Abstract
Recent studies have revealed the importance of feedbacks between contemporary rapid evolution (i.e. evolution that occurs through changes in allele frequencies) and ecological dynamics. Despite its inherent interdisciplinary nature, however, studies on eco-evolutionary feedbacks have been mostly ecological and tended to focus on adaptation at the phenotypic level without considering the genetic architecture of evolutionary processes. In empirical studies, researchers have often compared ecological dynamics when the focal species under selection has a single genotype with dynamics when it has multiple genotypes. In theoretical studies, common approaches are models of quantitative traits where mean trait values change adaptively along the fitness gradient and Mendelian traits with two alleles at a single locus. On the other hand, it is well known that genetic architecture can affect short-term evolutionary dynamics in population genetics. Indeed, recent theoretical studies have demonstrated that genetic architecture (e.g. the number of loci, linkage disequilibrium and ploidy) matters in eco-evolutionary dynamics (e.g. evolutionary rescue where rapid evolution prevents extinction and population cycles driven by (co)evolution). I propose that theoretical approaches will promote the synthesis of functional genomics and eco-evolutionary dynamics through models that combine population genetics and ecology as well as nonlinear time-series analyses using emerging big data.
This article is part of the theme issue ‘Genetic basis of adaptation and speciation: from loci to causative mutations’.
Keywords: allele dominance, epistasis, linkage disequilibrium, number of loci, phenotypic plasticity, rapid evolution
1. Introduction
The traditional assumption in ecology and evolutionary biology has been that evolutionary processes are much slower than contemporary ecological processes [1,2]. Recent studies, on the other hand, have demonstrated that rapid adaptive evolution (i.e. allele frequency changes in populations over just a few generations) is common and can be rapid enough to affect ongoing ecological processes including population, community and even ecosystem dynamics [3–9]. Selection pressure is often fluctuating [10] and temporally fluctuating selection can make evolution rapid over short time scales and can cancel out the evolutionary responses across longer time scales [5,8]. Because ecological processes alter fitness landscapes and drive adaptive evolution [11], there should be an interplay between ecological and evolutionary processes. The resultant feedback between ecological processes and rapid adaptive evolution is called eco-evolutionary dynamics [12]. Eco-evolutionary dynamics is one of the most active research areas in ecology and evolutionary biology [13–20] not only for the synthesis of these two basic sciences, but also for conservation and management of wild organisms rapidly evolving in response to drastic environmental changes [21–23].
Although studies of eco-evolutionary dynamics combine insights from ecology and evolutionary biology and are inherently interdisciplinary, it seems that research on eco-evolutionary feedbacks has been mostly conducted from the perspective of ecology. For example, the finding of evolutionary cycles where prey defence evolution changes the phase lag between predator and prey densities from a quarter-period to a half-period [24,25] was surprising for ecologists, but dynamics of prey defence traits there might not be so novel for evolutionary biologists. According to the Web of Science, about 58% of papers in a search of ‘eco-evo* dynamics’ were categorized as ‘Ecology’ whereas 30% were ‘Evolutionary Biology’ and 11% of papers were ‘Genetics Heredity’ (searched on 2 March 2022). Researchers have tended to focus on feedbacks between ecological dynamics and adaptation at the phenotypic level (the solid line surrounding these categories in figure 1) instead of including the genetic architecture (see Glossary) of evolutionary processes (the dashed line in figure 1) (see the following section). Treating genetic details as a black box (the ‘phenotypic gambit’ in evolutionary ecology [27]) is a powerful, simplifying and convincing approach for understanding complex long-term evolutionary dynamics. However, short-term evolutionary dynamics may be more constrained by the genetic architecture of phenotypic adaptation, especially because many such short-term adaptative changes are driven by a limited amount of standing genetic variation instead of a tremendous amount of de novo mutations [28]. Thus, understanding genetic architecture will be important for deepening our understanding of eco-evolutionary dynamics.
Figure 1.
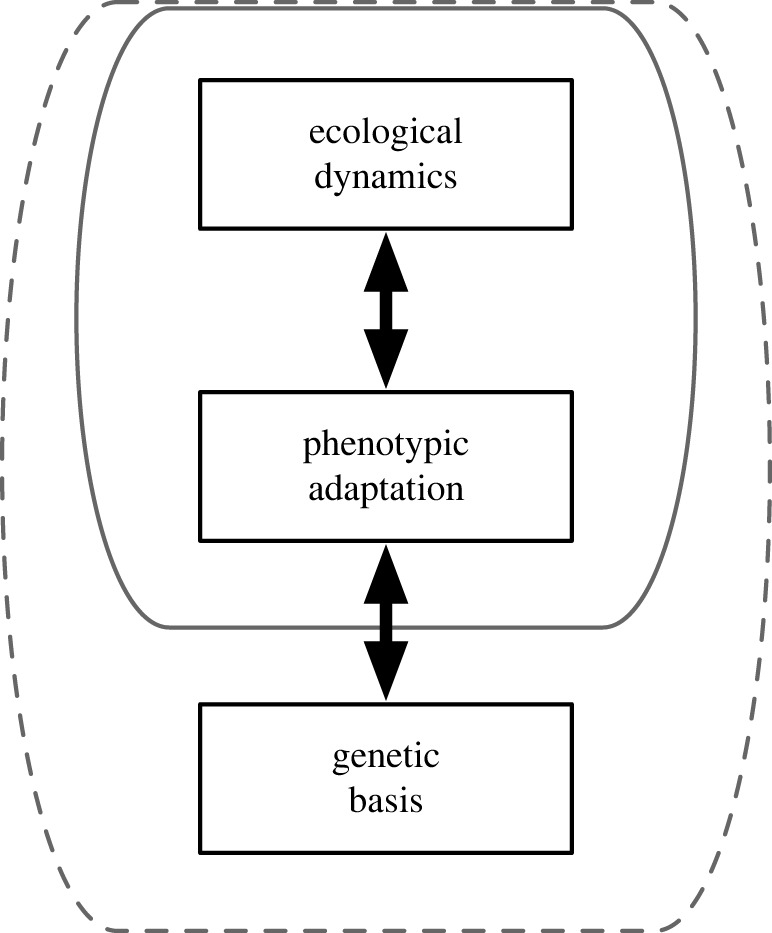
The conceptual framework of eco-evolutionary feedbacks (after [26]). Previous studies in eco-evolutionary dynamics tended to focus on feedbacks between ecological processes and phenotypic adaptation (indicated by the solid line). Including genetic basis of phenotypic adaptation (as indicated by the dashed line) may improve our understanding of eco-evolutionary dynamics. Note that the figure seems to be suggesting that the three components are separate, but they are confounded with one another.
A similar argument about the potential importance of mechanisms of adaptation has been made for the difference between rapid evolution and phenotypic plasticity. Both rapid evolution and phenotypic plasticity are trait changes that often increase an individual's fitness and are rapid enough to affect ecological dynamics [5,29,30]. It may be difficult for us to differentiate them when we observe adaptive trait changes in the wild (but see [31]), although plasticity is not necessarily adaptive. Some studies suggested that genetic evolution, phenotypic plasticity and even behavioural changes based on learning processes can be described by a quantitative trait model (see the following section) simply through changing the speed of trait adaptation (e.g. [32–34]). However, theoretical studies have proposed that phenotypic plasticity may be better at stabilizing population cycles due to faster responses to environmental changes [35–37] and may not cause antiphase predator–prey cycles unlike rapid evolution because plastic changes are not directly affected by the local fitness gradient [37,38]. Indeed, experimental studies on the rapid evolution of prey defence traits in zooplankton–phytoplankton microcosm systems showed antiphase cycles [24,39], whereas those on inducible defence did not find antiphase cycles [40].
While some studies have pointed out the potential importance of genomic studies in eco-evolutionary dynamics [41–44], the dynamic consequences of genetic architectures on eco-evolutionary dynamics have to date not been well recognized. Here I review theoretical results on the effects of genetic architecture on evolutionary and eco-evolutionary dynamics and propose a future direction where genetic and genomic studies deepen our understanding of eco-evolutionary dynamics by combining dynamic models and nonlinear time-series analyses.
