Abstract
Background
Heart involvement represents the most ominous prognostic factor in light-chain amyloidosis (AL), often foreclosing curative therapies such as high-dose chemotherapy followed by autologous stem cell transplantation (ASCT). Heart transplantation (HTx) may be considered before ASCT in rigorously selected cases of advanced AL cardiac amyloidosis (CA). In ASCT-ineligible patients, chemotherapy with cyclophosphamide, bortezomib, and dexamethasone combined (CyBorD) regimen, even at low-dose, is feasible and effective in obtaining hematological and organ response.
Case Summary
A previously healthy 50-year-old woman presented with severely symptomatic new-onset heart with preserved ejection fraction, significant cardiac hypertrophy, and an ‘apical sparing’ pattern. Bone marrow and abdominal fat biopsy revealed AL amyloidosis due to a smouldering micromolecular λ-type myeloma with severe cardiac involvement, and the patient was judged a good candidate to HTx followed by ASCT. Despite fragile conditions, she tolerated a full course of low-dose combination therapy with bortezomib and was withdrawn from HTx list because of unexpected persistent complete hematologic response and major cardiac improvement. Disease remission was achieved in the long term (>3 years).
Discussion
We report a case of exceptional persistent hematologic and cardiac response after CyBorD therapy in a patient with advanced AL-CA who left the transplantation lists (both HTx and ASCT). In ASCT-ineligible patients, chemotherapy with CyBorD regimen, even at low-dose, can lead to durable remission of the disease with excellent cardiac response.
Keywords: Light chain cardiac amyloidosis, Case report, Heart failure, Heart transplantation, Autologous stem cell transplantation, CyBorD therapy
Learning Points.
Early diagnosis of cardiac amyloidosis, an accurate multidisciplinary prognostic stratification and quantification of cardiac and extra-cardiac disease burden allow to select the best therapeutic strategy.
In well-selected cases of light-chain amyloidosis involving the heart, foreclosing high-dose chemotherapy followed by autologous stem cell transplantation (ASCT), heart transplantation may be considered before ASCT.
In ASCT-ineligible patients, chemotherapy with cyclophosphamide, bortezomib, and dexamethasone combined regimen, even at low-dose, can lead to complete hematologic response and exceptional cardiac improvement, thus avoiding transplantations.
Primary specialties involved other than cardiology
Hematology, nuclear medicine, pathological anatomy and histology
Introduction
Light-chain amyloidosis (AL) is the most common form of systemic amyloidosis, characterized by extracellular deposition of monoclonal light-chain immunoglobulins as insoluble beta-fibrillar proteins in various tissues, leading to progressive organ failure.1 Heart involvement is common (>50% of patients diagnosed with AL),2 representing the most ominous prognostic factor. Patients with advanced cardiac amyloidosis (CA) usually do not benefit from traditional heart failure (HF) treatments3,4 and, often, cannot access valuable curative therapies such as high-dose chemotherapy followed by autologous stem cell transplantation (ASCT). Bortezomib combination chemotherapy, mainly at low doses, is feasible and effective in obtaining hematological and organ response in ASCT ineligible patients,5 even though overall survival in advanced CA is still poor.6 In selected cases, heart transplantation (HTx) followed by ASCT might represent a suitable approach to achieve long-term survival and, sometimes, full recovery from hematologic disease.7,8 Therefore, accurate patient selection and risk stratification is essential.
Timeline
| AUGUST, 2017 |
|
| SEPTEMBER, 2017 |
|
| OCTOBER, 2017 |
|
| OCTOBER, 2018 |
|
| 2019 |
|
| FEBRUARY, 2020 |
|
Case presentation
A 50-year-old woman with a history of fatigue, worsening exertional dyspnoea, and weight increase (8 kg) in the last 3 months was referred to our Cardiovascular Department by her general practitioner. Clinical examination detected hypotension (90/60 mmHg), an holosystolic murmur best heard at the cardiac apex, bilateral pulmonary rales, lower extremity oedema, and macroglossia. Her past medical history was unremarkable except for bilateral carpal tunnel (CT) surgery some years before. She denied suffering from arterial hypertension, and an electrocardiogram (ECG) obtained 1 year earlier was reported normal (not available).
