Summary
Patients are at risk for several types of lung injury in the perioperative period including atelectasis, pneumonia, pneumothorax, acute lung injury, and acute respiratory distress syndrome. Anaesthetic management can cause, exacerbate, or ameliorate these injuries. This review examines the effects of perioperative mechanical ventilation and its role in ventilator-induced lung injury. Lung protective ventilatory strategies to specific clinical situations such as cardiopulmonary bypass and one-lung ventilation along with newer novel lung protective strategies are discussed.
Keywords: lung injury, acute; lung injury, ventilator-induced; ventilation, mechanical
Key points.
-
•
Mechanical ventilation can have adverse effects on pulmonary function by several distinct mechanisms.
-
•
Patients undergoing one-lung ventilation or cardiopulmonary bypass are at increased risk of developing acute lung injury (ALI).
-
•
Protective ventilatory strategies may prevent or reduce ALI.
-
•
There is a lack of randomized controlled trials to guide optimal intra-operative ventilation.
Anaesthetists manage patients with both normal and injured lungs in the perioperative setting. The incidence of pulmonary complications after non-cardiac surgery is comparable with that of cardiac complications (2.7% vs 2.5%, respectively).1 Pulmonary complications, specifically respiratory failure requiring ventilation, are associated with high morbidity and mortality, increased costs, and length of hospital stay. Normal lungs need protection from multiple potentially injurious factors including, but not limited to, cardiopulmonary bypass (CPB), sepsis, trauma, lung ischaemia–reperfusion, and blood product transfusion. Injured lungs need protection from ongoing injury. Anaesthetic management, particularly mechanical ventilation, can influence the extent and course of perioperative lung injury.
Mechanical ventilation
Historically, anaesthetists ventilate patients in the perioperative period with relatively large tidal volumes. Volumes as high as 15 ml kg−1 ideal body weight have been suggested to avoid intraoperative atelectasis.2 This far exceeds normal spontaneous tidal volumes (6 ml kg−1) common to most mammals.3 Recent studies have identified large tidal volumes as a major risk factor for the development of lung injury in mechanically ventilated patients without acute lung injury (ALI). Gajic and colleagues4 reported that 24% of the patients with normal lungs ventilated in an intensive care unit (ICU) setting for 2 days or longer developed ALI or acute respiratory distress syndrome (ARDS). The main risk factors for ALI were large tidal volumes (odds ratio 1.3 for each ml kg−1 above 6 ml kg−1 predicted body weight), blood product transfusion (odds ratio 3.0), and restrictive lung disease. A prospective study from the same group found that tidal volumes >700 ml and peak airway pressures >30 cm H2O were independently associated with the development of ARDS.5 A study of patients having oesophageal surgery compared the use of tidal volumes of 9 ml kg−1 without PEEP during two- and one-lung ventilation (OLV) vs 9 ml kg−1 during two-lung ventilation and 5 ml kg−1 during OLV with PEEP of 5 cm H2O.6 They found significantly lower serum makers of inflammation [interleukin (IL)-β, IL-6, and IL-8] in the lower tidal volume plus PEEP group. The study demonstrated better oxygenation in the lower tidal volume group during and immediately after OLV, with earlier extubation (postoperative mechanical ventilation duration, 115 vs 171 min).
In a study comparing conventional with protective ventilation in critically ill patients without lung injury, de Oliveira and colleagues7 randomized patients to ventilation with either 10–12 or 6–8 ml kg−1 predicted body weight. In both groups, a PEEP of 5 cm H2O applied and the inspired oxygen fraction (F i o2) titrated to keep haemoglobin oxygen saturations (Spo2) above 90%. At 12 h post-ventilation, inflammatory markers in bronchoalveolar lavage fluid [tumour necrosis factor-α (TNF-α) and IL-8] were significantly higher in the larger tidal volume group. Choi and colleagues8 compared 12 ml kg−1 without PEEP vs 6 ml kg−1 with 10 cm H2O PEEP and showed procoagulant changes in lavage fluid of the larger tidal volume group after 5 h of mechanical ventilation. A recent randomized controlled trial in 150 critically ill patients without ALI compared tidal volumes of 10 vs 6 ml kg−1 predicted body weight.9 The primary endpoints were cytokine levels in bronchoalveolar lavage fluid and plasma and the secondary endpoint was the development of lung injury. The trial was terminated early because the development of lung injury was significantly higher in the larger tidal volume group compared with the lower tidal volume group (13.5% vs 2.6%). The larger tidal volumes were also associated with sustained increase in plasma inflammatory cytokines.
Recent animal work suggests that even non-injurious or so-called protective ventilatory settings can induce lung injury in previously healthy lungs. An animal study using an elegant murine ‘one hit’ ventilator-induced lung injury (VILI) model showed that even the least injurious volume settings induced biochemical and histological changes consistent with lung injury.10 Work with rodents undergoing mechanical ventilation showed significant gene expression (including genes involved in immunity and inflammation such as IL-1β and IL-6) after only 90 min of protective ventilation in the absence of a primary pulmonary insult.11 Whether this has an impact on clinical outcomes is unknown, but we can surmise the following based on the preceding work.
-
(i)
Non-physiological ventilation (which is ventilation with larger tidal volumes) in healthy lungs induces ALI.
-
(ii)
Protective lung ventilation in non-injured lungs and in the absence of a primary pulmonary insult may initiate subclinical VILI, as evidenced by gene expression and inflammatory markers, and possibly sensitize the lung to a ‘second hit’.
How relevant is this to the practising anaesthetist? Millions of anaesthetics are given worldwide each year without apparent lung injury. As mentioned, pulmonary complications share an incidence that matches, if not exceeds cardiac complications, with ALI being the most common cause of postoperative respiratory failure and is associated with decreased postoperative survival.12 A prospective case-controlled study by Fernandez-Perez and colleagues looking at intraoperative ventilator settings and ALI after elective surgery in over 4000 patients showed a 3% incidence of ALI in high-risk elective surgeries. Compared with controls, patients with ALI had significantly lower 60 day and 1 year postoperative survival (99% vs 73% and 92% vs 56%, respectively) and had increased length of hospital stay. Interestingly, in this study, intraoperative peak airway pressure, but not tidal volume, PEEP, or (F I O2, was associated with ALI.