2. Common approaches in eco-evolutionary dynamics
For understanding the effects of rapid evolution on ecological dynamics, empirical researchers often compared ecological dynamics when the focal species under selection has only a single allele at the focal locus versus dynamics with multiple alleles and so can evolve (or, in the case of asexually reproducing species, dynamics with a single clonal genotype versus dynamics with multiple clonal genotypes; e.g. [24,45–47]). Even with a single genotype of asexual organisms, de novo mutations may produce genetic variation and eventually cause rapid evolution [39,48]. However, as long as experimental periods are short, mutation rates are small and generation time is not relatively short, it will be possible to observe ecological dynamics without rapid evolution [49].
It should be noted that there are three types of empirical studies: (1) studies examining the effects of ongoing rapid evolution on ecological dynamics (e.g. [24,39]), (2) studies examining the effects of evolved traits (usually after short evolution experiments) on ecological dynamics (e.g. [50–52]) and (3) studies examining the effects of genetic variation (without evolutionary changes) on ecological dynamics in short-term experiments (e.g. [45]). Case (1) may be further divided into (1a) continuous eco-evolutionary dynamics where genetic variation is maintained by selection (e.g. [24]) and (1b) transient eco-evolutionary dynamics where selection eventually removes genetic variation (e.g. [53]). Although genetic variation is a prerequisite of rapid evolution in most situations, rapid evolution does not always occur during the experiments in cases (2) and (3). Studies in ‘community genetics’ tend to use plant traits, and thus to consider cases (2) and (3) [54], while theoretical studies often consider case (1). Genetic architectures may become important in empirical studies of the case (1) type.
In theoretical studies, common approaches assume continuous quantitative traits controlled by many loci with small effects:
| 2.1 |
where is a mean value of a quantitative trait, N is a population density, and f and g represent their dynamics [12,18,20]. Mean trait dynamics is often represented by
| 2.2 |
where ν is additive genetic variance and is population mean fitness (i.e. the per capita growth rate: dN/Ndt) [32,55]. Here the mean trait changes along the local fitness gradient to increase the fitness (e.g. [33,34,38,56–59]).
Some studies employed models of discrete Mendelian traits with two alleles in a single locus (e.g. [60]) or a clonal model,
| 2.3 |
where Ni represents the density of a clone (genotype) i in an asexual organism such as bacteria and algae (e.g. [25,35,36,39,48]). This can be re-written as
| 2.4 |
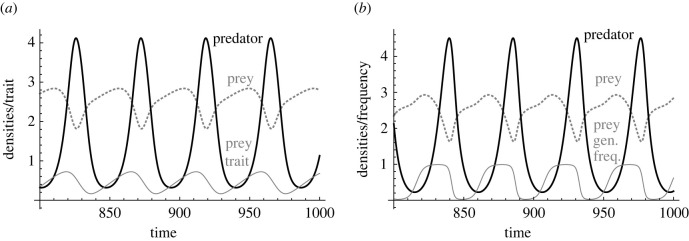
where NT = N1 + N2 and p = N1/NT. Note that equation (2.4) corresponds to equation (2.1): p(1 − p) is the additive genetic variance and the difference between the per capita growth rates represents the fitness gradient. While the additive genetic variance ν is often assumed to be a fixed parameter in equation (2.2), the variance p(1 − p) changes depending on the clonal frequency p in equation (2.4). Despite the difference, the two approaches can produce very similar dynamics [57,61]. For example, predator–prey antiphase cycles with quantitative traits [56], and those with two clonal genotypes [25,48] are basically very similar (figure 2). Theoreticians have sometimes used an Adaptive Dynamics approach (i.e. evolutionary invasion analysis) assuming asexual reproduction as well (e.g. [62]), but a common assumption seems to be that genetic architectures do not matter and can be safely ignored for understanding eco-evolutionary dynamics [63].
Figure 2.
Antiphase predator–prey cycles in (a) a quantitative trait model [56] and (b) a clonal model [25]. Black solid lines and grey dotted lines represent predator and prey densities, respectively. Grey solid lines are (a) prey trait and (b) prey genotype frequency, respectively, and higher values indicate less defended states.
3. Effects of genetic architecture on evolutionary dynamics
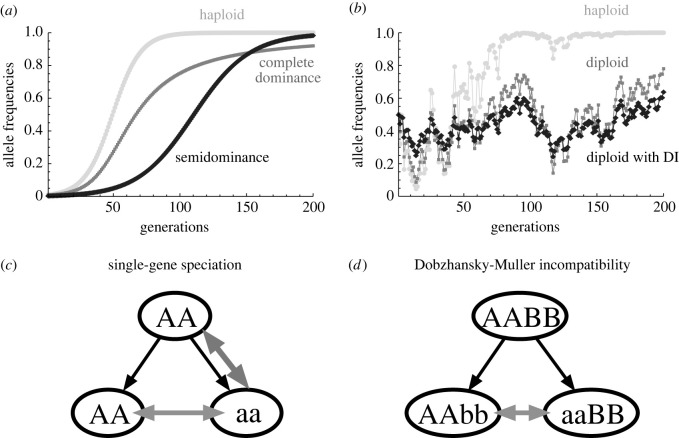
In evolutionary biology, especially in population genetics, it is well known that genetic architecture can affect evolutionary dynamics. Genetic architectures themselves can evolve in response to selection over long time scales (e.g. [64]), but short-term evolution is constrained by the relationships between genotypes and phenotypes. Previous studies demonstrated that a single gene can have large phenotypic consequences in insects [65,66], mollusks [67], fish [68,69], mammals [70–72] and plants [73,74]. Although there is likely to be publication bias and many adaptive traits are likely quantitative with many loci that have small effects [75,76], it is meaningful to start from models with one locus or two loci for heuristic purposes [77]. Here I outline three examples: the effects of ploidy and allele dominance on the speed of allele fixation (figure 3a), the effects of ploidy and maternal effects on the maintenance of genetic variation under temporally fluctuating selection (figure 3b), and the required number of loci in speciation (figure 3c,d).
Figure 3.
Examples of the effects of genetic architectures on evolutionary dynamics. (a) The effects of ploidy and allele dominance on evolutionary dynamics under directional selection. Haploid (light grey), diploid with complete dominance (grey) and diploid with semidominance (black) are shown. (b) The effects of ploidy and delayed inheritance (DI) on evolutionary dynamics under temporally fluctuating selection [78]. Haploid (light grey), diploid with complete dominance (grey) and diploid with DI (black) are shown. (c,d) The effects of the number of loci on speciation processes. (c) Single-gene speciation from an ancestral population with an allele A to two populations with alleles A and a where there is reproductive incompatibility between alleles A and a (shown by grey arrows). Because of the incompatibility, it is difficult for a mutant allele a to increase in an ancestral population with a resident allele A. (d) Speciation from an ancestral population with alleles A and B to two populations with alleles A, B, a and b where there is Dobzhansky–Muller incompatibility between alleles a and b due to epistasis. Mutant alleles a and b can increase in an ancestral population without incompatibility unlike the model of single-gene speciation.
Probably the simplest example is evolutionary dynamics under directional selection (figure 3a). Haploid inheritance is the most sensitive to selection, whereas complete dominance in diploid inheritance can delay evolutionary responses to selection due to a mismatch between genotypes and phenotypes: heterozygotes include a recessive allele but have a dominant phenotype. When dominant mutant alleles are selected for, they can quickly increase when rare, but it is difficult for them to remove the resident recessive alleles unlike semidominance. With genetic drift in finite populations, frequency dynamics when alleles are rare are important for fixation, and thus adaptive alleles are more likely to be dominant (i.e. Haldane's sieve: [79]).
