Abstract
Alzheimer’s disease is the leading cause of dementia and a growing worldwide problem, with its incidence expected to increase in the coming years. Since synapse loss is a major pathology and is correlated with symptoms in Alzheimer’s disease, synapse dysfunction and loss may underlie pathophysiology. In this context, this review focuses on emerging insights into synaptic changes at the ultrastructural level. The three-dimensional electron microscopy technique unequivocally detects all types of synapses, including multi-synapses, which are indicators of synaptic connectivity between neurons. In recent years it has become feasible to perform sophisticated three-dimensional electron microscopy analyses on post-mortem human Alzheimer’s disease brain as tissue preservation and electron microscopy techniques have improved. This ultrastructural analysis found that synapse loss does not always precede neuronal loss, as long believed. For instance, in the transentorhinal cortex and area CA1 of the hippocampus, synapse loss does not precede neuronal loss. However, in the entorhinal cortex, synapse loss precedes neuronal loss. Moreover, the ultrastructural analysis provides details about synapse morphology. For example, changes in excitatory synapses’ post-synaptic densities, with fragmented postsynaptic densities increasing at the expense of perforated synapses, are seen in Alzheimer’s disease brain. Further, multi-synapses also appear to be altered in Alzheimer’s disease by doubling the abundance of multi-innervated spines in the transentorhinal cortex of Alzheimer’s disease brain. Collectively, these recent ultrastructural analyses highlight distinct synaptic phenotypes in different Alzheimer’s disease brain regions and broaden the understanding of synapse alterations, which may unravel some new therapeutic targets.
Keywords: Alzheimer’s disease, three-dimensional electron microscopy, synapses, multi-spine bouton, multi-innervated spine
Martínez-Serra et al. report that synapse changes in post-mortem Alzheimer’s disease brain regions are heterogenous. Further, changes in multi synapses are most prominent in the disease, indicating that pre- and post-synapses degenerate independently and that a change in neuronal connectivity is relevant for cognitive decline in Alzheimer’s disease.
Graphical Abstract
Graphical Abstract.
Introduction
Alzheimer’s disease is the most common cause of dementia. Growing evidence suggests that memory impairment in Alzheimer’s disease correlates with synapse loss in the forebrain.1–5 For instance, synapse loss in the hippocampus, dentate gyrus, inferior temporal gyrus and superior frontal cortex negatively correlates with performance in various types of memory tasks.6–10 Given this correlation, it is important to understand how synapses are affected in Alzheimer’s disease in order to be able to intervene and reverse synaptic changes to possibly prevent and/or rescue cognitive and memory impairment.
To this date, several methods have been used to assess synapse density. For example, indirect quantification of pre- and post-synaptic proteins, such as synaptophysin, synapsin-1 and postsynaptic density protein 95 (PSD-95) by immunohistochemistry, ELISA, dot-blot and western blot.4,9,11–15 However, these methods estimate, at very best, the presence of specific synaptic proteins in the pre- or post-synaptic compartments, but they cannot provide the detailed context of pre- and post-synaptic architecture and determine the progression of pathology in the disease. For example, the presynaptic marker CSPalpha is reduced in Alzheimer’s disease before synaptophysin levels are affected,16 suggesting that at least some synaptic markers can have a reduction in expression without any impact on synapse numbers. Further, DeKosky and colleagues4 did not find a correlation between synaptophysin expression and cognitive function, even though synapse density correlated with cognitive abilities, showing the inaccuracy of relying on synaptic protein expression as markers for synapses.
It is also very common to assess synapse density and morphology by fluorescence imaging of dendritic spines. However, it should be noted that such imaging does not assure that the dendritic spines have a presynaptic input, and it can also not distinguish between multi-synapses and one-input-one-spine synapses, except for the recently developed super-resolution imaging with the DNA-paint method for cultured neurons.17,18 Further, subcellular components within the spines, such as the spine apparatus, cannot be identified with light microscopy in contrast with electron microscopy (EM).
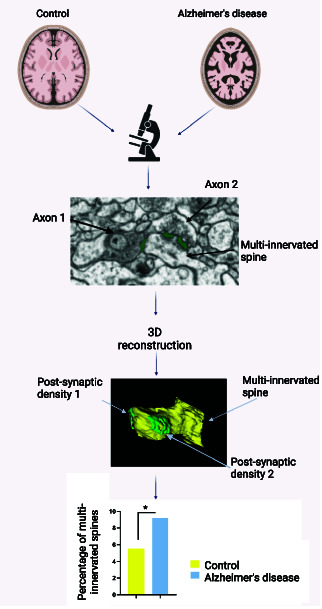
To study how synapses are changed in Alzheimer’s disease, it is important to use methods that allow for the unequivocal identification of synapses. EM is the gold standard for ultrastructure assay since it provides sufficient high resolution for a clear visualization of PSDs and presynaptic vesicles, making it possible to identify a synaptic connection on the nanometric scale (Fig. 1). EM also allows for the identification of synapses and their classification into asymmetric synapses (AS) and symmetric synapses (SS). This distinction is important as these two types of synapses correlate with different functions: AS are mostly glutamatergic and excitatory, while SS are mostly GABAergic and inhibitory.19
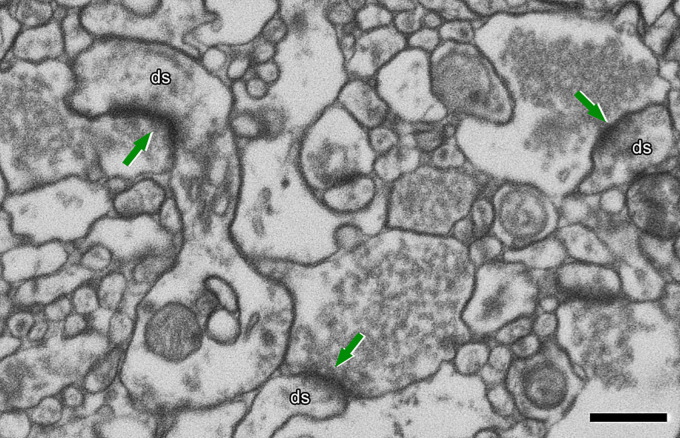
Figure 1.
