Abstract
Background
Low tidal volume (VT), PEEP, and low plateau pressure (PPLAT) are lung protective during acute respiratory distress syndrome (ARDS). This study tested the hypothesis that the aspiration of dead space (ASPIDS) together with computer simulation can help maintain gas exchange at these settings, thus promoting protection of the lungs.
Methods
ARDS was induced in pigs using surfactant perturbation plus an injurious ventilation strategy. One group then underwent 24 h protective ventilation, while control groups were ventilated using a conventional ventilation strategy at either high or low pressure. Pressure–volume curves (Pel/V), blood gases, and haemodynamics were studied at 0, 4, 8, 16, and 24 h after the induction of ARDS and lung histology was evaluated.
Results
The Pel/V curves showed improvements in the protective strategy group and deterioration in both control groups. In the protective group, when respiratory rate (RR) was ≈60 bpm, better oxygenation and reduced shunt were found. Histological damage was significantly more severe in the high-pressure group. There were no differences in venous oxygen saturation and pulmonary vascular resistance between the groups.
Conclusions
The protective ventilation strategy of adequate pH or PaCO2 with minimal VT, and high/safe PPLAT resulting in high PEEP was based on the avoidance of known lung-damaging phenomena. The approach is based upon the optimization of VT, RR, PEEP, I/E, and dead space. This study does not lend itself to conclusions about the independent role of each of these features. However, dead space reduction is fundamental for achieving minimal VT at high RR. Classical physiology is applicable at high RR. Computer simulation optimizes ventilation and limiting of dead space using ASPIDS. Inspiratory Pel/V curves recorded from PEEP or, even better, expiratory Pel/V curves allow monitoring in ARDS.
Key words: ARDS, computer simulation, dead space, pressure–volume curve, protective ventilation
Editor’s key points.
-
•
Protective strategies to minimize ventilator-induced lung injury (VILI) in acute respiratory distress syndrome (ARDS) are accepted.
-
•
Computer simulation was used to define the GOAL protective strategy.
-
•
The GOAL strategy was compared with the conventional protective ventilation at two plateau pressures in a pig model of ARDS with VILI.
-
•
Ventilation improved in the GOAL pigs and deteriorated in the other groups.
-
•
Computer simulation may be useful to optimize goal-orientated ventilation.
In patients with acute respiratory distress syndrome (ARDS), the risk of superimposed ventilator-induced lung injury (VILI) merits lung-protective ventilation (LPV) strategies.1, 2, 3, 4, 5, 6, 7, 8, 9 It has been recommended that a tidal volume (V T) ≤6 ml kg−1 body weight and plateau pressure ≤30 cm H2O is used, while PEEP is modified according to oxygenation. Respiratory acidosis after V T reduction may require increased respiratory rate (RR), the effect of which is limited by high dead space in ARDS.10, 11, 12, 13, 14, 15
Our concept for LPV, the so-called GOAL strategy, is to reach two goals: maintaining a target pH or Paco2 while providing optimal lung protection. The former goal is achieved by adequate alveolar ventilation ( ), while for LPV, barotrauma is avoided by limiting the plateau pressure (P PLAT) and volutrauma by minimizing V T. By combining a high but safe P PLAT with minimum V T, the total PEEP (PEEPTOT) will be as high as is compatible with a proper , a safe P PLAT, and a minimal V T. High PEEPTOT keeps the lungs open and ensures oxygenation at low inspired oxygen factions (F I O2). Dead space reduction lowers V T both directly and also indirectly, by allowing a higher RR.
Airway dead space in the present study was minimized by using aspiration of dead space (ASPIDS), such that the gas expired later during expiration is aspirated through an extra catheter from the tip of the tracheal tube and simultaneously replaced by fresh gas through the inspiratory line.16, 17, 18 With ASPIDS, dead space from the Y-piece to the trachea is completely removed without any other effect on ventilation.
In ARDS, even an experienced operator may not be able to define the optimal combination of V T, PEEP, RR, and the inspiratory/expiratory ratio for reaching all physiological goals. The use of computer simulation once mathematical characterization of lung mechanics and CO2 exchange has been achieved may identify beneficial combinations.19, 20, 21
In the present study, we modelled the common clinical scenario of VILI aggravation of ARDS after the initial lung insult using surfactant perturbation followed by high V T ventilation.22 23
The primary objective was to test the hypothesis that the GOAL strategy is more lung protective than conventional ventilation with either a low or a high plateau pressure. The endpoints were physiological parameters studied 16 h after ARDS. Both inspiratory and expiratory elastic pressure volumes were studied, plus lung histology at 24 h.
Methods
Subjects and preparation
The Local Ethics Board of Animal Research approved the study. The animals were fasted overnight with free access to water and premedicated with xylazine (2 mg kg−1). Anaesthesia was induced by ketamine (15 mg kg−1) and maintained with i.v. fentanyl (60 µg kg−1 h−1) and midazolam (0.7 mg kg−1 h−1). Vecuronium (0.3 mg kg−1) provided paralysis during measurements. Sudden modifications in the heart rate and arterial pressure and increases in muscular activity were considered as indicators of distress and inadequate anaesthesia, and in this case, a bolus dose of ketamine was administered, followed by an increase in midazolam and fentanyl infusion dose.
Animals were hydrated with a priming of 1000 ml of isotonic crystalloids followed by an infusion of 125 ml h−1. If mean arterial pressure (MAP) decreased below 40 mm Hg, dextran was given. Blood glucose was maintained at 3.6–5.3 mmol ml−1 by administration of i.v. glucose. Body temperature was controlled at 38.5–39.5°C. Prophylactic i.m. streptomycin and benzylpenicillin (0.50 g and 400 000 IU, respectively) were given and the animals were prepared under sterile conditions.
A balloon-tipped, multi-lumen catheter was sited, with its tip in a pulmonary artery. Arterial and venous catheters were inserted and pressure was monitored and recorded for the right atrium and pulmonary and peripheral arteries. Arterial and central venous blood gas samples were analysed immediately for P o 2, P co 2, pH, BE, Hb, and oxygen saturation (Radiometer ABL725, Copenhagen, Denmark).
Ventilation and monitoring
A ServoVentilator 900C (Siemens-Elema, Solna, Sweden) with a CO2 Analyzer 930 for monitoring CO2 elimination per minute ( ) was volume-controlled at baseline with square inspiratory flow at 20 min−1, 15 cm H2O PEEP, and an F I O2 of 1.0. Minute ventilation was set to maintain arterial pH at 7.30–7.45. Ventilator signals (flow rate, airway pressure, and P co 2) were sampled at 100 Hz.24 To minimize dead space, an active inspiratory line humidifier was used with minimal connectors to the tracheal tube.