With temporally fluctuating selection [10], haploid inheritance is so sensitive to selection pressure that it cannot maintain genetic variation: an allele with the highest geometric mean fitness dominates and other alleles will be lost from a population (figure 3b) [80]. On the other hand, the maintenance of genetic variation is possible in diploid inheritance with complete dominance because alleles can be stored in heterozygotes when they are not favoured [81,82]. This is what we call the storage effect [83]. These days, researchers tend to think that overlapping generations play a primary role for buffered population growth of the storage effect [83,84], but genetic architecture can also work for buffering. As like complete dominance, a maternal genetic effect where maternal genotypes determine offspring phenotypes (delayed inheritance (DI)) further blurs the relationship between genotypes and phenotypes and makes the maintenance of genetic variation easier [78]. Note that there are a few other mechanisms that have been demonstrated to maintain genetic diversity (e.g. reversal of dominance) and they are summarized in Bertram and Masel [85].
When an ancestral population splits into two populations, researchers have suggested speciation is unlikely when reproductive incompatibility is caused only by a single locus with two alleles. This is because there is reproductive incompatibility between alleles in this single-gene speciation scenario and hence it is difficult for a mutant allele to increase when rare (figure 3c) [86,87]. When there are two loci with epistasis, on the other hand, speciation can occur without difficulty: this is called Dobzhansky–Muller reproductive incompatibility (figure 3d) [88–90]. In this case, reproductive incompatibility occurs between mutant alleles at the two loci due to epistasis. Thus, the number of loci affecting reproductive incompatibility determines the outcome of the speciation processes.
4. Effects of genetic architecture on eco-evolutionary dynamics
As shown in the previous section, genetic architectures can affect evolutionary dynamics and thus eco-evolutionary dynamics as well. Here I introduce recent theoretical studies that showed the potential effects of the genetic architecture on eco-evolutionary dynamics. In future empirical studies, it may become possible to compare eco-evolutionary dynamics with different genetic architecture (e.g. dynamics with haploid inheritance versus dynamics with diploid inheritance) directly based on the following theoretical predictions as like studies on rapid evolution and phenotypic plasticity. There are many possible combinations of ecological dynamics (e.g. population extinction and population cycles) and genetic details (e.g. the number of loci and recombination), and there are a few studies that have explored some of them (table 1).
Table 1.
Theoretical studies that combine ecological dynamics and genetic structure. Note that sexual reproduction, recombination and ploidy are fundamentally tightly related.
| ecological dynamics |
|||
|---|---|---|---|
| population extinction (evolutionary rescue) | predator–prey cycles (including apparent and exploitative competition) | ||
| genetic structure | number of loci | Orr & Unckless [91], Gomulkiewicz et al. [92], Kardos & Luikart [93] | Yamamichi & Ellner [94] |
| recombination/epistasis | Schiffers et al. [95], Uecker & Hermisson [96] |
Patel & Bürger [97] | |
| clonal versus sexual reproduction/ploidy | Orive et al. [98], Uecker [99], Peniston et al. [100] | Schreiber et al. [60], Doebeli & Koella [101], Doebeli [102], Bolnick et al. [103] | |
Evolutionary rescue is probably the most interdisciplinary topic in eco-evolutionary dynamics, with work from ecologists, evolutionary biologists, population geneticists and medical researchers [104–106]. Evolutionary rescue is a phenomenon where rapid adaptive evolution prevents population extinction in the face of an environmental change [107]. It is not only important for conservation and wildlife management, but also for medicine where researchers seek to prevent evolutionary rescue of bacteria from suppression by antibiotics [104]. Gomulkiewicz & Holt [107] originally examined a quantitative-genetic model (as like equation (2.2)) and a one-locus model (as like equation (2.3)) and obtained qualitatively similar results. Orr & Unckless [91] showed that it is difficult for a single locus to adapt to rapid environmental change compared with the case for multiple loci where any one of them can rescue the population. On the other hand, Gomulkiewicz et al. [92] showed that increasing the number of loci can decrease the speed of adaptation and prevent the resultant rescue from extinction because selection per locus is weakened. More recently, Kardos & Luikart [93] demonstrated that population extinction is less likely in models with polygenic architectures compared with models with large-effect loci due to higher short-term evolutionary potential. Uecker & Hermisson [96] analysed a model where evolutionary rescue depends on mutations at two loci and found complex effects of recombination on extinction because recombination generates and breaks up favourable gene combinations. These studies suggest that models at the extremes of either a single locus or infinitely many loci behave similarly, whereas models with intermediate numbers of loci may show complex dynamics.
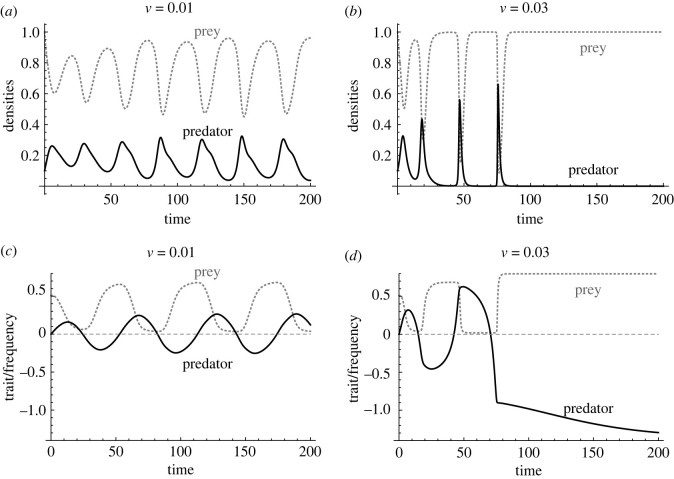
Predator–prey population dynamics has been a central topic in eco-evolutionary dynamics since the seminal experimental papers on antiphase and cryptic cycles driven by rapid evolution [24,48]. Because those studies considered defence evolution of asexually reproducing algae, genetic details have not been considered intensively [48,57]. Yamamichi & Ellner [94] modelled antagonistic coevolution between the Mendelian trait of a prey and the quantitative trait of its predator inspired by a snake-snail predator–prey system [67]. They found that rapid predator evolution can result in predator extinction (figure 4a,b) unlike coevolution between Mendelian traits or between quantitative traits. This is because evolution of the prey's discrete trait can throw off tracking by the predator's continuous trait as the amplitudes of coevolutionary cycles amplify, especially with complete allele dominance (figure 4c,d). On the other hand, Schreiber et al. [60] examined the effects of ploidy (haploid versus diploid) on species coexistence and showed that diploid inheritance can stabilize community dynamics with exploitative and apparent competition due to the inefficacy of selection. More recently, Patel & Bürger [97] explored how recombination in predator species affects apparent competition of two prey species and found a novel feedback between predator density, total prey density and linkage disequilibrium in the predator induced by epistatic fitness effects of linked loci.
Figure 4.
Rapid predator evolution can result in predator extinction in genetically asymmetric coevolution (coevolution between a prey's Mendelian trait and a predator's quantitative trait) [94]. Predation is more successful when traits match (i.e. a bidirectional axis of vulnerability [108]) due to, for example, handedness of snails and snakes. (a,c) Persistent predator–prey population cycles (a) and trait coevolution (c) when the additive genetic variance of the quantitative trait, v = 0.01. (b,d) Predator extinction (b) due to large amplitudes in trait coevolution (d) when additive genetic variance of the quantitative trait, v = 0.03. Black solid lines and grey dotted lines represent predator and prey species, respectively.
While previous studies of eco-evolutionary dynamics have tended to focus on evolutionary rescue and predator–prey interactions, it will be interesting to examine other ecological dynamics such as competitive and mutualistic interactions [109] as well as host–parasite dynamics [110]. In addition, speciation processes can be studied from the perspective of eco-evolutionary dynamics. For example, rapid evolution in reproductive character displacement (reinforcement) can prevent population extinction by weakening reproductive interference and positive frequency-dependence in community dynamics due to incomplete reproductive isolation [111,112]. It may be interesting to study how the genetic basis of speciation (speciation genes: figure 3c,d) affects eco-evolutionary dynamics.
