Abstract
COVID-19 is one of the viral diseases that has caused many deaths and financial losses to humans. Using the available information, this virus appears to activate the host cell-death mechanism through Calpain activation. Calpain inhibition can stop its downstream cascade reactions that cause cell death. Given the main roles of Calpain in the entry and pathogenicity of the SARS-CoV-2, its inhibition can be effective in controlling the COVID-19. This review describes how the virus activates Calpain by altering calcium flow. When Calpain was activated, the virus can enter the target cell. Subsequently, many complications of the disease, such as inflammation, cytokine storm and pulmonary fibrosis, are caused by virus-activated Calpain function. Calpain inhibitors appear to be a potential drug to control the disease and prevent death from COVID-19.
Keywords: Apoptosis, Calcium channel, Inflammation, Necrosis, SARS-CoV-2
Introduction
An enveloped virus with non-segmented positive-sense RNA, SARS-CoV-2, is grouped as a member of the Sarbecovirus subgenus in the Betacoronavirus genus Orthocoronavirinae subfamily (Qiu et al. 2020). There are several unique features to SARS-CoV-2 compared with other human coronaviruses. SARS-CoV-2 infection mortality rate approximates 3.06%, which is significantly low compared to MERS-CoV (37%) and SARS-CoV (10%). The SARS-CoV-2’s primary reproduction number (R0) is 3.3–5.5, while 2–5 for SARS-CoV and 2.7–3.9 for MERS-CoV, suggesting SARS-CoV-2 higher transmissibility among other human coronaviruses (Wang et al. 2020). Angiotensin-converting enzyme 2 (ACE2) is a well-identified cell surface receptor of SARS-CoV-2 (Qiu et al. 2020). The composition of SARS-CoV-2 is structurally well-defined comprised of 14 binding residues which are in direct interaction with human ACE2. Eight of these 14 amino acids are conserved in SARS-CoV-2 (Sohrabi et al. 2020). Initially, ACE2 was recognized as an exopeptidase in vascular endothelial cells in the kidney and the heart, catalyzing angiotensins’ conversion (Donoghue et al. 2000; Ferrario et al. 2005). However, it later appeared to function as the receptor for SARS-CoV-2 (Kuba et al. 2005). Knowing that SARS-CoV-2 utilizes ACE2 as the receptor is a convincing reason to consider SARS-CoV-2 one of the subgenus of SARS-CoV. In the vertebrates, ACE2 presents a global expression pattern, which can’t be employed as the SARS-CoV-2 receptor (Wang et al. 2020).
Symptoms of diseases caused by SARS-CoVs are tied to an immunopathological response regulated by pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β, which the latter is a critical mediator of the cascade. IL-1β overproduction is associated with inflammatory features such as those induced by respiratory syndrome-linked coronaviruses (Nieto-Torres et al. 2015).
Among the viral open reading frames (ORFs) involved in inflammation, ORF-7a is responsible for the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB); ORF3b induces the expression of several cytokines and chemokines; ORF-6 downregulates interferon (IFN) generation; ORF-8a initiates cell apoptosis, and ORF-8b decrements viral multiplication. Finally, ORF-9b is selective toward the mitochondrial antiviral signaling protein (MAVS) signalosome to activate the breakdown of MAVS, TNF Receptor Associated Factor (TRAF)3, and TRAF6, significantly restricting the response of host cell IFN (Yue et al. 2018). This functional complexity is an alarming signal to cause lysosomal damage and endoplasmic reticulum stress, leading to activation of the NLR family pyrin domain containing 3 (NLRP3) inflammasome (Zhao and Zhao 2020). All S proteins in coronaviruses have three extracellular domains, a transmembrane anchor domain, and a short intracellular tail. Two active subunits exist in EC called the receptor-binding subunit (S1) and membrane-fusion subunit (S2). S1 is composed of two independent domains; one is the N-terminal domain (S1-NTD), and the other receptor-binding domain (RBD), which has a crucial duty to recognize and bind to the receptor. In the course of host–virus membrane fusion, host proteases cleave spike proteins at the S1/S2 boundary, in turn releases the spike fusion peptide, which is a necessary component of virus entry. There are a variety of host proteases responsible for S protein cleavage among various coronaviruses, determining virus pathological, and epidemiological attributes such as tissue tropism, host range, mortality, and transmissibility (Wang et al. 2020).
There are some hypotheses behind SARS-CoV evolution for targeting the necroptotic pathway and the receptor-interacting protein kinase 3 (Rip3). One of the concerns is the criticality of late interferon response in SARS-CoV pathogenesis (Channappanavar et al. 2016). Interferons are proteins released initially by those immune cells, which highly express Rip3. So by targeting Rip3-expressing immune cells through 3a, SARS-CoV might suppress any interferon responses, thus, increasing its chances of survival in the early infection stages. Another possible theory is that the inflammatory process of immunomodulators (IMMs) necrotic death can suppress the interferon response. In the lungs, inflammatory cell death can cause severe lung damage, which pathophysiologically linked with coughing as a clinical manifestation (Lu et al. 2006). The viral infection affects the ionic efflux and integrity of the plasma membrane, resulting in apoptosis, thus activating the NLRP3 inflammasome. The viral replication process leads to lytic cell death and, subsequently, potassium efflux, which produces the second activation signal for NLRP3 inflammasome (da Costa et al. 2019).
In this review, we showed that how the SARS-CoV-2 enters the cell and caused cell death by activating a protein involved in apoptotic (Calpain). The pivotal role of activated Calpain in causing cellular and clinical damages and its association with the calcium pathway even in the absence of a virus is described. In addition, it has been suggested that Calpain inhibition may provide a promising perspective for treating COVID-19.
SARS-CoV-2 receptor
Spike (S) glycoprotein of SARS-CoV-2 is capable of binding to respiratory epithelial cells through ACE2. In the attachment process, S glycoprotein splits into the subunits of S1 and S2. S1 holds the receptor-binding domain (RBD) through which the virus binds to the ACE2 receptor peptidase domain. It has been shown that surface protease transmembrane serine protease 2 (TMPRSS2) by cleavage receptor-binding domain (RBD) of SARS-CoV-2 spike protein increases its affinity to ACE2 receptors and increases its efficiency in endocytosis the virus into lung epithelial and lung fibroblast cell lines (Shang et al. 2020). S2 interferes in plasma membrane fusion (Yan et al. 2020). ACE2 is active in most tissues, while endothelium, lungs, kidney, and heart display the highest expression levels of ACE2 (Tipnis et al. 2000). ACE2 reduces blood pressure through catalyzing angiotensin II (a vasoconstrictor peptide) and cleavage to angiotensin 1–7 (a vasodilator) (Rice et al. 2004). Endocytosis occurs when the S glycoprotein of SARS-CoV-2 is bound to the ACE2 at the site of endocytosis (Baglivo et al. 2020).
Voltage-gated Ca++ channels are especially vital in contractile cells because extracellular Ca++ influx is crucial for muscle contraction and tension maintenance. Ca++ channels have been extensively studied in vascular smooth muscle (VSM), in which calcium influx influences arterial vaso-relaxation and vaso-constriction and eventually affects systemic blood pressure (Cribbs 2001).
The chemistry of Calpain
Calpain is a member of the conserved group of intracellular cysteine proteinases of calcium regulation pathways, which catalyzes the proteolysis of many specific substrates. Among more than ten different members of the Calpain family, micro (μ)-Calpain and milli (m)-Calpain have been described more commonly. They are universally expressed and are named after their required amount of Ca++ concentration for their activity in vitro (Storr et al. 2011). μ-Calpain and m-Calpain are both heterodimers containing a catalytically active subunit and a regulatory subunit, 80 kDa and 28 kDa, respectively. The regulatory subunit is encoded by CAPSNS1 in both isoforms, whereas the catalytic subunits are encoded by CAPN1 for μ-Calpain and CAPN2 for m-Calpain (Aoki et al. 1986).
