Abstract
A pure culture of an obligately anaerobic marine bacterium was obtained from an anaerobic enrichment culture in which taurine (2-aminoethanesulfonate) was the sole source of carbon, energy, and nitrogen. Taurine fermentation resulted in acetate, ammonia, and sulfide as end products. Other sulfonates, including 2-hydroxyethanesulfonate (isethionate) and cysteate (alanine-3-sulfonate), were not fermented. When malate was the sole source of carbon and energy, the bacterium reduced sulfate, sulfite, thiosulfate, or nitrate (reduced to ammonia) but did not use fumarate or dimethyl sulfoxide as a terminal electron acceptor for growth. Taurine-grown cells had significantly lower adenylylphosphosulfate reductase activities than sulfate-grown cells had, which was consistent with the notion that sulfate was not released as a result of oxidative C-S bond cleavage and then assimilated. The name Desulforhopalus singaporensis is proposed for this sulfate-reducing bacterium, which is morphologically unusual compared to the previously described sulfate-reducing bacteria by virtue of the spinae present on the rod-shaped, gram-negative, nonmotile cells; endospore formation was not discerned, nor was desulfoviridin detected. Granules of poly-β-hydroxybutyrate were abundant in taurine-grown cells. This organism shares with the other member of the genus Desulforhopalus which has been described a unique 13-base deletion in the 16S ribosomal DNA. It differs in several ways from a recently described endospore-forming anaerobe (K. Denger, H. Laue, and A. M. Cook, Arch. Microbiol. 168:297–301, 1997) that reportedly produces thiosulfate but not sulfide from taurine fermentation. D. singaporensis thus appears to be the first example of an organism which exhibits sulfidogenesis during taurine fermentation. Implications for sulfonate sulfur in the sulfur cycle are discussed.
Organosulfur compounds are widely distributed in the environment (25), and the range of oxidation states of sulfur in these compounds (+6 in chondroitin sulfate to −1 in methanethiol) is similar to the range for inorganic sulfur (+6 for sulfate to −2 for hydrogen sulfide) (21, 50). The nomenclature of the organosulfur compounds differs according to the oxidation state of the sulfur (50). Sulfonic acids are a class of organosulfur compounds with the general structure R-H2C-SO3−, in which the sulfur is at an oxidation state of +5 (21, 47); the R represents a carbon-containing residue which can be aliphatic, aromatic, or more complex. Sulfonates are synthesized by diverse biota (33, 42) and are also synthesized chemically; the sulfonate moiety (-SO3−) is often added to compounds to increase water solubility (3, 50) or to enhance resistance to biodegradation (28, 32). Sulfonates are especially abundant in some environments (28, 33) and thus may serve as nutrients or sources of energy. The initial focus on the metabolism of sulfonates was primarily on aerobic utilization of these compounds (32, 42); cleavage of the carbon sulfur bond usually involves monooxygenases (16, 22) and sulfolyases (27). However, recent reports have demonstrated that sulfonates can be mineralized under strictly anaerobic conditions (8, 9, 29, 30, 33, 34). In initial studies of the use of sulfonates as terminal electron acceptors (TEA) by sulfate-reducing bacteria (SRB), we noted that none of the compounds tested served as a sole source of carbon and energy for growth of strain IC1 (34). Recently, however, cysteate fermentation (29) and taurine fermentation (9) have been described for bacteria belonging to two different genera. Although the sole structural difference between cysteate and taurine is the carboxyl group of cysteate, the end products formed from cysteate fermentation (ammonia, acetate, sulfide, sulfate) differed from the end products formed from taurine fermentation (ammonia, acetate, thiosulfate). Here we describe the ability of a morphologically unusual sulfate-reducing bacterium that ferments taurine to form ammonia, acetate, and sulfide as end products.
MATERIALS AND METHODS
Chemicals.
The chemicals used were analytical or reagent grade and were purchased from Fisher Scientific (Pittsburgh, Pa.), Fluka (Milwaukee, Wis.), and Sigma Chemical Co. (St. Louis, Mo.). Gases were purchased from (Northeast Airgas, Cheshire, Conn.).
Bacterial cultures.
Desulfovibrio desulfuricans IC1 (= DSM 12129) was obtained from our collection. Desulfitobacterium hafniense was kindly provided by Jan Gerritse of the University of Groningen, Groningen, The Netherlands.
Enrichment and cultivation of bacteria.
Desulfovibrio desulfuricans IC1 and strain T1 (see below) were maintained and grown in the mineral salts medium of Widdel and Pfennig (53); freshwater, saltwater, and brackish conditions were created by adjusting the NaCl and MgCl2 concentrations. Sulfate was omitted when cultures were grown with sulfonates. When organisms were tested for growth with taurine as the sole carbon, energy, and nitrogen source, NH4Cl was omitted and the gas used was a mixture containing 75% (vol/vol) Ar and 25% (vol/vol) CO2. Titanous chloride (the amount added was just sufficient to turn the redox indicator colorless) was used as a reductant when strain T1 was grown with nitrate as a TEA. Substrates were added from separately sterilized 0.5 to 1 M stock solutions.
Strain T1 was isolated from an enrichment culture by using sulfide-rich black marine mud obtained from a marsh (Marsh Gardens, East Coast Highway, Republic of Singapore). The primary liquid enrichment culture contained 10 mM malate and 10 mM taurine as the electron donor and the electron acceptor, respectively.
Solidified media in plastic petri dishes were used to obtain pure cultures. We added 10 mM taurine as the sole carbon and energy source and agarose (1.5%, wt/vol) as a solidifying agent to the mineral salts medium. After the pH was adjusted to 7 to 7.4 and after autoclaving, the medium was cooled to ca. 80°C, and the mineral mixture, vitamins, and resazurin were added (52). The preparation was immediately transferred into an anaerobic hood, and then bicarbonate buffer and sodium sulfide reductant were added to final concentrations of 30 and 1.5 mM, respectively. The medium was then mixed, immediately poured into petri dishes, allowed to solidify, and then stored in Brewer type jars in the anaerobic chamber. Enrichment cultures were streaked onto the media outside the anaerobic hood, and the plates were then immediately returned to the hood for incubation in jars. The plates were incubated for 1 to 2 weeks before bacteria from single colonies were streaked onto new media.