5. Conclusion and future directions
Previous studies have shown that genetic details can affect evolutionary and eco-evolutionary dynamics (figures 3 and 4). However, few theoretical studies have examined the effects of genetic architectures on eco-evolutionary dynamics (table 1). Therefore, more studies are needed of eco-evolutionary dynamics that integrate genetics, evolutionary biology and ecology (figure 1). Recent studies have emphasized the importance of the analogy between community ecology and population genetics [113,114], but the integrated eco-evolutionary framework (figure 1) will be another important step for population biology synthesizing population genetics and population ecology.
There are many ways to add genetic details to simple eco-evolutionary models such as equations (2.1)–(2.4), including epigenetics, pleiotropy and allele dominance in addition to the number of loci, varying phenotypic effects of loci, recombination, epistasis, ploidy and sexual versus asexual reproduction (table 1). Indeed some researchers made the quantitative trait model (equation (2.2)) more realistic by considering trait variance dynamics [115], bimodal trait distributions [116] and evolutionary diversification [117]. However, complex models are not always better than simple ones. All models are wrong, and hence it is important to ask when we need to care about genetic bases of ecologically important traits. Indeed, the quantitative trait and clonal models show similar eco-evolutionary dynamics (figure 2), and models with 20, 100 or 1000 loci may show very similar dynamics [92]. In this case, simply estimating additive genetic variance of fitness-associated traits may be better than considering genetic basis. Accumulating more theoretical studies should reveal conditions where the details can be safely ignored.
Because of rapid developments of molecular biological techniques, it is now possible to investigate genetic basis of ecologically important traits in non-model organisms [118,119]. This ecological and evolutionary functional genomics will not only promote our understanding of past evolutionary processes, but also contribute to studies on eco-evolutionary dynamics [41–44]. Some organisms are often used for genomic studies as well as studies on eco-evolutionary dynamics. Thus, it will be possible to connect genome structure and eco-evolutionary dynamics by using, for example, baker's yeast (Saccharomyces: [53]), green algae (Chlamydomonas: [120]), waterflea (Daphnia: [121]), threespine sticklebacks (Gasterosteus: [46]), thale cress (Arabidopsis: [122]) and poplars (Populus: [54]). Even with the genomic resources, however, genomics of eco-evolutionary dynamics is still in its infancy due to its inherent difficulty. How can we understand the relationship between fitness and traits in addition to the relationship between traits and genomes? When selective landscapes vary through time, when should the architecture be studied? If the architecture varies over time, what can be learned? Indeed, previous studies found that various genetic bases can exist behind the same evolutionary responses [119,120,123]. This may be a part of the reason why there are not so many empirical studies on genomics of eco-evolutionary dynamics despite the previous perspective papers [41–44].
Lastly, I propose three possible research directions that would combine genomic data and eco-evolutionary dynamics with a guide of theoretical modelling: backward inferences based on genomic data, nonlinear time-series data analyses and genome-wide association studies. First, if we know how eco-evolutionary dynamics affect genomic patterns (e.g. how evolutionary rescue affects selective sweep and genetic hitchhiking of linked neutral alleles: [124]) by using population genetic models, then it may even be possible for us to detect past eco-evolutionary dynamics from population genomic data. This may be an interesting approach for transient dynamics such as evolutionary rescue [124] as well as continuous dynamics such as coevolutionary cycles [125]. Second, when time series of genomic data are available (e.g. [126,127]), nonlinear time-series data analyses such as empirical dynamic modelling (EDM) [128] and transfer entropy [129] may make it possible to infer causal relationships between time-series data of allele frequencies in single-nucleotide polymorphisms (SNPs), expression patterns, fitness and population densities. Currently, it is very difficult to obtain such a huge amount of time-series data, but it may become possible to collect data of wild organism more easily in the near future through automated monitoring with advanced techniques such as environmental DNA [130], machine learning for camera trap data [131], mobile DNA sequencers and unmanned aerial vehicles [132]. Then, we may be able to use EDM to re-construct attractors from time-series data based on Takens’ theorem and to infer causal relationships between genomic data and ecological processes [128,133,134]. Based on the time-series analyses, we may be able to draw integrated networks of eco-evolutionary dynamics including gene interactions, trait interactions and species interactions [76,135]. Note that eco-evolutionary dynamics can be cryptic (i.e. eco-evolutionary dynamics may appear like purely ecological expectations) [48,136], and in this case, it may be difficult to infer causality solely from time-series analyses. In addition, because fitness is an emergent property of many traits, even when there are alleles of moderate effect on individual fitness-associated traits, their individual effect on resultant eco-evolutionary processes is likely to be quite small because of a polygenic basis [137]. This considerable hurdle in many empirical systems may be addressed by time-series analyses, if researchers can obtain big data from genome, epigenome, fitness, trait dynamics and ecological dynamics. Finally, even when the data are not especially rich, it will be interesting to examine associations between genetic markers (e.g. SNPs and structural variants) and key ecological parameters (e.g. population densities of the focal species or community compositions on the focal host species). This may be done by conducting genome-wide association studies that examine associations between genetic and epigenetic patterns with ecological dynamics (instead of phenotypic traits) [138] as well as differentiation outlier methods that screen for alleles that show large genetic differentiation between populations that exhibit different ecological patterns [139]. In this context, theoretical models will be useful for understanding the entangled interactions between genes, traits and species even in this era of big data.
Acknowledgements
I thank three anonymous reviewers, NG Hairston Jr and SP Ellner for their helpful comments.
Data accessibility
This article has no additional data.
Authors' contributions
M.Y.: conceptualization, funding acquisition, investigation, visualization, writing—original draft and writing—review and editing.
Conflict of interest declaration
I declare I have no competing interests.
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (KAKENHI) 19K16223, 20KK0169 and 21H02560.
Glossary
- Epistasis:
the phenomenon where the effects of one gene on a phenotype is affected by the expression of other gene(s).
- Genetic architecture:
the genetic basis underlying a phenotypic trait.
- Haldane's sieve:
the bias against the fixation of recessive beneficial mutations.
- Linkage disequilibrium:
the nonrandom assortment of alleles at different loci (i.e. the deviation from independent association).
- Pleiotropy:
the phenomenon where one gene affects two (or more) phenotypic traits.
- Ploidy:
the number of complete sets of chromosomes in the nucleus of a cell.
