 h of culture. Unlike parental strain MC4100, E. coli MKH13 (which lacks both GB porters [21]) could not grow in hyperosmotic M63 medium with or without GB, DMSA, or DMSP (Fig. 2). However, as observed in MC4100, DMSA and DMSP, like GB, were highly osmoprotective for E. coli GM50 (Fig. 2c) and BK32 (Fig. 2d), which express only the ProU and ProP porters, respectively (21, 37). Thus, the osmoprotective activity of DMSA and DMSP for E. coli was clearly dependent on the presence of functional ProP and ProU porters, and this activity did not appear to rely on other transport systems.
FIG. 2.
Effects of osmoprotectants on the final OD570 of cultures of E. coli strains expressing both ProP and ProU (strain MC4100) (a), neither ProP nor ProU (strain MKH13) (b), only ProP (strain GM50) (c), or only ProU (strain BK32) (d). Cultures were inoculated at an initial OD570 of 0.1, and the final OD570 were determined after 24 h of incubation in M63 medium containing no NaCl with or without 1 mM osmoprotectant (GB, DMSA, or DMSP) (bars 1), 0.8 M NaCl and no osmoprotectant (bars 2), 0.8 M NaCl plus 1 mM GB (bars 3), 0.8 M NaCl plus 1 mM DMSA (bars 4), or 0.8 M NaCl plus 1 mM DMSP (bars 5).
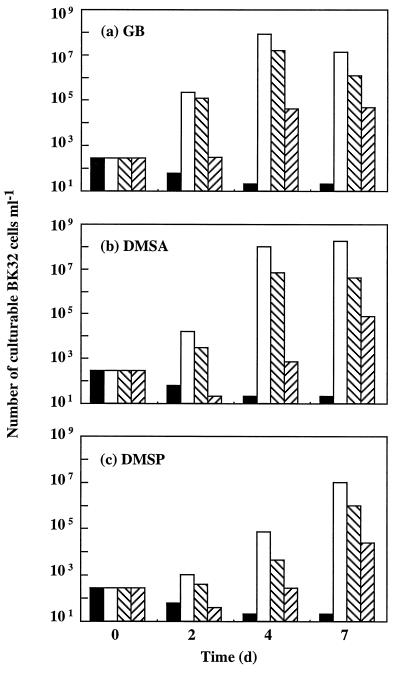
Strains GM50 and BK32 were also inoculated at low cell densities (200 to 400 CFU/ml) into M63 medium supplemented with 0.8 M NaCl and very low concentrations (1 nM to 1 μM) of GB, DMSA, or DMSP. The culturable cells were counted over a 7-day period. No growth was detected when salt-stressed strain GM50 (proP+ proU) was cultured for 7 days in the presence of any of the three osmoprotectants at a concentration of 1 μM or 1 nM (data not shown). In other words, ProP did not contribute to osmoprotection of E. coli when the concentrations of GB, DMSA, and DMSP were 1 μM or lower. In sharp contrast, growth of salt-stressed strain BK32 (proP proU+) was strongly stimulated by micromolar and even nanomolar concentrations of GB, DMSA, and DMSP. Indeed, after 4 days of growth, the total number of viable BK32 cells had increased as follows: (i) more than 5 orders of magnitude in the cultures supplemented with either 1 mM GB (Fig. 3a) or 1 mM DMSA (Fig. 3b); (ii) about 4 log factors in the cultures grown with either 1 μM GB or 1 μM DMSA; and (iii) about 2 log factors in the suspensions supplemented with either 1 mM DMSP (Fig. 3c) or as little as 1 nM GB (Fig. 3a). After this, no further increase in the number of BK32 culturable cells was observed with any of the three concentrations of GB (1 mM, 1 μM, or 1 nM) (Fig. 3a) or with either 1 mM or 1 μM DMSA (Fig. 3b). However, BK32 cells grown with either 1 nM DMSA (Fig. 3b) or 1 nM to 1 mM DMSP (Fig. 3c) continued to grow until the seventh day. Collectively, these growth data indicate that DMSP was about as effective as DMSA and GB were in increasing the final cell yields in stressed cultures of E. coli BK32; however, the maximal response of this proP proU+ strain to DMSP (Fig. 3c) was delayed about 3 days compared to the response to either GB or DMSA (Fig. 3a and b). These data suggested that DMSP was transported at lower rates than GB and DMSA were transported.
FIG. 3.
Comparative osmoprotective activities of a wide range of GB (a), DMSA (b), and DMSP (c) concentrations in the proP proU+ strain E. coli BK32. The experimental conditions were the same as those described in the legend to Fig. 1. d, day.
Characteristics of GB, DMSA, and DMSP uptake via ProP and ProU.
