Abstract
The novel coronavirus pandemic has led to morbidity and mortality throughout the world. Until now, it is a highly virulent contagion attacking the respiratory system in humans, especially people with chronic diseases and the elderly who are most vulnerable. A majority of afflicted are those suffering from cardiovascular and coronary diseases. In this review article, an attempt has been made to discuss and thoroughly review the mode of therapies that alleviate cardiac complications and complications due to hypercoagulation in patients infected with the SARS-CoV-2 virus. Presently a host of thrombolytic drugs are in use like Prourokinase, Retelapse, RhTNK-tPA and Urokinase. However, thrombolytic therapy, especially if given intravenously, is associated with a serious risk of intracranial haemorrhage, systemic haemorrhage, immunologic complications, hypotension and myocardial rupture. The effects of the SARS-CoV-2 virus upon the cardiovascular system and coagulation state of the body are being closely studied. In connection to the same, clinical prognosis and complications of thrombolytic therapy are being scrutinized. It is noteworthy to mention that myocardial oxygen supply/demand mismatch, direct myocardial cells injury and acute plaque rupture are the multiple mechanisms responsible for acute coronary syndrome and cardiac complications in Covid-19 infection. However, this review has limitations as data available in this context is limited, scattered and heterogenous that questions the reliability of the same. So, more multi-centric studies involving representative populations, carried out meticulously, could further assist in responding better to cardiac complications among Covid-19 patients.
Keywords: SARS-CoV-2, thrombolytic therapy, cardiovascular system, cardiovascular diseases, ST elevation myocardial infarction, hypercoagulation
What Do We Already Know About This Topic?
• The management of heart diseases is well described in literature and the practical treatment is in the least at an adequate standard in hospitals across the world.
How Does This Study Contribute to the Field?
• The Covid-19 pandemic struck suddenly and the world was not prepared. Doctors were facing several challenges to manage heart diseases during this SARS-CoV-2 crisis. This review describes in brief the impact of SARS-Cov-2 and heart diseases and also the shortcomings the medical system faced during that period.
What are the Implications Towards Practice?
• To facilitate the management of heart patients infected with SARs-CoV-2.
Introduction
Cardiovascular disease (CVD) has emerged as a leading cause of death in the world in the past decade. More than 80% of these deaths occur in countries with lower-income and middle-income countries (LMICs). 1 Myocardial infarction is the most common form of coronary heart disease (CHD). An occluded or about to occlude coronary artery resulting in severe reduction in the blood flow, causing the infarct in the heart muscle. 2 There are two types of myocardial infarction recognised by significant changes in the electrocardiography (ECG) findings; ST segment elevation myocardial infarction (STEMI), which is characterised by a typical elevation in the ‘ST segment’ of the ECG wave, and non-ST segment elevation myocardial infarction (NSTEMI). 3
Treatment modalities of STEMI include percutaneous coronary interventions (PCIs) and thrombolytic agents, which should be used expeditiously to improve the prognosis of patients. 4 The effectiveness of thrombolytic therapy in restoring the blood flow of the heart was established 20–30 years back. 5 However, thrombolytic therapy has some limitations as it cannot be used in a certain group of people due to the risk of excessive bleeding. Observations have been made that it is not 100% effective and in about one-third of patients in whom it is used, it does not achieve adequate reperfusion results. Furthermore, in 1% of the people it can cause fatal complications like haemorrhagic stroke; hence, mechanical techniques, like coronary angioplasty, thrombus extraction catheters and stenting have received their due attention by being termed as primary percutaneous coronary intervention (PPCI). 6 Nevertheless, both forms of treatment are being widely used for the management of myocardial infarction (MI) patients as both have their own indications and advantages.
Coronaviruses (CoVs) were discovered in the 1960s. They were classified under the familyCoronaviridae, which is the largest family in the order Nidovirales. 7 Prior to 2002, CoVs were not seen as a serious threat owing to the mild respiratory illness they caused but this perception of CoVs changed after the emergence of Severe acute respiratory syndrome coronavirus (SARS-CoV) in 2002–2003, which assumed the status of an epidemic quickly, claiming many lives due to its high mortality rate. 8 Though the majority of infections caused by CoVs are mild respiratory ones, serious diseases might occur in Middle East respiratory syndrome coronavirus (MERS-CoV), SARS-CoV and SARS-CoV-2 infections.8,9 A novel coronavirus (SARS-CoV-2) caused an outbreak of pneumonia in Wuhan, China in December 2019 that quickly spread to the rest of the world becoming a global public health emergency.10,11 Although the predominant symptoms of Covid-19 infection are respiratory, severe cardiovascular (CV) damage may occur in few patients, especially those already suffering from underlying CV diseases. 12
Cardiovascular injury may occur as a result of Covid-19 infection through direct myocardial injury due to hypoxemia, inflammatory myocarditis, hypercoagulopathy leading to microvascular dysfunction, thrombus formation, stress myocardiopathy, or cytokine storm resulting in systemic inflammation and destabilization of coronary artery plaques. 13 Pneumonia and influenza infections are proven factors responsible for a six-fold increase in the risk of acute myocardial infarction.14,15 The clinical impact of SARS-CoV-2 infection is likely to be greater in those suffering from underlying diseases and in elderly persons. In one study from China, it was seen that patients who had prior cardiovascular diseases made up 22.7% of all fatalities, and the fatality rate was 10.5% among CVD patients. 16
During the current Covid-19 pandemic, the association between SARS-CoV-2 and cardiac damage has been sporadically observed but a causal association is yet to be established. The limbo is further exaggerated by a lack of common guidelines to treat heart disease, like Acute Cardiac Syndrome (ACS) in Covid-19 and non-Covid-19 patients. The aim of this review is to shed some light on the upsurge of cardiac ailments, especially ACS, after the covid-19 pandemic, analysing the use of thrombolytic therapy or PCI as a mainstay of treatment of ACS patients in the times of Covid-19. This review has been carried out by conducting a literature search on PubMed using the terms ‘cardiac disease’, ‘MI’, ‘ACS’, SARS-CoV-2, in combination with ‘Covid-19’, ‘coronavirus’, or ‘pandemic’. We mainly selected studies published after 2010 but included several older publications to provide background and context to this review. Among recent publications, we included number of studies published after 2020 to consider the effect of the covid-19 pandemic on heart disease. The present review was carried out according to the Preferred Reporting Items for Systematic Review (PRISMA) standards.