References
- 1.Darwin C. 1859. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. London, UK: John Murray. [PMC free article] [PubMed] [Google Scholar]
- 2.Slobodkin LB. 1961. Growth and regulation of animal populations. New York, NY: Holt, Rinehart and Winston. [Google Scholar]
- 3.Thompson JN. 1998. Rapid evolution as an ecological process. Trends Ecol. Evol. 13, 329-332. ( 10.1016/S0169-5347(98)01378-0) [DOI] [PubMed] [Google Scholar]
- 4.Hendry AP, Kinnison MT. 1999. The pace of modern life: measuring rates of contemporary microevolution. Evolution 53, 1637-1653. ( 10.1111/j.1558-5646.1999.tb04550.x) [DOI] [PubMed] [Google Scholar]
- 5.Hairston NG Jr, Ellner SP, Geber MA, Yoshida T, Fox JA. 2005. Rapid evolution and the convergence of ecological and evolutionary time. Ecol. Lett. 8, 1114-1127. ( 10.1111/j.1461-0248.2005.00812.x) [DOI] [Google Scholar]
- 6.Johnson MTJ, Stinchcombe JR. 2007. An emerging synthesis between community ecology and evolutionary biology. Trends Ecol. Evol. 22, 250-257. ( 10.1016/j.tree.2007.01.014) [DOI] [PubMed] [Google Scholar]
- 7.Matthews B, et al. 2011. Toward an integration of evolutionary biology and ecosystem science. Ecol. Lett. 14, 690-701. ( 10.1111/j.1461-0248.2011.01627.x) [DOI] [PubMed] [Google Scholar]
- 8.Messer PW, Ellner SP, Hairston NG Jr. 2016. Can population genetics adapt to rapid evolution? Trends Genet. 32, 408-418. ( 10.1016/j.tig.2016.04.005) [DOI] [PubMed] [Google Scholar]
- 9.Sanderson S, et al. 2022. The pace of modern life, revisited. Mol. Ecol. 31, 1028-1043. ( 10.1111/mec.16299) [DOI] [PubMed] [Google Scholar]
- 10.Bell G. 2010. Fluctuating selection: the perpetual renewal of adaptation in variable environments. Phil. Trans. R. Soc. B 365, 87-97. ( 10.1098/rstb.2009.0150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutchinson GE. 1965. The ecological theater and the evolutionary play. New Haven, CT: Yale University Press. [Google Scholar]
- 12.Post DM, Palkovacs EP. 2009. Eco-evolutionary feedbacks in community and ecosystem ecology: interactions between the ecological theatre and the evolutionary play. Phil. Trans. R. Soc. B 364, 1629-1640. ( 10.1098/rstb.2009.0012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carroll SP, Hendry AP, Reznick DN, Fox CW. 2007. Evolution on ecological time-scales. Funct. Ecol. 21, 387-393. ( 10.1111/j.1365-2435.2007.01289.x) [DOI] [Google Scholar]
- 14.Schoener TW. 2011. The newest synthesis: understanding the interplay of evolutionary and ecological dynamics. Science 331, 426-429. ( 10.1126/science.1193954) [DOI] [PubMed] [Google Scholar]
- 15.Ellner SP. 2013. Rapid evolution: from genes to communities, and back again? Funct. Ecol. 27, 1087-1099. ( 10.1111/1365-2435.12174) [DOI] [Google Scholar]
- 16.Reznick DN. 2013. A critical look at reciprocity in ecology and evolution: introduction to the symposium. Am. Nat. 181, S1-S8. ( 10.1086/670030) [DOI] [PubMed] [Google Scholar]
- 17.Hendry AP. 2016. Eco-evolutionary dynamics. Princeton, NJ: Princeton University Press. [Google Scholar]
- 18.Govaert L, et al. 2019. Eco-evolutionary feedbacks—theoretical models and perspectives. Funct. Ecol. 33, 13-30. ( 10.1111/1365-2435.13241) [DOI] [Google Scholar]
- 19.Yamamichi M. 2020. Effects of rapid evolution on population cycles and extinction in predator–prey systems. In Diversity of functional traits and interactions (ed. Mougi A), pp. 19-49. Singapore, Singapore: Springer. [Google Scholar]
- 20.Bassar RD, Coulson T, Travis J, Reznick DN. 2021. Towards a more precise – and accurate – view of eco-evolution. Ecol. Lett. 24, 623-625. ( 10.1111/ele.13712) [DOI] [PubMed] [Google Scholar]
- 21.Palumbi SR. 2002. The evolution explosion: how humans cause rapid evolutionary change. New York, NY: W. W. Norton & Company. [Google Scholar]
- 22.Stockwell CA, Hendry AP, Kinnison MT. 2003. Contemporary evolution meets conservation biology. Trends Ecol. Evol. 18, 94-101. ( 10.1016/S0169-5347(02)00044-7) [DOI] [Google Scholar]
- 23.Heino M, Díaz Pauli B, Dieckmann U. 2015. Fisheries-induced evolution. Annu. Rev. Ecol. Evol. Syst. 46, 461-480. ( 10.1146/annurev-ecolsys-112414-054339) [DOI] [Google Scholar]
- 24.Yoshida T, Jones LE, Ellner SP, Fussmann GF, Hairston NG Jr. 2003. Rapid evolution drives ecological dynamics in a predator–prey system. Nature 424, 303-306. ( 10.1038/nature01767) [DOI] [PubMed] [Google Scholar]
- 25.Jones LE, Ellner SP. 2007. Effects of rapid prey evolution on predator–prey cycles. J. Math. Biol. 55, 541-573. ( 10.1007/s00285-007-0094-6) [DOI] [PubMed] [Google Scholar]
- 26.Bailey JK, Hendry AP, Kinnison MT, Post DM, Palkovacs EP, Pelletier F, Harmon LJ, Schweitzer JA. 2009. From genes to ecosystems: an emerging synthesis of eco-evolutionary dynamics. New Phytol. 184, 746-749. ( 10.1111/j.1469-8137.2009.03081.x) [DOI] [PubMed] [Google Scholar]
- 27.Grafen A. 1984. Natural selection, kin selection and group selection. In Behavioural ecology: an evolutionary approach (eds Krebs JR, Davies NB), pp. 62-84, 2nd edn. Oxford, UK: Blackwell Scientific. [Google Scholar]
- 28.Barrett RDH, Schluter D. 2008. Adaptation from standing genetic variation. Trends Ecol. Evol. 23, 38-44. ( 10.1016/j.tree.2007.09.008) [DOI] [PubMed] [Google Scholar]
- 29.Miner BG, Sultan SE, Morgan SG, Padilla DK, Relyea RA. 2005. Ecological consequences of phenotypic plasticity. Trends Ecol. Evol. 20, 685-692. ( 10.1016/j.tree.2005.08.002) [DOI] [PubMed] [Google Scholar]
- 30.Ellner SP, Geber MA, Hairston NG Jr. 2011. Does rapid evolution matter? Measuring the rate of contemporary evolution and its impacts on ecological dynamics. Ecol. Lett. 14, 603-614. ( 10.1111/j.1461-0248.2011.01616.x) [DOI] [PubMed] [Google Scholar]
- 31.Govaert L, Pantel JH, De Meester L. 2016. Eco-evolutionary partitioning metrics: assessing the importance of ecological and evolutionary contributions to population and community change. Ecol. Lett. 19, 839-853. ( 10.1111/ele.12632) [DOI] [PubMed] [Google Scholar]
- 32.Abrams PA, Matsuda H, Harada Y. 1993. Evolutionarily unstable fitness maxima and stable fitness minima of continuous traits. Evol. Ecol. 7, 465-487. ( 10.1007/BF01237642) [DOI] [Google Scholar]
- 33.Fox JW, Vasseur DA. 2008. Character convergence under competition for nutritionally essential resources. Am. Nat. 172, 667-680. ( 10.1086/591689) [DOI] [PubMed] [Google Scholar]
- 34.Mougi A, Iwasa Y. 2010. Evolution towards oscillation or stability in a predator–prey system. Proc. R. Soc. B 277, 3163-3171. ( 10.1098/rspb.2010.0691) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamichi M, Yoshida T, Sasaki A. 2011. Comparing the effects of rapid evolution and phenotypic plasticity on predator–prey dynamics. Am. Nat. 178, 287-304. ( 10.1086/661241) [DOI] [PubMed] [Google Scholar]
- 36.Kovach-Orr C, Fussmann GF. 2013. Evolutionary and plastic rescue in multitrophic model communities. Phil. Trans. R. Soc. B 368, 20120084. ( 10.1098/rstb.2012.0084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamichi M, Klauschies T, Miner BE, van Velzen E. 2019. Modelling inducible defences in predator–prey interactions: assumptions and dynamical consequences of three distinct approaches. Ecol. Lett. 22, 390-404. ( 10.1111/ele.13183) [DOI] [PubMed] [Google Scholar]