The kinetic parameters of [14C]GB, [14C]DMSA, and [14C]DMSP uptake via the ProP and ProU transport systems were first studied by using dense cell suspensions (OD570, 1) of strains GM50 and BK32, respectively. Double-reciprocal (Lineweaver-Burk) plots of transport rates versus substrate concentrations always yielded straight lines, indicating that uptake of the three osmoprotectants via ProU and ProP followed typical Michaelis-Menten kinetics (data not shown). Table 1 shows that ProP exhibited similar affinities for GB and DMSA (Km, 50 and 55 μM, respectively) but exhibited a much lower affinity for DMSP (Km, 1.13 mM). However, ProP transported the three osmoprotectants at similar rates (Vmax, 8 to 9 nmol · min−1 · mg of protein−1). The ProU transporter in strain BK32 also exhibited similar affinities and maximal transport rates for GB and DMSA (Table 1). However, the affinity of ProU for DMSP (Km, 32 μM) was significantly lower (5.8 times lower) than the affinity of ProU for GB (Km, 5.5 μM). Moreover, the maximal initial rate of DMSP uptake via ProU (Vmax, 12 nmol · min−1 · mg of protein−1) was almost six times higher than the maximal rate of GB uptake via this transport system (Vmax, 2 nmol · min−1 · mg of protein−1).
TABLE 1.
Kinetic parameters (Km and Vmax) of the ProU and ProP transporters of E. coli for GB, DMSA, and DMSPa
| Osmoprotectant | Strain GM50 ProP
|
Strain BK32 ProU
|
||
|---|---|---|---|---|
| Km (μM) | Vmax (nmol · min−1 · mg of protein−1) | Km (μM) | Vmax (nmol · min−1 · mg of protein−1) | |
| GB | 50 | 8 | 5.5 | 2 |
| DMSA | 55 | 9 | 9.5 | 1.5 |
| DMSP | 1,130 | 8.5 | 32 | 12 |
Transport assays were performed with dense suspensions (OD570, 1) in M63 medium containing 0.3 M NaCl and 10 mM glucose. Km and Vmax values were determined from double-reciprocal plots.
14C-labeled osmoprotectant uptake was also assayed in dilute suspensions of strains GM50 and BK32 in order to determine whether ProP and ProU could operate with nanomolar concentrations of osmoprotectants. Low-density cell suspensions were obtained by diluting dense suspensions (OD570, 1) 1:1,000 with transport medium before a radiolabeled osmoprotectant was added (final cell density, ca. 2 × 105 CFU/ml). DMSP uptake was not determined under these experimental conditions, because the specific radioactivity of [14C]DMSP was too low for the assays to be performed. Uptake of [14C]GB and [14C]DMSA via ProP in strain GM50 could not be detected at substrate concentrations of 200 nM or less. This observation is consistent with the fact that nanomolar concentrations of GB and DMSA were not osmoprotective for dilute suspensions of E. coli GM50. In contrast to the situation observed with ProP, the uptake of [14C]GB and the uptake of [14C]DMSA via ProU were still appreciable at concentrations as low as 1 nM (100 to 200 fmol/min/106 CFU). Moreover, we observed that the calculated affinity constants (Km values) of ProU for GB and DMSA in dilute suspensions of strain BK32 were ca. 1 μM; thus, these values were comparable to the Km values determined in dense cell suspensions of this strain. Together, these transport data indicated that ProU was operational at substrate (exogenous osmoprotectant) concentrations ranging from nanomolar to millimolar.
We also performed crossed competition uptake assays to evaluate the substrate specificities of ProP and ProU for DMSA, DMSP, and GB. DMSA was a very weak competitor of [14C]GB uptake via ProU in strain BK32. Indeed, a 10-fold excess of unlabeled DMSA over [14C]GB resulted in only 10% inhibition of GB uptake through ProU, whereas unlabeled GB inhibited virtually all uptake of [14C]DMSA via the high-affinity porter, even when the two compounds were supplied at the same concentration (10 μM) in the competition assay (Table 2). Thus, ProU apparently exhibited a much higher specificity for GB than for DMSA, although it exhibited similar Km values for these two substrates (Table 1). The probable basis for these apparently antagonistic biochemical features is discussed below. In contrast to the situation observed with ProU, unlabeled DMSA was an effective competitor of [14C]GB uptake via ProP (which was inhibited 60% by an equimolar amount of DMSA). Reciprocally, GB was also a potent competitor of [14C]DMSA uptake through ProP. Interestingly, the percentages of inhibition of [14C]DMSA uptake by GB via ProP were similar to the percentages of inhibition of [14C]GB uptake by DMSA via the same transporter (Table 2). These data were in complete agreement with the kinetic data which indicated that ProP exhibited similar affinities for DMSA and GB and took up these two osmoprotectants at similar rates (Table 1).
TABLE 2.