The regulatory and catalytic subunits contain two (DV and DVI) domains and four (DI to DIV), respectively. Once Calpains are activated by calcium, DI is autolyzed. The protease domain DII is split into IIa and IIb subdomains that, upon binding with calcium, protease domain form a signal domain (II) that holds the catalytic site. DIII possesses features of C2 domains, engaged in structural modifications in the course of calcium-binding. The two carboxy-terminal domains of DIV (catalytic subunit) and DVI (regulatory subunit) comprise five EF-hands. However, the fifth hand is not involved in binding with calcium and assists subunits dimerization. At the regulatory amino-terminal, DVI holds a chain of glycine residues that enables interaction with the plasma membrane. These residues are autolyzed in Calpain activation (Imajoh et al. 1986). Clear roles have been demonstrated for Calpains in several major cellular processes, such as cell apoptosis and motility (Sáez et al. 2007; Vosler et al. 2008; Kerbiriou et al. 2009; Mani et al. 2009). Calpain activation has been implicated in several pathological conditions such as neuronal death following spinal cord injury (Wingrave et al. 2003), cataracts, multiple sclerosis, neuronal ischemia, central nervous system, and Alzheimer’s (Tsuji et al. 1998), Parkinson’s (Mouatt-Prigent et al. 1996), and amyotrophic lateral sclerosis (Ueyama et al. 1998). Reportedly, Calpain is responsible for glial cell apoptosis (Ray et al. 1999, 2002). Activation of Calpain has been recently demonstrated in motor neurons of adult mice spinal cord, which indicates a potential role for Calpain in adult motor neuron apoptosis (Momeni 2011).
While the protease domains (DII) of different Calpains are alike, other domains might show variations between isoforms; thus, not all isoforms are calcium-dependent, nor do they need the regulatory subunit (Sorimachi et al. 2010). Some isoforms in the Calpain family are universally expressed, like m-Calpain and μ-Calpain. Some of them are expressed in specific tissues, like Calpain 9, described in the gastrointestinal tract (Sorimachi et al. 2010). Whereas mice with knockout Capn1- demonstrate the lake of phenotype, Capns1- and Capn2-knockout mice die in the embryonic stage, suggesting the critical role of Calpains in embryogenesis (Dutt et al. 2006). Some phospholipids facilitate Calpain autolysis in the plasma membrane, such as phosphatidylinositol, phosphatidylinositol-4-monophosphate, and phosphatidylinositol-4, 5-bisphosphate (PIP2). Protein kinase Cι (PKCι) phosphorylates both m-Calpain and μ-Calpain. This process is found to be linked with an enhancement of the migration of cells and the attacking of cancerous lung cells via the proteolysis of focal adhesion proteins (focal adhesion kinase and talin) and cleavage of inhibitors of nuclear factor-kB (IkB) respectively. Calpain is additionally apoptosis-linked by cleavage of BCL-2 family members, caspases, and apoptosis-inducing factors. ERK is directly involved in the phosphorylation of Calpain on a specific serine residue, affecting cellular adhesion and motility. It possibly reduces the concentration of calcium that is necessary for activating calpain. The activity of Calpain can be negatively affected by protein kinase A (PKA) as it can block the binding of PIP2 in the C2 domain of Calpain (Storr et al. 2011). The responsible mechanisms for Calpain binding and activation in the caveolae are known. Structural investigations might suggest an involvement from domain III of Calpain (Kifor et al. 2003). Like phospholipase A2, domain III holds a characteristic C2 region that might bind to phospholipids, for example, phosphatidylinositol 4, 5-bisphosphate (Goudenege et al. 2005).
These acidic loops of the C2 domain of phospholipase A2 might be concerned with targeting the enzyme to the plasma membrane in an analogous way to ER and Golgi apparatus (Hood et al. 2004). Because of the C2 region presence, Calpain might bind to PIP2 in pulmonary smooth muscle caveolae, where Calpain is positioned in the vicinity of its likely substrates of the cytoskeleton-associated proteins, such as myristoylated alanine-rich C-kinase substrate (MARCKS), calcium-sensing receptors (CaRs), and cell signaling molecules (Goudenege et al. 2005) in the caveolae (Kifor et al. 2003). During the endocytosis process, the active Calpain cleaves the Talin-2 (TLN2) protein, and the β-integrin linkage with the cytoskeletal actin is disrupted, which causes endocytosis. It has been found that Angiotensin 2 increases the activity of β-integrin (Kawano et al. 2000). Therefore, inhibition of Calpain is by preventing the cleavage of TLN2 and the activation of β-integrin, and subsequently, endocytosis could eliminate the effects of activation of Angiotensin 2. Angiotensin 2 actives Calpain (Letavernier et al. 2008), and Calpain inhibition can counteract the impact of its activation. Extracellular space Ca++ concentration is 1–10 mM, whereas cytosol Ca++ concentration (Ca++ i) is 0.1–l M. It makes an inward electrochemical gradient that forces Ca++ entry through the plasma membrane (Nakamura et al. 1999). Smooth muscle Caveolae has several mechanisms for increasing Ca++) such as reverse-mode NCX, voltage-gated Ca++ channels, and non-selective cation channels), which all guarantee sufficient transient peak Ca++ levels to be met during activation. Therefore, NCX cleavage by Calpain might result in Ca++ efflux inhibition, which can cause a persistent overload of calcium in the smooth muscle of the pulmonary artery, which leads to pulmonary hypertension (Ghosh et al. 2009). It has been shown that by Calpain inhibition, the influx of extracellular into the cells is also stopped (Waters et al. 1997).
Calpain key role in COVID-19
COVID-19-induced inflammation and cell death and its relationship with Calpain
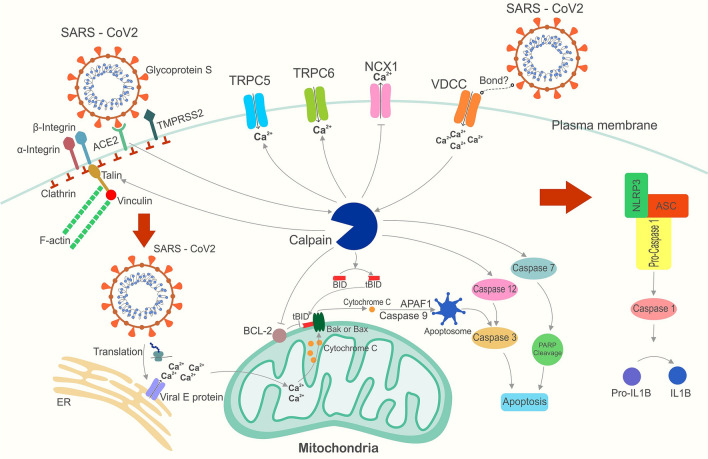
As confirmed for the influenza A virus, the calcium entry into the cytoplasm via sialic acid occurs by binding an unidentified viral protein to the calcium channel (Fujioka et al. 2018). In COVID-19, it has been shown that the decrease in serum calcium concentration can be a marker to determine the severity of the disease (Zhou et al. 2020a, b). Calpain is activated by elevated intracellular calcium, thereby triggering the viral endocytosis procedure by Talin cleavage in locations wherein the viral S protein couples to the ACE2 receptor (Franco et al. 2004). Activation of Calpain, in turn, leads to the increased entrance of calcium from the extracellular into the intracellular milieu by activating calcium channels, including TRPC5 (Kaczmarek et al. 2012) and TRPC6 (Verheijden et al. 2018). The activation of Calpain inhibits calcium efflux NCX1 cleavage (Bano et al. 2005), resulting in increased intracellular calcium and decreased extracellular calcium concentrations. A reduction in serum calcium concentrations in COVID-19 patients is one of the observed events. (Sun et al. 2020). Blood pressure in COVID-19 patients may decrease significantly due to this elevation along with the Ang-II factor. Some Calpain-digested extracellular calcium stays in the extracellular milieu through Calpain suppression. Extracellular Ca + + can prevent pressure falls due to the viral disease in the smooth muscle cells (Scholze et al. 2005). When SARS-CoV-2 enters the cytoplasm and its proteins are translated, the viral E protein creates a channel in the ER membrane. Activation of these channels is caused releasing calcium into the cytoplasm from the ER lumen, leading to calcium overloading, which causes the activation of the NLRP3 inflammasome (Fig. 1).
Fig. 1.
