Abstract
Different types of tissues respond differently to the action of oxidative stress. The visual system is very sensitive to oxidative action due to continuous exposure to light. In consideration of the growing interest of scientific studies towards various compounds endowed with antioxidant and anti-inflammatory properties, we performed a review of the literature focusing on the use of some antioxidant molecules for the treatment of conditions affecting the visual system. In this study, we focused on the ability of two antioxidant agents, the small molecule α-lipoic acid (ALA) and the enzyme superoxide dismutase (SOD), to influence the neurodegenerative physiological processes related to aging and oxidative stress affecting the ocular segment. The literature data report that ALA and SOD can protect against neurodegenerative effects both the optic nerve and retina and, if administered together, they are able to lower the levels of oxidative stress, thus preventing neurodegeneration and reducing the apoptotic process.
Keywords: alpha-lipoic acid (ALA), antioxidants, retina and optic nerve aging, superoxide dismutase (SOD)
Oxidative Stress
The term oxidative stress refers to the set of alterations that occurs after the exposure of biological tissues, cells, and macromolecules, to an excess of oxidizing agents (Kudryavtseva et al., 2016[30]; Sies, 1993[49]; Halliwell and Gutteridge, 2007[21]). Reactive radical species are physiologically produced by the cell during metabolism, however, their excessive concentration due to an over-production or as a consequence of decreased levels of antioxidants can be harmful to cells (Halliwell and Gutteridge, 2007[21]; Li et al., 2016[32]). The substances responsible for oxidative stress are not only free radicals, but also other elements that have a different chemical structure but are capable to exert an oxidative action on cells. These are the so-called "non-radical oxidizing chemical species" which, unlike free radicals, that possess an unpaired electron at the orbital level, have their electrons mostly distributed in pairs and engaged in covalent bonds. An example is represented by the hydrogen peroxide (H2O2) (Li et al., 2016[32]; Sies, 2015[48]). Despite being structurally different, free radicals and non-radical chemical species share an intrinsic characteristic which is their oxidizing capacity. Frequently, when addressing the subject of free radicals reference is made to molecules which contain oxygen such as the radical anion superoxide (O2-), hydrogen peroxide (H2O2), and hydroxyl radicals (Li et al., 2016[32]; Sies, 2015[48]; Davies, 1995[14]). However, the oxidizing action may be exerted even by free radicals containing carbon or nitrogen (the so-called oxygen-free radicals) (Sies, 2015[48]).
Reactive species (RS)
Based on their chemical nature, reactive species (RS) are divided into reactive oxygen species (ROS), reactive nitrogen species (RNS), reactive chlorine species (RClS), reactive sulfur species (RSS), and reactive bromine species (RBrS). The most important ones, in both quantitative and qualitative terms, are ROS and RNS. In all aerobic organisms there is a delicate redox balance between the oxidizing substances produced, including ROS, and the antioxidant defence system which has the function of preventing and repairing the damage induced by them (Sies, 2015[48]; Davies, 1995[14]; Denisov and Afanas'ev, 2005[15]; Oz et al., 2005[44]). The excessive production of oxidizing agents in the absence of an effective defensive system may, in the long term, trigger a cascade of oxidative reactions highly harmful to cells, there by compromising their integrity. However, not all free radicals have to be considered harmful to the body; in fact, a small amount is normally produced and plays a protective role for the organism. Numerous biochemical processes in our organism continuously produce ROS and other RS (Uttara et al., 2009[61]), and certain amounts of oxidizing substances are essential to maintain the correct cell functioning and the mechanisms of homeostasis (Alkadi, 2020[4]).
Oxidant/antioxidant balance
In physiological conditions there is an antioxidant barrier characterized by a balance between the quantity of free radicals that are produced and the quantity of free radicals that are physiologically eliminated from the body. The antioxidant barrier plays a key role against free radicals; the human body has highly complex antioxidant defence systems that cooperate with other cellular protection systems against the oxidative damage. The enzymatic system with the highest antioxidant activitis represented by the superoxide dismutase (SOD), belonging to a family of metallo-enzymes whose function is to eliminate the radical anion superoxide. Another antioxidant system is represented by the glutathione (GSH) peroxidase (GPx), which has a reductive action against organic hydroperoxides using GSH as co-substrate. Furthermore, catalase (CAT) induces a reduction of hydrogen peroxide (Holley et al., 2011[25]; Li, 1995[33]).