Effects of unlabeled analogs on uptake of [14C]GB, [14C]DMSA, and [14C]DMSP via the ProU and ProP transporters of E. colia
| Strain | Transport system | % Inhibition of 14C-labeled osmoprotectant uptake at two competitor/substrate ratiosb
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [14C]GB uptake with:
|
[14C]DMSA uptake with:
|
[14C]DMSP uptake with:
|
|||||||||||
| DMSA
|
DMSP
|
GB
|
DMSP
|
GB
|
DMSA
|
||||||||
| 1:1 | 1:10 | 1:1 | 1:10 | 1:1 | 1:10 | 1:1 | 1:10 | 1:1 | 1:10 | 1:1 | 1:10 | ||
| BK32 | ProU | 1 | 10 | 6 | 31 | 97 | 97 | 10 | 58 | 92 | 96 | 93 | 98 |
| GM50 | ProP | 60 | 94 | 5 | 22 | 60 | 96 | 1 | 33 | 82 | 99 | 70 | 96 |
Uptake assays were performed in M63 medium supplemented with 0.3 M NaCl and 10 mM glucose.
The concentrations of radiolabeled substrates used were 10 and 50 μM [14C]GB or 10 and 50 μM [14C]DMSA for ProU and ProP, respectively and 100 μM [14C]DMSP for both ProU and ProP. The results are expressed as percentages of reduction of the uninhibited transport rates (measured in the absence of a competitor). The uninhibited uptake rates were as follows: 1.2, 0.65, and 9.6 nmol · min−1 · mg of protein−1 for GB, DMSA, and DMSP, respectively, in the proP proU+ strain BK32; and 4.7, 4.60, and 6.5 nmol · min−1 · mg of protein−1 for GB, DMSA, and DMSP, respectively, in the proP+ proU strain GM50. Values are means based on triplicate determinations, and the standard deviations were less than 6%.
Uptake of radiolabeled GB and DMSA via ProU and ProP was not significantly inhibited by unlabeled DMSP (level of inhibition, less than 10%) when this compound was supplied at the same concentration as either [14C]GB or [14C]DMSA (Table 2). However, significant inhibition of GB and DMSA uptake via both transporters (levels of inhibition, 22 to 58%) was observed when a 10-fold excess of unlabeled DMSP over either [14C]GB or [14C]DMSA was used. By comparison, uptake of [14C]DMSP via ProU was virtually abolished by an equimolar concentration of unlabeled GB or DMSA. Meanwhile, transport of [14C]DMSP via ProP was also strongly inhibited by an equimolar concentration of GB or DMSA (levels of inhibition, 82 and 70%, respectively), but a 10-fold excess of these two osmoprotectants was necessary to fully inhibit DMSP uptake through ProP (Table 2). Collectively, these competition data are also consistent with the kinetic data (Table 1), which indicated that ProU and ProP exhibited higher affinities for GB and DMSA than for DMSP.
Fate of DMSA and DMSP.
Glucose, the carbon and energy source in M63 medium, was replaced by either DMSA (10 mM) or DMSP (10 mM) in order to determine if E. coli MC4100 could use these sulfonium compounds as growth substrates. No increases in culturable counts and turbidity were observed after 2 days of incubation (data not shown). Thus, E. coli could not grow at the expense of DMSA or DMSP. Also, no 14CO2 evolved from unstressed and stressed cultures of MC4100 that were grown overnight in M63 medium supplemented with 10 mM glucose plus either [methyl-14C]DMSA or [1-14C]DMSP. Moreover, a chromatographic and electrophoretic analysis of ethanolic extracts of these cultures (17, 41) showed that the radioactivity supplied to the cells was always quantitatively recovered in the cytoplasm in the form of either [14C]DMSA or [14C]DMSP (data not shown). Thus, E. coli was not able to catabolize DMSA and DMSP, just as this bacterium is not able to catabolize GB and many other betaines (8, 41, 47).
The maximal amounts of osmoprotectants accumulated by dense cell suspensions of E. coli MC4100 (ca. 2 × 108 CFU/ml) grown in hyperosmotic M63 medium supplemented with 1 mM [14C]DMSA, 1 mM [14C]DMSP, or 1 mM [14C]GB were easily determined by measuring the radioactivities of these compounds in filtered cell suspensions. Quantification of osmolytes was performed in the mid-exponential phase of growth. The amount of cytosolic [14C]DMSA in salt-stressed MC4100 increased with the osmolarity of the growth medium, starting at 65 nmol · mg of protein−1 in cells grown without NaCl and reaching 390, 575, and 685 nmol · mg of protein−1 in stressed cells grown in M63 medium supplemented with 0.3, 0.6, and 0.8 M NaCl, respectively. Likewise, the levels of cytosolic DMSP increased as the salinity of the growth medium increased and were comparable to the DMSA and GB levels measured in cells grown at the same salinities (Table 3). Thus, DMSA, DMSP, and GB contributed equally to osmotic adjustment in E. coli MC4100. The steady-state levels of accumulated GB and DMSA were also measured in dilute suspensions of MC4100 (105 CFU/ml) incubated in M63 medium containing 0.3 M NaCl and 150 nM [14C]GB or 150 nM [14C]DMSA. The maximal levels of these two osmoprotectants were comparable to the maximal levels found in dense cell suspensions incubated in isoosmotic M63 medium supplemented with either 1 mM [14C]GB or 1 mM [14C]DMSA (Table 1).
TABLE 3.