Calpain’s role in COVID-19-induced inflammation
Calpain and SARS-CoV-2 entry
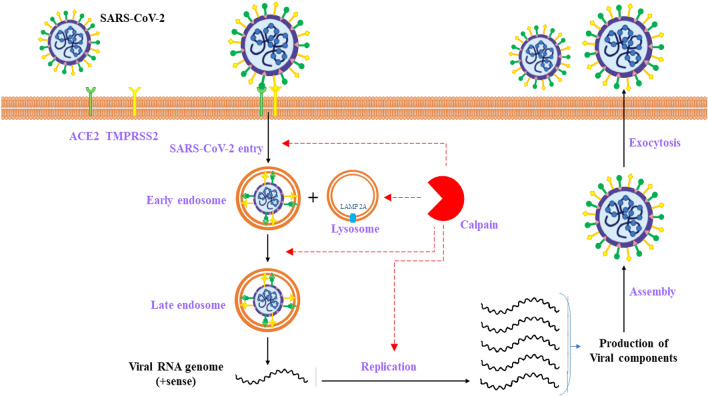
The proteolytic cleavage of coronavirus S proteins by host cell-originated proteases is critical for entering the virus in addition to receptor coupling. This procedure includes cleaving the S1/S2 site by serine protease (the surface trans-membrane protease serine two or TMPRSS2) which facilitates viral entrance at the plasma membrane surface. subsequently, cysteine protease activates SARS-CoV-2 Spike in endosomes (e.g., endolysosomal cathepsin L). This results in compensation for entrance into cells lacking TMPRSS2, thereby mediating the fusion of virus-cell membrane at the cell surface and endosomal compartments in respective order (Harrison et al. 2020). To determine the role of cysteine protease for the entrance of SARS-CoV-2, cells underwent treatment with inhibitors of cathepsin and Calpain. According to observations of cells treated with E64D inhibitors (cathepsin B, H, L, and Calpain), the entrance of SARS-CoV-2 decreased considerably by 92.5%. Nevertheless, cells treated with the inhibitor of cathepsin L and cathepsin B showed a decrease of more than 76% and the non-significant entrance of the virus in respective order. Besides demonstrating the crucial contribution of cathepsin to SARS-CoV-2 entry, the findings indicate the essential function of Calpain as well (Ou et al. 2020).
Notably, LAMP 2A is a substratum of Calpain 1 at the lysosomal membrane surface. Activated Calpain, leading to lysosomal stabilization, disrupts lysosomes, and suppression of cathepsins prevents cellular death (Villalpando Rodriguez and Torriglia 2013). Calpain has a significant function in the viral entrance phase (Fig. 2). Calpain's inhibitors can contribute to the suppression of viral entrance via plasma membrane or endocytosis, conversion of the early endosome to late endosome dynamics, and viral multiplication and propagation.
Fig. 2.
Calpain’s role in the conversion of the early endosome to late endosome dynamics and the viral multiplication and propagation
COVID-19-induced cytokine storm and its relationship with Calpain
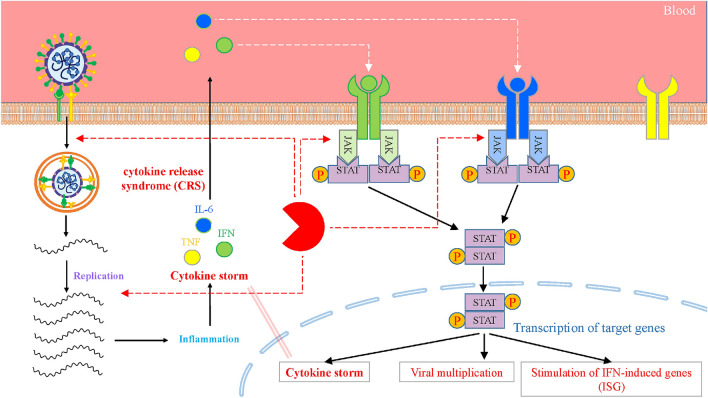
Acute respiratory distress syndrome (ARDS) mainly causes the demise of COVID-19 with cytokine storms (Fig. 3). A bulk of literature indicates that the acuteness of COVID-19 is accompanied by elevated levels of inflammatory mediators, such as cytokines and chemokines. Among the increased inflammatory mediators, the circulatory IL-6 concentration has a high correlation with the disease fatality in COVID-19 (Zhou et al. 2020a, b), implying that lethal COVID-19 is represented as a cytokine release syndrome (CRS) (Hirano and Murakami 2020; McGonagle et al. 2020). Intravascular coagulation is among the inducers of multi-organ dysfunction where the primary mediators are inflammatory cytokines, particularly IL-6 (Guillén et al. 2020). The induction of the endocytosis of ACE2 by SARS-CoV-2 infection and SARS-CoV in target cells, such as epithelial and endothelial cells, results in high concentrations of serum angiotensin II (Ang II) because of reduced ACE2 expressed at the surface. (Li et al. 2003; Kuba et al. 2005).
Fig. 3.
Schematic presentation of cytokine storm following the entry of SARS-CoV-2
Ang II functions in vaso-constriction and as a pro-inflammatory cytokine through Ang II type 1 receptor (AT1R). Thus, it has been hypothesized that a renin-angiotensin system (RAS) is responsible for the ARDS development after SARS-CoV-2 infection (Hirano and Murakami 2020). Treating mice with AT1R inhibitors or exogenic recombinant ACE2 inhibits the development of the ARDS caused by SARS-CoV infection (Kuba et al. 2005).
Furthermore, reports are available on the likely merit of RAS inhibitors in patients with COVID-19. The ADAM metallopeptidase domain 17 (ADAM17) is activated by the Ang II-AT1R signaling axis, which subsequently absorbs the membrane types of epidermal growth factor family members (EGF, TGF, etc.). TNF-α, and the NF-κB route is stimulated by the whole abovementioned factors. The ADAM17 enzyme also processes the membrane-bound IL-6Rα in a solvable type (sIL-6Rα) along with ADAM10 (Eguchi et al. 2018) (Scheller et al. 2011). Hence, the assumed serum Ang II and sIL-6Rα are potential markers predicting the COVID-19 acuteness.
Upon the generation of sIL-6Rα, the sIL-6R-IL-6 complex through its coupling to gp130 transduces the intracellular signaling. A signal transducer of IL-6, that shows expression in non-immune cells, such as endothelial cells, epithelial cells, and fibroblasts, even with no indication of membrane IL-6R, and the Janus kinase (JAK)/STAT3 is activated afterward. Ang II-AT1R signaling can form an IL-6-interceded positive feedback loop of signaling in NF-κB, a process recognized as the IL-6 intensifier, in the course of lung inflammation, after which the occurrence of ARDS accompanied by multi-organ dysfunction and clotting (Hojyo et al. 2020).
A crucial stage of rescue from acute COVID-19 has suggested being cytokine storm treatment. Some of the cytokines contributed to COVID-19 apply a distinctive intracellular signaling route in which Janus kinases (JAKs) have a mediatory role. JAK-STAT signaling pathway in cytokine and chemokine responses is an important immunological and immunopathological component in pathogenic viral infections in humans. (Channappanavar and Perlman 2017). Although the contribution of pro-inflammatory cytokines and chemokines in pathological studies of COVID-19 has not been evidenced directly, correlations have been observed between high levels of serum cytokines and chemokines had been observed to correlate with the disease acuteness and complications clinically (Huang et al. 2020). The JAK-STAT signaling route is critical in transducing the signaling of Type I IFNs, which are generated in responding to bacterial contagiousness. They are also the main contributors to preventing viral multiplication at the initial phase of infection (Mesev et al. 2019) (Perry et al. 2005).
The induction of Type II IFN (IFNγ) signaling via the mediation with JAK1–JAK2 complexes is reported to result in improved antibacterial immunity and upregulated the expression level of multiple genes induced by IFN, which play the primary role in viral clearance (Schoggins et al. 2011) (Decker et al. 2005). JAK suppression can affect these useful antibacterials and antiviral procedures by mediating Type I IFN and IFNγ.
A recent report by Blanco-Melo et al. indicates that SARS-CoV-2 induces partial IFN-I and -III responses but a robust chemotactic and inflammatory response, characterized by significant increases in IL-6, IL-1β, IL1RA, CCL2, and CCL8 levels. The authors showed that the decreased expression of IFN in COVID-19 patients might be an antagonistic mechanism of SARS-CoV-2, which evades the response of Type I IFN to prevent the instigation of immune cells and stimulation of IFN-induced genes (ISG) (Blanco-Melo et al. 2020).