Entry Mechanism of Human Coronaviruses
The novel coronavirus thrives on the proteins of a host cell to replicate. This characteristic also helps the virus to form glycoprotein spikes on its outer surface which in turn help it attach and enter the host cell. 17 Notably, the virus can infect multiple hosts owing to the loose attachment of the receptor-binding domain (RBD) to its body.18,19 SARS-CoV and MERS-CoV recognise exopeptidases as key receptors for entering the human cells and the mechanism of entry of the virus inside cells depends upon cellular proteases like human airway trypsin-like protease (HAT), cathepsins and transmembrane protease serine 2 (TMPRSS2), which assist in slitting of the spike protein and establish penetration.2,20,21 Various polyproteins, nucleoproteins and membrane proteins such as RNA polymerase, 3-chymotrypsin-like protease, papain-like protease, helicase, glycoprotein and accessory proteins are present on the outer coat of the virus.22,23 SARS-CoV-2 has a typical coronavirus structure with spike proteins having a three dimensional structure in the RBD region to facilitate Vander Waals forces. 24
Cardiovascular Effects of SARS-CoV-2
COVID-19 is an acute respiratory disease caused by a novel coronavirus, which first surfaced in Wuhan, China last year. Evidence suggests that cardiac involvement, particularly in patients hospitalized for COVID-19 is common. 25 If the same is true for all infected, symptomatic and asymptomatic persons, it is yet to be fully established due to lack of vigilance, given the widespread outbreak of the disease, forcing the current focus of healthcare providers (HCPs) on thousands of hospitalized patients. The majority of resources are being utilized to halt the overwhelming swell of the pandemic and flattening the curve of infections and fatalities in the world.11,12,26 People having underlying health problems like cardiovascular disease (CVD) or other cardiac complications are at a heightened risk to develop severe COVID-19 symptoms, with grim chances of recovery.
Cardiovascular disease has been frequently reported in patients hospitalised due to Covid-19. In various studies, the baseline prevalence of CVD in admitted patients ranges from 10 to 15%, whereas, the reported prevalence of hypertension is higher, ranging from 15 to 30%.11,12,26 Prognostic significance of CVD was amply illustrated by a cohort study of 191 patients, where 30% of patients having hypertension constituted 48% of non-survivors, and 8% of CVD patients constituted 13% of non-survivors. 27 A summary of reports of 72 314 Covid-19 patients from China has revealed that the overall case fatality rate (CFR) is higher in patients suffering from hypertension, diabetes and CVD (6%, 7.3% and 10.5%, respectively), as compared to the other members of the cohort (CFR 2.3%). 28 A high level of cytokine surge has also been documented in severe Covid-19 infections, resulting in multiple organ injury in these patients.29,30 Other studies on previous SARS and MERS outbreaks have also suggested multiple mechanisms for cardiac damage.29,31-33
Mechanisms Causing Development of Cardiac Damage in Covid-19 Infection
The occurrence of cardiac damage in Covid-19 patients remains enigmatic with a few possible explanations, which are
1. Direct myocardial cells injury: The SARS-CoV-2 virus uses angiotensin converting enzyme (ACE) 2 receptors to enter the host cells. These receptors are expressed in pneumocytes and other types of cells like endothelial cells. The interaction of the virus with the ACE2 receptors can change the ACE2 pathways resulting in acute injury to multiple organs like lung, heart and endothelial cells.29,34
2. Myocardial oxygen supply/demand mismatch: Severe pneumonia or acute respiratory distress syndrome caused by systemic infection and ongoing hypoxia prompts an increased demand for oxygen which if not supplied in adequate measure can lead to myocardial damage. 29
3. Acute plaque rupture: Acute plaque rupture leads to acute coronary syndrome due to systemic inflammation and surge in catecholamine, which is characteristic of this disease. This process increases plaque vulnerability and can further precipitate plaque rupture leading to acute coronary syndrome.29,35
A recent systematic review reports that the main cause of death among heart patients suffering from covid-19 was respiratory failure. 36 The second most common cause of death was acute heart failure and a few patients specifically died of myocarditis. Main cardiac pathological findings were cardiac dilatation, necrosis, lymphocytic infiltration of the myocardium and small coronary vessel microthrombosis. 36
The Pathogenesis of Hypercoagulability in COVID-19
Hypercoagulability can be termed Virchow’s triad. There are three major factors responsible for clot formation which happens in severe COVID-19 infection. These three factors are
1. Endothelial Injury – Invasion of SARS-CoV-2 virus, along with other factors like intravascular catheters and presence of mediators of an acute systemic inflammatory response (e.g. interleukin-6), can potentially cause fulminant endothelial cell injury. 37 Endothelial cells might also be injured through a complement-mediated pathway. 38
2. Stasis – Immobilization can cause stasis of blood flow in all hospitalized and critically ill patients, regardless of whether or not they are infected with COVID-19.
