Abstract
The global methane cycle includes both terrestrial and atmospheric processes and may contribute to feedback regulation of the climate. Most oxic soils are a net sink for methane, and these soils consume approximately 20 to 60 Tg of methane per year. The soil sink for atmospheric methane is microbially mediated and sensitive to disturbance. A decrease in the capacity of this sink may have contributed to the ∼1% · year−1 increase in the atmospheric methane level in this century. The organisms responsible for methane uptake by soils (the atmospheric methane sink) are not known, and factors that influence the activity of these organisms are poorly understood. In this study the soil methane-oxidizing population was characterized by both labelling soil microbiota with 14CH4 and analyzing a total soil monooxygenase gene library. Comparative analyses of [14C]phospholipid ester-linked fatty acid profiles performed with representative methane-oxidizing bacteria revealed that the soil sink for atmospheric methane consists of an unknown group of methanotrophic bacteria that exhibit some similarity to type II methanotrophs. An analysis of monooxygenase gene libraries from the same soil samples indicated that an unknown group of bacteria belonging to the α subclass of the class Proteobacteria was present; these organisms were only distantly related to extant methane-oxidizing strains. Studies on factors that affect the activity, population dynamics, and contribution to global methane flux of “atmospheric methane oxidizers” should be greatly facilitated by use of biomarkers identified in this study.
Methane is a radiatively active atmospheric trace gas whose concentration is increasing at a rate of ca. 1% · year−1 (∼40 Tg · year−1). Human activity is thought to be a causative factor in the rising methane concentration and, as such, may contribute to global warming (4, 8, 27). The global methane cycle consists of both atmospheric (mainly chemical) and terrestrial (mainly biological) processes (27). The observed increase in the methane concentration has been attributed to a combination of an increase in the number of sources of methane and a decrease in the number of sinks for methane (4).
The major sinks for methane are biological oxidation at or near the sites of production (∼700 Tg · year−1), uptake of methane from the atmosphere by aerobic soils (20 to 60 Tg · year−1), and photochemical oxidation in the atmosphere (∼450 Tg · year−1) (27). Soil uptake of atmospheric methane is significant since the magnitude of the soil sink is equivalent to the observed annual increase in the methane concentration and it is more susceptible to disturbance by human activities (16, 21, 24, 34). A change in the soil sink can have a significant effect on the atmospheric mixing ratios of methane.
Biological methane oxidation consists of both aerobic and anaerobic processes. The global methane sink is dominated by aerobic methane-oxidizing bacteria (MOB). The biochemical basis of methane oxidation in all known MOB is similar (1, 9, 13, 20). All MOB possess a membrane-bound monooxygenase whose substrate range includes both methane and ammonia (note that some MOB contain an additional, biochemically distinct enzyme designated the soluble methane monooxygenase [sMMO]) (1). The membrane-bound monooxygenases are thought to be evolutionarily related (15). The MOB exhibit limited physiological, structural, and phyletic diversity compared to other functionally defined groups of bacteria (13, 25). Of particular significance are differences in the fate of carbon, the kinetic properties of the monooxygenase, and the evolutionary separation of the four major phyletic groups.
On the basis of cell physiology, the MOB can be divided into the methane-assimilating bacteria (MAB) (methanotrophs) and bacteria which cooxidize methane (autotrophic ammonia-oxidizing bacteria [AAOB]). The former organisms use methane as a sole source of carbon and energy and are characterized by the presence of a complete pathway for methane oxidation, the ability to assimilate cell carbon as formaldehyde, and apparent Km values for methane in the micromolar range (1, 13). The AAOB use ammonia oxidation as an energy source for autotrophic growth; they are characterized by a complete pathway for oxidation of ammonia to nitrite and assimilation of cell carbon by the Benson-Calvin cycle. In most cases their apparent Km values for methane are in the millimolar range and methane is cooxidized with no apparent benefit to the cells (1).
Both phenotypic and phylogenetic data can be used to subdivide the methanotrophs and AAOB into two additional groups that are defined on the basis of intracellular membrane type, major membrane fatty acids, and genetic comparison data (5, 13, 33). Thus, there is very strong support for the existence of four monophyletic groups of MOB, two MAB groups and two AAOB groups. The phyletic distinctiveness of these four groups from each other, combined with the relatively shallow phylogenetic depths of the groups, has allowed the use of various biomarkers as signatures in ecological studies. These biomarkers have included oligonucleotide probes and phospholipid ester-linked fatty acids (PLFA) (6, 12, 14, 23, 28, 29, 37, 38).
Soil methane uptake has been demonstrated to be biological. Methane uptake activity shares many features with the known MOB activity but also exhibits traits which do not occur during methane oxidation by extant organisms. The differences include a >100-fold-greater affinity for methane but an apparently poor capacity for growth on this substrate (2, 9, 20, 21, 31, 35). Perhaps the most significant difference is a much lower threshold concentration for sustained methane uptake. Several explanations have been proposed to account for this, including (i) mixotrophic growth of methanotrophs, (ii) cooxidation of methane by ammonia oxidizers, (iii) induction of a high-affinity enzyme system in response to starvation, and (iv) activity of novel methanotrophic bacteria (2, 7, 9, 20, 31, 40). More recently, workers have shown that methanotrophs in pure cultures can exhibit sustained uptake of atmospheric methane at normal atmospheric concentrations if the cultures are supplemented with methanol (3, 18). These workers proposed that the presence of methanol in soil may provide a physiological basis for methane uptake by conventional methanotrophs in soil.