Furthermore, it is noteworthy that ACE2, the reputed receptor of SARS-CoV-2, is an ISG with predominant expression in epithelial cells of human airways (Ziegler et al. 2020). There is a need for additional studies to clarify if treating with the IFN-I could result in the upregulated ACE2 and have the potential to improve infection in reputed target cells for SARS-CoV-2 or using JAK inhibitors that target IFN signal transmission would lower the risk of SARS-CoV-2 disease. Thus, JAK suppression represents a fascinating therapeutical modality for CRS, which commonly causes unfavorable complications clinically in COVID-19 (Luo et al. 2020). Slight suppression of IFN by JAK inhibitors can lead to more positive aftermaths than severe inhibition of IFN, resulting in other viral and accompanying bacterial infections.
The VE-cadherin, an inter-endothelial adhesion molecule, and inhibitor of cytokine signaling 3 (SOCS3), an endogenic JAK/STAT cytokine signaling inhibitor, has been recognized as a substrate of the Calpain system (Calabrese et al. 2020). It can be said that if Calpain is inhibited, the JAK/STAT inhibitor will not be subjected to Calpain proteolytic action and prevents the JAK/STAT system from being over-activated. Given the function of IL-6 in the induction of pulmonary fibrosis, a strategy is to inhibit it for preventing fibrosis due to COVID-19 viral infection. The damaging impacts of IL-6 can be neutralized via inhibiting the JAK with Calpain inhibitors. Accordingly, Calpain inhibitors can be used as an effective strategy in (partial) inhibition of the JAK.
ANGII-mediated MMP induction and its association with Calpain
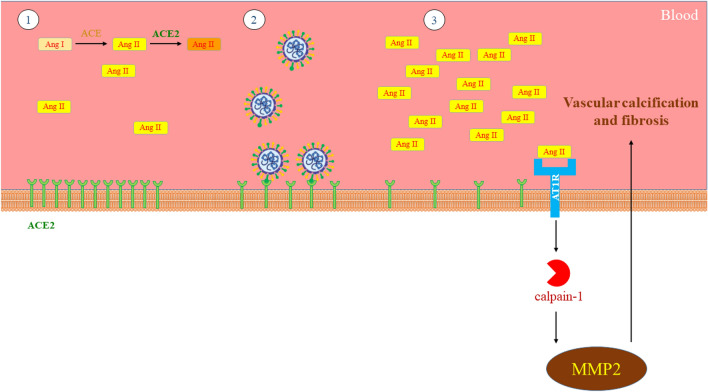
Angiotensin II is responsible for matrix metalloproteinase type 2 (MMP2) and the expression and activity of Calpain-1 in the arterial wall. Activated MMP2 is secreted by the cross-talk of two proteases, Calpain-1 and MMP2, leading to ECM remodeling modulation through enhancement of collagen synthesis and facilitation of vascular calcification (Fig. 4).
Fig. 4.
Relationship between Calpain and vascular calcification and fibrosis following COVID-19 1. Normal state of the vascular system 2. Binding of SARS-CoV-2 to the ACE2 receptor 3. Development of vascular calcification and fibrosis by Calpain and MMP2
MMP2 transcripts and protein concentrations and function are induced by overexpression of Calpain-1, partially by raising the portion of membrane-type 1 MMPs to tissue inhibitor of metalloproteinase 2. Such impacts of MMP2 activated by overexpression of Calpain-1 have a relationship with elevated collagen I, III, synthesis and vascular calcification. Transformation of growth factor-β1/Smad signaling, elastin dissociation, alkaline phosphatase activity and the total calcium level are also induced by the overexpression of Calpain-1 (Jiang et al. 2012).
Platelet activation in COVID-19 and its relationship with Calpain
According to a growing body of literature, COVID-19 sensitizes patients to thromboembolic ailments. It indicates (1) COVID-19 patients undergo elevated activity of in vivo platelet, as demonstrated by highly activated αIIbβ3 and expressed P-selectin; besides, viral RNA detected in the blood robustly associated with hyperactivity of platelet. (2) ACE2 and TMPRSS2 are strongly expressed in platelets. (3) Platelet activity and forming thrombus are promoted by SARS-CoV-2 and its spike protein through the mitogen-activated protein kinase (MAPK) route downstream of ACE2. And (4) platelet activity and forming thrombus caused by SARS-CoV-2 can be blocked by recombinant human ACE2 protein and anti-Spike monoclonal antibody treatments (Zhang et al. 2020).
A highly combined occurrence of thrombotic cases has increasingly been evidenced in COVID-19 patients with critical complications, namely venous and arterial thrombotic cases, and thrombocytopenia that is associated with elevated death rate (Klok et al. 2020; Yang et al. 2020). Anticoagulation is associated with a low death rate risk with no risk of bleeding diathesis amongst hospitalized patients with COVID-19 (Tang et al. 2020).
Platelets, which mainly mediate thrombosis, undergo hyperactivation in patients with COVID-19. According to other recently published reports, a variety of platelet-activating processes, such as aggregation, adhesion, infiltration, and inflammatory response, have contributed to lung damage and microvascular thrombosis in SARS-CoV-2-related pneumonia (Poissy et al. 2020) (Li et al. 2020), demonstrating the function of activated platelet in the pathogenicity of COVID-19.
Recently, investigations have shown the hyperactivation of platelets in COVID-19 patients. The biomarkers of platelet activation are linked to coagulation malfunction and the combined consequence of stroke or demise (Zhang et al. 2020). The emphasis of such investigations is the concept of likely activation of hyper inflammation and hypercoagulability by the cytokine storm.
The phosphorylation of MAPK was detected in SARS-CoV-2-induced platelets, and phosphorylation of ACE2 occurred sooner than MAPK signaling, suggesting MAPK activation downstream of ACE2 induction. In support of this observation, activated MAPK was attributed to ACE2 signaling in lung cells (Chen et al. 2010).
Based on findings, MAPK inhibitors can reserve SARS-CoV-2-stimulated platelet induction, which supports our assumption of the MAPK-mediated induction of platelets. Corresponding to these results, the promotion of thromboxane formation by MAPK phosphorylation via triggering cPLA2 in platelets has been reported in a recently published study (Manne et al. 2018). Besides, platelet secretion and clot recantation are facilitated by activating MAPK by inducing myosin light chain phosphorylation (Flevaris et al. 2009).
Furthermore, direct stimulation of releasing Factor V and XIII by SARS-CoV-2 and its Spike protein occurred at levels corresponding to thrombin induction and creation of leukocyte–platelet aggregates (LPAs) (Zhang et al. 2020).
As significant sources of coagulation factors V and XIII in α granules, platelets act as adhesion positions through exposing their surface phosphatidylserine (PS) (Golebiewska and Poole 2015). SARS-CoV-2 was found to stimulate platelets for releasing both Factor V and Factor XIII. Platelets contain a broad array of inflammatory cytokines in α granules capable of rapid secretion once activated to contribute to the immune response (Koenen 2016). PF4, TNF-α, IL-8, and IL-1β were secreted from platelets by stimulating SARS-CoV-2 and its spike protein (Zhang et al. 2020).
Consequently, the elevated expression of phosphatidylserine and P-selectin results from activated platelets. Following activated immunity, mitochondrial membrane depolarization, up-regulation of Bax, down-regulation of Bcl-XL, moderate induction of procaspase 3, and elevated Calpain action were biochemical indications of cell death. In the caspase three induction, the activity of Calpains has significant contributions to platelet roles, including aggregation, adhesion, dispersion, and platelet-induced shrinkage of blood clots (Mordakhanova et al. 2020).
Besides the Calpain inhibition effect on inhibition of MAPK, Calpain function inhibition confirmed the reversion of platelet hyperreactivity stimulated by hypoxia (Liu et al. 2016). An in vivo model of hypoxia-stimulated thrombosis additionally proved a prothrombotic function for Calpain. Plasma Calpain actions and soluble P-selectin levels rose in patients who presented thrombosis while at the endmost altitudes. The present research indicates that improved Calpain function is linked to a high occurrence of thrombosis under hypoxic settings (Tyagi et al. 2014).
This proves that platelet secretion, aggregation, and spreading are regulated by Calpain, indicating that Calpain has an initial contribution to platelet induction after stimulating thrombin receptors. Other regulative functions have also been recognized for Calpain in initial cytoskeletal remodeling, such as secretion, aggregation, and spreading (Croce et al. 1999).
The function of Calpain in the PIP2 pathway in COVID-19
Phosphoinositides have multiple critical functions in endocytosis, one of them is phosphatidylinositol-3,5-bisphosphate (PIP2), regulating early endosome to late endosome dynamics. This indicates that the PIP2 pathway could be deemed a promising general drug target for CoV infection. Due to possible Calpain binding to PIP2, this conversion can be disrupted by suppressing Calpain (Ou et al. 2020).