Lung recruitment at 50 cm H2O preceded recordings of multiple elastic pressure–volume (P el/V) curves as described.23 The loops started with inspiration from a PEEP level of, in order: 15, 11, 7, 4, and 0 cm H2O and ended at 50 cm H2O. Higher pressures were considered to be potentially harmful. The P el/V curves were modelled as a sigmoid curve.25 26 The volume was set to zero at zero PEEP during the last loop. The volume was expressed as a percentage of the initial volume at 50 cm H2O in each pig.
ARDS model
Lung surfactant was perturbed by inhalation of 200 breaths of 5% dioctyl sulphosuccinate aerosol.22 23 During pressure-controlled harmful ventilation, P PLAT was 50 cm H2O, RR was 10 bpm, and end-expiratory pressure was −10 cm H2O. Dead space was added to maintain normocapnia. Harmful ventilation was continued until compliance, that is, V T/(P PLAT–PEEPTOT) decreased by 25%, or until substantial exudates appeared in the tracheal tube. ARDS was diagnosed when the PaCO2/F I O2 was <27 kPa after 5 min at zero PEEP with a ventilator setting otherwise as they were at baseline. If this criterion was not met, harmful ventilation continued for one or more periods of 30 min. After the diagnosis of ARDS, the animals were randomized into three groups with different ventilation strategies: a GOAL group and two control groups with conventional ventilation at high plateau pressure (HP) or conventional ventilation plus low plateau pressure (LP) (Table 1 ).
Table 1.
Treatment goals and limitations after randomization
| Group | GOAL | LP | HP | |||
|---|---|---|---|---|---|---|
| VT | Minimal | |||||
| RR (bpm) | 40–60 | 6–35 | 6–35 | |||
| Arterial pH | 7.35 | 7.30–7.45 | 7.30–7.45 | |||
| PPLAT (cm H2O) | 30 | 25–30 | 40–45 | |||
| PEEPTOT (cm H2O) and FIO2 to achieve PaO2 9–12 kPa. For high- and low-pressure groups, allowable combinations are indicated | PEEPTOT | FIO2 | PEEPTOT | FIO2 | PEEPTOT | FIO2 |
| Maximal | Adjustable | 5 | 0.3 | As in group LP | ||
| 5 | 0.4 | |||||
| 8 | 0.4 | |||||
| 8 | 0.5 | |||||
| 10 | 0.5 | |||||
| 10 | 0.6 | |||||
| 10 | 0.7 | |||||
| 12 | 0.7 | |||||
| 14 | 0.7 | |||||
| 14 | 0.8 | |||||
| 14 | 0.9 | |||||
| 16 | 0.9 | |||||
| 18 | 0.9 | |||||
| 18 | 1.0 | |||||
| 20 | 1.0 | |||||
| 22 | 1.0 | |||||
| 24 | 1.0 | |||||
Protocol
Initially, and 0, 4, 8, 16, and 24 h after ARDS diagnosis, measurements of haemodynamics, blood gases, and P el/V loops were carried out after 20 min of baseline ventilator setting at an F I O2 of 1.0. In the GOAL group, the goals were to reach a pH of 7.35 and a P PLAT of 30 cm H2O at minimal V T. A decelerating inspiratory flow was applied with a post-inspiratory pause time of 5%. RR should not, for technical reasons, be higher than 60 bpm. To reduce V T, dead space down to the tip of the tracheal tube was eliminated using ASPIDS.16, 17, 18
The principle behind the computer simulation for identifying an optimal ventilator setting has been described previously.19, 20, 21 It is based upon a physiological profile comprising a description of the elastic pressure/volume diagram, inspiratory and expiratory resistance as a function of volume, and the tidal volume of CO2 eliminated in relation to V T.21 The simulation process is based upon the target immediate physiological goals that should be achieved by ventilation. These were pH=7.35 and P PLAT=30 cm H2O at minimal V T. Standard acid/base equations were used for the calculation of relationships between pH and PaCO2.
Ten consecutive breaths were simulated by dividing each breath into 100 steps in time, the last breath representing steady state. During each step, changes in volume and pressure were calculated from the action of the simulated ventilator and the physiological profile. The volume of CO2 eliminated per minute was calculated from the single breath test for CO2 describing the tidal volume of CO2 related to tidal volume.21 The stepwise simulation process that incorporates the ASPIDS function was also previously described in detail.21
The ventilator setting defined by the simulation was then implemented over 24 h, except for short periods of measurements as described. Hourly minor adjustments were carried out: PEEP to keep P PLAT at 30 cm H2O, minute ventilation to maintain the pH at 7.35, and F I O2 to keep PaO2 within 9–12 kPa.
In the LP and HP groups, the inspiratory flow profile was square, inspiration time was 33%, and pause time was 5%. To keep PaO2 within 9–12 kPa, F I O2 and PEEPTOT were adjusted as shown in Table 1, following the principles of ARDSnet.9 According to ARDSnet, pH should be within the range 7.30–7.45 in both these groups. P PLAT should be within the range 25–30 cm H2O for the LP group and 40–45 cm H2O in the HP group. However, pilot experiments indicated that the high metabolic rates in adolescent pigs with ARDS required significantly higher V T than recommended by ARDSnet, such that in order to keep the pH at 7.30–7.45 and P PLAT at the target value, the combination of RR and minute ventilation had to be titrated. RR up to 35 bpm was allowed.
After 24 h, animals were killed using a bolus dose of 1.2 mg fentanyl and 100 mg ketamine, followed by fast desanguination. The lungs were removed for histological examination,23 and eight healthy pigs were used as controls with respect to histology. Since the primary endpoint was at 16 h after ARDS, those pigs not surviving for 16 h were replaced with others, so each group had eight animals which survived to 16 h.
Histopathological processing
The left lung was removed, expanded, and fixed with intrabronchial formalin. A central sagittal slice was embedded in paraffin and 5 μm thick sections were cut with a whole mount microtome, mounted on large glass slides, and stained with haematoxylin/eosin. Thirty circular areas, of 5 mm diameter, were scored according to three factors (Table 2 ): hyaline membranes, bronchiolar neutrophil granulocyte infiltration, and bronchiolar epithelial damage. For each factor, the average areas were calculated.
Table 2.