However, no organism that has been isolated from soil has been conclusively demonstrated to account for soil methane uptake. Consequently, the biochemical, physiological, and phyletic relationships of soil high-affinity methane oxidizers to extant MOB are unknown. Demonstration and assessment of the biochemical and physiological relationships are important to the use of extant MOB as experimental models for soil methane uptake. The current mechanistic models for inhibition of high-affinity methane oxidation by soil additives are based on the assumption that there is biochemical similarity (7, 16, 21, 24, 34, 35). Understanding the phyletic relationships is important for using biomarkers to study the ecology of methane oxidizers.
MATERIALS AND METHODS
Cultures.
Methylosinus trichosporium OB3b and Methylomicrobium album BG8 were obtained from the culture collection at the University of Warwick, Coventry, United Kingdom.
Study sites.
Soil samples were collected from a beech forest in Denmark (Rold Forest), a rainforest in Brazil (Pantanal), and a mixed hardwood forest in the United States (Maine). Samples obtained from depths of 4 to 8 cm (Denmark and Brazil) and 6 to 10 cm (Maine) were used for analysis. All samples exhibited uptake of atmospheric methane. Characteristics of the three soils are given elsewhere (30).
Methane oxidation rates.
Sieved soil subsamples were assayed to determine oxidation of atmospheric methane (∼1.7 ppm of CH4) as previously described (28). Oxidation rates were estimated from first-order decreases in headspace methane concentrations. The affinity for methane (apparent Km) was determined by measuring methane uptake at methane concentrations between 1 and 200 ppm. Apparent Km values were estimated from direct nonlinear regression analysis results (uptake rates versus methane concentrations). Methane was analyzed with a Chrompak model 438A gas chromatograph equipped with a flame ionization detector. The detection limit for methane was 0.1 ppm.
Radiolabelling of methane oxidizers.
Soil samples were incubated with 14CH4 to specifically radiolabel the methanotrophic bacteria (29, 30). Intact soil samples (3 g) were incubated in 14-ml serum vials to which five aliquots consisting of 0.2 ml of 14CH4 (0.2 MBq · ml−1; Amersham, Amersham, England) were added at intervals. The methane concentration decreased from 50 to <0.5 ppm (CH4 plus 14CH4) between additions. The samples were aerated between additions to ensure that oxic conditions were present and to remove 14CO2. Labelling was terminated when the soil had consumed a total of 0.2 MBq of 14CH4 (after 3 to 4 days).
Pure cultures of methanotrophic bacteria (100 ml) were grown on 1% methane in a nitrate minimal medium (29). Cultures in the mid-logarithmic phase of growth were then radiolabelled with 14CH4 in 500-ml Erlenmeyer flasks as described above (five aliquots consisting of 0.2 ml of 14CH4). The initial methane concentration was adjusted to 1,000 ppm with unlabelled methane. The headspace methane concentration (CH4 plus 14CH4) decreased from 1,000 to <1 ppm between additions. Labelling was terminated when the cultures had consumed a total of 0.2 MBq of 14CH4 (after 2 to 3 days).
14C-PLFA analysis.
Extraction of total lipids, separation of lipid classes, and preparation of phospholipid ester-linked fatty acid methyl esters were carried out essentially as described previously (29, 30). Phospholipid ester-linked fatty acid methyl esters were separated with a model HP 5890 series II gas chromatograph equipped with a flame ionization detector and a 50-m type HP Ultra II fused-silica capillary column. Radiolabelled phospholipid fatty acids (14C-PLFAs) were detected as 14CO2 after combustion in the flame ionization detector (radio gas chromatography analysis). The 14C-PLFAs were separated into 15 fractions on the basis of their retention times and equivalent chain lengths (29, 30). Fatty acids were identified based on retention times relative to the retention times of authentic standards (Nu Chek Prep Inc.). The results were compared with data obtained with parallel samples analyzed by Microbial Insights Inc. (Knoxville, Tenn.).
DNA extraction.
Total DNA was extracted from the soil samples by using a hot sodium dodecyl sulfate lysis method derived from the method of Selenska and Klingmuller (32). Approximately 100 μg of DNA was obtained from a 2-g (fresh weight) portion of each soil sample. The protocol which we used has been demonstrated to be reliable for lysis of methanotrophs and to yield good-quality DNA from a variety of soils (23).
PCR, cloning, and sequencing.
Oligonucleotide primers that target universally conserved domains of the active site subunit (PmoA) (43) of all known particulate methane monooxygenase (pMMO) and ammonia monooxygenase (AMO) sequences have been described previously (15). A PCR in which this primer set is used amplifies homologs of pMMO and AMO genes (26) and can be used to create a library that is representative of the soil methane-oxidizing population. These primers were used to amplify and clone pmoA-amoA sequences from the soil horizons that exhibited atmospheric methane oxidation activity. Four libraries were constructed from DNA obtained from soil samples obtained from the Rold (Denmark), Maine (United States), and Pantanal (Brazil) forests. The PCR conditions used have been described previously (15). PCR products were cloned into the pCR II vector supplied with a T/A cloning kit (Invitrogen). Clones were also screened with environmental pmoA clone type RA14-specific primers (RAf380 [TGGGGCTGGACCTTCTATCC] and RAr541 [GCCATATTGCTCGGTCGGCTG]) and environmental pmoA clone type RA21-specific primers (RXf380 [CATATCTGGGCCTGGTTTCC] and RXr655 [CGGAATGGCCCCCGAAGGT]). Plasmids were purified, and sequencing reactions were carried out by cycle sequencing by using a dye terminator kit (PE Applied Biosystems, Cheshire, United Kingdom) as previously described (15).