The main downstream effectors of PIP2 are two-pore channel subtype 2 (TPC2) and TRPML1 in lysosomes. TPC2 action is blocked by tetrandrine, an inhibitor for TPC2, and declined entrance of SARS-CoV-2 S pseudovirions indicates the importance of TPC2 for SARS-CoV-2 entrance (Ou et al. 2020). PI (3, 5) P2 may contribute to SARS-CoV-2 entry through its main effectors in lysosomes.
Multiple cytoskeletal regulatory functions are responsible for endocytosis, one of which is talin triggered by PPIs, among the PIP2 importantly regulates cytoskeletal and membrane dynamics. The cellular impact of PIP2 level manipulation is rather evident in cells: its increased generation results in highly increased cellular Factin and decreased PIP2 concentrations or sequestration by overexpressed PIP2 scavengers, leading to diminished actin assemblage and separation of the membrane from the internal cytoskeleton. A proposed variant of coincidental induction is that PIP2 contributes to the localization of talin as an actin-membrane linker to the plasma membrane, which is activated afterward. Talin is a multi-domain protein maintained in an inactive cytosolic mode by inhibitive interplays amongst its different domains. Such an auto-inhibition is relievable by PIP2 for activating talin binding to both the F-actin and the transmembrane adhesion protein integrin. Talin also couples to phosphatidylinositol 4-phosphate 5-kinase gamma (PIPKIγ2) upon phosphorylation of the enzyme with AKT. PIPKIγ2 couples to the plasma membrane, wherein it generates PIP2 from its precursor PIP, after which the freshly produced PIP2 couples to neighboring talin (Janmey et al. 2018).
According to the abovementioned observations (cases), PIP2 in lysosomes and endosomes may also involve SARS-CoV-2 entrance (endocytosis). In animals, selective catalysis of the phospholipid PIP2 hydrolysis by PLC is done on the glycerol side of the phosphodiester bond (Kuchay and Chishti 2007). The phospholipase C-β (PLC-β) and protein kinase C (PKC) have also been identified as Calpain substrates. They have the potential of modulating downstream signaling routes of secretive procedures in responding to a variety of agonists (Kuchay and Chishti 2007). Researches showed, during cell attachment, 32P-labeled phosphatidic acid and PIP2, products of phospholipase C and phosphoinositide 3-kinase functions, increases. Adding the Calpain inhibitor led to substantial reductions in phosphatidic acid and PIP2. PLC induction regulated with Calpains could be critical for controlling PtdIns-4,5-P2 and diacylglycerols synthesis, having significant contributions to regulating actin-associated proteins (Paulhe et al. 2001).
Calpain’s role in SARS-CoV-2 replication
The SARS-CoV-2’s major protease, crucial to the viral multiplication and propagation in the host cells, is named Mpro. The inhibition of this protease blocks the multiplication; thus, it is an exciting target for antiviral drug innovation.
Among over 13,000 drugs present in the DrugBank, inhibitive compounds of Mpro were found through a virtual screening by the E-pharmacophore modeling approach according to an energy-optimized pharmacophore hypothesis. The development of this hypothesis was established on the crystallographic construct of Mpro in a complex with a non-covalent inhibitor. Accordingly, 1000 drugs were chosen as the best hits in the uppermost ranks concerning the fitness score. Later, exploiting molecular docking through high-throughput virtual screening (HTVS), standard precision (SP), and extra precision (XP) docking modules, additional screening of these hits was carried out. Only 30 drugs were chosen from this pool docked Mpro, for which binding free energy was estimated by the MMGBSA approach, with the final selection of eight drugs. Two (DB04653 and DB02243) of eight potential inhibitors of Mpro among these experimental drugs are cysteine protease inhibitors. DB04653, named Calpain inhibitor IV, is a reputed inhibitor of Calpain, a calcium-dependent cysteine protease (Fitness score = 0.536, Glide core (Kcal/mol) = − 8.342, and Binding Free Energy (kcal/mol) = − 81.28). DB02243 is an inhibitor of Cathepsin F, a protease that belongs to the papain family of cysteine proteases (Fitness score = 1.083, Glide core (Kcal/mol) = − 7.281, and Binding Free Energy (Kcal/mol) = − 101.52) (Abhithaj et al. 2020).
Since the Mpro has a vital contribution to coronavirus multiplication, Chunlong Ma et al. reportedly discovered inhibitors that target the SARS-CoV-2 key protease (Mpro), aiming at developing potent Mpro inhibitors as SARS-CoV-2 antivirals. Amongst a set of examined protease inhibitors, multiple inhibitors, such as boceprevir, GC-376, and Calpain inhibitors II, and XII, were detected to active enzymatic assay figure submicromolar IC50 levels. It is noteworthy that Calpain inhibitors II and XII characterize new chemotypes as being distinctive from reputed substrate-based peptidomimetic Mpro inhibitors.
Uncovering Calpain II and XII inhibitors as potential SARS-CoV-2 antivirals implies that it was possibly plausible to develop dual inhibitors against the viral Mpro and the host Calpains/cathepsins, which both are critical for viral multiplication. The inhibition constants (KI) are 1.18 ± 0.10 μM, 1.57 ± 0.13 μM, 0.40 ± 0.02 μM, and 0.13 ± 0.02 μM for boceprevir, MG-132, and Calpain inhibitors II and XII, respectively. The most potent compound was Calpain inhibitor XII, which displayed an EC50 of 0.49 μM in the primary CPE test and an EC50 of 0.78 μM in the secondary VYR test. Inhibitors of Calpain and cathepsin such as MDL28170 (Calpain inhibitor III), 33 MG-132, and 34 Calpain inhibitor VI previously have been identified to suppress SARS-CoV multiplication under in vitro conditions. In addition to the high potentiality of selectivity toward both Mpro and Calpain/cathepsin extra advantage of these dual inhibitors could be their high genetic barricade to drug resistance (Ma et al. 2020).
Putative Calpain inhibitors
Studies on Calpain inhibitors and knockdown experiments confirm that inhibition of Calpain inhibits SARS-CoV replication by MG132 (Schneider et al. 2012). It is reported that Calpain-1 is a defensive mechanism in neuronal cell activation through employing selective inhibitors. Insertion and activation of Calpain-2 on the plasma membrane are responsible for cell damage (Martines et al. 2017). Studies have previously shown that viral ORFs generate proteins that activate the cellular death mechanism. Calpain, activated by extracellular calcium increase caused by virus contamination, seems to play a regulating role in apoptosis or, at least, aggravates it. Calpain inhibition is capable of blocking this pathway to a great extent. Extracellular space calcium can serve as a vasoconstrictor against virus-mediated vasodilation. Calpain inhibition inhibits endocytosis through which SARS-CoV-2 gains entry to the host cell.
SNJ‐1945 is a metabolically stable and permeable compound demonstrated to reduce in vivo and in vitro retinal cell death. Another inhibitor of Calpain, MDL28170, has been shown to serve as a protective factor against excitotoxicity. E64, leupeptin, and BDA‐410 are Calpain inhibitors that have also been reported to exert protective effects on AD cell culture. Along with other inhibitors such as SJA6017 and ALLN, there is a recently manufactured A‐705053 that can be orally administrated and has enhanced pharmacokinetics. It indicates a preventive function against dynamin‐1 in primary hippocampal neurons culture mediated by Calpain, as well as being an effective preventive, simultaneous, or curative treatment when used for Aβ‐triggered neurodegeneration in nucleus basalis magnocellularis (Mahaman et al. 2019).
BLD-2660 is a new, artificial, orally active, small-molecule inhibitor of Calpain (CAPN) 1, 2, and 9, having selectivity over the cathepsins and other protease families. This compound is metabolically stable and permeable, orally bioavailable, besides has low cytochrome P450 (CYP) suppression. Thanks to these properties, it has been developed for the therapy of COVID-19, wherein there are considerable unfulfilled medical needs (ClinicalTrials.gov Identifier: NCT04334460).