3. Hypercoagulable State – A number of changes in circulating prothrombotic factors have been reported in patients with severe COVID-19.39,40
The Development of Coagulation Abnormalities in Covid-19
Patients with COVID-19 coagulation abnormalities have a hypercoagulable state that is consistent with an increased risk of venous thrombo-inflammation or, as some experts suggest, COVID-19 associated coagulopathy (CAC),41,42 This hypercoagulable state appears to be distinct from disseminated intravascular coagulation (DIC), though later has been reported in severely affected Covid-19 patients.
A hypercoagulable state has also been reported in similar findings, including very high D-dimer, VWF antigen and activity, and factor VIII activity.40,43 It has been further suggested that patients with COVID-19 have higher platelet counts than patients with other coronavirus infections. 44 Autopsy of some patients who succumbed to COVID-19 demonstrated microvascular thrombosis in the lungs.38,42 A recent systematic review also suggests that Covid-19 infection leads to diffuse alveolar damage with hyaline membrane formation, alongside microthrombi in small pulmonary vessels. 45 Hypoxic changes have also been reported alteration of brain tissue, followed by ischemic and hemorrhagic lesions and reactive astrogliosis and microgliosis. These findings do not seem to be specific to SARS-CoV-2 infection, they are more likely because of systemic inflammation and coagulopathy caused by COVID-19. 46
Findings from 183 patients from Wuhan, China, suggested that Covid-19 patients have more marked thrombocytopenia and prolonged prothrombin time (PT) and activated partial thromboplastin time (aPTT).47-50 However, these parameters improved as the pandemic progressed. The reason behind this difference in two findings could be attributed to the fact the patients were sicker, perhaps because at the initial stages of the pandemic the disease could not be recognized as quickly, thus, resulting in a delay in patient presentation and/or treatment.
The presence of a lupus anticoagulant (LA) could be another reason, possibly, for an isolated prolonged aPTT. Two studies have reported the presence of a high level of LA along with prolonged aPTT among 88% (50 out of 57 patients) and 91% (31 out of 34 patients) of Covid-19 patients, respectively.51,52 Patients detected with a LA should receive anticoagulation. Some other markers of deranged coagulation (e.g. D-dimer) appear to correlate with illness severity too. D-dimer is often increased, sometimes markedly, in individuals with overt DIC and those in the intensive care unit (ICU).
Distinction between Hyper Coagulation and Disseminated Intravascular Coagulation (DIC)
The state of hyper coagulation, associated with COVID-19, has been referred to by some as disseminated intravascular coagulation (DIC)-like state, since many individuals are ill and meet the criteria of DIC according to the scoring system published by the International Society on Thrombosis and Haemostasis (ISTH) in 2009. 53 It is notable that coagulopathy is commonly observed in SARS-CoV-2 infection, and an increase in D-dimer is the most characteristic finding. 54 Though high D-dimer levels correlate with the presence of DIC, other coagulation parameters in COVID-19 are distinct from DIC. In COVID-19, the typical findings include high fibrinogen and high factor VIII activity, suggesting scanty consumption of coagulation factors. 55 Low fibrinogen, due to consumption of clotting factors are, in contrast, associated with acute decompensated DIC. According to a report prepared on one of the largest series on thromboembolic events, none of the patients developed overt DIC. 55
Since bleeding is predominant in acute decompensated DIC and thrombosis predominates in chronic compensated DIC (without any significant overlap); the hypercoagulable state in patients with COVID-19 is thus more similar to compensated DIC than to acute DIC. However in COVID-19, the platelet count and aPTT are typically normal. Points are given for thrombocytopenia (1 point for platelet count 50 000–100,000/microL; 2 points for platelet count <50,000/microL), prolonged PT (1 point for 3–6 seconds of prolongation; 2 points for more than 6 seconds of prolongation), low fibrinogen (1 point for <100 mg/dL) and increased D-dimer (2 points for moderate increase; 3 points for ‘strong’ increase). A score of 5 or more points suggests possible DIC. Despite this scoring system, the diagnosis of DIC is made clinically. In comparison with the opinion of experts, the ISTH scoring system is reported to have a sensitivity of 91% and a specificity of 97%. 53 Truly speaking, there is no gold standard and no single test or combination of tests that is pathognomonic of DIC.