SOD is an important physiological antioxidant which is found in three isoforms: extracellular superoxide dismutase (ECSOD), manganese superoxide dismutase (MnSOD), and copper-zinc superoxide dismutase (Cu/ZnSOD). MnSOD is mainly present in mitochondria, while Cu/ZnSOD is found in the cytosol. In situations of oxidative stress, when an increase in H2O2 is recorded, Cu/ZnSOD enters the cell nucleus where it carries out its antioxidant activity through gene regulation (Holley et al., 2011[25]; Li, 1995[33]; Vanfleteren, 1993[63]; Reveillaud et al., 1991[47]; Tsang et al., 2014[58]). The main source of redox species is the mitochondrial respiratory chain while MnSOD is the major mitochondrial antioxidant enzyme. Cu/ZnSOD has two identical sub-units of approximately 32 kDa, each containing a metal cluster. The active site consists of a copper atom and a zinc atom (Vanfleteren, 1993[63]; Reveillaud et al., 1991[47]; Tsang et al., 2014[58]).
In addition, the non-enzymatic antioxidants are divided into directly or indirectly acting antioxidants. The former include lipoic and carotenoids, and play a relevant role in the protection against oxidative stress. The latter comprise chelating and binding agents, which can reduce metal ions and prevent radical formation (Uttara et al., 2009[61]). An important group of non-enzymatic antioxidants which play a protective role against the damaging effects of ROS are the thiol group (-SH) containing molecules (Valko et al.,2006[62]). The α-lipoic acid (ALA) is a disulfide derived from octanoic acid that is considered a free radical scavenger and a very important antioxidant thiol. Indeed, ALA possesses two oxidized or reduced thiol groups capable of neutralizing diverse reactive species of ROS and RNS. Therefore, ALA can rapidly induce an increase of the intracellular GSH and ascorbate levels in the liver, which usually tend to decrease with age (Nebbioso et al., 2013[41]; Lykkesfeldt and Ames, 1999[38]).
Oxidative Stress and the Visual System
Different types of tissues respond differently to the action of oxidative stress. In particular, the tissues of the central nervous system are highly sensitive to oxidative damage because they are characterized by a low level of antioxidant enzymes and a high content of oxidizable substrates. Furthermore, neuro-chemical reactions produce numerous free radicals. The oxidative stress has the undesirable effect of breaking a biochemical balance, can affect the onset or course of a large number of pathologies, and is responsible for both premature aging and neurodegeneration (Sies, 1993[49]; Tangvarasittichai and Tangvarasittichai, 2018[53]). Vitamins, polyphenols, and trace elements also intervene in this important defensive activity. In specific conditions, free radicals can cause cell and tissue damage, inducing chronic inflammatory reactions such as the typical alterations affecting the eye and the entire visual system (Ung et al., 2017[59]). Indeed, given its anatomical structure and constant contact with the external environment the eye is undoubtedly one of the organs most widely affected by free radicals (Tangvarasittichai and Tangvarasittichai, 2018[53]; Ung et al., 2017[59]; Nebbioso et al., 2013[42], 2012[40]). The appearance of inflammatory processes affecting the eye and the exposure to various types of radiations or chemical agents can be directly responsible for the high increase in hydrogen peroxide in the eye, with a consequent increase in ROS responsible for oxidative action. The oxidative action exerted by free radicals at ocular level manifests as structural and functional alterations in the corneal epithelium associated with apoptotic processes in which keratocytes and stromal fibroblasts play a central role (Ung et al., 2017[59]; Taurone et al., 2020[54]). In the light of such scientific evidence, the aim of this review was to evaluate the antioxidant effects of ALA and SOD in the visual system, and the possible induction capacity of tissue regeneration following neurodegenerative damage caused by the accumulation of free radicals.
Mechanisms of oxidative damage to the visual system
Oxidative stress is believed to be one of the mechanisms responsible for the cellular and neuronal damage observed both during the normal aging process and during development of neurodegenerative diseases (Good el al., 1996[19]) and affects all tissues including those of the visual system. Consequently, the role of oxidative stress continues to be the subject of numerous studies. It has long been established that an increase in reactive chemical species leads to damage at the level of biological macromolecules, including nucleic acids (DNA and RNA), lipids, and proteins. Once this damage has been triggered, its role in aging and neurodegenerative disorders may be critical. Indeed, many authors have suggested a correlation between increased damage, defects in the DNA repair system, aging, and neurodegenerative diseases (Du et al., 2009[17]). It has been demonstrated that DNA oxidation increases with age, with higher levels in the cerebral cortex and cerebellum of elderly subjects (Butterfield et al., 2007[8]).