Stock cultures were stored in liquid medium at 4°C and were transferred at 1- to 2-month intervals. A culture of isolate T1 has been deposited in the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ), Braunschweig, Germany, under accession no. DSM 12130.
Analytical conditions.
Organic acids, hydrogen sulfide, and growth were detected and quantified as described previously (34). Taurine was quantified by the high-performance liquid chromatography method used to quantify organic acids; a linear response was observed for taurine concentrations ranging from 2.5 to 20 mM. Nitrate concentrations were measured by suppressed ion chromatography with conductivity detection by using an IonPac AS4A-SC analytical column. The eluant was 1.8 mM Na2CO3–1.7 mM NaHCO3, and the flow rate was 2 ml/min. Protein was quantified by a modified Lowry procedure (35). Poly-β-hydroxybutyrate (PHB) was detected as crotonic acid and was quantified by using the assay of Law and Slepecky (31). Desulfoviridin was detected spectrophotometrically by determining the presence of a peak at 630 nm (26, 44, 53) and also by using the fluorescence test of Postgate (41). DNA G+C content and menaquinone analyses were performed by Hans Hippe of the DSMZ.
Electron microscopy. (i) Transmission electron microscopy.
Bacteria in the late exponential or early stationary phase were used for transmission electron microscopy. Cells grown in a liquid culture were concentrated by centrifugation and gently resuspended in a smaller volume of culture medium. The resulting concentrate was then pipetted onto UV-irradiated (15 min) Formvar- and carbon-coated grids and allowed to settle for 1 to 2 min. The cells were negatively stained with phosphotungstate for 1 min. Excess stain was removed by blotting, and the preparation was viewed with a Philips model EM 300 electron microscope at an accelerating voltage of 80 kV.
(ii) Scanning electron microscopy.
A few drops of a cell suspension in the mid-exponential to late exponential phase were placed onto cut silicon wafers (area, approximately 1 mm2) that had been coated with poly-l-lysine (0.1%, wt/vol). The cells were allowed to settle for 5 to 10 min. Attached cells were then fixed with a solution containing 1.5% (wt/vol) glutaraldehyde and 1.5% (wt/vol) formaldehyde in 0.1 M HEPES buffer (pH 7.6) containing 3 mM MgCl2 for 1 h. After two washes in distilled water, the cells were postfixed with 1% (wt/vol) OsO4 in distilled water overnight. Samples were washed three times in distilled water and then dehydrated twice in a graded series of ethanol solutions (50, 70, and 100% [vol/vol] ethanol). The wash solutions were partially drained in order to leave ca. 10% of the solution, so that the silicon wafers remained immersed. New solutions were added gently, so spinae were not detached from the cells. The wafers were then dried with a critical point dryer (Polaron model E3000) for 2 h, sputter coated (Polaron model E5100) with gold and palladium, and then viewed with a Zeiss model DSM 982 Gemini field emission scanning electron microscope operated at an accelerating voltage of 2 kV.
Isolation of nucleic acids and sequencing.
One milliliter of a culture of strain T1 was placed in a sterile microcentrifuge tube and centrifuged for 10 min at 13,500 × g. The supernatant was discarded, and the pellet was resuspended in water. The contents were then vortexed and boiled for 7 min, which yielded a crude DNA template. Nearly full-length 16S ribosomal DNA (rDNA) was amplified in four sets of 100-μl reaction mixtures containing 3 μl of DNA template, 0.2 μM universal primer fD1, 0.2 μM universal primer rD1 (49), 200 μM dATP, 200 μM dCTP, 200 μM dGTP, 200 μM dTTP, 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2.5 mM MgCl2, and 3.75 U of AmpliTaq DNA polymerase (Perkin-Elmer Corp., Norwalk, Conn.).
The PCR was performed with a Perkin-Elmer model 2400 thermal cycler by using the following conditions: primary denaturation at 94°C for 2 min and 35 cycles consisting of denaturation at 94°C for 30 s, primer annealing at 55°C for 30 s, and DNA extension at 72°C for 1 min. The reaction mixtures were kept at 72°C for 7 min, and then the PCR was terminated. The four PCR mixtures were combined. The presence of nearly full-length 16S rDNA was confirmed by horizontal agarose gel electrophoresis, which yielded a single band of the expected size. The PCR amplicon was then purified with a Qiagen (Chatsworth, Calif.) column and quantified with a model DyNA Quant 200 DNA fluorometer (Hoefer Pharmacia Biotech, Inc., San Francisco, Calif.).
The 16S rDNA amplicon was cycle sequenced and analyzed with an Applied Biosystems Prism sequencer (Perkin-Elmer). The initial sequence was generated with primers fD1 and rD1 by using an Applied Biosystems cycle sequencing kit. The entire amplicon was then cycle sequenced with primers spaced approximately 300 bases apart. The 16S rDNA amplicon was completely sequenced in both directions.
Phylogenetic inference.
Searches performed with FASTA (39) and BLAST (54) revealed that the 16S rDNA gene of strain T1 was closely related to the 16S rDNA genes of sulfate-reducing members of the δ subclass of the class Proteobacteria (δ-Proteobacteria). The GCG package (10) run on a VAX computer was used to retrieve the following 16S rDNA signatures: AB015241 of unidentified proteobacterial strain JTB20, L42613 of “Desulforhopalus vacuolatus,” X99707 of Desulfofustis glycolicus, X95181 of Desulfocapsa thiozymogenes, M34411 of Desulfobulbus sp., X95180 of Desulfobulbus elongatus, M34410 of Desulfobulbus propionicus, L07834 of Geobacter metallireducens, X70954 of Pelobacter propionicus, M26634 of Desulfuromonas acetoxidans, X83274 of Desulforhabdus amnigenus, L27426 of Desulfacinum infernum, M34403 of “Desulfoarculus baarsii,” X85131 of Syntrophus buswellii, X85132 of Syntrophus gentianae, X93994 of Desulfovibrio sp., and J01695 of Escherichia coli.