Regardless of differences and similarities, emphasis should be laid on basic principles of DIC management, including treatment of the underlying conditions. Interventions should be based on clinical findings rather than depending on laboratory testing alone, thus should consider the use of both fibrinolytic and haemostatic therapies, as mandated by the clinical condition of the patient.
Clinical Prognosis of Thrombolytic Treatment
A breakthrough in the treatment of thrombolytic therapy has saved countless precious lives, besides adding a new chapter in the reperfusion treatment of STEMI since 1980.
In 2019, China issued guidelines for the treatment of STEMI. Emphasis was given on the fact that both thrombolysis and PCI are indispensable in the treatment of STEMI if started early. In France FAST-2014 MI cohort study, which included 1492 patients with STEMI, 447 patients underwent thrombolysis treatment (295 patients were administered pre-hospital thrombolytic therapy (PHT)); 583 patients underwent primary PCI, and 462 patients received conservative treatment. The 5-year survival rate was 88%, 84% and 65% in the three groups, respectively. This study suggested that the long-term survival rate of STEMI patients receiving thrombolysis was similar to that of those receiving primary PCI. 56 One thousand eight hundred and twenty three patients included in the STREAM study done by Welsh et al 57 were divided into 3 groups: fibrinolysis requiring rescue (rescue, n = 348), fibrinolysis with scheduled angiography (scheduled, n = 516), and primary PCI (n = 927). The study concluded that early thrombolytic therapy can improve the survival rate of STEMI patients, as pharmaco-invasively treated patients requiring rescue angiography had greater baseline risk with more co-morbidities and worse 30-day outcomes as compared to successful fibrinolytic-treated patients. 57 Thus, Franzosi et al 58 whilst conducting a 10-year follow-up of patients with STEMI, found that intravenous infusion of streptokinase thrombolytic regimen could reduce 19 deaths per 1000 cases treated. Also, the benefits were more obvious for those who were hospitalized within 1 hour of onset of symptoms. Results from the nationwide French USIC 2000 registry suggested that patients treated with PHT very early have a much higher one year survival rate and the PHT treatment was comparable to other modes of reperfusion therapy. 59
After starting intravenous thrombolysis, clinical symptoms should be closely monitored for any ST segment changes and arrhythmia in the ECG. 60 ECG shows that the elevated ST segments decrease by at least 50% within 60–90 min after thrombolysis. 61 The symptoms of chest pain relieve or disappear within 2 hours after thrombolysis. 62 The peak value of myocardial necrosis markers is pushed ahead in time, such as the peak value of troponin I is advanced to within 12 hours of onset, and the peak value of creatine kinase isoenzyme is advanced to within l4 hours of onset. 56 Reperfusion arrhythmias occur within 2–3 hours after thrombolysis, such as accelerated ventricular autonomic rhythm, atrioventricular block or bundle branch block, which can suddenly improve or disappear; or transient sinus bradycardia, sinus atrial block with or without hypotension in patients with inferior wall myocardial infarction. The typical criteria for successful thrombolytic therapy are based on ≥50% resolution of elevation ST segment in ECG, accompanied by significant relief in chest pain and/or reperfusion arrhythmia. The success of thrombolytic treatment is based on coronary angiography; for example grade 2–3 of thrombolysis in myocardial infarction (TIMI) blood flow in myocardial infarction-related vessels represents vascular recanalization, grade 3 of TIMI means complete recanalization, and grade 0–1 of TIMI blood flow suggests thrombolysis failure and continuous occlusion of infarction-related vessels.60,63
Effective restoration of myocardial perfusion and early, complete and permanent patency of infarction-related blood vessels in patients with STEMI are vital. With the rapid development of PCI in recent years, the application of thrombolysis in the treatment of STEMI has been reduced. However, thrombolytic treatment acts rapidly, is simple, economical and easy to manage. It can dissolve thrombus and open occluded vessels in the shortest time. Even in the United States and Europe, the rate of thrombolytic therapy performed for AMI reperfusion is comparable to that of primary PCI. Although PCI is being widely used in clinical practice, nearly 40% of patients with AMI still receive thrombolytic therapy.63,64 Thrombolytic treatment could save 60 ∼ 100 min compared to PCI. In order to reduce the spread of Covid-19 and shorten the delay time, thrombolytic therapy may have obvious advantages during this pandemic. 60 Administration of thrombolytic therapy is simple and only one doctor with a nurse are required for the job, whereas performing PCI requires more medical staff and inter-departmental coordination. 61 Thrombolytic therapy is better than primary PCI in a few ways, as 60% of STEMI is caused by the rupture of mild to moderate vulnerable plaque and PCI is not necessary after thrombolytic therapy in these patients, especially if they are young. 62 During thrombolytic therapy, it is easier to protect medical staff and it further reduces the number of people needed to be sent for isolation. 26 The efficacy of thrombolysis within 3 hours of onset is also comparable to that of primary PCI. 57
Thrombolytic Drugs
Thrombolytic drugs play a crucial role in the management of thrombotic and thromboembolic complications. Once the arterial lumen is eroded and plaque ruptures, the lipid-rich core of the plaque triggers the formation of unstable platelet aggregates, leading to thrombus development and reduction or complete occlusion of the coronary blood flow, culminating in ST segment elevation myocardial infarction. 65 As discussed before, MI can be treated with the help of percutaneous coronary intervention and thrombolytic therapy. The comparison of the characteristics of different thrombolytic drugs is shown in Table 1.