Identification of synapses in EM image obtained by FIB/SEM on the transentorhinal cortex from post-mortem human brain (control). Excitatory synapses (arrows) on dendritic spines (ds) are shown. Presynaptic elements contain numerous and visible vesicles. The arrows point at the asymmetric PSDs. Scale bar: 500 nm.
Serial sectioning TEM is a well-established technique to obtain three-dimensional data from ultrathin sections of brain tissue. However, obtaining a long series of ultrathin sections is extremely time-consuming, difficult and requires labour-intensive human interaction that prevents this approach from being widely employed (reviewed in20). However, the development of automated EM techniques represents an important advance. One of these techniques of three-dimensional (3D)-EM, called dual-beam microscopy, combines a high-resolution field-emission SEM column with a focused gallium ion beam (FIB), which permits the removal of thin layers of material from the sample surface on a nanometer scale. As soon as one layer of material is removed by the FIB, the exposed surface of the sample is imaged by the SEM using a backscattered electron detector. The sequential automated use of FIB milling and SEM imaging allows for obtaining long series of photographs of a 3D sample of selected brain regions (e.g. see reference 21). The FIB/SEM microscopy offers the advantage that the process of obtaining serial images is fully automated, eliminating the need for serial sectioning, the collection of ultrathin sections and the manual acquisition of microphotographs. Indeed, FIB/SEM is an excellent tool to study in detail the ultrastructure and alterations of the synaptic organization of the human brain, as shown by Blazquez- Blazquez-Llorca et al.22 who studied AD human tissue for the first time using this technique.
Further, 3D EM is essential to identify synapses that have connections with multiple dendritic spines or with multiple presynaptic terminals, which can be considered as multi-output and multi-input, respectively (Fig. 2). Correspondingly, these synapse types are named multi-innervated spines (MIS) and multi-spine boutons (MSBs). 3D EM can identify and reconstruct the post-synaptic densities (PSDs) as independent elements, which can be achieved only by a 3D analysis at the EM level (Fig. 3) and will provide the ultrastructure synapse architecture in the brain.
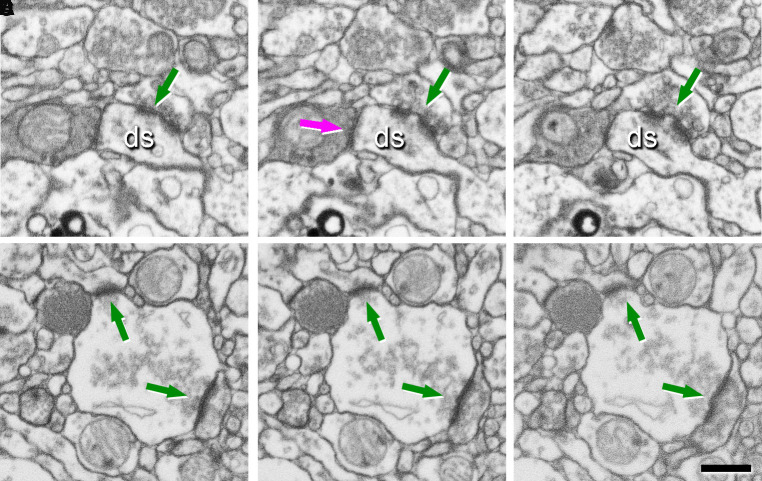
Figure 2.
Identification of synapses in EM serial images obtained by FIB/SEM on the transentorhinal cortex from post-mortem human brain (control). (A–C) A sequence of serial images showing a multi-innervated dendritic spine (ds) with an excitatory A–C and an inhibitory synapse B indicated by arrows. (D–F) A sequence of serial images to illustrate a multi-synaptic bouton establishing two excitatory synapses with two dendritic spines (arrows). Scale bar in F 500 nm.
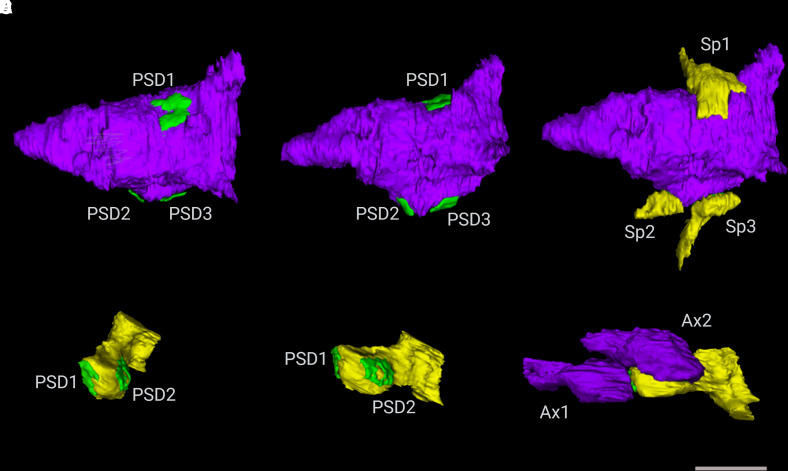
Figure 3.