An imbalance of the homeostatic mechanisms that regulate the equilibrium between oxidizing and antioxidant substances results in oxidative damage (Alderton et al., 2001[3]), and this imbalance also plays a fundamental role in the etiology of a great variety of inflammatory and neurodegenerative disorders (Abushouk et al., 2017[2]; Halliwell and Gutteridge, 2015.[22] Nowadays, growing attention is being paid to the cellular aging process and to the underlying factors (Hase et al., 2018[24]; Davalli et al., 2016[13]; Stefanatos and Sanz, 2018[51]), in an attempt to clarify the involutional and degenerative pathologies occurring in old age, which affect various organs and systems. In this context, the neurodegenerative processes impacting the visual system assume particular importance, owing to the frequency and the severity of the disability they may cause (Taurone et al., 2020[54]; Babizhayev and Yegorov, 2016[5]). The expression of the alterations and the severity of damage ranges from slight modifications of the main metabolic functions to more severe modifications of neurotransmitters with impairment of the neuronal function. The exact molecular mechanisms underlying these processes have still not been entirely clarified and little is known regarding their temporal sequence. In the light of these uncertainties, the etiopathogenesis of neurodegenerative processes must be considered heterogeneous and multifactorial. Recent experimental studies have been performed to understand whether the neurodegeneration mechanisms identified are specific for a single pathology or common to different conditions. Recently, the role played by oxidative stress in the pathogenesis of neurological disorders has been the subject of great interest. There is strong evidence that free radicals are involved in the onset and progression of many eye diseases (Ung et al., 2017[59]). Inflammation and oxidative stress have a crucial role in the etiology and progression of age-related eye diseases, which are the leading causes of blindness and include glaucoma, age-related macular degeneration, diabetic retinopathy, and dry eye disease (Taurone et al., 2015[56], 2019[57], 2020[55]; Fehér et al., 2018[18]). In recent years, many studies have focused their attention on phytochemical compounds with anti-inflammatory and antioxidant properties, which can be advantageously employed for treating and, in particular, for preventing several eye diseases. Some phytochemical compounds, such as carotenoids, resveratrol, and curcumin, can lower the production of ROS and consequently inhibit the activity of VEGF and TNF-α, as well as of other inflammatory cytokines (Rauf et al., 2017[45]; London and Beezhold, 2015[35]; Abu-Amero et al., 2016[1]). The term “antioxidant” covers all those molecules capable of stabilizing or deactivating free radicals before they produce cell damage. Several experimental models have been introduced to better understand the mechanisms responsible for the damage produced by ischemic phenomena affecting the retina. Numerous in vitro models of damage to retinal ganglion cells (RGC) based on the reduction of oxygen and glucose availability have been described in literature (Izumi et al., 2003[26]; Kinukawa et al., 2005[27]; Mastrodimou et al., 2005[39]). Ischemic diseases of the central nervous system are mediated by a complex cascade of biochemical events in which the excitatory neurotransmitter glutamate is a common element and plays a central role in the degenerative process. This is also true for the retina indeed, the excessive activation of NMDA (N-methyl-D-aspartate) and non-NMDA receptors, as well as the consequent accumulation of nitric oxide, are among the mechanisms responsible for the cell loss induced by ischemic phenomena. Furthermore, this loss may be prevented by systemic pre-treatment with NMDA and non-NMDA receptor antagonists and with L-NAME (L-Nitroarginine methyl ester) (Nucci et al., 2007[43]). In metabolic terms, the eye is a very active structure. Since it is mostly exposed to light, oxidative and, particularly, photo-oxidative processes play a critical role in the pathological conditions of the eye, in particular the ones associated with aging (Williams, 2008[64]).
A variety of interconnected pathways may lead to generation of ROS in the eye. The normal process of electron transport and the activity of cytochrome P450 can generate the superoxide anion. The production of free radicals may be inhibited by the enzymatic activity of molecules such as SOD, CAT, and GPx by restoring the body homeostasis (Lightfoot et al., 2006[34]). Oxidative stress is the result of free radicals failing to be inhibited by antioxidant enzymes (Zanza et al., 2019[68]). This deficit induces cellular oxidation which leads to tissue necrosis (Remacle et al., 1995[46]). This process becomes particularly accentuated with aging, where the damage induced by oxidative stress increases and the normal cell regeneration processes are less active (Cabrera and Chihuailaf, 2011[9]; Carneiro and Andrade, 2017[12]).