The sequences were aligned and edited by using ClustalW. Sites that did not contain at least 50% of one base were deleted from the alignment. Neighbor-joining and parsimony trees (Parsimony trees are not shown) were generated by using PHYLIP (18), and maximum-likelihood trees (data not shown) were generated by using PUZZLE (45). The trees were viewed in TREEVIEW (38). Phylogenetic trees were also constructed for an unedited alignment containing all sites.
Cell extracts and enzymology.
Cells were grown in 500-ml bottles capped with screw caps with butyl rubber stoppers, which allowed us to introduce or withdraw substrates with syringes. The cells were harvested by centrifugation and washed with marine buffer (343 mM NaCl, 6 mM MgCl2, 10 mM Tris-HCl; pH 7.2) three times. After resuspension in a small volume of the buffer, the cells were broken with a French pressure cell at 15,000 lb/in2. The preparation was centrifuged at 10,000 × g, and the supernatant (cell extract) was used for enzyme assays. Adenylylphosphosulfate (APS) reductase assays were performed as described previously (33).
Nucleotide sequence accession number.
The 16S rRNA-encoding DNA sequence of strain T1 has been deposited in the GenBank database under accession no. AF118453.
RESULTS
Enrichment cultures.
In the primary enrichment culture containing malate plus taurine, growth and hydrogen sulfide were detected after about 1 week of incubation at 28°C. The enrichment culture consisted predominantly of vibrios and some rods. A secondary enrichment culture containing taurine (10 mM) as the sole carbon and energy source contained predominantly rod-shaped bacteria after about 1 week; millimolar concentrations of hydrogen sulfide were detected. A pure culture of strain T1 was obtained from the taurine enrichment culture after five sequential streaking procedures to obtain well-separated colonies. Culture purity was confirmed microscopically and by the lack of aerobic or anaerobic growth in complex medium (AC medium [Difco]) under marine and nonmarine conditions.
Morphology and physical characteristics.
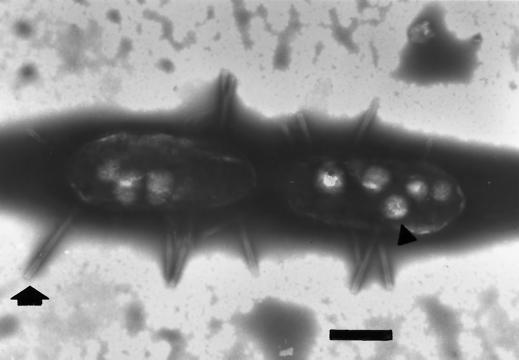
Cells of strain T1 were rod shaped; the cell lengths ranged from 1.7 to 2.3 μm, and the cell widths ranged from 0.9 to 1.2 μm. Often the cells were in chains containing up to six cells. As determined by electron microscopy, some cells contained nonprosthecate structures called spinae (14, 15) (Fig. 1 and 2). The spinae that were produced appeared to be the type of spinae produced by marine pseudomonad strain D7 (13). The bases of the spinae were flared, and the spinae appeared to be hollow from the base to the tip (Fig. 1, arrow). The spinae appeared to be somewhat flexible, and some of the spinae were as long as 2.5 μm. Spinae were observed on cells grown by anaerobic respiration with malate plus sulfate, as well as on cells fermenting taurine or pyruvate, although we noticed more spinae on cells grown with taurine; no detailed studies were made to determine which conditions resulted in maximum spina production. Cells were not motile, nor were flagella detected by electron microscopy. Spores were not observed, and no growth was detected after pasteurization at 80°C for 10 min; Desulfitobacterium hafniense (which forms endospores) was the positive control used.
FIG. 1.
(a) Scanning electron micrograph of strain T1 grown with taurine as the sole carbon and energy source. The arrow indicates a spina; the arrowhead indicates a truncated spina. Bar = 1 μm. (b) magnified image of the truncated spina in panel A. Bar = 200 nm.
FIG. 2.
Transmission electron micrograph of strain T1 grown with taurine as the sole carbon and energy source. Cells were negatively stained with phosphotungstate. The arrow indicates a representative spina; the arrowhead indicates an inclusion body. Bar = 2 μm.
Growth conditions and physiological characteristics.
The growth temperature range tested was 17 to 37°C. We found that strain T1 is a mesophile with an optimum growth temperature of about 31°C, and growth occurred at temperatures of 20 to 35°C. No growth occurred at 17 or 37°C. Growth occurred at pH 6.0 to 8.2, and the optimum pH was 7.4. Growth occurred in brackish medium but not in freshwater medium. The electron donors that supported growth with sulfate included formate (40 mM), ethanol (5 mM), propanol (10 mM), butanol (10 mM), lactate (10 mM), pyruvate (10 mM), propionate (10 mM), malate (10 mM), fumarate (10 mM), succinate (10 mM), Casamino Acids (0.2%, wt/vol), butyrate (5 mM), isobutyrate (5 mM), and alanine (10 mM). The electron donors tested that did not support growth included acetone (10 mM), acetate (10 mM), glycolate (5 mM), glyoxylate (5 mM), ethylene glycol (5 mM), tetraethylene glycol (5 mM), ethylamine (5 mM), ethanolamine (5 mM), cysteine (10 mM), glucose (10 mM), and benzoate (2.5 mM). The organic substrates that supported fermentative growth included taurine (5 mM) and pyruvate (10 mM). Growth occurred with sulfite (10 mM) as the sole source of energy when 2.5 mM acetate was a source of carbon; no growth occurred when thiosulate (20 mM) replaced sulfite. Fumarate, lactate, succinate, malate, isethionate, cysteate, coenzyme M, bromoethanesulfonate, sulfosuccinate, and 3-aminoethanesulfonate (each at a concentration of 10 mM) were not fermented. When malate was the carbon and energy source, the TEA that supported growth included sulfite (5 mM), thiosulfate (10 mM), sulfate (10 mM), and nitrate (10 mM). However, when sulfide (final concentration, 0.5 mM) was used as a reductant instead of titanous chloride, strain T1 did not grow with nitrate as the TEA. The TEA that did not support growth included dimethyl sulfoxide (10 mM), fumarate (10 mM), and the sulfonates isethionate (10 mM), cysteate (10 mM), coenzyme M (10 mM), bromoethanesulfonate (10 mM), and sulfosuccinate (10 mM).