Examples of 3D reconstructed axonal boutons establishing synapses with dendritic spines. (A–C) 3D reconstructions of a multi-spine bouton after axis rotation of the axon. The MSB includes one axonal bouton, three post-synaptic densities (PSD) on A–B and three dendritic spines (Sp) on C. (D–F) 3D reconstructions of a multi-innervated spine after axis rotation of the dendritic spine. The MIS consists of one dendritic spine, two post-synaptic densities (PSD) on D–E, and two axonal boutons (Ax) on F. Scale bar (in F) indicates 1 μm in A–F.
Multi-synapses in Alzheimer’s disease have been overlooked for many years, but it is paramount to study these types of synapses as the presence and/or proportions of both types of multi-synapses change the connectivity between neurons and seem to contribute to learning and memory.23 For instance, Geinisman24 reported that trace eyeblink conditioning in rabbits increases MSB density in hippocampal CA1 stratum radiatum. Similarly, aged mice, as well as mutants with impaired long-term potentiation, are able to form and store hippocampal-dependent memories through the formation of MIS.25–27 Therefore, given the contribution of multi-synapses to cognition and memory, it is important to be able to identify and analyse these types of synapses in Alzheimer’s disease.
Despite providing resolution at the nanoscale level, to undoubtedly identify synapses, synapse types and subcellular structures, EM has some limitations. For instance, it is not possible to use EM imaging in living organisms; therefore, longitudinal studies to assess synaptic alteration during disease progression are not possible. Also, 3D-EM synapse reconstruction and analysis are very time-consuming.
Reviewed data come from FIB/SEM studies performed on human brain samples from control and AD cases (for details, see references 28–32). Briefly, brain tissue samples with a very short post-mortem delay (less than 4 h) were fixed in cold 4% paraformaldehyde. After fixation, the tissue was coronally sectioned. Serial sections were post-fixed and stained with uranyl acetate and then dehydrated and flat-embedded in Araldite.33 Embedded sections were glued onto a block. Blocks were glued onto a sample stub, and the top surface was coated with a layer of gold/palladium to facilitate charge dissipation. The blocks were used to obtain images stacks using a dual-beam microscope (FIB/SEM; Crossbeam® 540 electron microscope, Carl Zeiss NTS GmbH, Oberkochen, Germany). FIB/SEM images were obtained avoiding the neuronal and glial somata, blood vessels and also Aβ plaques in order to eliminate the effect of alterations of synapses in the vicinity of Aβ-plaques, which has been described previously (e.g. see22) FIBSEM images were analysed using EspINA software, which allows for the 3-dimensional reconstruction of synapses (Video 1).
In this review, we will focus on the emerging insights of synaptic changes in post-mortem Alzheimer’s disease brain derived from recent 3D EM analyses, which became feasible due to very short post-mortem delay to assure high tissue preservation. An example of a post-mortem EM image is presented in Fig. 1. This analysis provides the best knowledge to date about ultrastructural changes in synapses in Alzheimer’s disease, which is essential for an understanding of the mechanisms underlying synaptic degeneration in the disease. There is a notion that 3D EM analysis occasionally contradicts what was observed with traditional EM analysis, such as in the CA1 region of the hippocampus.7 However, it is mandatory that more brain regions are analysed using 3D EM before reaching any general conclusion.
Does synapse loss precede neuronal depletion in post-mortem human Alzheimer’s disease brain?
An important question is whether synapse loss in Alzheimer’s disease is a cause or a consequence of neurodegeneration. The recent 3D EM analysis has revealed that there is no simple answer to this question. For instance, brain atrophy, which includes neuronal loss, is greater than 30% in the CA1 region of the hippocampus and the transentorhinal cortex in post-mortem human Alzheimer’s disease brain. However, synapse density is not reduced in the surviving tissue in the transentorhinal cortex and CA1 stratum pyramidale and stratum radiatum.30,31 In contrast, in layers II and III of the entorhinal cortex, synapse density is substantially reduced.29 Thus, in the transentorhinal cortex and in the CA1 region of the hippocampus, synapse loss appears to be associated with neuronal loss, whereas in the entorhinal cortex, synapse loss may precede neuronal loss.
Table 1 summarizes the relationship between synapse and neuronal loss in different post-mortem Alzheimer’s disease brain areas using 3D EM analysis. In most, but not all, analysed brain regions, synapse loss seems to be a consequence of neuronal death rather than the cause of neurodegeneration. The fact that this is not always the case, as in the analysed layers of the entorhinal cortex, suggests that synapse vulnerability may differ between brain areas and/or layers. For example, synapses in CA1 stratum pyramidale may be more resistant towards degeneration than synapses in layers II and III of the entorhinal cortex.
Table 1.
Summary of neuron and synapse density changes in Alzheimer’s disease
| Neuron density | Synapse density | Synapse loss precedes neuron loss | References | |
|---|---|---|---|---|
| CA1 stratum pyramidale (hippocampus) | ↓ | = | No | 31 |
| CA1 stratum radiatum (hippocampus) | ↓ | = | No | 31 |
| Layers 2 and 3 entorhinal cortex | = | ↓ | Yes | 29 |
| Layer 2 transentorhinal cortex | ↓ | = | No | 30 |
↓Means a decrease observed at the ultrastructure level and = shows no change.
Region-specific differences in alterations of calcium/calmodulin-dependent kinase II (CaMKII) expression may contribute to this range of synapse vulnerability.34 αCaMKII, which is known to be involved in synaptic plasticity and memory formation35 is also a tau kinase.36 Strikingly, only CA1 pyramidal neurons in Alzheimer’s disease hippocampus have elevated αCaMKII expression.34 CA3 pyramidal neurons and granule cells in dentate gyrus have no altered αCaMKII expression, but the activity of αCaMKII at synapses is impaired, affecting the functioning of these neurons.34 These distinct αCaMKII changes correlate with substantial loss of CA1 pyramidal neurons, but almost no loss of CA3 pyramidal neurons nor granule cells in the dentate gyrus of severe Alzheimer’s disease hippocampus.37
Which synapses are altered or missing in post-mortem human Alzheimer’s disease brain?