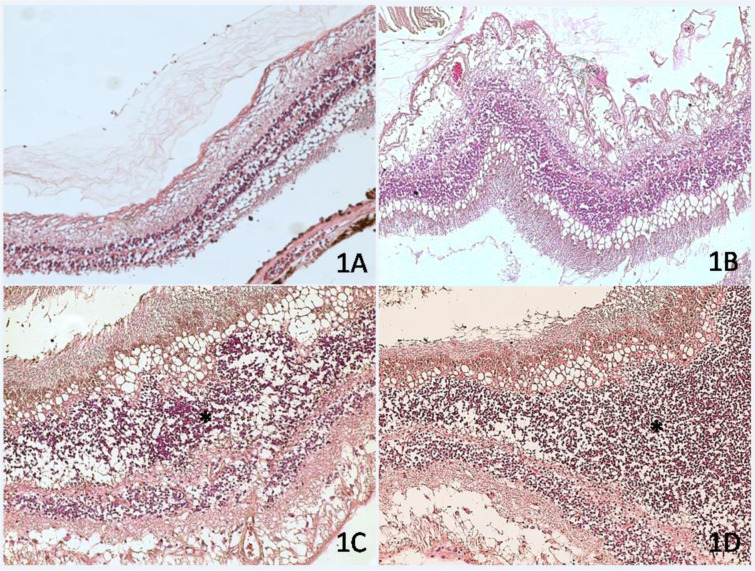
Oxidative stress plays an important role also in the development of diabetic retinopathy (Kubes, 2000[29]). The increase in blood glucose concentration determines numerous metabolic imbalances that contribute to the production of free radicals responsible for oxidative stress (Lopez-Galvez et al., 2014[37]). The high exposure of the delicate retinal tissues to the action of free radicals induces an increase in inflammatory cytokines such as IL-1β and TNF-α which act on the vascular cells inducing angiogenesis at the level of retinal lesions (Li et al., 2017[31]). The retinal photoreceptors carry out an intense metabolic activity and are therefore continuously exposed to the damage induced by free radicals (Figure 1(Fig. 1)). Following ischemia, a high amount of ROS is produced within the retina, with a consequent increase of damage induced by oxidative stress and loss of retinal cones (Gopinath et al., 2018[20]; Campochiaro and Mir, 2018[10]).
Figure 1. Human retina of patients affected by diabetic retinopathy. H&E stain. Within the retinal tissue, some microhemorrhages and cystoid degeneration of the inner layers of the retina with cellular apoptosis (C and D, asterisk) are visible. (1A) magnification 10×. (1B) Magnification 20 x; (1C-1D) Magnification 40 x.
The role of antioxidant molecules in the visual system
The aim of our study was to identify the factors responsible for progression of age-related inflammatory eye diseases in order to prevent the irreversible damage that causes blindness. Considering this, we have focused our attention on the antioxidant activity of ALA and SOD in the visual system. The aged tissues, namely those prone to neurodegeneration, significantly express iNOS. Once produced, NO can cause irreversible tissue injuries (Campochiaro et al., 2015[11]; Wu and Rao, 2008[67]; Steinert et al., 2010[52]). The toxic effects of iNOS have been documented in a wide range of degenerative and inflammatory disorders, including Parkinson's disease, Alzheimer's disease, and multiple sclerosis (Wu and Rao, 2008[67]; Upadhyay and Dixit, 2015[60]). The antioxidant action of SOD and GST enzymes seems to inhibit the production of ROS; moreover, the enzymatic activity of these enzymes has a protective action on the neurons (Simon et al., 2000[50]). Studies regarding iNOS over-expression in mice have demonstrated that increased NO levels could cause retinal photoreceptor apoptosis death (Wright et al., 2010[65]). ROS may induce or prevent the apoptotic process and such activity depends on their intracellular concentration (Handa, 2012[23]). Treatment with antioxidant molecules leads to a reduction in the oxidative damage (Wright et al., 2010[65]; Handa, 2012[23]). Furthermore, an increase in the stability of mitochondrial membranes, as shown by the diminution of the LOP reaction induced by free radicals, could be useful to reduce the release of some pro-apoptotic molecules that are responsible for the induction of the programmed cell death.
Some reports indicate that oxidative stress induces a deficiency of MnSOD and consequently an increase in the apoptotic process (Kokoszka et al., 2001[28]). Considering that ALA is also able to reduce cellular apoptosis, the combined use of the two agents seems to greatly strengthen their antioxidant effects, most likely as a consequence of a synergistic action of ALA and SOD. In particular, considering that SOD has an extracellular activity and ALA mainly an intracellular one (Nebbioso et al., 2013[42], 2012[40]; Bertolotto and Massone, 2012[6]), their combined use for anti-apoptotic purposes could be useful in reducing the neurodegeneration at retinal level.