Desulfoviridin was not detected in cell extracts; cell extracts of sulfate-grown strain IC1 cells were used as the positive control. The cells contained menaquinone 5 (H2), as revealed by high-performance liquid chromatography. The G+C content of the DNA was 50.6 ± 0.2 mol%.
Growth with taurine as a fermentable substrate and production of inclusion bodies.
Taurine served as a sole source of carbon, energy, and nitrogen for growth when it was used in the absence of ammonia and dinitrogen. The soluble end products obtained from taurine fermentation were ammonia, acetate, and hydrogen sulfide. At taurine concentrations of approximately 11 and 16 mM, about 83% of the carbon was recovered (Table 1). Cells grown with taurine also contained large numbers of inclusion bodies (Fig. 2), which were found to be PHB. Taurine-grown cells and cells grown on malate plus sulfate contained 50 mg of PHB per g (wet weight) (25% of the cell dry weight) and 14 mg of PHB per g (wet weight) (7% of the cell dry weight), respectively.
TABLE 1.
Stoichiometric relationship between substrates utilized and end products detected
| Substrate(s) provided | Substrate remaining | Acetate
|
Sulfidec
|
Ammoniac
|
|||
|---|---|---|---|---|---|---|---|
| Concn detected (mM) | % of theoretical valueb | Concn detected (mM) | % of theoretical valueb | Concn detected (mM) | % of theoretical valueb | ||
| Taurine (5.42 mM) | NDd | 1.61 | 59 | 5.44 | 100 | 3.8 | 70 |
| Taurine (11.33 mM) | ND | 4.73 | 83 | 11.58 | 102 | 11 | 95 |
| Taurine (16.33 mM) | Taurine (1.19 mM) | 6.22 | 82 | 14.26 | 94 | 14 | 98 |
| Taurine (11.63 mM)a | ND | 3.79 | 65 | 12.16 | 105 | 9.9 | 81 |
| Taurine (11.43 mM) + nitrate (10 mM)a | Nitrate (10 mM) | 3.19 | 56 | 12.64 | 111 | 11.43 | 90 |
Titanous chloride replaced sulfide as the reductant.
Percentages were calculated based on the following equation (see text): C2H7O3NS→0.5 C2H4O2 + CO2 + NH4+ + HS−.
Values were corrected for the initial sulfide concentration and the initial ammonia concentration used as the reductant and the nitrogen source, respectively.
ND, not detected.
The sulfur of taurine was quantitatively recovered as hydrogen sulfide, and most of the nitrogen of taurine was recovered as ammonia when the initial concentrations of taurine were ca. 5 and 11 mM and taurine was completely utilized (Table 1). At a taurine concentration of ca. 16 mM, about 7% of the taurine remained; no further increase in acetate production was observed. Sulfide at concentrations of about 15 mM appeared to inhibit growth.
When cells were grown with ca. 11 mM taurine and 10 mM nitrate as the electron donor and the electron acceptor respectively, nitrate was not utilized as a TEA (Table 1). The taurine, however, was completely consumed; sulfide, ammonia and acetate were detected at concentrations essentially identical to the concentrations obtained when approximately the same concentration of taurine was the sole carbon, energy, and nitrogen source in the absence of added nitrate (Table 1).
Stoichiometry of substrate conversion coupled to sulfate reduction.
Growth with fumarate (4.9 mM) plus sulfate (10 mM) resulted in production of sulfide (5.7 mM), but acetate was not detected. In cultures containing lactate (4.8 mM) plus sulfate (10 mM), sulfide (3.6 mM) and acetate (3.5 mM) were detected as end products (Table 2). The final optical densities at 650 nm after growth with lactate and after growth with fumarate were 0.17 and 0.40, respectively.
TABLE 2.
Stoichiometry of substrates utilized and end products formeda
| Carbon source utilized | Optical density at 650 nm | Acetate concn (mM) | Sulfide concn (mM) | Ratio of carbon source utilized to acetate produced to sulfide produced |
|---|---|---|---|---|
| Lactate (4.8 mM) | 0.17 | 3.5 | 3.6 | 1:0.73:0.75 |
| Fumarate (4.9 mM) | 0.40 | 0 | 5.7 | 1:0:1.16 |
The initial sulfate concentration was 10 mM.
APS reductase levels during growth with various substrates and effects of sulfate analogs on growth with taurine.
Cells grown with sulfate as the TEA had a APS reductase specific activity of 0.83 μmol of ferricyanide reduced/min/mg of protein, while taurine-fermenting cells had a lower specific activity (0.31 μmol of ferricyanide reduced/min/mg of protein). Molybdate at a concentration of 5 mM and 10 mM tungstate inhibited strain T1 growth with 10 mM taurine; no growth was observed when the culture was incubated for 1 month.
Phylogenetic characteristics.