It is of interest whether in Alzheimer’s disease brain particular synapse types are more prone to degeneration. Recent 3D EM analysis suggests that the ratio of excitatory towards inhibitory synapses is not altered in the transentorhinal cortex or in CA1 stratum pyramidale and stratum radiatum, where also no synapse loss on surviving neurons seems to occur.30,31 This ratio also remains unchanged in the entorhinal cortex in Alzheimer’s disease, despite synapse loss on surviving neurons.29,38 These findings suggest that both excitatory and inhibitory synapses are equally vulnerable in Alzheimer’s disease. However, due to the limited number of brain regions investigated, we cannot rule out that Alzheimer’s disease has a different impact on excitatory and inhibitory synapses in other brain regions.
Morphology of PSDs, which can be either macular or non-macular (horseshoe-shaped, perforated and fragmented)39,40 also seems to be altered in Alzheimer’s disease brain. Different alterations are detected in distinct brain areas, but some common traits can be observed. For instance, the morphology of inhibitory synapses does not seem to be modified in any of the analysed brain regions. As for excitatory synapses, those with fragmented PSDs are generally increased, while perforated synapses are more often decreased in Alzheimer’s disease.28,29,31
Synaptic connections can target different parts of the post-synaptic cell, which may have mechanistic implications. For instance, synapses can be seen in dendritic spines, further divided into spine heads or necks, and also in dendritic shafts. Considering these parameters, the synaptic location appears to be altered in Alzheimer’s disease, but only in brain regions where synapse loss does not precede neuronal loss, i.e. hippocampus and transentorhinal cortex.28,29,31 In these brain areas, the number of axonal boutons targeting dendritic spines is reduced, while excitatory synapses on dendritic shafts are increased.28,31 Inhibitory axodendritic synapses are reduced in CA1 stratum radiatum.31
In summary, synapse morphology and location are altered in Alzheimer’s disease (Table 2), but more research is needed to establish whether this is a general feature and/or if particular synapse types are predisposed to degeneration in Alzheimer’s disease. Especially research on brain regions where synapse loss precedes neuronal death in Alzheimer’s disease is essential because here, just one brain region with this characteristic has been considered, and this is not enough to reach a meaningful conclusion.
Table 2.
Summary of synapse subtype changes in Alzheimer’s disease
| AS:SS | Synapse morphology (AS) | Synapse targeting | References | |
|---|---|---|---|---|
| CA1 stratum pyramidale (hippocampus) | = | ↓ perforated | ↓ axospinous AS, ↑ axodendritic AS | 31 |
| CA1 stratum radiatum (hippocampus) | = | = | ↓ axodendritic SS | 31 |
| Layers 2 and 3 entorhinal cortex | = |
Layer 2: ↑ horseshoe-shaped Layer 3: ↑ fragmented and macular, ↓ perforated |
= | 29 |
| Layer 2 transentorhinal cortex | = | ↑ fragmented | ↓ axospinous AS, ↑ axodendritic AS | 28,30 |
↓ and ↑indicate changes observed at the ultrastructural levels, = means no change. AS, asymmetric synapses; SS, symmetric synapses.
What are the features of surviving synapses in late-stage Alzheimer’s disease brain?
Recent studies have also tested for possible differences in synaptic enlargement in relation to synapse type and PSD morphology rather than a general enlargement of synapses. In the entorhinal cortex, only excitatory synapses in layer II, and perforated excitatory synapses in layer III, are enlarged.29 However, enlarged synapses do not occur in the transentorhinal cortex or any hippocampal CA1 layer.28,31
Previously, the generally accepted idea was that a reduction in synapse number correlates with a significant increase in synapse size in the post-mortem Alzheimer’s disease brain.4,10,41–43 This previous work illustrated an increase of the PSD size and apposition length or synaptic apposition surface by about 25% in Alzheimer’s disease compared with age-matched control subjects.7,42–45 As a result of this correlation, a common inference was that the total synaptic contact area was maintained to a similar level than in controls.10,42,43 However, in contrast with this previous work, a general synaptic enlargement is not detected with recent 3D EM analysis in human samples from Alzheimer’s disease brains (Table 3), indicating that the total synaptic contact area may also be lost in Alzheimer’s disease.
Table 3.
Summary of synapse size change in Alzheimer’s disease
| Synapse enlargement | Maintenance of total synaptic contact area | References | |
|---|---|---|---|
| CA1 stratum pyramidale (hippocampus) | No | ? | 31 |
| CA1 stratumradiatum (hippocampus) | No | ? | 31 |
| Layers 2 and 3 entorhinal cortex | Only excitatory synapses in layer 2 and excitatory perforated synapses in layer 3 | Maybe | 29 |
| Layer 2 transentorhinal cortex | No | ? | 28 |
Does Alzheimer’s disease alter synapse connectivity?