Sequence analysis.
Nucleic acid sequences and inferred peptide sequences were aligned manually with the sequences in a database containing pmoA sequences. Bootstrapping, evolutionary distance calculation, and tree construction were performed by using the programs SEQBOOT, PROTDIST, DNADIST, FITCH, DNAPARS, PROTPARS, and CONSENSE of the PHYLIP package (version 3.5) (11). PAM distances were used for peptide trees, and Kimura distances were used for nucleic acid trees.
Nucleotide sequence accession numbers.
Sequences of partial pmoA gene fragments (RA14, RA21, Rold 1, Rold 2, Rold 3, Rold 4, Rold 5, Maine 6, Maine 7, Maine 8, and Maine 9) have been deposited in the GenBank database under accession no. AF148521 through AF148531.
RESULTS
Methane oxidation.
In all of the soils examined, the greatest atmospheric methane oxidation activity was found in the mineral soil below the organic horizon (depth, <4 cm). The potential activities for oxidation of atmospheric methane were 229, 752, and 21 pmol · g−1 · h−1 for the soils from the Rold, Maine, and Pantanal forests, respectively. The apparent Km for methane oxidation was determined for the Rold forest soil. Fresh soil exhibited an apparent Km of 10.7 ppm of CH4, which corresponded to approximately 14.7 nM CH4 in the soil water.
PLFA analysis.
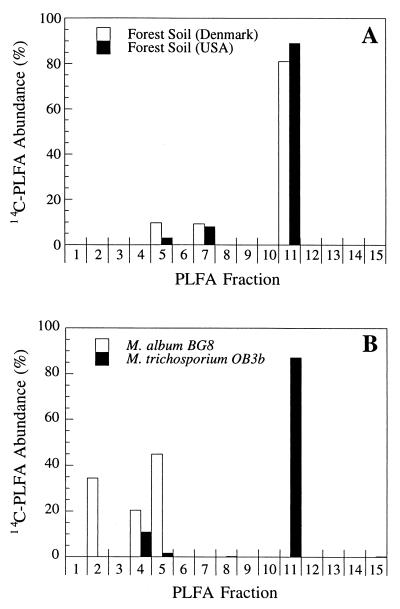
Soil samples were incubated with 14CH4 (<50 ppm) in order to radiolabel the microorganisms that metabolize methane at near-atmospheric concentrations. Phospholipids extracted from soil samples incubated with 14CH4 were assayed for the presence of 14C-PLFAs. Figure 1A shows the relative levels of 14C-PLFAs in radiolabelled samples obtained from the Rold and Maine forest soils. Significant amounts of radioactivity were detected in 3 of the 15 fractions (fractions 5, 7, and 11). These PLFA fractions represent fatty acids with equivalent chain lengths of 15.7 to 16.1 (fraction 5), 16.5 to 16.8 (fraction 7), and 17.7 to 17.9 (fraction 11) (see references 29 and 30 for details).
FIG. 1.
14C-PLFA fingerprints for MOB. (A) Unknown methanotrophs that metabolized atmospheric methane in two forest soils in Denmark (Rold) and the United States (Maine). (B) Type I methanotroph Methylomicrobium album BG8 and type II methanotroph Methylosinus trichosporium OB3b. Each bar represents the amount of radiolabel in a fraction as a percentage of the radioactivity in all fractions. The following reference standard PLFAs coeluted with the 14C-PLFAs: 14:0, i15:1, and a15:1 in fraction 2; 16:1w9 and 16:1w8 in fraction 4; 16:1w7, 16:1w5, and 16:0 in fraction 5; i17:0, a17:0, and 17:1w8 in fraction 7; and 18:1w9, 18:1w8, and 18:1w7 in fraction 11.
The inferred PLFA signature for the soil methanotrophic community under these labelling conditions was different from the PLFA signature reported for known MOB, including autotrophic ammonia oxidizers and type I or type II methanotrophic bacteria (12, 30, 41). The distinction from methanotrophs was confirmed by the results of a 14C-PLFA analysis of a representative type I methanotroph (Methylomicrobium album BG8) and a type II methanotroph (Methylosinus trichosporium OB3b) labelled under comparable conditions (Fig. 1B). The 14C-PLFA(s) present in fraction 7 (Fig. 1A) had a retention time that was significantly different from the retention time of any fatty acid labelled in the control methanotrophs (the values were >5 min different). The fatty acids in fraction 7 coeluted with the reference standard fatty acids i17:0, a17:0, and 17:1w8.
pmoA analysis.
The presence of uncharacterized MOB in soil was confirmed by using a molecular biology approach. The Rold library contained 14 clones of methane monooxygenase (MMO) type sequences, and 4 of these clones (designated RA9, RA14, RA21, and RA26) could not be placed in any known group of MMO or AMO sequences in our database. The remaining clones were identified as Methylococcus sp. (four clones), Nitrosospira sp. (three clones), Nitrosococcus sp. (one clone), or Nitrosomonas sp. (two clones). Clones RA9, RA14, and RA26 were very similar, exhibiting 98% identity.
pmoA libraries were also constructed by using three soils obtained from the Rold, Maine, and Pantanal forests. Forty clones from each library were screened by PCR with RA14- and RA21-specific PCR primers. A total of 15 clones were identified and sequenced. Three clones each from the Rold, Maine, and Pantanal libraries were similar to RA14 (Rold 1, Rold 3, and Rold 5; Maine 6, Maine 8, and Maine 10; and Pantanal 11, Pantanal 13, and Pantanal 14), and a tenth clone which was amplified with the RA14 primers, Rold 4, was similar to the pmoA sequence of Methylocystis sp. strain M. However, the five clones identified with the RA21 primers were all similar to the Nitrosomonas amoA sequences.