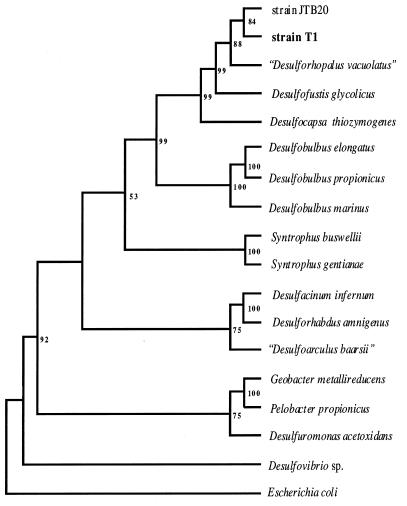
The phylogenetic trees generated by the maximum-likelihood, neighbor-joining, and parsimony algorithms were in virtual agreement for both edited and nonedited alignments. Agreement of phylogenetic trees for members of the δ-Proteobacteria when edited or nonedited alignments were used has been described previously (20). All of the trees placed strain T1 close to unidentified strain JTB20 and “Desulforhopalus vacuolatus” (23). Desulfofustis glycolicus (20) also branched close to strain T1. The maximum-likelihood tree (data not shown) suggested that “Desulforhopalus vacuolatus,” strain T1, and strain JTB20 had a common ancestor. The neighbor-joining tree in Fig. 3 shows that the bootstrap value for separation of Desulfofustis glycolicus from “Desulforhopalus vacuolatus,” strain T1, and JTB20 was 99%. The levels of similarity between strain T1 and JTB20, “Desulforhopalus vacuolatus,” and Desulfofustis glycolicus were 95.3, 93.0, and 91.5%, respectively. Additional phylogenetic support for clustering “Desulforhopalus vacuolatus” with strain T1 and JTB20 includes the presence of a unique 13-base deletion found in the unedited 16S rDNA alignment for only these three organisms. The 16S rDNA of Desulfofustis glycolicus does not contain this deletion. Many of the members of the δ-Proteobacteria in our alignment have an approximately 18-base insertion between E. coli nucleotide positions 186 and 187. The 13-base deletion is found in this insertion region. Because this stretch of 16S rDNA was found to be hypervariable, these sites were not used in the alignments used to produce Fig. 3. However, we consider the deletion significant and believe that it may be a phylogenetic characteristic of species belonging to the genus “Desulforhopalus.”
FIG. 3.
Neighbor-joining cladogram based on δ-proteobacterial 16S rDNA sequences. Sequences were aligned and edited in ClustalW. Hypervariable sites (as determined by less than 50% consensus of one base per alignment site) were removed. The aligned sequences were analyzed in PHYLIP. Seqboot was used to generate 500 bootstrap data sets. The bootstrap data sets were used to produce 500 distance matrices in DNADIST. The Kimura two-parameter model was used in DNADIST to account for probable variance in base substitution rates. Transversions were weighted twice as heavily as transitions were weighted. Neighbor-joining trees were constructed from the matrices by using NEIGHBOR. A consensus tree was generated in CONSENSE. The consensus tree was rooted with E. coli and was viewed in TREEVIEW. The numbers are bootstrap percentages. The levels of similarity between strain T1 and JTB20, “Desulforhopalus vacuolatus”, and Desulfofustis glycolicus were 95.3%, 93.0, and 91.5%, respectively.
DISCUSSION
Taurine fermentation.
The detected products of taurine fermentation by strain T1 were acetate, ammonia, and sulfide. Based on measurements of the substrates utilized and the products detected, we believe that the fermentation is represented by the following equation: C2H7O3NS→0.5 C2H4O2 + CO2 + NH4+ + HS−, where ΔG°′ = −136.99 kJ/mol of taurine (46). This contrasts with taurine fermentation in another obligate anaerobe, in which the sulfurous end product was thiosulfate, not sulfide (9). Whether taurine fermentation in strain T1 proceeds by the initial reactions demonstrated for the taurine-fermenting syntrophomonad (9) remains to be determined. The proposal of Cook (6) that transamination of taurine to sulfoacetaldehyde is followed by hydrolytic release of sulfite from sulfoacetaldehyde and that this process occurs during taurine metabolism in both aerobes and anaerobes, either for assimilatory or dissimilatory purposes, is an attractive proposal and is consistent with the overall stoichiometry proposed above.
PHB was produced in cells of strain T1 during taurine fermentation, as well as when malate oxidation was coupled to sulfate reduction. The amount of PHB produced during taurine fermentation was approximately three times more than the amount of PHB produced by cells grown on malate plus sulfate per gram (wet weight) of cells. A more detailed study of PHB production as it relates to taurine carbon assimilation is warranted.
Taurine-grown cells had an APS reductase specific activity that was less than one-half the APS reductase specific activity of sulfate-grown cells, suggesting that the sulfur of taurine is not released as sulfate and then assimilated. Low APS reductase activity was also exhibited by SRB utilizing TEA other than sulfate, including isethionate (33) or nitrate (12), for growth. Despite the fact that taurine-grown cells had a reduced level of APS reductase activity (and presumably ATP sulfurylase activity as well), it was surprising that this organism failed to grow with taurine in the presence of either molybdate or tungstate (which are considered competitive inhibitors of ATP sulfurylase and inhibit growth by effecting degradation of intracellular ATP [37]). It seems probable that the decreased ATP sulfurylase activity is nonetheless sufficient to deplete the ATP in cells.
Growth with taurine under nitrate-reducing conditions.
When sulfide (ca. 0.5 mM) was used as a reductant, strain T1 did not grow with malate plus nitrate; when titanous chloride was used as a reductant, growth via nitrate reduction to ammonia occurred. The apparent inhibition by sulfide is consistent with the sulfide inhibition of nitrate ammonification reported for a freshwater Desulfovibrio isolate (7).