Is there evidence that anaesthetists are ventilating patients in a potentially injurious manner? A retrospective cohort study conducted at a large tertiary medical centre provides some insight.13 This study looked at over 11 000 patients receiving general anaesthesia between 2005 and 2009 who had at least one arterial blood gas measurement. Patients undergoing OLV and CPB, and those at extremes of height and weight, were excluded. The authors examined intraoperative ventilator management of hypoxia by assessing ventilation settings at different Pao2/F i o2 ratios (P/F ratios). Their aim was to determine the current management of hypoxaemic patients and frequency at which lung protective strategies are implemented in response to low P/F ratios and to assess if other methods were preferentially used. They found that similar ventilation strategies were used regardless of P/F ratios, with tidal volumes ranging between 8.6 and 9.1 ml kg−1 predicted body weight and an average PEEP of 2.5–5.5 cm H2O. The results of the study suggest that anaesthetists are treating hypoxaemia with higher (F I O2 and increasing peak inspiratory pressure (PIP). There was little evidence that in patients with lower P/F ratios, ALI/ARDS was considered in the differential diagnosis or that lung protective strategies were used. There was a trend over the 5 yr, however, to lower tidal volume (decrease by 2 ml kg−1 predicted body weight), lower PIP (decreased by 5 cm H2O), and higher PEEP (increased by 2 cm H2O). Thus, lung protective strategies from critical care medicine are beginning to influence anaesthetic management. A key point highlighted by this study is that it is important to use predicted body weight rather than actual body weight in determining tidal volume. Although there are several limitations to this study, it provides some evidence of evolving anaesthetic ventilator management.
Ventilator-induced lung injury
The phenomenon of VILI is well recognized. VILI can exacerbate existing lung injury or sensitize the lung to further injury (the so-called two-hit model, which is significant with large transfusions, CPB, and associated lung ischaemia–reperfusion injury). VILI involves a complex interaction of over-distension (volutrauma), increased transpulmonary pressure (barotrauma), cyclic opening and closing of alveoli (atelectotrauma), and inflammatory mediators (biotrauma).14 15 This interaction involves the alveolar epithelium, vascular endothelium, polymorphonuclear leucocyte (PMN) recruitment and activation, and apoptosis/necrosis balance. Mechano-transduction is the key link between the physical forces (such as stress and strain) imposed on the lung and intracellular signalling pathways leading to the production of cytokines. Shear stress induces pro-inflammatory cytokines [specifically up-regulation of nuclear factor (NF)-ĸB] in endothelial, epithelial, and macrophage cells. Although a degree of lung stretch is important for surfactant production, the pattern and magnitude of stretch is critical to the activation of an inflammatory cascade.
PMNs play a central role in mediating tissue injury, being primed and activated by the inflammatory mediators. These mediators can be released from cells even without tissue disruption. This so-called ‘loss of compartmentalization’ results in spillover of these mediators into the systemic circulation. Thus, this ‘biotrauma’ not only aggravates ongoing lung injury but can also induce or exacerbate remote organ dysfunction. A study looking at novel mechanisms of remote organ injury resulting from VILI showed that mechanical ventilation can lead to epithelial cell apoptosis in the kidney and small intestine with accompanying biochemical evidence of organ dysfunction.16 In mice undergoing injurious mechanical ventilation, alveolar stretch-induced adhesion molecules are expressed not only in the lung but also in the liver and kidney. In addition, cytokine and chemokine expression in the pulmonary, hepatic, and renal tissue after mechanical ventilation is accompanied by an enhanced recruitment of PMNs to these organs.17
The mechanism of injury-induced cell death is important in the pulmonary response to an insult. Apoptosis is an organized, programmed cell death mechanism without the release of cell contents while necrosis involves cell membrane disruption and subsequent inflammation. Low mechanical stress causes primarily pulmonary apoptosis, whereas high stress changes the balance between apoptosis and necrosis, leading to more necrosis. Apoptotic cell death plays a role in protecting the lung against mechanical stress and is vital in maintaining alveolar epithelial integrity. Apoptosis is suppressed by the mitogen-activated protein kinase (MAPK) pathway, a key transducer between the cell membrane and the nucleus. Overexpression of MAPKs, as seen in high-stretch ventilation strategies, results in the inhibition of less damaging pro-apoptotic mechanisms.18
Atelectasis
Atelectasis occurs frequently after open surgical procedures and in up to 90% of the patients undergoing general anaesthesia.19 It is a pathological state that has direct and indirect effects on the development or aggravation of ALI.
Direct, stretch-independent effects include (i) a propensity to infection due to impaired alveolar macrophage function and (ii) local hypoxia and hyperoxia. Atelectasis causes local alveolar hypoxia due to collapse and systemic hypoxaemia due to increased pulmonary shunt. An increase in (F i o2 causes hyperoxia in the aerated lung. Alveolar hypoxia can induce lung inflammation, and alveolar hyperoxia can worsen atelectasis by absorption and lead to an excess of reactive oxygen species, all contributing to ALI.
Indirect, stretch-dependent effects include mechanical stress-induced injury discussed above. Interestingly, alveolar injury is maximal in the non-atelectatic regions of the lung, consistent with a shift of tidal volume to the aerated lung units with subsequent over-inflation.
There is concern that lower tidal volumes advocated by protective ventilation strategies might predispose to atelectasis and subsequent ALI. There is conflicting evidence on the influence of tidal volumes on atelectasis and de-recruitment. A study of 15 ICU patients with ALI showed evidence of de-recruitment with a change in tidal volume from 10 to 6 ml kg−1 which was reversed with recruitment manoeuvres and an increase in PEEP.20 In 16 ASA class I and II patients undergoing general anaesthesia without lung injury, there was no difference between conventional tidal volume (10 ml kg−1) and low tidal volume (6 ml kg−1) groups in terms of atelectasis.21 It is clear that techniques to avoid or treat atelectasis, including recruitment manoeuvres and application of appropriate PEEP, are effective in the setting of low tidal volumes.22 The prevention of atelectasis during emergence and after general anaesthesia is often overlooked; a combination of low F i o2 (where appropriate) and continuous positive airway pressure after tracheal extubation decreases atelectasis and leads to better lung function in the early postoperative period.23 24
One-lung ventilation
Patients requiring OLV are heterogeneous, both in terms of underlying pathology and surgical procedure. Both the pathology and the surgical procedure can predispose to or cause ALI. ALI after pulmonary resection was recognized since the beginning of OLV for thoracic surgery. The most well-known report is Zeldin and colleagues’25 compilation of 10 pneumonectomy cases published in 1984 that focused on the role of i.v. overhydration as a cause of post-pneumonectomy pulmonary oedema. Much work has subsequently followed, and our understanding of risk factors, mechanisms of injury, and management strategies for what is now termed post-thoracotomy ALI has greatly advanced.