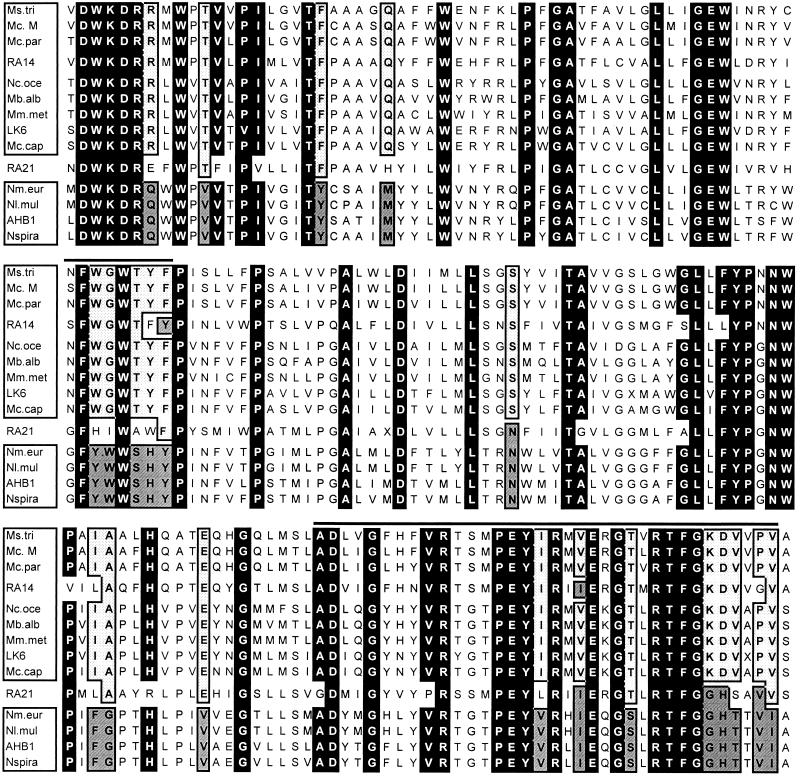
Alignments of available PmoA and AmoA sequence information (Fig. 2) revealed 52 universally conserved amino acid residues. Members of the RA14 group possessed 50 of these 52 universally conserved residues, and clone RA21 had 45 matches with these 52 conserved amino acid residues. While the new sequences clearly belong to the same protein family, they are significantly different at sites which are otherwise highly conserved in physiologically and phyletically diverse organisms.
FIG. 2.
Alignment of predicted peptide sequences of PmoA and AmoA from representative methanotrophic and nitrifying bacteria with soil clone RA14 and RA21 sequences. Residues which are universally conserved in extant MOB are highlighted with black. Putative MMO signatures are highlighted with dots, and putative AMO signatures are highlighted with grey. The horizontal lines indicate predicted hydrophilic domains conserved in all of the peptides (15). The sequences shown (and their accession numbers) are as follows: Ms.tri, Methylosinus trichosporium (U31650); Mc. M, Methylocystis sp. strain M (U81596); Mc.par, Methylocystis parvus (U31651); Nc.oce, Nitrosococcus oceanus (U31652); Mb.alb, Methylomicrobium album BG8 (U31654); Mm.met, Methylomonas methanica S1 (U31653); LK6, Methylocaldum tepidum LK6 (U89304); Mc.cap, Methylococcus capsulatus Bath (L40804); Nm.eur, Nitrosomonas europaea (L08050); Nl.mul, Nitrosolobus multiformis (U31649); AHB1, Nitrosospira sp. strain AHB1 (X90821); Nspira, Nitrosospira sp. strain Np22 (U31655).
Sequence analysis.
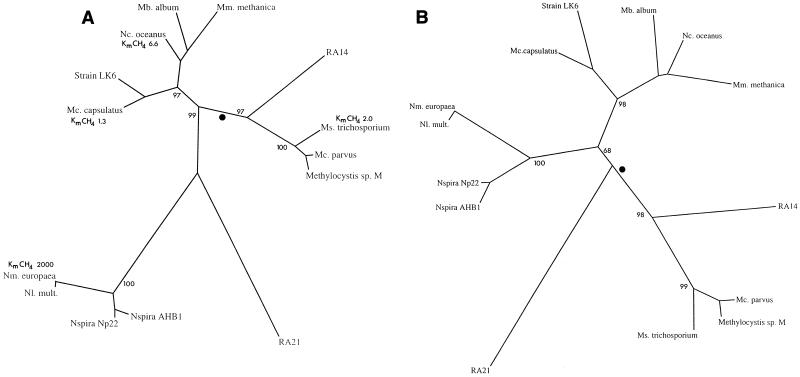
Dendrograms constructed from the inferred peptide sequences of PmoA and AmoA showed that there were three strongly supported monophyletic groups: (i) the representatives of the α subclass of the class Proteobacteria (α-Proteobacteria) (type II methanotrophs) and RA14, (ii) the γ-Proteobacteria representatives (type I methanotrophs and Nitrosococcus oceanus), and (iii) the β-Proteobacteria ammonia oxidizers (Fig. 3). The relationships among the cultivated strains were broadly similar to the relationships based on an analysis of several other genes, including the 16S rRNA gene (26). We did not observe a significant relationship between RA21 and any of the other PmoA or AmoA sequences.