Lung injury score
| Score | Hyaline membranes | Bronchiolar granulocyte infiltration | Bronchiolar epithelial damage |
|---|---|---|---|
| 0 | No hyaline membranes | No granulocytes | No epithelial damage |
| 1 | A few, just detectable membranes | Luminar granulocytes | Single patch of epithelial denudation in a bronchiole |
| 2 | Evident membranes in <50% of alveoli | Luminar granulocytes and patches of transluminar granulocyte infiltration | >1 patch of epithelial denudation in a bronchiole |
| 3 | Evident membranes in >50% of alveoli | Luminar granulocytes and circumferential transluminar granulocyte infiltration | Denudation of >50% of circumference of the bronchiole |
Data analysis
Gas exchange
VDALV and CO2 elimination ( ) were calculated from the single-breath test for CO2.11 Qs/Qt was calculated from end-capillary oxygen content (CcO2), arterial oxygen content (CaO2), and mixed venous oxygen content (CvO2):
Haemodynamics
Heart rate (HR), MAP, mean central venous pressure, mean pulmonary artery pressure (MPAP), and pulmonary capillary wedge pressure (PCWP) were measured.
Cardiac output (CO) was calculated according to Fick:
where is O2 delivery calculated as , assuming that RQ=0.85.
Pulmonary vascular resistance (PVR) was calculated as (MPAP–PCWP)/CO.
Statistical analysis
Data are shown as mean and standard deviation (sd). Two-way variance on ranked data was used to analyse P el/V curves at different PEEP levels and different times. The Kruskal–Wallis test was used to detect differences between the groups in PaO2, SvO2, Qs/Qt, V DALV, PVR, and lung damage score. The Mann–Whitney test was used to further analyse the groups that differed. A P-value of <0.05 was considered significant. XLSTAT 7.5.2 (Addinsoft, New York, NY, USA) was used for analysis.
Results
ARDS was diagnosed after 114 (41) min of harmful ventilation. PaCO2/F I O2 was 11 (5 kPa) at zero PEEP and 47 (17 kPa) at a PEEP level of 15 cm H2O, and was similar between the groups. Two animals which died after 2.5 h in the LP group and 10 h in the HP group were substituted. Also in the HP group, two animals died (after 18 and 23 h) due to arrhythmia and tension pneumothorax with hypoxia, respectively. There was no death at any time in the GOAL group. In all animals and at all times, P PLAT and pH were within the limits given by the protocol, while PaO2 was slightly higher (Table 3 ).
Table 3.
Average of ventilation parameters measured each hour during 24 h (16 h for two animals in HP)
| GOAL |
LP |
HP |
||||
|---|---|---|---|---|---|---|
| Mean | sd | Mean | sd | Mean | sd | |
| RR (bpm) | 58 | 5 | 19 | 9 | 7.5 | 2 |
| VT (ml) | 119 | 9 | 268 | 107 | 538 | 108 |
| VT (ml kg−1) | 5.8 | 0.4 | 13.2 | 5.1 | 27 | 4.8 |
| PPLAT (cm H2O) | 30 | 0.3 | 29 | 2.8 | 41 | 1.3 |
| PEEPI (cm H2O) | 0.9 | 1.0 | 0.5 | 0.4 | 0.7 | 0.6 |
| PEEPTOT (cm H2O) | 19 | 1.7 | 8 | 2.4 | 8 | 1.3 |
| FIO2 | 0.35 | 0.06 | 0.47 | 0.10 | 0.49 | 0.10 |
| pH | 7.36 | 0.02 | 7.38 | 0.02 | 7.38 | 0.03 |
| PaCO2 (kPa) | 6.5 | 0.8 | 7.1 | 0.9 | 6.4 | 0.8 |
| PaO2 (kPa) | 16.4 | 3.7 | 12.6 | 1.6 | 13.4 | 1.7 |
In GOAL, the settings according to computer simulation resulted in an RR of 60 bpm in most pigs, which was the maximum allowed according to the protocol. V T in the GOAL group was less than half that seen in the LP group, while PEEPTOT more than twice that in the LP and HP groups (Table 3). Also in GOAL animals, the I:E ratio was 1:1 in all but one of the animals. Intrinsic PEEP (PEEPI) was ∼1 cm H2O (Table 3).
Mechanics of the respiratory system
At ARDS diagnosis (0 h), the inspiratory and expiratory P el/V curves were similar in all groups (Fig. 1 ). In all the groups and at all times, inspiratory P el/V curves starting at PEEP levels from 15 to 0 cm H2O showed a loss of volume, reflecting progressive de-recruitment for each lower PEEP level.
Fig 1.
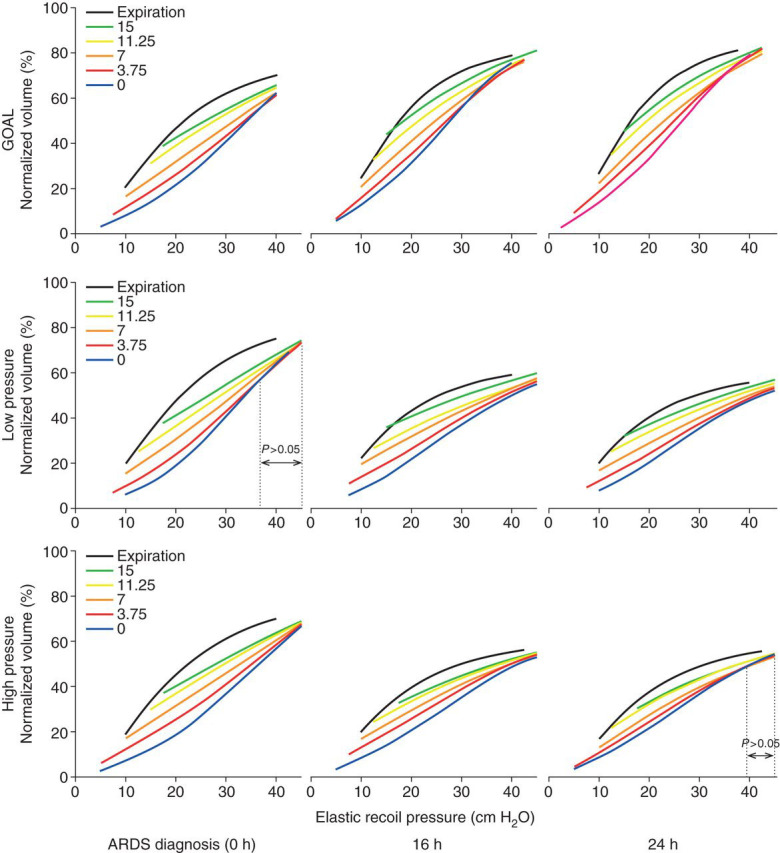
Inspiratory Pel/V curves recorded from PEEP 15 to 0 cm H2O and expiratory Pel/V curves recorded from 50 cm H2O; average in each group at 0, 16, and 24 h. Inspiratory curves showed significant differences related to starting pressure over the full pressure range apart from LP at 0 h and in HP at 24 h (two-way analysis of variance).