The patient is a previously asymptomatic young adult without comorbidities, presenting with severe new-onset HF. The differential diagnosis includes cardiomyopathies, hypertensive, ischaemic, and valvular heart diseases. Macroglossia and CT syndrome suggest an infiltrative disease,3 while the heart murmur endorses the possibility of a severe valve disease precipitating acute HF. The absence of any cardiovascular risk factor makes the diagnosis of ischaemic heart disease unlikely.
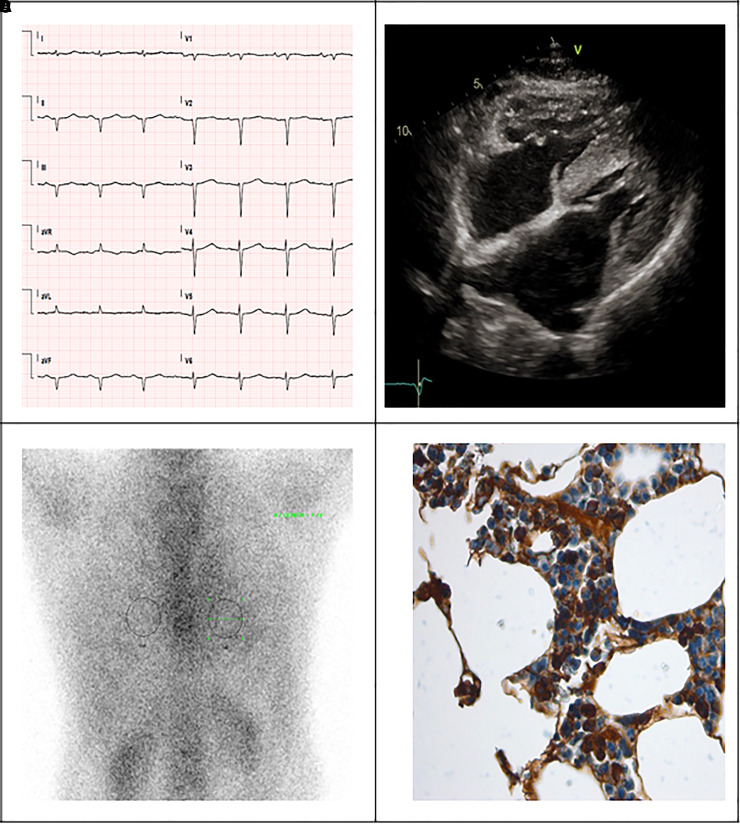
The ECG showed sinus rhythm with normal QRS voltages and Q-waves in anterior and inferior leads (Figure 1A) not consistent with the degree of left ventricle (LV) hypertrophy (max 17 mm) and normal wall motion evident at echocardiography (Figure 1B). In addition, grade III diastolic dysfunction, reduced LV global longitudinal strain (GLS –14%) with an ‘apical sparing’ pattern, thickened mitral valve leaflets, right ventricle hypertrophy and dilated inferior vena cava were found (Video 1). Laboratory tests revealed high brain natriuretic peptide (BNP) levels (618 pg/L, normal reference value <100 pg/L) and persistent mild increase in troponin T (0.34 ng/mL, normal reference value <0.25 ng/mL). An invasive angiography excluded coronary artery disease. Clinical and instrumental findings rose the suspicion of CA, and the Gillmore’s algorithm was followed9: serum λ free light chains (FLCs) returned pathologically increased (108 mg/dL, delta FLCs 93 mg/dL) and Perugini grade 1 myocardial uptake was found at diphosphonate scintigraphy (Figure 1C). The diagnosis of AL-CA was confirmed through detection and typing of amyloid fibrils at Congo red staining and electron microscopy of abdominal fat specimen. A concomitant diagnosis of a smouldering micromolecular λ-type myeloma was made on the basis of 22% clonal plasma cell in the bone marrow biopsy (BMB) without signs of end-organ (classic ‘calcium elevation, renal insufficiency, anemia, and bone abnormalities’ criteria) damage (Figure 1D).