Conclusions
Oxidative stress has a notable role in pathological conditions affecting the visual system. Light passes through all layers of the eye and generates high amounts of ROS during aging. If this condition is accompanied by pathological stimuli such as high IOP or increase blood sugar concentrations, there is an even greater production of ROS. Some studies have reported that the onset of eye diseases is more frequent when there is an imbalance between the production of free radicals and antioxidants by the body's defence mechanisms (Di Marco et al., 2015[16]).
Another step of damage could be the imbalance of cerebral autoregulation, a mechanism that prevents the accumulation of free radicals overall in the central nervous system where the blood stream is finely regulated (Longhitano et al., 2021[36]).
From the data available in the literature, it appears evident that during physiological aging the neuronal cells of the eye undergo a neurodegenerative process mainly due to the accumulation of free radicals, especially considering that the normal antioxidant defence systems become less effective with age. In this context, the use of antioxidant molecules such as ALA and SOD could significantly protect against retinal degeneration. Consequently, there would be an improvement in the conditions of the ocular nervous tissue. Further future studies are needed not only for a broader case analysis but also to evaluate other markers capable of inducing oxidative stress to define whether oxidative damage is an immediate cause or a consequence in the pathogenesis of ocular diseases.
Notes
Samanta Taurone, Massimo Ralli, Marco Artico, Marcella Nebbioso, Roberta Costi and Alessandra Micera contributed equally to this publication.
Declaration
Funding
This paper was financially supported by Ministry of Health (grant no. RC 2765943) and Fondazione Roma.
Author contributions
Conceptualization, S.T., M.R. and M.A.; methodology, V.M.N. software, S.S.; validation, A.M., M.B. and P.F., investigation, M.N., data curation, A.M. and S.A.N.; writing-original draft preparation, S.T., M.R. and. A.M., writing-review and editing, M.A. and S.A.N.; visualization, R.C.; supervision, A.M.; funding acquisition, A.M. All authors have read and agreed to the published version of the manuscript.
Institutional review board statement
Not applicable.
Informed consent statement
Not applicable.
Data availability statement
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Abu-Amero KK, Kondkar AA, Chalam KV. Resveratrol and ophthalmic diseases. Nutrients. 2016;8:200. doi: 10.3390/nu8040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abushouk AI, Ismail A, Salem AMA, Afifi AM, Abdel-Daim MM. Cardioprotective mechanisms of phytochemicals against doxorubicin-induced cardiotoxicity. Biomed Pharmacother. 2017;90:935–46. doi: 10.1016/j.biopha.2017.04.033. [DOI] [PubMed] [Google Scholar]
- 3.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alkadi HA. Review on free radicals and antioxidants. Infect Disord Drug Targets. 2020;20:16–26. doi: 10.2174/1871526518666180628124323. [DOI] [PubMed] [Google Scholar]
- 5.Babizhayev MA, Yegorov YE. Reactive oxygen species and the aging eye: specific role of metabolically active mitochondria in maintaining lens function and in the initiation of the oxidation-induced maturity onset cataract - a novel platform of mitochondria-targeted antioxidants with broad therapeutic potential for redox regulation and detoxification of oxidants in eye diseases. Am J Ther. 2016;23(1):e98–117. doi: 10.1097/MJT.0b013e3181ea31ff. [DOI] [PubMed] [Google Scholar]
- 6.Bertolotto F, Massone A. Combination of alpha lipoic acid and superoxide dismutase leads to physiological and symptomatic improvements in diabetic neuropathy. Drugs R D. 2012;12:29–34. doi: 10.2165/11599200-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busse E, Zimmer G, Schopohl B, Kornhuber B. Influence of alpha-lipoic acid on intracellular glutathione in vitro and in vivo. Arzneimittel-Forschung. 1992;42:829–831. [PubMed] [Google Scholar]