Table 1.
Comparison of Characteristics of Different Thrombolytic Drugs.
| Thrombolytic drugs | Routine dose | Fibrin specificity | Antigenicity or allergic reaction | Fibrinogen consumption | Reperfusion rate within 90 minutes of thrombolysis | TIMI 3 flow (%) |
|---|---|---|---|---|---|---|
| Prourokinase | 50 mg | Yes | No | Mild | 85.4 | 60.08 |
| Reteplase | 10MU*2, >2 min per time | Yes | No | Medium | 85.2 | 60 |
| Alteplase | 100 mg, 90 min | Yes | No | Mild | 75 | 54 |
| RhTNK-tPA | 16 mg (5∼10s) projectile intravenous injection | Yes | No | Minimum | 85 | 63 |
| Urokinase | 1.5 million u, 30 min | No | No | Obvious | Unknown | Unknown |
Source: Original.
Complications of Thrombolytic Therapy
As pertinent as it is, thrombolytic therapy is not without risks. There are 5 major risk factors that should be kept in mind whilst using it, which are intracranial haemorrhage, systemic haemorrhage, immunologic complications, hypotension and myocardial rupture. Theoretically, thromboembolism is also a risk but it is rarely come across in clinical practice. Similarly, reperfusion arrhythmias, which are cardiac rhythm disturbances at the time of reperfusion, also do not pose a significant risk to the lives of the patients on thrombolytic therapy. Systemic haemorrhages are also uncommon, especially in the absence of major vascular punctures, and the chances of fatal outcomes are rare.
The risk with using streptokinase or agents with a streptokinase moiety, including anistreplase (an isolated plasminogen – streptokinase activator complex, APSAC), is the development of anaphylaxis which, again is rare. Contrarily, hypotension is much more common in these patients. 66 However, most clinicians are worried about devastating intracranial haemorrhage, which occurs in 0.2–1% of the patients on thrombolytic therapy.67,68 Clinicians should be wary of using thrombolysis late, as myocardial rupture is increasingly being recognised as an associated outcome of the same.
Clinical risks should always be weighed against the potential benefits and thus it is often seen that those at the highest risk are the ones who gain the most from the therapy.
Critical Issues in Management of Cardiac Patients during Covid-19 Pandemic
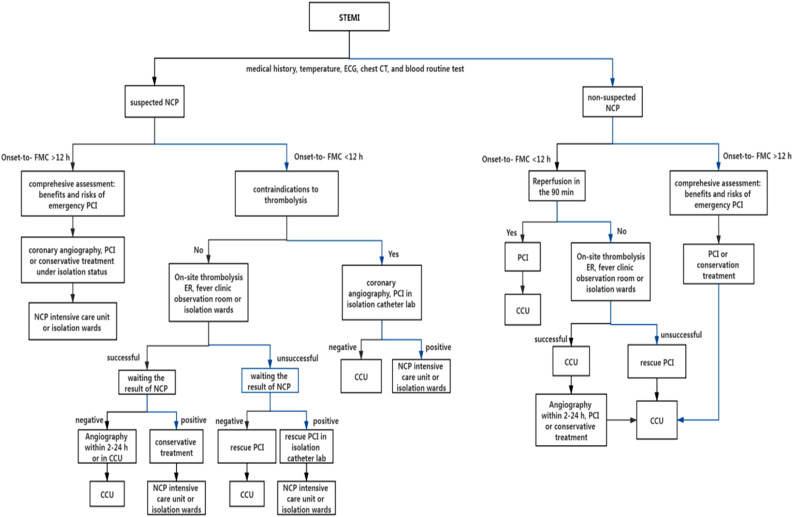
In many parts of the world, hospital admissions have been greatly reduced, reducing the number of admissions from cardiac complaints by 75% in the worst hit areas. 69 On the other hand, lack of healthcare manpower and/or fear of Covid-19 precludes the delivery of efficient healthcare services to cardiac patients in many regions. 70 There is a need for a protocol indicating the feasibility of performing PCI procedures on ACS patients, keeping into consideration the safety of healthcare workers and welfare of the patient. Thrombolytic agents, though safe to use, have their limitations in presence of a Covid infection. Clopidogrel and ticagrelor showed interaction with anti-viral drugs used to treat coronavirus infections in animal models, however, these claims are yet to be replicated in a randomised control trial.71,72 Additionally, aspirin and NSAIDS can also be used without a threat of an adverse reaction in these patients. 73 Critically ill ACS patients irrespective of their Covid-19 status should be sent for angiography with the intent to revascularize mechanically and for clinically stable patients too primary PCI remains the treatment of choice. The reperfusion strategies among STEMI patients having suspected Novel Coronavirus Pneumonia (NCP) has been depicted in detail in (Figure 1).
Figure 1.