FIG. 3.
Unrooted phylogenetic trees showing relationships of RA14 and RA21 to representative methanotrophs and ammonia-oxidizing bacteria. (A) Tree constructed by using a 171-amino-acid segment of the 27-kDa subunit of MMO (PmoA or AmoA). (B) Tree constructed by using a restricted sequence alignment (125 amino acids) in which putative signature residues (see Fig. 2) were omitted from the analysis. On the basis of the bacterial phylogeny derived from rRNA (10, 26, 42), the root of the trees is predicted to lie on the branch indicated by a dot. The numbers at the nodes indicate the numbers of times the groups occurred in 100 bootstrap replicates. Approximate Km values for CH4 (Km CH4) of the holoenzymes are indicated as micromolar concentrations (Table 1). For the sequence accession numbers see the legend to Fig. 2. Abbreviations: Mb, Methylomicrobium; Mm., Methylomonas; Nc., Nitrosococcus; Mc., Methylocystis; Ms., Methylosinus; Nm., Nitrosomonas; Nl., Nitrosolobus; Nspira, Nitrosospira.
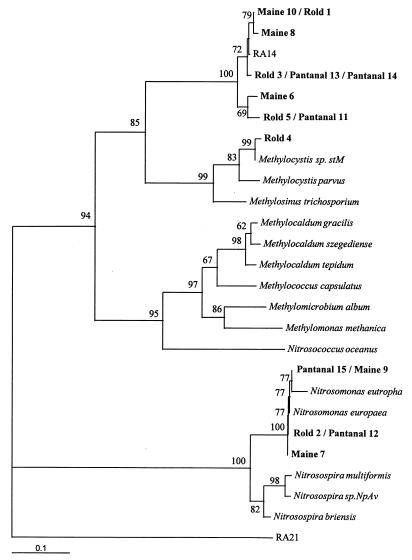
Phylogenetic analysis of the new Rold, Maine, and Pantanal PmoA sequences (Fig. 4) revealed a number of clones from each soil which grouped with the RA14 sequence, but we detected no clones which grouped with the RA21 sequence.
FIG. 4.
Phylogenetic analysis of the derived amino acid sequences encoded by the pmoA genes of methanotrophs, nitrifiers, and Rold, Maine, and Pantanal soil DNA samples. The dendrogram shows the results of an analysis in which PROTDIST was used. Bootstrap values greater than 50% derived from 100 replicates are also shown. The bar represents 10% sequence divergence, as determined by measuring the lengths of the horizontal lines connecting any two species.
DISCUSSION
The MOB are a physiologically (functionally) defined group of bacteria. Four subgroups of MOB have been recognized; there are two groups of MAB (type I and II methanotrophs) and two groups of AAOB (β- and γ-subdivision ammonia oxidizers). Soil methane uptake activity experiments typically reveal biochemical characteristics with broad similarity to characteristics of the known members of these four phyletic groups (Table 1). The debate concerning the nature of the organisms responsible for soil methane uptake has focused on three distinct issues. Do all soil methane oxidizers possess the same biochemistry for methane oxidation? What is the physiological role of methane in soil methane oxidizers? Do soil methane oxidizers belong to any of the four currently known phyletic groups of MOB?
TABLE 1.
Some characteristics of AAOB (nitrifying bacteria), methanotrophic bacteria, and unknown high-affinity methane oxidizers in soila
| Bacteria | Phylogenetic affiliation | Membrane type | Major fatty acidsb | Key enzyme | Apparent Km for CH4 (μM)c | Vmax for CH4 (mmol · g of cells−1 · h−1) | Oxidation inhibited by C2H2, C2H4, DME, and COd | Growth inhibited by elevated NH3 concn | CH4 required to maintain activity | % Assimilation of oxidized CH4e |
|---|---|---|---|---|---|---|---|---|---|---|
| MAB | ||||||||||
| Group 1 | γ-Proteobacteria | Stacked, central | 14:0, 16:0, and 16:1 species | pMMO (+ some sMMO) | 0.8–32 | 10–31 | + | + | + | 29–62 |
| Group 2 | α-Proteobacteria | Flat, peripheral | 18:1 species | pMMO (+ some sMMO) | 0.8–32 | 10–31 | + | + | + | 29–62 |
| Soil high affinity | ? | ? | 18:1 species | ? | 0.01–0.06 | ? | + | + | + | 20–54 |
| AAOB | ||||||||||
| Group 3 | β-Proteobacteria | Flat, peripheral | 16:0 and 16:1 species | AMO | 2,000 | 0.03–0.59 | + | − | − | 0–17 |
| Group 4 | γ-Proteobacteria | Stacked, central | 16:0 and 16:1 species | AMO | 6.6 | 0.01–0.08 | + | − | − | 1–47 |
A major fatty acid is present at a concentration of >10% in at least one member of each group.
The values for nitrifiers were based on the Ki of CH4 for ammonia oxidation.
DME, dimethyl ether.
The fate of labelled methane in nitrifiers is not known. The apparent assimilation could be due to nonspecific labelling by formaldehyde or assimilation at the level of CO2.