Since strain T1 could ferment taurine but also reduced nitrate, we attempted to grow this isolate with taurine and nitrate as the electron donor and the electron acceptor, respectively (these conditions supported growth of a strain of Acaligenes sp. [8]). Titanous chloride was used as a reductant, and the inoculum had previously been grown with nitrate so as to not carry over any contaminating sulfide. We were surprised to observe that coupling of taurine oxidation to nitrate reduction did not occur and that instead taurine was fermented preferentially. Whether the sulfur of taurine is released as sulfide, thereby inhibiting the ability to grow with nitrate, or whether the sulfur is released as sulfite, which is then reduced in preference to nitrate, remains to be studied. Desulfovibrio desulfuricans Essex 6 (43) reduced nitrate in preference to sulfate when both TEA were present; however, other Desulfovibrio spp. could reduce nitrate and sulfate simultaneously (24, 36). Clearly, regulation of the metabolism of nitrate and sulfur compounds needs further study.
Oxidation of substrates during sulfate reduction.
The fact that acetate accumulated during lactate oxidation and fermentation of taurine by strain T1 suggests that this isolate should be considered an incomplete oxidizer; the lack of acetate accumulation when fumarate was oxidized indicates otherwise. Other than Desulfobacter spp., SRB that are considered complete oxidizers are known to excrete acetate, or not to excrete acetate, depending on the electron donor respired (51); one such SRB had (19) a ratio of amount of lactate consumed to amount of acetate (and sulfide) accumulated similar to the ratio obtained for strain T1.
Spinae.
Spina production is not limited to a particular physiological group (13), and thus far spinae have been found in Pseudomonas spp. (15), a Chlorobium sp. (4, 5), and a Synechococcus sp. (40). This apparently is the first report of spina production by a SRB. Whether the spinae in this SRB play roles that have been proposed for spinae in other bacteria (2, 13) remains to be determined.
Ecological significance.
Significant concentrations of sulfonates occur in the environment; these compounds account for 20 to 40% of the total organic sulfur in marine sediments (48) and at least 50% of the total organic sulfur in a variety of forest soils (1). This report and other reports of sulfidogenesis from anaerobic sulfonate dissimilation (29, 30, 33, 34) underscore the potential for sulfonates to be significant sources of hydrogen sulfide, as well as carbon sources, in various habitats. Sulfonate sulfur reduction may thus account for reported sulfide concentrations that are significantly higher than the concentrations expected based on the pool of sulfate present (11, 17). Since taurine fermentation by members of two physiologically distinct bacterial groups leads to production of different sulfurous end products (thiosulfate [9] and the bisulfide ion [this study]), important implications for the participation of sulfonate sulfur in the global sulfur cycle are evident.
Phylogenetic position.
The results of the phylogenetic analyses, as well as the presence of a unique 13-base deletion in both “Desulforhopalus vacuolatus” and strain T1, indicate strongly that strain T1 should be considered a member of the genus “Desulforhopalus.” The fact that a third uncharacterized bacterium (strain JTB20) may also fall into this genus is interesting; unfortunately, no information concerning this bacterium’s physiology is available.
Description of Desulforhopalus singaporensis sp. nov.
Desulforhopalus singaporensis (sin.ga.po′rensis. M.L. n. Singapore, Republic of Singapore; L. suff. -ensis, native of; M.L. adj. Singaporensis, native of Singapore, referring to the place of isolation). Gram-negative cells that are 1.7 to 2.3 μm long and 0.9 to 1.2 μm wide. Endospores not detected. Cells may grow as chains containing two to six cells. No flagella. Cells able to produce the nonprosthecate structures called spinae. Strict anaerobe. Grows with 2-aminoethanesulfonate (taurine) as a sole carbon, energy, and nitrogen source without an additional TEA. Grows with sulfite as the sole source of energy with acetate as the source of assimilatory carbon. Grows chemotrophically with oxidation of formate, lactate, pyruvate, fumarate, succinate, butyrate, ethanol, butanol, or Casamino Acids coupled to reduction of sulfate; slow growth occurs with propionate, isobutyrate, and caproate. Acetate accumulates from lactate oxidation but not from fumarate oxidation. H2 and acetate are not utilized. Pyruvate is fermented. Sulfite, thiosulfate, and nitrate serve as alternate TEA for growth; 0.5 mM sulfide inhibits growth on nitrate. Desulfoviridin not detected. Cells contain menaquinone 5(H2). The pH range for growth is 6.0 to 8.2, and the optimum pH is 7.4. The temperature range for growth is 20 to 35°C, and the optimum temperature is 31°C. Isolated from marine sulfidogenic mud from a saltwater marsh in the Republic of Singapore. The G+C content of the genomic DNA is 50.6 ± 0.2 mol%. The type strain is S’pore T1, which has been deposited in the DSMZ as strain DSM 12130.
ACKNOWLEDGMENTS
We thank Terry Beveridge of the University of Guelph, Guelph, Ontario, Canada, for comments on bacterial spinae and David Benson and Jeffrey Gawronski for helpful advice and discussions about the phylogeny of strain T1. We thank Marie Cantino, Lamia Khairallah, Steve Daniels, and Jim Romanow of the Electron Microscopy Facility (University of Connecticut) for the excellent electron microscans. We appreciate helpful discussions with Pieter Visscher, the use of his ion chromatograph, and the technical assistance of Shelley Hoeft and Dan Rogers. Valuable comments from anonymous reviewers improved the manuscript.