Families of inspiratory P el/V curves preceded by expirations to different PEEP levels did not completely converge, indicating that lung units collapsing at low pressures did not fully re-expand at 50 cm H2O (Fig. 1).
Inspiratory P el/V curves starting at zero PEEP showed, in GOAL animals, progressively higher volumes during the 24 h after the onset of ARDS. In the LP group, no significant change was seen over time. In HP animals, volumes decreased slightly, but only above 35 cm H2O (Fig. 2 , upper panels).
Fig 2.
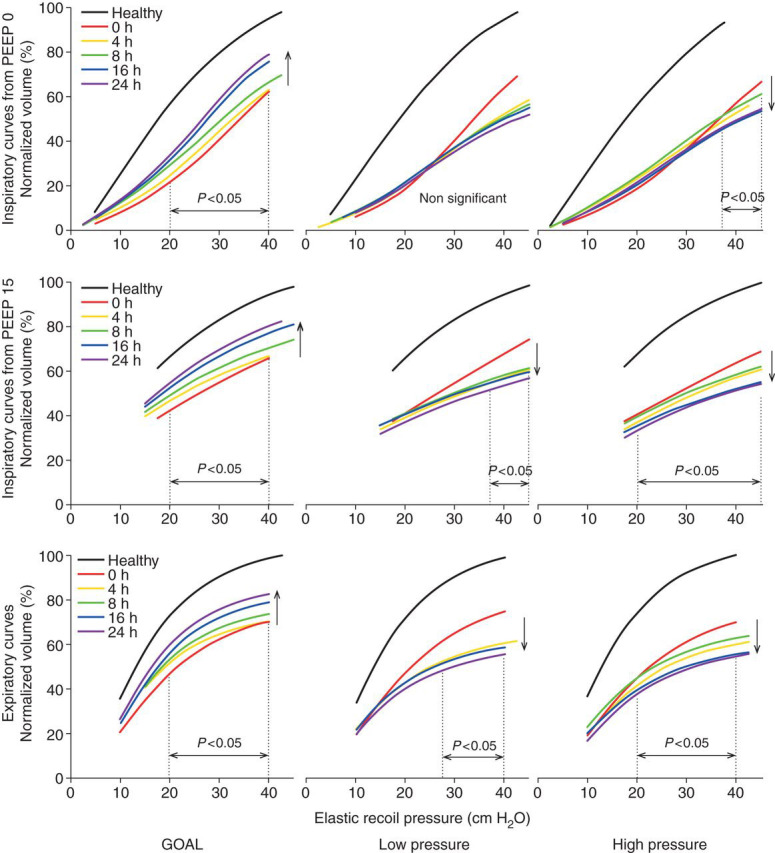
Upper panels: average inspiratory Pel/V curves recorded from zero PEEP in the three groups. Middle panels: average inspiratory Pel/V curves recorded from PEEP 15 cm H2O. Lower panels: average expiratory Pel/V curves recorded from 50 cm H2O. Volume trends over time in the direction of the arrows were significant over the indicated pressure ranges.
In GOAL animals, inspiratory P el/V curves starting at a PEEP of 15 mm H2O showed progressively higher volumes during the 24 h after ARDS, while in the LP group, and particularly in the HP group, progressively lower volumes were recorded (Fig. 2, middle panels) such that the volumes in LP pigs were higher than in HP animals (P<0.001).
In GOAL-treated pigs expiratory P el/V curves, increasing volumes were seen in the 24 h after ARDS, while decreasing volumes were seen in LP and HP animals (Fig. 2, lower panels).
At 16 and 24 h, the volumes in inspiratory and expiratory P el/V curves were much higher in GOAL than in LP and HP animals (P<0.0001) (Fig. 1) and in the LP group volumes were slightly higher than in the HP group (P<0.02).
Gas exchange and haemodynamics
At 0 h, there were no differences between the groups in terms of PaO2, SvO2, Qs/Qt, V DALV, CO, PVR, or any other haemodynamic and gas exchange parameter. In GOAL animals at an F I O2 of 1.0, PaO2 was higher than in LP animals from 4 h onwards, and in HP animals from 8 h. After 4 h, PaO2 was not different from the pre-ARDS values in the GOAL animals (Fig. 3 ). Throughout the study period, CO and SvO2 did not differ between the groups. Accordingly, PaO2 inversely reflected variations in Qs/Qt.
Fig 3.
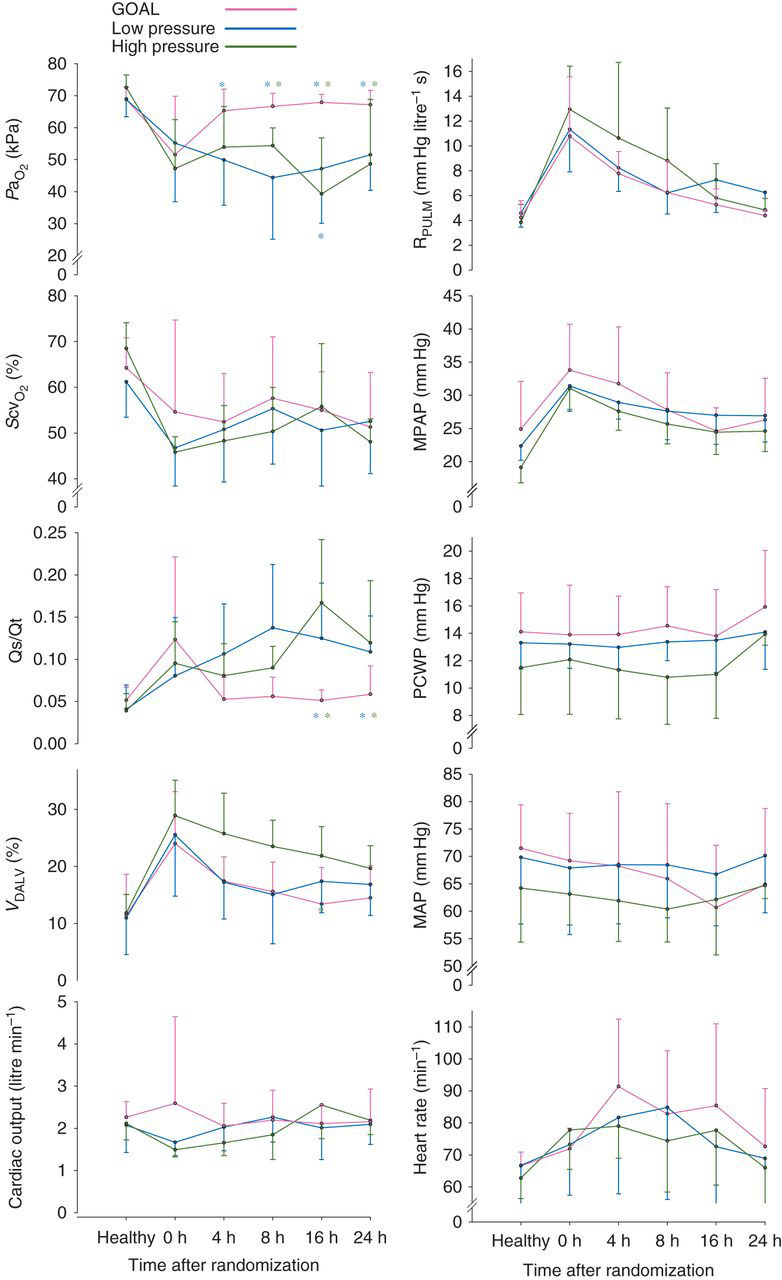
Haemodynamics and gas exchange during the study. GOAL differed from the LP and HP as indicated by asterisks in blue and green colours, respectively. Data are expressed as mean and sd.