Although various compounds have been proposed for the treatment of COVID-19, the necessary and appropriate efficacy to combat the effects of this disease has not yet been achieved. Due to the wide range of symptoms in patients with this disease, it seems that a compound that can disrupt various aspects of the virus in the cell has a better chance of clinical success. This compound can be used as a treatment and front line against this virus along with the use of vaccines. As mentioned, Calpain has a variety of roles in the pathogenesis of COVID-19. Thus, inhibition of Calpain leads to a reduction or inhibition of processes such as SARS-CoV-2 entry into the cell, maturation of the primary endosome to the secondary endosome, virus replication, development of ARDS, CRS, MMP induction, inflammation, and platelet activation. This therapeutic strategy has the potential to dramatically reduce the morbidity and mortality of COVID-19.
Author contributions
ADJ: investigation and data curation. MHR: supervision and conceptualization. MS: writing–original draft preparation and visualization.
Funding
Not applicable.
Data availability
Enquiries about data availability should be directed to the authors.
Declarations
Competing interests
The authors declare that they have no conflict of interest, financial or otherwise.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aref Doozandeh Juibari, Email: arefdoozandeh@yahoo.com.
Mohammad Hossein Rezadoost, Email: rezadoostmh@guilan.ac.ir.
Masoud Soleimani, Email: soleim_m@modares.ac.ir.
References
- Abhithaj J, Francis D, Sharanya CS, Arun KG, Sadasivan C, Variyar EJ. Repurposing simeprevir, calpain inhibitor IV and a cathepsin F inhibitor against SARS-CoV-2 and insights into their interactions with Mpro. J Biomol Struct Dyn. 2020;40(1):325–336. doi: 10.1080/07391102.2020.1813200. [DOI] [PubMed] [Google Scholar]
- Aoki K, Imajoh S, Ohno S, Emori Y, Koike M, Kosaki G, Suzuki K. Complete amino acid sequence of the large subunit of the low-Ca2+-requiring form of human Ca2+-activated neutral protease (muCANP) deduced from its cDNA sequence. FEBS Lett. 1986;205(2):313–317. doi: 10.1016/0014-5793(86)80919-X. [DOI] [PubMed] [Google Scholar]
- Baglivo M, Baronio M, Natalini G, Beccari T, Chiurazzi P, Fulcheri E, Petralia PP, Michelini S, Fiorentini G, Miggiano GA, Morresi A, Tonini G, Bertelli M. Natural small molecules as inhibitors of coronavirus lipid-dependent attachment to host cells: a possible strategy for reducing SARS-COV-2 infectivity? Acta Biomed. 2020;91(1):161–164. doi: 10.23750/abm.v91i1.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bano D, Young KW, Guerin CJ, Lefeuvre R, Rothwell NJ, Naldini L, Rizzuto R, Carafoli E, Nicotera P. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell. 2005;120(2):275–285. doi: 10.1016/j.cell.2004.11.049. [DOI] [PubMed] [Google Scholar]
- Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Møller R, Jordan TX, Oishi K, Panis M, Sachs D, Wang TT, Schwartz RE, Lim JK, Albrecht RA, tenOever BR. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–1045.e1039. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese LH, Lenfant T, Calabrese C. Cytokine storm release syndrome and the prospects for immunotherapy with COVID-19, part 4: the role of JAK inhibition. Cleve Clin J Med. 2020 doi: 10.3949/ccjm.87a.ccc060. [DOI] [PubMed] [Google Scholar]
- Channappanavar R, Fehr AR, Vijay R, Mack M, Zhao J, Meyerholz DK, Perlman S. Dysregulated type I Interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19(2):181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen IY, Chang SC, Wu HY, Yu TC, Wei WC, Lin S, Chien CL, Chang MF. Upregulation of the chemokine (C-C motif) ligand 2 via a severe acute respiratory syndrome coronavirus spike-ACE2 signaling pathway. J Virol. 2010;84(15):7703–7712. doi: 10.1128/JVI.02560-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs LL. Vascular smooth muscle calcium channels: could “T” be a target? Circ Res. 2001;89(7):560–562. doi: 10.1161/res.89.7.560. [DOI] [PubMed] [Google Scholar]
- Croce K, Flaumenhaft R, Rivers M, Furie B, Furie BC, Herman IM, Potter DA. Inhibition of calpain blocks platelet secretion, aggregation, and spreading. J Biol Chem. 1999;274(51):36321–36327. doi: 10.1074/jbc.274.51.36321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa LS, Outlioua A, Anginot A, Akarid K, Arnoult D. RNA viruses promote activation of the NLRP3 inflammasome through cytopathogenic effect-induced potassium efflux. Cell Death Dis. 2019;10(5):346. doi: 10.1038/s41419-019-1579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker T, Müller M, Stockinger S. The yin and yang of type I interferon activity in bacterial infection. Nat Rev Immunol. 2005;5(9):675–687. doi: 10.1038/nri1684. [DOI] [PubMed] [Google Scholar]
- Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87(5):E1–9. doi: 10.1161/01.RES.87.5.e1. [DOI] [PubMed] [Google Scholar]
- Dutt P, Croall DE, Arthur JS, Veyra TD, Williams K, Elce JS, Greer PA. m-Calpain is required for preimplantation embryonic development in mice. BMC Dev Biol. 2006;6:3. doi: 10.1186/1471-213X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi S, Kawai T, Scalia R, Rizzo V. Understanding angiotensin II type 1 receptor signaling in vascular pathophysiology. Hypertension. 2018;71(5):804–810. doi: 10.1161/HYPERTENSIONAHA.118.10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1–7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. 2005;289(6):H2281–H2290. doi: 10.1152/ajpheart.00618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flevaris P, Li Z, Zhang G, Zheng Y, Liu J, Du X. Two distinct roles of mitogen-activated protein kinases in platelets and a novel Rac1-MAPK-dependent integrin outside-in retractile signaling pathway. Blood. 2009;113(4):893–901. doi: 10.1182/blood-2008-05-155978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco SJ, Rodgers MA, Perrin BJ, Han J, Bennin DA, Critchley DR, Huttenlocher A. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat Cell Biol. 2004;6(10):977–983. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- Fujioka Y, Nishide S, Ose T, Suzuki T, Kato I, Fukuhara H, Fujioka M, Horiuchi K, Satoh AO, Nepal P, Kashiwagi S, Wang J, Horiguchi M, Sato Y, Paudel S, Nanbo A, Miyazaki T, Hasegawa H, Maenaka K, Ohba Y. A sialylated voltage-dependent Ca(2+) channel binds hemagglutinin and mediates influenza A virus entry into mammalian cells. Cell Host Microbe. 2018;23(6):809–818.e805. doi: 10.1016/j.chom.2018.04.015. [DOI] [PubMed] [Google Scholar]
- Ghosh B, Kar P, Mandal A, Dey K, Chakraborti T, Chakraborti S. Ca2+ influx mechanisms in caveolae vesicles of pulmonary smooth muscle plasma membrane under inhibition of alpha2beta1 isozyme of Na+/K+-ATPase by ouabain. Life Sci. 2009;84(5–6):139–148. doi: 10.1016/j.lfs.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Golebiewska EM, Poole AW. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev. 2015;29(3):153–162. doi: 10.1016/j.blre.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudenege S, Poussard S, Dulong S, Cottin P. Biologically active milli-calpain associated with caveolae is involved in a spatially compartmentalised signalling involving protein kinase C alpha and myristoylated alanine-rich C-kinase substrate (MARCKS) Int J Biochem Cell Biol. 2005;37(9):1900–1910. doi: 10.1016/j.biocel.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Guillén L, Padilla S, Fernández M, Agulló V, García JA, Telenti G, García-Abellán J, Botella Á, Gutiérrez F, Masiá M. Preemptive interleukin-6 blockade in patients with COVID-19. Sci Rep. 2020;10(1):16826. doi: 10.1038/s41598-020-74001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AG, Lin T, Wang P. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends Immunol. 2020;41(12):1100–1115. doi: 10.1016/j.it.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52(5):731–733. doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojyo S, Uchida M, Tanaka K, Hasebe R, Tanaka Y, Murakami M, Hirano T. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 2020;40:37. doi: 10.1186/s41232-020-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood JL, Brooks WH, Roszman TL. Differential compartmentalization of the calpain/calpastatin network with the endoplasmic reticulum and Golgi apparatus. J Biol Chem. 2004;279(41):43126–43135. doi: 10.1074/jbc.M408100200. [DOI] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imajoh S, Kawasaki H, Suzuki K. The amino-terminal hydrophobic region of the small subunit of calcium-activated neutral protease (CANP) is essential for its activation by phosphatidylinositol. J Biochem. 1986;99(4):1281–1284. doi: 10.1093/oxfordjournals.jbchem.a135593. [DOI] [PubMed] [Google Scholar]