ST segment elevation myocardial infarction reperfusion treatment strategy for suspected Novel Coronavirus Pneumonia (NCP) patients during the Covid-19 epidemic. Source: Original. In STEMI patient the course of action differs if the patient receives first medical contact before or after 12 hours, irrespective of suspicion of NCP. If the patient is a suspected NCP patient, and he receives medical care before 12 hours then contraindication to thrombolysis is assessed. If the patient has contraindications then PCI is done in isolation catheter lab and if there are no contraindications to thrombolytic therapy then onsite thrombolysis is done. However, if the patients presents later than 12 hours then emergency PCI is done after assessing the risks and benefits of the same, or conservative therapy is given and the patient is kept in a NCP CCU. If the patient is a non-suspected NCP, presenting before 12 hours then reperfusion is done with the help of PCI within 90 minutes. However, if the patients presents later than 12 hours then emergency PCI is done after assessing the risks and benefits of the same, or conservative therapy is given. First Medical Contact, PCI: Percutaneous Coronary Intervention, ER: Emergency Room, CCU: Cardiac Care Unit.
Critical Issues in Management of Cardiac Complications in Covid-19 Patients
Elevated troponin levels are sometimes (7%–27.8%) reported in patients with Covid-19 infections, and are associated with increased morbidity and mortality among these patients.11,74,75 The pathogenesis for high troponin levels in these patients is multi-factorial, however, common clinical conditions should be first ruled out in these patients such as MI, arrhythmia, heart failure, renal failure, hypotension and hypoxemia. 74 The widespread inflammation in the body of a Covid-19 patient might theoretically increase the risk of MI but there is no direct evidence available for this assumption, though, this is the case in other viral infections like influenza. 76 Just like high troponin levels, hypertension is also commonly seen to be co-existing in as many as 15%–35% of Covid-19 patients. 77 There are two confounding factors that account for this observation – first is the high prevalence of hypertension in the population and second is the people admitted with Covid-19 are usually of advanced age. 27 There is a major knowledge gap about the role of hypertension as a risk factor for Covid-19 and it will take large, representative, population-based, prospective studies to fill this gap. Likewise, there is a paucity of good epidemiological data reporting the increased risk of a cardiac event during Covid-19 infection, and just one study reported an increased risk of stroke during the infection. 78 As discussed earlier in this review, cytokine storm in Covid-19 may lead to a systemic inflammatory response and multi-organ failure. But though cardiovascular complications are recognised, the incidence of cardiac arrhythmias in affected patients is infrequently reported among these patients, despite of many Covid-19 drugs having a pro-arrhythmic potential.
Even so, the use of both (hydroxy)chloroquine and lopinavir/ritonavir should be avoided in patients at increased risk of arrhythmias like the ones having a congenital or acquired long-QT syndrome, and there is a need to keep their electrolyte imbalance in check in order to timely detect hypokalemia. 79 The concomitant use of other QT-interval prolonging drugs should also be avoided. 79
Statins, however, have shown no deleterious effect on the cardiovascular system in Covid-19 infection, rather they may be beneficial owing to their innate ability to regulate immune response through post-translational modification of intracellular signalling molecules. 74 Additionally, other COVID-19–related effects on the heart, or massive pulmonary embolism remains elusive.
Conclusions and Perspectives
Despite of the low admission rates, cardiac illness still accounts for a large proportion of the morbidity and mortality of the population in both the developed and developing world. COVID-19 infection can lead to different cardiac complications by way of different inflammatory mechanisms which can increase the mortality, especially in patients with risk factors. Indeed, there are various thrombolytic therapies available in the world today that can greatly improve the prognosis of patients with STEMI, but we should not downplay or underestimate complications these therapies present, like intracranial haemorrhage, systemic haemorrhage, immunologic complications, hypotension and myocardial rupture. Hence, during this pandemic, finding a balance between risks related to the untimely treatment of STEMI patients and SARS-CoV-2 infection, control has become a global challenge. To summarise, there is a dire need for more studies to explain the cardiac effects of Covid-19. It was also seen that the data available in this context is limited, scattered and heterogenous that questions the reliability of the same. So, more multi-centre studies involving representative population, carried out meticulously could further assist in alleviation of the current crisis. Till then, the patients who are on cardio-protective therapies should continue as such and patients developing cardiovascular complications owing to Covid-19 should be put on tried and tested therapies if no contraindications are there.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Statement: Our study did not require an ethical board approval because it is not applicable.
ORCID iD
Yoosuf Ali Ashraf Muhammad Hussenbocus https://orcid.org/0000-0002-3650-269X
References
- 1.World Health Organization . Cardiovascular diseases (CVDs). Fact sheet No. 317. 2011. http://www.who.%20int/mediacentre/factsheets/fs317/en/index.html. Accessed October 10 2020.