Histopathology
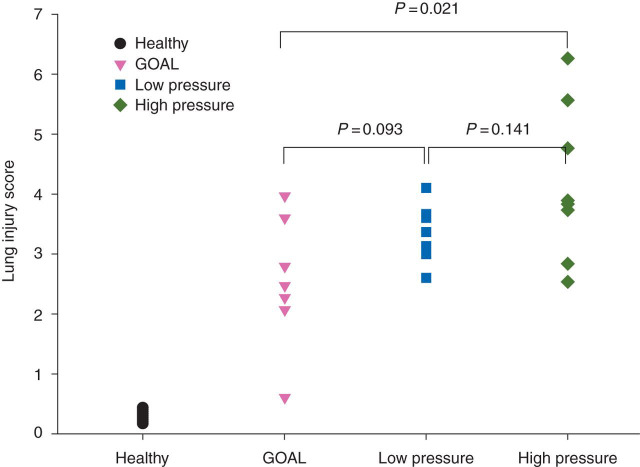
In untreated healthy pigs, the lung injury score was close to 0. At 24 h, scores were higher than in healthy pigs in all the groups (P<0.001) and differed between the groups (anova, P=0.033) (Fig. 4 ).
Fig 4.
Lung injury score in individual pigs at the end of the study.
Discussion
In an ARDS pig model, induced by VILI after surfactant perturbation, the GOAL strategy based upon proper pH, safe P PLAT, and minimal V T, together implying high PEEPTOT, resulted in improvement over 24 h after initiation of ARDS. Accordingly, the GOAL strategy was shown to be lung protective relative to an alternative strategy with the same P PLAT in which deterioration was observed.
The tools used were goal-orientated ventilation facilitated by computer simulation, dead space reduction by ASPIDS, and for monitoring, advanced P el/V recording. On the basis of the characterization of the physiological status of the animal with respect to mechanics and CO2 exchange, the setting of several ventilator parameters, which together were expected to minimize lung injury, was defined. This approach is motivated by the judgement that there is no ‘best PEEP’ without taking into account V T and no ideal V T without regarding P PLAT, etc. Trying to identify the role of specific steps of ventilator setting may, in this respect, be futile. The GOAL strategy obviates the need for titration of the ‘best PEEP’.
In GOAL, a P PLAT of 30 cm H2O and an arterial pH of 7.35 were chosen according to ARDSnet.9 We refrained from practising permissive hypercapnia to further reduce V T, as this remains a controversial issue.27 A decelerating flow was accomplished by computer control of ventilator function. 24 25 The reason for using the decelerating flow was to improve CO2 exchange by prolonging the mean distribution time.28, 29, 30 In the light of recent findings, a constant flow, a short inspiratory time, and a post-inspiratory pause time of more 5% would have been chosen as this has been found to further enhance CO2 exchange at constant V T.30 31 It was considered that minimal V T is a central issue in LPV. Recently, Bruhn and colleagues32 confirmed, by dynamic CT, that cyclic collapse and opening is reduced by lower V T, providing direct evidence that low V T ventilation may be lung protective by reducing this phenomenon. Very recently, it has also been experimentally proven that an open lung strategy, aimed at reducing dynamic strain, improves arterial oxygenation, but fails to positively affect respiratory mechanics and pulmonary inflammation compared with mechanical ventilation with lower PEEP according to ARDSnet.33
A high dead space pre-empts isocapnic ventilation at a very low V T. An active humidifier and minimal connectors between the y-piece and the tracheal tube were used in all the groups in our study. In GOAL, ASPIDS was applied for optimal dead space reduction.16, 17, 18 The ventilator setting defined with computer simulation resulted in V T≈5.8 ml kg−1, RR≈58 min−1, and I:E ratio≈1:1. That V T could not be further reduced reflects the high CO2 production in these adolescent pigs and very high physiological dead space in the ARDS model. The high I:E ratio reflects that computer simulation recognized that a short mean distribution time would not otherwise allow efficient CO2 exchange.21 As stated, recent findings would have encouraged the use of a longer pause time.
In GOAL, an open lung strategy comprised an initial recruitment manoeuvre followed by a high-PEEP and low-V T ventilation. The recruitment manoeuvre to 45–50 cm H2O may have left some units collapsed which might be recruited at a higher, potentially injurious pressure. A recruitment manoeuvre has a transient effect, while a high PEEP combined with a low V T maintains recruitment and enhances arterial oxygenation.34 35 Hence, the strategy was based on moderate recruitment, low V T, and high PEEP.