Figure 1.
Main diagnostic tests used for diagnosing cardiac amyloidosis: electrocardiogram with normal QRS voltage and evidence of pseudonecrosis (A), echocardiography reporting biventricular hypertrophy, valvular thickening and pericardial effusion (B), grade 1 Perugini at diphosphonate scintigraphy (C) and atypical plasmacellular infiltrate with free light chains lambda restriction at bone marrow biopsy (D).
The advanced heart involvement (Mayo cardiac stage III)10 and the micromolecular myeloma contraindicated ASCT as up-front therapy. Therefore, low-dose cytoreductive therapy with cyclophosphamide, bortezomib, and dexamethasone (CyBorD) was started, halving the standard dose of the proteasome inhibitor and monitoring troponin serum concentrations and fluid status on a daily basis. Up-titration of diuretic dose up to 200 mg/die of furosemide was necessary due to frequent readmissions for decompensated HF with haemodynamic deterioration. No other HF medications were started. Low cardiac index (2.5 l/min/m2) at right heart catheterization and severely reduced maximal oxygen consumption during exercise (VO2) peak (15.4 ml/kg/min, 50% predicted value) with impaired ventilatory efficiency at cardiopulmonary exercise test (CPET) were documented. After one CyBorD cycle, therapeutic strategies were collegially discussed in a multidisciplinary team, considering three main variables: young age, severe cardiac involvement with ominous prognosis, and absence of significant extra-cardiac involvement, which was systematically investigated. The patient was judged a good candidate for HTx followed by ASCT. Thus, she entered on the HTx waiting list within 2 months from HF diagnosis. She experienced two syncopal episodes without clear prodromal symptoms and received an implantable cardioverter defibrillator for the prevention of arrhythmic death as bridge to HTx.
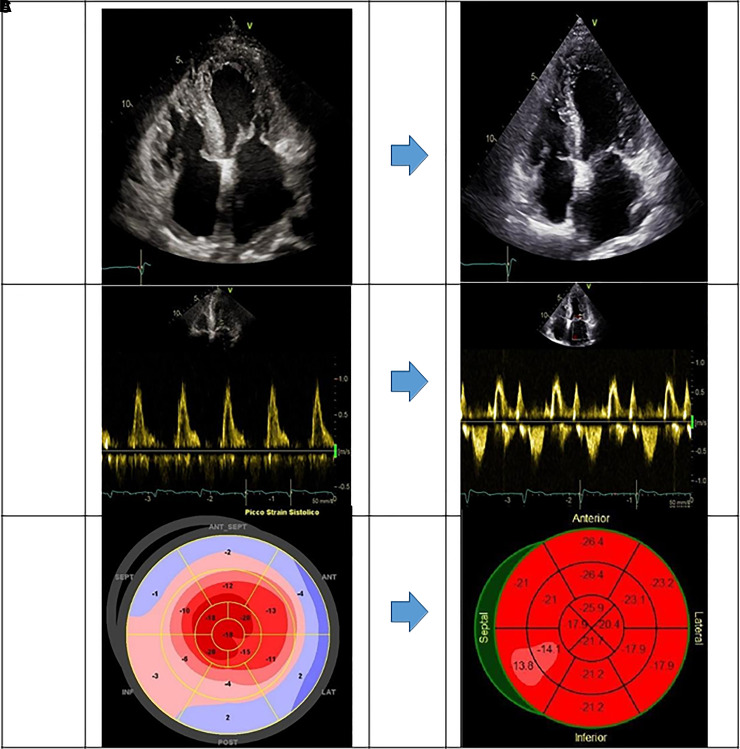
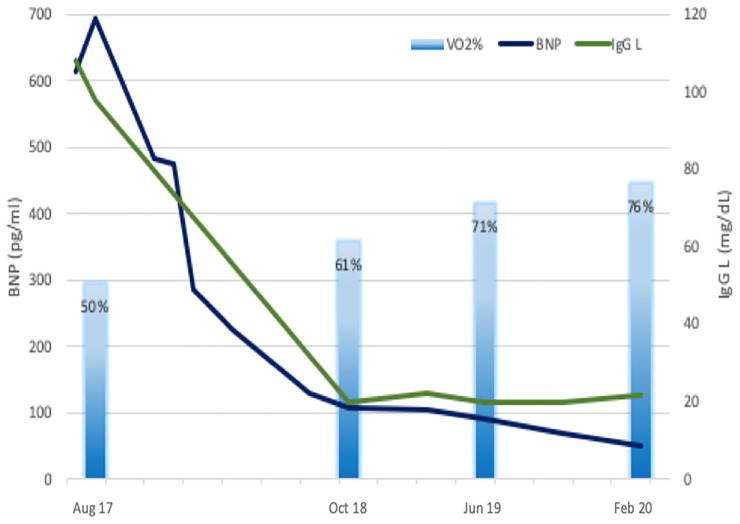
Despite fragile conditions, she succeeded in having a full course of chemotherapy with unexpected progressive reduction in serum values of BNP and FLCs. After 1 year of CyBorD therapy (6 cycles), serum FLCs normalized without residual hematological disease at BMB. At cardiological evaluation, the patient was in New York Heart Failure (NYHA) I functional class with normal BNP value (70 pg/L), lower diuretic dose (125 mg/die), recovered LV systolic function (GLS −20%) (Figure 2), increased exercise capacity (VO2 peak of 20.8 ml/kg/min, 76% predicted value), and normalization of ventilation and carbon dioxide production slope at CPET (Figure 3). Due to significantly improved cardiac performance and the complete hematological response, CyBorD therapy was discontinued, and the patient was withdrawn from HTx waiting list and the planned ASCT. She underwent apheresis and cryopreservation of hematopoietic stem cells in case of future need of ASCT. At device interrogations, no record of ventricular arrhythmic events or ICD discharge was found. She remained stable in NYHA I functional class after >3 years from HF onset under regular cardiological and hematological follow-up.
Figure 2.
Echocardiographic findings pre (left column) and post (right column) chemotherapy with cyclophosphamide, bortezomib, and dexamethasone combined protocol: four-chamber view (A), mitral inflow posterior wall (B) and global longitudinal strain (C).
Figure 3.
Trends of brain natriuretic peptide (pg/mL), immunoglobulin G lambda (mg/dL) and peak maximal oxygen consumption during exercise (% of predicted value) during follow up. cyclophosphamide, bortezomib, and dexamethasone combined therapy administered between February and June 2018.
Discussion
We reported an unusual case of AL-CA that left the transplantation lists (both HTx and ASCT) due to persistent (>3 years) complete hematologic response and exceptional cardiac improvement under CyBorD chemotherapy. The following remarkable issues emerged from this case:
Maintaining a high suspicion of CA in patients with HF with preserved ejection fraction and specific red flags (i.e. bilateral CT syndrome) is essential to reach an early diagnosis and start therapies with proven impact on survival.
Selecting the best therapeutic strategy in CA relies on accurate multidisciplinary prognostic stratification and quantification of cardiac and extra-cardiac disease burden.
Treatment for AL must be patient-tailored: in this case, the patient was initially considered ASCT-ineligible because of severe cardiac involvement and, therefore, entered the HTx list; however, low-dose combination therapy (i.e. CyBorD) was effective enough to induce long-term hematological remission, significant cardiac improvement (NYHA I, BNP normalization, normal cardiac catheterization, no HF hospitalizations for >3 years), and to avoid transplantations.