Accumulation of osmoprotectants by salt-stressed E. coli MC4100a
| NaCl concn in M63 medium (M) | Amt of osmoprotectant accumulated (nmol mg of protein−1)
|
||
|---|---|---|---|
| DMSA | DMSP | GB | |
| 0 | 65 | 85 | 40 |
| 0.3 | 390 | 435 | 250 |
| 0.6 | 575 | 580 | 525 |
| 0.8 | 685 | 700 | 630 |
Cultures were grown to the mid-exponential phase in M63 medium supplemented with NaCl. The steady-state levels of accumulated osmoprotectants were measured after dense cell suspensions (OD570, 1) were incubated in the presence of [14C]GB, [14C]DMSA, or [14C]DMSP at a concentration of 1 mM (about 100,000 dpm in 200 μl).
Scavenging of nanomolar concentrations of osmoprotectants by confined cultures of E. coli exposed to seawater.
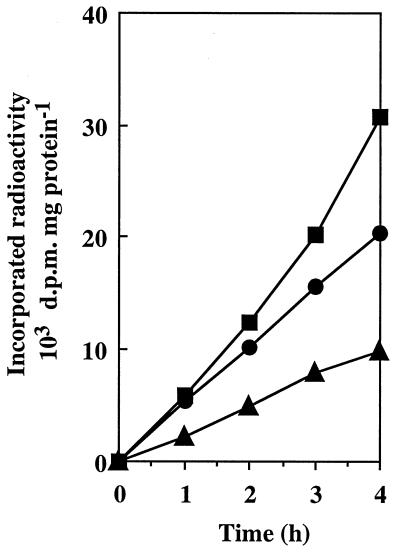
Natural populations of free-living marine bacteria can salvage nanomolar levels of environmental GB and DMSP from seawater and can accumulate these compounds as cytosolic osmolytes (28, 29, 52). However, it is not known if E. coli (which is a common indicator of contamination of estuarine and coastal waters [17, 18]) can take up very low levels of osmoprotectants under conditions that approach natural conditions. Therefore, we designed an experiment to evaluate the ability of E. coli to scavenge nanomolar concentrations of GB and its two sulfonium analogs from seawater devoid of an exogenously added source of energy. Specifically, osmoadapted cells were maintained in a diffusion microchamber which was immersed in autoclaved seawater containing either 1 nM [14C]GB, 1 nM [14C]DMSA, or 50 nM [14C]DMSP. Uptake of the radiolabeled osmoprotectants by the confined cells was determined at 1-h intervals over a 4-h period. The amounts of radioactivity retained on Whatman GF/F filters after the external seawater was filtered were always negligible (data not shown). This indicated that the bacteria remained confined in the diffusion chamber throughout the experiment. Figure 4 shows that uptake of the three osmoprotectants by confined cells of wild-type E. coli MC4100 was linear with time throughout the 4-h uptake period. MC4100 cells scavenged [14C]DMSP, [14C]DMSA, and [14C]GB from oligotrophic seawater at constant rates of 2,500, 6,400, and 5,200 dpm h−1 mg of protein−1, respectively. Similar uptake rates were obtained for the proP proU+ strain BK32, but no uptake of radiolabeled osmoprotectants was detected with the proP+ proU strain GM50. Thus, ProU was apparently the sole transporter involved in uptake of nanomolar concentrations of osmoprotectants from seawater.
FIG. 4.
Uptake of nanomolar concentrations of osmoprotectants by confined cultures of E. coli MC4100 exposed to natural seawater. Osmoadapted cultures (ca. 109 CFU ml−1) were pregrown in M63 medium supplemented with 0.3 M NaCl and were incubated in the presence of 1 nM [14C]GB (●), 1 nM [14C]DMSA (■), or 50 nM [14C]DMSP (▴). Uptake assays were performed as described in Materials and Methods.
DISCUSSION
We demonstrated in this study that osmoprotection of E. coli by DMSA and DMSP depends on the functionality of the ProU and ProP osmoporters. Moreover, we found that the minimal concentration of GB, DMSA, and DMSP that is sufficient to relieve osmotic inhibition of growth of E. coli cells expressing the high-affinity ProU general osmoporter is 1 nM (19, 21, 34). In contrast, ProP, the low-affinity GB-proline porter (10), does not provide osmoprotection to E. coli at very low concentrations (1 nM to 1 μM) of GB or its sulfonium analogs. The 1 nM threshold concentration required by proU+ strains is considerably lower than the minimal concentrations of GB and DMSP previously shown to confer maximal osmoprotection to E. coli and many other bacteria (usually 10 to 500 μM [11, 12, 31, 41, 42]). The striking differences between the previously published values and our data are not contradictory. They reflect (i) the fact that the cultures in this study were inoculated at very low initial cell densities (300 ± 100 CFU/ml) and (ii) the fact that we monitored bacterial growth by a colony enumeration technique instead of commonly used spectrophotometric methods, which require much higher initial cell densities (ca. 5 × 107 CFU/ml) to measure bacterial growth. Previously, cultures with low initial cell densities and enumeration techniques have rarely been used together to evaluate the biological activity of bacterial osmoprotectants. Koo and Booth (31) combined these experimental approaches and showed that ProU can effect osmoprotection of S. typhimurium by as little as 100 nM GB. This value is also consistent with our data (which showed that osmoprotection of proU+ strains of E. coli occurred with only 1 nM GB), because Koo and Booth (31) enumerated viable Salmonella cells, which were inoculated at densities that were about 100 times higher than the initial densities of E. coli cells used in this study. Thus, the osmoprotective activity of GB (and its sulfonium analogs) for enteric bacteria is primarily determined by the functionality of the ProU high-affinity osmoporter, as well as the relative proportions of molecules of osmoprotectants and bacterial cells, rather than by the concentration of the osmoactive solutes in the growth medium. This interpretation was validated by the fact that similar steady-state levels of [14C]GB and [14C]DMSA accumulated in dense MC4100 cell suspensions that were supplemented with the osmoprotectants at a high concentration (1 mM) and in dilute suspensions that were supplemented with the osmoprotectants at a low concentration (150 nM).
