Abstract
In recent functional genomics studies, a large number of non-coding RNAs have been identified. It has become increasingly apparent that noncoding RNAs are crucial players in a wide range of cellular and physiological functions. They have been shown to modulate gene expression on different levels, including transcription, post-transcriptional processing, and translation. This review aims to highlight the diverse mechanisms of the regulation of gene expression by small noncoding RNAs in different conditions and different types of human cells. For this purpose, various cellular functions of microRNAs (miRNAs), circular RNAs (circRNAs), snoRNA-derived small RNAs (sdRNAs) and tRNA-derived fragments (tRFs) will be exemplified, with particular emphasis on the diversity of their occurrence and on the effects on gene expression in different stress conditions and diseased cell types. The synthesis and effect on gene expression of these noncoding RNAs varies in different cell types and may depend on environmental conditions such as different stresses. Moreover, noncoding RNAs play important roles in many diseases, including cancer, neurodegenerative disorders, and viral infections.
Keywords: noncoding RNAs, gene expression, human diseases
1. Introduction
The human genome encodes only ~20,000 protein-coding genes, representing <2% of the total genome sequence. Most of the human genome consists of noncoding fragments, which have historically been regarded as junk DNA. However, the development of high-throughput technologies, such as next-generation sequencing, have allowed an in-depth examination of the noncoding genome, which revealed that the majority of genomic DNA is transcribed under some conditions. Indeed, most of the human genome is transcribed into noncoding RNAs. Moreover, some protein-coding loci also generate noncoding RNAs by alternative splicing, and in some cases the major transcript does not code a protein. Additionally, many RNAs, usually regarded as strictly protein-coding mRNAs, may also fulfill non-coding functions. Evidence from whole-genome and transcriptome sequencing suggests that the complexity of an organism may be regulated by noncoding RNAs. Consequently, the central dogma ‘DNA→RNA→protein’, which proposes that genetic information is stored in protein-coding genes, has been undermined. RNA is no longer considered to be merely an intermediary between genes and proteins. It has become increasingly apparent that noncoding RNAs are crucial players in a variety of cellular and physiological functions [1,2,3,4].
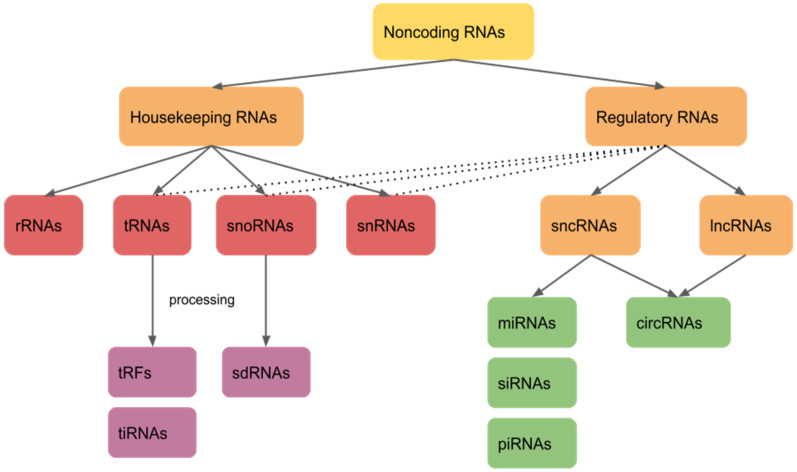
Noncoding RNAs can generally be divided into two groups: housekeeping RNAs and regulatory RNAs (Figure 1). Housekeeping RNAs are abundantly and ubiquitously expressed in cells, as they primarily regulate basic cellular functions. They include ribosomal RNAs (rRNAs) and transfer RNAs (tRNAs), which are essential elements of the translation machinery, as well as small nuclear RNAs (snRNAs) and small nucleolar RNAs (snoRNAs) implicated in diverse functions, including splicing regulation and rRNA modifications. Regulatory RNAs function as regulators of gene expression at epigenetic, transcriptional, and post-transcriptional levels. Based on their size, regulatory RNAs can be divided into two groups: small noncoding RNAs (sncRNAs), which are shorter than 200 nt, and long noncoding RNAs (lncRNAs), longer than 200 nt. Regulatory small noncoding RNAs are important regulators of gene expression in diverse biological contexts. They include, among others, microRNAs (miRNAs), small interfering RNAs (siRNAs), Piwi-associated RNAs (piRNAs), and circular RNAs (circRNAs). Furthermore, tRNAs and snoRNAs are processed into smaller fragments: tRNA-derived fragments (tRFs) and snoRNA-derived small RNAs (sdRNAs), which also have regulatory functions [5,6,7,8].
Figure 1.
Classification of noncoding RNAs. Noncoding RNAs are divided into housekeeping RNAs and regulatory noncoding RNAs. Housekeeping RNAs include rRNAs, tRNAs, snoRNAs, and snRNAs. tRNAs and snoRNAs can be further processed into tRFs and sdRNAs, respectively. Regulatory RNAs are divided into sncRNAs and lncRNAs. sncRNAs include miRNAs, siRNAs, and piRNAs. Furthermore, circRNAs can be categorized as either sncRNAs or lncRNAs based on their size.
This review aims to highlight the diverse mechanisms of the regulation of gene expression by small noncoding RNAs in different conditions and different types of human cells. For this purpose, various cellular functions of miRNAs, circRNAs, sdRNAs and tRFs will be exemplified, with particular emphasis on the diversity of their occurrence and on the effects on gene expression in different stress conditions and diseased cell types.
2. microRNAs
MicroRNAs (miRNAs) are small, evolutionary conserved, noncoding RNAs, with an average length of 22 nucleotides. miRNAs are involved in many physiological processes, such as differentiation, proliferation, apoptosis, and development. It is estimated that 60% of human mRNAs are modulated by miRNAs [9].
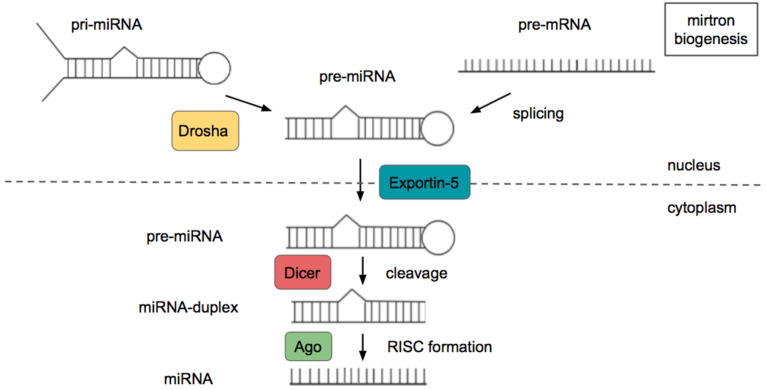
About half of miRNAs are intragenic and therefore are derived from introns of protein-coding genes. The remaining miRNAs are intergenic, derived from independent transcriptional units [10]. Biogenesis of miRNAs occurs in a two-step process requiring a nuclear and cytoplasmic cleavage event (Figure 2). Firstly, primary miRNAs (pri-miRNAs) are transcribed by RNA polymerases II (Pol II) or III (Pol III). pri-miRNAs are processed into hairpin-shaped pre-miRNAs by the nuclear “microprocessor” complex, containing the RNase III endonuclease Drosha and its interacting partner DGCR8. A subset of non-canonical miRNAs called mirtrons are produced in a splicing-dependent and Drosha-independent manner. Thus, they bypass Drosha cleavage and instead depend on splicing to produce pre-miRNA molecules. The pre-miRNA is then exported to the cytoplasm by exportin-5 and is further processed by the RNase III endonuclease Dicer, which removes the terminal loop generating a miRNA duplex. This duplex is loaded onto the Argonaute (Ago) protein. Argonaute proteins are highly conserved proteins that contain domains responsible for both binding of small noncoding RNAs and cleavage of RNA substrates, and therefore constitute a main component of the RNA-induced silencing complex (RISC). At this point the passenger strand is removed or degraded, while the other is deemed the guide strand. The selection depends on the thermodynamic properties of the miRNA [11,12,13].
Figure 2.
miRNA biogenesis.
The guide strand and Ago form the minimal RISC (miRISC). miRNA guides miRISC to specifically recognize mRNA and post-transcriptionally regulate gene expression. The recognition occurs by the miRNA seed region, bases 2–7 or 8 of miRNA, interacting through base-pairing with complementary sequences on target mRNA, called miRNA response elements (MREs). Most miRNA binding sites lie at the 3′ untranslated region (UTR) of the target mRNA. However, miRNA binding sites have also been detected in 5′ UTR sequences, as well as in coding regions, and within promoter regions. The degree of MRE complementarity determines the gene silencing mechanism. A near-perfect base-pairing enables the Ago2 endonuclease cleavage, whereas a central mismatch induces translation inhibition [11,13].
miRNA-directed mRNA cleavage induced by a high degree of sequence complementarity is catalyzed by Ago2. However, a central mismatch in the base-pairing prevents Ago2 endonuclease activity but initiates the recruitment of proteins promoting mRNA decay through deadenylation, decapping, and exonucleolytic digestion [14,15]. Moreover, miRISC can inhibit translation initiation by releasing the eukaryotic initiation factors 4A (eIF4AI and eIF4aII) from mRNA, which impedes the assembly of the eIF4F translation initiation complex [16]. Another mechanism of miRNA-mediated translation repression is the sequestering of mRNAs away from translational machinery into cytoplasmic processing bodies (P-bodies), which are the functional site of miRNA-mediated gene silencing. P-bodies lack any translational machinery and therefore are not involved in the translation process [14,15].
Mechanisms involving miRNAs can not only inhibit but also increase translation rates of proteins. miRNA-directed translation activation can depend on the cell cycle state and proteins bound to the Ago2-miRNA complex. In serum-starved cells, the Ago2-miRNA complex associated with AU-rich elements at the 3′ UTR recruited the FXR1 (Fragile-X-mental retardation related protein 1) and activated translation [17]. Several miRNAs associate with Ago2 and FXR1 to activate translation during cell cycle arrest, but they inhibit translation in proliferating cells [10]. Specific mRNAs are also translationally upregulated in quiescent cells, such as oocytes, by an FXR1a-associated miRNA-protein complex [18]. Furthermore, miRNAs that bind to the 5′ UTR can also enhance protein synthesis. For example, miR-10a interacts with the 5′ UTR of mRNAs encoding ribosomal proteins and enhances their translation during amino acid starvation [19].
Most of the miRNA-mediated regulation of gene expression occurs in the cytoplasm and the P-bodies. However, miRNAs and Ago have also been discovered in the nucleus of human cells [20]. Human promoters contain miRNA-seed matching sites, which suggests that miRNA-mediated transcription regulation may be a common phenomenon [21]. An interaction between the promoter and miRNA may either suppress or activate transcription depending on the location of the target region and epigenetic status of the promoter [22], which is called transcriptional gene silencing (TGS) or transcriptional gene activation (TGA), respectively (Table 1). As an example, Ago2 and let-7 are involved in transcriptional repression of proliferation-promoting genes in senescence [23]. Moreover, nuclear miR-522 suppresses transcription of CYP2E1 gene by interacting with its promoter forming a DNA:RNA hybrid to prevent Pol II and transcription factor binding to the promoter [24]. miR-223 also forms a DNA:RNA hybrid by targeting the NFI-A promoter region containing miR-223 complementary sequences during human granulopoiesis. It represses transcription of the NFI-A gene, which is an important step of granulocytic differentiation [25]. On the other hand, several miRNAs including let-7i, miR-138, miR-92a, and miR-181d bind to the TATA-box and enhance the promoter activities of interleukin-2, insulin, calcitonin, or c-myc, respectively [26]. Additionally, miRNA-373 activates transcription of E-cadherin and CSDC2 genes through enrichment of Pol II at their promoters [27]. Moreover, a subset of miRNAs deriving from the enhancer loci can induce expression of neighboring genes and function as enhancer regulators. For example, miR-24-1 increases expression of neighboring genes FBP1 and FANCC by targeting enhancers. Increased miR-24-1 expression causes direct chromatin state alteration of the FBP1 enhancer, which activates transcription. Interestingly, when miR-24-1 is located in the cytoplasm it would still be able to function as a repressor for its target genes. Thus, miRNA can carry a dual function ability: activation in the nucleus and repression in the cytoplasm [28].
Table 1.
Examples of miRNA-mediated transcription regulation in human cells.
| miRNA | Function | miRNA Target | miRNA Mechanism of Action | References |
|---|---|---|---|---|
| miR-522 | TGS | CYP2E1 promoter | DNA:RNA hybrid with promoter | [24] |
| miR-223 | TGS | NFI-A promoter | DNA:RNA hybrid with promoter | [25] |
| let-7i | TGA | Interleukin-2 promoter | Binds to TATA-box and enhances promoter activities | [26] |
| miR-138 | TGA | Insulin promoter | Binds to TATA-box and enhances promoter activities | [26] |
| miR-92a | TGA | Calcitonin promoter | Binds to TATA-box and enhances promoter activities | [26] |
| miR-181d | TGA | C-myc promoter | Binds to TATA-box and enhances promoter activities | [26] |
| miR-373 | TGA | E-cadherin, CSDC2 | Pol II enrichment at promoters | [27] |
| miR-24-1 | TGA | FBP1, FANCC | Chromatin state alteration of the FBP1 enhancer | [28] |
TGS-transcriptional gene silencing, TGA-transcriptional gene activation.
Furthermore, miRNAs are also involved in the nuclear splicing program. In one mechanism, they can indirectly modulate alternative splicing by regulating translation of various splicing factors. In another pathway, Ago-miRNA complexes can regulate alternative splicing directly in the nucleus by epigenetic and non-epigenetic mechanisms. Ago2 and miRNAs binding sites were identified in the intronic sequences in human brain samples and human myocardial cells [29,30]. The proposed mechanism involves miRNA-mediated compaction of chromatin structure at specific intron-exon junctions, which slows the rate of Pol II elongation favoring exon inclusion [31].
2.1. miRNAs in Stress Conditions
miRNAs play key roles in mediating cellular stress responses to pathophysiological and physiological conditions, including oxidative stress, DNA damage, and oncogenic stress. miRNA biogenesis and regulating networks may be disrupted by stressors and the resulting changes in miRNA levels may induce significant physiological effects by regulating transcription factors and other signaling molecules [32,33]. An example of this mechanism is the DNA damage response. The p53 tumor suppressor gene is a key regulator of the miR-34 family genes [34]. p53 expression level is regulated by its ubiquitin-mediated degradation and repression by miR-125b [35]. A wide range of stresses, including DNA double-strand breaks, activate p53 to arrest the cell cycle for DNA repair. Active p53 induces the transcription of miR-34a and miR-34b/c genes, which repress the expression of target genes to promote the induction of apoptosis, cell cycle arrest, and senescence [36]. Therefore, miR-34a has a complex effect on the p53 response. Moreover, miR-34a targets TP53 and MDM4, a strong p53 transactivation inhibitor, and four other post-translational inhibitors of p53. In HCT116 cells, miR-34a overexpression increases p53 protein levels and stability. However, the p53-mediated response to genotoxic stress is unimpaired in HCT116 and MCF7 miR-34a knock-out cells. The complex functional relationship between miR-34a and p53 suggests that miR-34a might act at a systems level to stabilize the p53 response to genotoxic stress [37].
Furthermore, stress conditions affecting the endoplasmic reticulum (ER) can lead to an imbalance between the demand for protein folding and the capacity of the ER protein folding, causing ER stress. Due to the accumulation of misfolded or unfolded proteins, affected cells launch the unfolded protein response (UPR). This intracellular signaling mechanism involving miRNAs can have either pro-adaptive or pro-apoptotic roles. X-box binding protein 1 (XBP1) is a pro-adaptive transcription factor that enhances secretory capacity and promotes cell survival in the adaptive UPR [32]. In a prolonged UPR, ER stress-induced apoptosis is initiated [38]. miR-30c-2-3p is upregulated during UPR and targets the 3′ UTR of XBP1 mRNA, thereby limiting the expression of XBP1 and the survival of cells experiencing ER stress. By limiting the induction of XBP1 mRNA, XBP1 protein, and XBP1-dependent target genes, miR-30c-2-3p could contribute to the balance between pro- and maladaptive outcomes in the UPR, considering the possible deleterious effects of excessive XBP1 expression [39]. Furthermore, several miRNAs influence apoptotic signaling pathways in ER stress. miR-211-5p and miR-204-5p regulate the expression of several ER stress markers, particularly the pro-apoptotic transcription factor CHOP. Blocking of endogenous miRNA-211-5p and miR-204-5p increases human beta cell apoptosis [40]. Furthermore, miR-204 was implicated in ER stress-responsive gene modulation and apoptosis susceptibility in human trabecular meshwork cells. miR-204 inhibited the induction of ER-stress response markers. Overexpression of miR-204 increased apoptosis and cell death in response to oxidative stress and ER stress [41]. miR-96-5p, another miRNA involved in the response to ER stress, is involved in the non-cholinergic toxicity of malathion in normal human kidney cells (HK-2 cells). miR-96-5p protects HK-2 cells from malathion-induced ER stress-dependent apoptosis by targeting DDIT3, a well-known marker of ER stress [42].