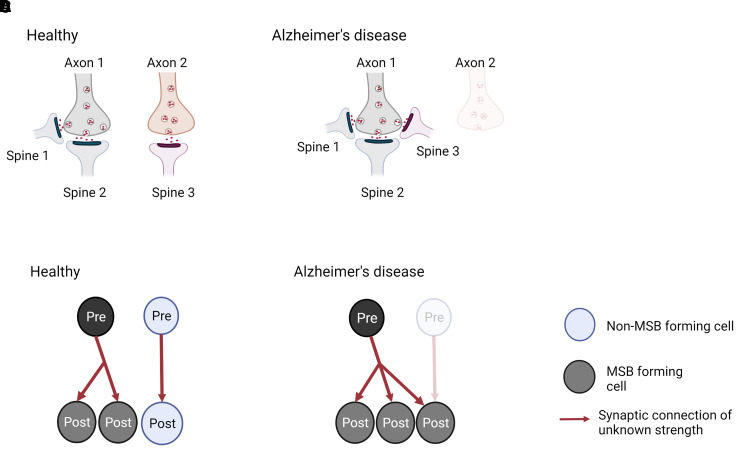
The remaining question is whether synapse alterations in Alzheimer’s disease affect connectivity between pre- and post-synaptic neurons. It is known that synaptic connections can be clustered into MSBs and MIS (Fig. 2).46,47 Alterations in MSB and MIS numbers and complexity change brain connectivity and are thought to contribute to memory.23 For instance, the number of MSBs is increased in rabbit hippocampal CA1 stratum radiatum after eyeblink conditioning.24 Further, MISs have been suggested to be the mechanism responsible for hippocampal memory storage in aged mice as well as in LTP-impaired mouse.25–27 Despite the evidence linking MSB and MIS with memory, research on these types of synapses has been overlooked for many years, and they have not been considered much in studies of Alzheimer’s disease. Recently, however, MISs have been investigated in post-mortem Alzheimer’s disease brains. They are not altered in the CA1 region of the hippocampus, but they are doubled in the transentorhinal cortex of the Alzheimer’s disease brain.28,31 It is possible that in Alzheimer’s disease, some post-synaptic terminals become dysfunctional, degenerate or just disconnect from presynapses across a synapse, and then these presynaptic terminals would get a signal to find another existing spine to establish a new synapse, inducing the formation of MIS, and explaining the lack of synapse loss. Alternatively, new axonal terminals may be formed and incorporated into existing SSB, these becoming an MIS (Fig. 4).
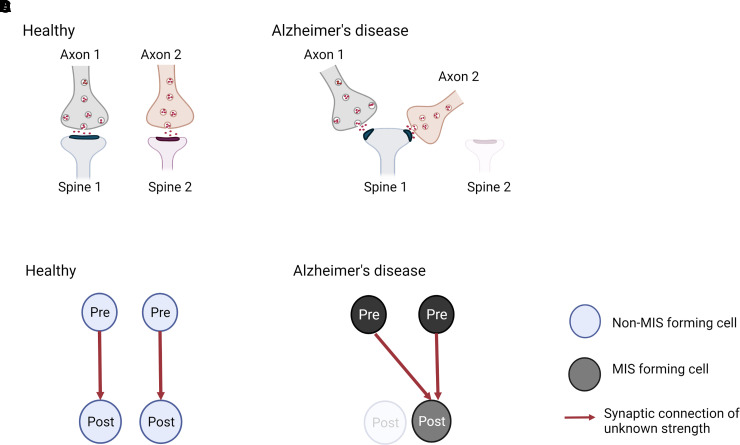
Figure 4.
Model for generation of multi-innervated dendritic spines (MIS) in Alzheimer’s disease brains and impact on synaptic connectivity. (A and B) Two synapses in a healthy brain and Alzheimer’s disease brain are shown. (A) In a healthy brain, one synapse is formed between axon 1 and spine 1, while the other synapse is made between axon 2 and spine 2. (B) In Alzheimer’s disease, spine 2 has degenerated, and its presynaptic input from axon 2 established a new connection with spine 1, generating an MIS. As a consequence, synapse density is maintained, but MIS number is increased. (C and D) Illustration of the difference in synaptic connectivity and resulting information flow as a consequence of dendritic spine loss and MIS generation in Alzheimer’s disease. It is less likely that the higher MIS number increases connectivity between two neurons (scenario not shown), as two axonal branches from one pre-synaptic neuron would have to connect to one spine. - Created with BioRender.
Further, our lab has found that the number of MSBs in the transentorhinal cortex, entorhinal cortex and stratum pyramidale superior of Alzheimer’s disease brains is similar to control levels (unpublished data). We hypothesize that though some spine-forming synapses in this region may become dysfunctional, degenerate, or disconnect from axons across the synapse, other spines that may have lost their presynaptic partners would get a signal to find another existing axonal bouton to establish a new synapse and explain the lack of synapse loss, the maintenance of the proportion of MSBs and maybe increase the number of connections per bouton. An alternative explanation is that new spines may be formed and incorporated into existing boutons to form new MSBs (Fig. 5).
Figure 5.
Model for generation of multi-spine boutons in Alzheimer’s disease brains and impact on synaptic connectivity. (A and B) Three synapses in a healthy and Alzheimer’s disease brain are shown. (A) An MSB is formed between axon 1 and spines 1 and 2, and a single synapse between axon 2 and spine 3 is shown for a healthy brain. (B) In Alzheimer’s disease, the presynaptic input from axon 2 has degenerated, and spine 3 from the single synapse established a new connection with the existing MSB. As a consequence, synapse density and MSB number are maintained, but MSB’s complexity is increased. (C and D) Illustration of the difference in synaptic connectivity and resulting information flow as a consequence of dendritic spine loss and MIS generation in Alzheimer’s disease. Note that in a healthy brain, the vast majority of most MSBs are formed between one presynaptic neuron and two post-synaptic neurons. Thus, the higher MSB complexity in Alzheimer’s disease brain is unlikely to include spines from the same dendrite, which would not lead to connecting of previously unconnected neurons (scenario not shown). Created with BioRender.