The fact that nanomolar levels of osmoprotectants confer a considerable growth advantage (enhanced salinity tolerance) to proU+ strains but not proP+ strains of E. coli (Fig. 1 and 3) is completely consistent with the transport data presented in this study. Indeed, we found that uptake of GB and DMSA via ProP is undetectable at substrate concentrations of 200 nM or less. Moreover, we demonstrated that ProU operates over a broad spectrum of substrate concentrations that encompass the nanomolar levels of GB and DMSP found in marine environments (27, 30, 51) and the millimolar concentrations of osmoprotectants commonly used in standard laboratory osmoprotection bioassays (11, 41, 42). Thus, aside from MC4100, other wild-type strains of E. coli and S. typhimurium, which generally possess both ProU and ProP porters (4, 5, 7, 9, 47), should also take advantage of a wide range of osmoprotectant concentrations for osmoregulation purposes. Moreover, it is noteworthy that the micromolar Km values obtained for GB and DMSA uptake via ProP and ProU in E. coli are comparable to the Km values reported for GB uptake via ProP and ProU in S. typhimurium (4, 5), as well as for uptake of GB and other osmoprotectants in numerous species of bacteria (1, 8, 22, 23, 42, 45). Furthermore, the multiplicity of osmoporters is a genetic feature common to many bacterial species (4, 5, 9, 23, 26). Future research should determine which of the carriers previously characterized as high-affinity osmoporters can also mediate the uptake of nanomolar concentrations of environmental osmoprotectants.
Crossed competition assays showed that ProU and ProP exhibit higher specificities for GB than for DMSA and DMSP (Table 2). Globally, the results of these competition assays are consistent with the results of transport kinetic studies. However, the fact that ProU has a higher specificity for GB than for DMSA apparently is not consistent with the observation that ProU has similar kinetic parameters for GB and DMSA (Table 1). Nonetheless, the fact that DMSA is a poor competitor of GB uptake via ProU is consistent with the observation that DMSA does not compete with [14C]GB for binding to the periplasmic GB-binding protein (GBBP) encoded by ProU (43). Moreover, in accordance with these observations, [14C]DMSA does not bind to the GBBP of E. coli MC4100 under any of the well-established experimental conditions (2, 19, 21) that allow effective binding of [14C]GB to this protein (43). This indicates that the GBBP does not recognize DMSA as a substrate, as it also fails to recognize other structural analogs of GB (19, 21, 22). Therefore, the ProU-encoded GBBP of E. coli is highly substrate specific. The very narrow substrate specificity of the GBBP could explain why DMSA is a weak competitor of GB uptake via ProU. However, it is not clear how ProU can compensate for a lack of DMSA binding activity (compared to a high-affinity GB binding activity [KD, 1.4 μM] [43]) and still take up DMSA and GB at similar rates with similar affinities.
Osmoprotection of proU+ strains of E. coli by nanomolar concentrations of GB and its sulfonium analogs is of prime ecological interest. This ecological interest arises from the presence of nanomolar concentrations of GB and DMSP in marine ecosystems, such as shallow coastal waters (concentrations, 1 to 10 nM) and estuarine sediments (DMSP concentrations, up to 200 nM) (27, 30, 51), which are often colonized by thick mats of GB- or DMSP-producing algae (24, 25, 48, 49). Obviously, these levels of environmental osmoprotectants are compatible with the operation of ProU but are not compatible with the functioning of ProP. Furthermore, it has been shown recently that natural populations of as-yet-unspecified marine bacteria can also salvage nanomolar levels of GB, choline (a natural precursor of GB), and DMSP from seawater and can accumulate these compounds as cytosolic osmolytes (28, 29, 52). Here, we found that proU+ strains of E. coli (MC4100 and BK32) can take up nanomolar levels of GB and DMSA (1 nM), as well as DMSP (50 nM), from oligotrophic seawater. This observation is consistent with the fact that proU::lacZ gene fusions, but not proP::lacZ fusions, are expressed at appreciable levels when E. coli MC4100 is incubated in seawater (16). Collectively, these data indicate that ProU is apparently the sole uptake route that is physiologically and ecologically relevant for stressed E. coli cells in natural environments with very low concentrations of osmoprotectants.