A thorough retrospective study of 806 pneumonectomies found a 2.5% incidence of post-pneumonectomy pulmonary oedema with 100% mortality in affected patients.26 There was no difference in perioperative fluid balance between post-pneumonectomy ALI cases (24 h fluid balance 10 ml kg−1) compared with matched pneumonectomy controls without ALI (13 ml kg−1). This study used rigorous fluid restriction compared with other reports, which suggests that limiting intraoperative fluids might reduce but not eliminate ALI.27 Post-pneumonectomy ALI has a bimodal time of onset.28 Late cases present 3–10 days after operation and are secondary to obvious causes such as bronchopneumonia or aspiration. Early or ‘primary’ ALI present on postoperative days 0–3. Four factors were independent significant predictors of primary ALI: high intraoperative ventilation pressures, excessive i.v. volume replacement, pneumonectomy, and preoperative alcohol abuse. Looking specifically at ventilation pressures, Licker and colleagues used a ventilatory pressure index taking into account both the duration of OLV and increased inspiratory pressure. This index represented the strongest risk factor for ALI (approximately three-fold increased risk of PIP ≥25 vs 15 cm H2O).
Features of ALI after lung surgery include: an incidence after pneumonectomy of 2–4%, greater frequency after right compared with left pneumonectomy, onset 1–3 days post-surgery, high associated mortality (25–50%), and resistance to standard therapies. Although ALI occurs after lesser resections (e.g. lobectomy), it has a much lower mortality rate. Of interest, in eight of nine cases of unilateral ALI after lobectomy, the affected lung was the non-operated (i.e. the ventilated) lung.29 Although there is an association between postoperative ALI and fluid overload, the non-cardiogenic nature of the pulmonary oedema (low/normal pulmonary occlusion pressures) and the protein-rich oedema fluid is more consistent with an ARDS-type mechanism, with endothelial damage playing a key role.
Postoperative increases in lung permeability of the non-operated lung have been demonstrated after pneumonectomy but not lobectomy.30 This capillary-leak injury might be due to an inflammatory cascade affecting even the non-operative lung that is triggered by lung resection and is proportional to the amount of lung resected.31 32 Oxygen free radical generation in lung cancer patients is related to the duration of OLV.33 Although there is no single mechanism to explain ALI after lung resection, it appears that a spectrum of ALI occurs during all lung resections proportional to the extent of the resection.
End-inspiratory lung volume is a key factor in VILI.34 Many patients, especially emphysema patients, develop auto-PEEP with OLV, thus inspiration begins at a lung volume above functional residual capacity (FRC).35 Using large tidal volumes (10–12 ml kg−1) during OLV in such patients produces end-inspiratory volumes that might cause or contribute to ALI. The effects of PEEP during OLV are variable and very much dependent on the lung mechanics of the individual patient. Initial studies suggested that PEEP led to a deterioration of arterial oxygenation.36 Most chronic obstructive pulmonary disease (COPD) patients develop auto-PEEP during OLV, leading to hyperinflation and increased shunt (Fig. 1 a).37 However, patients with normal lung parenchyma or those with restrictive lung diseases tend to decrease below their FRC at end-expiration during OLV and benefit from external PEEP (Fig. 1 b). Just as in two-lung ventilation, high tidal volumes in OLV cause or contribute to ALI. In a rabbit model of OLV during isolated perfusion, large tidal volume (8 ml kg−1) ventilation produced a picture of ALI absent in animals randomized to a lung protective ventilation pattern (4 ml kg−1 plus PEEP).38
Fig 1.
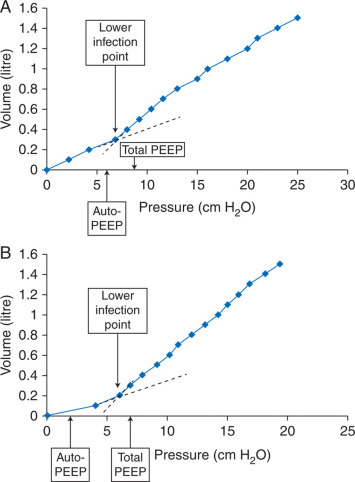
(a) An inspiratory compliance curve (lung volume vs airway pressure) during OLV as the lung is slowly inflated by 100 ml increments in a patient with mild COPD. The lower inflection point of the curve (thought to represent FRC) is at 7 cm H2O. During OLV, this patient developed intrinsic PEEP (measured by the end-expiratory airway occlusion plateau pressure ‘auto-PEEP’) of 6 cm H2O. The addition of 5 cm H2O of PEEP in this patient raised the end-expiratory lung volume above FRC, thus raising pulmonary vascular resistance in the ventilated lung and caused a deterioration in oxygenation. (b) The inspiratory compliance curve during OLV in a patient with normal pulmonary function. The lower inflection point of the curve is at 6 cm H2O. During OLV, this patient developed intrinsic PEEP of 2 cm H2O. The addition of 5 cm H2O of PEEP raised the end-expiratory lung volume to FRC, thus decreasing pulmonary vascular resistance in the ventilated lung and caused an improvement in oxygenation. Based on data from Slinger and colleagues.37
Large resections (pneumonectomy or bi-lobectomy) should be considered to be associated with some degree of ALI. Forty-two per cent of pneumonectomy patients who had been ventilated with peak airway pressures >40 cm H2O had ALI diagnosed radiographically.39 A retrospective study found that post-pneumonectomy respiratory failure was associated with the use of higher intraoperative tidal volumes (8.3 vs 6.7 ml kg−1).40
Current understanding of OLV-associated ALI supports management strategies to minimize lung injury: maintain F I O2 as low as possible, use variable tidal volumes, begin inspiration at FRC, and avoid atelectasis with frequent recruitment manoeuvres.41 42 An observational study in patients undergoing lung cancer surgery appears to confirm this strategy.43 Using a protective lung ventilation strategy (tidal volume <8 ml kg−1 predicted body weight, pressure control ventilation, PIPs <35 cm H2O, external PEEP of 4–10 cm H2O, and frequent recruitment manoeuvres) in a protocol group compared with conventional ventilation in an historical group showed a decreased incidence of ALI (0.9–3.7%), atelectasis (5.0–8.8%), and ICU admissions (9.4–2.5%) and shorter hospital stay (Fig. 2 ). Minimizing pulmonary capillary pressure by avoiding overhydration in patients undergoing pneumonectomy is reasonable while acknowledging that not all perioperative increases in pulmonary artery pressures are due to intravascular volume replacement. Finally, it must be appreciated that not all hyperinflation of the residual lung occurs in the operating theatre. The use of a balanced chest drainage system after pneumonectomy to keep the mediastinum in a neutral position and avoid hyperinflation of the residual lung has been suggested to decrease ALI.44
Fig 2.