- 2.Goldstein JA, Demetriou D, Grines CL, et al. Multiple complex coronary plaques in patients with acute myocardial infarction. N Eng J Med. 2000;343(13):915-922. [DOI] [PubMed] [Google Scholar]
- 3.O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:529-555. [DOI] [PubMed] [Google Scholar]
- 4.Baralis G, Rossini R, Musumeci G. Antiplatelet therapy in STEMI undergoing primary PCI: when, which one and how long. Minerva Cardioangiol. 2018;66(4):422-428. [DOI] [PubMed] [Google Scholar]
- 5.Rentrop KP, Blanke H, Karsch KR, et al. Acute myocardial infarction: intracoronary application of nitroglycerin and streptokinase. Clin Cardiol. 1979;2(5):354-363. [DOI] [PubMed] [Google Scholar]
- 6.Carville SF, Henderson R, Gray H. The acute management of ST-segment-elevation myocardial infarction. Clin Med. 2015;15(4):362-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woo PC, Huang Y, Lau SK, et al. Coronavirus genomics and bioinformatics analysis. Viruses. 2010;2:1804-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drosten C, Gunther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Eng J Med. 2003;348:1967-1976. [DOI] [PubMed] [Google Scholar]
- 9.Zaki AM, van Boheemen S, Bestebroer TM, et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Eng J Med. 2012;367:1814-1820. [DOI] [PubMed] [Google Scholar]
- 10.Zhu N, Zhang D, Wang W, et al. China novel coronavirus investigating and research team. a novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Libby P, Loscalzo J, Ridker PM, et al. Inflammation, immunity, and infection in atherothrombosis: JACC review topic of the week. J Am Coll Cardiol. 2018;72:2071-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musher DM, Abers MS, Corrales-Medina VF. Acute infection and myocardial infarction. N Engl J Med. 2019;380:171-176. [DOI] [PubMed] [Google Scholar]
- 15.Kwong JC, Schwartz KL, Campitelli MA. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378:2540-2541. [DOI] [PubMed] [Google Scholar]
- 16.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team . The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19): China. China CDC Weekly. 2020;2:113-122. [PMC free article] [PubMed] [Google Scholar]
- 17.van Boheemen S, de Graaf SM, Lauber C, et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. mBio. 2012;3(6):e00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raj VS, Mou H, Smits SL, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495(7440):251-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perlman S, Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbial. 2009;7(6):439-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glowacka I, Bertram S, Müller MA, et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85(9):4122-4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertram S, Glowacka I, Müller MA, et al. Cleavage and activation of the severe acute respiratory syndrome coronavirus spike protein by human airway trypsin-like protease. Virol. 2011;85(24):13363-13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou P, Yang X, Wang X, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63(3):457-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020;14(3):247-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese centre for disease control and prevention. JAMA. 2020;323(13):1239-1242. [DOI] [PubMed] [Google Scholar]
- 29.Xiong TY, Redwood S, Prendergast B, et al. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J. 2020;41(19):1798-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frisoni P, Neri M, D’Errico S, et al. Cytokine storm and histopathological findings in 60 cases of COVID-19-related death: from viral load research to immunohistochemical quantification of major players IL-1β, IL-6, IL-15 and TNF-α. Forensic Sci Med Pathol. 2021;18(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng YY, Ma YT, Zhang JY, et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clerkin KJ, Fried JA, Raikhelkar J, et al. Coronavirus Disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020;141(20):1648-1655. [DOI] [PubMed] [Google Scholar]
- 34.Babapoor-Farrokhran S, Gill D, Walker J, et al. Myocardial injury and COVID-19: possible mechanisms. Life Sci. 2020;253:117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schoenhagen P, Tuzcu EM, Ellis SG. Plaque vulnerability, plaque rupture, and acute coronary syndromes: (multi)-focal manifestation of a systemic disease process. Circulation. 2002;106(7):760-762. [DOI] [PubMed] [Google Scholar]
- 36.Maiese A, Frati P, Del Duca F, et al. Myocardial pathology in COVID-19-associated cardiac injury: a systematic review. Diagnostics. 2021;11(9):1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Begbie M, Notley C, Tinlin S, et al. The Factor VIII acute phase response requires the participation of NFkappaB and C/EBP. ThrombHaemost . 2000;84:216-222. [PubMed] [Google Scholar]
- 38.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID-19 patients in Intensive Care Unit. A Report of Thrombo-elastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(17):1738-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18(7):1747-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Covid 19 Resources, American Society of Haematology. COVID-19 and VTE/Anticoagulation: Frequently Asked Questions. https://www.hematology.org/covid-19/covid-19-and-coagulopathy. Accessed April 20 2020.