To determine how diverse the soil methane oxidizers are, we analyzed the distribution of radiolabelled PLFAs in soil samples after incubation with 14CH4. This analysis was based on the assumption that atmospheric methane is assimilated into biomass by the same population of organisms that oxidize it. This has been shown previously to be the most likely scenario for methane oxidizers in a forest soil (28). In the Rold, Maine, and Pantanal forest soils, 36, 39, and 35%, respectively, of the atmospheric methane consumed was assimilated into microbial biomass (30). This relatively high level of carbon conversion efficiency is consistent with the hypothesis that soil methane uptake is dominated by MAB. The relatively simple 14C-PLFA profile obtained from soils supplied with low methane concentrations (Fig. 1) suggests that the diversity of the organisms that incorporate the label is low (i.e., all members of the population are likely to be related at the generic level). In broad terms the PLFA profile of the soil methanotroph community was comparable to the PLFA profiles of members of the α-Proteobacteria, including the type II methanotrophs (Fig. 1). However, of particular significance is the peak associated with fraction 7, which represents 8 to 9% of the total. The labelled PLFAs in this fraction coeluted with reference standards i17:0, a17:0, and 17:1w8, which are scarce (<1%) or absent in previously studied methanotrophs and autotrophic ammonia oxidizers (5, 12, 30, 41). We have recently obtained similar 14C-PLFA profiles for high-affinity methane oxidizers in other soils, including soils from Brazil, the United States, Denmark, and Greenland (30).
Some bacteria are known to produce culture-dependent PLFA profiles, and it could be argued that the differences between the profiles obtained for the type II methanotrophs and the soil methane oxidizers (principally the fraction 7 fatty acid) reflect metabolic stresses on cells in the soil. However, soils labelled in the presence of saturating concentrations of methane (10,000 ppm) still contained significant amounts of these PLFAs, which could not be detected in pure cultures of methanotrophs labelled at low methane concentrations (30).
Soil samples that actively oxidized atmospheric methane contained phyletically distinct pMMO-like enzymes. On the basis of the relationships between PmoA sequences, clone RA14 may be considered a deep-branching member of the type II methanotroph group. Clone RA21 is not significantly related to any known pMMO-like sequence (Fig. 3 and 4). While RA14 is strongly related to type II methanotroph PmoA sequences, the evolutionary distance of this relationship is relatively large compared to the distance between known type II methanotroph PmoA sequences. On this basis, while it is probable that the RA14 organism is also a member of the α-Proteobacteria, it is unlikely to be sufficiently closely related to type II methanotrophs to form a monophyletic group. Other RA14 type sequences were detected from Maine, Pantanal, and additional Rold soil samples that had 14C-PLFA profiles similar to that of the Rold forest soil (Fig. 4). These sequences formed a cluster with the RA14 sequence, indicating that these types of sequences are found in a range of soils that oxidize atmospheric methane.
The topology of the monooxygenase trees appears to primarily reflect the phylogenetic relationships of the organisms and not the physiological roles of the enzymes (viz., the N. oceanus AmoA clusters with PmoA sequences). However, a comparison of the monooxygenase tree with trees derived from 16S rRNA (26, 42) or 23S rRNA (10) did indicate that the monooxygenase tree may reflect some specialization for using either methane or ammonia as a substrate for the enzyme (26). Phylogenies inferred from rRNAs indicate that the β- and γ-Proteobacteria are sister groups, whereas the monooxygenase tree (Fig. 3A) revealed that there is a comparatively close relationship between the α- and γ-Proteobacteria and that the β-Proteobacteria appear to be a highly divergent group. This could reflect selection for ammonia-specialized enzymes in the β-Proteobacteria and/or convergence to methane-specialized monooxygenases in the α- and γ-Proteobacteria. This implies that some sites in the protein sequence alignments are subject to positive selection, which results in bias in the monooxygenase tree. This hypothesis is consistent with current data on apparent Km and Vmax values for methane for members of the α- and γ-Proteobacteria (Table 1 and Fig. 3A) and the apparent ability of N. oceanus to use methane as an alternative carbon source (1, 19, 39). The relationship of RA14 to the α- and γ-Proteobacteria suggests that this clone represents a methane-specialized form. In the absence of pure cultures, confirmation of this will require demonstration that a radiotracer is incorporated into the RA14 pmoA gene or gene product. We have been unable to recover sufficient labelled genetic material from soil to demonstrate this to date.
Identification of amino acid residues which indicate that an organism is adapted to methane as the preferred substrate (i.e., signature residues for methane-specialized enzymes) may provide circumstantial evidence which supports the positive selection hypothesis. An amino acid was considered a putative signature residue if it satisfied the following criteria: it had to be universally conserved in all known members of the α- and γ-Proteobacteria, and at the same position in the alignment there had to be universal conservation of a different residue in all known members of the β-Proteobacteria. Sites fulfilling these criteria were found to be concentrated in two regions of the peptides. Interestingly, these two regions lie outside potential membrane-spanning domains (Fig. 2) and correlate closely with domains of the AMO which have been predicted to form the active site of the enzyme (36). The RA14 cluster sequences contained 74% (16 of 21) of the putative MMO signature residues and only 10% (2 of 21) of the putative AMO signatures. In contrast, RA21 exhibited no clear bias toward either substrate; it contained 33% (7 of 21) of the putative MMO signatures and 24% (5 of 21) of the putative AMO signatures. The effect of this putative positive selection on tree topology was investigated by constructing trees for which all such residues were omitted from the analysis. The resulting tree (Fig. 3B) still strongly supported the hypothesis that RA14 is related to the α-Proteobacteria (98% of bootstrap replicates), and RA21 branched at the predicted position for the root of the tree.