- 38.Cortez MH. 2011. Comparing the qualitatively different effects rapidly evolving and rapidly induced defences have on predator–prey interactions. Ecol. Lett. 14, 202-209. ( 10.1111/j.1461-0248.2010.01572.x) [DOI] [PubMed] [Google Scholar]
- 39.Becks L, Ellner SP, Jones LE, Hairston NG Jr. 2010. Reduction of adaptive genetic diversity radically alters eco-evolutionary community dynamics. Ecol. Lett. 13, 989-997. ( 10.1111/j.1461-0248.2010.01490.x) [DOI] [PubMed] [Google Scholar]
- 40.Verschoor AM, Vos M, van der Stap I. 2004. Inducible defences prevent strong population fluctuations in bi- and tritrophic food chains. Ecol. Lett. 7, 1143-1148. ( 10.1111/j.1461-0248.2004.00675.x) [DOI] [Google Scholar]
- 41.Hendry AP. 2013. Key questions in the genetics and genomics of eco-evolutionary dynamics. Heredity 111, 456-466. ( 10.1038/hdy.2013.75) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodríguez-Verdugo A, Buckley J, Stapley J. 2017. The genomic basis of eco-evolutionary dynamics. Mol. Ecol. 26, 1456-1464. ( 10.1111/mec.14045) [DOI] [PubMed] [Google Scholar]
- 43.Matthews B, Best RJ, Feulner PGD, Narwani A, Limberger R. 2018. Evolution as an ecosystem process: insights from genomics. Genome 61, 298-309. ( 10.1139/gen-2017-0044) [DOI] [PubMed] [Google Scholar]
- 44.Rudman SM, et al. 2018. What genomic data can reveal about eco-evolutionary dynamics. Nat. Ecol. Evol. 2, 9-15. ( 10.1038/s41559-017-0385-2) [DOI] [PubMed] [Google Scholar]
- 45.Crutsinger GM, Collins MD, Fordyce JA, Gompert Z, Nice CC, Sanders NJ. 2006. Plant genotypic diversity predicts community structure and governs an ecosystem process. Science 313, 966-968. ( 10.1126/science.1128326) [DOI] [PubMed] [Google Scholar]
- 46.Harmon LJ, Matthews B, Des Roches S, Chase JM, Shurin JB, Schluter D. 2009. Evolutionary diversification in stickleback affects ecosystem functioning. Nature 458, 1167-1170. ( 10.1038/nature07974) [DOI] [PubMed] [Google Scholar]
- 47.Turcotte MM, Reznick DN, Hare JD. 2011. The impact of rapid evolution on population dynamics in the wild: experimental test of eco-evolutionary dynamics. Ecol. Lett. 14, 1084-1092. ( 10.1111/j.1461-0248.2011.01676.x) [DOI] [PubMed] [Google Scholar]
- 48.Yoshida T, Ellner SP, Jones LE, Bohannan BJM, Lenski RE, Hairston NG Jr. 2007. Cryptic population dynamics: rapid evolution masks trophic interactions. PLoS Biol. 5, 1868-1879. ( 10.1371/journal.pbio.0050235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hiltunen T, Hairston NG Jr, Hooker G, Jones LE, Ellner SP. 2014. A newly discovered role of evolution in previously published consumer–resource dynamics. Ecol. Lett. 17, 915-923. ( 10.1111/ele.12291) [DOI] [PubMed] [Google Scholar]
- 50.Hiltunen T, Becks L. 2014. Consumer co-evolution as an important component of the eco-evolutionary feedback. Nat. Commun. 5, 5226. ( 10.1038/ncomms6226) [DOI] [PubMed] [Google Scholar]
- 51.Declerck SAJ, Malo AR, Diehl S, Waasdorp D, Lemmen KD, Proios K, Papakostas S. 2015. Rapid adaptation of herbivore consumers to nutrient limitation: eco-evolutionary feedbacks to population demography and resource control. Ecol. Lett. 18, 553-562. ( 10.1111/ele.12436) [DOI] [PubMed] [Google Scholar]
- 52.Hart SP, Turcotte MM, Levine JM. 2019. Effects of rapid evolution on species coexistence. Proc. Natl Acad. Sci. USA 116, 2112-2117. ( 10.1073/pnas.1816298116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bell G, Gonzalez A. 2009. Evolutionary rescue can prevent extinction following environmental change. Ecol. Lett. 12, 942-948. ( 10.1111/j.1461-0248.2009.01350.x) [DOI] [PubMed] [Google Scholar]
- 54.Whitham TG, et al. 2006. A framework for community and ecosystem genetics: from genes to ecosystems. Nat. Rev. Genet. 7, 510-523. ( 10.1038/nrg1877) [DOI] [PubMed] [Google Scholar]
- 55.Lande R. 1976. Natural selection and random genetic drift in phenotypic evolution. Evolution 30, 314-334. ( 10.1111/j.1558-5646.1976.tb00911.x) [DOI] [PubMed] [Google Scholar]
- 56.Abrams PA, Matsuda H. 1997. Prey adaptation as a cause of predator–prey cycles. Evolution 51, 1742-1750. ( 10.1111/j.1558-5646.1997.tb05098.x) [DOI] [PubMed] [Google Scholar]
- 57.Cortez MH, Weitz JS. 2014. Coevolution can reverse predator–prey cycles. Proc. Natl Acad. Sci. USA 111, 7486-7491. ( 10.1073/pnas.1317693111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Velzen E, Gaedke U. 2017. Disentangling eco-evolutionary dynamics of predator-prey coevolution: the case of antiphase cycles. Sci. Rep. 7, 17125. ( 10.1038/s41598-017-17019-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamamichi M, Letten AD. 2021. Rapid evolution promotes fluctuation-dependent species coexistence. Ecol. Lett. 24, 812-818. ( 10.1111/ele.13707) [DOI] [PubMed] [Google Scholar]
- 60.Schreiber SJ, Patel S, terHorst C. 2018. Evolution as a coexistence mechanism: does genetic architecture matter? Am. Nat. 191, 407-420. ( 10.1086/695832) [DOI] [Google Scholar]
- 61.Yamamichi M, Meunier CL, Peace A, Prater C, Rúa MA. 2015. Rapid evolution of a consumer stoichiometric trait destabilizes consumer–producer dynamics. Oikos 124, 960-969. ( 10.1111/oik.02388) [DOI] [Google Scholar]
- 62.Ferriere R, Legendre S. 2013. Eco-evolutionary feedbacks, adaptive dynamics and evolutionary rescue theory. Phil. Trans. R. Soc. B 368, 20120081. ( 10.1098/rstb.2012.0081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Geritz SAH, Kisdi É, Meszéna G, Metz JAJ. 1998. Evolutionarily singular strategies and the adaptive growth and branching of the evolutionary tree. Evol. Ecol. 12, 35-57. ( 10.1023/A:1006554906681) [DOI] [Google Scholar]
- 64.Kopp M, Hermisson J. 2006. The evolution of genetic architecture under frequency-dependent disruptive selection. Evolution 60, 1537-1550. ( 10.1111/j.0014-3820.2006.tb00499.x) [DOI] [PubMed] [Google Scholar]
- 65.Takahashi Y, Kagawa K, Svensson EI, Kawata M. 2014. Evolution of increased phenotypic diversity enhances population performance by reducing sexual harassment in damselflies. Nat. Commun. 5, 4468. ( 10.1038/ncomms5468) [DOI] [PubMed] [Google Scholar]
- 66.Nishikawa H, et al. 2015. A genetic mechanism for female-limited Batesian mimicry in Papilio butterfly. Nat. Genet. 47, 405-409. ( 10.1038/ng.3241) [DOI] [PubMed] [Google Scholar]
- 67.Hoso M, Kameda Y, Wu SP, Asami T, Kato M, Hori M. 2010. A speciation gene for left-right reversal in snails results in anti-predator adaptation. Nat. Commun. 1, 133. ( 10.1038/ncomms1133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Colosimo PF, et al. 2005. Widespread parallel evolution in sticklebacks by repeated fixation of Ectodysplasin alleles. Science 307, 1928-1933. ( 10.1126/science.1107239) [DOI] [PubMed] [Google Scholar]
- 69.Chan YF, et al. 2010. Adaptive evolution of pelvic reduction in sticklebacks by recurrent deletion of a Pitx1 enhancer. Science 327, 302-305. ( 10.1126/science.1182213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoekstra HE, Hirschmann RJ, Bundey RA, Insel PA, Crossland JP. 2006. A single amino acid mutation contributes to adaptive beach mouse color pattern. Science 313, 101-104. ( 10.1126/science.1126121) [DOI] [PubMed] [Google Scholar]