- 42.Connors JM, Levy JH. Thrombo-inflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020;18(7):1559-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yin S, Huang M, Li D, et al. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2021;51:1107-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maiese A, Manetti AC, La Russa R, et al. Autopsy findings in COVID-19-related deaths: a literature review. Forensic Sci Med Pathol. 2021;17:279-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maiese A, Manetti AC, Bosetti C, et al. SARS-CoV-2 and the brain: a review of the current knowledge on neuropathology in COVID-19. Brain Pathol. 2021;31(6):e13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amgalan A, Othman M. Exploring possible mechanisms for COVID-19 induced thrombocytopenia: unanswered questions. J Thromb Haemost. 2020;18(6):1514-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thachil J. The versatile heparin in COVID-19. J Thromb Haemost. 2020;18:1020-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas W, Varley J, Johnston A, et al. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb Res. 2020;191:76-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bowles L, Platton S, Yartey N, et al. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. N Engl J Med. 2020;383:288-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levi M, Toh CH, Thachil J, et al. Guidelines for the diagnosis and management of disseminated intravascular coagulation. Br J Haematol. 2009;145:24-33. [DOI] [PubMed] [Google Scholar]
- 54.Iba T, Levy JH, Levi M, et al. Coagulopathy of coronavirus disease 2019. Crit Care Med. 2020;48(9):1358-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Danchin N, Puymirat E, Steg PG, et al. FAST-MI 2005 investigators. five-year survival in patients with ST-segment-elevation myocardial infarction according to modalities of reperfusion therapy: the French registry on acute ST-Elevation and non-ST-Elevation Myocardial Infarction (FAST-MI) 2005 Cohort. Circulation. 2014;129(16):1629-1636. [DOI] [PubMed] [Google Scholar]
- 57.Welsh RC, Van de Werf F, Westerhout CM, et al. Outcomes of a pharmacoinvasive strategy for successful versus failed fibrinolysis and primary percutaneous intervention in acute myocardial infarction (from the Strategic Reperfusion Early after Myocardial Infarction [STREAM] study). Am J Cardiol. 2014;114(6):811-819. [DOI] [PubMed] [Google Scholar]
- 58.Franzosi MG, Santoro E, De Vita C, et al. Ten-year follow-up of the first mega-trial testing thrombolytic therapy in patients with acute myocardial infarction: results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto-1 Study. Circulation. 1998;98(24):2659-2665. [DOI] [PubMed] [Google Scholar]
- 59.Danchin N, Blanchard D, Steg PG, et al. Impact of prehospital thrombolysis for acute myocardial infarction on 1-year outcome results from the French Nationwide USIC 2000 Registry. Circulation. 2004;110(14):1909-1915. [DOI] [PubMed] [Google Scholar]
- 60.Yerasi C, Case BC, Forrestal BJ, et al. Treatment of ST-segment elevation myocardial infarction during COVID-19 pandemic. Cardiovasc Revasc Med. 2020;21(8):1024-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chinese Society of Cardiology of Chinese Medical Association . 2019 Chinese Society of Cardiology (CSC) guidelines for the diagnosis and management of patients with ST-segment elevation myocardial infarction [J]. Chin J Cardiol. 2019;47(10):766-783. [DOI] [PubMed] [Google Scholar]
- 62.Zhao YY, Yang JG, Xu HB, et al. Association between the composite measures of hospital performance and in-hospital mortality for patients with acute ST-segment elevation myocardial infarction in china correlational analysis of mortality (CAMI study) [J]. Chin Circ J. 2019;34(5):437-443. [Google Scholar]
- 63.Zhang Y, Gao C, Duan G, et al. Survey on the early reperfusion therapy status in patients with ST-segment elevation myocardial infarction hospitalized in tertiary and secondary hospitals in Henan province, Chin J Cardiol. 2015;43(10):858-862. [PubMed] [Google Scholar]
- 64.Xinyun L, You Z, Muwei L, et al. Efficacy of thrombolytic therapy using reteplase in cases with acute ST-segment elevation myocardial infarction: results from a multicenter clinical trial, Chin J Cardiol. 2016;44(9):766-770. [DOI] [PubMed] [Google Scholar]
- 65.Lakshmanadoss U. Novel strategies in ischemic heart disease. Intech; 2012:123-134. [Google Scholar]
- 66.Kase CS, O’Neal AM, Fisher M, et al. Intracranial haemorrhage after use of tissue plasminogen activator for coronary thrombolysis. Ann Intern Med. 1990;112:17-21. [DOI] [PubMed] [Google Scholar]
- 67.Srinivasan G, Boschman C, Roth SI, et al. Unsuspected vasculitis and intracranial hemorrhage following thrombolysis. Clin Cardiol. 1997;20(1):84-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sloan M, Gore JM. Ischemic stroke and intracranial haemorrhage following thrombolytic therapy for acute myocardial infarction: a risk-benefit analysis. Am J Cardiol. 1992;69:21a-38a. [DOI] [PubMed] [Google Scholar]
- 69.De Rosa S, Spaccarotella C, Basso C, et al. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J. 2020;41(22):2083-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tam CF, Cheung KS, Lam S, et al. Impact of coronavirus disease 2019 (COVID-19) outbreak on ST-segment-elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13(4):e006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chu CM, Cheng VC, Hung IF, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cao B, Wang Y, Wen D, et al. A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.World Health Organisation . The use of non-steroidal anti-inflammatory drugs (NSAIDs) in Patients with COVID-19. https://www.who.int/news-room/commentaries/detail/the-use-of-non-steroidal-anti-inflammatory-drugs-(nsaids)-in-patients-with-covid-19. Accessed May 18 2020.
- 74.Gupta AK, Jneid H, Addison D, et al. Current perspectives on coronavirus disease 2019 and cardiovascular disease: a white paper by the JAHA Editors. J Am Heart Assoc. 2020;9(12):e017013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARSCoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kwong JC, Schwartz KL, Campitelli MA, et al. Acute myocardial infarction after laboratory‐confirmed influenza infection. N Engl J Med. 2018;378:345-353. [DOI] [PubMed] [Google Scholar]
- 77.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu CI, Postema PG, Arbelo E, et al. SARS-CoV-2, COVID-19, and inherited arrhythmia syndromes. Heart Rhythm 2020;17:1456-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]