Unfortunately, it is not possible to use 3D EM to investigate whether these alterations in MIS and MSB are caused by particular terminals degenerating or if they are newly formed since longitudinal studies are not possible. Either way, it is mandatory to investigate whether these new connections forming MIS or complex MSBs originate from the same neuron, hence increasing the connectivity in the brain, or if they arise from different axons or dendrites and therefore connect more cells. If the latter is true, and the connected cells have unrelated activity, memories encoded at different synapses might be ‘mixed’, possibly affecting memory and cognition.
Given the implications that changes in MIS and/or MSB appear to have in brain connectivity and cognition, it should be investigated whether MIS and MSB alterations are seen throughout the Alzheimer’s disease brain or just in the transentorhinal cortex. Further, the functional impact of such changes in synaptic connectivity needs to be analysed in model systems.
Concluding remarks and outstanding questions
Despite all research looking at synaptic alterations in Alzheimer’s disease, many outstanding questions remain to be addressed. With the development of newer and more advanced techniques, such as 3D EM and super-resolution imaging, along with the possibility of obtaining post-mortem brain tissue with minimal post-mortem delay and ensuring better tissue preservation, more detailed analyses can be carried out. Detailed synapse analysis is feasible, for instance, looking at specific synapse types (excitatory and inhibitory, macular and non-macular, etc), the location of the synapse within the post-synaptic cell (spine head, neck or dendritic shaft) as well as the quantification of multi-synapses (MIS and MSB). Therefore, recent studies using this technique have overcome some of the previous limitations and will provide a better and more accurate understanding of the disease pathology.
Regarding synapse and neuronal loss, it seems there is no homogeneity throughout the post-mortem Alzheimer’s disease brain, with synapse loss being associated with neuronal death in some but not all brain regions.29–31 This suggests different levels of resilience against synaptic dysfunction and degeneration between brain subregions. In order to target synaptic pathology in the most vulnerable regions, we need to understand what causes synapses to be more resistant towards dysfunction and degeneration in these areas. Excitatory and inhibitory synapses seem to be equally lost in Alzheimer’s disease, but more studies in other brain regions are needed in order to find whether this is a general principle in Alzheimer’s disease.
There is also no uniformity regarding changes in PSD morphology and synapse location within the post-synaptic cell in Alzheimer’s disease brains. These alterations appear to happen in specific neurons, with excitatory neurons being more affected.28,29,31 Changes in PSD morphology and synapse location could affect synapse function, maybe altering the excitatory-inhibitory balance, and affecting the cellular mechanism of learning and memory. Therefore, it is important to investigate why these particular synapses are altered and the implications of these changes.
Notably, 3D EM studies did not detect any synapse enlargement in most areas of the Alzheimer’s disease brain, in contrast to what has been reported before.10,41–44 Therefore, the maintenance of the total synaptic contact area also appears not to be a general feature of Alzheimer’s disease. However, these studies looked at neuropil synapses; therefore, a compensatory enlargement of perisomatic synapses cannot be ruled out. Unfortunately, studies using 3D EM to analyse post-mortem Alzheimer’s disease brains are still scarce, and this makes it difficult to know if synaptic enlargement may be a feature of synapses on cell bodies and/or in other brain regions. It is also important to know why in the transentorhinal cortex specific neuropil synapses and not others get bigger, what mechanisms underlie the size alteration and if these enlarged synapses are conserved, maybe in an attempt to store memories, or per contra, if they are in the process of dying.
Finally, MIS and MSBs also seem to be altered in Alzheimer’s disease. The reported changes could be part of a compensatory mechanism, trying to regain brain connectivity, leading to the disruption of stored memories. However, more 3D-EM analyses should be done to investigate whether these are common alterations in different brain areas and also if they represent an increased connectivity between the same neurons or a higher connectivity between different cells.
Despite the detailed ultrastructural analyses that 3D EM can offer, many questions remain unanswered. It is not yet clear why resilience towards synapse degeneration appears to be higher in some brain regions or why PSD morphology and synapse location are more commonly altered in excitatory synapses. Further, the role of abnormally enlarged neuropil synapses in the transentorhinal cortex and whether their formation should be enhanced or prevented or how this can be done also remains unknown. In addition, MIS and MSBs appear to be vulnerable in the transentorhinal cortex, but it still is unclear what their exact role is and whether they are important for memory storage or retrieval.
In order to tackle these questions, more studies are still needed in more brain regions to further investigate synaptic changes. Analyses of post-mortem brain tissue at the early stages of Alzheimer’s disease will also be necessary to discern between primary and secondary changes in association with pathology. Further, while 3D-EM is the gold standard for synapse identification, molecular mechanisms cannot be very well studied using this technique, as it involves immuno-EM, a laborious procedure for which it may be difficult to find suitable antibodies. However, single-molecule imaging techniques, such as super resolution microscopy (SRM), stochastic optical reconstruction microscopy (STORM) or DNA paint, are more suitable to study molecular mechanisms.48 On the other hand, these techniques cannot be used to identify multiple synapses, and they are not very well established for in vivo or in situ work. Therefore, findings using 3D-EM could be translated and looked at with a single-molecule imaging technique in order to unravel the molecular mechanisms underlying synaptic changes. For instance, findings using array tomography suggest that synapse density is decreased in proximity to amyloid oligomers surrounding plaques, and it increases in an approximately linear manner to reach similar levels as controls at 50 µm from a plaque.49 Similarly, FIB/SEM imaging has shown that the closer to an amyloid plaque, the smaller the number of synapses.22 Therefore, in the future, these techniques could be combined to investigate synaptic changes within 50 µm from an amyloid plaque and also to study the effects of hyperphosphorylated tau and neurofibrillary tangles in synapses. This can aid in finding the most accurate readout of pathophysiology to identify and study molecular and mechanistic processes happening in Alzheimer’s disease, which will deepen the understanding of disease-associated pathology and facilitate the development of new therapeutic targets.