REFERENCES
- 1.Autry A R, Fitzgerald J W. Sulfonate S: a major form of forest soil organic sulfur. Biol Fertil Soils. 1990;10:50–56. [Google Scholar]
- 2.Bayer M E, Easterbrook K. Tubular spinae are long-distance connectors between bacteria. J Gen Microbiol. 1991;137:1081–1086. doi: 10.1099/00221287-137-5-1081. [DOI] [PubMed] [Google Scholar]
- 3.Bickerton J, MacNab J I, Skinner H A, Pilcher G. Enthalpies of solution of some aromatic sulphonic acids and of some aminosulphonic acids. Thermochim Acta. 1993;222:69–71. [Google Scholar]
- 4.Brooke J S, Koval S F, Beveridge T J. Unusually stable spinae from a freshwater Chlorobiumsp. Appl Environ Microbiol. 1995;61:130–137. doi: 10.1128/aem.61.1.130-137.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooke J S, Thompson J B, Beveridge T J, Koval S F. Frequency and structure of spinae on Chlorobiumspp. Arch Microbiol. 1992;157:319–322. [Google Scholar]
- 6.Cooke A M. Sulfonated surfactants and related compounds: facets of their desulfonation by aerobic and anaerobic bacteria. Tenside Surfact Deterg. 1998;35:52–56. [Google Scholar]
- 7.Dalsgaard T, Bak F. Nitrate reduction in a sulfate-reducing bacterium, Desulfovibrio desulfuricans, isolated from rice paddy soil: sulfide inhibition, kinetics, and regulation. Appl Environ Microbiol. 1994;60:291–297. doi: 10.1128/aem.60.1.291-297.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denger K, Laue H, Cook A M. Anaerobic taurine oxidation: a novel reaction by a nitrate-reducing Alcaligenessp. Microbiology. 1997;143:1919–1924. doi: 10.1099/00221287-143-6-1919. [DOI] [PubMed] [Google Scholar]
- 9.Denger K, Laue H, Cook A M. Thiosulfate as a metabolic product: the bacterial fermentation of taurine. Arch Microbiol. 1997;168:297–301. doi: 10.1007/s002030050502. [DOI] [PubMed] [Google Scholar]
- 10.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunnette D A, Chynoweth D P, Mancy K H. The source of hydrogen sulfide in anoxic sediment. Water Res. 1985;19:875–884. [Google Scholar]
- 12.Dzierżewicz Z, Cwalina B, Chodurek E, Bulaś L. Differences in hydrogenase and APS-reductase activity between Desulfovibrio desulfuricansstrains growing on sulphate or nitrate. Acta Biol Cracov Ser Bot. 1997;39:9–15. [Google Scholar]
- 13.Easterbrook K B. Spinate bacteria. In: Staley J T, Bryant M P, Pfennig N, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 3. Baltimore, Md: The Williams & Wilkins Co.; 1989. pp. 1991–1993. [Google Scholar]
- 14.Easterbrook K B, Coombs R W. Spinin: the subunit protein of bacterial spinae. Can J Microbiol. 1976;22:438–440. doi: 10.1139/m76-067. [DOI] [PubMed] [Google Scholar]
- 15.Easterbrook K B, Willison J H M, Coombs R W. Arrangement of morphological subunits in bacterial spinae. Can J Microbiol. 1976;22:619–629. doi: 10.1139/m76-092. [DOI] [PubMed] [Google Scholar]
- 16.Eichhorn E, van der Ploeg J R, Kertesz M A, Leisinger T. Characterization of α-ketoglutarate-dependent taurine dioxygenase from Escherichia coli. J Biol Chem. 1997;272:23031–23036. doi: 10.1074/jbc.272.37.23031. [DOI] [PubMed] [Google Scholar]
- 17.Federle T W, Schwab B S. Mineralization of surfactants in anaerobic sediments of a laundromat waste pond. Water Res. 1992;26:123–127. [Google Scholar]
- 18.Felsenstein J. PHYLIP (phylogeny inference package), version 3.5c. Seattle: Department of Genetics, University of Washington; 1993. [Google Scholar]
- 19.Friedrich M, Schink B. Isolation and characterization of a desulforubidin-containing sulfate-reducting bacterium growing with glycolate. Arch Microbiol. 1995;164:217–279. [Google Scholar]
- 20.Friedrich M, Springer N, Ludwig W, Schink B. Phylogenetic positions of Desulfofustis glycolicus gen. nov., sp. nov., and Syntrophobotulus glycolicusgen. nov., sp. nov., two new strict anaerobes growing with glycolic acid. Int J Syst Bacteriol. 1996;46:1065–1069. doi: 10.1099/00207713-46-4-1065. [DOI] [PubMed] [Google Scholar]
- 21.Hanselmann K W. Microbial energetics applied to waste repositories. Experientia. 1991;47:645–687. [Google Scholar]
- 22.Higgins T P, Davey M, Trickett J, Kelly D P, Murrell J C. Metabolism of methansulfonic acid involves a multicomponent monooxygenase enzyme. Microbiology. 1996;142:1–10. doi: 10.1099/13500872-142-2-251. [DOI] [PubMed] [Google Scholar]
- 23.Isaksen M F, Teske A. Desulforhopalus vacuolatusgen. nov., sp. nov., a new moderately psychrophilic sulfate-reducing bacterium with gas vacuoles isolated from a temperate estuary. Arch Microbiol. 1996;166:160–168. [Google Scholar]
- 24.Keith S M, Herbert R A. Dissimilatory nitrate reduction by a strain of Desulfovibrio desulfuricans. FEMS Microbiol Lett. 1983;18:55–59. [Google Scholar]
- 25.Kelly D P, Smith N A. Organic sulfur compounds in the environment. Biogeochemistry, microbiology and ecological aspects. Adv Microb Ecol. 1990;11:345–385. [Google Scholar]
- 26.Kobayashi K, Takahashi E, Ishimoto M. Biochemical studies on sulfate-reducing bacteria. XI. Purification and some properties of sulfate-reductase, desulfoviridin. J Biochem (Tokyo) 1972;72:879–887. doi: 10.1093/oxfordjournals.jbchem.a129982. [DOI] [PubMed] [Google Scholar]