2.2. miRNAs in Cancer
Cancer progression is a multistep process including mutation and selection for cells with increasingly enhanced proliferative, survival, invasion, and metastatic capacities. Cancer cells have several distinct characteristics enabling their autonomous excessive proliferation. They can proliferate independently of growth signals and are unresponsive to inhibitory growth signals, resulting in limitless replicative potential. Furthermore, cancer cells evade apoptosis, induce and sustain angiogenesis, and form new colonies discontinuous with the primary tumor [43].
All tumors present specific signatures of altered miRNAs expression. Generally, downregulation of miRNA expression is observed in many cancers, although upregulated expression of some miRNAs can also be directly correlated with tumor development. In addition to the distinction of tumors from normal tissues, miRNA expression is characteristic for a cancer type, stage, and other clinical variables [44]. Different miRNAs can act as tumor suppressor genes and/or oncogenes (Table 2).
Table 2.
Examples of miRNAs as tumor suppressors and oncogenes in cancer.
| miRNA | miRNA Expression in Cancer | miRNA Target | Function | Type of Cancer | References |
|---|---|---|---|---|---|
| let-7 | Downregulated | PD-L1, HMGA1, apoptotic genes | tumor suppressor | various cancers | [45] |
| miR15/16 | Downregulated | BCL2 | tumor suppressor | CLL | [46] |
| miR-21 | Upregulated | Maps, PDCD4, TPM1, PTEN | oncogene | lung, breast, and bladder cancer | [47,48,49] |
| miR-155 | Upregulated | signaling pathways (TGF-β, JAK-STAT) | oncogene | breast cancer | [50,51] |
| miR-125b | upregulated/downregulated | multiple mRNAs with diverse functions in different tissues | oncogene/tumor suppressor | colon cancer, hematopoietic tumors/NSCLC, breast cancer | [52,53,54] |
| miR-17-92 | upregulated/downregulated | E2F transcription factors/AIB1, ETV1, ERBB3 | oncogene/tumor suppressor | lymphoma, lung cancer, colon cancer, pancreatic cancer, prostate cancer, HNSCC/breast cancer, HCC | [55,56,57,58,59] |
let-7 is one of the earliest discovered miRNAs. It is considered a tumor suppressor gene partly due to its normal physiological role in arresting cell development [45]. While let-7 is almost absent during embryonic stages or tissues, it is highly expressed in most differentiated tissues. The reduction of let-7 in cancers is similar to let-7 expression during development, as its expression is lowest in less differentiated, advanced stages of cancer [60]. let-7 targets transcripts critical for DNA replication such as PD-L1 (programmed cell death ligand 1) and HMGA2 (high mobility group AT-hook 2), as well as apoptotic genes [45]. Moreover, the miR15/16 cluster acts as a tumor suppressor by targeting BCL2 mRNA. The oncogenic BCL2 protein, commonly overexpressed in various cancers, promotes cell survival by evading apoptosis. In normal conditions, miR-15/16 directly targets and negatively regulates BCL2, inducing intrinsic apoptosis pathways [46]. However, the miR-15a/16-1 cluster is a frequently deleted region in B cell chronic lymphocytic leukemia (CLL) and other cancers, resulting in higher expression of BCL2 oncogene [61].
On the contrary, some miRNAs have been found to function as oncogenes. miR-21 downregulates four tumor suppressors: mapsin, PDCD4 (programmed cell death 4), TMP1 (tropomyosin1), and PTEN (phosphatase and tensin homolog). miR-21 binds to the 3′ UTR of the gene transcripts, preventing their translation. This in turn promotes cell transformation, tumor growth, invasion, and metastasis [55]. miR-21 overexpression was observed in a variety of cancers including lung, breast, and bladder cancer [47,48,49]. miR-155 is also known to be oncogenic in multiple tumors. Several signaling pathways, such as TGF-β and JAK-STAT, are under the control of miR-155 [50]. In breast cancer cells, miR-155 was shown to enhance tumor growth, promote cell proliferation and inhibit apoptosis [51]. Stimulation of breast cancer cells by inflammatory cytokines significantly upregulates miR-155 expression, suggesting that miR-155 may serve as a bridge between inflammation and cancer [62].
Because of the miRNA expression patterns, which can differ for specific tissues and differentiation states, and the fact that a single miRNA can regulate multiple targets, some miRNAs can function as both tumor suppressors and oncogenes in different contexts. miRNA-125b is upregulated in some tumor types, such as colon cancer and hematopoietic tumors, and displays oncogenic potential, as it induces cell growth and proliferation while blocking the apoptotic machinery. However, in other tumor entities, including non-small cell lung cancer (NSCLC) and breast cancer, miRNA-125b is heavily downregulated, which is accompanied by de-repression of cellular proliferation and anti-apoptotic programs, contributing to malignant transformation. These opposing roles might be explained in that miR-125b targets multiple mRNAs, which have diverse functions in individual tissues. miR-125b is regulated by multiple factors, and the interaction of miR-125b with its targets constitutes their regulatory network. Therefore, because of the complexity of this network, miRNA-125b has a wide range of cellular functions, depending on the context [52,53,54].
Similarly, the miR-17-92 cluster also has a dual role as both an oncogene and tumor suppressor. This cluster is highly elevated in a variety of lymphomas, as well as lung, colon, pancreas, and prostate cancers [55]. The miR-17-92 cluster constitutes a very complex regulatory network with c-myc and E2F transcription factors, which are critical regulatory components for apoptosis and cell proliferation. miR-17-5p and miR-20a of the miR-17-92 cluster inhibit the translation of E2F mRNA [56]. Furthermore, miR-17-5p acts as a tumor promoter and prognostic factor for recurrence in head and neck squamous cell carcinoma (HNSCC) [57]. However, in some cases, the miR-17-92 cluster was found to act as a tumor suppressor. miR-17-5p downregulates the proto-oncogenic transcriptional activator AIB1 (amplified in breast cancer 1), which is involved in breast cancer proliferation, growth, and hormone signaling [55]. miR-17-5p also acts as a tumor suppressor by targeting the ETV1 (ETS variant 1) transcription factor, which promotes triple-negative breast tumor cell proliferation, invasion, and migration. The expression of miR-17-5p in triple-negative breast cancer (TNBC) cell lines reduced the expression of ETV1 and consequently inhibited cell proliferation, migration, and invasion, which might suppress the development of TNBC. High miR-17-5p expression was associated with a significantly favorable prognosis [58]. Moreover, miR-17-5p and miR-20a-5p from the miR-17-92 cluster play inhibitory roles on hepatocellular carcinoma (HCC) metastasis. miR-17-5p and miR-20a-5p both target the 3′ UTR of the oncogene ERBB3. They could suppress postoperative metastasis of HCC [59].
Aside from the miRNA-mediated post-transcriptional regulation, nuclear miRNAs, which mainly act upon the promoter region, can regulate the transcription of tumor-suppressor genes, oncogenes, or other cancer-related genes. The miR-877-3p binding site was found on the promoter of tumor suppressor gene p16. Enforced expression of miR-877-3p can increase the expression of p16, which inhibits proliferation and tumorigenicity of bladder cancer through cell cycle G-1 phase arrest [63]. In turn, miR-6734 inhibits the growth of colon cancer cells by upregulating p21 gene expression, which leads to induction of cell cycle arrest and apoptosis [64]. On the other hand, miR-483 is an oncogenic intronic miRNA that binds to IGF2 (imprinted insulin-like growth factor 2) gene promoter. Ectopic expression of miR-483 induces upregulation of IGF2 expression, as well as an increase in tumor cell proliferation, migration, invasion, and tumor colony formation in HCC [65].
2.3. miRNAs in Viral Infections
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in December 2019 in China and caused the global pandemic of coronavirus disease 2019 (COVID-19) [66]. The plasma miRNA profile is deeply disrupted by SARS-CoV-2 infection, making miRNAs highly valuable as early prognostic biomarkers of severity and mortality [67]. The differential miRNA expression in COVID-19 patients may regulate the immune responses and viral replication during infection. COVID-19 exploits the interplay between miRNA and other biomolecules to avoid being effectively recognized and attacked by the host’s immune system. SARS-CoV-2 adsorbs host immune-related miRNAs and participates in the maladjustment of the host’s immune systems. The virus also encodes its own miRNAs, which can enter the host cell and are not perceived by the host’s immune system [68,69,70].
Viral miRNAs can either interact with specific regions of their own genome or transcript or bind to host miRNAs and genes during virus infection [70]. Six SARS-CoV-2 miRNAs were predicted to interact with human miRNAs targeting immune genes and result in cytokine storm, characterized by the excessive release of pro-inflammatory cytokines [71,72]. SARS-CoV-2 miRNAs have been shown to inhibit ribosomal translation of some key human proteins by hybridizing their mRNAs. This inhibition concerns, for example, the hemoglobin and type I interferon protein synthesis, hence highly perturbing oxygen distribution in vital organs and immune response in COVID-19 patients [73]. Furthermore, a virus-encoded miRNA MR147-3p can enhance the TMPRSS2 gene expression in the gut. TMPRSS2 is involved in the activation of the spike protein of the virus binding to the ACE2 (angiotensin-converting enzyme 2) receptor. Consequently, the virus-derived MR147-3p facilitates effective evasion of the virus into gut cells [74]. Host miRNAs might block attachment and entry of SARS-CoV-2 because several miRNA families target ACE2 and TMPRSS2 genes in different tissues. For instance, miR-200c suppresses ACE2 mRNA and protein expression in cardiomyocytes [75]. hsa-miR-98-5p targets TMPRSS2 3′ UTR inhibiting transcription of TMPRSS2 gene in human lung microvascular endothelial cells and human umbilical vein endothelial cells [76]. In addition, many human miRNAs target and inhibit viral genes involved in replication, translation, and protein synthesis by interacting with MREs within the 5′ UTRs and 3′ UTRs of the viral genome [77]. For instance, miR-1307-3p and miR-3613-5p were predicted to prevent virus replication by binding to 3′ UTRs of its genome [78].
However, SARS-CoV-2 infection-induced host miRNAs can also function as pro-viral factors by targeting several important host immune surveillance pathways [79,80]. One of the pathways downregulated by host-miRNAs during SARS-CoV-2 infection is the signaling of different toll-like receptors (TLRs), which are the primary stimulatory molecules for producing host antiviral responses. Other receptor signaling involved in antiviral responses, including uPA-UPAR signaling, TRAF6 signaling, S1P1 signaling, Estrogen receptor signaling, protease-activated receptor (PAR) signaling, and bone morphogenetic protein (BMP) signaling, can also be deregulated by the host miRNAs, leading to the host’s immune suppression [69].
Alterations in the expression of miR-200c-3p and miR-421-5p may influence the outcomes of COVID-19 infection. These miRNAs are considered to modulate the expression of the ACE2 gene. The expression levels of miR-200c-3p and miR-421-5p were significantly decreased, while inflammatory cytokine IL-6 expression was enhanced in COVID-19 patients at the time of admission. Targeting miR-200c-3p and miR-421-5p can maintain the level of ACE2 and modulate the inflammation [81].
2.4. miRNAs in Neurological Disorders
miRNAs are important regulators of intracellular pathways for various neurodegenerative conditions. Altered levels of numerous miRNAs were found in several pathological conditions including Alzheimer’s disease (AD) and Parkinson’s disease (PD) [82].
Alzheimer’s disease (AD) is the most common age-related neurodegenerative disorder. In AD, the accumulation of amyloid β peptides in plaques and the presence of phosphorylated Tau aggregates in neurofibrillary tangles are observed. miRNAs were found to regulate the expression levels of amyloid beta precursor protein (APP) [83]. miR-106a, miR-520c, miR-20a, miR-17-5p, and miR-106b bind to the 3′ UTR of APP mRNA to inhibit its translation, and thus reducing APP protein levels [84,85]. On the contrary, miR-346 promotes APP translation through interaction with its 5′ UTR. This interaction prevents the recruitment of a translation suppressor aconitase 1, indirectly stimulating APP translation [86]. Furthermore, miRNAs can regulate different steps of Tau processing. miR-15 members downregulate the mitogen-activated protein kinases 1 and 3 (MAPD1/3), which phosphorylate Tau [83]. miR-26a regulates another protein kinase, the glycogen synthetase kinase 3 beta (GSK3B), which also hyperphosphorylates Tau. GSK3B is associated with the generation of amyloid β and formation of neurofibrillary tangles in AD brains [87]. Moreover, miRNAs may be promising biomarkers in the early diagnosis of AD. For instance, the expression levels of miR-4722-5p, miR-615-3p, hsa-let7d-5p, and hsa-let7g-5p were significantly increased in AD patients. Therefore, measuring the transcript levels of these miRNAs can be used as a diagnostic biomarker for AD [88,89].
Parkinson’s disease (PD) is characterized by bradykinesia, muscle stiffness, postural instability, and involuntary tremors. These signs are related to the loss of dopaminergic neurons in the substantia nigra and the pathology spreading to other regions of the brain. The α-synuclein protein elicits the insoluble aggregates that compose the main structure of Lewy bodies leading to the death of dopaminergic neurons [90]. miR-7 inhibits α-synuclein expression directly through the 3′ UTR of α-synuclein mRNA. By downregulating the α-synuclein level, miR-7 protects cells against oxidative stress. In PD, miR-7 expression decreases, possibly contributing to increased α-synuclein expression [91]. Moreover, overactivation of microglia is one of the critical pathophysiological mechanisms underlying PD. miRNAs can affect the progress of PD by regulating the expression of various microglia genes and the polarization state of the microglia [92]. For example, miR-132-3p promotes neuroinflammation and dopaminergic neurodegeneration by enhancing the activation of microglia cells. Elevated expression of miR-132-3p, as well as decreased expression of its target GLRX, were found in PD patients and cell models. miR-132-3p-induced activation of microglia cells can be reversed by GLRX overexpression. Suppression of miR-132-3p alleviates neuroinflammation and dopaminergic neurodegeneration in PD mouse models [93].
3. Circular RNAs
Circular RNAs (circRNAs) are single-stranded RNAs with the 3′ and 5′ ends covalently linked. They originate from over 14% of transcribed genes in human fibroblasts. circRNAs are abundant and in some cases, they demonstrate higher expression than the associated mRNA. Large numbers of circRNAs can accumulate in the cytoplasm of cells because of their resistance to degradation by mechanisms recognizing the ends of linear RNAs. The lack of 5′–3′ polarity and a polyadenylated tail gives them a higher tolerance to endonucleases, making them highly stable, with transcript half-lives exceeding 48 h [94,95].
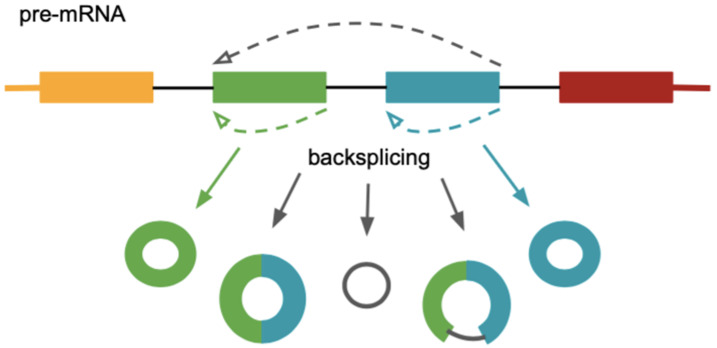
Circular RNAs are generally formed by alternative splicing of pre-mRNA in a process known as ‘backsplicing’, in which an upstream splice acceptor is joined to a downstream splice donor (Figure 3). Nearly all circular RNAs contain exonic sequences, usually between one and five exons. The canonical splicing machinery functions in their biogenesis as both canonical splice sites are required for exon circularization. However, the exact mechanism of circRNA biogenesis and regulatory factors involved in circularization is still unclear. Proposed models include exon skipping and intron lariat formation, the presence of inverted repeats and intron pairing, as well as interaction with RNA-binding proteins (RBPs). Based on the biogenesis features and their structural domains, circRNAs can be classified into three types: exonic circRNAs (ecircRNAs), localized predominantly in the cytoplasm, circular intronic RNAs (ciRNAs), and exon-intron circRNAs (EIcircRNAs), both found mainly in the nucleus [96,97].
Figure 3.
circRNAs formation by backsplicing (pre-mRNA splicing at a reversed order in which a downstream splice donor is joined to an upstream splice acceptor).
circRNAs are produced in a tissue-specific and developmental stage-regulated manner. Over 50% of them are tissue-specific and their number and expression levels are higher in fetal tissue than in adult tissues. Many circRNAs are highly abundant in the brain and their levels increase during neuronal differentiation and development. Large amounts of circRNAs are accumulated in neuronal tissues with aging. Consistent with these findings, a negative correlation of global circRNA abundance and proliferation was suggested, which seems to be a general principle in human tissues. Therefore, circRNAs have higher expression levels in low-proliferating cells such as cardiomyocytes compared to the high-proliferating cells of the liver [98,99,100].