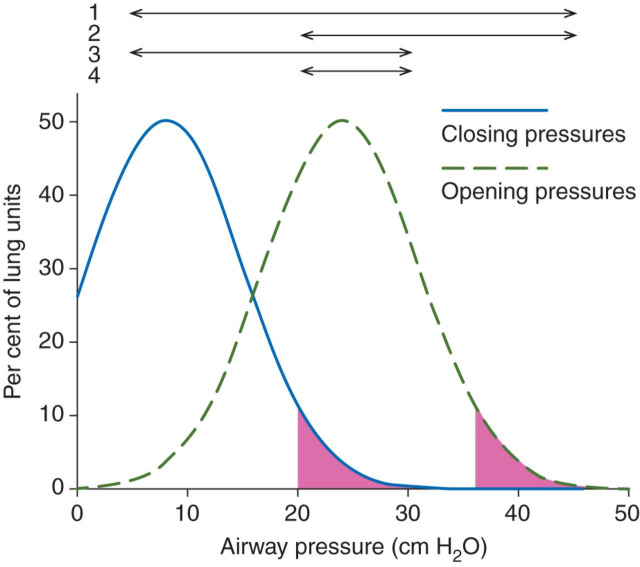
In an open lung, oxygenation can be achieved at reduced ventilation just by increasing F I O2.36 However, CO2 exchange depends strictly upon alveolar ventilation. At high V T, shear forces in lung zones undergoing repeated collapse and re-expansion (RECOREX) lead to VILI.37, 38, 39 Closing pressures are much lower than opening pressures, but their distributions overlap.17 40 41 At a safe P PLAT, it appears impossible to maintain complete aeration. Even at a PEEP of 20 cm H2O, some units will close. Reasonably, these units also have particularly high opening pressures. If V T is so low that the difference between PEEP and P PLAT is less than the difference between closing and opening pressures, these units remain closed during breaths. Accordingly, both volutrauma related to RECOREX and barotrauma due to high P PLAT may be abolished at very low V T and high PEEP. This contention is underpinned in Figure 5 , in which distributions of opening and closing pressures refer to the study by Crotti and colleagues40 and which are in line with patterns of inspiratory and expiratory P el/V curves in the present study. The conclusion is that it is rational to reduce V T to the lowest possible level.
Fig 5.
Realistic distributions of opening and closing pressures of lung units in ARDS. Arrows illustrate the range PEEPTOT to PPLAT under different ventilation regimes. Arrow 1 refers to obsolete high VT/low PEEP ventilation at which most lung units undergo RECOREX, while high PPLAT causes barotrauma. Arrow 2 represents moderate VT/high PEEP ventilation. Only units with high closing pressure undergo RECOREX, but barotrauma remains important. Arrow 3 illustrates moderate VT/low PEEPTOT. Low PPLAT prevents barotrauma, but many lung units collapsing during expiration undergo RECOREX. Arrow 4 illustrates very low VT/high PEEP ventilation. Low PPLAT prevents barotrauma. Only those units with particularly high closing pressures represented by the pink area will close. If these units also have high opening pressures (pink area), they will remain collapsed throughout the breath so as to fully prevent RECOREX.
For each lower level of PEEP, families of inspiratory P el/V curves showed, consistently in each animal, a loss of volume, confirming wide distributions of closing pressure in ARDS below a PEEP of 15 cm H2O. During insufflations, the P el/V curves started in each animal to approach one another at pressures above ∼20 cm H2O, but the volume losses were incompletely regained during insufflation up to PEEP of 40–50 cm H2O. The findings indicate a wide range of opening pressures that are higher than closing pressures. The estimated opening and closing pressures agreed with previous observations.17 40 41 In this respect, the ARDS pig model is similar to that seen in ARDS in patients.
An RR as high as 60 bpm overlaps with high-frequency ventilation. Simulation was based upon classical models of mechanics and CO2 exchange plus the concept of mean distribution time.28, 29, 30, 31 The settings defined by computer simulation led to predefined goals, implying that classical physiology is valid in ARDS up to at least 60 bpm.21 In spite of short expirations at high RR and high I:E ratio, PEEPTOT was close to the set PEEP.
In GOAL, PaO2 became normal as early as 4 h. The P el/V curves showed a continuous improvement until 24 h (Fig. 2). In the LP and HP groups, inspiratory P el/V curves recorded at a PEEP of 15 cm H2O, and expiratory P el/V curves showed a clear continuous deterioration. These types of curves starting when the lungs are reasonably well aerated reveal mainly elastic properties. In contrast, the inspiratory P el/V curves recorded from zero PEEP are influenced by continuous inspiratory re-opening.26 42 43 In ARDS, they are not useful in monitoring elastic properties.
In the LP group, P PLAT was similar to that in GOAL, while V T was twice as high. The non-favourable evolution in LP is therefore explained by volutrauma rather than barotrauma. Much lower PEEP in LP than in GOAL favours RECOREX, leading to lung injury. Results in HP and LP were, in general, similar. The histology suggested more pronounced changes in HP in spite of much higher V T and P PLAT. Similar findings in LP and HP suggest that in both the groups, injurious effects of ventilation were high enough to maintain a vicious circle in which shear forces caused by RECOREX led to lung damage, leakage of plasma proteins, and increasing surfactant inactivation, thereby accentuating lung collapse and VILI.22
Like in ARDSnet, P PLAT in LP should not exceed 30 cm H2O and the pH should be about 7.35.9 In LP, V T was much higher compared with the 6 ml kg−1 body weight recommended by ARDSnet. A limitation of our study is that we did not achieve titration to a low enough V T to mitigate lung injury. The GOAL strategy is to find a truly minimal V T. When, from the end-inspiratory situation, a minimal V T is expired, the end-expiratory elastic recoil pressure, that is, PEEPTOT, will be the highest one compatible with the selected P PLAT and minimal V T. This will keep the lung open, so as to minimize the required F I O2 and reduce oxytrauma. In this study, even in the GOAL group, PEEPI was quite low in spite of the short expirations at a high I:E ratio and a high RR. If PEEPI would be important under other circumstances, the set PEEP should be reduced to balance this effect.
In this study, an optimal technique for airway dead space reduction was applied, that is, ASPIDS. An advanced technique was used for the characterization of respiratory mechanics and of dead space in order to allow identification of a ventilator setting adapted to the prevalent physiology and the goals, namely minimal V T, isocapnia, and optimal lung protection.
Although some techniques used for the GOAL strategy are not generally available, the basic principle to use the lowest feasible V T at high but safe P PLAT to keep the lung open may be applied in clinical studies. Dead space should be minimized by not using humidifying filters or connectors with unnecessarily high volumes. Reduced dead space paves the way for higher than conventional RR. At a higher RR, V T may be lowered much more than the primary dead space reduction itself. At high RR, an I:E ratio of up to 1:1 should be applied in order to improve alveolar equilibration of CO2 by diffusion. Lessons learned may be applied in different clinical settings. It is possible that improved mechanical ventilation may lessen the need for extreme rescue therapies such as extra extracorporeal gas exchange in viral pneumonia. More commonly, improved mechanical ventilation during anaesthesia may mitigate perioperative lung injury.44
Conclusions
A valid strategy for LPV in ARDS is to define the immediate physiological goals, which may be adequate pH or PaCO2, safe P PLAT, and minimal V T. With these, high PEEP will keep the lung open and reduce the required F I O2. Dead space reduction is fundamental for higher than ordinary RR and for enhanced V T reduction. Classical physiology is applicable at rates overlapping high-frequency ventilation. Computer simulation may guide ventilator settings so as to optimize goal-orientated ventilation. Inspiratory elastic pressure–volume curves recorded from high PEEP or even better, expiratory curves, allow the monitoring of improvements and deteriorations in ARDS.