- 8.Butterfield D, Reed T, Newman, SF, Sultana R. Roles of amyloid-peptide associated oxidative stress and brain protein modifications in the pathogenesis of alzheimer's disease and mild cognitive impairment. Free Radic Biol Med. 2007;43:658–77. doi: 10.1016/j.freeradbiomed.2007.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabrera MP, Chihuailaf RH. Antioxidants and the integrity of ocular tissues. Vet Med Int. 2011;2011:905153. doi: 10.4061/2011/905153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campochiaro PA, Mir TA. The mechanism of cone cell death in Retinitis Pigmentosa. Prog Retin Eye Res. 2018;62:24–37. doi: 10.1016/j.preteyeres.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Campochiaro PA, Strauss RW, Lu L, Hafiz G, Wolfson Y, Shah SM, et al. Is there excess oxidative stress and damage in eyes of patients with retinitis pigmentosa? Antioxid Redox Signal. 2015;23:643–8. doi: 10.1089/ars.2015.6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carneiro Â, Andrade JP. Nutritional and lifestyle interventions for age-related macular degeneration: a review. Oxid Med Cell Longev. 2017;2017:6469138. doi: 10.1155/2017/6469138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davalli P, Mitic T, Caporali A, Lauriola A, D'Arca D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid Med Cell Longev. 2016;2016:3565127. doi: 10.1155/2016/3565127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies KJA. Oxidative stress: the paradox of aerobic life. Biochem Soc Symp. 1995;61:1–31. doi: 10.1042/bss0610001. [DOI] [PubMed] [Google Scholar]
- 15.Denisov E, Afanas’ev I. Oxidation and antioxidants in organic chemistry and biology. London: Taylor & Francis; 2005. [Google Scholar]
- 16.Di Marco E, Jha JC, Sharma A, Wilkinson-Berka JL, Jandeleit-Dahm KA, Haan JB. Are reactive oxygen species still the basis for diabetic complications? Clin Sci. 2015;129:199–216. doi: 10.1042/CS2015009. [DOI] [PubMed] [Google Scholar]
- 17.Du Y, Wooten MC, Gearing M, Wooten MW. Age-associated oxidative damage to the p62 promoter: implications for Alzheimer disease. Free Radic Biol Med. 2009;46:492–501. doi: 10.1016/j.freeradbiomed.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fehér J, Taurone S, Spoletini M, Biró Z, Varsányi B, Scuderi G, et al. Ultrastructure of neurovascular changes in human diabetic retinopathy. Int J Immunopathol Pharmacol. 2018;31:394632017748841. doi: 10.1177/0394632017748841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Good PF, Werner P, Hus A, Olanow CW, Perl DP. Evidence for neuronal oxidative damage in Alzheimer’s disease. Am J Pathol. 1996;149:21–28. [PMC free article] [PubMed] [Google Scholar]
- 20.Gopinath B, Liew G, Kifley A, Flood VM, Joachim N, Lewis JR, et al. Dietary flavonoids and the prevalence and 15-y incidence of age-related macular degeneration. Am J Clin Nutr. 2018;108:381–7. doi: 10.1093/ajcn/nqy114. [DOI] [PubMed] [Google Scholar]
- 21.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 4th ed. Oxford: Oxford Univ. Press; 2007. [Google Scholar]
- 22.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 5th ed. Oxford: Oxford Univ. Press; 2015. [Google Scholar]
- 23.Handa JT. How does the macula protect itself from oxidative stress? Mol Aspects Med. 2012;33:418–35. doi: 10.1016/j.mam.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hase Y, Horsburgh K, Ihara M, Kalaria RN. White matter degeneration in vascular and other ageing-related dementias. J Neurochem. 2018;144:617–633. doi: 10.1111/jnc.14271. [DOI] [PubMed] [Google Scholar]
- 25.Holley AK, Bakthavatchalu V, Velez-Roman JM, Clair DKSt. Manganese superoxide dismutase: guardian of the powerhouse. Int J Mol Sci. 2011;12:7114–7162. doi: 10.3390/ijms12107114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izumi Y, Hammerman SB, Kirby CO, Benz AM, Olney JW, Zorumski CF. Involvement of glutamate in ischemic neurodegeneration in isolated retina. Vis Neurosci. 2003;20:97‐107. doi: 10.1017/s0952523803202017. [DOI] [PubMed] [Google Scholar]
- 27.Kinukawa J, Shimura M, Harata N, Tamai M. Gliclazide attenuates the intracellular Ca2+ changes induced in vitro by ischemia in the retinal slices of rats with streptozotocin‐induced diabetes. Curr Eye Res. 2005;30:789‐98. doi: 10.1080/02713680591002808. [DOI] [PubMed] [Google Scholar]