Circular RNAs can regulate gene expression at both the transcriptional and post-transcriptional levels. ciRNA and EIcircRNA are mostly localized in the nucleus, where they can act as transcriptional regulators. EIcircRNAs can regulate transcription because they retain the intronic sequences from their parental gene [101]. Two EIcircRNAs, circEIF3J and circPAIP2 can interact with U1 small nuclear ribonucleoproteins (U1 snRNPs) and promote transcription of host genes by binding to Pol II [102]. In addition, ciRNA were shown to associate with the elongation Pol II machinery to activate the transcription of their parent genes [103]. For circRNAs containing intronic sequences, transcriptional activation may be their general function, which would likely explain their nuclear localization [104]. ecircRNAs that consist of exons can play a role in alternative splicing since circularization competes with canonical splicing and thus impacts linear RNA formation to modulate the transcription of related genes. The disturbed balance between circular and linear splicing can promote aberrant transcription of oncogenes or tumor-suppressor genes in cancer. Moreover, the formation of an ecircRNAs can act as an ‘mRNA trap’ by sequestering the translation start site and leaving a noncoding linear transcript. This mechanism could be very widespread, as many single ecircRNAs in human fibroblasts contain a translation start [105].
circRNAs were found to act as protein sponges, scaffolds, decoys, and recruiters. Some of them can regulate gene expression by interacting with proteins to either promote or inhibit translation. Many circRNAs contain miRNA response elements and act as competing endogenous RNAs to restrain miRNAs from negatively regulating their target mRNAs. Thus, by acting as miRNA sponges, circRNA can indirectly regulate the translation of mRNAs [100,105].
3.1. circRNAs in Stress Conditions
Circ_0010729 participates in the regulation of hypoxia-induced cardiomyocyte injuries by mediating the miR-27a-3p/TRAF5 axis. Hypoxia inhibits cell viability, induces apoptosis, and blocks glycolysis. Circ_0010729 targets miR-27a-3p, which in turn upregulates the expression of the tumor necrosis factor receptor-associated factor 5 (TRAF5). Therefore, miR-27a-3p inhibition leads to the deterioration of hypoxia-induced cardiomyocyte injuries and consequently, these injuries were alleviated by circ_0010729 knockdown [106]. Moreover, the hypoxia-induced upregulation of circ-0010729 was shown to regulate vascular endothelial cell proliferation and apoptosis via targeting the miR-186/HIF1α axis. Knockdown of circ-0010729 suppressed the proliferation and migration ability and enhanced apoptosis [107].
Hsa_circ_0005915 is significantly upregulated in HL-7702 liver cells under oxidative stress. Hsa_circ_0005915 downregulates the expression of nuclear factor erythroid-2-related factor 2 (NRF2) by promoting its ubiquitination and degradation, which leads to increased accumulation of reactive oxygen species (ROS). Overexpression of has_circ_0005915 causes a decline of the expression levels of NRF2-regulated antioxidative genes: heme oxygenase 1 (HO1) and NAD(P)H quinone dehydrogenase 1 (NQO1) [108].
Circ-RBMS1 was found to be highly expressed in chronic obstructive pulmonary disease (COPD) patients. Cigarette smoke extract (CSE) increases expression of circ-RBMS1 in a dose-dependent manner. Knockdown of circ-RBMS1 alleviates CSE-induced oxidative stress, inflammation, and apoptosis. Circ-RBMS1 targets miR-197-3p, which in turn targets the F-box only protein 11 (FBXO11). FBXO11 promotes the degradation of proteins critical in regulation of the cell cycle, such as BCL6, SNAIL and p53. Therefore, by acting as a sponge for miR-197-3p, circ-RBMS1 positively regulates FBXO11 expression in bronchial epithelial cells, enhancing CSE-induced apoptosis, inflammation, and oxidative stress [109,110].
3.2. circRNAs in Cancer
FLI1 exonic circular RNA (FECR1) formed by the proto-oncogene Friend leukemia virus integration 1 (FLI1) was identified upregulated in advanced metastatic breast cancer. FECR1 uses a positive feedback mechanism to activate FLI1 by inducing DNA hypomethylation in CpG islands of the promoter. FECR1 recruits TET1, a demethylase to the promoter, and downregulates DNMT1, a methyltransferase essential for the maintenance of DNA methylation. By regulating the DNA methylating and demethylating enzymes, FECR1 acts as an upstream regulator to control breast cancer tumor growth. Overexpression of FECR1 was shown to enhance the invasiveness of breast cancer cells [111]. Similarly, in gastric cancer (GC), circ-DONSON recruits the NURF complex to the SOX4 promoter and initiates its transcription, thus promoting the proliferation, migration, and invasion of GC cells [112]. circAMOTL1 can interact with the proto-oncogene transcription factor c-myc increasing its retention in the nucleus and promoting its stability. Consequently, circAMOTL1 upregulates the expression of c-myc targets, including HIF-1α, Cdc25a, ELK-1, and JUN [113]. circYAP negatively regulates the yes-associated protein (Yap) translation by suppressing the assembly of Yap translation initiation machinery [114]. Yap activation can promote proliferation, inhibit apoptosis, and facilitate the metastasis of cancer cells [105].
ciRS-7 (also known as CDR1as), the circRNA sponge for miR-7, contains over 70 miR-7 binding sites and is expressed in different tissues and organs. By recruiting miR-7, ciRS-7 inhibits its function and upregulates the expression of related genes, which are central oncogenic factors in cancer-associated signaling pathways. Therefore, ciRS-7 acts as an oncogene and promotes tumor progression through competitively inhibiting miR-7 (Table 3). ciRS-7 is upregulated in several cancers [94,115]. ciRS-7 levels increase with the development of NSCLC and negatively correlate with the expression of miR-7 but positively correlate with the expression of its key target genes and consequently promote the tumor progression in NSCLC [116]. ciRS-7 is also upregulated in colorectal cancer (CRC). ciRS-7 overexpression impairs tumor suppressive effects of miR-7 in CRC cells, resulting in a more aggressive oncogenic phenotype. Overexpression of ciRS-7 was associated with poor patient survival and emerged as an important risk factor for overall survival [117].
Table 3.
Examples of circRNAs acting as miRNA sponges and their functions in various types of cancer.
| circRNA | miRNA Target | Disease Associated | circRNA Expression | circRNA Function | References |
|---|---|---|---|---|---|
| ciRS-7 (CDR1as) | miR-7 | Several cancers including NSCLC and CRC | upregulated | oncogene | [94,115,116,117] |
| circHIPK3 | miR-7, miR-124, miR-506 | CRC, GBC, HCC | upregulated | oncogene | [118,119,120] |
| circITCH | miR-7, miR-214 | CRC, lung cancer, NPC | downregulated | tumor suppressor | [104,121,122] |
| circNT5E | miR-422a, miR-134, miR-502-5p | Glioma, NSCLC, bladder cancer | upregulated | oncogene | [123,124,125] |
| circDENND2A | miR-625-5p | Glioma | upregulated | oncogene | [126] |
| circDENND4C | miR-200b, miR-200c, miR-141-3p | Breast cancer, NSCLC, lung cancer | upregulated | oncogene | [127,128] |
Likewise, circHIPK3 is also upregulated in CRC and was found to sponge the tumor suppressor miR-7. Consequently, circHIPK3 has a tumor-promoting role in CRC [118]. Furthermore, elevated expression of circHIPK3 was found in gallbladder cancer (GBC) cell lines. circHIPK3 sponges the tumor-suppressive miR-124 leading to an increased expression of miR-124 targets, including ROCK1 (rho-associated protein kinase 1) and CDK6 (rho-associated protein kinase) [119]. In HCC, circHIPK3 sponges miR-124 and miR-506 to upregulate PDK2, which results in accelerated proliferation and invasion of HCC cells [120].
circITCH derives from the ITCH gene, which encodes a member of the E3 ubiquitin-protein ligase family and some of its targets are associated with tumor formation and cancer progression. circITCH has multiple binding sites, which were also found in the 3′ UTRs of ITCH transcripts. Therefore, the sponging of target miRNAs by circITCH regulates ITCH levels. The presence of circITCH in cells leads to an increase in ITCH mRNA levels resulting in greater ITCH ubiquitin activity, which decreases the activity of oncogenic factors [129]. In CRC, the expression of circITCH was downregulated in cancerous cells, resulting in low levels of ITCH gene expression. In addition, circITCH is likely involved in a signaling pathway regulating cell proliferation and migration and may have an anti-proliferative role in CRC [121]. Furthermore, circITCH plays a crucial role in suppressing lung cancer progression by functioning as a sponge for miR-7 and miR-214 [104]. The circITCH/miR-214 axis regulates nasopharyngeal carcinoma (NPC) proliferation, migration, and invasion through regulating the expression of PTEN, a direct target of miR-214. circITCH suppresses NPC tumorigenesis by upregulating PTEN expression through inhibiting miR-214. In NPC, the level of circITCH was decreased, while the level of miR-214 was increased [122].
Another circRNA, circNT5E, is an oncogene in glioma. circNT5E acts as a sponge of the brain-enriched miR-422a, which acts as a tumor suppressor by suppressing proliferation and invasion and inducing apoptosis in glioblastoma cells. circNT5E binds miR-422a and inhibits its activity, thus promoting the pathological development of human glioblastoma [123]. In NSCLC, circNT5E levels are significantly elevated, while its target miR-134 is downregulated, which promotes progression of NSCLC [124]. circNT5E was also found to be elevated in bladder cancer tissues and its expression level correlated with larger tumor size and lower survival rate. In this case, circNT5E sponges miR-502-5p to increase the expression of HOXC8, which promotes tumor growth and metastasis [125].
The expression of circRNAs can be induced by hypoxia, which is a key feature of the tumor microenvironment and impacts the cancer aggressiveness and therapy [130]. Hypoxia induces the expression of circDENND2A in glioma, which sponges the tumor suppressor miR-625-5p. miR-625-5p inhibits proliferation and increases the chemosensitivity of glioma cells. By sponging miR-625-5p, circDENND2A promotes the migration and invasion of glioma cells [126]. circDENND4C, highly expressed in breast cancer, is upregulated in response to hypoxia. It regulates breast cancer progression by sponging miR-200b and miR-200c. miR-200b and miR-200c serve as important tumor suppressors by inhibiting cell proliferation, migration, and invasion in breast cancer. Knockdown of circDENND4C suppresses glycolysis, migration, and invasion in breast cancer cells under hypoxia by increasing miR-220b and miR-200c [127]. circDENND4C was also found to act as an oncogene in other types of cancer. For example, in lung cancer circDENND4C upregulates BRD4 by sponging miR-141-3p, thereby promoting metastasis and proliferation of NSCLC [128].
3.3. circRNAs in Viral Infections
Viral infections can change the expression profile of circRNAs in the host cells. However, the genome of many viruses can induce circRNAs to regulate viral infections and pathogenesis.
Altered expression of circRNAs was found during human cytomegalovirus (HCMV) latent infection. 1421 differentially expressed circRNAs were identified in latently infected human leukemia monocytes (THP-1 cells). Those circRNAs mainly targeted genes involved in the regulation of secretion pathways, cell cycle, and apoptosis. A potential target of the differentially expressed circRNAs could be hsa-miR-21, an important intrinsic antiviral factor that is regulated by HCMV infection. hsa-miR-21 plays an important role in regulating the cell cycle, controlling tumor growth, and reducing apoptosis [131].
circPSD3 is upregulated in hepatitis C virus (HCV) infected liver cells. circPSD3 displays a pro-viral effect and promotes replication of HCV genotype 1 and 2. circPSD3 sequesters eIF4A3, thereby inhibiting the cellular nonsense-mediated decay (NMD) pathway in HCV-infected liver cells and aiding in viral replication [132].
Recently, 3437 circRNAs encoded by SARS-CoV-2 were identified. In SARS-CoV-2, viral circRNAs downregulate genes associated with metabolic processes of cholesterol, alcohol, sterol, and fatty acid and upregulate genes associated with cellular responses to oxidative stress in the late stage of viral infection. Several genes regulated by viral circRNAs participate in biological processes such as response to reactive oxygen and centrosome localization [133]. Moreover, 6118 circRNAs were identified in human lung epithelial cells infected with SARS-CoV-2, including 477 novel circRNAs. These circRNAs are derived mainly from genes involved in immune and inflammatory responses. Therefore, SARS-CoV-2 infection significantly impacts the circRNA expression profiles of the host, suggesting their potential roles during virus infection. A total of 43 circRNAs were significantly dysregulated following infection at multiple phases. The differential expression of circRNAs was gradually increased with progression of infection. The dysregulated circRNAs could regulate mRNA stability, immunity, and cell death by binding specific proteins [134].
3.4. circRNAs in Neurological Disorders
circRNAs are specifically enriched in brain tissue. Intriguingly, they are differentially expressed in various brain regions and subcellular compartments as well as at specific embryonic and postnatal stages. circRNAs were found to be highly enriched in synapses, implicating a role for circRNAs in neuronal development and plasticity. Furthermore, circRNAs can be up- or downregulated in cultured neurons in response to fluctuations in neuronal activity, suggesting that neuronal activity, which affects gene expression in multiple ways, could potentially also do so by interfering with circRNA levels [135,136].
circRNAs may be involved in a wide range of neuronal stress responses and their aberrant expression or function may contribute to the pathogenesis and progression of neurological diseases. In AD, functional deficiency of ciRS-7 can upregulate miR-7 expression and may lead to downregulation of AD-relevant targets, such as ubiquitin protein ligase A. This autophagic protein is important for clearing amyloid peptides and is less abundant in the AD brain. Consequently, ciRS-7 may participate in AD pathogenesis [135,137,138].
Aberrant expression of circRNAs was also found in PD. In the healthy substantia nigra, circRNAs accumulate in an age-dependent manner. However, in the PD substantia nigra this correlation is lost and the number of circRNAs is reduced. In other studied brain regions of PD patients, the levels of circRNAs are increased. cirSLC8A1 increases in the substantia nigra of PD individuals and sponges miR-128. Targets of miR-128 include the neurodegeneration and aging-related BMI1, SIRT1, and AXIN1 transcripts. Concordantly, these transcripts are increased in PD patients [136,139].
4. snoRNA-Derived Small RNAs
Small nucleolar RNAs (snoRNAs) are 60–300 nt noncoding RNAs that accumulate in the nucleolus. snoRNAs comprise two families, C/D and H/ACA box RNA, that function as ribonucleoprotein (RNP) complexes to guide modification and processing of other RNAs, mainly ribosomal RNAs (rRNAs). Over 400 different snoRNA species have been identified in the human genome. However, only half of them have predicted target sites. The remaining and increasing number of snoRNAs, which could have different functions, is referred to as “orphan snoRNAs”. Other functions fulfilled by snoRNAs include metabolic stress regulation and modulation of alternative splicing. Furthermore, snoRNAs undergo further processing into stable shorter fragments called snoRNA-derived RNAs (sdRNAs). sdRNA production is widespread, as over half of all snoRNAs produce smaller fragments. sdRNAs are similar in size to miRNAs as they typically vary from 20 to 30 nt. snoRNA-derived RNAs larger than 22 nt are also referred to as processed snoRNAs (psnoRNAs). The term sdRNAs describes both psnoRNAs and snoRNA-derived miRNAs [140,141,142,143].
sdRNAs associate with different proteins to snoRNAs, suggesting that they fulfill distinct cellular functions. They can regulate alternative splicing events and have miRNA-like abilities [144,145]. sdRNAs were observed in most if not all species’ genomes containing snoRNA genes with conserved processing profiles, which suggests their functional relevance [146]. Common processing patterns have been observed across snoRNAs and the processing extent of different snoRNAs may differ in the same cell line, suggesting that sdRNAs are not simply degradation products [147]. sdRNAs derived from C/D snoRNAs are predominantly ~17–19 nt and ~30 nt and originate from the 5′ end, whereas H/ACA box snoRNAs generate 20–24 nt-sized fragments mainly derived from the 3′ end [148]. Many H/ACA sdRNAs seem to follow the canonical miRNA pathway, as they are produced in a Dicer-dependent manner and are associated with Ago proteins. However, C/D box-derived sdRNAs are Dicer-independent [149].
Numerous C/D box-derived sdRNAs that exhibit miRNA silencing features were identified in human cell lines: HeLa, Jurkat (T cells), and RPMI8866 (B cell). Importantly, the silencing activity differed amongst all three cell types [150]. Generally, sdRNAs exhibit tissue-specific expression [141]. In addition, several snoRNA-derived miRNAs originate from orphan snoRNAs, suggesting orphan snoRNAs function as a substrate for miRNA production [140]. In turn, several known and described miRNAs genes were found to have snoRNAs precursors. Some pre-miRNAs are H/ACA-derived snoRNA, such as miR-1291/ACA34, miR-1248/HBI-6, and miR-664/ACA36b [151].