- 71.Hedrick PW, Ritland K. 2012. Population genetics of the white-phased ‘Spirit’ black bear of British Columbia. Evolution 66, 305-313. ( 10.1111/j.1558-5646.2011.01463.x) [DOI] [PubMed] [Google Scholar]
- 72.Johnston SE, Gratten J, Berenos C, Pilkington JG, Clutton-Brock TH, Pemberton JM, Slate J. 2013. Life history trade-offs at a single locus maintain sexually selected genetic variation. Nature 502, 93-95. ( 10.1038/nature12489) [DOI] [PubMed] [Google Scholar]
- 73.Bradshaw HD Jr, Schemske DW. 2003. Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature 426, 176-178. ( 10.1038/nature02106) [DOI] [PubMed] [Google Scholar]
- 74.Tsuchimatsu T, et al. 2010. Evolution of self-compatibility in Arabidopsis by a mutation in the male specificity gene. Nature 464, 1342-1346. ( 10.1038/nature08927) [DOI] [PubMed] [Google Scholar]
- 75.Barton NH, Etheridge AM, Véber A. 2017. The infinitesimal model: definition, derivation, and implications. Theor. Popul. Biol. 118, 50-73. ( 10.1016/j.tpb.2017.06.001) [DOI] [PubMed] [Google Scholar]
- 76.Boyle EA, Li YI, Pritchard JK. 2017. An expanded view of complex traits: from polygenic to omnigenic. Cell 169, 1177-1186. ( 10.1016/j.cell.2017.05.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oomen RA, Kuparinen A, Hutchings JA. 2020. Consequences of single-locus and tightly linked genomic architectures for evolutionary responses to environmental change. J. Hered. 111, 319-332. ( 10.1093/jhered/esaa020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamamichi M, Hoso M. 2017. Roles of maternal effects in maintaining genetic variation: maternal storage effect. Evolution 71, 449-457. ( 10.1111/evo.13118) [DOI] [PubMed] [Google Scholar]
- 79.Orr HA, Betancourt AJ. 2001. Haldane's sieve and adaptation from the standing genetic variation. Genetics 157, 875-884. ( 10.1093/genetics/157.2.875) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gillespie JH. 1973. Natural selection with varying selection coefficients – a haploid model. Genet. Res. 21, 115-120. ( 10.1017/S001667230001329X) [DOI] [Google Scholar]
- 81.Dempster ER. 1955. Maintenance of genetic heterogeneity. Cold Spring Harb. Symp. Quant. Biol. 20, 25-32. ( 10.1101/SQB.1955.020.01.005) [DOI] [PubMed] [Google Scholar]
- 82.Haldane JBS, Jayakar SD. 1963. Polymorphism due to selection of varying direction. J. Genet. 58, 237-242. ( 10.1007/BF02986143) [DOI] [Google Scholar]
- 83.Chesson PL, Warner RR. 1981. Environmental variability promotes coexistence in lottery competitive systems. Am. Nat. 117, 923-943. ( 10.1086/283778) [DOI] [Google Scholar]
- 84.Ellner S, Hairston NG Jr. 1994. Role of overlapping generations in maintaining genetic variation in a fluctuating environment. Am. Nat. 143, 403-417. ( 10.1086/285610) [DOI] [Google Scholar]
- 85.Bertram J, Masel J. 2019. Different mechanisms drive the maintenance of polymorphism at loci subject to strong versus weak fluctuating selection. Evolution 73, 883-896. ( 10.1111/evo.13719) [DOI] [PubMed] [Google Scholar]
- 86.Orr HA. 1991. Is single-gene speciation possible? Evolution 45, 764-769. ( 10.1111/j.1558-5646.1991.tb04345.x) [DOI] [PubMed] [Google Scholar]
- 87.Yamamichi M, Sasaki A. 2013. Single-gene speciation with pleiotropy: effects of allele dominance, population size, and delayed inheritance. Evolution 67, 2011-2023. ( 10.1111/evo.12068) [DOI] [PubMed] [Google Scholar]
- 88.Dobzhansky T. 1936. Studies on hybrid sterility. II. Localization of sterility factors in Drosophila pseudoobscura hybrids. Genetics 21, 113-135. ( 10.1093/genetics/21.2.113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Muller HJ. 1942. Isolating mechanisms, evolution, and temperature. Biol. Symp. 6, 71-125. [Google Scholar]
- 90.Orr HA. 1996. Dobzhansky, Bateson, and the genetics of speciation. Genetics 144, 1331-1335. ( 10.1093/genetics/144.4.1331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Orr HA, Unckless RL. 2008. Population extinction and the genetics of adaptation. Am. Nat. 172, 160-169. ( 10.1086/589460) [DOI] [PubMed] [Google Scholar]
- 92.Gomulkiewicz R, Holt RD, Barfield M, Nuismer SL. 2010. Genetics, adaptation, and invasion in harsh environments. Evol. Appl. 3, 97-108. ( 10.1111/j.1752-4571.2009.00117.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kardos M, Luikart G. 2021. The genetic architecture of fitness drives population viability during rapid environmental change. Am. Nat. 197, 511-525. ( 10.1086/713469) [DOI] [PubMed] [Google Scholar]
- 94.Yamamichi M, Ellner SP. 2016. Antagonistic coevolution between quantitative and Mendelian traits. Proc. R. Soc. B 283, 20152926. ( 10.1098/rspb.2015.2926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schiffers K, Bourne EC, Lavergne S, Thuiller W, Travis JMJ. 2013. Limited evolutionary rescue of locally adapted populations facing climate change. Phil. Trans. R. Soc. B 368, 20120083. ( 10.1098/rstb.2012.0083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Uecker H, Hermisson J. 2016. The role of recombination in evolutionary rescue. Genetics 202, 721-732. ( 10.1534/genetics.115.180299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Patel S, Bürger R. 2019. Eco-evolutionary feedbacks between prey densities and linkage disequilibrium in the predator maintain diversity. Evolution 73, 1533-1548. ( 10.1111/evo.13785) [DOI] [PubMed] [Google Scholar]
- 98.Orive ME, Barfield M, Fernandez C, Holt RD. 2017. Effects of clonal reproduction on evolutionary lag and evolutionary rescue. Am. Nat. 190, 469-490. ( 10.1086/693006) [DOI] [PubMed] [Google Scholar]
- 99.Uecker H. 2017. Evolutionary rescue in randomly mating, selfing, and clonal populations. Evolution 71, 845-858. ( 10.1111/evo.13191) [DOI] [PubMed] [Google Scholar]
- 100.Peniston JH, Barfield M, Holt RD, Orive ME. 2021. Environmental fluctuations dampen the effects of clonal reproduction on evolutionary rescue. J. Evol. Biol. 34, 710-722. ( 10.1111/jeb.13778) [DOI] [PubMed] [Google Scholar]
- 101.Doebeli M, Koella JC. 1994. Sex and population dynamics. Proc. R. Soc. B 257, 17-23. ( 10.1098/rspb.1994.0088) [DOI] [Google Scholar]
- 102.Doebeli M. 1996. Quantitative genetics and population dynamics. Evolution 50, 532-546. ( 10.1111/j.1558-5646.1996.tb03866.x) [DOI] [PubMed] [Google Scholar]
- 103.Bolnick DI, et al. 2011. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 26, 183-192. ( 10.1016/j.tree.2011.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alexander HK, Martin G, Martin OY, Bonhoeffer S. 2014. Evolutionary rescue: linking theory for conservation and medicine. Evol. Appl. 7, 1161-1179. ( 10.1111/eva.12221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carlson SM, Cunningham CJ, Westley PAH. 2014. Evolutionary rescue in a changing world. Trends Ecol. Evol. 29, 521-530. ( 10.1016/j.tree.2014.06.005) [DOI] [PubMed] [Google Scholar]
- 106.Bell G. 2017. Evolutionary rescue. Annu. Rev. Ecol. Evol. Syst. 48, 605-627. ( 10.1146/annurev-ecolsys-110316-023011) [DOI] [Google Scholar]