- 27.Kondo H, Ishimoto M. Purification and properties of sulfoacetaldehyde sulfo-lyase, a thiamine pyrophosphate-dependent enzyme forming sulfite and acetate. J Biochem (Tokyo) 1975;78:317–325. doi: 10.1093/oxfordjournals.jbchem.a130910. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn E P, Suflita J M. Anaerobic biodegradation of nitrogen-substituted and sulfonated benzene aquifer contaminants. Haz Waste Haz Mater. 1989;6:121–133. [Google Scholar]
- 29.Laue H, Denger K, Cook A M. Fermentation of cysteate by a sulfate-reducing bacterium. Arch Microbiol. 1997;168:210–214. doi: 10.1007/s002030050502. [DOI] [PubMed] [Google Scholar]
- 30.Laue H, Denger K, Cook A M. Taurine reduction in anaerobic respiration of Bilophila wadsworthiaRZATAU. Appl Environ Microbiol. 1997;63:2016–2021. doi: 10.1128/aem.63.5.2016-2021.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Law J H, Slepecky R A. Assay of poly-β-hydroxybutyric acid. J Bacteriol. 1961;82:33–36. doi: 10.1128/jb.82.1.33-36.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leidner H, Gloor R, Wüest D, Wuhrmann K. The influence of the sulphonic group on the biodegradability of n-alkylbenzene sulphonates. Xenobiotica. 1980;10:47–56. doi: 10.3109/00498258009033730. [DOI] [PubMed] [Google Scholar]
- 33.Lie T J, Leadbetter J R, Leadbetter E R. Metabolism of sulfonic acids and other organosulfur compounds by sulfate-reducing bacteria. Geomicrobiol J. 1998;15:135–149. [Google Scholar]
- 34.Lie T J, Pitta T, Leadbetter E R, Godchaux III W, Leadbetter J R. Sulfonates: novel electron acceptors in anaerobic respiration. Arch Microbiol. 1996;166:204–210. doi: 10.1007/s002030050376. [DOI] [PubMed] [Google Scholar]
- 35.Markwell M A K, Hass S M, Bieber L L, Tolbert N E. A modification of Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 36.McCready R G L, Gould W D, Cook F D. Respiratory nitrate reduction by Desulfovibriosp. Arch Microbiol. 1983;146:63–67. [Google Scholar]
- 37.Oremland R S, Capone D G. Use of specific inhibitors in biogeochemistry and microbial ecology. Adv Microb Ecol. 1988;10:285–383. [Google Scholar]
- 38.Page R D M. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput Applic Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 39.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perkins F O, Haas L W, Phillips D E, Webb K L. Ultrastructure of a marine Synechococcuspossessing spinae. Can J Microbiol. 1981;27:318–329. doi: 10.1139/m81-049. [DOI] [PubMed] [Google Scholar]
- 41.Postgate J R. The sulphate-reducing bacteria. 2nd ed. Cambridge, United Kingdom: Cambridge University Press; 1984. [Google Scholar]
- 42.Seitz A P, Leadbetter E R. Microbial assimilation and dissimilation of sulfonate sulfur. In: Vairavamurthy M A, Schoonen A A, editors. Geochemical transformation of sedimentary sulfur. American Chemical Society Symposium Series, no. 612. Washington, D.C: American Chemical Society; 1995. pp. 365–376. [Google Scholar]
- 43.Seitz H-J, Cypionka H. Chemolithotrophic growth of Desulfovibrio desulfuricanswith hydrogen coupled to ammonification of nitrate or nitrite. Arch Microbiol. 1986;146:63–67. [Google Scholar]
- 44.Steuber J, Cypionka H, Kroneck P M H. Mechanism of dissimilatory sulfite reduction by Desulfovibrio desulfuricans: purification of a membrane-bound sulfite reductase and coupling with cytochrome c3and hydrogenase. Arch Microbiol. 1994;162:255–260. [Google Scholar]
- 45.Strimmer K, Haeseler A V. Quartet puzzling: a quartet maximum likelihood method for reconstructing tree topologies. Mol Biol Evol. 1996;13:964–969. [Google Scholar]
- 46.Thauer R K, Jungermann K, Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977;41:100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vairavamurthy A, Manowitz B, Luther III G W, Jeon Y. Oxidation state of sulfur in thiosulfate and implications for anaerobic energy metabolism. Geochim Cosmochim Acta. 1993;57:1619–1623. [Google Scholar]
- 48.Vairavamurthy A, Zhou W, Eglinton T, Manowitz B. Sulfonates: a novel class of organic sulfur compounds in marine sediments. Geochim Cosmochim Acta. 1994;58:4681–4687. [Google Scholar]
- 49.Weisburg W G, Barns S M, Pelletier D A, Lane D J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whitham G H. Organosulfur chemistry. New York, N.Y: Oxford University Press; 1995. [Google Scholar]
- 51.Widdel F. Microbiology and ecology of sulfate- and sulfur-reducing bacteria. In: Zehnder A J B, editor. Biology of anaerobic microorganisms. New York, N.Y: John Wiley and Sons; 1988. pp. 469–585. [Google Scholar]
- 52.Widdel F, Bak F. Gram-negative mesophilic sulfate-reducing bacteria. In: Balows A, Truper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. A handbook on the biology of bacteria: ecophysiology, isolation, identification, applications. 2nd ed. Vol. 4. New York, N.Y: Springer-Verlag; 1992. pp. 33353–3378. [Google Scholar]
- 53.Widdel F, Pfennig N. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. I. Isolation of new sulfate-reducing bacteria enriched with acetate from saline environments. Description of Desulfobacter postgateigen. nov., sp. nov. Arch Microbiol. 1981;129:395–400. doi: 10.1007/BF00406470. [DOI] [PubMed] [Google Scholar]
- 54.Zhang J, Madden T L. PowerBLAST: a new network BLAST application for interactive or automated sequence analysis and annotation. Genome Res. 1997;7:649–656. doi: 10.1101/gr.7.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]