First sdRNA that was found to have miRNA-like activity is derived from the H/ACA box snoRNA ACA45. This sdRNA processing requires Dicer activity but is independent of Drosha/DGCR8. ACA45 sdRNA recognizes 3′ UTR sequences of CDC2L6 mRNA and has the ability to post-transcriptionally silence the expression of the CDC2L6 gene by binding to Ago1 and Ago2 proteins. Since the gene product is a component of the Mediator complex responsible for the regulation of transcription, the silencing of this gene is important for the general transcription process. Ender et al. proposed a model in which a minor portion of full-length snoRNA ACA45 is transported from the nucleus to the cytoplasm, where it is further processed to a sdRNA by Dicer [145].
Besides miRNA-like functions, numerous noncanonical functions, such as mediating RNA editing and splicing, have been ascribed to sdRNAs. The loss of HBII-52 and related C/D box snoRNA expression units have been implicated as a cause for the Prader-Willi syndrome (PWS). HBII-52 was found to regulate the alternative splicing of HTR2C (serotonin receptor 2C) pre-mRNA [152]. However, it is not the full-length cluster but sdRNAs derived from this cluster that recruit spliceosomal factors and regulate the alternative splicing of HTR2C. Thus, the loss of the regulatory psnoRNAs could contribute to the etiology of PWS [144]. Furthermore, over a hundred alternative splicing target sites for HBII-85 and five other orphan snoRNAs were predicted to have a significant association with alternatively spliced genes [153]. SNORD88C (HBII-180C) is another snoRNA that is processed and shows complementarity to a pre-miRNA. HBII-180C produces sdRNAs containing the M-box that is complementary to several pre-mRNAs including FGFR3 and can regulate splicing through interactions with pre-mRNA regulatory elements [154]. The interaction between the HBII-180C M-box and an intronic FGFR3 element leads to increased exon inclusion [155].
Recently, a new class of nucleus-localized small RNAs called snRNA/snoRNA-derived nuclear RNAs (sdnRNAs) was identified. The most abundant one, sdnRNA-3, was shown to inhibit the transcription of the Nos2 gene by decreasing chromatin accessibility of the gene promoter in macrophages. sdnRNA-3 promotes the enrichment of the repressive chromatin-remodeling regulator Mi-2β and the repressive histone modification H3K27me3 at the Nos2 gene promoter [156].
4.1. sdRNAs in Stress Conditions
The production of sdRNAs increases during stress conditions, suggesting potential roles in stress regulation. Under stress conditions, snoRNAs were shown to be significantly less abundant, while sdRNAs were significantly more abundant, suggesting stress-dependent regulation of sdRNA excision. However, snoRNA levels did not always correlate with sdRNA abundance. Interestingly, sdRNAs produced under stress conditions were found to be associated with ribosomes, suggesting yet unidentified roles of sdRNAs in translation. Considering that translation typically decreases under stress conditions, sdRNAs could potentially participate in the downregulation of protein synthesis [143,157].
4.2. sdRNAs in Cancer
Different cancer types are associated with unique sdRNA signatures. Even in the case of cancers from a similar tissue of origin, different cancer types exhibit divergent sdRNA expression patterns. Moreover, specific sets of sdRNAs are coordinately expressed across cancers from different tissues of origin, which suggests that sdRNA biogenesis from snoRNAs is coordinately regulated in cancer [158].
Deep sequencing of small noncoding RNAs from patients with normal prostate and prostate cancer in different stages revealed sdRNAs production from the majority of human snoRNAs. In general, sdRNAs display stronger differential expression than miRNAs and are massively upregulated in prostate cancer. At least 78 of the detected sdRNAs, including sdRNAs derived from SNORD44, SNORD78, SNORD74, and SNORD81, demonstrate strong differential expression in this cancer. Furthermore, the expression of SNORD78 and its sdRNA is significantly higher in patients that developed metastatic disease [159]. The full-length SNORD44 is encoded in one of the introns of the noncoding GAS5 (growth arrest-specific) transcript shown to be downregulated in breast tumors [141,160]. Decreased levels of SNORD44 are associated with poor prognosis. In contrast, sdRNAs derived from SNORD44, SNORD78, and other snoRNAs encoded in GAS5, are upregulated in prostate cancer, which suggests separate mechanisms controlling the post-transcriptional levels of snoRNA products from the same precursor transcript [141].
The most differentially expressed sdRNA in breast cancer was found to be sdRNA-93. Increased sdRNA-93 expression in breast cancer results in enhanced invasion, while its inhibition leads to a loss of invasiveness. sdRNA-93 regulates the expression of Pipox, a sarcosine metabolism-related protein whose expression correlates with specific breast cancer subtypes and prognosis. sdRNA-93 targets Pipox 3′ UTR and inhibits its expression. Therefore, through participating in miRNA-like regulation of the Pipox gene, sdRNA-93 expression actively contributes to the malignant phenotype of breast cancer [161].
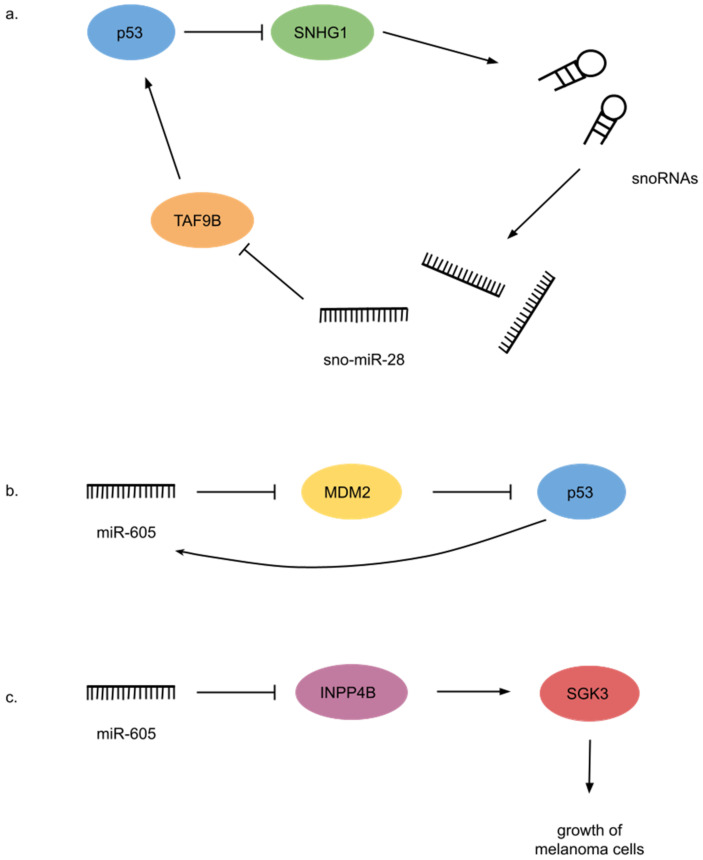
The tumor suppressor protein p53 negatively regulates transcription of the snoRNA host gene SNHG1, which produces several sdRNAs. The most frequently expressed sdRNA from SNHG1, sno-miR-28 produced from SNORD28, directly targets the p53-stabilizing gene TAF9B. sno-miR-28 recognizes the 3′ UTR sequence of TAF9B and together with the Ago protein inhibits its expression. The reduced expression in turn impairs the stability of p53. In this regulatory feedback loop p53 represses sno-miR-28 via SNHG1 and sno-miR-28 plays an oncogenic role by targeting TAF9B to negatively regulate p53 stability (Figure 4a). Importantly, SNHG1, SNORD28, and sno-miR-28 are overexpressed in breast tumors. Moreover, overexpression of sno-miR-28 accelerates breast epithelial cell proliferation and colony formation [162].
Figure 4.
sdRNA mode of action. (a) p53 inhibits sno-miR-28 formation via repressing SNGH1. sno-miR-28 targets TAF9B to negatively regulate p53 stability. (b) Positive feedback loop involving miR-605, MDM2 and p53. (c) miR-605 represses INPP4B, and consequently SGK3, which is responsible for promoting the growth of melanoma cells.
Another regulatory feedback loop affecting p53 involves miR-605, the precursor of which resembles the structure of known box H/ACA snoRNAs [146,151]. miR-605 targets and post-transcriptionally represses the ubiquitin ligase MDM2, a direct inhibitor of p53. Activation of p53 upregulates miR-605 by interacting with the promoter region of the gene. Consequently, miR-605 interrupts the interaction between p53 and MDM2 to create a positive feedback loop (Figure 4b). Under stress conditions, this feedback loop could be a way for 53 to rapidly accumulate [163]. miR-605 also targets INPP4B, a phosphatase that functions as an oncogenic driver through activating SGK3 kinase in melanoma tissue. miR-605 negatively regulates INPP4B expression by repressing its mRNA translation. Thus, miR-605 functions as a tumor suppressor by inhibiting INPP4B expression and SGK3 activity in melanoma (Figure 4c). However, miR-605 is significantly downregulated in melanoma cells and tissues and therefore does not suppress the growth of melanoma cells. Generally, miR-605 functions as a tumor suppressor in various cancers [164].
Several sdRNAs are significantly correlated with features of the tumor-immune microenvironment, such as immunosuppressive markers, cytolytic T cell activity, and tumor vasculature. Many sdRNAs, including sdRNAs derived from SNORA36B in thymoma and SNORA44 in LGG, are significantly correlated with the immunosuppressive biomarker PD-L1. Various sdRNAs, including those derived from SCARNA5, SNORD6, and SNORD114-22, are strongly positively correlated with intratumoral T cell-mediated cytotoxicity by granzyme. Moreover, sdRNA expression signatures are connected to tumor vascularization. 449 sdRNAs were correlated with endothelial cell abundance in at least one cancer type. sdRNAs produced from the C/D snoRNA SNORD114-1 were found to be positively correlated with endothelial cell abundance in 16 different cancer types, suggesting that these sdRNAs play highly conserved roles in tumor vascularization across different tissues [158].
sdRNAs can also modulate cellular drug disposition. hsa-miR-1291 is a small noncoding RNA derived from SNORA34. SNORA34 was found to be processed into hsa-miR-1291 in human pancreatic carcinoma PANC-1 cells. hsa-miR01291 targets the 3′ UTR of ABCC1 (multidrug resistance-associated protein 1) and downregulates its expression leading to a greater intracellular drug accumulation and chemosensitivity. Furthermore, hsa-miR-1291 is significantly downregulated in pancreatic ductal adenocarcinoma, compared to normal pancreas. Lower expression of hsa-miR-1291 was also found in other cancers, suggesting a common downregulation of hsa-miR-1291 in cancerous tissues [165].
5. tRNA-Derived Small RNAs
Transfer RNAs (tRNAs) are 76–93 nt long noncoding RNAs, essential for mRNA translation. They are the most abundant small noncoding RNAs, constituting 4–10% of all cellular RNA. During translation, tRNAs deliver amino acids to the growing polypeptide chains synthesized on ribosomes. tRNA genes are transcribed by RNA polymerase III into precursor tRNAs (pre-tRNAs), which undergo several modifications during tRNA maturation. The 5′ leader and the 3′ trailer sequences are enzymatically digested and the ‘CCA’ sequence is attached at the 3′ ends. Pre-tRNAs undergo splicing and post-transcriptional modifications. Both pre-tRNAs and mature tRNAs can be specifically cleaved into tRNA-derived small RNAs (tsRNAs), which are then modified at the 5′- and 3′-ends to stabilize them. Although tsRNAs were already discovered in the 1970s in the urine of cancer patients, it was determined only over a decade ago that they are not a degradation product, but have biological roles under different physiological and pathological conditions. tsRNAs can be divided into two main groups: tRNA-derived fragments (tRFs) and tRNA-derived stress-induced RNA (tiRNA), which are also called tRNA halves [166].
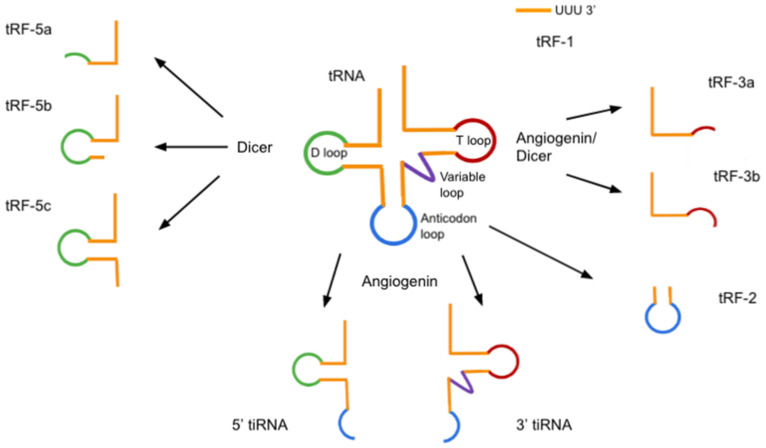
tRNA-derived fragments (tRFs) are produced from pre-tRNAs or mature tRNAs. With lengths of 14–32 nucleotides and a 5′ phosphate and a 3′ hydroxyl group, they show similarities to microRNAs. tRFs can be divided into five classes: tRF-1, tRF-2, tRF-3, tRF-5 and i-tRF (Figure 5). i-tRFs originate from the internal region of mature tRNAs and do not include the 5′-terminal and 3′-terminal regions. The exact mechanism of their biogenesis is still unclear. In addition to the above-mentioned tRFs, other tRNA fragments can also be identified by high-throughput sequencing, indicating greater diversity than existing classification [166,167,168].
Figure 5.
Classification of tRFs and tiRNAs. tRF-1, also called 3′ U-tRF, is generated from the 3′ untranslated region during pre-tRNA maturation and is cleaved by RNase Z or its cytoplasmic homolog ribonuclease Z 2 (ELAC2) at the 5′ end. Therefore, the 3′ end of tRF-1 contains a poly U sequence. tRF-2 contains the anticodon loop and excludes the 5′ and 3′ end structures. tRF-2s are derived from tRNA-Glu, tRNA-Asp, tRNA-Gly, and tRNA-Tyr and are induced in hypoxic conditions. tRF-3s are generated from the 3′ end of mature tRNAs by cleavage in the T-loop by angiogenin or other members of the Ribonuclease A superfamily. tRF-3s can be further divided into two subclasses: tRF3a and tRF-3b, based on their lengths of either ~18 or ~22 nucleotides, respectively. tRF-5s are produced by Dicer cutting the D-loop or the stem position between the D-loop and the anticodon loop of the mature tRNA transcript. Depending on the cleavage sites and therefore different lengths, tRF-5s are further divided into three subtypes: tRF-5a (14–16 nt), tRF-5b (22–24 nt), and tRF-5c (28–30 nt). The oligonucleotide fragments shown as stem loops, for example, tRF-2, may or may not be thermodynamically stable but are drawn as such, primarily to show the tRNA regions they are derived from [166,168,169].
Due to the structure and size similarity to miRNAs, tRFs have been hypothesized to have similar functions in inhibiting the mRNA translation. Indeed, both tRFs and miRNAs interact with Ago proteins and are involved in translation silencing. Furthermore, both are produced under stress conditions contributing to the global decrease in the translation rate during stress [170]. Some tRF-3s and tRF-5s may interact with complementary sequences on target RNAs and recruit them to Ago protein-containing complexes, which regulate the expression of these targets. The anatomy of a tRF-Target-Ago complex is similar to that of a microRNA-Target-Ago complex. tRF-3s and tRF-5s binding to Ago proteins use their 5′ seed sequence to bring the target mRNA into the Ago complexes [169]. The Argonaute-loading of tRFs is cell type-dependent, suggesting differential functional roles through the RNA interference pathway in different cell types [171].