The presence of physiologically active concentrations of bacterial osmoprotectants in marine ecosystems may also have sanitary implications. These implications stem from the ubiquity of the ProU osmoporter in E. coli strains, including clinical isolates (9), and from the episodic occurrence of enterotoxigenic E. coli contaminants in seafood and recreational waters, where these strains may pose a hazard to human health (17, 18, 44). Finally, it has been reported previously that exogenously added GB enhances the survival of E. coli MC4100 and other members of the family Enterobacteriaceae in seawater and marine sediments (14, 15, 17). However, most of the survival studies were performed with dense bacterial suspensions and near-millimolar levels of GB, two factors that are not likely to occur simultaneously under natural conditions. Therefore, it will be interesting to determine whether nanomolar levels of environmental GB and DMSP can also confer a selective advantage (i.e., enhance survival and growth in hyperosmotic ecosystems) to populations of bacteria expressing high-affinity osmoporters over species or strains lacking such transporters.
ACKNOWLEDGMENTS
We thank E. Bremer and M. Villarejo for providing bacterial strains and C. Blanco for helpful discussions.
This work was supported by grants from the Direction de la Recherche et des Etudes Doctorales and the Centre National de la Recherche Scientifique (DSV). V. Pichereau was the recipient of a research fellowship from the Ministère de l’Education Nationale et de la Recherche Scientifique.
REFERENCES
- 1.Bae J-H, Anderson S H, Miller K J. Identification of a high-affinity glycine betaine transport system in Staphylococcus aureus. Appl Environ Microbiol. 1993;59:2734–2736. doi: 10.1128/aem.59.8.2734-2736.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barron A, Jung J U, Villarejo M. Purification and characterization of a glycine betaine binding protein from Escherichia coli. J Biol Chem. 1987;262:11841–11846. [PubMed] [Google Scholar]
- 3.Blunden G, Smith B E, Irons M W, Yang M-H, Roch O G, Patel A V. Betaines and tertiary sulphonium compounds from 62 species of marine algae. Biochem Syst Ecol. 1992;20:373–388. [Google Scholar]
- 4.Cairney J, Booth I R, Higgins C F. Osmoregulation of gene expression in Salmonella typhimurium: proU encodes an osmotically induced betaine transport system. J Bacteriol. 1985;164:1224–1232. doi: 10.1128/jb.164.3.1224-1232.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cairney J, Booth I R, Higgins C F. Salmonella typhimurium proP gene encodes a transport system for the osmoprotectant betaine. J Bacteriol. 1985;164:1218–1223. doi: 10.1128/jb.164.3.1218-1223.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers S T, Kunin C M, Miller D, Hamada A. Dimethylthetin can substitute for glycine betaine as an osmoprotectant molecule for Escherichia coli. J Bacteriol. 1987;169:4845–4847. doi: 10.1128/jb.169.10.4845-4847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csonka L N, Epstein W. Osmoregulation. In: Neidhardt F C, editor. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: ASM Press; 1996. pp. 1210–1223. [Google Scholar]
- 8.Csonka L N, Hanson A D. Prokaryotic osmoregulation: genetics and physiology. Annu Rev Microbiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- 9.Culham D E, Emmerson K S, Lasby B, Mamelak D, Steer B A, Gyles C L, Villarejo M, Wood J M. Genes encoding osmoregulatory proline/glycine betaine transporters and the proline catabolic system are present and expressed in diverse clinical Escherichia coli isolates. Can J Microbiol. 1994;40:397–402. doi: 10.1139/m94-065. [DOI] [PubMed] [Google Scholar]
- 10.Culham D E, Lasby B, Marangoni A G, Milner J L, Steer B A, Van Nues R W, Wood J M. Isolation and sequencing of Escherichia coli gene proP reveals unusual structural features of the osmoregulatory proline/betaine transporter, ProP. J Mol Biol. 1993;229:268–276. doi: 10.1006/jmbi.1993.1030. [DOI] [PubMed] [Google Scholar]
- 11.Diaz M R, Taylor B F. Metabolism of methylated osmolytes by aerobic bacteria from Mono Lake, a moderately hypersaline, alkaline environment. FEMS Microbiol Ecol. 1996;19:239–247. [Google Scholar]
- 12.D’Souza-Ault M R, Smith L T, Smith G M. Roles of N-acetylglutaminylglutamine amide and glycine betaine in adaptation of Pseudomonas aeruginosa to osmotic stress. Appl Environ Microbiol. 1993;59:473–478. doi: 10.1128/aem.59.2.473-478.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galinski E A, Stein M, Amendt B, Kinder M. The kosmotropic (structure-forming) effect of compensatory solutes. Comp Biochem Physiol A. 1997;117A:357–365. [Google Scholar]