- 28.Kokoszka JE, Coskun P, Esposito LA, Wallace DC. Increased mitochondrial oxidative stress in the Sod2+/− mouse results in the age related decline of mitochondrial function culminating in increased apoptosis. Proc Natl Acad Sci USA. 2001;98:2278–83. doi: 10.1073/pnas.051627098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kubes P. Inducible nitric oxide synthase: a little bit of good in all of us. Gut. 2000;47:6–9. doi: 10.1136/gut.47.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kudryavtseva AV, Krasnov GS, Dmitriev AA, Alekseev BY, Kardymon OL, Sadritdinova AF, et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget. 2016;7(29):44879–44905. doi: 10.18632/oncotarget.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li C, Miao X, Li F, Wang S, Liu Q, Wang Y, et al. Oxidative stress-related mechanisms and antioxidant therapy in diabetic retinopathy. Oxid Med Cell Longev. 2017;2017:9702820. doi: 10.1155/2017/9702820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li R, Jia Z, Trush MA. Defining ROS in biology and medicine. React Oxyg Species (Apex) 2016;1(1):9–21. doi: 10.20455/ros.2016.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;1:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 34.Lightfoot T, Skibola C, Smith A, Forrest MS, Adamson PJ, Morgan GJ, et al. Polymorphisms in the oxidative stress genes, superoxide dismutase, glutathione peroxidase and catalase and risk of non-Hodgkin’s lymphoma. Haematologica. 2006;91:1222–7. [PubMed] [Google Scholar]
- 35.London DS, Beezhold B. A phytochemical-rich diet may explain the absence of age- related decline in visual acuity of Amazonian hunter-gatherers in Ecuador. Nutr Res. 2015;35:107–17. doi: 10.1016/j.nutres.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Longhitano Y, Iannuzzi F, Bonatti G, Zanza C, Messina A, Godoy D, et al. Cerebral autoregulation in non-brain injured patients: a systematic review. Front Neurol. 2021;12:732176. doi: 10.3389/fneur.2021.732176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez-Galvez MI, Lavado FM, Pastor JC. Diabetic retinopathy: an overview. In: Preedy VR, editor. Handbook of nutrition, diet and the eye. San Diego, CA: Academic Press; 2014. pp. 41–51. [Google Scholar]
- 38.Lykkesfeldt J, Ames BN. Ascorbic acid recycling in rathe patocytes as measurement of antioxidant capacity: Decline with age. Methods Enzymol. 1999;299:83–88. doi: 10.1016/s0076-6879(99)99011-0. [DOI] [PubMed] [Google Scholar]
- 39.Mastrodimou N, Lambrou GN, Thermos K. Effect of somatostatin analogues on chemically induced ischaemia in the rat retina. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:44‐53. doi: 10.1007/s00210-004-1011-9. [DOI] [PubMed] [Google Scholar]
- 40.Nebbioso M, Pascarella A, Cavallotti C, Pescosolido N. Monoamine oxidase-enzymes and oxidative stress in the rat optic nerve: Age-related changes. Int J Exp Path. 2012;93:401–405. doi: 10.1111/j.1365-2613.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nebbioso M, Pranno F, Pescosolido N. Lipoic acid in animal models and clinical use in diabetic retinopathy. Expert Opin Pharmacother. 2013;14:1829–1838. doi: 10.1517/14656566.2013.813483. [DOI] [PubMed] [Google Scholar]
- 42.Nebbioso M, Scarsella G, Tafani M, Pescosolido N. Mechanisms of ocular neuroprotection by antioxidant molecules in animal models. J Biol Regul Homeost Agents. 2013;27:197–209. [PubMed] [Google Scholar]
- 43.Nucci C, Tartaglione R, Cerulli A, Mancino R, Spanò A, Cavaliere F, et al. Retinal damage caused by high intraocular pressure-induced transient ischemia is prevented by coenzyme Q10 in rat. Int Rev Neurobiol. 2007;82:397‐406. doi: 10.1016/S0074-7742(07)82022-8. [DOI] [PubMed] [Google Scholar]
- 44.Oz HS, Chen TS, McClain CJ, de Villiers WJS. Antioxidants as novel therapy in a murine model of colitis. J Nutr Biochem. 2005;16:297–304. doi: 10.1016/j.jnutbio.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Rauf A, Imran M, Suleria HAR, Ahmad B, Peters DG, Mubarak MS. A comprehensive review of the health perspectives of resveratrol. Food & Function. 2017;8:4284–305. doi: 10.1039/c7fo01300k. [DOI] [PubMed] [Google Scholar]