5.1. tsRNAs in Stress Conditions
tRNA-derived stress-induced RNAs (tiRNAs, tRNA halves) are produced as a result of tRNA cleavage under stress conditions, such as nutrition deficiency, hypoxia, and hypothermia. Angiogenin, a stress-activated ribonuclease, cleaves tRNAs to create tiRNAs (Figure 5). The cleavage in or near the anticodon loop results in generating 5′- and 3′-fragments, which are 31-40 nt long [168].
tRNA cleavage under stress conditions was first regarded as a cellular process to decrease the mature tRNA levels. However, the pool of mature tRNAs did not significantly change with the formation of tRNA halves. Consequently, tiRNAs have an independent role in global translation inhibition [172,173]. tRNA halves are induced under oxidative stress, arsenite, heat shock, and UV radiation [174]. They inhibit translation by forming cytoplasmic stress granules (SGs) [175,176]. SGs are cytoplasmic ribonucleoprotein complexes that promote cell survival by sequestering pro-apoptotic signaling proteins while promoting the production of pro-survival proteins. Furthermore, SGs conserve anabolic energy by selectively repressing the synthesis of housekeeping proteins. There are two pathways inducing SG formation: phosphorylation of the eukaryotic translation initiation factor 2α (eIF2α) and an eIF2α phosphorylation-independent pathway [177]. Only 5′ tiRNAs, but not 3′ tiRNAs, induce the eIF2α phosphorylation-independent formation of SGs. In turn, Ala 5′ tRNA halves and Cys 5′ tRNA halves with terminal oligonucleotide motifs (four to five guanine residues) at their 5′ termini form intermolecular RNA G-quadruplexes (RG4). Then, RG4 competitively binds to translation initiation factor eIF4G in the translation initiation complex. The binding of RG4 to the translation initiation complex impairs 40S ribosome scanning on mRNA, leading to the formation of eIF2α-independent stress granules [178]. Furthermore, 5′ tRNA halves cooperate with the YB-1 protein to promote stress granules assembly. YB-1 directly binds to tiRNAs via its cold shock domain and thus enables the tiRNA-induced assembly of SGs [175].
Stress signals can also affect the stability of mitochondrial tRNAs leading to their cleavage and the production of mitochondrial tRFs (mt-tRFs). Several mt-tRFs are produced because of the excessive mitochondrial stress affecting mitochondrial DNA integrity [179].
5.2. tsRNAs in Cancer
tRNA halves can also be found under specific non-stress conditions, indicating that they may regulate other processes besides cellular stress responses. A type of tRNA halves, the sex hormone-dependent tRNA-derived RNA (SHOT-RNA) is induced by sex hormones, not by stress. SHOT-RNAs are produced by angiogenin-mediated anticodon cleavage promoted by sex hormones and their receptors. 5′ SHOT-RNA contains a cyclic phosphate at the 3′ end, while the 3′ terminus of the 3′ SHOT-RNA contains an amino acid, as it derives from aminoacylated tRNA. SHOT-RNAs are specifically expressed in estrogen receptor (ER)-positive breast cancer and androgen receptor (AR)-positive prostate cancer cells and are not expressed in other examined cancer cell lines [167,180].
tRF-2s derived from tRNA-Glu, tRNA-Asp, tRNA-Gly, and tRNA-Tyr are induced in hypoxic conditions prevalent in tumors and act as tumor suppressors in breast cancer. They displace the 3′ UTRs from the RNA-binding protein YBX1 that normally stabilizes oncogenic transcripts. Consequently, they cause destabilization of multiple oncogenic transcripts in breast cancer cells and contain tumor-suppressive and metastasis-suppressive activity [170]. These tRF-2s may be generated under oncogenic stress as an internal mechanism for tumor suppression. Goodarzi et. al., propose two mechanisms, possibly countering the tRF-2-mediated tumor-suppressive mechanism: avoidance of the hypoxia-evoked induction of tumor-suppressive tRFs and the upregulation of YBX1 [181]. This hypothesis is supported by findings showing decreased expression of tRF-2s in metastatic breast cancer and the upregulation of YBX1 during cancer progression [182].
tRF-3027b, also called CU1276, is a DICER1-dependent, tRNA Gly-GCC-derived tRNA fragment expressed in human mature B lymphocytes. tRF-3027b is downregulated in germinal center-derived lymphomas, suggesting a role in the pathogenesis. It associates with Ago proteins and functions as an miRNA. tRF-3027b represses endogenous RPA1, a gene essential for many aspects of DNA dynamics, including genome replication. Stable expression of tRF-3027b in a lymphoma cell line suppresses proliferation and modulates the molecular response to DNA damage [183].
tRF-3019a is upregulated in GC tissues and cell lines and promotes GC cell proliferation, migration, and invasion. tRF-3019a binds to the 3′ UTR of tumor suppressor gene F-box protein 47 (FBXO47) and interacts with Ago2 to inhibit FBXO47 expression [184].
Abnormal expression of tRFs has been found in several cancers. For example, in NPC, 158 differentially expressed tRFs were identified, of which 88 are upregulated and 70 are downregulated [185]. In CRC, the upregulation of tRF-phe-GAA-031 and tRF-VAL-TCA-002 is correlated with distant metastasis and clinical stage. These tRFs might play an important role in the metastasis of CRC [186]. Furthermore, elevated levels of i-tRF-GlyGCC were correlated with an aggressive phenotype of ovarian tumor and were linked to adverse survival outcomes [187].
5.3. tsRNAs in Viral Infections
tRF-3006, a tRNA-Lys-derived tRF, was found in HIV-1-infected cells. It binds to the primer-binding site (PBS) in the genomic RNA of HIV, serving as the primer for reverse transcription. The prevalence of tRF-3006 in the infected cells is positively correlated with the replication of HIV [170,188].
tRF-3019 is perfectly complementary to the PBS of the human T cell leukemia virus type 1 (HTLV-1). This tRF has been shown to prime HTLV-1 reverse transcriptase in an in vitro assay. Therefore, tRF-3019 could initiate reverse transcription and increase virus amplification [189].
tRF-5 GluCTC is induced by the human respiratory syncytial virus (RSV). Its suppression leads to a reduction in RSV viral particle production. tRF-5 GluCTC silences the apolipoprotein E receptor 2 (APOER2), which is required for activation of host immune responses. tRF-5 GluCTC binds to APOER2 3′ UTR, and thus suppresses immune responses and promotes RSV replication in RSV-infected human airway epithelial cells. Moreover, RSV leads to the induction of tRF-5 GlyCCC and tRF-5 LysCTT, which promote RSV replication and impact RSV-induced cytokines/chemokines [167].
5.4. tsRNAs in Neurological Disorders
In cases of neurodegenerative diseases, several mutations have been found in genes associated with tsRNA biogenesis, among them over 40 angiogenin mutants [176]. For instance, ALS-linked mutant angiogenin has limited catalytic activity and fails to induce tRNA cleavage [190]. Under stress conditions, tiRNAs (such as 5′-tiRNA Ala and 5′-tiRNA Cys) or their DNA analog inhibit protein synthesis and trigger the assembly of stress granules. They form RG4 structures, which are required for translation inhibition. The RG4 structure allows them to enter the motor neurons spontaneously and trigger a neuroprotective response [191]. However, Angiogenin can promote the accumulation of tiRNAs due to defects in tRNA methyltransferases Dnmt2 and Nsun2. The accumulation of tiRNAs triggers a stress response and cell death in the nervous system [192,193]. Therefore, tiRNAs seem to have opposite effects, either protecting neurons or promoting neuronal damage. A possible explanation could be that the roles of tiRNAs in neurons are determined by their levels and types. Production of tiRNAs at early stages could activate a stress response protecting cell survival, while in later stages of cell damage tiRNAs could lead to sustained stress and could eventually damage the cells [176].
Recently, tRFs were found to be involved in human AD. tRF-5 expression is significantly altered in the hippocampus of AD patients. Angiogenin is also enhanced in AD, suggesting its role in tRNA cleavage and tRF induction. tRF5-ProAGG is enhanced in AD and its expression is stage-dependent, suggesting its potential role as a biomarker and therapeutic target [194].
6. Conclusions
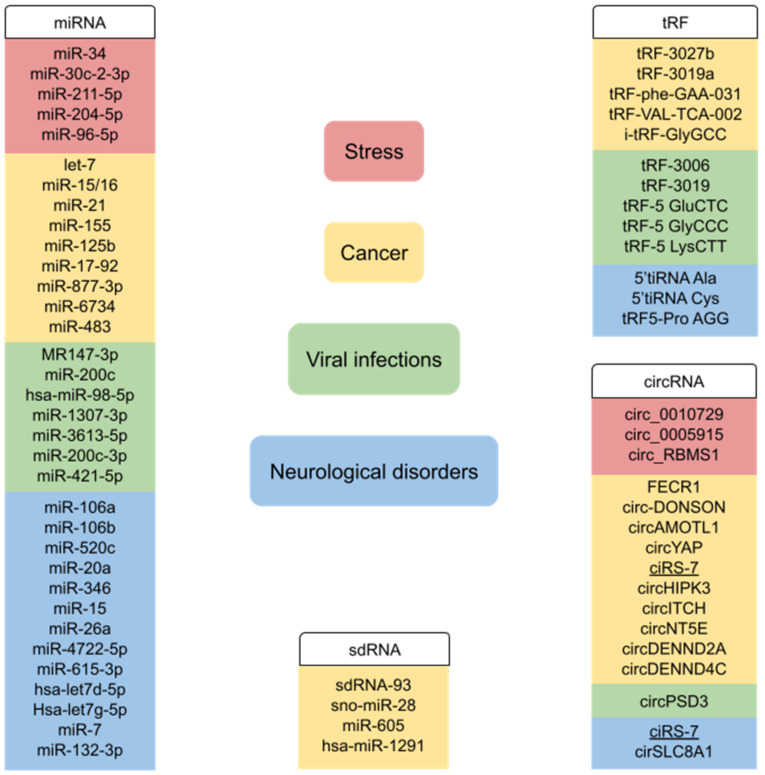
Noncoding RNAs regulate a large variety of cellular processes in different types of human cells. Their expression and function may depend on environmental conditions such as various stresses. Noncoding RNAs also play important roles in the pathogenesis of various diseases, including cancer, neurodegenerative disorders, and viral infections (Figure 6). Because of their involvement in pathogenesis, often in a specific manner, they may potentially serve as novel therapeutic targets. In addition, circulating noncoding RNAs could serve as diagnostic or prognostic tools, since noncoding RNA expression profiles reflect numerous pathological variables. It should be noted with respect to the tRNA fragments that they are also found in prokaryotes and appear to be involved in bacterial pathogenesis [195]. It appears that tRNA fragments function in a wide range of life forms.
Figure 6.
Examples of noncoding RNAs involved in stress-related processes, cancer, viral infections, and neurological disorders. RNAs involved in more than one disease are underlined.
The functions and detailed mechanisms of action of many noncoding RNAs are not yet completely understood. Further development of next-generation sequencing technologies and the expanding data on the expression of noncoding RNAs will clarify cellular functions and mechanistic roles of a larger spectrum of noncoding RNAs.
Author Contributions
Conceptualization, K.D.R. and K.P.; resources, K.P. and K.D.R.; data curation, K.P. and K.D.R.; writing—original draft preparation, K.P.; writing—review and editing, K.D.R. and K.P.; visualization, K.P.; supervision, K.D.R.; funding acquisition, K.D.R. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by: Polish National Science Centre, Narodowe Centrum Nauki grant number 2018/30/E/NZ2/00295 (to KDR).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ponting C.P., Belgard T.G. Transcribed Dark Matter: Meaning or Myth? Hum. Mol. Genet. 2010;19:R162–R168. doi: 10.1093/hmg/ddq362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ulveling D., Francastel C., Hubé F. When One Is Better than Two: RNA with Dual Functions. Biochimie. 2011;93:633–644. doi: 10.1016/J.BIOCHI.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Gonzàlez-Porta M., Frankish A., Rung J., Harrow J., Brazma A. Transcriptome Analysis of Human Tissues and Cell Lines Reveals One Dominant Transcript per Gene. Genome Biol. 2013;14:R70. doi: 10.1186/gb-2013-14-7-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beermann J., Piccoli M.T., Viereck J., Thum T. Non-Coding Rnas in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol. Rev. 2016;96:1297–1325. doi: 10.1152/physrev.00041.2015. [DOI] [PubMed] [Google Scholar]
- 5.Światowy W., Jagodzińśki P.P. Molecules Derived from TRNA and SnoRNA: Entering the Degradome Pool. Biomed. Pharmacother. 2018;108:36–42. doi: 10.1016/j.biopha.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Dai X., Kaushik A.C., Zhang J. The Emerging Role of Major Regulatory RNAs in Cancer Control. Front. Oncol. 2019;9:920. doi: 10.3389/FONC.2019.00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang P., Wu W., Chen Q., Chen M. Non-Coding RNAs and Their Integrated Networks. J. Integr. Bioinform. 2019;16:20190027. doi: 10.1515/JIB-2019-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romano G., Veneziano D., Acunzo M., Croce C.M. Small Non-Coding RNA and Cancer. Carcinogenesis. 2017;38:485–491. doi: 10.1093/carcin/bgx026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catalanotto C., Cogoni C., Zardo G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int. J. Mol. Sci. 2016;17:1712. doi: 10.3390/ijms17101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien J., Hayder H., Zayed Y., Peng C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rani V., Sengar R.S. Biogenesis and Mechanisms of MicroRNA-Mediated Gene Regulation. Biotechnol. Bioeng. 2022;119:685–692. doi: 10.1002/bit.28029. [DOI] [PubMed] [Google Scholar]
- 12.Salim U., Kumar A., Kulshreshtha R., Vivekanandan P. Biogenesis, Characterization, and Functions of Mirtrons. Wiley Interdiscip. Rev. RNA. 2022;13:e1680. doi: 10.1002/wrna.1680. [DOI] [PubMed] [Google Scholar]
- 13.Kilikevicius A., Meister G., Corey D.R. Reexamining Assumptions about MiRNA-Guided Gene Silencing. Nucleic Acids Res. 2022;50:617–634. doi: 10.1093/nar/gkab1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wahid F., Shehzad A., Khan T., Kim Y.Y. MicroRNAs: Synthesis, Mechanism, Function, and Recent Clinical Trials. Biochim. Biophys. Acta. 2010;1803:1231–1243. doi: 10.1016/j.bbamcr.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Naeli P., Winter T., Hackett A.P., Alboushi L., Jafarnejad S.M. The Intricate Balance between MicroRNA-Induced MRNA Decay and Translational Repression. FEBS J. 2022 doi: 10.1111/febs.16422. [DOI] [PubMed] [Google Scholar]