The existing biomarkers used to study MOB ecology include signature lipids for both type I and type II methanotrophs (12, 13), as well as several phylogenetic group-specific oligonucleotide probes that target the 16S rRNA (6, 14, 23, 37, 38). The evolutionary distances of the RA14 and RA21 PmoA sequences suggest that previously described phylogenetic group-specific biomarkers are not likely to detect these new groups of putative MOB.
In conclusion, using radiotracers and functional gene probes, we obtained substantial evidence that there are phyletically distinct populations of MOB in soils. The results of a comparative analysis of two distinct biomarkers (PLFA and PmoA) suggested that the high-affinity methane oxidizers are a novel group of α-Proteobacteria methanotrophs. Studies on factors that affect the activity, population dynamics, and contribution to global methane flux of atmospheric methane oxidizers should be facilitated by use of these markers.
ACKNOWLEDGMENTS
This work was supported by grant ERBIO4CT960419 from the European Union, by grant GST/02/622 from NERC, and by the Danish Research Council.
REFERENCES
- 1.Bedard C, Knowles R. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol Rev. 1989;53:68–84. doi: 10.1128/mr.53.1.68-84.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bender M, Conrad R. Kinetics of CH4 oxidation in oxic soils exposed to ambient air or high CH4 mixing ratios. FEMS Microbiol Ecol. 1992;101:261–270. [Google Scholar]
- 3.Benstead J, King G M, Williams H G. Methanol promotes atmospheric methane oxidation by methanotrophic cultures and soils. Appl Environ Microbiol. 1998;64:1091–1098. doi: 10.1128/aem.64.3.1091-1098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blake D R, Rowland F S. Continuing worldwide increase in tropospheric methane, 1978–1987. Science. 1988;239:1129–1131. doi: 10.1126/science.239.4844.1129. [DOI] [PubMed] [Google Scholar]
- 5.Bowman J P, Sly L I, Nicholls P D, Hayward A C. Revised taxonomy of the methanotrophs: description of Methylobacter gen. nov., emendation of Methylococcus, validation of Methylosinus and Methylocystis species, and a proposal that the family Methylococcaceae includes only the group I methanotrophs. Int J Syst Bacteriol. 1993;43:735–753. [Google Scholar]
- 6.Brusseau G A, Bulygina E S, Hanson R S. Phylogenetic analysis and development of probes for differentiating methylotrophic bacteria. Appl Environ Microbiol. 1994;60:626–636. doi: 10.1128/aem.60.2.626-636.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro M S, Peterjohn W T, Melilo J M, Steudler P A. Effects of nitrogen fertilization on the fluxes of N2O, CH4, and CO2 from soils in a Florida slash pine plantation. Can J For Res. 1994;24:9–13. [Google Scholar]
- 8.Chappellaz J, Barnola J M, Raynaud D, Korotkevich Y S, Lorius C. Ice-core record of atmospheric methane over the past 160,000 years. Nature (London) 1990;345:127–131. [Google Scholar]
- 9.Conrad R. Soil microbial processes involved in production and consumption of atmospheric trace gases. Adv Microb Ecol. 1995;14:207–250. [Google Scholar]
- 10.De Rijk P, Van de Peer Y, Van den Broeck I, De Wachter R. Evolution according to large subunit ribosomal RNA. J Mol Evol. 1995;41:366–375. doi: 10.1007/BF01215184. [DOI] [PubMed] [Google Scholar]
- 11.Felsenstein J. PHYLIP—phylogeny inference package (version 3.2) Cladistics. 1989;5:164–166. [Google Scholar]
- 12.Guckert J B, Ringleberg D B, White C C, Hanson R S, Bratina B J. Membrane fatty acids as phenotypic markers for the polyphasic approach to taxonomy of methylotrophs within the Proteobacteria. J Gen Microbiol. 1991;137:2631–2641. doi: 10.1099/00221287-137-11-2631. [DOI] [PubMed] [Google Scholar]
- 13.Hanson R S, Hanson T E. Methanotrophic bacteria. Microbiol Rev. 1996;60:439–471. doi: 10.1128/mr.60.2.439-471.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes A J, Owens N J P, Murrell J C. Detection of novel marine methanotrophs using phylogenetic and functional gene probes after methane enrichment. Microbiology. 1995;141:1947–1955. doi: 10.1099/13500872-141-8-1947. [DOI] [PubMed] [Google Scholar]
- 15.Holmes A J, Costello A, Lidstrom M E, Murrell J C. Evidence that particulate methane monooxygenase and ammonia monooxygenase may be evolutionarily related. FEMS Microbiol Lett. 1995;132:203–208. doi: 10.1016/0378-1097(95)00311-r. [DOI] [PubMed] [Google Scholar]
- 16.Hutsch B W, Webster C P, Powlson D S. Long-term effects of nitrogen fertilization on methane oxidation in soil of the Broadbalk wheat experiment. Soil Biol Biochem. 1993;25:1307–1315. [Google Scholar]
- 17.Hyman M R, Wood P M. Methane oxidation by Nitrosomonas europaea. Biochem J. 1983;212:31–37. doi: 10.1042/bj2120031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen S, Priemé A, Bakken L. Methanol improves methane uptake in starved methanotrophic microorganisms. Appl Environ Microbiol. 1998;64:1143–1146. doi: 10.1128/aem.64.3.1143-1146.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones R D, Morita R Y. Methane oxidation by Nitrosococcus oceanus and Nitrosomonas europaea. Appl Environ Microbiol. 1983;45:401–410. doi: 10.1128/aem.45.2.401-410.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King G. Ecological aspects of methane oxidation, a key determinant of global methane dynamics. Adv Microb Ecol. 1992;12:431–468. [Google Scholar]