The suspicion of CA arose from the integration of clinical, echocardiographic, and laboratory data.2,11–13 Discrepancy between QRS voltages at ECG and the degree of LV wall thickness measured at echocardiography, severe diastolic dysfunction with apical sparing pattern, persistently elevated troponin, and history of bilateral CT surgery were major clues for CA diagnosis in this case.
Prognostic stratification of patients with AL-CA is estimated by dedicated scores integrating N-terminal brain natriuretic peptide (NT-proBNP), troponin, and FLCs values,10 which can be used to monitor treatment response.14 Our patient was scored Mayo stage III, outlining advanced cardiac involvement which bears high early death rate. Frequently, patients with these characteristics do not have enough time to respond to therapy. Accurate and prompt selection of the best treatment option for the individual case maximizes survival chances.
In the acute phase of AL amyloidosis, cardiac injury and dysfunction result from the myocardial deposition of amyloid fibrils and the toxic effect of light chains on cardiomyocytes, which is a reversible component under effective chemotherapy. Therefore, overall survival in AL amyloidosis is critically dependent on the presence and magnitude of hematologic response. Chemotherapy is directed towards the underlying clone and is aimed at suppressing the production of FLCs causing organ dysfunction.2 Currently, CyBorD is the standard of care for AL amyloidosis providing a partial hematological response (≥50% decrease in FLCs concentrations) and a cardiac response (>30% or >300 pg/mL decrease in NT-proBNP levels) in about 60% and 25% of treated patients, respectively.6,15 A 5-year overall survival of 80% with a median duration of response of 4.5 years was recently reported for patients who achieved satisfactory response after CyBorD alone, similarly to patients sequentially treated with CyBorD and ACST.5 Improved survival was also reported in stage IIIb patients who were able to receive enough CyBorD treatment to achieve a hematologic response.6
Commonly, even low-dose CyBorD regimen is hardly tolerated in presence of hypotension and severely symptomatic HF.
In the present case, the patient was ASCT-ineligible due to severe cardiac amyloid infiltration and was started on HTx list in light of no significant extra-cardiac involvement, absence of other major comorbidities, and young age. Although HTx enables ASCT and may confer survival advantage,7,8 this approach carries a non-negligible rate of complications, including death, and can be performed only in a few experienced centres. Of note, the correct timing for ASCT after HTx is still under investigation. In recent transplant series, some patients did not have time to receive ASCT after HTx due to AL amyloidosis progression.16 Therefore, this treatment option is feasible in well-selected AL-CA patients without clinically significant extra-cardiac amyloid where ASCT is expected to confer substantial survival benefit and can be performed with relatively little risk for treatment-related mortality.17
Unexpectedly, low-dose CyBorD regimen was effective in obtaining a complete hematological and an impressive cardiac response, with long-term good clinical status. Nevertheless, frequent clinical reassessment, strict monitoring of response to treatments, and therapy adjustments were essential in the management of our patient.
Recently, the combination of daratumumab and bortezomib has proven effective in the treatment of AL amyloidosis, holding promise for a future re-definition of the standard of care in AL amyloidosis.18,19 Further research is required as advances in pharmacotherapy of AL amyloidosis will have a great impact on clinical management patients, possibly reducing the need for ASCT.
Conclusion
Early detection of cardiac involvement in AL provides the widest access to therapeutic options, improving survival. HTx may be considered before ASCT in rigorously selected cases when advanced AL-CA precludes eligibility to ASCT. Interestingly, in ASCT-ineligible patients, chemotherapy with CyBorD regimen, even at low-dose, can lead to durable remission of the disease with excellent cardiac response.