- 16.Fukao A., Mishima Y., Takizawa N., Oka S., Imataka H., Pelletier J., Sonenberg N., Thoma C., Fujiwara T. MicroRNAs Trigger Dissociation of EIF4AI and EIF4AII from Target MRNAs in Humans. Mol. Cell. 2014;56:79–89. doi: 10.1016/j.molcel.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Vasudevan S., Steitz J.A. AU-Rich-Element-Mediated Upregulation of Translation by FXR1 and Argonaute 2. Cell. 2007;128:1105–1118. doi: 10.1016/j.cell.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bukhari S.I.A., Truesdell S.S., Lee S., Kollu S., Classon A., Boukhali M., Jain E., Mortensen R.D., Yanagiya A., Sadreyev R.I., et al. A Specialized Mechanism of Translation Mediated by FXR1a-Associated MicroRNP in Cellular Quiescence. Mol. Cell. 2016;61:760–773. doi: 10.1016/j.molcel.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ørom U.A., Nielsen F.C., Lund A.H. MicroRNA-10a Binds the 5′UTR of Ribosomal Protein MRNAs and Enhances Their Translation. Mol. Cell. 2008;30:460–471. doi: 10.1016/j.molcel.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Liao J.-Y., Ma L.-M., Guo Y.-H., Zhang Y.-C., Zhou H., Shao P., Chen Y.-Q., Qu L.-H. Deep Sequencing of Human Nuclear and Cytoplasmic Small RNAs Reveals an Unexpectedly Complex Subcellular Distribution of MiRNAs and TRNA 3′ Trailers. PLoS ONE. 2010;5:e10563. doi: 10.1371/journal.pone.0010563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts T.C. The MicroRNA Biology of the Mammalian Nucleus. Mol. Ther.-Nucleic Acids. 2014;3:e188. doi: 10.1038/mtna.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H., Lei C., He Q., Pan Z., Xiao D., Tao Y. Nuclear Functions of Mammalian MicroRNAs in Gene Regulation, Immunity and Cancer. Mol. Cancer. 2018;17:64. doi: 10.1186/s12943-018-0765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benhamed M., Herbig U., Ye T., Dejean A., Bischof O. Senescence Is an Endogenous Trigger for MicroRNA-Directed Transcriptional Gene Silencing in Human Cells. Nat. Cell Biol. 2012;14:266–275. doi: 10.1038/ncb2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miao L., Yao H., Li C., Pu M., Yao X., Yang H., Qi X., Ren J., Wang Y. A Dual Inhibition: MicroRNA-552 Suppresses Both Transcription and Translation of Cytochrome P450 2E1. Biochim. Biophys. Acta. 2016;1859:650–662. doi: 10.1016/j.bbagrm.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Zardo G., Ciolfi A., Vian L., Starnes L.M., Billi M., Racanicchi S., Maresca C., Fazi F., Travaglini L., Noguera N., et al. Polycombs and MicroRNA-223 Regulate Human Granulopoiesis by Transcriptional Control of Target Gene Expression. Blood. 2012;119:4034–4046. doi: 10.1182/blood-2011-08-371344. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y., Fan M., Zhang X., Huang F., Wu K., Zhang J., Liu J., Huang Z., Luo H., Tao L., et al. Cellular MicroRNAs Up-Regulate Transcription via Interaction with Promoter TATA-Box Motifs. RNA. 2014;20:1878–1889. doi: 10.1261/rna.045633.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Place R.F., Li L.-C., Pookot D., Noonan E.J., Dahiya R. MicroRNA-373 Induces Expression of Genes with Complementary Promoter Sequences. Proc. Natl. Acad. Sci. USA. 2008;105:1608. doi: 10.1073/pnas.0707594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao M., Li J., Li W., Wang Y., Wu F., Xi Y., Zhang L., Ding C., Luo H., Li Y., et al. MicroRNAs Activate Gene Transcription Epigenetically as an Enhancer Trigger. RNA Biol. 2017;14:1326–1334. doi: 10.1080/15476286.2015.1112487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boudreau R.L., Jiang P., Gilmore B.L., Spengler R.M., Tirabassi R., Nelson J.A., Ross C.A., Xing Y., Davidson B.L. Transcriptome-Wide Discovery of MicroRNA Binding Sites in Human Brain. Neuron. 2014;81:294–305. doi: 10.1016/j.neuron.2013.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spengler R.M., Zhang X., Cheng C., McLendon J.M., Skeie J.M., Johnson F.L., Davidson B.L., Boudreau R.L. Elucidation of Transcriptome-Wide MicroRNA Binding Sites in Human Cardiac Tissues by Ago2 HITS-CLIP. Nucleic Acids Res. 2016;44:7120–7131. doi: 10.1093/nar/gkw640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadakierska-Chudy A. Micrornas: Diverse Mechanisms of Action and Their Potential Applications as Cancer Epi-Therapeutics. Biomolecules. 2020;10:1285. doi: 10.3390/biom10091285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Byrd A.E., Brewer J.W. Micro(RNA)Managing Endoplasmic Reticulum Stress. IUBMB Life. 2013;65:373–381. doi: 10.1002/iub.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olejniczak M., Kotowska-Zimmer A., Krzyzosiak W. Stress-Induced Changes in MiRNA Biogenesis and Functioning. Cell. Mol. Life Sci. 2018;75:177–191. doi: 10.1007/s00018-017-2591-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L., Liao Y., Tang L. MicroRNA-34 Family: A Potential Tumor Suppressor and Therapeutic Candidate in Cancer. J. Exp. Clin. Cancer Res. 2019;38:53. doi: 10.1186/s13046-019-1059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le M.T.N., Teh C., Shyh-Chang N., Xie H., Zhou B., Korzh V., Lodish H.F., Lim B. MicroRNA-125b Is a Novel Negative Regulator of P53. Genes Dev. 2009;23:862–876. doi: 10.1101/gad.1767609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki H.I., Yamagata K., Sugimoto K., Iwamoto T., Kato S., Miyazono K. Modulation of MicroRNA Processing by P53. Nature. 2009;460:529–533. doi: 10.1038/nature08199. [DOI] [PubMed] [Google Scholar]
- 37.Navarro F., Lieberman J. MiR-34 and P53: New Insights into a Complex Functional Relationship. PLoS ONE. 2015;10:e0132767. doi: 10.1371/journal.pone.0132767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tabas I., Ron D. Integrating the Mechanisms of Apoptosis Induced by Endoplasmic Reticulum Stress. Nat. Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byrd A.E., Aragon I.V., Brewer J.W. MicroRNA-30c-2* Limits Expression of Proadaptive Factor XBP1 in the Unfolded Protein Response. J. Cell Biol. 2012;196:689. doi: 10.1083/jcb.201201077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grieco F.A., Schiavo A.A., Brozzi F., Juan-Mateu J., Bugliani M., Marchetti P., Eizirik D.L. The MicroRNAs MiR-211-5p and MiR-204-5p Modulate ER Stress in Human Beta Cells. J. Mol. Endocrinol. 2019;63:139. doi: 10.1530/JME-19-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li G., Luna C., Qiu J., Epstein D.L., Gonzalez P. Role of MiR-204 in the Regulation of Apoptosis, Endoplasmic Reticulum Stress Response, and Inflammation in Human Trabecular Meshwork Cells. Investig. Ophthalmol. Vis. Sci. 2011;52:2999. doi: 10.1167/iovs.10-6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li S., Yang Y., Shi M.H., Wang J.F., Ran X.Q. MiR-96-5p Attenuates Malathion-Induced Apoptosis of Human Kidney Cells by Targeting the ER Stress Marker DDIT3. J. Environ. Sci. Health Part B. 2020;55:1080–1086. doi: 10.1080/03601234.2020.1816092. [DOI] [PubMed] [Google Scholar]
- 43.Si L., Yang Z., Ding L., Zhang D. Regulatory Effects of LncRNAs and MiRNAs on the Crosstalk between Autophagy and EMT in Cancer: A New Era for Cancer Treatment. J. Cancer Res. Clin. Oncol. 2022;148:547–564. doi: 10.1007/S00432-021-03892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dragomir M.P., Knutsen E., Calin G.A. Classical and Noncanonical Functions of MiRNAs in Cancers. Trends Genet. 2022;38:379–394. doi: 10.1016/J.TIG.2021.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Bernstein D.L., Jiang X., Rom S. Let-7 MicroRNAs: Their Role in Cerebral and Cardiovascular Diseases, Inflammation, Cancer, and Their Regulation. Biomedicines. 2021;9:606. doi: 10.3390/biomedicines9060606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pekarsky Y., Balatti V., Croce C.M. BCL2 and MiR-15/16: From Gene Discovery to Treatment. Cell Death Differ. 2017;25:21–26. doi: 10.1038/cdd.2017.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dai L., Chen F., Zheng Y., Zhang D., Qian B., Ji H., Long F., Cretoiu D. MiR-21 Regulates Growth and EMT in Lung Cancer Cells via PTEN/Akt/GSK3β Signaling. Front. Biosci. 2019;24:1426–1439. doi: 10.2741/4788. [DOI] [PubMed] [Google Scholar]
- 48.Wang H., Tan Z., Hu H., Liu H., Wu T., Zheng C., Wang X., Luo Z., Wang J., Liu S., et al. MicroRNA-21 Promotes Breast Cancer Proliferation and Metastasis by Targeting LZTFL1. BMC Cancer. 2019;19:738. doi: 10.1186/s12885-019-5951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin F., Yin H.B., Li X.Y., Zhu G.M., He W.Y., Gou X. Bladder Cancer Cell-secreted Exosomal MiR-21 Activates the PI3K/AKT Pathway in Macrophages to Promote Cancer Progression. Int. J. Oncol. 2020;56:151–164. doi: 10.3892/ijo.2019.4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J., Wu J. Role of MiR-155 in Breast Cancer. Front. Biosci. 2012;17:2350–2355. doi: 10.2741/4056. [DOI] [PubMed] [Google Scholar]
- 51.Mattiske S., Suetani R.J., Neilsen P.M., Callen D.F. The Oncogenic Role of MiR-155 in Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2012;21:1236–1243. doi: 10.1158/1055-9965.EPI-12-0173. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y., Wei Y., Fan X., Zhang P., Wang P., Cheng S., Zhang J. MicroRNA-125b as a Tumor Suppressor by Targeting MMP11 in Breast Cancer. Thorac. Cancer. 2020;11:1613. doi: 10.1111/1759-7714.13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peng B., Theng P.Y., Le M.T.N. Essential Functions of MiR-125b in Cancer. Cell Prolif. 2021;54:e12913. doi: 10.1111/cpr.12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang L., Yuan Y., Zhai H., Wang J., Zhang D., Liang H., Shi Y., Duan L., Jiang X. MicroRNA-125b-5p Correlates with Prognosis and Lung Adenocarcinoma Progression. Front. Mol. Biosci. 2021;8:788690. doi: 10.3389/fmolb.2021.788690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacFarlane L.-A., Paul R.M. MicroRNA: Biogenesis, Function and Role in Cancer. Curr. Genom. 2010;11:537–561. doi: 10.2174/138920210793175895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gruszka R., Zakrzewski K., Liberski P.P., Zakrzewska M. MRNA and MiRNA Expression Analyses of the MYC/E2F/MiR-17-92 Network in the Most Common Pediatric Brain Tumors. Int. J. Mol. Sci. 2021;22:543. doi: 10.3390/ijms22020543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang Q., Shen Y.J., Hsueh C.Y., Guo Y., Zhang Y.F., Li J.Y., Zhou L. MiR-17-5p Drives G2/M-Phase Accumulation by Directly Targeting CCNG2 and Is Related to Recurrence of Head and Neck Squamous Cell Carcinoma. BMC Cancer. 2021;21:1074. doi: 10.1186/s12885-021-08812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li J., Lai Y., Ma J., Liu Y., Bi J., Zhang L., Chen L., Yao C., Lv W., Chang G., et al. MiR-17-5p Suppresses Cell Proliferation and Invasion by Targeting ETV1 in Triple-Negative Breast Cancer. BMC Cancer. 2017;17:745. doi: 10.1186/s12885-017-3674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu D.L., Lu L.L., Dong L.L., Liu Y., Bian X.Y., Lian B.F., Xie L., Wen D., Gao D.M., Ke A.W., et al. MiR-17-5p and MiR-20a-5p Suppress Postoperative Metastasis of Hepatocellular Carcinoma via Blocking HGF/ERBB3-NF-ΚB Positive Feedback Loop. Theranostics. 2020;10:3668–3683. doi: 10.7150/thno.41365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shell S., Park S.-M., Radjabi A.R., Schickel R., Kistner E.O., Jewell D.A., Feig C., Lengyel E., Peter M.E. Let-7 Expression Defines Two Differentiation Stages of Cancer. Proc. Natl. Acad. Sci. USA. 2007;104:11400–11405. doi: 10.1073/pnas.0704372104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lovat F., Fassan M., Sacchi D., Ranganathan P., Palamarchuk A., Bill M., Karunasiri M., Gasparini P., Nigita G., Distefano R., et al. Knockout of Both MiR-15/16 Loci Induces Acute Myeloid Leukemia. Proc. Natl. Acad. Sci. USA. 2018;115:13069–13074. doi: 10.1073/pnas.1814980115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang S., Zhang H.-W., Lu M.-H., He X.-H., Li Y., Gu H., Liu M.-F., Wang E.-D. MicroRNA-155 Functions as an OncomiR in Breast Cancer by Targeting the Suppressor of Cytokine Signaling 1 Gene. Cancer Res. 2010;70:3119–3127. doi: 10.1158/0008-5472.CAN-09-4250. [DOI] [PubMed] [Google Scholar]
- 63.Li S., Zhu Y., Liang Z., Wang X., Meng S., Xu X., Xu X., Wu J., Ji A., Hu Z., et al. Up-Regulation of P16 by MiR-877-3p Inhibits Proliferation of Bladder Cancer. Oncotarget. 2016;7:51773. doi: 10.18632/oncotarget.10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang M.R., Park K.H., Yang J.-O., Lee C.W., Oh S.J., Yun J., Lee M.Y., Han S.-B., Kang J.S. MiR-6734 Up-Regulates P21 Gene Expression and Induces Cell Cycle Arrest and Apoptosis in Colon Cancer Cells. PLoS ONE. 2016;11:e0160961. doi: 10.1371/journal.pone.0160961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang S., Chen Y., Feng S., Yi T., Liu X., Li Q., Liu Z., Zhu C., Hu J., Yu X., et al. MiR-483-5p Promotes IGF-II Transcription and Is Associated with Poor Prognosis of Hepatocellular Carcinoma. Oncotarget. 2017;8:99871–99888. doi: 10.18632/oncotarget.21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fernández-Pato A., Virseda-Berdices A., Resino S., Ryan P., Martínez-González O., Pérez-García F., Martin-Vicente M., Valle-Millares D., Brochado-Kith O., Blancas R., et al. Plasma MiRNA Profile at COVID-19 Onset Predicts Severity Status and Mortality. Emerg. Microbes Infect. 2022;11:676–688. doi: 10.1080/22221751.2022.2038021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li C., Hu X., Li L., Li J. Differential MicroRNA Expression in the Peripheral Blood from Human Patients with COVID-19. J. Clin. Lab. Anal. 2020;34:e23590. doi: 10.1002/jcla.23590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khan M.A.A.K., Sany M.R.U., Islam M.S., Islam A.B.M.M.K. Epigenetic Regulator MiRNA Pattern Differences Among SARS-CoV, SARS-CoV-2, and SARS-CoV-2 World-Wide Isolates Delineated the Mystery Behind the Epic Pathogenicity and Distinct Clinical Characteristics of Pandemic COVID-19. Front. Genet. 2020;11:765. doi: 10.3389/fgene.2020.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang S., Amahong K., Sun X., Lian X., Liu J., Sun H., Lou Y., Zhu F., Qiu Y. The MiRNA: A Small but Powerful RNA for COVID-19. Brief Bioinform. 2021;22:1137–1149. doi: 10.1093/bib/bbab062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Satyam R., Bhardwaj T., Goel S., Jha N.K., Jha S.K., Nand P., Ruokolainen J., Kamal M.A., Kesari K.K. MiRNAs in SARS-CoV 2: A Spoke in the Wheel of Pathogenesis. Curr. Pharm. Des. 2020;27:1628–1641. doi: 10.2174/1381612826999201001200529. [DOI] [PubMed] [Google Scholar]
- 72.Turnquist C., Ryan B.M., Horikawa I., Harris B.T., Harris C.C. Cytokine Storms in Cancer and COVID-19. Cancer Cell. 2020;38:598–601. doi: 10.1016/j.ccell.2020.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Demongeot J., Seligmann H. SARS-CoV-2 and MiRNA-like Inhibition Power. Med. Hypotheses. 2020;144:110245. doi: 10.1016/j.mehy.2020.110245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abedi F., Rezaee R., Hayes A.W., Nasiripour S., Karimi G. MicroRNAs and SARS-CoV-2 Life Cycle, Pathogenesis, and Mutations: Biomarkers or Therapeutic Agents? Cell Cycle. 2021;20:143–153. doi: 10.1080/15384101.2020.1867792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu D., Chatterjee S., Xiao K., Riedel I., Wang Y., Foo R., Bär C., Thum T. MicroRNAs Targeting the SARS-CoV-2 Entry Receptor ACE2 in Cardiomyocytes. J. Mol. Cell. Cardiol. 2020;148:46–49. doi: 10.1016/j.yjmcc.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Matarese A., Gambardella J., Sardu C., Santulli G. MiR-98 Regulates TMPRSS2 Expression in Human Endothelial Cells: Key Implications for COVID-19. Biomedicines. 2020;8:462. doi: 10.3390/biomedicines8110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rizkita L.D., Astuti I. The Potential of MiRNA-Based Therapeutics in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Review. J. Pharm. Anal. 2021;11:265–271. doi: 10.1016/j.jpha.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen L., Zhong L. Genomics Functional Analysis and Drug Screening of SARS-CoV-2. Genes Dis. 2020;7:542–550. doi: 10.1016/j.gendis.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bruscella P., Bottini S., Baudesson C., Pawlotsky J.-M., Feray C., Trabucchi M. Viruses and MiRNAs: More Friends than Foes. Front. Microbiol. 2017;8:824. doi: 10.3389/FMICB.2017.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saksena N., Bonam S.R., Miranda-Saksena M. Epigenetic Lens to Visualize the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Infection in COVID-19 Pandemic. Front. Genet. 2021;12:581726. doi: 10.3389/fgene.2021.581726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abdolahi S., Hosseini M., Rezaei R., Mohebbi S.R., Rostami-Nejad M., Mojarad E.N., Mirjalali H., Yadegar A., Asadzadeh Aghdaei H., Zali M.R., et al. Evaluation of MiR-200c-3p and MiR-421-5p Levels during Immune Responses in the Admitted and Recovered COVID-19 Subjects. Infect. Genet. Evol. 2022;98:105207. doi: 10.1016/j.meegid.2022.105207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khezri M.R., Yousefi K., Zolbanin N.M., Ghasemnejad-Berenji M. MicroRNAs in the Pathophysiology of Alzheimer’s Disease and Parkinson’s Disease: An Overview. Mol. Neurobiol. 2022;59:1589–1603. doi: 10.1007/S12035-022-02727-4. [DOI] [PubMed] [Google Scholar]