- 21.King G, Schnell S. Effect of increasing atmospheric methane concentrations on ammonium inhibition of soil methane consumption. Nature (London) 1994;370:282–284. [Google Scholar]
- 22.Leak D J, Dalton H. Growth yields of methanotrophs. Appl Microbiol Biotechnol. 1986;23:470–476. [Google Scholar]
- 23.McDonald I R, Holmes A J, Kenna E M, Murrell J C. Molecular methods for the detection of methanotrophs. In: Sheehan D, editor. Methods in biotechnology. 2. Bioremediation protocols. Totowa, N.J: Humana Press Inc.; 1997. pp. 111–126. [Google Scholar]
- 24.Mosier A, Schimel D, Valentine D, Bronson K, Parton W. Methane and nitrous oxide fluxes in native, fertilized and cultivated grasslands. Nature (London) 1991;350:330–332. [Google Scholar]
- 25.Murrell J C, Holmes A J, McDonald I R, Kenna E M. Molecular ecology of methanotrophs. In: Murrell J C, Kelly D P, editors. Microbiology of atmospheric trace gases. Berlin, Germany: Springer-Verlag; 1996. pp. 135–152. [Google Scholar]
- 26.Murrell J C, Holmes A J. Molecular biology of particulate methane monooxygenase. In: Lidstrom M E, Tabita F R, editors. Microbial growth on C1 compounds. Dordrecht, The Netherlands: Kluwer; 1996. pp. 133–140. [Google Scholar]
- 27.Reeburgh W S, Whalen S C, Alperin M J. The role of methylotrophy in the global methane budget. In: Murrell J C, Kelly D P, editors. Microbial growth on C1 compounds. Andover, England: Intercept; 1993. pp. 1–14. [Google Scholar]
- 28.Roslev P, Iversen N, Henricksen K. Oxidation and assimilation of atmospheric methane by soil methane oxidizers. Appl Environ Microbiol. 1997;63:874–880. doi: 10.1128/aem.63.3.874-880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roslev P, Iversen N, Henricksen K. Direct fingerprinting of metabolically active bacteria in environmental samples by substrate specific radiolabelling and lipid analysis. J Microbiol Methods. 1998;31:99–111. [Google Scholar]
- 30.Roslev, P., and N. Iversen. Radioactive fingerprinting of microorganisms that oxidize atmospheric methane in soils. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 31.Schnell S, King G. Stability of methane oxidation capacity to variations in methane and nutrient concentrations. FEMS Microbiol Ecol. 1995;17:285–294. [Google Scholar]
- 32.Selenska S, Klingmuller W. DNA recovery and direct detection of Tn5 sequences from soil. Lett Appl Microbiol. 1991;13:21–24. doi: 10.1111/j.1472-765x.1991.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 33.Stephen J R, McCaig A E, Smith Z, Prosser J I, Embley T M. Molecular diversity of soil and marine 16S rDNA sequences related to β-subgroup ammonia-oxidizing bacteria. Appl Environ Microbiol. 1996;62:4147–4154. doi: 10.1128/aem.62.11.4147-4154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steudler P A, Jones R D, Castro M S, Melilo J M, Lewis D S. Influence of nitrogen fertilization on methane uptake in temperate forest soils. Nature (London) 1989;341:314–316. [Google Scholar]
- 35.Striegl R G, McConnaughey T A, Thorstenson D C, Weeks E P, Woodward J C. Consumption of atmospheric methane by desert soils. Nature. 1992;357:145–147. [Google Scholar]
- 36.Vannelli T, Bergmann D, Arciero D M, Hooper A B. Mechanism of N-oxidation and electron transfer in the ammonia oxidizing autotrophs. In: Lidstrom M E, Tabita F R, editors. Microbial growth on C1 compounds. Dordrecht, The Netherlands: Kluwer; 1996. pp. 80–87. [Google Scholar]
- 37.Voytek M A, Ward B B. Detection of ammonium-oxidizing bacteria in the beta-subclass of the class Proteobacteria in aquatic samples with the PCR. Appl Environ Microbiol. 1995;61:1444–1450. doi: 10.1128/aem.61.4.1444-1450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner M, Rath G, Koops H-P, Flood J, Amann R. In situ identification of ammonia-oxidizing bacteria. Syst Appl Microbiol. 1995;18:251–264. [Google Scholar]
- 39.Ward B B. Kinetics of ammonia oxidation by a marine nitrifying bacterium: methane as a substrate analog. Microb Ecol. 1990;19:211–225. doi: 10.1007/BF02017166. [DOI] [PubMed] [Google Scholar]
- 40.Whalen S C, Reeburgh W S. Consumption of atmospheric methane to subambient concentrations by tundra soils. Nature (London) 1990;346:160–162. [Google Scholar]
- 41.Wilkinson S G. Gram negative bacteria. In: Ratledge C, Wilkinson S G, editors. Microbial lipids. Vol. 1. London, United Kingdom: Academic Press; 1988. pp. 299–488. [Google Scholar]
- 42.Woese C R. Bacterial evolution. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zahn J A, DiSpirito A A. Membrane-associated methane monooxygenase from Methylococcus capsulatus (Bath) J Bacteriol. 1996;178:1018–1029. doi: 10.1128/jb.178.4.1018-1029.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]