- 14.Gauthier M J, Flatau G N, Breittmayer V A. Protective effect of glycine betaine on survival of Escherichia coli cells in marine environments. Water Sci Technol. 1991;24:129–132. [Google Scholar]
- 15.Gauthier M J, Flatau G N, Le Rudulier D, Clément R L, Combarro Combarro M-P. Intracellular accumulation of potassium and glutamate specifically enhances survival of Escherichia coli in seawater. Appl Environ Microbiol. 1991;57:272–276. doi: 10.1128/aem.57.1.272-276.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gauthier M J, Le Rudulier D. Survival in seawater of Escherichia coli cells grown in marine sediments containing glycine betaine. Appl Environ Microbiol. 1990;56:2915–2918. doi: 10.1128/aem.56.9.2915-2918.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghoul M, Bernard T, Cormier M. Evidence that Escherichia coli accumulates glycine betaine from marine sediments. Appl Environ Microbiol. 1990;56:551–554. doi: 10.1128/aem.56.2.551-554.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghoul M, Minet J, Bernard T, Dupray E, Cormier M. Marine macroalgae as osmoprotective source for Escherichia coli. Microb Ecol. 1995;30:171–181. doi: 10.1007/BF00172572. [DOI] [PubMed] [Google Scholar]
- 19.Gouesbet G, Jebbar M, Talibart R, Bernard T, Blanco C. Pipecolic acid is an osmoprotectant for Escherichia coli taken up by the general osmoporters ProU and ProP. Microbiology. 1994;140:2415–2422. doi: 10.1099/13500872-140-9-2415. [DOI] [PubMed] [Google Scholar]
- 20.Gröne T, Kirst G O. Aspects of dimethylsulfoniopropionate effects on enzymes isolated from the marine phytoplankter Tetraselmis subcordiformis (Stein) J Plant Physiol. 1991;138:85–91. [Google Scholar]
- 21.Haardt M, Kempf B, Faatz E, Bremer E. The osmoprotectant proline betaine is a major substrate for the binding-protein-dependent transport system ProU of Escherichia coli K-12. Mol Gen Genet. 1995;246:783–786. doi: 10.1007/BF00290728. [DOI] [PubMed] [Google Scholar]
- 22.Jebbar M, Talibart R, Gloux K, Bernard T, Blanco C. Osmoprotection of Escherichia coli by ectoine: uptake and accumulation characteristics. J Bacteriol. 1992;174:5027–5035. doi: 10.1128/jb.174.15.5027-5035.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kappes R M, Kempf B, Bremer E. Three transport systems for the osmoprotectant glycine betaine operate in Bacillus subtilis: characterization of OpuD. J Bacteriol. 1996;178:5071–5079. doi: 10.1128/jb.178.17.5071-5079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karsten U, Kirst G O. Intracellular solutes, photosynthesis and respiration of the green alga Blidingia minima in response to salinity stress. Bot Acta. 1989;102:123–128. [Google Scholar]
- 25.Karsten U, Wiencke C, Kirst G O. The effect of salinity changes upon the physiology of eulittoral green macroalgae from Antarctica and southern Chile. 2. Intracellular inorganic ions and organic compounds. J Exp Bot. 1991;42:1533–1539. [Google Scholar]
- 26.Kempf B, Bremer E. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolarity environments. Arch Microbiol. 1998;170:319–330. doi: 10.1007/s002030050649. [DOI] [PubMed] [Google Scholar]
- 27.Kiene R P. Turnover of dissolved DMSP in estuarine and shelf waters from the northern Gulf of Mexico. In: Kiene R P, Visscher P, Keller M, Kirst G O, editors. Environmental and biological chemistry of dimethylsulfoniopropionate and related sulfonium compounds. New York, N.Y: Plenum Press; 1996. pp. 337–349. [Google Scholar]
- 28.Kiene R P. Uptake of choline and its conversion to glycine betaine by bacteria in estuarine waters. Appl Environ Microbiol. 1998;64:1045–1051. doi: 10.1128/aem.64.3.1045-1051.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiene R P, Hoffmann Williams L P, Walker J E. Sea water microorganisms have a high-affinity glycine betaine uptake system which also recognizes dimethylsulfoniopropionate. Aquat Microb Ecol. 1998;15:39–51. [Google Scholar]
- 30.King G M. Distribution and metabolism of quaternary amines in marine sediments. In: Blackburn T H, Sorensen J, editors. Nitrogen cycling in coastal marine environments. New York, N.Y: John Wiley & Sons, Inc.; 1988. pp. 143–173. [Google Scholar]
- 31.Koo S P, Booth I R. Quantitative analysis of growth stimulation by glycine betaine in Salmonella typhimurium. Microbiology. 1994;140:617–621. doi: 10.1099/00221287-140-3-617. [DOI] [PubMed] [Google Scholar]