- 83.Rybak-Wolf A., Plass M. RNA Dynamics in Alzheimer’s Disease. Molecules. 2021;26:5113. doi: 10.3390/molecules26175113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Patel N., Hoang D., Miller N., Ansaloni S., Huang Q., Rogers J.T., Lee J.C., Saunders A.J. MicroRNAs Can Regulate Human APP Levels. Mol. Neurodegener. 2008;3:10. doi: 10.1186/1750-1326-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hébert S.S., Horré K., Nicolaï L., Bergmans B., Papadopoulou A.S., Delacourte A., de Strooper B. MicroRNA Regulation of Alzheimer’s Amyloid Precursor Protein Expression. Neurobiol. Dis. 2009;33:422–428. doi: 10.1016/j.nbd.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 86.Long J.M., Maloney B., Rogers J.T., Lahiri D.K. Novel Upregulation of Amyloid-β Precursor Protein (APP) by MicroRNA-346 via Targeting of APP MRNA 5′-Untranslated Region: Implications in Alzheimer’s Disease. Mol. Psychiatry. 2018;24:345–363. doi: 10.1038/s41380-018-0266-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maqbool M., Mobashir M., Hoda N. Pivotal Role of Glycogen Synthase Kinase-3: A Therapeutic Target for Alzheimer’s Disease. Eur. J. Med. Chem. 2016;107:63–81. doi: 10.1016/J.EJMECH.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 88.Liu Y., Xu Y., Yu M. MicroRNA-4722-5p and MicroRNA-615-3p Serve as Potential Biomarkers for Alzheimer’s Disease. Exp. Ther. Med. 2022;23:241. doi: 10.3892/etm.2022.11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Poursaei E., Abolghasemi M., Bornehdeli S., Shanehbandi D., Asadi M., Sadeghzadeh M., Rahmanpour D., Sadeh R.N. Evaluation of Hsa-Let-7d-5p, Hsa-Let-7g-5p and Hsa-MiR-15b-5p Plasma Levels in Patients with Alzheimer’s Disease. Psychiatry Genet. 2022;32:25–29. doi: 10.1097/YPG.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 90.García-Fonseca Á., Martin-Jimenez C., Barreto G.E., Pachón A.F.A., González J. The Emerging Role of Long Non-Coding RNAs and MicroRNAs in Neurodegenerative Diseases: A Perspective of Machine Learning. Biomolecules. 2021;11:1132. doi: 10.3390/biom11081132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Choi D.C., Yoo M., Kabaria S., Junn E. MicroRNA-7 Facilitates the Degradation of Alpha-Synuclein and Its Aggregates by Promoting Autophagy. Neurosci. Lett. 2018;678:118. doi: 10.1016/j.neulet.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li S., Bi G., Han S., Huang R. MicroRNAs Play a Role in Parkinson’s Disease by Regulating Microglia Function: From Pathogenetic Involvement to Therapeutic Potential. Front. Mol. Neurosci. 2022;14:358. doi: 10.3389/fnmol.2021.744942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gong X., Huang M., Chen L. Mechanism of MiR-132-3p Promoting Neuroinflammation and Dopaminergic Neurodegeneration in Parkinson’s Disease. eNeuro. 2022;9:1–17. doi: 10.1523/ENEURO.0393-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tang X., Ren H., Guo M., Qian J., Yang Y., Gu C. Review on Circular RNAs and New Insights into Their Roles in Cancer. Comput. Struct. Biotechnol. J. 2021;19:910–928. doi: 10.1016/j.csbj.2021.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nisar S., Bhat A.A., Singh M., Karedath T., Rizwan A., Hashem S., Bagga P., Reddy R., Jamal F., Uddin S., et al. Insights into the Role of CircRNAs: Biogenesis, Characterization, Functional, and Clinical Impact in Human Malignancies. Front. Cell Dev. Biol. 2021;9:617281. doi: 10.3389/fcell.2021.617281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Y., Wang Z. Efficient Backsplicing Produces Translatable Circular MRNAs. RNA. 2015;21:172–179. doi: 10.1261/rna.048272.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tao M., Zheng M., Xu Y., Ma S., Zhang W., Ju S. CircRNAs and Their Regulatory Roles in Cancers. Mol. Med. 2021;27:94. doi: 10.1186/S10020-021-00359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Patop I.L., Wüst S., Kadener S. Past, Present, and Future of Circ RNA s. EMBO J. 2019;38:e100836. doi: 10.15252/embj.2018100836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang A., Zheng H., Wu Z., Chen M., Huang Y. Circular RNA-Protein Interactions: Functions, Mechanisms, and Identification. Theranostics. 2020;10:3506–3517. doi: 10.7150/thno.42174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Misir S., Wu N., Yang B.B. Specific Expression and Functions of Circular RNAs. Cell Death Differ. 2022;29:481–491. doi: 10.1038/s41418-022-00948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang M., Yu F., Li P. Circular RNAs: Characteristics, Function and Clinical Significance in Hepatocellular Carcinoma. Cancers. 2018;10:258. doi: 10.3390/cancers10080258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Z., Huang C., Bao C., Chen L., Lin M., Wang X., Zhong G., Yu B., Hu W., Dai L., et al. Exon-Intron Circular RNAs Regulate Transcription in the Nucleus. Nat. Struct. Mol. Biol. 2015;22:256–264. doi: 10.1038/nsmb.2959. [DOI] [PubMed] [Google Scholar]
- 103.Zhang Y., Zhang X.O., Chen T., Xiang J.F., Yin Q.F., Xing Y.H., Zhu S., Yang L., Chen L.L. Circular Intronic Long Noncoding RNAs. Mol. Cell. 2013;51:792–806. doi: 10.1016/j.molcel.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 104.Wang Y., Lu T., Wang Q., Liu J., Jiao W. Circular RNAs: Crucial Regulators in the Human Body (Review) Oncol. Rep. 2018;40:3119–3135. doi: 10.3892/or.2018.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Geng X., Jia Y., Zhang Y., Shi L., Li Q., Zang A., Wang H. Circular RNA: Biogenesis, Degradation, Functions and Potential Roles in Mediating Resistance to Anticarcinogens. Epigenomics. 2020;12:267–283. doi: 10.2217/epi-2019-0295. [DOI] [PubMed] [Google Scholar]
- 106.Lei D., Wang Y., Zhang L., Wang Z. Circ_0010729 Regulates Hypoxia-Induced Cardiomyocyte Injuries by Activating TRAF5 via Sponging MiR-27a-3p. Life Sci. 2020;262:118511. doi: 10.1016/j.lfs.2020.118511. [DOI] [PubMed] [Google Scholar]
- 107.Dang R.Y., Liu F.L., Li Y. Circular RNA Hsa_circ_0010729 Regulates Vascular Endothelial Cell Proliferation and Apoptosis by Targeting the MiR-186/HIF-1α Axis. Biochem. Biophys. Res. Commun. 2017;490:104–110. doi: 10.1016/j.bbrc.2017.05.164. [DOI] [PubMed] [Google Scholar]
- 108.Liu Z., He Q., Liu Y., Zhang Y., Cui M., Peng H., Wang Y., Chen S., Li D., Chen L., et al. Hsa_circ_0005915 Promotes N,N-Dimethylformamide-Induced Oxidative Stress in HL-7702 Cells through NRF2/ARE Axis. Toxicology. 2021;458:152838. doi: 10.1016/j.tox.2021.152838. [DOI] [PubMed] [Google Scholar]
- 109.Vikhe P.P., Tateossian H., Bharj G., Brown S.D.M., Hood D.W. Mutation in Fbxo11 Leads to Altered Immune Cell Content in Jeff Mouse Model of Otitis Media. Front. Genet. 2020;11:50. doi: 10.3389/fgene.2020.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Qiao D., Hu C., Li Q., Fan J. Circ-Rbms1 Knockdown Alleviates CSE-Induced Apoptosis, Inflammation and Oxidative Stress via up-Regulating FBXO11 through MiR-197-3p in 16HBE Cells. Int. J. COPD. 2021;16:2105–2118. doi: 10.2147/COPD.S311222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen N., Zhao G., Yan X., Lv Z., Yin H., Zhang S., Song W., Li X., Li L., Du Z., et al. A Novel FLI1 Exonic Circular RNA Promotes Metastasis in Breast Cancer by Coordinately Regulating TET1 and DNMT1. Genome Biol. 2018;19:218. doi: 10.1186/s13059-018-1594-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ding L., Zhao Y., Dang S., Wang Y., Li X., Yu X., Li Z., Wei J., Liu M., Li G. Circular RNA Circ-DONSON Facilitates Gastric Cancer Growth and Invasion via NURF Complex Dependent Activation of Transcription Factor SOX4. Mol. Cancer. 2019;18:45. doi: 10.1186/s12943-019-1006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang Q., Du W.W., Wu N., Yang W., Awan F.M., Fang L., Ma J., Li X., Zeng Y., Yang Z., et al. A Circular RNA Promotes Tumorigenesis by Inducing C-Myc Nuclear Translocation. Cell Death Differ. 2017;24:1609–1620. doi: 10.1038/cdd.2017.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu N., Yuan Z., Du K.Y., Fang L., Lyu J., Zhang C., He A., Eshaghi E., Zeng K., Ma J., et al. Translation of Yes-Associated Protein (YAP) Was Antagonized by Its Circular RNA via Suppressing the Assembly of the Translation Initiation Machinery. Cell Death Differ. 2019;26:2758–2773. doi: 10.1038/s41418-019-0337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rahmati Y., Asemani Y., Aghamiri S., Ezzatifar F., Najafi S. CiRS-7/CDR1as; An Oncogenic Circular RNA as a Potential Cancer Biomarker. Pathol. Res. Pract. 2021;227:153639. doi: 10.1016/j.prp.2021.153639. [DOI] [PubMed] [Google Scholar]
- 116.Zhang X., Yang D., Wei Y. Overexpressed CDR1as Functions as an Oncogene to Promote the Tumor Progression via MiR-7 in Non-Small-Cell Lung Cancer. OncoTargets Ther. 2018;11:3979–3987. doi: 10.2147/OTT.S158316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Weng W., Wei Q., Toden S., Yoshida K., Nagasaka T., Fujiwara T., Cai S., Qin H., Ma Y., Goel A. Circular RNA CiRS-7—A Promising Prognostic Biomarker and a Potential Therapeutic Target in Colorectal Cancer. Clin. Cancer Res. 2017;23:3918–3928. doi: 10.1158/1078-0432.CCR-16-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zeng K., Chen X., Xu M., Liu X., Hu X., Xu T., Sun H., Pan Y., He B., Wang S. CircHIPK3 Promotes Colorectal Cancer Growth and Metastasis by Sponging MiR-7. Cell Death Dis. 2018;9:417. doi: 10.1038/s41419-018-0454-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 119.Kai D., Yannian L., Yitian C., Dinghao G., Xin Z., Wu J. Circular RNA HIPK3 Promotes Gallbladder Cancer Cell Growth by Sponging MicroRNA-124. Biochem. Biophys. Res. Commun. 2018;503:863–869. doi: 10.1016/j.bbrc.2018.06.088. [DOI] [PubMed] [Google Scholar]
- 120.Yu Q., Chen W., Li Y., He J., Wang Y., Yang S., Zhou J. The Novel Circular RNA HIPK3 Accelerates the Proliferation and Invasion of Hepatocellular Carcinoma Cells by Sponging the Micro RNA-124 or Micro RNA-506/Pyruvate Dehydrogenase Kinase 2 Axis. Bioengineered. 2022;13:4717–4729. doi: 10.1080/21655979.2022.2031398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang G., Zhu H., Shi Y., Wu W., Cai H., Chen X. Cir-ITCH Plays an Inhibitory Role in Colorectal Cancer by Regulating the Wnt/β-Catenin Pathway. PLoS ONE. 2015;10:e0131225. doi: 10.1371/journal.pone.0131225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang L., Sang J., Zhang Y., Gao L., Zhao D., Cao H. Circular RNA ITCH Attenuates the Progression of Nasopharyngeal Carcinoma by Inducing PTEN Upregulation via MiR-214. J. Gene Med. 2022;24 doi: 10.1002/jgm.3391. [DOI] [PubMed] [Google Scholar]
- 123.Wang R., Zhang S., Chen X., Li N., Li J., Jia R., Pan Y., Liang H. CircNT5E Acts as a Sponge of MiR-422a to Promote Glioblastoma Tumorigenesis. Cancer Res. 2018;78:4812–4825. doi: 10.1158/0008-5472.CAN-18-0532. [DOI] [PubMed] [Google Scholar]
- 124.Dong L., Zheng J., Gao Y., Zhou X., Song W., Huang J. The Circular RNA NT5E Promotes Non-Small Cell Lung Cancer Cell Growth via Sponging MicroRNA-134. Aging. 2020;12:3936. doi: 10.18632/aging.102861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang J., Liu X., Dai G., Qu L., Tan B., Zhu B., Qi F., Gai X., Cheng B. CircNT5E Promotes the Proliferation and Migration of Bladder Cancer via Sponging MiR-502-5p. J. Cancer. 2021;12:2430. doi: 10.7150/jca.53385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Su H., Zou D., Sun Y., Dai Y. Hypoxia-Associated CircDENND2A Promotes Glioma Aggressiveness by Sponging MiR-625-5p. Cell. Mol. Biol. Lett. 2019;24:24. doi: 10.1186/s11658-019-0149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ren S., Liu J., Feng Y., Li Z., He L., Li L., Cao X., Wang Z., Zhang Y. Knockdown of CircDENND4C Inhibits Glycolysis, Migration and Invasion by up-Regulating MiR-200b/c in Breast Cancer under Hypoxia. J. Exp. Clin. Cancer Res. 2019;38:388. doi: 10.1186/s13046-019-1398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ma D., Qin Y., Li S., Li L., He J., Chen Y., Zhou X., Liu H. CircDENND4C Promotes Proliferation and Metastasis of Lung Cancer by Upregulating BRD4 Signaling Pathway. J. Oncol. 2021;2021:2469691. doi: 10.1155/2021/2469691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ghafouri-Fard S., Khoshbakht T., Taheri M., Jamali E. CircITCH: A Circular RNA With Eminent Roles in the Carcinogenesis. Front. Oncol. 2021;11:4304. doi: 10.3389/fonc.2021.774979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang Q., Wang W., Zhou Q., Chen C., Yuan W., Liu J., Li X., Sun Z. Roles of CircRNAs in the Tumour Microenvironment. Mol. Cancer. 2020;19:14. doi: 10.1186/s12943-019-1125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lou Y.Y., Wang Q.D., Lu Y.T., Tu M.Y., Xu X., Xia Y., Peng Y., Lai M.M., Zheng X.Q. Differential CircRNA Expression Profiles in Latent Human Cytomegalovirus Infection and Validation Using Clinical Samples. Physiol. Genom. 2019;51:51–58. doi: 10.1152/physiolgenomics.00096.2018. [DOI] [PubMed] [Google Scholar]
- 132.Chen T.-C., Tallo-Parra M., Cao Q.M., Kadener S., Böttcher R., Pérez-Vilaró G., Boonchuen P., Somboonwiwat K., Díez J., Sarnow P. Host-Derived Circular RNAs Display Proviral Activities in Hepatitis C Virus-Infected Cells. PLOS Pathog. 2020;16:e1008346. doi: 10.1371/journal.ppat.1008346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cai Z., Lu C., He J., Liu L., Zou Y., Zhang Z., Zhu Z., Ge X., Wu A., Jiang T., et al. Identification and Characterization of CircRNAs Encoded by MERS-CoV, SARS-CoV-1 and SARS-CoV-2. Brief. Bioinform. 2021;22:1297–1308. doi: 10.1093/bib/bbaa334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang M., Qi M., Xu L., Huang P., Wang X., Sun J., Shi J., Hu Y. Differential Host CircRNA Expression Profiles in Human Lung Epithelial Cells Infected with SARS-CoV-2. Infect. Genet. Evol. 2021;93:104923. doi: 10.1016/j.meegid.2021.104923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang M., Bian Z. The Emerging Role of Circular RNAs in Alzheimer’s Disease and Parkinson’s Disease. Front. Aging Neurosci. 2021;13:426. doi: 10.3389/FNAGI.2021.691512/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Doxakis E. Insights into the Multifaceted Role of Circular RNAs: Implications for Parkinson’s Disease Pathogenesis and Diagnosis. NPJ Parkinson’s Dis. 2022;8:7. doi: 10.1038/s41531-021-00265-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cochran K.R., Veeraraghavan K., Kundu G., Mazan-Mamczarz K., Coletta C., Thambisetty M., Gorospe M., De S. Systematic Identification of CircRNAs in Alzheimer’s Disease. Genes. 2021;12:1258. doi: 10.3390/genes12081258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ghafouri-Fard S., Safari M., Taheri M., Samadian M. Expression of Linear and Circular LncRNAs in Alzheimer’s Disease. J. Mol. Neurosci. 2021;72:187–200. doi: 10.1007/s12031-021-01900-z. [DOI] [PubMed] [Google Scholar]