Data availability
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Abbreviations
- 3D-EM =
three-dimensional electron microscopy
- AS =
asymmetric synapses
- CaMKII =
calcium/calmodulin-dependent kinase II
- EM =
electron microscopy
- MIS =
multi-innervated spines
- MSB =
multi-synaptic boutons
- PSD =
postsynaptic density
- PSD-95 =
postsynaptic density protein 95
- SRM =
super-resolution microscopy
- SS =
symmetric synapses
- STORM =
stochastic optical reconstruction microscopy
Funding
This work was supported by a Medical Research Council Doctoral Training Partnership and PhD scholarship (grant code: ST12205) to K.P.G. and K.C. L.A.-N. is supported by a grant (PGC2018-094307-B-I00) funded by the Spanish Ministry of Science and Innovation (MCIN/AEI/10.13039/501100011033).
Competing interests
The authors report no competing interests.
References
- 1.DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: Correlation with cognitive severity. Ann Neurol 1990;27(5):457–464. [DOI] [PubMed] [Google Scholar]
- 2.Scheff SW, DeKosky ST, Price DA. Quantitative assessment of cortical synaptic density in Alzheimer’s disease. Neurobiol Aging 1990;11(1):29–37. [DOI] [PubMed] [Google Scholar]
- 3.Shankar GM, Walsh DM. Alzheimer’s disease: synaptic dysfunction and Aβ. Mol Neurodegener 2009;4(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeKosky ST, Scheff SW, Styren SD. Structural correlates of cognition in dementia: Quantification and assessment of synapse change. Neurodegeneration 1996;5(4):417–421. [DOI] [PubMed] [Google Scholar]
- 5.Terry RD, Masliah E, Salmon DP, et al. Physical basis of cognitive alterations in alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol 1991;30(4):572–580. [DOI] [PubMed] [Google Scholar]
- 6.Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 2006;27(10):1372–1384. [DOI] [PubMed] [Google Scholar]
- 7.Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology 2007;68(18):1501–1508. [DOI] [PubMed] [Google Scholar]
- 8.Scheff SW, Price DA, Schmitt FA, Scheff MA, Mufson EJ. Synaptic loss in the inferior temporal gyrus in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis 2011;24(3):547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheff SW, Price DA, Ansari MA, et al. Synaptic change in the posterior cingulate gyrus in the progression of Alzheimer’s disease. J Alzheimers Dis 2014;43(3):1073–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheff S P, A D. Synaptic pathology in Alzheimer’s disease: A review of ultrastructural studies. Neurobiol Aging 2003;24(8):1029–1046. [DOI] [PubMed] [Google Scholar]
- 11.Honer WG, Dickson DW, Gleeson J, Davies P. Regional synaptic pathology in Alzheimer’s disease. Neurobiol Aging 1992;13(3):375–382. [DOI] [PubMed] [Google Scholar]
- 12.Masliah E, Terry RD, Alford M, DeTeresa R. Quantitative immunohistochemistry of synaptophysin in human neocortex: an alternative method to estimate density of presynaptic terminals in paraffin sections. J Histochem Cytochem 1990;38(6):837–844. [DOI] [PubMed] [Google Scholar]
- 13.Masliah E, Mallory M, Alford M, et al. Altered expression of synaptic proteins occurs early during progression of Alzheimer’s disease. Neurology 2001;56(1):127–129. [DOI] [PubMed] [Google Scholar]
- 14.Sherwood CC, Miller SB, Karl M, et al. Invariant synapse density and neuronal connectivity scaling in primate neocortical evolution. Cereb Cortex 2020;30(10):5604–5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sultana R, Banks WA, Butterfield DA. Decreased levels of PSD95 and two associated proteins and increased levels of BCl2 and caspase 3 in hippocampus from subjects with amnestic mild cognitive impairment: Insights into their potential roles for loss of synapses and memory, accumulation of Aβ, and neurodegeneration in a prodromal stage of Alzheimer’s disease. J Neurosci Res 2009;88(3):469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiwari SS, d’Orange M, Troakes C, et al. Evidence that the presynaptic vesicle protein CSPalpha is a key player in synaptic degeneration and protection in Alzheimer’s disease. Mol Brain 2015;8(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo SM, Veneziano R, Gordonov S, et al. Multiplexed and high-throughput neuronal fluorescence imaging with diffusible probes. Nat Commun 2019;10(1):4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schnitzbauer J, Strauss MT, Schlichthaerle T, Schueder F, Jungmann R. Super-resolution microscopy with DNA-PAINT. Nat Protoc 2017;12(6):1198–1228. [DOI] [PubMed] [Google Scholar]
- 19.DeFelipe J, Fariñas I. The pyramidal neuron of the cerebral cortex: Morphological and chemical characteristics of the synaptic inputs. Prog Neurobiol 1992;39(6):563–607. [DOI] [PubMed] [Google Scholar]
- 20.DeFelipe J. From the connectome to the synaptome: An epic love story. Science 2010;330(6008):1198–1201. [DOI] [PubMed] [Google Scholar]
- 21.Merchan-Pérez A. Counting synapses using FIB/SEM microscopy: A true revolution for ultrastructural volume reconstruction. Front Neuroanat 2009;3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blazquez-Llorca L, DeFelipe J. FIB/SEM technology and Alzheimer’s disease: Three-dimensional analysis of human cortical synapses. J Alzheimers Dis 2013;34(4):995–1013. [DOI] [PubMed] [Google Scholar]
- 23.Borczyk M, Radwanska K, Giese KP. The importance of ultrastructural analysis of memory. Brain Res Bull 2021;173:28–36. [DOI] [PubMed] [Google Scholar]