Declaration of interest
None declared.
Funding
The study was funded by the Swedish Heart Lung Foundation, the Medical Faculty of Lund University, and Region Skåne.
Acknowledgements
We thank Ingegerd Göransson, Elisabet Åström, and Berit Olsson for their extensive assistance throughout the study, in particular their skilful experimental work and data acquisition; Lisbet Niklason, Björn Drefeldt, and Gerth-Inge Jönsson for help with software and hardware; Sten Blomquist and Edgar Grins for valuable assistance during animal preparation; Peter Dahm and Lena Åkesson for help with equipment; and Anders Larsson and Thomas Dyhr for valuable advice.
References
- 1.Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am Rev Respir Dis. 1974;110:556–565. doi: 10.1164/arrd.1974.110.5.556. [DOI] [PubMed] [Google Scholar]
- 2.Parker JC, Townsley MI, Rippe B, Taylor AE, Thigpen J. Increased microvascular permeability in dog lungs due to high peak airway pressures. J Appl Physiol. 1984;57:1809–1816. doi: 10.1152/jappl.1984.57.6.1809. [DOI] [PubMed] [Google Scholar]
- 3.Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis. 1988;137:1159–1164. doi: 10.1164/ajrccm/137.5.1159. [DOI] [PubMed] [Google Scholar]
- 4.Corbridge TC, Wood LD, Crawford GP, Chudoba MJ, Yanos J, Sznajder JI. Adverse effects of large tidal volume and low PEEP in canine acid aspiration. Am Rev Respir Dis. 1990;142:311–315. doi: 10.1164/ajrccm/142.2.311. [DOI] [PubMed] [Google Scholar]
- 5.Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 6.Brochard L, Roudot-Thoraval F, Roupie E, et al. Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. The Multicenter Trail Group on Tidal Volume reduction in ARDS. Am J Respir Crit Care Med. 1998;158:1831–1838. doi: 10.1164/ajrccm.158.6.9801044. [DOI] [PubMed] [Google Scholar]
- 7.Stewart TE, Meade MO, Cook DJ, et al. Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome. Pressure- and Volume-Limited Ventilation Strategy Group. N Engl J Med. 1998;338:355–361. doi: 10.1056/NEJM199802053380603. [DOI] [PubMed] [Google Scholar]
- 8.Brower RG, Shanholtz CB, Fessler HE, et al. Prospective, randomized, controlled clinical trial comparing traditional versus reduced tidal volume ventilation in acute respiratory distress syndrome patients. Crit Care Med. 1999;27:1492–1498. doi: 10.1097/00003246-199908000-00015. [DOI] [PubMed] [Google Scholar]
- 9.ARDSnetwork Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 10.Nuckton TJ, Alonso JA, Kallet RH, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346:1281–1286. doi: 10.1056/NEJMoa012835. [DOI] [PubMed] [Google Scholar]
- 11.Beydon L, Uttman L, Rawal R, Jonson B. Effects of positive end-expiratory pressure on dead space and its partitions in acute lung injury. Intensive Care Med. 2002;28:1239–1245. doi: 10.1007/s00134-002-1419-y. [DOI] [PubMed] [Google Scholar]
- 12.Raurich JM, Vilar M, Colomar A, et al. Prognostic value of the pulmonary dead-space fraction during the early and intermediate phases of acute respiratory distress syndrome. Respir Care. 2010;55:282–287. [PubMed] [Google Scholar]
- 13.Lucangelo U, Bernabe F, Vatua S, et al. Prognostic value of different dead space indices in mechanically ventilated patients with acute lung injury and ARDS. Chest. 2008;133:62–71. doi: 10.1378/chest.07-0935. [DOI] [PubMed] [Google Scholar]
- 14.Kallet RH, Alonso JA, Pittet JF, Matthay MA. Prognostic value of the pulmonary dead-space fraction during the first 6 days of acute respiratory distress syndrome. Respir Care. 2004;49:1008–1014. [PubMed] [Google Scholar]
- 15.Cepkova M, Kapur V, Ren X, et al. Pulmonary dead space fraction and pulmonary artery systolic pressure as early predictors of clinical outcome in acute lung injury. Chest. 2007;132:836–842. doi: 10.1378/chest.07-0409. [DOI] [PubMed] [Google Scholar]
- 16.De Robertis E, Sigurdsson SE, Drefeldt B, Jonson B. Aspiration of airway dead space. A new method to enhance CO2 elimination. Am J Respir Crit Care Med. 1999;159:728–732. doi: 10.1164/ajrccm.159.3.9712140. [DOI] [PubMed] [Google Scholar]
- 17.De Robertis E, Servillo G, Tufano R, Jonson B. Aspiration of dead space allows isocapnic low tidal volume ventilation in acute lung injury. Intensive Care Med. 2001;27:1496–1503. doi: 10.1007/s001340101046. [DOI] [PubMed] [Google Scholar]
- 18.De Robertis E, Uttman L, Jonson B. Re-inspiration of CO2 from ventilator circuit: effects of circuit flushing and aspiration of dead space up to high respiratory rate. Crit Care. 2010;14:R73. doi: 10.1186/cc8986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uttman L, Jonson B. Computer-aided ventilator resetting is feasible on the basis of a physiological profile. Acta Anaesthesiol Scand. 2002;46:289–296. doi: 10.1034/j.1399-6576.2002.460311.x. [DOI] [PubMed] [Google Scholar]
- 20.Uttman L, Beydon L, Jonson B. Effects of positive end-expiratory pressure increments can be predicted by computer simulation based on a physiological profile in acute respiratory failure. Intensive Care Med. 2003;29:226–232. doi: 10.1007/s00134-002-1620-z. [DOI] [PubMed] [Google Scholar]
- 21.Uttman L, Ögren H, Niklason L, Drefeldt B, Jonson B. Computer simulation allows goal-oriented mechanical ventilation in acute respiratory distress syndrome. Crit Care. 2007;11:R36. doi: 10.1186/cc5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taskar V, John J, Evander E, Robertson B, Jonson B. Surfactant dysfunction makes lungs vulnerable to repetitive collapse and reexpansion. Am J Respir Crit Care Med. 1997;155:313–320. doi: 10.1164/ajrccm.155.1.9001330. [DOI] [PubMed] [Google Scholar]