- 139.Hanan M., Simchovitz A., Yayon N., Vaknine S., Cohen-Fultheim R., Karmon M., Madrer N., Rohrlich T.M., Maman M., Bennett E.R., et al. A Parkinson’s Disease Circ RNA s Resource Reveals a Link between Circ SLC 8A1 and Oxidative Stress. EMBO Mol. Med. 2020;12:e11942. doi: 10.15252/emmm.201911942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Falaleeva M., Stamm S. Processing of SnoRNAs as a New Source of Regulatory Non-Coding RNAs: SnoRNA Fragments Form a New Class of Functional RNAs. BioEssays. 2013;35:46–54. doi: 10.1002/bies.201200117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Martens-Uzunova E.S., Olvedy M., Jenster G. Beyond MicroRNA-Novel RNAs Derived from Small Non-Coding RNA and Their Implication in Cancer. Cancer Lett. 2013;340:201–211. doi: 10.1016/j.canlet.2012.11.058. [DOI] [PubMed] [Google Scholar]
- 142.Mleczko A.M., Bąkowska-Żywicka K. When Small RNAs Become Smaller: Non-Canonical Functions of SnoRNAs and Their Derivatives. Acta Biochim. Pol. 2016;63:601–607. doi: 10.18388/abp.2016_1330. [DOI] [PubMed] [Google Scholar]
- 143.Wajahat M., Bracken C.P., Orang A. Emerging Functions for SnoRNAs and SnoRNA-Derived Fragments. Int. J. Mol. Sci. 2021;22:10193. doi: 10.3390/ijms221910193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kishore S., Khanna A., Zhang Z., Hui J., Balwierz P.J., Stefan M., Beach C., Nicholls R.D., Zavolan M., Stamm S. The SnoRNA MBII-52 (SNORD 115) Is Processed into Smaller RNAs and Regulates Alternative Splicing. Hum. Mol. Genet. 2010;19:1153–1164. doi: 10.1093/hmg/ddp585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ender C., Krek A., Friedländer M.R., Beitzinger M., Weinmann L., Chen W., Pfeffer S., Rajewsky N., Meister G. A Human SnoRNA with MicroRNA-Like Functions. Mol. Cell. 2008;32:519–528. doi: 10.1016/j.molcel.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 146.Abel Y., Rederstorff M. SnoRNAs and the Emerging Class of SdRNAs: Multifaceted Players in Oncogenesis. Biochimie. 2019;164:17–21. doi: 10.1016/j.biochi.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 147.Bratkovič T., Rogelj B. The Many Faces of Small Nucleolar RNAs. Biochim. Et Biophys. Acta. 2014;1839:438–443. doi: 10.1016/j.bbagrm.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 148.Taft R.J., Glazov E.A., Lassmann T., Hayashizaki Y., Carninci P., Mattick J.S. Small RNAs Derived from SnoRNAs. RNA. 2009;15:1233–1240. doi: 10.1261/rna.1528909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Langenberger D., Çakir M.V., Hoffmann S., Stadler P.F. Dicer-Processed Small RNAs: Rules and Exceptions. J. Exp. Zool. B Mol. Dev. Evol. 2013;320:35–46. doi: 10.1002/jez.b.22481. [DOI] [PubMed] [Google Scholar]
- 150.Brameier M., Herwig A., Reinhardt R., Walter L., Gruber J. Human Box C/D SnoRNAs with MiRNA like Functions: Expanding the Range of Regulatory RNAs. Nucleic Acids Res. 2011;39:675–686. doi: 10.1093/nar/gkq776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Scott M.S., Avolio F., Ono M., Lamond A.I., Barton G.J. Human MiRNA Precursors with Box H/ACA SnoRNA Features. PLOS Comput. Biol. 2009;5:e1000507. doi: 10.1371/journal.pcbi.1000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sahoo T., del Gaudio D., German J.R., Shinawi M., Peters S.U., Person R.E., Garnica A., Cheung S.W., Beaudet A.L. Prader-Willi Phenotype Caused by Paternal Deficiency for the HBII-85 C/D Box Small Nucleolar RNA Cluster. Nat. Genet. 2008;40:719–721. doi: 10.1038/ng.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bazeley P.S., Shepelev V., Talebizadeh Z., Butler M.G., Fedorova L., Filatov V., Fedorov A. SnoTARGET Shows That Human Orphan SnoRNA Targets Locate Close to Alternative Splice Junctions. Gene. 2008;408:172–179. doi: 10.1016/j.gene.2007.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Scott M.S., Ono M., Yamada K., Endo A., Barton G.J., Lamond A.I. Human Box C/D SnoRNA Processing Conservation across Multiple Cell Types. Nucleic Acids Res. 2012;40:3676–3688. doi: 10.1093/nar/gkr1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ono M., Yamada K., Avolio F., Scott M.S., van Koningsbruggen S., Barton G.J., Lamond A.I. Analysis of Human Small Nucleolar RNAs (SnoRNA) and the Development of SnoRNA Modulator of Gene Expression Vectors. Mol. Biol. Cell. 2010;21:1569–1584. doi: 10.1091/mbc.e10-01-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shi Y., Shi Q., Shen Q., Zhang Q., Cao X. Dicer-Independent SnRNA/SnoRNA-Derived Nuclear RNA 3 Regulates Tumor-Associated Macrophage Function by Epigenetically Repressing Inducible Nitric Oxide Synthase Transcription. Cancer Commun. 2021;41:140–153. doi: 10.1002/cac2.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mleczko A.M., Machtel P., Walkowiak M., Wasilewska A., Pietras P.J., Bąkowska-Żywicka K. Levels of SdRNAs in Cytoplasm and Their Association with Ribosomes Are Dependent upon Stress Conditions but Independent from SnoRNA Expression. Sci. Rep. 2019;9:18397. doi: 10.1038/s41598-019-54924-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chow R.D., Chen S. Sno-Derived RNAs Are Prevalent Molecular Markers of Cancer Immunity. Oncogene. 2018;37:6442–6462. doi: 10.1038/s41388-018-0420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Martens-Uzunova E.S., Hoogstrate Y., Kalsbeek A., Pigmans B., Vredenbregt-Van Den Berg M., Dits N., Nielsen S.J., Baker A., Visakorpi T., Bangma C., et al. C/D-Box SnoRNA-Derived RNA Production Is Associated with Malignant Transformation and Metastatic Progression in Prostate Cancer. Oncotarget. 2015;6:17430–17444. doi: 10.18632/oncotarget.4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mourtada-Maarabouni M., Pickard M.R., Hedge V.L., Farzaneh F., Williams G.T. GAS5, a Non-Protein-Coding RNA, Controls Apoptosis and Is Downregulated in Breast Cancer. Oncogene. 2008;28:195–208. doi: 10.1038/onc.2008.373. [DOI] [PubMed] [Google Scholar]
- 161.Patterson D.G., Roberts J.T., King V.M., Houserova D., Barnhill E.C., Crucello A., Polska C.J., Brantley L.W., Kaufman G.C., Nguyen M., et al. Human SnoRNA-93 Is Processed into a MicroRNA-like RNA That Promotes Breast Cancer Cell Invasion. NPJ Breast Cancer. 2017;3:25. doi: 10.1038/s41523-017-0032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yu F., Bracken C.P., Pillman K.A., Lawrence D.M., Goodall G.J., Callen D.F., Neilsen P.M. P53 Represses the Oncogenic Sno-MiR-28 Derived from a SnoRNA. PLoS ONE. 2015;10:e0129190. doi: 10.1371/JOURNAL.PONE.0129190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xiao J., Lin H., Luo X., Luo X., Wang Z. MiR-605 Joins P53 Network to Form a P53:MiR-605:Mdm2 Positive Feedback Loop in Response to Stress. EMBO J. 2011;30:524. doi: 10.1038/emboj.2010.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen L., Cao Y., Rong D., Wang Y., Cao Y. MicroRNA-605 Functions as a Tumor Suppressor by Targeting INPP4B in Melanoma. Oncol. Rep. 2017;38:1276–1286. doi: 10.3892/or.2017.5740. [DOI] [PubMed] [Google Scholar]
- 165.Pan Y.-Z., Zhou A., Hu Z., Yu A.-M. Small Nucleolar RNA-Derived MicroRNA Hsa-MiR-1291 Modulates Cellular Drug Disposition through Direct Targeting of ABC Transporter ABCC1. Drug Metab. Dispos. 2013;41:1744–1751. doi: 10.1124/dmd.113.052092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zong T., Yang Y., Zhao H., Li L., Liu M., Fu X., Tang G., Zhou H., Aung L.H.H., Li P., et al. TsRNAs: Novel Small Molecules from Cell Function and Regulatory Mechanism to Therapeutic Targets. Cell Prolif. 2021;54:e12977. doi: 10.1111/cpr.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yu X., Xie Y., Zhang S., Song X., Xiao B., Yan Z. TRNA-Derived Fragments: Mechanisms Underlying Their Regulation of Gene Expression and Potential Applications as Therapeutic Targets in Cancers and Virus Infections. Theranostics. 2020;11:461–469. doi: 10.7150/thno.51963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Liu B., Cao J., Wang X., Guo C., Liu Y., Wang T. Deciphering the TRNA-Derived Small RNAs: Origin, Development, and Future. Cell Death Dis. 2022;13:24. doi: 10.1038/S41419-021-04472-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kumar P., Kuscu C., Dutta A. Biogenesis and Function of Transfer RNA-Related Fragments (TRFs) Trends Biochem. Sci. 2016;41:679–689. doi: 10.1016/j.tibs.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pan Q., Han T., Li G. Novel Insights into the Roles of TRNA-Derived Small RNAs. RNA Biol. 2021;18:2157–2167. doi: 10.1080/15476286.2021.1922009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Telonis A.G., Loher P., Honda S., Jing Y., Palazzo J., Kirino Y., Rigoutsos I. Dissecting TRNA-Derived Fragment Complexities Using Personalized Transcriptomes Reveals Novel Fragment Classes and Unexpected Dependencies. Oncotarget. 2015;6:24797–24822. doi: 10.18632/oncotarget.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Thompson D.M., Lu C., Green P.J., Parker R. TRNA Cleavage Is a Conserved Response to Oxidative Stress in Eukaryotes. RNA. 2008;14:2095–2103. doi: 10.1261/rna.1232808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kim H.K., Yeom J.H., Kay M.A. Transfer RNA-Derived Small RNAs: Another Layer of Gene Regulation and Novel Targets for Disease Therapeutics. Mol. Ther. 2020;28:2340–2357. doi: 10.1016/j.ymthe.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yamasaki S., Ivanov P., Hu G.F., Anderson P. Angiogenin Cleaves TRNA and Promotes Stress-Induced Translational Repression. J. Cell Biol. 2009;185:35–42. doi: 10.1083/jcb.200811106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lyons S.M., Achorn C., Kedersha N.L., Anderson P.J., Ivanov P. YB-1 Regulates TiRNA-Induced Stress Granule Formation but Not Translational Repression. Nucleic Acids Res. 2016;44:6949–6960. doi: 10.1093/nar/gkw418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li S., Xu Z., Sheng J. TRNA-Derived Small RNA: A Novel Regulatory Small Non-Coding RNA. Genes. 2018;9:246. doi: 10.3390/genes9050246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Farny N.G., Kedersha N.L., Silver P.A. Metazoan Stress Granule Assembly Is Mediated by P-EIF2α-Dependent and -Independent Mechanisms. RNA. 2009;15:1814–1821. doi: 10.1261/rna.1684009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lyons S.M., Kharel P., Akiyama Y., Ojha S., Dave D., Tsvetkov V., Merrick W., Ivanov P., Anderson P. EIF4G Has Intrinsic G-Quadruplex Binding Activity That Is Required for TiRNA Function. Nucleic Acids Res. 2020;48:6223–6233. doi: 10.1093/nar/gkaa336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shaukat A.N., Kaliatsi E.G., Stamatopoulou V., Stathopoulos C. Mitochondrial TRNA-Derived Fragments and Their Contribution to Gene Expression Regulation. Front. Physiol. 2021;12:1425. doi: 10.3389/fphys.2021.729452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Honda S., Loher P., Shigematsu M., Palazzo J.P., Suzuki R., Imoto I., Rigoutsos I., Kirino Y. Sex Hormone-Dependent TRNA Halves Enhance Cell Proliferation in Breast and Prostate Cancers. Proc. Natl. Acad. Sci. USA. 2015;112:E3816–E3825. doi: 10.1073/pnas.1510077112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Goodarzi H., Liu X., Nguyen H.C.B., Zhang S., Fish L., Tavazoie S.F. Endogenous TRNA-Derived Fragments Suppress Breast Cancer Progression via YBX1 Displacement. Cell. 2015;161:790–802. doi: 10.1016/j.cell.2015.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lasham A., Samuel W., Cao H., Patel R., Mehta R., Stern J.L., Reid G., Woolley A.G., Miller L.D., Black M.A., et al. YB-1, the E2F Pathway, and Regulation of Tumor Cell Growth. J. Natl. Cancer Inst. 2012;104:133. doi: 10.1093/jnci/djr512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Maute R.L., Schneider C., Sumazin P., Holmes A., Califano A., Basso K., Dalla-Favera R. TRNA-Derived MicroRNA Modulates Proliferation and the DNA Damage Response and Is down-Regulated in B Cell Lymphoma. Proc. Natl. Acad. Sci. USA. 2013;110:1404–1409. doi: 10.1073/pnas.1206761110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhang F., Shi J., Wu Z., Gao P., Zhang W., Qu B., Wang X., Song Y., Wang Z. A 3′-TRNA-Derived Fragment Enhances Cell Proliferation, Migration and Invasion in Gastric Cancer by Targeting FBXO47. Arch. Biochem. Biophys. 2020;690:108467. doi: 10.1016/j.abb.2020.108467. [DOI] [PubMed] [Google Scholar]
- 185.Lu Z., Su K., Wang X., Zhang M., Ma S., Li H., Qiu Y. Expression Profiles of TRNA-Derived Small RNAs and Their Potential Roles in Primary Nasopharyngeal Carcinoma. Front. Mol. Biosci. 2021;8:780621. doi: 10.3389/fmolb.2021.780621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chen H., Xu Z., Cai H., Peng Y., Yang L., Wang Z. Identifying Differentially Expressed TRNA-Derived Small Fragments as a Biomarker for the Progression and Metastasis of Colorectal Cancer. Dis Markers. 2022;2022:8. doi: 10.1155/2022/2646173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Panoutsopoulou K., Dreyer T., Dorn J., Obermayr E., Mahner S., van Gorp T., Braicu I., Zeillinger R., Magdolen V., Avgeris M., et al. TRNAGlyGCC-Derived Internal Fragment (i-TRF-GlyGCC) in Ovarian Cancer Treatment Outcome and Progression. Cancers. 2021;14:24. doi: 10.3390/cancers14010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yeung M.L., Bennasser Y., Watashi K., Le S.-Y., Houzet L., Jeang K.-T. Pyrosequencing of Small Non-Coding RNAs in HIV-1 Infected Cells: Evidence for the Processing of a Viral-Cellular Double-Stranded RNA Hybrid. Nucleic Acids Res. 2009;37:6575–6586. doi: 10.1093/nar/gkp707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ruggero K., Guffanti A., Corradin A., Sharma V.K., de Bellis G., Corti G., Grassi A., Zanovello P., Bronte V., Ciminale V., et al. Small Noncoding RNAs in Cells Transformed by Human T-Cell Leukemia Virus Type 1: A Role for a TRNA Fragment as a Primer for Reverse Transcriptase. J. Virol. 2014;88:3612–3622. doi: 10.1128/JVI.02823-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang E., Thombre R., Shah Y., Latanich R., Wang J. G-Quadruplexes as Pathogenic Drivers in Neurodegenerative Disorders. Nucleic Acids Res. 2021;49:4816. doi: 10.1093/nar/gkab164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ivanov P., O’Day E., Emara M.M., Wagner G., Lieberman J., Anderson P. G-Quadruplex Structures Contribute to the Neuroprotective Effects of Angiogenin-Induced TRNA Fragments. Proc. Natl. Acad. Sci. USA. 2014;111:18201–18206. doi: 10.1073/pnas.1407361111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Schaefer M., Pollex T., Hanna K., Tuorto F., Meusburger M., Helm M., Lyko F. RNA Methylation by Dnmt2 Protects Transfer RNAs against Stress-Induced Cleavage. Genes Dev. 2010;24:1590–1595. doi: 10.1101/gad.586710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Blanco S., Dietmann S., Flores J.V., Hussain S., Kutter C., Humphreys P., Lukk M., Lombard P., Treps L., Popis M., et al. Aberrant Methylation of TRNAs Links Cellular Stress to Neuro-Developmental Disorders. EMBO J. 2014;33:2020–2039. doi: 10.15252/embj.201489282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wu W., Lee I., Spratt H., Fang X., Bao X. TRNA-Derived Fragments in Alzheimer’s Disease: Implications for New Disease Biomarkers and Neuropathological Mechanisms. J. Alzheimers Dis. 2021;79:793. doi: 10.3233/JAD-200917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Li Z., Stanton B.A. Transfer RNA-Derived Fragments, the Underappreciated Regulatory Small RNAs in Microbial Pathogenesis. Front. Microbiol. 2021;12:687632. doi: 10.3389/fmicb.2021.687632. [DOI] [PMC free article] [PubMed] [Google Scholar]