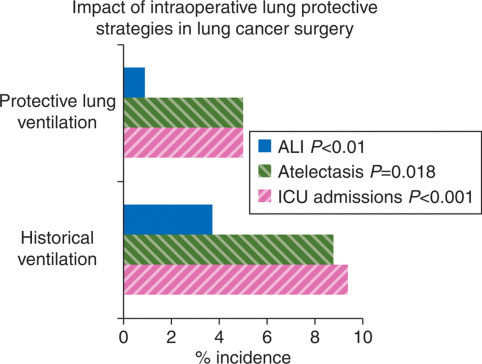
Impact of intraoperative lung protective strategies in lung cancer surgery. Comparison of historical control group vs lung protective ventilation group in patients undergoing OLV for lung cancer surgery showed significant benefits in terms of reduced ALI, atelectasis, and ICU admissions.42
Hypercapnia
Permissive hypercapnia, or hypercapnic acidosis (HCA), is an accepted consequence of lung protective ventilation in patients with ALI/ARDS. Although conventional wisdom holds that HCA is a consequence of relative hypoventilation, HCA per se has protective effects on the pathogenesis of ALI. Secondary analysis of the original ARDSnet data showed that HCA itself was protective in the 12 ml kg−1 tidal volume group.45 There was no additional benefit in the 6 ml kg−1 group. HCA is protective in many models of ALI.46 Beneficial effects include attenuation of lung PMN recruitment, pulmonary and systemic cytokine concentrations, cell apoptosis, and free radical injury. In several animal models, HCA attenuated free radical-mediated lung injury by inhibiting endogenous xanthine oxidase and attenuated lung injury in both early and prolonged sepsis.47 48 Acidosis and hypercapnia might exert distinct biological effects, but this remains to be elucidated.
Experimental hypocapnia causes profound acute parenchymal lung injury and worsens ischaemia–reperfusion injury. However, acceptable limits for both pH and CO2 in the intraoperative setting have not been well defined. Anaesthetists are rightly concerned about: (i) potential adverse effects of high tissue P CO2 including, but not limited to, haemodynamic compromise, increased intracranial blood flow, and increased pulmonary vascular resistance; and (ii) inconsistent correlation between Pao2 and E′CO2. Reassuringly, humans are remarkably tolerant of even extreme hypercarbia and the accompanying acidosis.49 How HCA will translate into beneficial clinical practice remains to be seen, with issues such as timing and patient recovery remaining to be resolved.
Cardiopulmonary bypass
Pulmonary dysfunction after CPB is well described but poorly understood.50 Although the incidence of ARDS after CPB is low (<2%), the mortality associated with it is high (>50%).51 Systemic inflammatory response syndrome (SIRS) initiated by CPB plays a major role, but the pulmonary insult is multifactorial and not all related to CPB itself. Additional factors are general anaesthesia, sternotomy, and breaching of the pleura. CPB-related factors include hypothermia, blood contact with artificial surfaces, ischaemia–reperfusion injury, administration of blood products, and ventilatory arrest. Strategies to potentially limit lung injury during CPB are shown in Table 1 . The strategies in Table 1, while having good theoretical basis, have shown inconsistent results in terms of improving pulmonary outcome. Protective postoperative ventilatory strategies of ‘at risk’ lungs is key. A randomized controlled trial compared the use of high tidal volumes (10–12 ml kg−1) plus low PEEP (2–3 cm H2O) with that of lung protective low tidal volumes (8 ml kg−1) plus high PEEP (10 cm H2O) in patients ventilated for 6 h after CPB for coronary artery bypass surgery.52 Serum and bronchiolar lavage levels of the inflammatory cytokines IL-6 and IL-8 were significantly increased at 6 h only in the high tidal volume ventilation group.
Table 1.
| Intervention | Mechanism of action |
|---|---|
| Off-pump surgery | Reduced cytokine and SIRS response |
| Drugs (steroids, aprotinin) | Reduced pro-inflammatory cytokine release |
| Biocompatible circuits | Mimics endothelial surface. Reduces complement activation and inflammatory response |
| Leucocyte filters | Preferentially removes activated leucocytes, attenuates ischaemia–reperfusion injury |
| Ultrafiltration | Removal of destructive and inflammatory substances reducing SIRS response |
| Protective ventilation strategies | Prevents atelectasis, development of hydrostatic oedema, and pulmonary ischaemia |
| Pulmonary perfusion techniques (e.g. Drew–Anderson technique) | Continuous perfusion of lungs |
| Avoid use of oxygenator | |
| Reduced pro-inflammatory cytokines | |
| Meticulous myocardial protection | Limit ischaemia–reperfusion injury to lungs |
Role of anaesthetic agents in lung protection
Volatile agents
Volatile anaesthetic agents have immune-modulatory effects. Much work, especially in the cardiac setting, has been done on the role of volatiles in ischaemia–reperfusion injury and in pre- and post-conditioning. Recent studies in models of ALI, during OLV and in cases of lung ischaemia–reperfusion, suggest that volatile anaesthetics might act as pre- and post-conditioning agents inducing lung protection by the inhibition of the expression of pro-inflammatory mediators.53 Isoflurane pretreatment in an endotoxin-mediated animal model of lung injury exerted protective effects, as evidenced by reduced PMN recruitment and microvascular protein leakage.54 Post-conditioning with sevoflurane attenuated lung damage and preserved lung function in an in vivo rat ALI model.55 In a prospective study, patients undergoing thoracic surgery with OLV were randomized to either propofol or sevoflurane anaesthesia.56 Inflammatory markers in the non-ventilated lung were reduced and the sevoflurane group had improved outcome and fewer adverse events. A study comparing OLV (tidal volume 10 ml kg−1) with desflurane or propofol anaesthesia examined the inflammatory response in the ventilated lung.57 The inflammatory markers IL-8, IL-10, PMN elastase, and TNF-α were significantly lower in the desflurane group. This exciting work points towards a role for volatile anaesthetics in attenuating the pro-inflammatory response in the lungs to a host of insults.
Nitrous oxide
Owing to its relatively higher solubility compared with oxygen and nitrogen, nitrous oxide plays a role in absorption atelectasis.58 Although this may be helpful in aiding lung collapse in the setting of OLV, there is no strong evidence for or against this agent for lung protection.59
Nitric oxide
Nitric oxide (NO) is implicated in both pro- and anti-inflammatory processes in the lung. It has been shown to attenuate ALI via inhibition of NF-ĸB.60 Although it has theoretical benefits in ALI/ARDS and can temporarily improve oxygenation, it has shown no mortality benefit and is thus not currently recommended for routine use.61 NO is an important mediator in ischaemia–reperfusion injury and might have a role in attenuating this in the setting of lung transplantation.62 Optimal timing and dose are uncertain. As a selective pulmonary vasodilator, it is effective in lowering pulmonary arterial pressure.
I.V. agents
Agents as diverse as local anaesthetics (ropivacaine), induction agents (ketamine, propofol, and thiopental), and α-2-agonists (dexmedetomidine) have shown potential anti-inflammatory effects.63 64 This work is still very preliminary and its clinical significance and application are unknown.