Lead author biography

Aldostefano Porcari graduated from the University ‘Campus Bio-Medico di Roma’ in 2015 and completed the cardiology residency led by Prof. G. Sinagra at the Trieste University in 2020, where he works as a Clinical and Research Fellow in Cardiology. He has a keen interest in the diagnosis and treatment of cardiac amyloidosis. He is the administrator of the ‘Cardiac Amyloidosis Registry’ that includes data of >200 patients with AL and ATTR amyloidosis, followed at the Cardiothoracovascular Department, Trieste University Hospital, and the coordinator of a national survey investigating the prevalence of the disease in Italy. He is currently collaborating with the National Amyloidosis Centre, London, to deepen his clinical and research knowledge.
Supplementary Material
Supplementary material
Supplementary material is available at the European Heart Journal – Case Reports online.
Slide sets: A fully edited slide set detailing these cases and suitable for local presentation is available online as Supplementary data.
Consent: The study was conducted according to the Declaration of Helsinki and received institutional review board approval (identifier 43_2009). The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance and under the institutional review board policies of the hospital administration.
Conflict of interest: None declared.
Funding: None declared.
References
- 1.Porcari A, Falco L, Lio V, Merlo M, Fabris E, Bussani R, Sinagra G. Cardiac amyloidosis: do not forget to look for it. Eur Hear J Suppl 2020;22:E142–E147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falk RH, Alexander KM, Liao R, Dorbala S. AL (light-chain) cardiac amyloidosis: a review of diagnosis and therapy. J Am Coll Cardiol 2016;68:1323–1341. [DOI] [PubMed] [Google Scholar]
- 3.Porcari A, Merlo M, Rapezzi C, Sinagra G. Transthyretin amyloid cardiomyopathy: an uncharted territory awaiting discovery. Eur J Intern Med 2020;82:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tini G, Cappelli F, Biagini E, Musumeci B, Merlo M, Crotti L, et al. Current patterns of beta-blocker prescription in cardiac amyloidosis: an Italian nationwide survey. ESC Hear Fail 2021;8:3369–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basset M, Milani P, Nuvolone M, Benigna F, Rodigari L, Foli A, et al. Sequential response-driven bortezomib-based therapy followed by autologous stem cell transplant in AL amyloidosis. Blood Adv 2020;4:4175–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palladini G, Sachchithanantham S, Milani P, Gillmore J, Foli A, Lachmann H, et al. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood 2015;126:612–615. [DOI] [PubMed] [Google Scholar]
- 7.Gillmore JD, Goodman HJ, Lachmann HJ, Offer M, Wechalekar AD, Joshi J, Pepys MB, Hawkins PN. Sequential heart and autologous stem cell transplantation for systemic AL amyloidosis. Blood 2006;107:1227–1229. [DOI] [PubMed] [Google Scholar]
- 8.Barrett CD, Alexander KM, Zhao H, Haddad F, Cheng P, Liao R, Wheeler MT, Liedtke M, Schrier S, Arai S, Weisshaar D, Witteles RM. Outcomes in patients with cardiac amyloidosis undergoing heart transplantation. JACC Heart Fail 2020;8:461–468. [DOI] [PubMed] [Google Scholar]