- 107.Gomulkiewicz R, Holt RD. 1995. When does evolution by natural selection prevent extinction? Evolution 49, 201-207. ( 10.1111/j.1558-5646.1995.tb05971.x) [DOI] [PubMed] [Google Scholar]
- 108.Abrams PA. 2000. The evolution of predator–prey interactions: theory and evidence. Annu. Rev. Ecol. Syst. 31, 79-105. ( 10.1146/annurev.ecolsys.31.1.79) [DOI] [Google Scholar]
- 109.Jones EI, Ferrière R, Bronstein JL. 2009. Eco-evolutionary dynamics of mutualists and exploiters. Am. Nat. 174, 780-794. ( 10.1086/647971) [DOI] [PubMed] [Google Scholar]
- 110.Frickel J, Sieber M, Becks L. 2016. Eco-evolutionary dynamics in a coevolving host–virus system. Ecol. Lett. 19, 450-459. ( 10.1111/ele.12580) [DOI] [PubMed] [Google Scholar]
- 111.Liou LW, Price TD. 1994. Speciation by reinforcement of premating isolation. Evolution 48, 1451-1459. ( 10.1111/j.1558-5646.1994.tb02187.x) [DOI] [PubMed] [Google Scholar]
- 112.Morita K, Yamamichi M. In press How does the magnitude of genetic variation affect ecological and reproductive character displacement? Popul. Ecol. ( 10.1002/1438-390X.12097) [DOI] [Google Scholar]
- 113.Hairston NG Jr, Ellner S, Kearns CM. 1996. Overlapping generations: the storage effect and the maintenance of biotic diversity. In Population dynamics in ecological space and time (eds Rhodes OE Jr, Chesser RK, Smith MH), pp. 109-145. Chicago, IL: University of Chicago Press. [Google Scholar]
- 114.Vellend M. 2016. The theory of ecological communities. Princeton, NJ: Princeton University Press. [Google Scholar]
- 115.Tirok K, Bauer B, Wirtz K, Gaedke U. 2011. Predator–prey dynamics driven by feedback between functionally diverse trophic levels. PLoS ONE 6, e27357. ( 10.1371/journal.pone.0027357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Klauschies T, Coutinho RM, Gaedke U. 2018. A beta distribution-based moment closure enhances the reliability of trait-based aggregate models for natural populations and communities. Ecol. Model. 381, 46-77. ( 10.1016/j.ecolmodel.2018.02.001) [DOI] [Google Scholar]
- 117.Sasaki A, Dieckmann U. 2011. Oligomorphic dynamics for analyzing the quantitative genetics of adaptive speciation. J. Math. Biol. 63, 601-635. ( 10.1007/s00285-010-0380-6) [DOI] [PubMed] [Google Scholar]
- 118.Feder ME, Mitchell-Olds T. 2003. Evolutionary and ecological functional genomics. Nat. Rev. Genet. 4, 649-655. ( 10.1038/nrg1128) [DOI] [PubMed] [Google Scholar]
- 119.Wilder AP, Palumbi SR, Conover DO, Therkildsen NO. 2020. Footprints of local adaptation span hundreds of linked genes in the Atlantic silverside genome. Evol. Lett. 4, 430-443. ( 10.1002/evl3.189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Becks L, Ellner SP, Jones LE, Hairston NG Jr. 2012. The functional genomics of an eco-evolutionary feedback loop: linking gene expression, trait evolution, and community dynamics. Ecol. Lett. 15, 492-501. ( 10.1111/j.1461-0248.2012.01763.x) [DOI] [PubMed] [Google Scholar]
- 121.Miner BE, De Meester L, Pfrender ME, Lampert W, Hairston Jr NG. 2012. Linking genes to communities and ecosystems: Daphnia as an ecogenomic model. Proc. R. Soc. B 279, 1873-1882. ( 10.1098/rspb.2011.2404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barbour MA, Kliebenstein DJ, Bascompte J. 2022. A keystone gene underlies the persistence of an experimental food web. Science 376, 70-73. ( 10.1126/science.abf2232) [DOI] [PubMed] [Google Scholar]
- 123.Good BH, McDonald MJ, Barrick JE, Lenski RE, Desai MM. 2017. The dynamics of molecular evolution over 60,000 generations. Nature 551, 45-50. ( 10.1038/nature24287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Osmond MM, Coop G. 2020. Genetic signatures of evolutionary rescue by a selective sweep. Genetics 215, 813-829. ( 10.1534/genetics.120.303173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ebert D, Fields PD. 2020. Host–parasite co-evolution and its genomic signature. Nat. Rev. Genet. 21, 754-768. ( 10.1038/s41576-020-0269-1) [DOI] [PubMed] [Google Scholar]
- 126.Bergland AO, Behrman EL, O'Brien KR, Schmidt PS, Petrov DA. 2014. Genomic evidence of rapid and stable adaptive oscillations over seasonal time scales in Drosophila. PLoS Genet. 10, e1004775. ( 10.1371/journal.pgen.1004775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rudman SM, Greenblum SI, Rajpurohit S, Betancourt NJ, Hanna J, Tilk S, Yokoyama T, Petrov DA, Schmidt P. 2022. Direct observation of adaptive tracking on ecological time scales in Drosophila. Science 375, eabj7484. ( 10.1126/science.abj7484) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ushio M, Hsieh CH, Masuda R, Deyle ER, Ye H, Chang CW, Sugihara G, Kondoh M. 2018. Fluctuating interaction network and time-varying stability of a natural fish community. Nature 554, 360-363. ( 10.1038/nature25504) [DOI] [PubMed] [Google Scholar]
- 129.Runge J, et al. 2019. Inferring causation from time series in Earth system sciences. Nat. Commun. 10, 2553. ( 10.1038/s41467-019-10105-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bálint M, Pfenninger M, Grossart HP, Taberlet P, Vellend M, Leibold MA, Englund G, Bowler D. 2018. Environmental DNA time series in ecology. Trends Ecol. Evol. 33, 945-957. ( 10.1016/j.tree.2018.09.003) [DOI] [PubMed] [Google Scholar]
- 131.Tabak MA, et al. 2019. Machine learning to classify animal species in camera trap images: applications in ecology. Methods Ecol. Evol. 10, 585-590. ( 10.1111/2041-210X.13120) [DOI] [Google Scholar]
- 132.Toju H, et al. 2018. Core microbiomes for sustainable agroecosystems. Nat. Plants 4, 247-257. ( 10.1038/s41477-018-0139-4) [DOI] [PubMed] [Google Scholar]
- 133.Sugihara G, May R, Ye H, Hsieh CH, Deyle E, Fogarty M, Munch S. 2012. Detecting causality in complex ecosystems. Science 338, 496-500. ( 10.1126/science.1227079) [DOI] [PubMed] [Google Scholar]
- 134.Chang CW, Ushio M, Hsieh CH. 2017. Empirical dynamic modeling for beginners. Ecol. Res. 32, 785-796. ( 10.1007/s11284-017-1469-9) [DOI] [Google Scholar]
- 135.Toju H, Yamamichi M, Guimarães PR Jr, Olesen JM, Mougi A, Yoshida T, Thompson JN. 2017. Species-rich networks and eco-evolutionary synthesis at the metacommunity level. Nat. Ecol. Evol. 1, 0024. ( 10.1038/s41559-016-0024) [DOI] [PubMed] [Google Scholar]
- 136.Kinnison MT, Hairston NG Jr, Hendry AP. 2015. Cryptic eco-evolutionary dynamics. Ann. N Y Acad. Sci. 1360, 120-144. ( 10.1111/nyas.12974) [DOI] [PubMed] [Google Scholar]
- 137.Crutsinger GM, et al. 2014. Testing a ‘genes-to-ecosystems’ approach to understanding aquatic–terrestrial linkages. Mol. Ecol. 23, 5888-5903. ( 10.1111/mec.12931) [DOI] [PubMed] [Google Scholar]
- 138.Tam V, Patel N, Turcotte M, Bossé Y, Paré G, Meyre D. 2019. Benefits and limitations of genome-wide association studies. Nat. Rev. Genet. 20, 467-484. ( 10.1038/s41576-019-0127-1) [DOI] [PubMed] [Google Scholar]
- 139.Hoban S, et al. 2016. Finding the genomic basis of local adaptation: pitfalls, practical solutions, and future directions. Am. Nat. 188, 379-397. ( 10.1086/688018) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.