- 24.Geinisman Y, Berry RW, Disterhoft JF, Power JM, Van der Zee EA. Associative learning elicits the formation of multiple-synapse boutons. J Neurosci 2001;21(15):5568–5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aziz W, Kraev I, Mizuno K, et al. Multi-input synapses, but not LTP-strengthened synapses, correlate with hippocampal memory storage in aged mice. Curr Biol 2019;29(21):3600–3610.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giese KP, Aziz W, Kraev I, Stewart MG. Generation of multi-innervated dendritic spines as a novel mechanism of long-term memory formation. Neurobiol Learn Mem 2015;124:48–51. [DOI] [PubMed] [Google Scholar]
- 27.Radwanska K, Medvedev NI, Pereira GS, et al. Mechanism for long-term memory formation when synaptic strengthening is impaired. Proc Natl Acad Sci 2011;108(45):18471–18475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Domínguez-Álvaro M, Montero-Crespo M, Blazquez-Llorca L, DeFelipe J, Alonso-Nanclares L. 3D electron microscopy study of synaptic organization of the normal human transentorhinal cortex and its possible alterations in Alzheimer’s disease. eNeuro 2019;6(4):ENEURO.0140-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Domínguez-Álvaro M, Montero-Crespo M, Blazquez-Llorca L, et al. 3D analysis of the synaptic organization in the entorhinal cortex in Alzheimer’s disease. eNeuro 2021;8(3):ENEURO.0504-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Domínguez-Álvaro M, Montero-Crespo M, Blazquez-Llorca L, Insausti R, DeFelipe J, Alonso-Nanclares L. Three-dimensional analysis of synapses in the transentorhinal cortex of Alzheimer’s disease patients. Acta Neuropathol Commun 2018;6(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montero-Crespo M, Domínguez-Álvaro M, Alonso-Nanclares L, DeFelipe J, Blazquez-Llorca L. Three-dimensional analysis of synaptic organization in the hippocampal CA1 field in Alzheimer’s disease. Brain 2021;144(2):553–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montero-Crespo M, Dominguez-Alvaro M, Rondon-Carrillo P, Alonso-Nanclares L, DeFelipe J, Blazquez-Llorca L. Three-dimensional synaptic organization of the human hippocampal CA1 field. eLife 2020;9:e57013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeFelipe J, Fairén A. A simple and reliable method for correlative light and electron microscopic studies. J Histochem Cytochem 1993;41(5):769–772. [DOI] [PubMed] [Google Scholar]
- 34.Reese LC, Laezza F, Woltjer R, Taglialatela G. Dysregulated phosphorylation of Ca2+/calmodulin-dependent protein kinase II-α in the hippocampus of subjects with mild cognitive impairment and Alzheimer’s disease: Dysregulated phosphorylation of CaMKII in MCI and AD. J Neurochem 2011;119(4):791–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giese KP, Mizuno K. The roles of protein kinases in learning and memory. Learn Mem 2013;20(10):540–552. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh A, Giese KP. Calcium/calmodulin-dependent kinase II and Alzheimer’s disease. Mol Brain 2015;8(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. The Lancet 1994;344(8925):769–772. [DOI] [PubMed] [Google Scholar]
- 38.Gómez-Isla T, Hollister R, West H, et al. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease: Neuronal loss in the superior temporal sulcus in Alzheimer’s disease. Ann Neurol 1997;41(1):17–24. [DOI] [PubMed] [Google Scholar]
- 39.Nikonenko I, Jourdain P, Alberi S, Toni N, Muller D. Activity-induced changes of spine morphology. Hippocampus 2002;12(5):585–591. [DOI] [PubMed] [Google Scholar]
- 40.Peters A, Palay SL. The morphology of synapses. J Neurocytol 1996;25(1):687–700. [DOI] [PubMed] [Google Scholar]
- 41.Scheff SW, Sparks DL, Price DA. Quantitative assessment of synaptic density in the entorhinal cortex in Alzheimer’s disease. Ann Neurol 1993;34(3):356–361. [DOI] [PubMed] [Google Scholar]
- 42.Scheff SW, Price DA. Synaptic density in the inner molecular layer of the hippocampal dentate gyrus in Alzheimer disease. J Neuropathol Exp Neurol 1998;57(12):1146–1153. [DOI] [PubMed] [Google Scholar]
- 43.Scheff SW, Price DA. Alzheimer’s disease-related synapse loss in the cingulate cortex. J Alzheimers Dis 2001;3(5):495–505. [DOI] [PubMed] [Google Scholar]
- 44.Androuin A, Potier B, Nägerl UV, et al. Evidence for altered dendritic spine compartmentalization in Alzheimer’s disease and functional effects in a mouse model. Acta Neuropathol (Berl) 2018;135(6):839–854. [DOI] [PubMed] [Google Scholar]
- 45.Neuman KM, Molina-Campos E, Musial TF, et al. Evidence for Alzheimer’s disease-linked synapse loss and compensation in mouse and human hippocampal CA1 pyramidal neurons. Brain Struct Funct 2015;220(6):3143–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J Neurosci 1998;18(21):8900–8911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sorra K, Harris K. Occurrence and three-dimensional structure of multiple synapses between individual radiatum axons and their target pyramidal cells in hippocampal area CA1. J Neurosci 1993;13(9):3736–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Padmanabhan P, Kneynsberg A, Götz J. Super-resolution microscopy: A closer look at synaptic dysfunction in Alzheimer disease. Nat Rev Neurosci 2021;22(12):723–740. [DOI] [PubMed] [Google Scholar]
- 49.Koffie RM, Meyer-Luehmann M, Hashimoto T, et al. Oligomeric amyloid β associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci 2009;106(10):4012–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.