Alternative lung protective strategies
Despite modern lung protective ventilation strategies, some patients have refractory hypoxaemia and hypercarbia. These patients have renewed interest in salvage or rescue therapy for non-responsive ALI/ARDS. The practising anaesthetist needs to be aware of the therapies available, as these patients can present to the operating theatre. Although many of these therapies are aimed at increasing oxygenation, improved oxygenation does not correlate with improved outcome. The goal of rescue strategies for hypoxaemia is to support the patient without inducing further injury, specifically to the lungs.
Novalung iLA membrane ventilator®
Along the continuum of lung protective ventilation therapy in ALI/ARDS is the concept of ultra-protective ventilation utilizing pumpless extracorporeal lung assist and near-static ventilation. The Novalung membrane ventilator allows O2 and CO2 gas exchange via simple diffusion.65 The membranes are biocompatible and provide a non-thrombogenic surface. It is designed to work without a mechanical pump in an arteriovenous configuration, thus requiring an adequate mean arterial pressure to drive flow. Flow rates are typically 1–2 litre min−1, or ∼15% of cardiac output. CO2 clearance is controlled by varying the oxygen flow rate. Oxygenation is variable and might not be sufficient in severe hypoxic disorders. Compared with conventional extracorporeal membrane oxygenation (ECMO), the Novalung is a simple, pumpless, and, very importantly, portable device. Anti-coagulation requirements are much reduced and bleeding complications and blood product requirements are subsequently less.
ARDSnet and animal data demonstrate that lower tidal volumes (3 ml kg−1) compared with even 6 ml kg−1 significantly reduce endothelial and epithelial injury.66 67 In other words, ‘protective’ tidal volumes can still induce VILI. However, the clearance of CO2 and oxygenation become an issue at these lower minute volumes. The Novalung allows for this marked reduction in minute ventilation and the simultaneous correction of PaCO2 and pH. An animal model of post-pneumonectomy lung injury using the Novalung with tidal volumes of 2.2 ml kg−1 and ventilatory frequency of 6 showed significantly better outcomes compared with conventional lung protective strategies.68 Numerous case reports in humans in a variety of clinical scenarios have been very encouraging.69, 70, 71, 72 Tidal volumes ≤3 ml kg−1, low inspiratory plateau pressure, high PEEP, and low ventilatory frequencies are all possible with the Novalung® in situ, causing less VILI and subsequent remote secondary organ failure.
Extracorporeal membrane oxygenation
ECMO has been used successfully for severe respiratory failure.73 It is highly specialized, resource intensive and expensive, and hence limited to specialized centres. It also allows for ultra-protective lung ventilation as described with the Novalung, but has significantly more side-effects, higher cost, and less portability. Although having a place in the ICU setting, it is unlikely to be of much relevance in the operating theatre.
High-frequency oscillatory ventilation
The potential benefits of high-frequency oscillatory ventilation (HFOV) include small tidal volumes, higher mean airway pressure, and maintenance of a constant airway pressure. This should translate to less shear stress and atelectotrauma. HFOV has been shown to improve oxygenation, albeit temporarily, but has not been shown to improve mortality. The few studies that have been performed compared HFOV with conventional ventilation with tidal volumes in the range of 8–10 ml kg−1.74 75 One study showed higher bronchoalveolar inflammatory cytokine levels (IL-8) in the HFOV group compared with conventional ventilation.76 The practicalities of applying HFOV in the operating theatre coupled with little obvious benefit imply that this strategy will not play a large role in lung protective ventilation.
Future lung protection therapies
Beyond those already discussed, there are several therapies that could play a future role in lung protection. Inhaled hydrogen sulphide shows beneficial effects in a model of VILI via inhibition of inflammatory and apoptotic responses independent of its effects on body temperature.77 Inhaled, aerosolized, activated protein C in a sheep model of ALI demonstrated improved oxygenation and lung aeration.78 The use of β-adrenergic agonists has potential benefits by increasing the rate of alveolar fluid clearance and anti-inflammatory effects.79 A randomized controlled trial in 40 patients with ALI showed a decrease in extravascular lung water and plateau airway pressure with i.v. salbutamol, although it showed no difference in outcome.80 Although it is unreasonable to expect there to be a single therapy that will prevent ALI, these exciting findings hold promise in furthering our understanding and management of injured or at risk lungs.
Conclusions
Anaesthetists manage patients with various degrees of pulmonary function including patients with healthy lungs at risk of lung injury to patients with established ALI/ARDS. More patients are at risk for ALI during surgery than previously appreciated. Appropriate perioperative management can prevent or ameliorate lung injury. Are the proven lung protective strategies in ARDS applicable to the perioperative environment, specifically in patients with healthy lungs?81 There is a lack of randomized controlled trials to define optimal intraoperative tidal volume, PEEP, and the use of intraoperative lung recruitment.82 However, applying protective ventilatory strategies in the intraoperative period seems reasonable based on our current understanding of mechanical ventilation and ALI.
Conflict of interest
None declared.