- 32.Ledyard K M, Dacey J W H. Dimethylsulfide production from dimethylsulfoniopropionate by a marine bacterium. Mar Ecol Prog Ser. 1994;110:95–103. [Google Scholar]
- 33.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 34.Lucht J M, Bremer E. Adaptation of Escherichia coli to high osmolarity environments: osmoregulation of the high-affinity glycine betaine transport system ProU. FEMS Microbiol Rev. 1994;14:3–20. doi: 10.1111/j.1574-6976.1994.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 35.Mason T G, Blunden G. Quaternary ammonium and tertiary sulphonium compounds of algal origin as alleviators of osmotic stress. Bot Mar. 1989;32:313–316. [Google Scholar]
- 36.Matrai P A, Keller M D. Total organic sulfur and dimethylsulfoniopropionate in marine phytoplankton: intracellular variations. Mar Biol. 1994;119:61–68. [Google Scholar]
- 37.May G, Faatz E, Villarejo M, Bremer E. Binding protein dependent transport of glycine betaine and its osmotic regulation in Escherichia coli K-12. Mol Gen Genet. 1986;205:225–233. doi: 10.1007/BF00430432. [DOI] [PubMed] [Google Scholar]
- 38.Nishiguchi M K, Somero G N. Temperature- and concentration-dependence of compatibility of the organic osmolyte β-dimethylsulfoniopropionate. Cryobiology. 1992;29:118–124. doi: 10.1016/0011-2240(92)90011-p. [DOI] [PubMed] [Google Scholar]
- 39.Paquet L, Lafontaine P J, Saini H, James F, Hanson A D. Evidence en faveur de la présence du 3-dimethylsulfoniopropionate (DMSP) chez une large gamme d’angiospermes. Can J Bot. 1995;73:1889–1896. [Google Scholar]
- 40.Paquet L, Rathinasabapathi B, Saini H, Zamir L, Gage D A, Huang Z-H, Hanson A D. Accumulation of the compatible solute 3-dimethylsulfoniopropionate in sugarcane and its relatives, but not other gramineous crops. Aust J Plant Physiol. 1994;21:37–48. [Google Scholar]
- 41.Perroud B, Le Rudulier D. Glycine betaine transport in Escherichia coli: osmotic modulation. J Bacteriol. 1985;161:393–401. doi: 10.1128/jb.161.1.393-401.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pichereau V, Pocard J-A, Hamelin J, Blanco C, Bernard T. Differential effects of dimethylsulfoniopropionate, dimethylsulfonioacetate, and other S-methylated compounds on the growth of Sinorhizobium meliloti at low and high osmolarities. Appl Environ Microbiol. 1998;64:1420–1429. doi: 10.1128/aem.64.4.1420-1429.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pichereau, V., and A. Cosquer. Unpublished data.
- 44.Pommepuy M, Butin M, Derrien A, Gourmelon M, Colwell R R, Cormier M. Retention of enteropathogenicity by viable but nonculturable Escherichia coli exposed to seawater and sunlight. Appl Environ Microbiol. 1996;62:4621–4626. doi: 10.1128/aem.62.12.4621-4626.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Proctor L M, Lai R, Gunsalus R P. The methanogenic archaeon Methanosarcina thermophila TM-1 possesses a high-affinity glycine betaine transporter involved in osmotic adaptation. Appl Environ Microbiol. 1997;63:2252–2257. doi: 10.1128/aem.63.6.2252-2257.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Randall K, Lever M, Peddie B A, Chambers S T. Accumulation of natural and synthetic betaines by a mammalian renal cell line. Biochem Cell Biol. 1996;74:283–287. doi: 10.1139/o96-030. [DOI] [PubMed] [Google Scholar]
- 47.Randall K, Lever M, Peddie B A, Chambers S T. Competitive accumulation of betaines by Escherichia coli K-12 and derivative strains lacking betaine porters. Biochim Biophys Acta. 1995;1245:116–120. doi: 10.1016/0304-4165(95)00071-i. [DOI] [PubMed] [Google Scholar]
- 48.Reed R H. Measurement and osmotic significance of β-dimethylsulphoniopropionate in marine macroalgae. Mar Biol Lett. 1983;34:173–181. [Google Scholar]
- 49.Reed R H. The osmotic responses of Polysiphonia lanosa (L.) Tandy from marine and estuarine sites: evidence for incomplete recovery of turgor. J Exp Mar Biol Ecol. 1983;68:169–193. [Google Scholar]
- 50.Rhodes D, Hanson A D. Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:357–384. [Google Scholar]
- 51.Turner S M, Malin G, Liss P S, Harbour D S, Holligan M H. The seasonal variation of dimethyl sulfide and dimethylsulfoniopropionate concentrations in nearshore waters. Limnol Oceanogr. 1988;33:364–375. [Google Scholar]
- 52.Wolfe G V. Uptake and retention of dissolved DMSP by marine bacteria with subsequent degradation during bacterivory. In: Kiene R P, Visscher P, Keller M, Kirst G O, editors. Environmental and biological chemistry of dimethylsulfoniopropionate and related sulfonium compounds. New York, N.Y: Plenum Press; 1996. pp. 277–291. [Google Scholar]