- 9.Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, Wechalekar AD, Berk JL, Quarta CC, Grogan M, Lachmann HJ, Bokhari S, Castano A, Dorbala S, Johnson GB, Glaudemans AWJM, Rezk T, Fontana M, Palladini G, Milani P, Guidalotti PL, Flatman K, Lane T, Vonberg FW, Whelan CJ, Moon JC, Ruberg FL, Miller EJ, Hutt DF, Hazenberg BP, Rapezzi C, Hawkins PN. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016;133:2404–2412. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C, Laumann K, Zeldenrust SR, Leung N, Dingli D, Greipp PR, Lust JA, Russell SJ, Kyle RA, Rajkumar SV, Gertz MA. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol 2012;30:989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witteles RM, Bokhari S, Damy T, Elliott PM, Falk RH, Fine NM, Gospodinova M, Obici L, Rapezzi C, Garcia-Pavia P. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Hear Fail 2019;7:709–716. [DOI] [PubMed] [Google Scholar]
- 12.Porcari A, Pagura L, Longo F, Sfriso E, Barbati G, Murena L, Longo E, Ramella V, Arnež ZM, Rapezzi C, Merlo M, Sinagra G. Prognostic significance of unexplained left ventricular hypertrophy in patients undergoing carpal tunnel surgery. ESC Hear Fail 2021;9:751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merlo M, Porcari A, Pagura L, Cameli M, Vergaro G, Musumeci B, Biagini E, Canepa M, Crotti L, Imazio M, Forleo C, Cappelli F, Favale S, Di Bella G, Dore F, Lombardi CM, Pavasini R, Rella V, Palmiero G, Caiazza M, Albanese M, Guaricci AI, Branzi G, Caponetti AG, Saturi G, La Malfa G, Merlo AC, Andreis A, Bruno F, Longo F, Sfriso E, Di Ienno L, De Carli G, Giacomin E, Spini V, Milidoni A, Limongelli G, Autore C, Olivotto I, Badano L, Parati G, Perlini S, Metra M, Emdin M, Rapezzi C, Sinagra G. A national survey on prevalence of possible echocardiographic red flags of amyloid cardiomyopathy in consecutive patients undergoing routine echocardiography: study design and patients characterization—the first insight from the AC-TIVE Study. Eur J Prev Cardiol 2021. 10.1093/eurjpc/zwab127. [DOI] [PubMed] [Google Scholar]
- 14.Pregenzer-Wenzler A, Abraham J, Barrell K, Kovacsovics T, Nativi-Nicolau J. Utility of Biomarkers in Cardiac Amyloidosis. JACC Hear Fail 2020;8:701–711. [DOI] [PubMed] [Google Scholar]
- 15.Palladini G, Milani P, Merlini G. Management of AL amyloidosis in 2020. Blood 2020;136:2620–2627. [DOI] [PubMed] [Google Scholar]
- 16.Di Nora C, Sponga S, Ferrara V, Patriarca F, Fanin R, Nalli C, Lechiancole A, Vendramin I, Livi U. Emerging therapy in light-chain and acquired transthyretin-related amyloidosis: an Italian single-centre experience in heart transplantation. J Cardiovasc Med (Hagerstown) 2021;22:261–267. [DOI] [PubMed] [Google Scholar]
- 17.Di Nora C, Livi U. Heart transplantation in cardiac storage diseases: data on Fabry disease and cardiac amyloidosis. Curr Opin Organ Transplant 2020;25:211–217. [DOI] [PubMed] [Google Scholar]
- 18.Kastritis E, Palladini G, Minnema MC, Wechalekar AD, Jaccard A, Lee HC., Sanchorawala V, Gibbs S, Mollee P, Venner CP, Lu J, Schönland S, Gatt ME, Suzuki K, Kim K, Cibeira MT, Beksac M, Libby E, Valent J, Hungria V, Wong SW, Rosenzweig M, Bumma N, Huart A, Dimopoulos MA, Bhutani D, Waxman AJ, Goodman SA, Zonder JA, Lam S, Song K, Hansen T, Manier S, Roeloffzen W, Jamroziak K, Kwok F, Shimazaki C, Kim J-S, Crusoe E, Ahmadi T, Tran N, Qin X, Vasey SY, Tromp B, Schecter JM, Weiss BM, Zhuang SH, Vermeulen J, Merlini G, Comenzo RL. Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med 2021;385:46–58. [DOI] [PubMed] [Google Scholar]
- 19.Mikhael JR, Schuster SR, Jimenez-Zepeda VH, Bello N, Spong J, Reeder CB, Stewart AK, Bergsagel PL, Fonseca R. Cyclophosphamide-bortezomib-dexamethasone (CyBorD) produces rapid and complete hematologic response in patients with AL amyloidosis. Blood 2012;119:4391–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.