References
- 1.Smetana G. Postoperative pulmonary complications: An update on risk assessment and reduction. Cleve Clin J Med. 2009;76:60–65. doi: 10.3949/ccjm.76.s4.10. [DOI] [PubMed] [Google Scholar]
- 2.Bendixen HH, Hedley-White J, Laver MB. Impaired oxygenation in surgical patients during general anesthesia with controlled ventilation: a concept of atelectasis. N Engl J Med. 1963;96:156–166. doi: 10.1056/NEJM196311072691901. [DOI] [PubMed] [Google Scholar]
- 3.Tenny SM, Remmers JE. Comparative quantitative morphology of the mammalian lung: diffusing area. Nature. 1963;196:54–56. doi: 10.1038/197054a0. [DOI] [PubMed] [Google Scholar]
- 4.Gajic O, Dara SI, Mendez JL, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med. 2004;32:1817–1824. doi: 10.1097/01.CCM.0000133019.52531.30. [DOI] [PubMed] [Google Scholar]
- 5.Gajic O, Frutos-Vivar F, Esteban A, et al. Ventilator settings as a risk factor for acute respiratory distress syndrome in mechanically ventilated patients. Intensive Care Med. 2005;31:922–926. doi: 10.1007/s00134-005-2625-1. [DOI] [PubMed] [Google Scholar]
- 6.Michelet P, D’Journo X-B, Roch A, et al. Protective ventilation influences systemic inflammation after esophagectomy: a randomized controlled study. Anesthesiology. 2006;105:911–919. doi: 10.1097/00000542-200611000-00011. [DOI] [PubMed] [Google Scholar]
- 7.de Oliveira RP, Hetzel MP, Silva M, Dallegrave D, Friedman G. Mechanical ventilation with high tidal volume induces inflammation in patients without lung disease. Crit Care. 2010;14:R39. doi: 10.1186/cc8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi G, Wolthuis EK, Bresser P, et al. Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents alveolar coagulation in patients without lung injury. Anesthesiology. 2006;105:689–695. doi: 10.1097/00000542-200610000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Determann R, Royakkers A, Wolthuis EK, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care. 2010;14:R1. doi: 10.1186/cc8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolthuis EK, Vlaar APJ, Choi G, Roelofs JJTH, Juffermans NP, Schults MJ. Mechanical ventilation using non-injurious ventilation settings causes lung injury in the absence of pre-existing lung injury in healthy mice. Crit Care. 2009;13:R1. doi: 10.1186/cc7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng CSH, Song Wan, Ho AMH, Underwood MJ. Gene expression changes with a ‘non-injurious’ ventilation strategy. Crit Care. 2009;13:403. doi: 10.1186/cc7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-Perez ER, Sprung J, Alessa B, et al. Intraoperative ventilator settings and acute lung injury after elective surgery: a nested case control study. Thorax. 2009;64:121–127. doi: 10.1136/thx.2008.102228. [DOI] [PubMed] [Google Scholar]
- 13.Blum JM, Fetterman DM, Park PK, Morris M, Rosenberg AL. A description of intraoperative ventilator management and ventilation strategies in hypoxic patients. Anesth Analg. 2010;110:1616–1622. doi: 10.1213/ANE.0b013e3181da82e1. [DOI] [PubMed] [Google Scholar]
- 14.Lionetti V, Recchia FA, Ranieri VM. Overview of ventilator-induced lung injury mechanisms. Curr Opin Crit Care. 2005;11:82–86. doi: 10.1097/00075198-200502000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Dos Santos CC, Slutsky AS. Cellular responses to mechanical stress. Invited review: mechanisms of ventilator-induced lung injury: a perspective. J Appl Physiol. 2000;89:1645–1655. doi: 10.1152/jappl.2000.89.4.1645. [DOI] [PubMed] [Google Scholar]
- 16.Imai Y, Parodo J, Kajikawa O, et al. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. J Am Med Assoc. 2003;280:2104–2112. doi: 10.1001/jama.289.16.2104. [DOI] [PubMed] [Google Scholar]
- 17.Hegeman MA, Henmus MP, Heijnen CJ, et al. Ventilator-induced endothelial activation and inflammation in the lung and distal organs. Crit Care. 2009;13:R182. doi: 10.1186/cc8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muders T, Wrigge H. New insights into experimental evidence on atelectasis and causes of lung injury. Best Prac Res Clin Anaesthesiol. 2010;24:171–182. doi: 10.1016/j.bpa.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Duggan M, Kavanagh B. Pulmonary atelectasis: a pathogenic perioperative entity. Anesthesiology. 2005;102:834–854. doi: 10.1097/00000542-200504000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Richard JC, Maggiore SM, Jonson B, et al. Influence of tidal volume on alveolar recruitment. Am J Respir Crit Care Med. 2001;163:1609–1613. doi: 10.1164/ajrccm.163.7.2004215. [DOI] [PubMed] [Google Scholar]
- 21.Cai H, Gong H, Zhang L, Wang Y, Tian Y. Effect of low tidal volume ventilation on atelectasis in patients during general anesthesia: a computed tomographic scan. J Clin Anesth. 2007;19:125–129. doi: 10.1016/j.jclinane.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Tusman G, Bohm SH, Suarez-Shipman F. Alveolar recruitment improves ventilatory efficiency of the lungs during anesthesia. Can J Anaesth. 2004;51:723–727. doi: 10.1007/BF03018433. [DOI] [PubMed] [Google Scholar]
- 23.Tusman G, Bohm SH. Prevention and reversal of lung collapse during the intra-operative period. Best Prac Res Clin Anaesth. 2010;24:183–197. doi: 10.1016/j.bpa.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Squadrone V, Coha M, Cerutti E, et al. Continuous positive airway pressure for the treatment of postoperative hypoxemia: a randomized controlled trial. J Am Med Assoc. 2005;293:589–595. doi: 10.1001/jama.293.5.589. [DOI] [PubMed] [Google Scholar]
- 25.Zeldin RA, Normadin D, Landtwing BS, Peters RM. Postpneumonectomy pulmonary edema. J Thorac Cardiovasc Surg. 1984;87:359–365. [PubMed] [Google Scholar]
- 26.Turnage WS, Lunn JL. Postpneumonectomy pulmonary edema. A retrospective analysis of associated variables. Chest. 1993;103:1646–1650. doi: 10.1378/chest.103.6.1646. [DOI] [PubMed] [Google Scholar]
- 27.Waller DA, Gebitekin C, Saundres NR, Walker DR. Noncardiogenic pulmonary edema complicating lung resection. Ann Thorac Surg. 1993;55:140–143. doi: 10.1016/0003-4975(93)90490-9. [DOI] [PubMed] [Google Scholar]
- 28.Licker M, de Perrot M, Spiliopoulos A, et al. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg. 2003;97:1558–1565. doi: 10.1213/01.ANE.0000087799.85495.8A. [DOI] [PubMed] [Google Scholar]
- 29.Padley SPG, Jordan SJ, Goldstraw P, Wells AU, Hansell DM. Asymmetric ARDS following pulmonary resection: CT findings initial observations. Radiology. 2002;223:468–473. doi: 10.1148/radiol.2232010721. [DOI] [PubMed] [Google Scholar]
- 30.Waller DA, Keavey P, Woodfine L, Dark JH. Pulmonary endothelial permeability changes after major resection. Ann Thorac Surg. 1996;61:1435–1440. doi: 10.1016/0003-4975(96)00103-8. [DOI] [PubMed] [Google Scholar]
- 31.Williams EA, Quinlan GJ, Goldstraw P, Gothard JW, Evans TW. Postoperative lung injury and oxidative damage in patients undergoing pulmonary resection. Eur Respir J. 1998;11:1028–1034. doi: 10.1183/09031936.98.11051028. [DOI] [PubMed] [Google Scholar]
- 32.Tayama K, Takamori S, Mitsuoka M, et al. Natriuretic peptides after pulmonary resection. Ann Thorac Surg. 2002;73:1582–1586. doi: 10.1016/S0003-4975(02)03417-3. [DOI] [PubMed] [Google Scholar]
- 33.Misthos P, Katsaragakis S, Milingos N, et al. Postresectional pulmonary oxidative stress in lung cancer patients. The role of one-lung ventilation. Eur J Cardiothorac Surg. 2005;27:370–383. doi: 10.1016/j.ejcts.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 34.Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis. 1988;137:1159–1164. doi: 10.1164/ajrccm/137.5.1159. [DOI] [PubMed] [Google Scholar]