- 23.Bitzen U, Enoksson J, Uttman L, Niklason L, Johansson L, Jonson B. Multiple pressure–volume loops recorded with sinusoidal low flow in a porcine acute respiratory distress syndrome model. Clin Physiol Funct Imaging. 2006;26:113–119. doi: 10.1111/j.1475-097X.2006.00660.x. [DOI] [PubMed] [Google Scholar]
- 24.Svantesson C, Drefeldt B, Sigurdsson S, Larsson A, Brochard L, Jonson B. A single computer-controlled mechanical insufflation allows determination of the pressure–volume relationship of the respiratory system. J Clin Monit Comput. 1999;15:9–16. doi: 10.1023/a:1009916905078. [DOI] [PubMed] [Google Scholar]
- 25.Bitzen U, Drefeldt B, Niklason L, Jonson B. Dynamic elastic pressure–volume loops in healthy pigs recorded with inspiratory and expiratory sinusoidal flow modulation. Relationship to static pressure–volume loops. Intensive Care Med. 2004;30:481–488. doi: 10.1007/s00134-003-2156-6. [DOI] [PubMed] [Google Scholar]
- 26.Jonson B, Richard JC, Straus C, Mancebo J, Lemaire F, Brochard L. Pressure–volume curves and compliance in acute lung injury: evidence of recruitment above the lower inflection point. Am J Respir Crit Care Med. 1999;159:1172–1178. doi: 10.1164/ajrccm.159.4.9801088. [DOI] [PubMed] [Google Scholar]
- 27.Laffey JG, O’Croinin D, McLoughlin P, Kavanagh BP. Permissive hypercapnia—role in protective lung ventilatory strategies. Intensive Care Med. 2004;30:347–356. doi: 10.1007/s00134-003-2051-1. [DOI] [PubMed] [Google Scholar]
- 28.Uttman L, Jonson B. A prolonged postinspiratory pause enhances CO2 elimination by reducing airway dead space. Clin Physiol Funct Imaging. 2003;23:252–256. doi: 10.1046/j.1475-097X.2003.00498.x. [DOI] [PubMed] [Google Scholar]
- 29.Aboab J, Niklason L, Uttman L, Kouatchet A, Brochard L, Jonson B. CO2 elimination at varying inspiratory pause in acute lung injury. Clin Physiol Funct Imaging. 2007;27:2–6. doi: 10.1111/j.1475-097X.2007.00699.x. [DOI] [PubMed] [Google Scholar]
- 30.Åström E, Uttman L, Niklason L, Aboab J, Brochard L, Jonson B. Pattern of inspiratory gas delivery affects CO2 elimination in health and after acute lung injury. Intensive Care Med. 2008;34:377–384. doi: 10.1007/s00134-007-0840-7. [DOI] [PubMed] [Google Scholar]
- 31.Devaquet J, Jonson B, Niklason L, et al. Effects of inspiratory pause on CO2 elimination and arterial PCO2 in acute lung injury. J Appl Physiol. 2008;105:1944–1949. doi: 10.1152/japplphysiol.90682.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruhn A, Bugedo D, Riquelme F, et al. Tidal volume is a major determinant of cyclic recruitment–derecruitment in acute respiratory distress syndrome. Minerva Anestesiol. 2011;77:418–426. [PubMed] [Google Scholar]
- 33.Spieth PM, Güldner A, Carvalho AR, et al. Open lung approach vs acute respiratory distress syndrome network ventilation in experimental acute lung injury. Br J Anaesth. 2011;107:388–397. doi: 10.1093/bja/aer144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richard JC, Brochard L, Vandelet P, et al. Respective effects of end-expiratory and end-inspiratory pressures on alveolar recruitment in acute lung injury. Crit Care Med. 2003;31:89–92. doi: 10.1097/00003246-200301000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Richard JC, Maggiore SM, Jonson B, Mancebo J, Lemaire F, Brochard L. Influence of tidal volume on alveolar recruitment. Respective role of PEEP and a recruitment maneuver. Am J Respir Crit Care Med. 2001;163:1609–1613. doi: 10.1164/ajrccm.163.7.2004215. [DOI] [PubMed] [Google Scholar]
- 36.Kolobow T, Gattinoni L, Tomlinson T, Pierce JE. An alternative to breathing. J Thorac Cardiovasc Surg. 1978;75:261–266. [PubMed] [Google Scholar]
- 37.Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol. 1970;28:596–608. doi: 10.1152/jappl.1970.28.5.596. [DOI] [PubMed] [Google Scholar]
- 38.Jonson B. In: Applied Physiology in Clinical Respiratory Care. Prakash O, editor. Martinus Nihoff Publishers; The Hague: 1982. Positive airway pressure: some physical and biological effects; pp. 125–139. [Google Scholar]
- 39.Ranieri VM, Zhang H, Mascia L, et al. Pressure–time curve predicts minimally injurious ventilatory strategy in an isolated rat lung model. Anesthesiology. 2000;93:1320–1328. doi: 10.1097/00000542-200011000-00027. [DOI] [PubMed] [Google Scholar]
- 40.Crotti S, Mascheroni D, Caironi P, et al. Recruitment and derecruitment during acute respiratory failure: a clinical study. Am J Respir Crit Care Med. 2001;164:131–140. doi: 10.1164/ajrccm.164.1.2007011. [DOI] [PubMed] [Google Scholar]
- 41.Maggiore SM, Jonson B, Richard JC, Jaber S, Lemaire F, Brochard L. Alveolar derecruitment at decremental positive end-expiratory pressure levels in acute lung injury: comparison with the lower inflection point, oxygenation, and compliance. Am J Respir Crit Care Med. 2001;164:795–801. doi: 10.1164/ajrccm.164.5.2006071. [DOI] [PubMed] [Google Scholar]
- 42.Hickling KG. The pressure–volume curve is greatly modified by recruitment. A mathematical model of ARDS lungs. Am J Respir Crit Care Med. 1998;158:194–202. doi: 10.1164/ajrccm.158.1.9708049. [DOI] [PubMed] [Google Scholar]
- 43.Jonson B, Svantesson C. Elastic pressure–volume curves: what information do they convey? Thorax. 1999;54:82–87. doi: 10.1136/thx.54.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kilpatrick B, Slinger P. Lung protective strategies in anaesthesia. Br J Anaesth. 2010;105(Suppl. 1):i108–i116. doi: 10.1093/bja/aeq299. [DOI] [PMC free article] [PubMed] [Google Scholar]