- 46.Remacle J, Raes M, Toussaint O, Renard P, Rao G. Low levels of reactive oxygen species as modulators of cell function. Mutat Res. 1995;316:103–22. doi: 10.1016/0921-8734(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 47.Reveillaud I, Niedzwiecki A, Bensch KG, Fleming JE. Expression of bovine superoxide dismutase in Drosophila melanogaster augments resistance of oxidative stress. Mol Cell Biol. 1991;11:632–640. doi: 10.1128/mcb.11.2.632-640.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sies H. Strategies of antioxidant defense. Eur J Biochem. 1993;215:213–21. doi: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]
- 50.Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–8. doi: 10.1371/journal.pone.0043089. [DOI] [PubMed] [Google Scholar]
- 51.Stefanatos R, Sanz A. The role of mitochondrial ROS in the aging brain. FEBS Lett. 2018;592:743–758. doi: 10.1002/1873-3468.12902. [DOI] [PubMed] [Google Scholar]
- 52.Steinert JR, Chernova T, Forsythe ID. Nitric oxide signaling in brain function, dysfunction, and dementia. Neuroscientist. 2010;16:435–52. doi: 10.1177/1073858410366481. [DOI] [PubMed] [Google Scholar]
- 53.Tangvarasittichai O, Tangvarasittichai S. Oxidative stress, ocular disease and diabetes retinopathy. Curr Pharm Des. 2018;24:4726–4741. doi: 10.2174/1381612825666190115121531. [DOI] [PubMed] [Google Scholar]
- 54.Taurone S, Miglietta S, Spoletini M, Feher J, Artico M, Papa V, et al. Age related changes seen in human cornea in formalin fixed sections and on biomicroscopy in living subjects: A comparison. Clin Anat. 2020;33:245–256. doi: 10.1002/ca.23488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taurone S, Ralli M, Nebbioso M, Greco A, Artico M, Attanasio G, et al. The role of inflammation in diabetic retino-pathy: a review. Eur Rev Med Pharmacol Sci. 2020;24:10319–10329. doi: 10.26355/eurrev_202010_23379. [DOI] [PubMed] [Google Scholar]
- 56.Taurone S, Ripandelli G, Pacella E, Bianchi E, Plateroti AM, De Vito S, et al. Potential regulatory molecules in the human trabecular meshwork of patients with glaucoma: immunohistochemical profile of a number of inflammatory cytokines. Mol Med Rep. 2015;11:1384–1390. doi: 10.3892/mmr.2014.2772. [DOI] [PubMed] [Google Scholar]
- 57.Taurone S, Spoletini M, Ralli M, Gobbi P, Artico M, Imre L, et al. Ocular mucous membrane pemphigoid: a review. Immunol Res. 2019;67:280–9. doi: 10.1007/s12026-019-09087-7. [DOI] [PubMed] [Google Scholar]
- 58.Tsang CK, Liu Y, Thomas J, Zhang Y, Zheng XS. Superoxide dismutase 1 acts as a nuclear transcription factor to regulate oxidative stress resistance. Nat Commun. 2014;5:3446. doi: 10.1038/ncomms4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ung L, Pattamatta U, Carnt N, Wilkinson-Berka JL, Liew G, White AJR. Oxidative stress and reactive oxygen species: a review of their role in ocular disease. Clin Sci (Lond) 2017;131:2865–2883. doi: 10.1042/CS20171246. [DOI] [PubMed] [Google Scholar]
- 60.Upadhyay S, Dixit M. Role of polyphenols and other phytochemicals on molecular signaling. Oxid Med Cell Longev. 2015;2015:504253. doi: 10.1155/2015/504253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 63.Vanfleteren JR. Oxidative stress and ageing in Caenorhabditis elegans. Biochem J. 1993;292:605–8. doi: 10.1042/bj2920605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams DL. Oxidative stress and the eye. Vet Clin North Am Small Anim Pract. 2008;38:179–192. doi: 10.1016/j.cvsm.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 65.Wright AF, Chakarova CF, Abd El-Aziz MM, Bhattacharya SS. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat Rev Genet. 2010;11:273–284. doi: 10.1038/nrg2717. [DOI] [PubMed] [Google Scholar]
- 66.Wu GS. Phenotype of transgenic mice over expressed with inducible nitric oxide synthase in the retina. PloS One. 2012;7:e43089. doi: 10.1371/journal.pone.0043089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu GS, Rao NA. Photoreceptor mitochondrial oxidative stress in uveitis. Expert Rev Ophthalmol. 2008;3:299–310. doi: 10.1586/17469899.3.3.299. [DOI] [Google Scholar]
- 68.Zanza C, Thangathurai J, Audo A, Muir HA, Candelli M, Pignataro G, et al. Oxidative stress in critical care and vitamins supplement therapy: "a beneficial care enhancing". Eur Rev Med Pharmacol Sci. 2019;23:7703–7712. doi: 10.26355/eurrev_201909_18894. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.