- 35.Slinger P, Hickey DR. The interaction between applied PEEP and auto-PEEP during one-lung ventilation. J Cardiothorac Vasc Anesth. 1998;12:133–136. doi: 10.1016/S1053-0770(98)90318-4. [DOI] [PubMed] [Google Scholar]
- 36.Capan LM, Turndorf H, Patel C, Ramanathan S, Acinapura A, Chalon J. Optimization of arterial oxygenation during one-lung anesthesia. Anesth Analg. 1980;59:847–851. [PubMed] [Google Scholar]
- 37.Slinger PD, Kruger M, McRae K, Winton T. Relation of the static compliance curve and positive end-expiratory pressure to oxygenation during one-lung ventilation. Anesthesiology. 2001;95:1096–1102. doi: 10.1097/00000542-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Gama de Abreu M, Heintz M, Heller A, Széchényi R, Albrecht DM, Koch T. One-lung ventilation with high tidal volumes and zero positive end-expiratory pressure is injurious in the isolated rabbit lung model. Anesth Analg. 2003;96:220–228. doi: 10.1097/00000539-200301000-00045. [DOI] [PubMed] [Google Scholar]
- 39.van der Werff YD, van der Houwen HK, Heijmans PJ, et al. Postpneumonectomy pulmonary edema. A retrospective analysis of incidence and possible risk factors. Chest. 1997;111:1278–1284. doi: 10.1378/chest.111.5.1278. [DOI] [PubMed] [Google Scholar]
- 40.Fernández-Pérez ER, Keegan MT, Brown DR, Hubmayr RD, Gajic O. Intraoperative tidal volume as a risk factor for respiratory failure after pneumonectomy. Anesthesiology. 2006;105:14–18. doi: 10.1097/00000542-200607000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Boker A, Haberman CJ, Girling L, et al. Variable ventilation improves perioperative lung function in patients undergoing abdominal aortic aneurysmectomy. Anesthesiology. 2004;100:608–616. doi: 10.1097/00000542-200403000-00022. [DOI] [PubMed] [Google Scholar]
- 42.Mols G, Priebe HJ, Guttmann J. Alveolar recruitment in acute lung injury. Br J Anaesth. 2006;96:156–166. doi: 10.1093/bja/aei299. [DOI] [PubMed] [Google Scholar]
- 43.Licker M, Diaper J, Villiger Y, et al. Impact of intraoperative lung-protective interventions in patients undergoing lung cancer surgery. Crit Care. 2009;13:R41. doi: 10.1186/cc7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alvarez JM, Panda RK, Newman MA, Slinger P, Deslauriers J, Ferguson M. Postpneumonectomy pulmonary edema. J Cardiothorac Vasc Anesth. 2003;17:388–395. doi: 10.1016/S1053-0770(03)00071-5. [DOI] [PubMed] [Google Scholar]
- 45.Kregenow DA, Rubenfeld GD, Hudson LD, Swenson ER. Hypercapnic acidosis and mortality in acute lung injury. Crit Care Med. 2006;34:1–7. doi: 10.1097/01.CCM.0000194533.75481.03. [DOI] [PubMed] [Google Scholar]
- 46.Curley G, Laffey JG, Kavanagh BP. Bench-to-bedside: carbon dioxide. Crit Care. 2010;14:220. doi: 10.1186/cc8926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shibata K, Cregg N, Engleberts D, et al. Hypercapnic acidosis may attenuate acute lung injury by inhibition of endogenous xanthine oxidase. Am J Resp Crit Care Med. 1998;158:1578–1584. doi: 10.1164/ajrccm.158.5.9804039. [DOI] [PubMed] [Google Scholar]
- 48.Costello J, Higgins B, Contreras M, et al. Hypercapnic acidosis attenuates shock and lung injury in early and prolonged systemic sepsis. Crit Care Med. 2009;37:2412–2420. doi: 10.1097/CCM.0b013e3181a385d3. [DOI] [PubMed] [Google Scholar]
- 49.Ni Chonghaile M, Higgins B, Laffey JG. Permissive hypercapnia: role in protective lung ventilatory strategies. Curr Opin Crit Care. 2005;11:56–62. doi: 10.1097/00075198-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Apostolakis EE, Koletsis EN, Baikoussis NG, Siminelakis SN, Papadopoulos GS. Strategies to prevent intraoperative lung injury during cardiopulmonary bypass. J Cardiothorac Surg. 2010;5:1. doi: 10.1186/1749-8090-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ng CS, Wan S, Yim AP, Arifi AA. Pulmonary dysfunction after cardiac surgery. Chest. 2002;121:1269–1277. doi: 10.1378/chest.121.4.1269. [DOI] [PubMed] [Google Scholar]
- 52.Zupancich E, Paparella D, Turani F, et al. Mechanical ventilation affects inflammatory mediators in patients undergoing cardiopulmonary bypass for cardiac surgery: a randomized clinical trial. J Thorac Cardiovasc Surg. 2005;130:378–383. doi: 10.1016/j.jtcvs.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 53.Fujinaga T, Nakamura T, Fukuse T, et al. Isoflurane inhalation after circulatory arrest protects against warm ischemia reperfusion injury of the lungs. Transplantation. 2006;82:1168–1174. doi: 10.1097/01.tp.0000237207.73439.2e. [DOI] [PubMed] [Google Scholar]
- 54.Reutershan J, Chang D, Hayes JK, Ley K. Protective effects of isoflurane pretreatment in endotoxin-induced lung injury. Anesthesiology. 2006;104:511–517. doi: 10.1097/00000542-200603000-00019. [DOI] [PubMed] [Google Scholar]
- 55.Voigtsberger S, Lachmann RA, Leutert AC, et al. Sevoflurane ameliorates gas exchange and attenuates lung damage in experimental lipopolysaccharide-induced lung injury. Anesthesiology. 2009;111:1238–1248. doi: 10.1097/ALN.0b013e3181bdf857. [DOI] [PubMed] [Google Scholar]
- 56.De Conno E, Steurer MP, Wittlinger M, et al. Anesthetic-induced improvement of the inflammatory response to one-lung ventilation. Anesthesiology. 2009;110:1316–1326. doi: 10.1097/ALN.0b013e3181a10731. [DOI] [PubMed] [Google Scholar]
- 57.Schilling T, Kozian A, Kretzschmar M, et al. Effects of propofol and desflurane anaesthesia on the alveolar inflammatory response to one-lung ventilation. Br J Anaesth. 2007;99:368–375. doi: 10.1093/bja/aem184. [DOI] [PubMed] [Google Scholar]
- 58.Joyce JC, Baker AB, Kennedy RR. Gas uptake from an unventilated area of lung: computer model of absorption atelectasis. J Appl Physiol. 1993;74:1107–1116. doi: 10.1152/jappl.1993.74.3.1107. [DOI] [PubMed] [Google Scholar]
- 59.Ko R, McCrae K, Darling G, et al. The use of air in the inspired gas mixture during two-lung ventilation delays lung collapse during one-lung ventilation. Anesth Analg. 2009;108:1092–1096. doi: 10.1213/ane.0b013e318195415f. [DOI] [PubMed] [Google Scholar]
- 60.Kang LJ, Park W, Pack IS, et al. Inhaled nitric oxide attenuates acute lung injury via inhibition of nuclear factor-ĸB and inflammation. J Appl Physiol. 2002;92:795–801. doi: 10.1152/japplphysiol.00202.2001. [DOI] [PubMed] [Google Scholar]
- 61.Adhikari NK, Burns KEA, Friedrich JO, et al. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. Br Med J. 2007;334:757–765. doi: 10.1136/bmj.39168.568692.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCrae K. Pulmonary transplantation. Curr Opin Anaesth. 2000;13:53–59. doi: 10.1097/00001503-200002000-00009. [DOI] [PubMed] [Google Scholar]
- 63.Blumenthal S, Borgeat A, Pasch T, et al. Ropivacaine decreases inflammation in experimental endotoxin-induced lung injury. Anesthesiology. 2006;104:961–969. doi: 10.1097/00000542-200605000-00012. [DOI] [PubMed] [Google Scholar]
- 64.Yang C-H, Tsai P-S, Wang T-Y, Huang C-J. Dexmedetomidine–ketamine combination mitigates acute lung injury in haemorrhagic shock rats. Resuscitation. 2009;80:1204–1210. doi: 10.1016/j.resuscitation.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 65.The Cardiothoracic Surgery Network website www.ctsnet.org/portals/thoracic/newtechnology/article-9.html Available from.