- Janmey PA, Bucki R, Radhakrishnan R. Regulation of actin assembly by PI(4,5)P2 and other inositol phospholipids: an update on possible mechanisms. Biochem Biophys Res Commun. 2018;506(2):307–314. doi: 10.1016/j.bbrc.2018.07.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Zhang J, Monticone RE, Telljohann R, Wu J, Wang M, Lakatta EG. Calpain-1 regulation of matrix metalloproteinase 2 activity in vascular smooth muscle cells facilitates age-associated aortic wall calcification and fibrosis. Hypertension. 2012;60(5):1192–1199. doi: 10.1161/HYPERTENSIONAHA.112.196840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek JS, Riccio A, Clapham DE. Calpain cleaves and activates the TRPC5 channel to participate in semaphorin 3A-induced neuronal growth cone collapse. Proc Natl Acad Sci U S A. 2012;109(20):7888–7892. doi: 10.1073/pnas.1205869109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano H, Cody RJ, Graf K, Goetze S, Kawano Y, Schnee J, Law RE, Hsueh WA. Angiotensin II enhances integrin and alpha-actinin expression in adult rat cardiac fibroblasts. Hypertension. 2000;35(1 Pt 2):273–279. doi: 10.1161/01.HYP.35.1.273. [DOI] [PubMed] [Google Scholar]
- Kerbiriou M, Teng L, Benz N, Trouve P, Ferec C. The calpain, caspase 12, caspase 3 cascade leading to apoptosis is altered in F508del-CFTR expressing cells. PLoS ONE. 2009;4(12):e8436. doi: 10.1371/journal.pone.0008436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kifor O, Kifor I, Moore FD, Jr, Butters RR, Jr, Brown EM. m-Calpain colocalizes with the calcium-sensing receptor (CaR) in caveolae in parathyroid cells and participates in degradation of the CaR. J Biol Chem. 2003;278(33):31167–31176. doi: 10.1074/jbc.M303377200. [DOI] [PubMed] [Google Scholar]
- Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen RR. The prowess of platelets in immunity and inflammation. Thromb Haemost. 2016;116(4):605–612. doi: 10.1160/TH16-04-0300. [DOI] [PubMed] [Google Scholar]
- Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchay SM, Chishti AH. Calpain-mediated regulation of platelet signaling pathways. Curr Opin Hematol. 2007;14(3):249–254. doi: 10.1097/MOH.0b013e3280ef68f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letavernier E, Perez J, Bellocq A, Mesnard L, de Castro Keller A, Haymann JP, Baud L. Targeting the calpain/calpastatin system as a new strategy to prevent cardiovascular remodeling in angiotensin II-induced hypertension. Circ Res. 2008;102(6):720–728. doi: 10.1161/CIRCRESAHA.107.160077. [DOI] [PubMed] [Google Scholar]
- Li C, Li J, Ni H. Crosstalk between platelets and microbial pathogens. Front Immunol. 2020;11:1962. doi: 10.3389/fimmu.2020.01962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZF, Zheng D, Fan GC, Peng T, Su L. Heat stress prevents lipopolysaccharide-induced apoptosis in pulmonary microvascular endothelial cells by blocking calpain/p38 MAPK signalling. Apoptosis. 2016;21(8):896–904. doi: 10.1007/s10495-016-1263-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Zheng BJ, Xu K, Schwarz W, Du L, Wong CK, Chen J, Duan S, Deubel V, Sun B. Severe acute respiratory syndrome-associated coronavirus 3a protein forms an ion channel and modulates virus release. Proc Natl Acad Sci U S A. 2006;103(33):12540–12545. doi: 10.1073/pnas.0605402103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Li YX, Jiang LJ, Chen Q, Wang T, Ye DW. Targeting JAK-STAT signaling to control cytokine release syndrome in COVID-19. Trends Pharmacol Sci. 2020;41(8):531–543. doi: 10.1016/j.tips.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Sacco MD, Hurst B, Townsend JA, Hu Y, Szeto T, Zhang X, Tarbet B, Marty MT, Chen Y, Wang J. Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. Cell Res. 2020;30(8):678–692. doi: 10.1038/s41422-020-0356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaman YAR, Huang F, Kessete Afewerky H, Maibouge TMS, Ghose B, Wang X. Involvement of calpain in the neuropathogenesis of Alzheimer's disease. Med Res Rev. 2019;39(2):608–630. doi: 10.1002/med.21534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SK, Balasubramanian S, Zavadzkas JA, Jeffords LB, Rivers WT, Zile MR, Mukherjee R, Spinale FG, Kuppuswamy D. Calpain inhibition preserves myocardial structure and function following myocardial infarction. Am J Physiol Heart Circ Physiol. 2009;297(5):H1744–1751. doi: 10.1152/ajpheart.00338.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manne BK, Münzer P, Badolia R, Walker-Allgaier B, Campbell RA, Middleton E, Weyrich AS, Kunapuli SP, Borst O, Rondina MT. PDK1 governs thromboxane generation and thrombosis in platelets by regulating activation of Raf1 in the MAPK pathway. J Thromb Haemost. 2018;16(6):1211–1225. doi: 10.1111/jth.14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martines A, Stifanese R, Faelli EL, Perasso L, Melloni I, Ruggeri P, Averna M. Calpain-1 resident in lipid raft/caveolin-1 membrane microdomains plays a protective role in endothelial cells. Biochimie. 2017;133:20–27. doi: 10.1016/j.biochi.2016.12.002. [DOI] [PubMed] [Google Scholar]
- McGonagle D, Sharif K, O'Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6):102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesev EV, LeDesma RA, Ploss A. Decoding type I and III interferon signalling during viral infection. Nat Microbiol. 2019;4(6):914–924. doi: 10.1038/s41564-019-0421-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momeni HR. Role of calpain in apoptosis. Cell J. 2011;13(2):65–72. [PMC free article] [PubMed] [Google Scholar]
- Mordakhanova ER, Nevzorova TA, Synbulatova GE, Rauova L, Weisel JW, Litvinov RI. Platelet activation in heparin-induced thrombocytopenia is followed by platelet death via complex apoptotic and non-apoptotic pathways. Int J Mol Sci. 2020;21(7):2556. doi: 10.3390/ijms21072556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouatt-Prigent A, Karlsson JO, Agid Y, Hirsch EC. Increased M-calpain expression in the mesencephalon of patients with Parkinson's disease but not in other neurodegenerative disorders involving the mesencephalon: a role in nerve cell death? Neuroscience. 1996;73(4):979–987. doi: 10.1016/0306-4522(96)00100-5. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Minamisawa H, Katayama Y, Ueda M, Terashi A, Nakamura K, Kudo Y. Increased intracellular Ca2+ concentration in the hippocampal CA1 area during global ischemia and reperfusion in the rat: a possible cause of delayed neuronal death. Neuroscience. 1999;88(1):57–67. doi: 10.1016/S0306-4522(98)00207-3. [DOI] [PubMed] [Google Scholar]
- Nieto-Torres JL, Verdiá-Báguena C, Jimenez-Guardeño JM, Regla-Nava JA, Castaño-Rodriguez C, Fernandez-Delgado R, Torres J, Aguilella VM, Enjuanes L. Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology. 2015;485:330–339. doi: 10.1016/j.virol.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, Guo L, Guo R, Chen T, Hu J, Xiang Z, Mu Z, Chen X, Chen J, Hu K, Jin Q, Wang J, Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11(1):1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulhe F, Bogyo A, Chap H, Perret B, Racaud-Sultan C. Vascular smooth muscle cell spreading onto fibrinogen is regulated by Calpains and phospholipase C. Biochem Biophys Res Commun. 2001;288(4):875–881. doi: 10.1006/bbrc.2001.5859. [DOI] [PubMed] [Google Scholar]
- Perry AK, Chen G, Zheng D, Tang H, Cheng G. The host type I interferon response to viral and bacterial infections. Cell Res. 2005;15(6):407–422. doi: 10.1038/sj.cr.7290309. [DOI] [PubMed] [Google Scholar]
- Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, Jeanpierre E, Rauch A, Labreuche J, Susen S. Pulmonary embolism in patients with COVID-19: awareness of an Increased Prevalence. Circulation. 2020;142(2):184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Zhao YB, Wang Q, Li JY, Zhou ZJ, Liao CH, Ge XY. Predicting the angiotensin converting enzyme 2 (ACE2) utilizing capability as the receptor of SARS-CoV-2. Microbes Infect. 2020;22(4–5):221–225. doi: 10.1016/j.micinf.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SK, Neuberger TJ, Deadwyler G, Wilford G, DeVries GH, Banik NL. Calpain and calpastatin expression in primary oligodendrocyte culture: preferential localization of membrane calpain in cell processes. J Neurosci Res. 2002;70(4):561–569. doi: 10.1002/jnr.10414. [DOI] [PubMed] [Google Scholar]
- Ray SK, Shields DC, Saido TC, Matzelle DC, Wilford GG, Hogan EL, Banik NL. Calpain activity and translational expression increased in spinal cord injury. Brain Res. 1999;816(2):375–380. doi: 10.1016/S0006-8993(98)01128-7. [DOI] [PubMed] [Google Scholar]
- Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383(Pt 1):45–51. doi: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáez ME, Martínez-Larrad MT, Ramírez-Lorca R, González-Sánchez JL, Zabena C, Martinez-Calatrava MJ, González A, Morón FJ, Ruiz A, Serrano-Ríos M. Calpain-5 gene variants are associated with diastolic blood pressure and cholesterol levels. BMC Med Genet. 2007;8:1. doi: 10.1186/1471-2350-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller J, Chalaris A, Garbers C, Rose-John S. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 2011;32(8):380–387. doi: 10.1016/j.it.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Schneider M, Ackermann K, Stuart M, Wex C, Protzer U, Schätzl HM, Gilch S. Severe acute respiratory syndrome coronavirus replication is severely impaired by MG132 due to proteasome-independent inhibition of M-calpain. J Virol. 2012;86(18):10112–10122. doi: 10.1128/JVI.01001-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, Rice CM. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472(7344):481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholze A, Maier A, Stocks F, Karamohamad F, Vetter R, Zidek W, Tepel M. Sustained increase of extracellular calcium concentration causes arterial vasoconstriction in humans. J Hypertens. 2005;23(11):2049–2054. doi: 10.1097/01.hjh.0000186831.41125.51. [DOI] [PubMed] [Google Scholar]
- Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, Li F. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci. 2020;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabi C, Alsafi Z, O'Neill N, Khan M, Kerwan A, Al-Jabir A, Iosifidis C, Agha R. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorimachi H, Hata S, Ono Y. Expanding members and roles of the calpain superfamily and their genetically modified animals. Exp Anim. 2010;59(5):549–566. doi: 10.1538/expanim.59.549. [DOI] [PubMed] [Google Scholar]
- Storr SJ, Carragher NO, Frame MC, Parr T, Martin SG. The calpain system and cancer. Nat Rev Cancer. 2011;11(5):364–374. doi: 10.1038/nrc3050. [DOI] [PubMed] [Google Scholar]
- Sun JK, Zhang WH, Zou L, Liu Y, Li JJ, Kan XH, Dai L, Shi QK, Yuan ST, Yu WK, Xu HY, Gu W, Qi JW. Serum calcium as a biomarker of clinical severity and prognosis in patients with coronavirus disease 2019. Aging (albany NY) 2020;12(12):11287–11295. doi: 10.18632/aging.103526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275(43):33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Shimohama S, Kimura J, Shimizu K. m-Calpain (calcium-activated neutral proteinase) in Alzheimer's disease brains. Neurosci Lett. 1998;248(2):109–112. doi: 10.1016/S0304-3940(98)00348-6. [DOI] [PubMed] [Google Scholar]
- Tyagi T, Ahmad S, Gupta N, Sahu A, Ahmad Y, Nair V, Chatterjee T, Bajaj N, Sengupta S, Ganju L, Singh SB, Ashraf MZ. Altered expression of platelet proteins and calpain activity mediate hypoxia-induced prothrombotic phenotype. Blood. 2014;123(8):1250–1260. doi: 10.1182/blood-2013-05-501924. [DOI] [PubMed] [Google Scholar]
- Ueyama H, Kumamoto T, Fujimoto S, Murakami T, Tsuda T. Expression of three calpain isoform genes in human skeletal muscles. J Neurol Sci. 1998;155(2):163–169. doi: 10.1016/S0022-510X(97)00309-2. [DOI] [PubMed] [Google Scholar]
- Verheijden KAT, Sonneveld R, Bakker-van Bebber M, Wetzels JFM, van der Vlag J, Nijenhuis T. The calcium-dependent protease Calpain-1 Links TRPC6 activity to podocyte injury. J Am Soc Nephrol. 2018;29(8):2099–2109. doi: 10.1681/ASN.2016111248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalpando Rodriguez GE, Torriglia A. Calpain 1 induce lysosomal permeabilization by cleavage of lysosomal associated membrane protein 2. Biochim Biophys Acta. 2013;1833(10):2244–2253. doi: 10.1016/j.bbamcr.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Vosler PS, Brennan CS, Chen J. Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol Neurobiol. 2008;38(1):78–100. doi: 10.1007/s12035-008-8036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Qiu Y, Li JY, Zhou ZJ, Liao CH, Ge XY. A unique protease cleavage site predicted in the spike protein of the novel pneumonia coronavirus (2019-nCoV) potentially related to viral transmissibility. Virol Sin. 2020;35(3):337–339. doi: 10.1007/s12250-020-00212-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters SL, Sarang SS, Wang KK, Schnellmann RG. Calpains mediate calcium and chloride influx during the late phase of cell injury. J Pharmacol Exp Ther. 1997;283(3):1177–1184. [PubMed] [Google Scholar]
- Wingrave JM, Schaecher KE, Sribnick EA, Wilford GG, Ray SK, Hazen-Martin DJ, Hogan EL, Banik NL. Early induction of secondary injury factors causing activation of calpain and mitochondria-mediated neuronal apoptosis following spinal cord injury in rats. J Neurosci Res. 2003;73(1):95–104. doi: 10.1002/jnr.10607. [DOI] [PubMed] [Google Scholar]
- Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Yang Q, Wang Y, Wu Y, Xu J, Yu Y, Shang Y. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18(6):1469–1472. doi: 10.1111/jth.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y, Nabar NR, Shi C-S, Kamenyeva O, Xiao X, Hwang I-Y, Wang M, Kehrl JH. SARS-coronavirus open reading frame-3a drives multimodal necrotic cell death. Cell Death Dis. 2018;9(9):904. doi: 10.1038/s41419-018-0917-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Liu Y, Wang X, Yang L, Li H, Wang Y, Liu M, Zhao X, Xie Y, Yang Y, Zhang S, Fan Z, Dong J, Yuan Z, Ding Z, Zhang Y, Hu L. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol. 2020;13(1):120. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Zhao W. NLRP3 inflammasome-a key player in antiviral responses. Front Immunol. 2020;11:211. doi: 10.3389/fimmu.2020.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Chen D, Wang L, Zhao Y, Wei L, Chen Z, Yang B. Low serum calcium: a new, important indicator of COVID-19 patients from mild/moderate to severe/critical. Biosci Rep. 2020;40(12):BSR20202690. doi: 10.1042/BSR20202690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, Cao Y, Yousif AS, Bals J, Hauser BM, Feldman J, Muus C, Wadsworth MH, 2nd, Kazer SW, Hughes TK, Doran B, Gatter GJ, Vukovic M, Taliaferro F, Mead BE, Guo Z, Wang JP, Gras D, Plaisant M, Ansari M, Angelidis I, Adler H, Sucre JMS, Taylor CJ, Lin B, Waghray A, Mitsialis V, Dwyer DF, Buchheit KM, Boyce JA, Barrett NA, Laidlaw TM, Carroll SL, Colonna L, Tkachev V, Peterson CW, Yu A, Zheng HB, Gideon HP, Winchell CG, Lin PL, Bingle CD, Snapper SB, Kropski JA, Theis FJ, Schiller HB, Zaragosi LE, Barbry P, Leslie A, Kiem HP, Flynn JL, Fortune SM, Berger B, Finberg RW, Kean LS, Garber M, Schmidt AG, Lingwood D, Shalek AK, Ordovas-Montanes J. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5):1016–1035.e1019. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Enquiries about data availability should be directed to the authors.