- 66.Hager DN, Krishnan JA, Hayden DL, Brower RG, ARDS Clinical Trials Network Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. Am J Respir Crit Care Med. 2005;10:1241–1245. doi: 10.1164/rccm.200501-048CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frank JA, Gutierrez JA, Jones KD, Allen L, Dobbs L, Matthay MA. Low tidal volume reduces epithelial and endothelial injury in acid-injured rat lungs. Am J Respir Crit Care Med. 2002;165:242–249. doi: 10.1164/ajrccm.165.2.2108087. [DOI] [PubMed] [Google Scholar]
- 68.Iglesias M, Jungebluth P, Petit C, et al. Extracorporeal lung membrane provides better lung protection than conventional treatment for severe postpneumonectomy noncardiogenic acute respiratory distress syndrome. J Thorac Cardiovasc Surg. 2008;6:1362–1371. doi: 10.1016/j.jtcvs.2007.08.074. [DOI] [PubMed] [Google Scholar]
- 69.Mallick A, Elliot S, McKinlay J, Bodenham A. Extracorporeal carbon dioxide removal using the Novalung in a patient with intracranial bleeding. Anaesthesia. 2007;62:72–74. doi: 10.1111/j.1365-2044.2006.04863.x. [DOI] [PubMed] [Google Scholar]
- 70.McKinlay J, Chapman G, Elliot S, Mallick A. Pre-emptive Novalung-assisted carbon dioxide removal in a patient with chest, head and abdominal injury. Anaesthesia. 2008;63:767–770. doi: 10.1111/j.1365-2044.2008.05484.x. [DOI] [PubMed] [Google Scholar]
- 71.Hammell C, Forrest M, Barrett P. Clinical experience with a pumpless extracorporeal lung assist device. Anaesthesia. 2008;63:1241–1244. doi: 10.1111/j.1365-2044.2008.05582.x. [DOI] [PubMed] [Google Scholar]
- 72.Elliot SC, Paramasivam K, Oram J, Bodenham AR, Howell SJ, Mallick A. Pumpless extracorporeal carbon dioxide removal for life-threatening asthma. Crit Care Med. 2007;35:945–948. doi: 10.1097/01.CCM.0000257462.04514.15. [DOI] [PubMed] [Google Scholar]
- 73.Liu LL, Aldrich JM, Shimabukuro DW, et al. Rescue therapies for acute hypoxemic respiratory failure. Anesth Analg. 2010;111:693–702. doi: 10.1213/ANE.0b013e3181e9c356. [DOI] [PubMed] [Google Scholar]
- 74.Bollen CW, van Well GT, Sherry T, et al. High frequency oscillatory ventilation compared with conventional ventilation in adult respiratory distress syndrome: a randomized controlled trial. Crit Care. 2005;9:R430–R439. doi: 10.1186/cc3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Derdak S, Mehta S, Stewart TE, et al. High-frequency oscillatory ventilation in patients with acute respiratory syndrome: a randomized, controlled trial. Am J Respir Crit Care Med. 2002;166:801–808. doi: 10.1164/rccm.2108052. [DOI] [PubMed] [Google Scholar]
- 76.Papazian L, Gainner M, Marin V, et al. Comparison of prone positioning and high-frequency oscillatory ventilation in patients with acute respiratory distress syndrome. Crit Care Med. 2005;33:2162–2171. doi: 10.1097/01.CCM.0000181298.05474.2B. [DOI] [PubMed] [Google Scholar]
- 77.Faller S, Ryter SW, Choi AMK, Loop T, Schmodt R, Hoetsel A. Inhaled hydrogen sulfide protects against ventilator-induced lung injury. Anesthesiology. 2010;113:104–115. doi: 10.1097/ALN.0b013e3181de7107. [DOI] [PubMed] [Google Scholar]
- 78.Waerhaug K, Kuzkov VV, Kuklin VN, et al. Inhaled aerosolised recombinant human activated protein C ameliorates endotoxin-induced lung injury in anesthetised sheep. Crit Care. 2009;13:R51. doi: 10.1186/cc7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matthay M. β-Adrenergic agonist therapy as a potential treatment for acute lung injury. Am J Respir Crit Care Med. 2006;173:254–255. doi: 10.1164/rccm.rccm2511003. [DOI] [PubMed] [Google Scholar]
- 80.Perkins GD, McAuley DF, Thickett DR, Gao F. The β-agonist lung injury trial. Am J Respir Crit Care Med. 2006;173:281–287. doi: 10.1164/rccm.200508-1302OC. [DOI] [PubMed] [Google Scholar]
- 81.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 82.Beck-Schimmer B, Schimmer RC. Perioperative tidal volume and intraoperative open lung strategy in healthy lungs: where are we going? Best Pract Res Clin Anaesthesiol. 2010;24:199–210. doi: 10.1016/j.bpa.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carvalho EMF, Gabriel EA, Salerno TA. Pulmonary protection during cardiac surgery: systematic literature review. Asian Cardiovasc Thorac Ann. 2008;16:503–507. doi: 10.1177/021849230801600617. [DOI] [PubMed] [Google Scholar]
- 84.Paparella D, Yau TM, Young E. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardiothorac Surg. 2002;21:232–244. doi: 10.1016/s1010-7940(01)01099-5. [DOI] [PubMed] [Google Scholar]


